Abstract
In the developing world, most patients with hepatocellular carcinoma present with advanced-stage disease, considered to be incurable based on current therapeutic algorithms. Here, we demonstrate that curative liver resection is achievable in a portion of Peruvian patients not addressed by these treatment algorithms. We conducted a retrospective cohort study of 253 hepatocellular carcinoma patients that underwent a curative hepatectomy between 1991 and 2011 at the National Cancer Institute of Peru. The median age of the cohort was 36 years, and merely 15.4% of the patients displayed cirrhosis. The average tumor size was over 14 cm in diameter, resulting in 76.3% of major hepatectomies performed. The 5- and 10-year survival probability estimates were 37.5% and 26.2%, respectively. Age (>44 vs. ≤44 years old; P = 0.005), tumor size (>10 cm vs. ≤10 cm in diameter; P = 0.009), cirrhosis (P < 0.001), satellite lesions (P < 0.001), macroscopic vascular invasion (P < 0.001), allogeneic blood transfusion (P = 0.011), and spontaneous rupture of the tumor (P = 0.006) were independent predictive factors for prognosis. Hepatocellular carcinomas in Peru are characterized by a distinct clinical presentation with notable features compared with those typically described throughout relevant literature. Despite a large number of advanced-stage hepatocellular carcinomas, the outcomes of liver resection observed in the present study were in good standing with the results previously described in other series. It thus appears that staging systems and associated therapeutic algorithms designed for use in the developed world remain inadequate in certain populations, especially in the context of Peruvian patients. Our findings suggest that clinicians in the developing world should reconsider management guidelines pertaining to hepatocellular carcinoma. Indeed, we hypothesize that, in developing countries, a strict adherence to these therapeutic algorithms might create a selection bias resulting in the dismissal of patients who could eventually be treated.
Keywords: Health sciences, Pathobiology of cancer, Cancer, Treatment of cancer
1. Introduction
We have described the peculiar clinical presentation of hepatocellular carcinoma (HCC) observed in the patients examined at the National Cancer Institute of Peru [1]. Remarkably, half of these patients are under 40 years of age presenting with massive primary tumors in the liver uniquely characterized by extremely low rate of cirrhosis and a relatively low rate of tumor invasiveness. This unique pathophysiological pattern of HCC coincides with a distinct mutation spectrum featuring genetic insertions and deletions, in contrast with the transitions and transversions paramount to the mutation spectra of HCCs from North Africa, North America, East Asia, and Europe [2]. These findings suggest that an uncommon and unique tumor process occurs in the Peruvian patient population. Likewise, the distribution of the HCC-related risk factors in Peru is rather unusual with low prevalence of alcohol abuse, non-alcoholic steatohepatitis, and chronic hepatitis C. In addition, hepatitis B virus (HBV), the major etiologic agent of primary liver cancer in the country, follows an unconventional pattern: while primary liver cancer risk is correlated with elevated HBV viral loads in Eastern Asia, almost half of HBV-associated HCCs develop in a context of occult infection in Peru [1].
The global incidence of HCC has doubled during the last two decades, with the highest burden arising in the developing world [3, 4]. To date, curative treatment of HCC relies primarily on surgical intervention, and particular for developing countries, on rarely performed liver resections. In this context, a vast majority of patients visit physicians with advanced-stage cancer, drastically limiting the use of liver transplantation and percutaneous ablation for treatment. As a consequence, HCC is particularly lethal in the Global South. Worldwide, 82.8% of liver cancer cases occur in the developing world, which also accounts for 83.6% of liver cancer-related deaths [4]. Thus, new affordable methods for early detection and intervention are needed to improve patient outcomes.
In order to guide practitioners in clinical management strategies, various therapeutic algorithms based on the stratification of the patients with HCC have been elaborated by groups of experts from North America, East Asia, and Europe. These staging systems have been compared to each other using cohort studies, conducive to build international consensus [5, 6]. However, the at times limited performance of the current therapeutic algorithms probably stems in part from their lack of interoperability in different populations of patients. Based on our long-term practice in primary liver cancer management in Peru, we suspect that some of these algorithms do not account for patients' groups, notably those from developing countries.
Here we present the data from 20 years of liver resection practice for HCC in Peru, in which massive tumors on non-cirrhotic livers in juvenile patients were prevailing. Specifically, this study was conducted retrospectively within a cohort assembled by analyzing the medical records of patients with HCC who underwent curative liver resection. Taking into consideration the peculiar clinical presentation encountered in Peru (a country in a hitherto neglected world region regarding studies of primary liver cancer), we think that our experience in the management of HCC can provide valuable information for the international community of health professionals on the development and usage of such staging systems. We are also convinced that our experience can lead in many instances to local reconsiderations of the strategy for the surgical management of HCC. Finally, we emphasize the necessity of searching for a truly multiregional consensus, taking into account local contexts.
2. Materials and methods
2.1. Ethics statement
Written consent was given by the patients for their information to be stored in the Department of Cancer Statistics and Epidemiology of the National Cancer Institute of Peru, and used for research. The study conforms to the ethical principles contained in the Declaration of Helsinki, and was approved by the Human Subjects Committee of the National Cancer Institute of Peru (Protocol Number #INEN10-05).
