Abstract
Background
The need for documentation in cartilage defects is as obvious as in other medical specialties. Cartilage defects can cause significant pain, and lead to reduced quality of life and loss of function of the affected joint. The risk of developing osteoarthritis is high. Therefore, the socioeconomic burden of cartilage defects should not be underestimated.
Objective
The objective of our study was to implement and maintain a registry of all patients undergoing surgical treatment of cartilage defects.
Methods
We designed this multicenter registry for adults whose cartilage defects of a knee, ankle, or hip joint are treated surgically. The registry consists of two parts: one for the physician and one for the patient. Data for both parts will be gathered at baseline and at 6-, 12-, 24-, 36-, 60-, and 120-month follow-ups.
Results
To date, a wide range of German, Swiss, and Austrian trial sites are taking part in the German Cartilage Registry, soon to be followed by further sites. More than 2124 (as of January 31, 2016) cases are already documented and the first publications have been released.
Conclusions
The German Cartilage Registry addresses fundamental issues regarding the current medical care situation of patients with cartilage defects of knee, ankle, and hip joints. In addition, the registry will help to identify various procedure-specific complications, along with putative advantages and disadvantages of different chondrocyte products. It provides an expanding large-scale, unselected, standardized database for cost and care research for further retrospective studies.
Trial Registration
German Clinical Trials Register: DRKS00005617; https://drks-neu.uniklinik-freiburg.de/ drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00005617 (Archived by WebCite at http://www.webcitation.org/6hbFqSws0)
Keywords: ankle joint, cartilage defect, chondral defect, hip joint, knee joint, patient registry
Introduction
Isolated cartilage defects are common orthopedic disorders in middle-aged patients that are typically associated with pain, reduced quality of life, and loss of function of the affected joint [1,2]. In fact, chondral defects have been described in 34% to 62% of knee arthroscopies [3-6]. They tend to progress to osteoarthritis, as spontaneous healing is rare, and can therefore be considered a potential risk factor or precondition for joint degeneration [7].
As of 2008, nearly 27 million US adults aged 25 and older have clinical osteoarthritis [8]. Osteoarthritis is the fourth most frequent cause of hospital admission in the United States and the leading cause of joint replacement surgery [9]. In 2009, in the United States 905,000 knee or hip replacements were conducted, resulting in treatment costs of US $42.3 billion [9]. In sum, osteoarthritis is one of the major causes of global disability and is a socioeconomic burden that will most likely soon become a substantial problem for global health systems [10,11]. Therefore, it is very important to cure cartilage defects in the first place.
Concerning cartilage repair techniques, several therapies have been established, which can be divided into two major groups: bone marrow stimulation techniques and transplantation techniques [12,13]. Despite the fact that the number of randomized controlled trials (RCTs) on cartilage repair has increased significantly over the years, RCTs aim only at direct comparison between two surgical procedures, such as the comparison between arthroscopic microfracturing and autologous chondrocyte implantation [14-18]. In addition, only a highly selected patient population is considered in these trials. Real-life clinical data are hardly ever considered.
The group of Engen et al [11] published a study on this issue and came to the final conclusion that only approximately 4.5% of patients with cartilage defects are represented by RCTs. Jakobsen et al [19] stated that promising results of cartilage repair studies have to be interpreted carefully due to their low methodological quality. Against this background and based on the fact that some scientific questions, such as a detailed analysis of surgical complication, and the influence of sex, overweight, and other factors, cannot be investigated in RCTs, many experts think that RCTs should be supplemented by well-designed observational studies [20-24]. Thus, we have initiated this multicenter patient registry to 1) systematically describe the current medical care situation of patients undergoing surgical treatment of their cartilage defect, 2) compare competing cartilage therapies regarding their outcomes, procedure-specific complication rates, and symptom relief by collecting real-life clinical data, 3) identify putative advantages and disadvantages of various chondrocyte products in daily clinical care, 4) develop new hypotheses on cartilage repair techniques as a basis for future RCTs and to test outcomes of former RCTs in a larger and more representative population, and 5) evaluate the efficiency and safety of surgically treated cartilage defects of knee, hip, and ankle joints, independent of strict patient characteristics or surgical procedure.
