Abstract
The search for novel anticancer therapeutics with the ability to overcome multi-drug resistance (MDR) mechanisms is of high priority. A class of molecules that show potential in overcoming MDR are the N-alkylated isatins. In particular 5,7-dibromo-N-alkylisatins are potent microtubule destabilizing agents that act to depolymerize microtubules, induce apoptosis and inhibit primary tumor growth in vivo. In this study we evaluated the ability of four dibrominated N-alkylisatin derivatives and the parent compound, 5,7-dibromoisatin, to circumvent MDR. All of the isatin-based compounds examined retained potency against the MDR cell lines; U937VbR and MES-SA/Dx5 and displayed bioequivalent dose-dependent cytotoxicity to that of the parental control cell lines. We show that one mechanism by which the isatin-based compounds overcome MDR is by circumventing P-glycoprotein (P-gp) mediated drug efflux. Thus, as the isatin-based compounds are not susceptible to extrusion from P-gp overexpressing tumor cells, they represent a promising alternative strategy as a stand-alone or combination therapy for treating MDR cancer.
Keywords: Medicinal chemistry, Cancer treatment, Cell biology
1. Introduction
Advances in molecular targeted anticancer agents over the past two decades have significantly improved outcomes for cancer patients. However, a major clinical obstacle to successful treatment has been the emergence of pleiotropic or multi-drug resistance (MDR), a phenomenon responsible for the decrease in efficacy of clinically used chemotherapeutics [1, 2]. MDR occurs when cancer cells become resistant to a variety of structurally and functionally unrelated anticancer agents even if the patient has not previously been exposed to that drug. An important molecular driver for MDR is overexpression of the plasma membrane glycoprotein (P-gp) [3]. P-gp is the gene product of ABCB1, a member of the ABC (ATP binding cassette) superfamily of transporter proteins, that acts as an ATP-dependent efflux pump preventing adequate intracellular accumulation of a large number of cytotoxic drugs including vinca alkaloids [4], taxanes [5, 6], anthracyclines [7], epipodophyllotoxins, camptothecins [8] and some of the newer molecular targeted anticancer drugs such as imatinib [9, 10], sorafenib [11] and everolimus [12]. Innate or acquired overexpression of P-gp is now a marker of chemoresistance and disease progression in a growing number of solid and hematologic malignancies [13, 14, 15].
The classic pharmacological strategy to overcome P-gp mediated efflux has been the co-administration of efflux pump inhibitors and cytotoxic agents in an effort to increase the intracellular concentration of cytotoxic drugs to lethal doses. Although several P-gp inhibitors have been evaluated in clinical trials they have all failed to date, attributable to lack of specificity, resulting in off-target organ accumulation and intolerable side effects [16]. For example, verapamil, a calcium channel blocker that also competitively inhibits P-gp transport of other substrates, is associated with cardiotoxicity at doses required to reverse MDR [17]. Cyclosporin A (CSA) causes increased nausea, vomiting, hypomagnesemia and myelosuppression [18] and second generation P-gp modulators such as valspodar, a cyclosporin D derivative that inhibits P-gp with 10- to 20-fold greater activity than CSA, significantly increases toxicity in patients with recurring and refractory multiple myeloma [19]. Given this, the search for novel therapeutics that are not substrates for drug efflux mechanisms and therefore not susceptible to extrusion from P-gp overexpressing tumor cells is of high priority and a promising alternative strategy [20].
A group of cytotoxins that show potential in overcoming MDR are those based on the isatin (1H-indole-2,3-dione) scaffold. For example, isatin-β-thiosemicarbazones have recently been identified to selectively kill P-gp overexpressing tumor cells in vitro [21, 22]. The lead compound (NSC73306) was effective in the National Cancer Institute 60 cell line (NCI-60) drug screen with de novo or acquired P-gp induced MDR and acted indirectly to resensitize cells to previously ineffective agents [23, 24]. Other isatin derivatives that display promising anticancer activities include those derived from 5,7-dibromoisatin (1; Fig. 1). The synthesis and subsequent cytotoxic evaluation of a library of N-alkylisatins (mainly N-arylalkylisatins) led to the discovery that a number of these compounds possess sub-micromolar cytotoxic activity (IC50) against a panel of human cancer cell lines [25, 26, 27]. One compound, 5,7-dibromo-N-(p-trifluoromethylbenzyl)isatin, was even shown to preferentially kill lymphoma and leukemic cancer cell lines over freshly isolated, non-transformed human peripheral blood lymphocytes [26]. Mode of action studies revealed that N-alkyl-5,7-dibromoisatins arrest cells in the G2/M phase and induce apoptosis via activation of the caspase 3/7 cascade [25, 26]. Furthermore, tubulin binding experiments demonstrated that N-alkyl-5,7-dibromoisatins destabilize microtubule growth as evidenced by the inhibition of microtubule polymerization in the presence of structurally diverse N-alkylisatins. Considering the role microtubule targeting chemotherapeutics play in the treatment and control of cancer, the development of novel microtubule agents that retain potency against MDR tumors is likely to have significant clinical impact in the future.