2.2. Patients' data collection
Between January 1991 and December 2011, a total of 339 patients with HCC were operated on in the Department of Abdominal Surgery of the National Cancer Institute of Peru. From this initial cohort, we selected a series of 253 patients with no extrahepatic metastases who underwent curative hepatectomy, i.e. complete (R0) resection of the tumoral liver lesions, ensuring tumor-free margins. The 86 patients excluded from this study were individuals who underwent partial (R2) resection (n = 70) or orthotopic liver transplantation (n = 2), with synchronous and secondary malignancies (n = 12), or for whom the medical record was not exhaustive (n = 2). The dataset included the patients' medical records, hepatitis B and C serology, preoperative liver function, alpha-fetoprotein (AFP) serum level, perioperative morbidity and mortality, hospital length of stay, as well as HCC recurrence and survival until August 2015.
2.3. Surgical procedure
Anatomical resection was the preferential treatment, but was not performed on patients with cirrhosis. In cases of cirrhotic liver, only patients with Child-Pugh class A were considered eligible for major hepatic resection, whereas patients with Child-Pugh class B were offered minor hepatic resection. Interventions were performed through midline, J-shaped, or bilateral subcostal abdominal incision, according to the tumor location and the physical characteristics of the patient. After incision, the abdominal cavity was extensively explored in order to discard any suggestions of additional hepatic disease, and the status of the future remnant liver was evaluated. This examination has been complemented by ultrasound exploration since 2000. After detaching the hilar plate, we started the afferent vascular control with the ligation and the division of the arterial and portal pedicles at the hilum. Suprahepatic veins were controlled outside the liver. Pringle or hemi-Pringle maneuvers were performed pro re nata. Total hepatic vascular exclusion was performed for tumors involving inferior vena cava or hepatic veins. Since 2001, we employed the anterior approach with or without a hanging maneuver in order to prevent tumor embolism. Liver parenchymal transection was performed by crush-clamping. Medium-sized blood vessels and bile ducts were ligated, while the smallest ones were cauterized. After the removal of the surgical piece, we achieved hemostasis by cauterizing liver bed bleeding by using an argon plasma coagulator or a hemostatic dissection device. Finally, a closed drainage system was installed. After surgical intervention, it was mandatory to admit cirrhotic patients to the intensive care unit of the National Cancer Institute of Peru. Patients without cirrhosis, however, were transferred to the intensive care unit at the discretion of the surgical team, based on the patient's post-operative health status. Patients were monitored throughout their hospital stay, and the drain was removed when the biliary fistula was discarded. Adjuvant chemotherapy was not routinely administered.
2.4. Patients' follow-up
Patients had an extensive checkup twice during the first month after leaving hospital, then every two months during the first year, and finally every four months from the third year onward. Liver regeneration and function were respectively assessed by abdominal computed tomography scan and liver function tests, including monitoring of the AFP serum level. While AFP concentration was above 10 ng/mL, the eventuality of recurrent or metastatic HCC was assessed by abdominal ultrasound and chest and bone radiographs. If necessary, the exam was completed by computed and positron emission tomographies. In cases of intrahepatic recurrence, the feasibility of surgical intervention was promptly evaluated, and when possible, tumor re-resection was performed quam primum. When recurrent HCC was unresectable, palliative treatments, such as intra-arterial chemoembolization, percutaneous ethanol injection, radiofrequency ablation, or tyrosine-kinase inhibitor chemotherapy, were applied. Surgical pulmonary resection was carried out in cases of single lung metastasis. Bone metastases were treated with radiation therapy. In cases without any follow-up, the National Registry of Identification and Civil Status of Peru was solicited in order to determine the fate of the patient.
2.5. Pathology report
Pathologists determined the macroscopic tumor size (i.e., longest chord measured), the nodule number, the presence of vascular invasion, and the structure of the non-tumoral liver parenchyma. HCC and non-tumoral liver diagnostic features were confirmed on hematoxylin and eosin-stained liver sections. The tumor grade was assessed according to the American Joint Committee on Cancer [7].
2.6. Statistical analysis
Survival probability estimates were calculated by the Kaplan–Meier method from the date of surgery [8]. Log-rank test was used for survival distribution comparison [9]. Postoperative deaths were included in the survival analysis, and subsequent decease from any cause was considered an event. Potential predictors of survival were evaluated using the Cox proportional-hazards regression model [10]. Statistical analyses were performed with an alpha significance level 0.05, using IBM SPSS Statistics software version 19.0.