Here we describe the study design and layout of the German Cartilage Registry, which is to our knowledge the first patient registry for this indication worldwide.
Methods
Study Design
The German Cartilage Registry is an observational and international multicenter registry that was initiated by the Arbeitsgemeinschaft Klinische Geweberegeneration (Working Group Clinical Tissue Regeneration) of the German Society for Orthopaedics and Trauma (DGOU) in 2013. It is a purely scientifically motivated project and as a consequence independent of the interests of industrial partners. The study is conducted in accordance with the Declaration of Helsinki and registered at germanctr.de (DRKS00005617).
The registry investigates the efficiency and safety of surgical treatment of cartilage defects in patients under real-life conditions. In October 2013, the assessment started with the documentation of cartilage defects of the knee. The modules for cartilage defects of the ankle and hip joint were implemented 1 year later.
Ethics Approval
Depending on individual state’s law, investigators consult the responsible ethics committee before starting the study at their site. At their request, investigators are supported by the Clinical Trials Unit (CTU; Medical Center - University of Freiburg, Freiburg, Germany) in preparing the essential documents for submission (first approval in Freiburg on March 13, 2013, internal number 105/13). So far, 33 ethics committees have welcomed the implementation of the German Cartilage Registry in their jurisdiction.
After consulting the ethics committee, investigators are allowed to take part in the German Cartilage Registry.
Study Population
All patients aged ≥18 years who meet the following criteria are eligible to take part in the German Cartilage Registry: 1) they have had surgical treatment of cartilage defects of a knee, ankle, or hip joint at a participating site, 2) they have given written informed consent, 3) they have a personal email address.
Procedure and Data Collection
Only after the patient has signed the written informed consent the investigator is allowed to register the patient in the database. We recommend that this registration procedure takes place immediately after the surgery is completed. Thus, the investigator can type in the following mandatory baseline data at the same time: initially, the date of surgery, the patient’s identification, and the patient’s email address to generate a new case; and subsequently, basic information concerning patient history and treatment technique. In the course of 6-, 12-, 24-, 36-, 60-, and 120-month follow-ups, the physician can document further optional data.
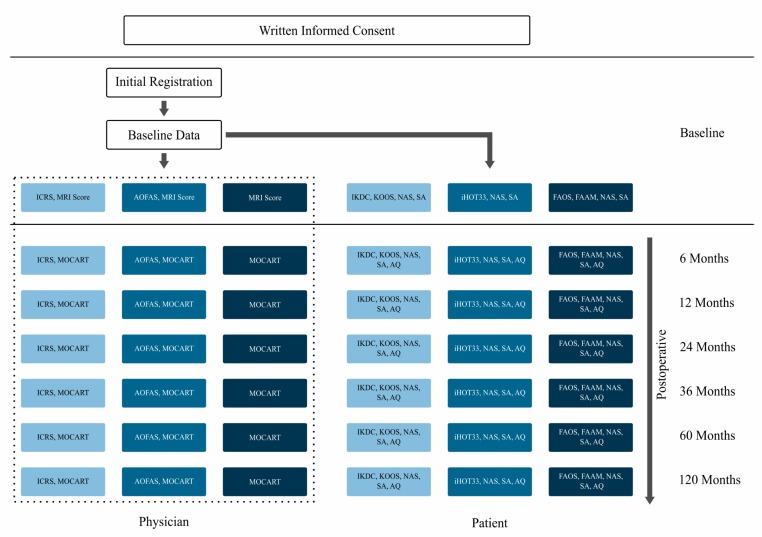
The day following initial data entry by the physician, the patient automatically receives an email inviting him or her to fill in a questionnaire for baseline data. Additionally, the patient receives an invitational email at 6-, 12-, 24-, 36-, 60-, and 120-month follow-ups to complete the questionnaire. If the patient does not complete the form within a given time limit, an email reminder is sent automatically. If the patient still does not fill in the questionnaire, the trial site seeks personal contact. Figure 1 shows the flow chart of the German Cartilage Registry in detail, naming all deployed questionnaires. Light blue represents all questionnaires that are used in the knee part, medium blue shows the questionnaires deployed in the hip part, and dark blue displays all questionnaires used in the ankle part of the German Cartilage Registry. Completion of the questionnaires shown in the dotted box is optional. Answering all other questionnaires is mandatory.