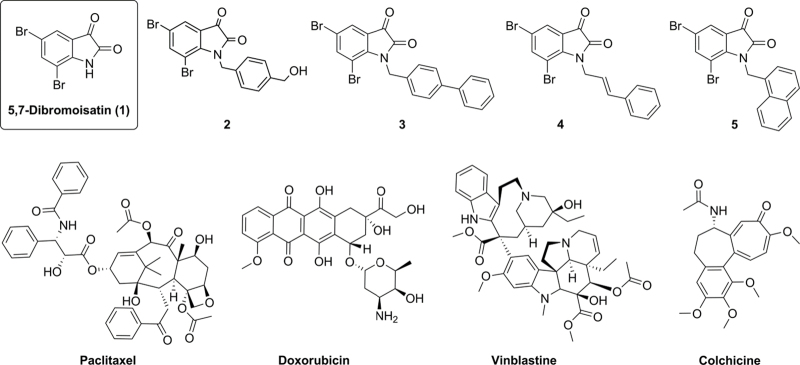
Fig. 1.
Chemical structures of 5,7-dibromoisatin (1) and the N-alkylisatin derivatives 5,7-dibromo-N-(p-hydroxymethylbenzyl)isatin (2); 5,7-dibromo-N-(p-phenylbenzyl)isatin (3); 5,7-dibromo-N-(p-cinnamyl)isatin (4) and 5,7-dibromo-N-(napthalen-1-ylmethyl)isatin (5) and the commercial anticancer agents paclitaxel, doxorubicin, vinblastine and colchicine.
In this study we sought to evaluate the cytotoxicity of a number of 5,7-dibromo-N-alkylisatins (2-5; Fig. 1) with varied substituents at the isatin nitrogen, against the human lymphoma (U937) and uterine sarcoma (MES-SA) cell lines and their corresponding MDR sublines (U937VbR and MES-SA/Dx5, respectively). The ability of these compounds to modulate P-gp ATPase activity was also studied to further elucidate direct drug - P-gp interactions in an effort to assess their potential as new drug candidates for treating MDR cancer.
2. Materials and methods
2.1. Reagents and chemicals
5,7-Dibromoisatin (1) and the N-alkylated derivatives 5,7-dibromo-N-(p-hydroxymethylbenzyl)isatin (2); 5,7-dibromo-N-(p-phenylbenzyl)isatin (3); 5,7-dibromo-N-(p-cinnamyl)isatin (4) and 5,7-dibromo-N-(-naphthalen-1-ylmethyl)isatin (5) were synthesized as described previously [25, 26]. Vinblastine sulfate, colchicine, paclitaxel, doxorubicin, rhodamine-123, calcein-AM, cyclosporine A, verapamil, anti-P-gp monoclonal antibody F4 and FITC-conjugated goat anti-mouse IgG were purchased from Sigma-Aldrich (Australia). The Pgp-Glo™ assay and CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) were purchased from Promega Corporation (Australia).
2.2. Cell lines and culture conditions
The human histiocytic lymphoma (U937), uterine sarcoma parental (MES-SA) and multi-drug resistant subline (MES-SA/Dx5) were purchased from the American Type Culture Collection (ATCC, USA), distributed by Cryosite, Australia. The MES-SA/Dx5 cell line was selected by continuous in vitro exposure to doxorubicin [28] and was chosen for use in this study as its P-gp-mediated resistance by over-expression of ABCB1 is well documented [28, 29]. U937 cells were routinely cultured in RPMI-1640 medium (Invitrogen, USA) containing 5–10% (v/v) fetal calf serum (FCS, Thermo, USA) and the MES-SA cell lines were cultured in DMEM/F12 medium (Invitrogen, USA) containing 10% FCS and supplemented with 2 mM L-glutamine. Cells were maintained in culture at 37 °C, 95% humidity, 5% CO2, in a Heracell incubator (Kendro Laboratory Products, Germany). The drug resistant U937 subline, U937VbR, was generated in-house through continuous low dose exposure (beginning with 60 nM and increasing to 120 nM) of vinblastine sulfate in culture media. The U937VbR cells underwent >20 passages in the presence of vinblastine in order to develop and maintain a resistant phenotype. Cells were harvested by centrifugation at 300 × g for 5 min and viable cells, based on trypan blue exclusion, counted using a hemocytometer. All cell lines were confirmed negative for mycoplasma contamination.