3. Results
3.1. Clinical presentation of hepatocellular carcinoma
Table 1 shows an overview of the clinical features of the 253 patients with complete resection at the time of surgery operated in the Department of Abdominal Surgery of the National Cancer Institute of Peru between January 1991 and December 2011. Over this 20-year period, an examination of the patient population structure did not reveal a noticeable evolution in terms of tumor presentation and clinical pathology and chemistry. The mean patient age was low; half of the patients were less than 36 years old. The average tumor size was over 14 cm in diameter, 73% of the patients presented with a tumor larger than 10 cm. The predominant histopathological architecture of the tumors was the trabecular pattern (70%), whereas fibrolamellar carcinomas represented a small minority of the cases (1.6%) despite the overall young age of the cohort. Major vascular invasion occurred only in 11% of the cases, all of them with a tumor larger than 10 cm in diameter. Merely 15% of the resections were performed on cirrhotic livers.
Table 1.
Baseline demographic and clinical features of the patient population investigated.
| Feature | Parameter | Number | Percentage |
|---|---|---|---|
| Cohort | Headcount | 253 | 100 |
| Age | Mean ± SD | 41.9 ± 21.4 | n/a |
| Median | 36 | n/a | |
| Range | [3–89] | n/a | |
| Interquartile range | 39 | n/a | |
| Gender | Female | 103 | 40.7 |
| Male | 150 | 59.3 | |
| Cirrhosis | Absent | 214 | 84.6 |
| Present | 39 | 15.4 | |
| HBsAg | Negative | 141 | 55.7 |
| Positive | 112 | 44.3 | |
| Anti-HCV* | Negative | 194 | 96.5 |
| Positive | 7 | 3.5 | |
| Tumor size (cm) | Mean ± SD | 14.2 ± 5.9 | n/a |
| Median | 14.5 | n/a | |
| Range | [2–33] | n/a | |
| Interquartile range | 8.4 | n/a | |
| <5 | 15 | 5.9 | |
| [5–10] | 53 | 20.9 | |
| >10 | 185 | 73.2 | |
| Multinodular tumors | Present | 78 | 30.8 |
| Bilobar tumors | Present | 80 | 31.6 |
| Child-Pugh class** | A | 30 | 76.9 |
| B | 9 | 23.1 | |
| Vascular invasion | Macro | 29 | 11.5 |
| Micro | 64 | 25.3 | |
| Negative | 160 | 63.2 | |
| Histopathology | Trabecular | 177 | 70 |
| Acinar | 10 | 4 | |
| Compact | 17 | 6.7 | |
| Mixed trabecular and acinar | 36 | 14.2 | |
| Sarcomatoid | 9 | 3.5 | |
| Fibrolamellar | 4 | 1.6 | |
| AFP (ng/mL) | Mean ± SD | 93,026 ± 241,792 | n/a |
| Median | 2651 | n/a | |
| Interquartile range | 56,826.8 | n/a | |
| Albumin (g/L) | Mean ± SD | 38.7 ± 9.7 | n/a |
| Median | 39 | n/a | |
| Interquartile range | 10 | n/a | |
| Alkaline phosphatase (U/L) | Mean ± SD | 187.3 ± 205.8 | n/a |
| Median | 129 | n/a | |
| Interquartile range | 159 | n/a | |
| Alanine transaminase (U/L) | Mean ± SD | 63.2 ± 90.2 | n/a |
| Median | 40 | n/a | |
| Interquartile range | 77 | n/a | |
| Aspartate transaminase (U/L) | Mean ± SD | 100.2 ± 115.6 | n/a |
| Median | 60 | n/a | |
| Interquartile range | 40 | n/a | |
| Direct bilirubin (μmol/L) | Mean±SD | 7.3 ± 29.7 | n/a |
| Median | 2 | n/a | |
| Interquartile range | 4.1 | n/a | |
| Indirect bilirubin (μmol/L) | Mean±SD | 10.2 ± 13.5 | n/a |
| Median | 8 | n/a | |
| Interquartile range | 18.5 | n/a | |
| Total bilirubin (μmol/L) | Mean ± SD | 18.2 ± 41 | n/a |
| Median | 12 | n/a | |
| Interquartile range | 14.2 | n/a | |
| Gamma-glutamyl transferase (U/L) | Mean ± SD | 288 ± 1,210 | n/a |
| Median | 124 | n/a | |
| Interquartile range | 158 | n/a | |
| Prothrombin time (s) | Mean ± SD | 13.1 ± 1.7 | n/a |
| Median | 12.8 | n/a | |
| Interquartile range | 2.1 | n/a | |
| International normalized ratio | Mean ± SD | 1.1 ± 0.2 | n/a |
| Median | 1.1 | n/a | |
| Interquartile range | 0.2 | n/a |
Percentages are expressed as ratio of the 253 patients investigated for the considered parameter, except for (*) hepatitis C infection (n = 201) and (**) Child-Pugh score in cirrhotic patients (n = 39). Histopathological architecture was defined according to the classification of tumors of the digestive system of the World Health Organization [11]. Mean values are presented with ± Standard Deviation (SD). AFP = alpha-fetoprotein; HBsAg = HBV surface antigen; HCV = hepatitis C virus; n/a = not applicable.
3.2. Hepatic resection category
Table 2 presents descriptive statistical results for the resection category. The largest majority of the liver cancer resections, i.e. 76.3%, were major hepatectomies, frequently extended to segment 1. Such a high ratio is rarely found in relevant literature, in which major hepatectomy represents often a minority of intervention [13].