Figure 1.
Flow chart of the German Cartilage Registry and questionnaires deployed to physicians and patients. AOFAS: American Orthopaedic Foot & Ankle Society; AQ: additional questions; FAAM: Foot and Ankle Ability Measure; FAOS: Foot and Ankle Outcome Score; ICRS: International Cartilage Repair Society; iHOT33: International Hip Outcome Tool-33; IKDC: International Knee Documentation Committee; KOOS: Knee injury and Osteoarthritis Outcome Score; MOCART: magnetic resonance observation of cartilage repair tissue; MRI: magnetic resonance imaging; NAS: numeric analog scale for pain description; SA: sports activities.
Instruments
The German Cartilage Registry consists of two parts: one for the physician and one for the patient. At baseline, the physician section includes mandatory information on patient-specific characteristics (age, sex, smoking behavior, weight and height, as well as varus or valgus malalignment), the preliminary operation(s), all surgical procedures performed on the injured joint (including defect-specific characteristics), and therapy characteristics.
Furthermore, the physician can fill in a premagnetic resonance imaging score (similar to the magnetic resonance observation of cartilage repair tissue, or MOCART, score), as well as joint-specific scores, such as the International Cartilage Repair Society (ICRS) score (equal to International Knee Documentation Committee, IKDC, objective score) for the knee joint and American Orthopaedic Foot & Ankle Society (AOFAS) for the ankle joint [25,26]. Investigator’s data entry at 6-, 12-, 24-, 36-, 60-, and 120-month follow-ups is optional. But there is the opportunity to document joint-specific scores, such as MOCART [27,28], AOFAS, and ICRS (see Figure 1).
At all times, the patient’s questionnaire consists of a numeric analog scale for pain description, a few questions about sports activities, and joint-specific, validated, and standardized instruments, such as IKDC subjective score and Knee injury and Osteoarthritis Outcome Score (for the knee part) [29-33], International Hip Outcome Tool-33 (hip part) [34,35], Foot and Ankle Outcome Score, and Foot and Ankle Ability Measure (ankle part) [36-38]. At 6-, 12-, 24-, 36-, 60-, and 120-month follow-ups, 3 additional questions ask about the patient’s satisfaction with the surgical treatment at baseline and further surgeries (see Figure 1).
Data Entry
The Web-based remote data entry system called RDE-LIGHT was developed by the CTU of the Medical Center - University of Freiburg as an electronic data entry interface and data management system for clinical studies and other projects in clinical research. Data are collected paperless and directly on site via an Internet browser. The RDE-LIGHT system displays the questionnaires in a structured view in the main window, indicating the status of the questionnaires as traffic light colors. Questionnaires are based on HTML. RDE-LIGHT is available in various languages and validated according to GAMP 5 (ISPE, Tampa, FL, USA). Furthermore, it fulfills all requirements of good clinical practice.
The RDE-LIGHT system applies established security standards such as cryptographic security protocols (secure socket layer/transport layer security), user authentication protocols, and authorization concepts. For example, investigators can access data only of their own site, while the system denies unauthorized access. Data transfer to the database is encrypted and secured. The server is located in the Medical Computer Department of the Medical Center - University of Freiburg, with strict access control. Hence, common concepts of data protection are implemented. Changes to the database and the underlying system are logged, saved, and archived regularly to ensure end-to-end tracking.