2.3. PCR and cell-based analyses of resistance mechanisms
2.3.1. Real-time quantitative PCR (RT-qPCR)
Analysis of ABCB1 mRNA levels was performed by RT-qPCR. Total RNA was extracted from cells using the ISOLATE RNA mini kit (Bioline, Australia) as per manufacturer's instructions. Aliquots of RNA (1 μg) were reverse transcribed using SensiFAST™ cDNA Synthesis Kit (Bioline, Australia) as per manufacturer's instructions. The 5x TransAmp™ Buffer provided in the kit included a blend of anchored oligo dT and random hexamer primers to ensure unbiased 3′ and 5′ coverage for enhanced data accuracy. The cDNA synthesis reaction was carried out in the total volume of 20 μL in the Eppendorf Mastercycler Pro (Eppendorf, Australia) with the following program: 25 °C for 10 min (primer annealing), 42 °C for 15 min (reverse transcription), 85 °C for 5 min (inactivation), 4 °C hold (or chill on ice). The RT-qPCR was performed on all cell lines using SYBR® Green PCR master mix (Applied Biosystems, Australia) and run on the Lightcycler480 (Roche, Australia). Primers used for the amplification of the target ABCB1 gene (forward 5′-AGTGAAAAGGTTGTCCAAG-3′ and reverse 5′-AGTCTGCATTCTGGATGG-3′) and the internal reference gene, β-actin (forward 5′-GAATTCTGGCCACGGCTGCTTC-3′ and reverse 5′-AAGCTTTTTCGTGGATGCCACA-3′), were the predesigned KiCqStart® SYBR® Green Primers purchased from Sigma, Australia. The RT-qPCR reaction was conducted in the total volume of 20 μL in a 96 well Plate (LightCycler® 480 Multiwell Plate 96 white, Roche Diagnostics, Australia) with the primer concentration of 440 nM. Cycle conditions were as follows: pre-incubation 1 cycle (95 °C for 5 min), amplification 45 cycles (95 °C for 10 sec, 55 °C for 10 sec and 72 °C for 20 sec), melting curve 1 cycle (95 °C for 5 sec and 65 °C for 1 min), cooling 1 cycle (40 °C for 10 sec). The amount of ABCB1 mRNA expression was normalised to β-actin and the relative quantification of ABCB1 was calculated according to the comparative quantification cycle (Cq) method as described by Applied Biosystems using RNA isolated from MES-SA/Dx5 cell line as the internal calibrator. PCR products were subjected to electrophoresis on a 2% agarose gel and were visualized by ethidium bromide staining to confirm size (data not shown).
2.3.2. Cell surface P-gp expression
Cell surface expression of P-gp was analyzed by flow cytometry. Briefly, 1 × 105 cells/100 μL in PBS (1% w/v BSA) were incubated with either anti-P-gp monoclonal antibody F4 (diluted 1:1000) or a matched mouse isotype control in the absence (MES cell lines) or presence (U937 cell lines) of FcR block (20% v/v) for 30 min on ice, followed by three washes with ice cold PBS with 1% BSA and 0.1% NaN3. Cells were then incubated with FITC-conjugated goat anti-mouse IgG (diluted 1:200) for 30 min on ice in the dark, again followed by three washes with PBS. Cells were resuspended in PBS and the fluorescence intensity of FITC-conjugated antibody analyzed by flow cytometry (LSR II flow cytometer; BD Biosciences, San Diego, CA) (excitation 488 nm, emission collected with 515/20 band-pass filter), using FlowJo software (version 10; Tree Star Inc., Ashland, OR) to evaluate cell surface expression of P-gp. Analysis was made comparing anti-P-gp monoclonal antibody F4 to the isotype control to account for nonspecific binding and presented as a fold increase compared to parental cell lines.
2.4. Cell viability assays
In vitro cell viability assays were performed in 96-well microtiter plates as described by Vine et al. [26] using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay, employing [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] (MTS). Compounds 1-5 and commercial anticancer drugs were serially diluted in DMSO (final concentration 2.5%) or PBS and incubated with sensitive or drug resistant cell lines for 48 h. IC50 values (the dose required to inhibit the metabolic activity of 50% of the cell population) were calculated from logarithmic sigmoidal dose response curves generated using GraphPad Prism v6 software (GraphPad Inc.). Data is presented as a mean ± standard deviation (SD) from ≥2 independent experiments, performed in triplicate. The resistance index (RI) was calculated to determine the degree of acquired resistance of each cell line to its selective drug and the cross resistance developed to other drugs. RI was calculated based on the following equation:
2.5. P-gp mediated efflux assays
2.5.1. Rhodamine 123
The presence of an active efflux pump, P-gp, was probed on U937VbR cells using the fluorescent P-gp substrate, rhodamine 123 (R123), in the absence or presence of cyclosporine A (CSA) or verapamil, specific P-gp inhibitors. Cells (5000–10,000/well) were seeded in 96-well microplates at a volume of 100 μL and incubated at 37 °C for 24 h prior to addition of R123 (5 μM) in the absence or presence of CSA (20 μM), verapamil (20 μM), PBS or DMSO vehicle control (final concentration 2.5% v/v). Cells were incubated at 37 °C for a further 40 min in the dark. Culture media was removed and the cells washed with ice-cold PBS (pH 7.4). Cells were lysed with 1% Triton-X, and an aliquot (80 μL) added to wells of a 96-well black plate. Fluorescence was then measured using an excitation wavelength of 485 nm, and emission wavelength of 530 nm using a FLUOstar Optima (BMG Labtech, Germany). Fluorescence measurements were normalised to protein content in each well as determined by a Lowry protein determination assay (BioRad, Australia).