Table 2.
Hepatic resection categories.
| Hepatic resection category | Subcategory | Number | Extended to segment 1 |
|---|---|---|---|
| Major hepatectomy (76.3%) | Right trisectionectomy | 37 | 7 |
| Left trisectionectomy | 16 | 12 | |
| Right hepatectomy | 85 | 6 | |
| Left hepatectomy | 55 | 36 | |
| Minor hepatectomy (23.7%) | Right anterior and left medial sectionectomies | 9 | |
| Right posterior sectionectomy | 1 | ||
| Left lateral sectionectomy | 12 | ||
| Left medial sectionectomy | 5 | ||
| Segmentectomy 1 | 2 | ||
| Segmentectomy 3 | 1 | ||
| Segmentectomy 5 | 1 | ||
| Segmentectomy 6 | 2 | ||
| Segmentectomy 8 | 2 | ||
| Bisegmentectomy 4,5 | 4 | ||
| Bisegmentectomy 5,6 | 14 | ||
| Bisegmentectomy 7,8 | 1 | ||
| Wedge | 6 | ||
| Total | 253 | 61 |
Hepatic resection categories were defined according to the Brisbane 2000 Terminology of Liver Anatomy and Resections [12].
3.3. Postoperative period, recurrence pattern, and cancer survival
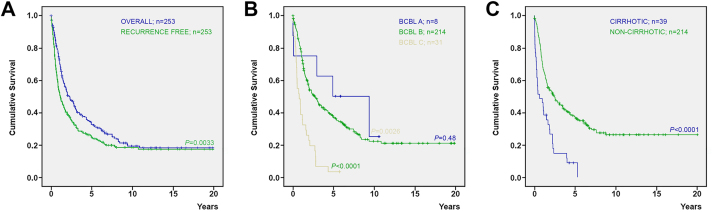
Table 3 details the postoperative morbidity (grades I to IV) and mortality (grade V). Immediate postoperative bleeding and liver failure were the most frequent and severe complications. The 30- and 90-day postoperative mortality rates were of 5.1% and 7.1%, respectively. Table 4 summarizes the descriptive statistic results for recurrence. In the follow-up period, 59% of the patients developed recurrence, and 15.5% of them underwent re-resection. Fig. 1A shows the overall and recurrence-free survival rates in the 20 years post-surgery (P = 0.0033). The 5- and 10-year survival probability estimates were 37.5% and 26.2%, respectively. The 5- and 10-year recurrence-free survival probability estimates were 22.9% and 16.2%, respectively. Fig. 1B displays the survival rates in the 20 years post-surgery with patients stratified according to the Barcelona-Clinic Liver Cancer (BCLC) stage disease; eight (3.2%) patients having BCLC A stage disease, 214 (84.6%) having BCLC B, and 31 (12.3%) having BCLC C [15, 16]. The 5-year survival probability estimates for patients with BCLC stages A and B were 50% and 36.8%, respectively; while the 10-year survival probability estimates were 25% and 22.1%, respectively (P = 0.48). The 5-year survival probability estimate for patients with BCLC stage C was only 3.2% and differed significantly from the one of both BCLC stages A and B (P = 0.0026 and <0.0001, respectively). Fig. 1C shows the survival rates of patients with or without cirrhosis in the 20 years post-surgery (P < 0.0001). The 5-year survival probability estimate among cirrhotic patients was 11.4%; whereas the 5- and 10-year survival probability estimates among non-cirrhotic patients were 40.1% and 27.9%, respectively.
Table 3.
Postoperative morbidity and mortality.
| Complication | Grade I | Grade II | Grade IIIa | Grade IIIb | Grade IV | Grade V | Total |
|---|---|---|---|---|---|---|---|
| Bile leakage | 2 | 2 | 2 | 1 | 7 | ||
| Cerebrovascular accident | 1 | 1 | |||||
| Evisceration | 2 | 2 | |||||
| Hemorrhaging | 1 | 2 | 5 | 3 | 11 | ||
| Intestinal obstruction | 1 | 1 | |||||
| Intra-abdominal abscess | 4 | 4 | |||||
| Liver failure | 1 | 8 | 6 | 15 | |||
| Pleural effusion | 1 | 1 | |||||
| Pneumonia | 1 | 2 | 3 | ||||
| Pulmonary embolism | 8 | 1 | 9 | ||||
| Total | 8 | 24 | 2 | 7 | 13 | 54 |
Postoperative morbidity and mortality were categorized according to the Dindo–Clavien classification [14].
Table 4.
Pattern of recurrence after hepatic resection.
| Recurrence pattern | Status | Number | Percentage |
|---|---|---|---|
| Recurrence* | Yes | 142 | 59.2 |
| No | 98 | 40.8 | |
| Recurrence presentation** | Intrahepatic | 72 | 50.7 |
| Extrahepatic | 47 | 33.1 | |
| Intra- and extra-hepatic | 23 | 16.2 | |
| Re-resection*** | Intrahepatic | 14 | 19.4 |
| Extrahepatic | 7 | 14.9 | |
| Intra- and extra-hepatic | 1 | 4.3 |
Any reappearance of HCC was considered as a recurrence regardless the time period after the initial intervention. Percentages were calculated with (*) 240 patients, excluding the 13 patients who died during the postoperative period (Table 3); (**) 142 patients with recurrent HCC; (***) the number of patients in relation to the site of recurrence, i.e. 72 for intrahepatic recurrence, 47 for extrahepatic recurrence, and 23 for intra- and extra-hepatic recurrence.