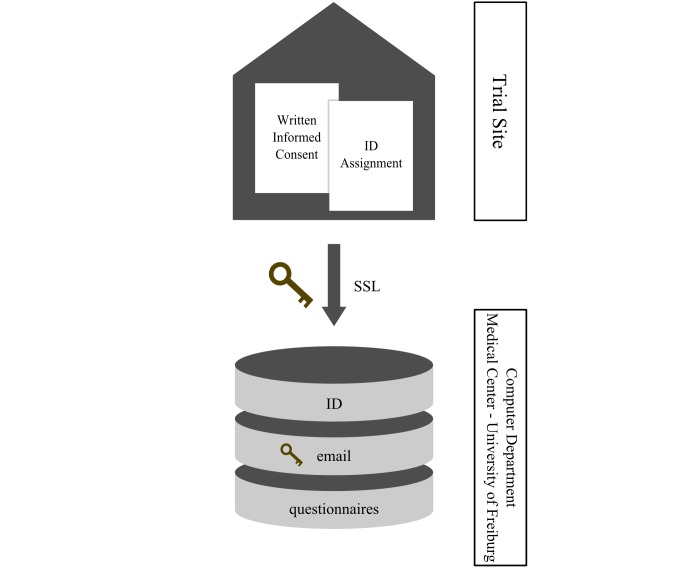
When working with personal data, the CTU encourages involved researchers to use pseudonyms to prohibit the identification of their patients. The patients’ names and contact details (email address) will be kept confidential and are available to the research team only for contact purposes. Any data presented publically will ensure participants' anonymity. In order to be able to automatically send emails to the patients when new questionnaires have to be completed, it is necessary to access the patients’ email addresses in the system’s database. As the email address is part of the patients’ personal information, it is stored in an encrypted way in the database using password-based encryption with MD5 and Triple Data Encryption Standard. Figure 2 illustrates the data storage location and clearly shows that the email addresses are separated from the physicians’ and patients’ questionnaires, as well as the patients’ identification, in a well-protected way.
Figure 2.
Data storage location for the German Cartilage Registry. ID: identification; SSL: secure socket layer.
Statistical Analysis
After approval from the Arbeitsgemeinschaft Klinische Geweberegeneration (Working Group Clinical Tissue Regeneration), every participating physician is allowed to publish the full set of anonymized data available at that time. Data will always be prepared by an experienced biostatistician of the CTU. We are planning several descriptive analyses concerning the structure and composition of the registry. Analyses will be done by first specifying several different research questions (eg, efficacy of certain therapies in real-life datasets) and prespecifying inclusion criteria for these specific questions. Independently, every investigator is allowed to download his or her own data set for anonymized statistical evaluations. However, it is important to keep in mind that registry data need special care in the analysis, as populations are unbalanced and several sources of bias can be present, such as in confounding variables. Therefore, the results must be interpreted very carefully.
Quality Assurance
The registry is being implemented and maintained by the CTU of the Medical Center- University of Freiburg. The CTU is member of the network of coordinating centers for clinical trials in Germany [39] and offers profound expertise in all areas of clinical trial planning, conduct, and analysis, in both universities and industry. The CTU is involved in about 250 trials a year.
Skilled and experienced staff of the CTU offer email and telephone support for any emerging problems. Additionally, documents, user manuals, and Web-based instructions via video may help to assist physicians and other personnel at the site. A query management system helps to identify patients and physicians who did not fill in the mandatory questionnaires.
Results
At time of data collection for this paper, 100 German trial sites and 5 trial sites in Austria and Switzerland are taking part in the German Cartilage Registry. Among these are university medical centers and private hospitals, doctors’ surgeries, and outpatient surgical centers. As of January 31, 2016, a total of 2124 patients have been registered (see Figure 3) and the first clinical results have been published [40-42].
Figure 3.
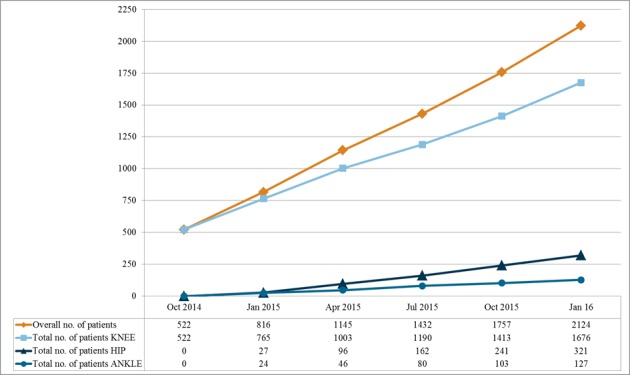
Total number of patients in the German Cartilage Registry as of January 31, 2016.
Discussion
The primary aim of this multicenter registry is to assess the efficiency and safety of surgically treated cartilage defects of knee, hip, and ankle joints and to subsequently provide future patients with their best treatment option. Therefore, we are collecting as much valuable information as possible on a preferably heterogeneous group of people who have been treated in day-to-day clinical practice.