2.5.2. Calcein-AM
Non-fluorescent calcein-AM is converted to a green-fluorescent calcein by intracellular esterases. The degree of inhibition of P-gp activity can be quantified by measuring the increase in intracellular calcein fluorescence. MES-SA/Dx5 cells (25,000/well) were seeded in 96-well microplates at a volume of 100 μL and incubated at 37 °C, 5% CO2 for 24 h prior to addition of CSA (40 μM), compounds 1-5 (40 μM) or DMSO vehicle control (final concentration 2% v/v), with calcein-AM (1 μM) added immediately after. Cells were further incubated for 40 min at 37 °C before being imaged on the IncuCyte ZOOM (Essen BioScience USA) using the phase and green filters at 20 × magnification (1392 × 1040, 0.61 μm/pixel, acquisition time 400 ms). All treatments were in triplicate and 4 images per well were acquired (total n = 12). The approximate number of cells scanned was 300-400 cells per image. IncuCyte ZOOM 2015A software was used to determine the fraction of calcein positive cells (defined as cells with a green pixel intensity greater than that observed for cells not incubated with calcein (i.e. green pixel intensity >10 GCU)) by dividing the average green fluorescence confluence by the average cell confluence.
2.6. Cell morphology
The ability of the N-alkylisatins to maintain their mode of action and disrupt microtubule dynamics was determined in the MDR resistant U937VbR subline using light microscopy. Vinblastine (60 nM), colchicine (10 nM) or representative N-alkylisatins 2, 4 and 5 (2.5 μM) were incubated with U937 or U937VbR cells for 24 h and changes in cell morphology compared to control treated cells.
2.7. P-gp ATPase assay
The effect of compounds 1-5 on P-gp ATPase activity was measured using the Pgp-Glo™ assay system. Verapamil, a P-gp substrate and competitive inhibitor for other substrates, was used as a positive control. Sodium orthovanadate (Na3VO4), a selective inhibitor of P-gp ATPase, was used to account for P-gp-independent ATP consumption in the reactions. All compounds and controls were tested at either 40 μM or 200 μM (in 1% DMSO) as per the manufacturer's protocol. Sample luminescence was recorded after 50 min incubation with the ATP detection reagent using a luminometer (POLARstar Omega, BMG Labtech, Ortenberg, Germany). Change in luminescence in relative light units (RLU; RLUNa3VO4–RLUcompound) is inversely proportional to ATP levels, which are negatively correlated with the activity of P-gp ATPase and therefore P-gp-mediated transport.
2.8. Statistical analysis
Data is presented as a mean ± SD based on two or more independent experiments, performed in triplicate. One-way analysis of variance (ANOVA) using a Dunnett's multiple comparisons post-test or t-tests were used to determine the significance among groups (GraphPad Prism 6.01). P-values < 0.05 were considered significant.
3. Results
3.1. U937VbR and MES-SA/Dx5 overexpress ABCB1 mRNA and its protein product P-gp
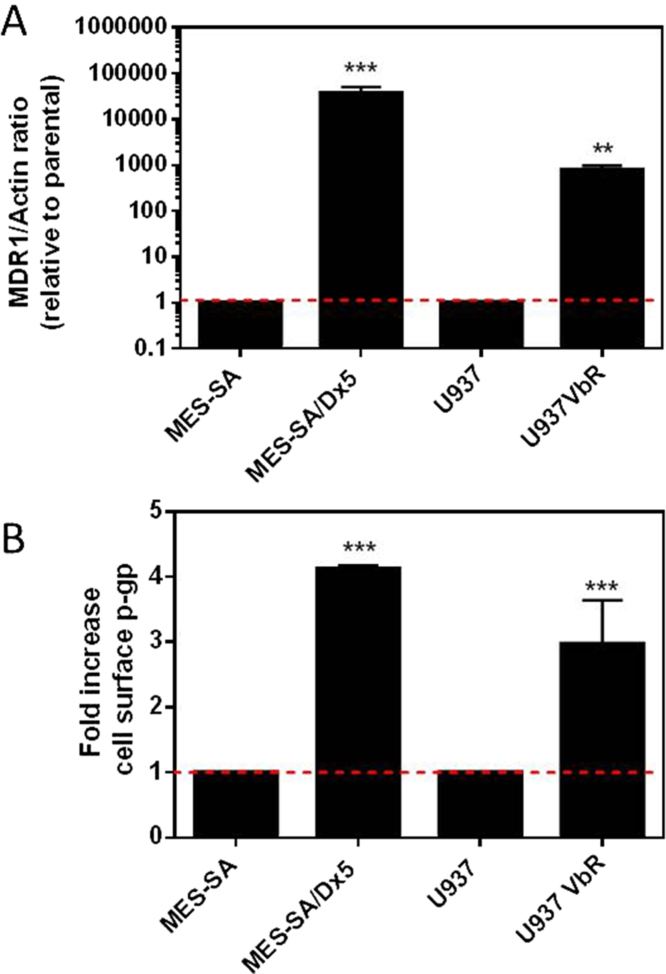
In order to ascertain if P-gp mediated efflux contributes to the mechanism of resistance of our newly generated U937VbR cell line to the commercial anticancer drugs vinblastine, colchicine, paclitaxel and doxorubicin, as well as compounds 1-5, we probed for expression of the ABCB1 gene, coding for P-gp, by RT-qPCR. The U937VbR cells exhibited a 765-fold increase in ABCB1 mRNA transcript levels compared to the parental U937 cells (Fig. 2A), which were similar to that expressed in the control MES-SA/Dx5 multi-drug resistant cell line, previously reported to highly express P-gp [29]. Increased ABCB1 mRNA expression was associated with a 3-4-fold increase in cell surface expression of P-gp on both resistant cell lines compared to their parental controls (Fig. 2B) as determined by immunofluorescence staining and flow cytometry. This result was also confirmed by assessing the uptake and retention of the fluorescent P-gp substrate R123 (Supplementary Information, Fig. S1). The fluorescent compound was incubated with cells in the presence or absence of two P-gp inhibitors. Extrusion of R123 substantially decreased in the presence of CSA or verapamil in the U937VbR subline.