Fig. 1.
Kaplan–Meier cumulative survival curves. (A) Overall and recurrence-free survivals are represented by blue and green curves, respectively. (B) Survivals for patients with BCBL A (n = 8), B (n = 214), and C (n = 31) stages disease are represented in green, blue, and yellow curves, respectively. (C) Survivals for patients with (n = 39) and without (n = 214) cirrhosis are represented in blue and green curves, respectively.
3.4. Potential predictors of survival
Table 5 presents the results of the multivariate analysis for factors related to survival. Among all the parameters tested, the age, the size of the tumor, the presence of cirrhosis, satellite lesions, or macroscopic vascular invasion, as well as allogeneic blood transfusion and spontaneous rupture of the liver tumor before surgery, were independent predictive factors for prognosis (P < 0.05).
Table 5.
Multivariate analysis for factors related to survival.
| Variable | Odds ratio | 95% confidence interval (lower – upper) |
P value |
|---|---|---|---|
| Age (>44 vs. ≤44 years old) | 1.54 | 1.140 – 2.103 | 0.005 |
| Tumor size (>10 cm vs. ≤10 cm in diameter) | 1.74 | 1.152 – 2.638 | 0.009 |
| Cirrhosis (presence vs. absence) | 2.92 | 1.927 – 4.447 | 0.000 |
| Macroscopic portal vein tumor thrombosis (presence vs. absence) | 2.73 | 1.767 – 4.220 | 0.000 |
| Allogeneic blood transfusion (yes vs. no) | 1.48 | 1.094 – 2.017 | 0.011 |
| Satellite lesions (presence vs. absence) | 2.25 | 1.634 – 3.120 | 0.000 |
| Spontaneous rupture of the liver tumor before surgery (yes vs. no) | 2.14 | 1.244 – 3.689 | 0.006 |
The age cutoff was chosen according to the in-between age calculated previously [1]. The odds ratio represents the exponentiation of the intercept in the null model.
4. Discussion
The aims of cancer staging systems and associated therapeutic algorithms are to rationally predict patient prognosis and to guide practitioners in deciding treatment allocation. Multiple staging systems have been developed and compared for HCC cases in order to determine which ones best predict prognosis. Indeed, the intended purpose of international collaboration between experts is to move towards an international consensus on the management of HCC [5, 6]. As a consequence of the neoplastic heterogeneity of HCC, this objective has not yet been achieved.
Since the first classification by Okuda and colleagues [17], various staging systems have been developed and notably include those of the American Joint Committee on Cancer [7], the Cancer of the Liver Italian Program [18], the French Group for the Study and the Treatment of HCC [19], the Chinese University of Hong Kong [20], the Japan Society of Hepatology [21], and the Liver Cancer Study Group of Japan [22]. The BCLC staging system is currently the most widely accepted therapeutic flow-chart across the world [15, 16], adopted by the European Association for the Study of the Liver and the American Association for the Study of Liver Disease [23, 24]. In these therapeutic algorithms, several clinico-biological parameters, such as AFP and bilirubin serum levels and presence of vascular invasion and metastases are taken into account to stratify patients and decide treatment allocation. Among those parameters, tumor features, and notably tumor size, are certainly the most critical in choosing between curative treatment and palliative care. Commonly, only early stage HCCs, up to 2 or 3 cm in diameter (depending on which flow-chart is used), are considered to be candidates for resection [25, 26].
In the last couple of years, a debate has taken place about the necessity of adherence to such staging systems [27, 28, 29, 30, 31]. In fact, most of these systems have been conceived by experts attending patients affected with HCC in a clinical context where cirrhotic liver represents the very large majority of the cases. However, HCC is among the most diversified types of cancer in terms of clinical presentation and molecular signature [32, 33], due in part to the variety of associated risk factors.
The team of the Department of Abdominal Surgery at the National Cancer Institute of Peru attends more than 120 patients a year diagnosed with primary liver cancer, and performs about 20 liver resections annually for HCC. These numbers have been steadily increasing during the last decades, due in part to the improvements to the national healthcare system in Peru that allow earlier patient detection, but also to the increasing trend in incidence of primary liver cancer in Latin America [4]. We recently described Peruvian patients developing HCC at a young age, and then correlated this clinical presentation to a peculiar mutation spectrum [1, 2]. In comparison with literature stating that fibrolamellar carcinoma occurs commonly in children and young adults [34], the very large majority of our patients developed HCC with a trabecular pattern (Table 1). To the best of our knowledge, Peruvian patients are remarkable for the consistent presentation of massive HCC, exceeding AFP serum level, and very low rate of cirrhosis, in distinct contrast with most observational studies described in cancer literature [1]. According to the staging systems currently available, the large majority of our patients would be scored at an advanced stage of disease with a poor prognosis (Table 1) [15, 16]. As a consequence, these patients would be mostly considered ineligible for curative liver resection.