In the following section we highlight the strengths of the German Cartilage Registry and discuss the known limitations to this project.
Complementing Data from RCTs
In recent years, there has been a focus on RCTs in cartilage repair, since they still are the highest level of clinical research [43]. Nevertheless, due to strict inclusion and exclusion criteria, study populations in many RCTs do not completely represent clinical routine and the entire population of patients with cartilage defects. In fact, the number of patients who are eligible for RCTs in the field of cartilage defects is estimated to be only around 4.5% [11]. Hence, the vast majority of patients are not represented by RCTs, since they do not qualify for different reasons, such as an increased body mass index or concomitant pathologies. In addition, important patient-related factors such as smoking and being overweight have been proven to significantly influence the outcome of cartilage repair techniques, but they cannot be analyzed by RCTs for methodological reasons [44-46]. This also applies to pathology-related parameters such as the influence of defect size or detailed defect location [46]. Furthermore, concomitant pathologies are considered to be exclusion criteria in most RCTs but are frequently present in cartilage repair patients. All of these factors underline the necessity of not exclusively relying on findings of RCTs, but to complement the results of RCTs with data from well-designed observational studies (eg, registries), and, therefore, to reassess findings of RCTs in daily clinical use.
Selection Bias
Due to organizational or other limitations, we cannot guarantee that every single patient with a surgically treated chondral defect of a knee, hip, or ankle joint will be documented in the system. For instance, for small- or medium-sized medical health providers, the additional workload for data input may seem too high. But we tried to keep the administrative effort as small as possible by allowing the physicians to register a patient immediately after surgery has been completed, although the first patient questionnaire refers to the complaints before surgery. In this way, the physician can record all mandatory data at once. Furthermore, we tried to include as many patient characteristics as possible that are thought to affect outcomes.
Data Quality
No onsite clinical monitoring is provided to assure the quality of entered data, and the respective sites are solely responsible for data input. Nevertheless, quality parameters need to be established and carefully applied. For instance, we have to observe the follow-up rate, which is crucial in any type of clinical research. Therefore, a validation study of recorded data will have to follow.
Expansion of the Registry
Additional sites in German-speaking countries, namely Austria and Switzerland, have already been affiliated and others will be approached to join the registry.
Further information is available on the KnorpelRegister website [47].
Acknowledgments
We thank all participating sites and the Arbeitsgemeinschaft Klinische Geweberegeneration (Working Group Clinical Tissue Regeneration) of the German Society for Orthopaedics and Trauma who have contributed to the successful implementation of the German Cartilage Registry. Special thanks to Deutsche Arthrose-Hilfe e.V. and Stiftung Oskar-Helene-Heim, who supported this registry financially.
The article processing charge was funded by the German Research Foundation (DFG) and the Albert-Ludwigs University Freiburg in the funding program Open Access Publishing.
Abbreviations
- AOFAS
American Orthopaedic Foot & Ankle Society
- CTU
Clinical Trials Unit
- DGOU
German Society for Orthopaedics and Trauma
- ICRS
International Cartilage Repair Society
- IKDC
International Knee Documentation Committee
- MOCART
magnetic resonance observation of cartilage repair tissue
- RCT
randomized controlled trial
Footnotes
Conflicts of Interest: None declared.