Fig. 2.
U937VbR and MES-SA/Dx5 overexpress ABCB1 mRNA and its protein product P-gp. (A) Real-time quantitative PCR (RT-qPCR) of the ABCB1 mRNA transcript level in MES-SA, MES-SA/Dx5, U937 and U937VbR cell lines. Raw expression values were normalized to the internal reference gene, β-actin and expressed as a fold increase to respective cells lines. (B) Fold-increase in P-gp. Immunofluorescent staining of P-gp on the surface of parental and resistant cell lines as determined by flow cytometry. Values are the mean (± SD) of triplicates.
3.2. N-Alkylisatins retain potency against MDR cancer cell lines
3.2.1. Cell viability
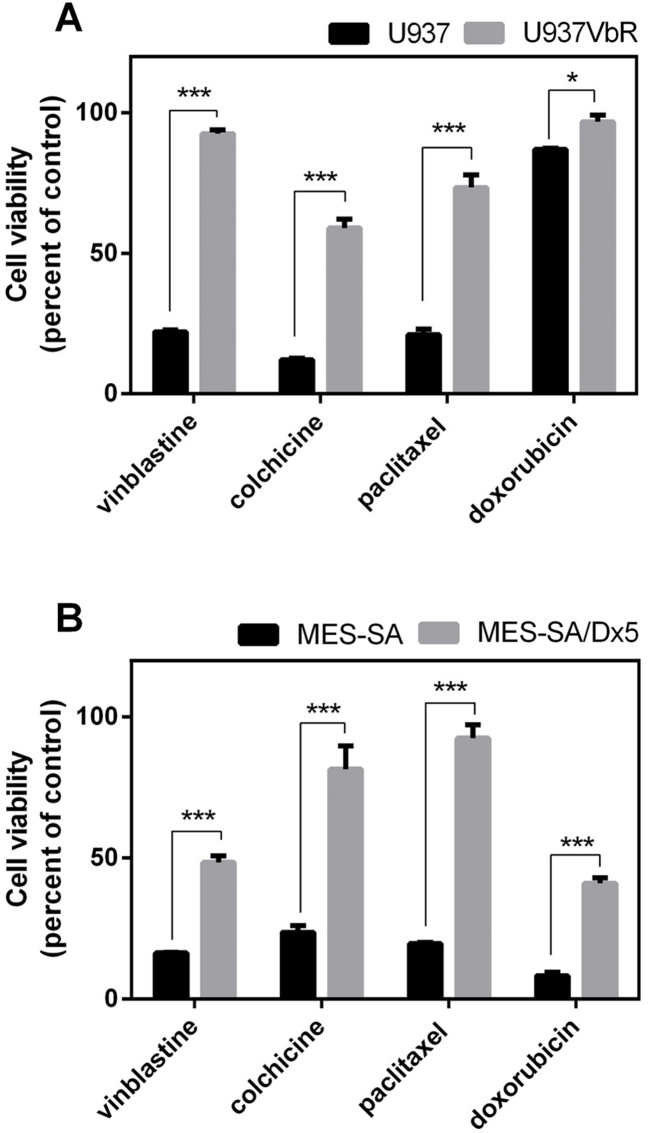
The degree of cross resistance to a single concentration of the commercial anticancer agents vinblastine, colchicine, paclitaxel and doxorubicin was determined in the U937VbR and MES-SA/Dx5 MDR sublines using the MTS cell proliferation assay, prior to screening the isatin compounds 1-5. Both the U937VbR and MES-SA/Dx5 cell lines exhibited a significant decrease in sensitivity compared to parental control cell lines (U937 and MES-SA) to all four cytotoxic chemotherapeutics at 100 nM, with a 3-5-fold decrease in potency for the microtubule targeting drugs (vinblastine, colchicine and paclitaxel) and 1.1-5.0-fold decrease in potency for the topoisomerase inhibitor (doxorubicin) (Fig. 3A & B). The U937VbR subline exhibited the greatest resistance to vinblastine and colchicine with a 4.2 ± 0.3 and 4.9 ± 0.5-fold decrease in drug sensitivity, respectively. Conversely the MES-SA/Dx5 subline displayed the greatest resistance to doxorubicin and paclitaxel with a 5.0 ± 0.6 and 4.7 ± 0.3-fold decrease in drug sensitivity, respectively after 48 h.