Indeed, the Latin American Association for the Study of the Liver has recently published clinical practice guidelines for the management of HCC in the region [35]. According to the authors, these guidelines are based on an international consensus, outlining the BCLC staging system. In our case, the application of these guidelines would result in the dismissal of those patients who received surgery. For instance, if we had applied the criteria of the BCLC staging system, only eight patients from our initial cohort would have been considered as candidate for liver resection, whereas our surgical strategy allowed us to treat 214 additional patients leading to a 5-year survival ratio of 36.8% (Fig. 1B). This result has to be offset with the fact that we performed intervention only on 15% of the overall HCC patients visiting the National Cancer Institute of Peru during this period. This is why we are convinced that the authority of such prescribed guidelines should be discussed and their contextual applications should be explicitly addressed. In fact, the adherence to international guidelines has been already questioned in real-life practice by some physicians [29, 30, 31], while a more contextualist approach has been suggested by others [36, 37].
From our perspective, only the recommendations of the Asian Pacific Association for the Study of the Liver would reflect our patient population [38]. This treatment algorithm developed in 2010 is distensible and eventually considers liver resection for a patient with HCC that is confined to the liver and technically, surgically resectable with sufficient remnant healthy liver to insure the vital hepatic functions. Through the evolution of our treatment, no suitable therapeutic flow-charts were identified; therefore, we have developed our own specific approach in the management of HCC based on our long-term experience in clinical practice in Peru. Our decision tree for curative liver resection was relatively concise, with few nodes. In using this approach, we first determined the tumor location site, i.e. whether the liver tumor is extra- or intra-hepatic. In cases with an intrahepatic tumor, we assessed the size of the remnant liver and its function by a blood test, which included measurement of the serum levels of albumin, alanine and aspartate aminotransferases, total and direct bilirubin, alkaline phosphatase, and gamma-glutamyl transpeptidase serum levels (Table 1). In addition, we calculated the prothrombin time and the international normalized ratio (Table 1). When this was conclusive, the patient was considered as a candidate for curative liver resection, regardless of the size of the tumor. In our hands, this approach, based on the absence of cirrhosis and the lack of evidence of extrahepatic tumor lesions, made liver resection the most effective treatment for nearly 15% of our patients. Unlike other cohort studies found in the literature, most resections performed in our study were major hepatectomies due to the size and the location of the tumors exsected (Table 2). The surgical outcomes observed in our resected patient cohort, as well as survival rates, were in good standing compared with other cohort studies found in relevant literature (Fig. 1, Table 3 and Table 4) [13]. Furthermore, this result was attained with a cohort of patients who were mostly less than 37 years old, in spite of the tumor size and the fact that carcinomas had virtually no fibrolamellar variants (Table 1), a histotype reputed to be less disseminative [39].
Several regions of the world, encompassing most of the so-called Global South, i.e. Africa, Latin America and Caribbean, Central, West, and Southeast Asia, Melanesia, and Polynesia (as defined by the International Agency for Research on Cancer [4]), have not been closely examined regarding the natural history and the most common clinical presentation of HCC. Most of these countries do not have the benefit of population-based cancer registries, and the coverage of cancer information is obtained sparsely by extracting data from patients' medical records. Yet, in developing countries, patients with liver cancer which would be considered advanced are rapidly dismissed from the chain of care [40]. This situation is partly due to the lack of alternative therapeutic support after a basic doctor's visit, during which a poor prognosis may be given according to the current staging systems. These poorly documented patients are often among the most deprived people, frequently living in remote areas [4, 40]. It is thus difficult to determine their fate once dismissed from the chain of care. As a consequence, a frequently unusual clinical presentation of HCC, as reported in Peru [1, 2], that contradicts the situation commonly described in available staging systems, could easily go unnoticed, despite the fact that some of these tumors would eventually be treatable. A rigid adherence to the current therapeutic algorithms in developing countries, without taking into account both local epidemiology and clinical presentation [3], might thus lead to a selection bias culminating in damaging adverse effect on a fraction of the local patients, and then, self-perpetuating the local application of these algorithms. Brought back to the Peruvian context, predictive factors such as those listed in Table 5 could be of special interest to build the foundations of a context-related interventional flow-chart.
Current data reports discrepancies in the performance of therapeutic algorithms typically used for patient populations originating in North America, East Asia, or Europe. Thus, it is highly plausible that these differences exist when these therapeutic algorithms are applied to regions which are underserviced with poor documentation of liver cancer outcomes. Therefore, there must be an in-depth discussion for a contextualist approach to HCC surgery in those regions. In this regard, new considerations for tumor size and morphology reflected in recent, 7th edition update of the tumor-node-metastasis classification [41, 42], as well as the recommendations of the Asian Pacific Association for the Study of the Liver [38], represent interesting advances, as they open up new intervention possibilities.