References
- 1.Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, Arøen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010 Feb;38(2):231–7. doi: 10.1177/0363546509352157.0363546509352157 [DOI] [PubMed] [Google Scholar]
- 2.Solheim E, Krokeide AM, Melteig P, Larsen A, Strand T, Brittberg M. Symptoms and function in patients with articular cartilage lesions in 1,000 knee arthroscopies. Knee Surg Sports Traumatol Arthrosc. 2014 Dec 13;:1–7. doi: 10.1007/s00167-014-3472-9. [DOI] [PubMed] [Google Scholar]
- 3.Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211–5. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]
- 4.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997 Aug;13(4):456–60. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 5.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002 Sep;18(7):730–4. doi: 10.1053/jars.2002.32839.S0749806302000257 [DOI] [PubMed] [Google Scholar]
- 6.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007 Jun;14(3):177–82. doi: 10.1016/j.knee.2007.02.001.S0968-0160(07)00027-0 [DOI] [PubMed] [Google Scholar]
- 7.Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005 Jul;52(7):2033–9. doi: 10.1002/art.21148. doi: 10.1002/art.21148. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F, National Arthritis Data Workgroup Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008 Jan;58(1):26–35. doi: 10.1002/art.23176. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012 Mar;112(3 Suppl 1):S13–9. doi: 10.1097/01.NAJ.0000412646.80054.21.00000446-201203001-00003 [DOI] [PubMed] [Google Scholar]
- 10.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, Bridgett L, Williams S, Guillemin F, Hill CL, Laslett LL, Jones G, Cicuttini F, Osborne R, Vos T, Buchbinder R, Woolf A, March L. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014 Jul;73(7):1323–30. doi: 10.1136/annrheumdis-2013-204763.annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 11.Engen CN, Engebretsen L, Årøen A. Knee cartilage defect patients enrolled in randomized controlled trials are not representative of patients in orthopedic practice. Cartilage. 2010 Oct;1(4):312–9. doi: 10.1177/1947603510373917. http://europepmc.org/abstract/MED/26069562 .10.1177_1947603510373917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niemeyer P, Kreuz PC, Steinwachs M, Südkamp NP. [Operative treatment of cartilage lesions in the knee joint] Sportverletz Sportschaden. 2007 Mar;21(1):41–50. doi: 10.1055/s-2007-963030. [DOI] [PubMed] [Google Scholar]
- 13.Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009 Jul;91(7):1778–90.91/7/1778 [PubMed] [Google Scholar]
- 14.Basad E, Ishaque B, Bachmann G, Stürz H, Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010 Apr;18(4):519–27. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 15.Vanlauwe J, Saris DB, Victor J, Almqvist KF, Bellemans J, Luyten FP, TIG/ACT/01/2000&EXT Study Group Five-year outcome of characterized chondrocyte implantation versus microfracture for symptomatic cartilage defects of the knee: early treatment matters. Am J Sports Med. 2011 Dec;39(12):2566–74. doi: 10.1177/0363546511422220.0363546511422220 [DOI] [PubMed] [Google Scholar]
- 16.Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, Emans P, Podskubka A, Tsuchida A, Kili S, Levine D, Brittberg M. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014 Jun;42(6):1384–94. doi: 10.1177/0363546514528093.0363546514528093 [DOI] [PubMed] [Google Scholar]
- 17.Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP, TIG/ACT/01/2000&EXT Study Group Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009 Nov;37 Suppl 1:10S–19S. doi: 10.1177/0363546509350694.0363546509350694 [DOI] [PubMed] [Google Scholar]