Fig. 3.
U937VbR and MES-SA/Dx5 cell lines exhibit cross resistance to the anticancer drugs vinblastine, colchicine, paclitaxel and doxorubicin. Cell viability of (A) U937 (black bars) or U937VbR (grey bars) and (B) MES-SA (black bars) or MES-SA/Dx5 (grey bars) after 48 h treatment with 0.1 μM of the commercial anticancer agents vinblastine, colchicine, paclitaxel and doxorubicin. Values are the mean (± SEM) of triplicates.
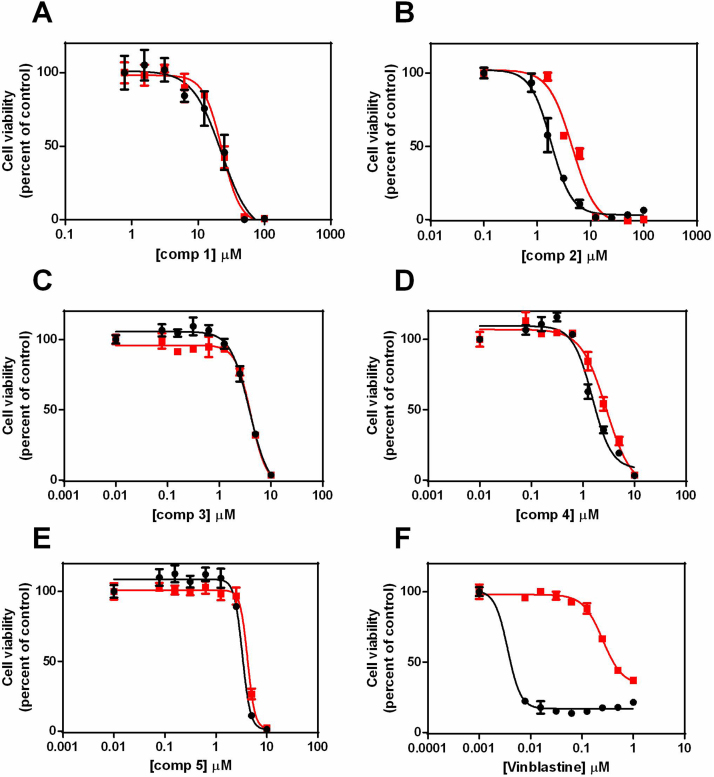
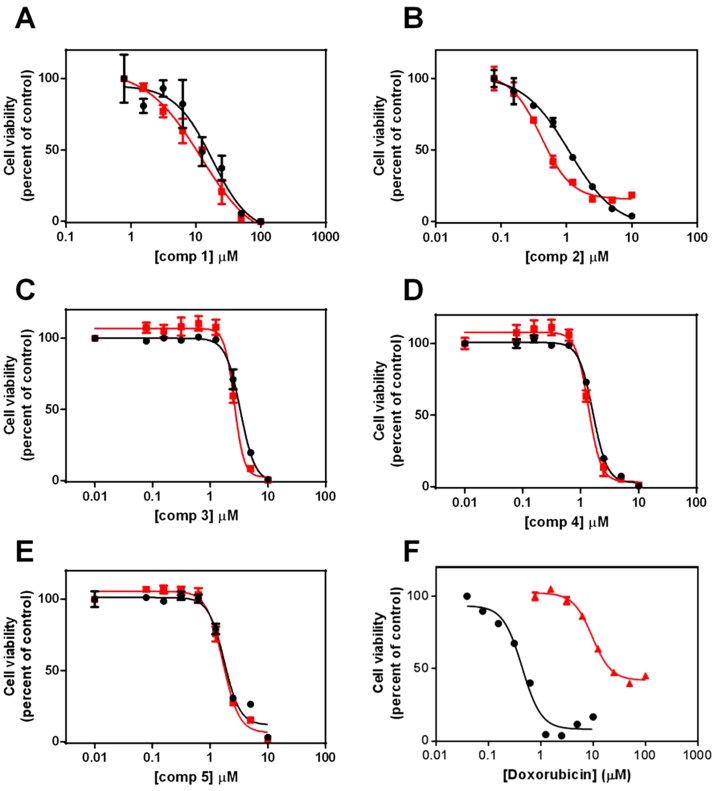
Comparison of the dose response curves for the isatin derivatives (1-5) against the MDR cell lines U937VbR and MES-SA/Dx5 found them to be bioequivalent to their respective chemosensitive cell lines at both 24 h (Fig. S2; Supplementary Information) and 48 h (Fig. 4 and Fig. 5). This was irrespective of the compounds' molecular weight, lipophilicity, N-alkylation and substitution pattern. This was in stark contrast to the commercial anticancer agents vinblastine (Fig. 4F) and doxorubicin (Fig. 5F). No significant difference in the IC50 values of compounds 1-5 were observed between resistant and parental cells lines, while vinblastine, paclitaxel and doxorubicin all exhibited a significant decrease in potency against the P-gp overexpressing U937VbR and MES-SA/Dx5 MDR cell lines (Table 1).