Declarations
Author contribution statement
Eloy Ruiz: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Teresa Rojas Rojas: Analyzed and interpreted the data.
Francisco Berropsi, Ivan Chavez, Carlos Luque, Luis Cano: Performed the experiments.
Franco Doimi: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Pascal Pineau, Eric Deharo, Stéphane Bertani: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
Eloy Ruiz, Luis Cano, and Franco Doimi were supported by the Young Research Teams Associated to IRD Program (INCAnCER). Teresa Rojas Rojas was supported by a doctoral fellowship from the Peruvian Fund for Innovation, Science, and Technology Program's Scholarships for Doctoral Studies Abroad (BECA-1-P-155-13). Pascal Pineau, Eric Deharo, and Stéphane Bertani were supported by the Third Cancer Plan of the French National Alliance for Life Sciences and Health (ENV201408).
Conflict of interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to acknowledge all patients whose participation was essential to the achievement of this study. The authors are grateful to Karina Cancino, Juan-Pablo Cerapio, Dany Cordova, Macarena Farías, Marlene Nuñez, Maria Solis, and Maricarmen Valera from the Department of Pathology of the National Cancer Institute of Peru for their leadership in aggregating the medical records; Jacques Gardon from the Institut de Recherche pour le Développement and Agnès Marchio from the Institut Pasteur for their critical discussions; and Elizabeth Elliott, Brian Gadd, and David D. Parker Jr. for their suggestions in preparing the manuscript. The authors thank the Ando-Amazonian International Mix Laboratory of Life Chemistry (LMI-LAVI), IRD-UPCH, for its logistic support.
References
- 1.Bertani S., Pineau P., Loli S., Moura J., Zimic M., Deharo E. An atypical age-specific pattern of hepatocellular carcinoma in Peru: a threat for Andean populations. PLoS One. 2013;8:e67756. doi: 10.1371/journal.pone.0067756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchio A., Bertani S., Rojas Rojas T., Doimi F., Terris B., Deharo E. A peculiar mutation spectrum emerging from young Peruvian patients with hepatocellular carcinoma. PLoS One. 2014;9:e114912. doi: 10.1371/journal.pone.0114912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender E. Developing world: global warning. Nature. 2014;509:S64–S65. doi: 10.1038/509S64a. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 5.Henderson J.M., Sherman M., Tavill A., Abecassis M., Chejfec G., Gramlich T. AHPBA/AJCC consensus conference on staging of hepatocellular carcinoma: consensus statement. HPB (Oxford) 2003;5:243–250. doi: 10.1080/13651820310015833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vauthey J.N., Dixon E., Abdalla E.K., Helton W.S., Pawlik T.M., Taouli B. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford) 2010;12:289–299. doi: 10.1111/j.1477-2574.2010.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge S.B., Byrd D.R., Compton C.C., Fritz A.G., Greene F.L., Trotti A., editors. AJCC cancer staging manual. 7th ed. Springer; New York: 2010. Liver; pp. 191–195. [Google Scholar]
- 8.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. [Google Scholar]
- 9.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 10.Cox D.R. Regression models and life-tables. J. R. Stat. Soc. B. 1972;34:187–220. [Google Scholar]
- 11.Bosman F.D., Carneiro F., Hruban R.H., Theise N.D., editors. Classification of tumours of the digestive system. 4th ed. WHO classification of tumours. Lyon: IARC Press; 2010. Tumours of the liver and intrahepatic bile ducts; pp. 205–216. [Google Scholar]
- 12.Terminology Committee of the International Hepato-Pancreato-Biliary Association The Brisbane 2000 terminology of hepatic anatomy and resections. HPB (Oxford) 2000;2:333–339. [Google Scholar]
- 13.Zhou Y., Lei X., Wu L., Wu X., Xu D., Li B. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg. Oncol. 2014;23:136–142. doi: 10.1016/j.suronc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llovet J.M., Brú C., Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin. Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 16.Forner A., Reig M.E., de Lope C.R., Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin. Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 17.Okuda K., Ohtsuki T., Obata H., Tomimatsu M., Okazaki N., Hasegawa H. Natural history of hepatocellular carcinoma and prognosis in relation to treatment study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.The Cancer of the Liver Italian Program (CLIP) Investigators A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 19.Chevret S., Trinchet J.C., Mathieu D., Rached A.A., Beaugrand M., Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. J. Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 20.Leung T.W.T., Tang A.M.Y., Zee B., Lau W.Y., Lai P.B., Leung K.L. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the cancer of the liver Italian program staging system. Cancer. 2002;94:1760–1769. doi: 10.1002/cncr.10384. [DOI] [PubMed] [Google Scholar]