- 18.Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, Vandekerckhove B, Almqvist KF, Claes T, Handelberg F, Lagae K, van der Bauwhede J, Vandenneucker H, Yang KG, Jelic M, Verdonk R, Veulemans N, Bellemans J, Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008 Feb;36(2):235–46. doi: 10.1177/0363546507311095.36/2/235 [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen RB, Engebretsen L, Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005 Oct;87(10):2232–9. doi: 10.2106/JBJS.D.02904.87/10/2232 [DOI] [PubMed] [Google Scholar]
- 20.Concato J, Lawler EV, Lew RA, Gaziano JM, Aslan M, Huang GD. Observational methods in comparative effectiveness research. Am J Med. 2010 Dec;123(12 Suppl 1):e16–23. doi: 10.1016/j.amjmed.2010.10.004.S0002-9343(10)00846-6 [DOI] [PubMed] [Google Scholar]
- 21.Ligthelm RJ, Borzì V, Gumprecht J, Kawamori R, Wenying Y, Valensi P. Importance of observational studies in clinical practice. Clin Ther. 2007 Jun;29(6 Pt 1):1284–92. doi: 10.1016/j.clinthera.2007.07.004.S0149-2918(07)00184-1 [DOI] [PubMed] [Google Scholar]
- 22.Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv. 2008 Jun;1(3):211–7. doi: 10.1016/j.jcin.2008.01.008. http://linkinghub.elsevier.com/retrieve/pii/S1936-8798(08)00170-2 .S1936-8798(08)00170-2 [DOI] [PubMed] [Google Scholar]
- 23.Silverman SL. From randomized controlled trials to observational studies. Am J Med. 2009 Feb;122(2):114–20. doi: 10.1016/j.amjmed.2008.09.030.S0002-9343(08)00952-2 [DOI] [PubMed] [Google Scholar]
- 24.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996 May 11;312(7040):1215–8. doi: 10.1136/bmj.312.7040.1215. http://europepmc.org/abstract/MED/8634569 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994 Jul;15(7):349–53. doi: 10.1177/107110079401500701. [DOI] [PubMed] [Google Scholar]
- 26.Kostuj T, Schaper K, Baums Mh, Lieske S. Eine Validierung des AOFAS-Ankle-Hindfoot-Scale für den deutschen Sprachraum. Fuß & Sprunggelenk. 2014 Jun;12(2):100–6. doi: 10.1016/j.fuspru.2014.02.002. [DOI] [Google Scholar]
- 27.Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, Trattnig S. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004 Dec;52(3):310–9. doi: 10.1016/j.ejrad.2004.03.014.S0720048X04000944 [DOI] [PubMed] [Google Scholar]
- 28.Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006 Jan;57(1):16–23. doi: 10.1016/j.ejrad.2005.08.007.S0720-048X(05)00288-3 [DOI] [PubMed] [Google Scholar]
- 29.Irrgang JJ, Anderson AF, Boland AL, Harner CD, Kurosaka M, Neyret P, Richmond JC, Shelborne KD. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600–13. doi: 10.1177/03635465010290051301. [DOI] [PubMed] [Google Scholar]
- 30.Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ, International Knee Documentation Committee The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med. 2006 Jan;34(1):128–35. doi: 10.1177/0363546505280214.0363546505280214 [DOI] [PubMed] [Google Scholar]
- 31.Higgins LD, Taylor MK, Park D, Ghodadra N, Marchant M, Pietrobon R, Cook C, International Knee Documentation Committee Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine. 2007 Dec;74(6):594–9. doi: 10.1016/j.jbspin.2007.01.036.S1297-319X(07)00226-6 [DOI] [PubMed] [Google Scholar]
- 32.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)--development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998 Aug;28(2):88–96. doi: 10.2519/jospt.1998.28.2.88. [DOI] [PubMed] [Google Scholar]
- 33.Kessler S, Lang S, Puhl W, Stöve J. [The Knee Injury and Osteoarthritis Outcome Score--a multifunctional questionnaire to measure outcome in knee arthroplasty] Z Orthop Ihre Grenzgeb. 2003;141(3):277–82. doi: 10.1055/s-2003-40083. [DOI] [PubMed] [Google Scholar]
- 34.Baumann F, Weber J, Zeman F, Müller M, Lahner M, Nerlich M, Fickert S. Validation of a German version of the International Hip Outcome Tool (G-iHOT33) according to the COSMIN checklist: how much improvement is clinically relevant? Arch Orthop Trauma Surg. 2016 Jan;136(1):83–91. doi: 10.1007/s00402-015-2336-1.10.1007/s00402-015-2336-1 [DOI] [PubMed] [Google Scholar]