Fig. 4.
Dose-dependent cytotoxicity of the isatin derivatives 1-5 is maintained in the vinblastine resistant U937 (U937VbR) subline. Cells were incubated with increasing concentrations of either (A) 5,7-dibromoisatin (1), (B) 5,7-dibromo-N-(p-hydroxymethylbenzyl)isatin (2), (C) 5,7-dibromo-N-(p-phenylbenzyl)isatin (3), (D) 5,7-dibromo-N-(p-cinnamyl)isatin (4), (E) 5,7-dibromo-N-(naphthalen-1-ylmethyl)isatin (5) or (F) vinblastine (as a control). The relative sensitivity of the U937 (black) and U937VbR (red) cell lines to the drugs was determined by MTS assay after 48 h incubation. Values are the mean (± SEM) of triplicates.
Fig. 5.
Dose-dependent cytotoxicity of the isatin derivatives 1-5 is maintained in doxorubicin resistant MES-SA (MES-SA/Dx5) subline. Cells were incubated with increasing concentrations of either (A) 5,7-dibromoisatin (1), (B) 5,7-dibromo-N-(p-hydroxymethylbenzyl)isatin (2), (C) 5,7-dibromo-N-(p-phenylbenzyl)isatin (3), (D) 5,7-dibromo-N-(p-cinnamyl)isatin (4), (E) 5,7-dibromo-N-(napthalen-1-ylmethyl)isatin (5) or (F) colchicine (as a control). The relative sensitivity of the MES-SA (black) and MES-SA/Dx5 (red) cell lines to the drugs was determined by MTS assay after 48 h incubation. Values are the mean (± SEM) of triplicates.
Table 1.
N-alkylisatins retain potency against the MES-SA/Dx5 and U937VbR MDR cancer cell lines.
| IC50 (μM) ± SD | RI | |||||
|---|---|---|---|---|---|---|
| Compound | U937 | U937VbR | MES-SA | MES-SA/Dx5 | U937VbR:U937 | MES-SA/Dx5:MES-SA |
| 1 | 16.00 (± 5.57) |
24.64 (± 2.13) |
17.25 (± 2.51) |
15.01 (± 0.22) |
1.5 | 0.9 |
| 2 | 1.52 (±1.44) |
2.84 (±2.38) |
0.49 (± 0.55) |
0.39 (±0.31) |
1.9 | 0.8 |
| 3 | 2.02 (±1.64) |
2.22 (±1.73) |
2.74 (± 2.42) |
1.89 (±1.48) |
1.1 | 0.7 |
| 4 | 1.48 (± 0.05) |
2.24 (± 0.83) |
1.08 (± 0.77) |
0.90 (± 0.64) |
1.5 | 0.8 |
| 5 | 1.81 (± 1.49) |
2.83 (± 2.11) |
0.241 (± 0.02) |
0.311 (± 0.01) |
1.6 | 1.3 |
| Vinblastine | 0.0032 (± 0.00) |
0.241 (± 0.02) |
0.0034 (± 0.02) |
0.082 (± 0.02) |
75 | 24 |
| Doxorubicin | – | – | 0.727 (± 0.20) |
6.70 (± 2.57) |
– | 9.2 |
| Paclitaxel | 0.001 (± 0.001) |
0.122 (±0.033) |
– | – | 122 | – |
- Not tested.
3.2.2. Cell morphology
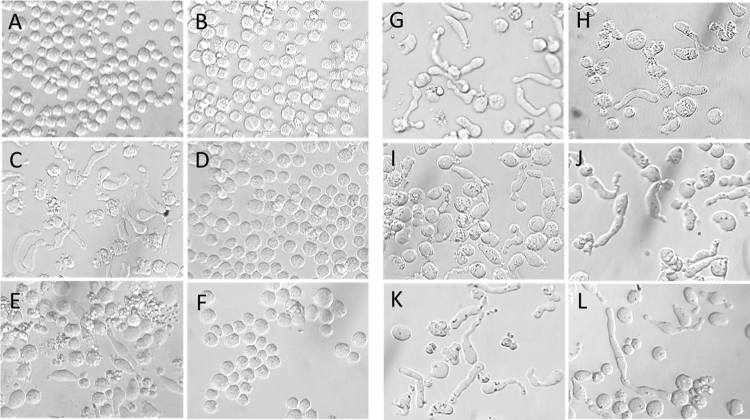
We have previously reported that lymphocytic cell lines display characteristic changes and elongated cell morphology after treatment with N-alkylisatins targeting tubulin polymerization [25, 26, 30]. We therefore monitored the morphology of resistant and parental U937 cell lines after treatment with the commercial microtubule targeting drugs, vinblastine and colchicine and compared this to three representative N-alkylisatins which are known to alter cell shape. Chemoresistant U937VbR cells treated with 60 nM vinblastine or 10 nM colchicine exhibited morphologies identical to vehicle control treated U937 and U937VbR cells (Fig. 6) indicating abrogation of activity. Conversely, the morphology of U937VbR cells treated with the N-alkylisatin based microtubule destabilizers 2, 4 and 5 was consistent with that observed for the chemosensitive parental U937 cell line (Fig. 6G–L) and demonstrates maintenance of activity and mode of action against this MDR cell line.