- 21.Kokudo N., Hasegawa K., Akahane M., Igaki H., Izumi N., Ichida T. Evidence-based clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2013 update (3rd JSH-HCC guidelines) Hepatol. Res. 2015;45:123–127. doi: 10.1111/hepr.12464. [DOI] [PubMed] [Google Scholar]
- 22.Kudo M., Matsui O., Izumi N., lijima H., Kadoya M., Imai Y. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology. 2014;87(Suppl. 1):7–21. doi: 10.1159/000368141. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J., Sherman M., Llovet J.M., Beaugrand M., Lencioni R., Burroughs A.K. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J., Sherman M., Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 25.Shindoh J., Andreou A., Aloia T.A., Zimmitti G., Lauwers G.Y., Laurent A. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2cm: Reappraisal of the staging system for solitary tumors. Ann. Surg. Oncol. 2012;20:1223–1229. doi: 10.1245/s10434-012-2739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guglielmi A., Ruzzenente A., Conci S., Valdegamberi A., Vitali M., Bertuzzo F. Hepatocellular carcinoma: surgical perspectives beyond the Barcelona clinic liver cancer recommendations. World J. Gastroenterol. 2014;20:7525–7533. doi: 10.3748/wjg.v20.i24.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livraghi T., Brambilla G., Carnaghi C., Tommasini M.A., Torzili G. Is it time to reconsider the BCLC/AASLD therapeutic flow-chart? J. Surg. Oncol. 2010;102:868–876. doi: 10.1002/jso.21733. [DOI] [PubMed] [Google Scholar]
- 28.Tsoulfas G., Mekras A., Agorastou P., Kiskinis D. Surgical treatment for large hepatocellular carcinoma: does size matter? ANZ J. Surg. 2012;82:510–517. doi: 10.1111/j.1445-2197.2012.06079.x. [DOI] [PubMed] [Google Scholar]
- 29.Trovato M.A., Pesce A., Sofia M., Montineri A., Basile A., Palermo F. Is BCLC algorithm useful in clinical practice? Study on 164 HCC patients. Hepatogastroenterology. 2013;60:1742–1745. [PubMed] [Google Scholar]
- 30.Leoni S., Piscaglia F., Serio I., Terzi E., Pettinari I., Croci L. Adherence to AASLD guidelines for the treatment of hepatocellular carcinoma in clinical practice: experience of the Bologna Liver Oncology Group. Dig. Liver Dis. 2014;46:549–555. doi: 10.1016/j.dld.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Hernández-Guerra M., Hernández-Camba A., Turnes J., Ramos L.M., Arranz L., Mera J. Application of the Barcelona Clinic Liver Cancer therapeutic strategy and impact on survival. United Eur. Gastroenterol. J. 2015;3:284–293. doi: 10.1177/2050640615575971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nault J.C., Villanueva A. Intratumor molecular and phenotypic diversity in hepatocellular carcinoma. Clin. Cancer Res. 2015;21:1786–1788. doi: 10.1158/1078-0432.CCR-14-2602. [DOI] [PubMed] [Google Scholar]
- 34.Schlageter M., Terracciano L.M., D'Angelo S., Sorrentino P. Histopathology of hepatocellular carcinoma. World J. Gastroenterol. 2014;20:15955–15964. doi: 10.3748/wjg.v20.i43.15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Méndez-Sánchez N., Ridruejo E., Alves de Mattos A., Chávez-Tapia N.C., Zapata R., Paraná R. Latin American Association for the Study of the Liver (LAASL) clinical practice guidelines: Management of hepatocellular carcinoma. Ann. Hepatol. 2014;13:S4–S40. [PubMed] [Google Scholar]
- 36.Kim S.E., Lee H.C., Kim K.M., Lim Y.S., Chung Y.H., Lee Y.S. Applicability of the BCLC staging system to patients with hepatocellular carcinoma in Korea: analysis at a single center with a liver transplant center. Korean J. Hepatol. 2011;17:113–119. doi: 10.3350/kjhep.2011.17.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J.J., Yan T., Zhao H., Zhou J.G., Huang Z., Zhang Y.F. Evaluation of eight different clinical staging systems associated with overall survival of Chinese patients with hepatocellular carcinoma. Chin. Med. J. (Engl.) 2015;128:316–321. doi: 10.4103/0366-6999.150095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Omata M., Lesmana L.A., Tateishi R., Chen P.J., Lin S.M., Yoshida H. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol. Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okuda K. Natural history of hepatocellular carcinoma including fibrolamellar and hepato-cholangiocarcinoma variants. J. Gastroenterol. Hepatol. 2002;17:401–405. doi: 10.1046/j.1440-1746.2002.02734.x. [DOI] [PubMed] [Google Scholar]
- 40.Farmer P., Frenk J., Knaul F.M., Shulman L.N., Alleyne G., Armstrong L. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010;376:1186–1193. doi: 10.1016/S0140-6736(10)61152-X. [DOI] [PubMed] [Google Scholar]
- 41.Edge S.B., Compton C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 42.Kee K.M., Wang J.H., Lin C.Y., Wang C.C., Cheng Y.F., Lu S.N. Validation of the 7th edition TNM staging system for hepatocellular carcinoma: an analysis of 8,828 patients in a single medical center. Dig. Dis. Sci. 2013;58:2721–2728. doi: 10.1007/s10620-013-2716-8. [DOI] [PubMed] [Google Scholar]