- 35.Mohtadi NG, Griffin DR, Pedersen ME, Chan D, Safran MR, Parsons N, Sekiya JK, Kelly BT, Werle JR, Leunig M, McCarthy JC, Martin HD, Byrd JW, Philippon MJ, Martin RL, Guanche CA, Clohisy JC, Sampson TG, Kocher MS, Larson CM, Multicenter Arthroscopy of the Hip Outcomes Research Network The development and validation of a self-administered quality-of-life outcome measure for young, active patients with symptomatic hip disease: the International Hip Outcome Tool (iHOT-33) Arthroscopy. 2012 May;28(5):595–605. doi: 10.1016/j.arthro.2012.03.013.S0749-8063(12)00205-8 [DOI] [PubMed] [Google Scholar]
- 36.van Bergen CJ, Sierevelt IN, Hoogervorst P, Waizy H, van Dijk CN, Becher C. Translation and validation of the German version of the foot and ankle outcome score. Arch Orthop Trauma Surg. 2014 Jul;134(7):897–901. doi: 10.1007/s00402-014-1994-8. [DOI] [PubMed] [Google Scholar]
- 37.Nauck T, Lohrer H. Translation, cross-cultural adaption and validation of the German version of the Foot and Ankle Ability Measure for patients with chronic ankle instability. Br J Sports Med. 2011 Aug;45(10):785–90. doi: 10.1136/bjsm.2009.067637.bjsm.2009.067637 [DOI] [PubMed] [Google Scholar]
- 38.Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM) Foot Ankle Int. 2005 Nov;26(11):968–83. doi: 10.1177/107110070502601113.905748 [DOI] [PubMed] [Google Scholar]
- 39.KKS Netzwerk . Koordinierungszentren für Klinische Studien. Cologne, Germany: KKS Netzwerk; [2016-05-19]. http://www.kks-netzwerk.de/ [Google Scholar]
- 40.Niemeyer P, Schweigler K, Grotejohann B, Maurer J, Angele P, Aurich M, Becher C, Fay J, Feil R, Fickert S, Fritz J, Hoburg A, Kreuz P, Kolombe T, Laskowski J, Lützner J, Marlovits S, Müller PE, Niethammer T, Pietschmann M, Ruhnau K, Spahn G, Tischer T, Zinser W, Albrecht D. [The German Cartilage Registry (KnorpelRegister DGOU) for evaluation of surgical treatment for cartilage defects: experience after six months including first demographic data] Z Orthop Unfall. 2015 Feb;153(1):67–74. doi: 10.1055/s-0034-1383222. [DOI] [PubMed] [Google Scholar]
- 41.Spahn G, Fritz J, Albrecht D, Hofmann GO, Niemeyer P. Characteristics and associated factors of Klee cartilage lesions: preliminary baseline-data of more than 1000 patients from the German cartilage registry (KnorpelRegister DGOU) Arch Orthop Trauma Surg. 2016 Mar 21;:1–6. doi: 10.1007/s00402-016-2432-x.10.1007/s00402-016-2432-x [DOI] [PubMed] [Google Scholar]
- 42.Niemeyer P, Feucht MJ, Fritz J, Albrecht D, Spahn G, Angele P. Cartilage repair surgery for full-thickness defects of the knee in Germany: indications and epidemiological data from the German Cartilage Registry (KnorpelRegister DGOU) Arch Orthop Trauma Surg. 2016 Apr 9;:1–7. doi: 10.1007/s00402-016-2453-5.10.1007/s00402-016-2453-5 [DOI] [PubMed] [Google Scholar]
- 43.Obremskey WT, Pappas N, Attallah-Wasif E, Tornetta P 3rd, Bhandari M. Level of evidence in orthopaedic journals. J Bone Joint Surg Am. 2005 Dec;87(12):2632–8. doi: 10.2106/JBJS.E.00370.87/12/2632 [DOI] [PubMed] [Google Scholar]
- 44.Jaiswal PK, Bentley G, Carrington RW, Skinner JA, Briggs TW. The adverse effect of elevated body mass index on outcome after autologous chondrocyte implantation. J Bone Joint Surg Br. 2012 Oct;94(10):1377–81. doi: 10.1302/0301-620X.94B10.29388.94-B/10/1377 [DOI] [PubMed] [Google Scholar]
- 45.Jaiswal PK, Macmull S, Bentley G, Carrington RW, Skinner JA, Briggs TW. Does smoking influence outcome after autologous chondrocyte implantation?: A case-controlled study. J Bone Joint Surg Br. 2009 Dec;91(12):1575–8. doi: 10.1302/0301-620X.91B12.22879.91-B/12/1575 [DOI] [PubMed] [Google Scholar]
- 46.Niemeyer P, Salzmann GM, Hirschmüller A, Südkamp NP. [Factors that influence clinical outcome following autologous chondrocyte implantation for cartilage defects of the knee] Z Orthop Unfall. 2012 Feb;150(1):83–8. doi: 10.1055/s-0030-1270894. [DOI] [PubMed] [Google Scholar]
- 47.KnorpelRegister DGOU. AG Klinische Geweberegeneration . KnorpelRegister DGOU. Berlin, Germany: Deutsche Gesellschaft für Orthopädie und Unfallchirurgie; 2016. [2016-05-19]. http://www.knorpelregister-dgou.de/start.html . [Google Scholar]