Fig. 6.
N-alkylisatins retain their mode of action against the vinblastine resistant U937VbR subline. Morphological effects of commercial and N-alkylisatin based microtubule destabilizers on U937 and U937VbR cells. Cells were treated with either (A/B) DMSO vehicle control, (C/D) 60 nM vinblastine, (E/F) 10 nM colchicine or 2.5 μM of the N-alkylisatins (G/H) 5,7-dibromo-N-(p-hydroxymethylbenzyl)isatin (2), (I/J) 5,7-dibromo-N-(p-cinnamyl)isatin (4) and (K/L) 5,7-dibromo-N-(naphthalen-1-ylmethyl)isatin (5) for 24 h. Images were obtained by brightfield microscopy on an inverted light microscope at 40x magnification.
3.3. N-Alkylisatins are not substrates or inhibitors of P-gp
3.3.1. Calcein-AM efflux assay
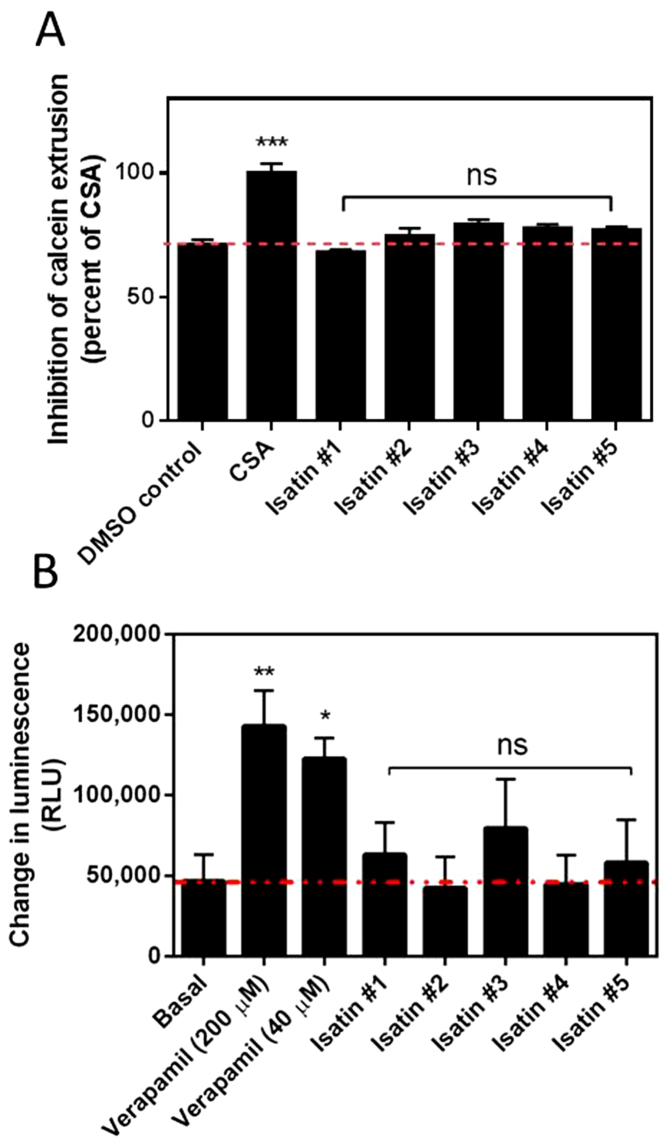
The intracellular accumulation of the fluorescent reporter calcein was used to assess the functional activity of P-gp in the MDR MES-SA/Dx5 cell line in the presence of compounds 1-5. Non-fluorescent, hydrophobic calcein-AM enters the lipid bilayer and is pumped out of the cell by P-gp, reducing the intracellular concentration of calcein-AM. Modulators of P-gp activity reduce the rate of calcein AM efflux, leading to increased intracellular calcein AM, which is then hydrolyzed by intracellular esterases, converting it to the fluorescent product calcein. Free calcein is hydrophilic and thus retained inside the cells. After 40 min the proportion of calcein positive cells was 1.5 times greater for cells treated with the P-gp inhibitor CSA than for cells treated with the vehicle control (P = 0.0014; Fig 7A). No significant increase in intracellular accumulation of calcein was seen after incubation with compounds 1-5 compared to the vehicle control, indicating that isatins do not inhibit P-gp.
Fig. 7.
N-Alkylisatins are not substrates or inhibitors of P-gp. (A) Relative effect of the isatins on Pgp-mediated extrusion of Calcein AM. Cells (MES-SA/Dx5) were incubated with compounds 1-5 (40 μM), CSA (40 μM) or vehicle control (2% v/v) for 40 min. Data is normalized to CSA treated cells (100% inhibition) and presented as the mean ± SD (n = 12) determined using live cell imaging (IncuCyte ZOOM). ***P < 0.001; ns = not significant. (B) Effect of test compounds on P-gp ATPase activity. P-gp membranes in the presence of compounds 1-5 (in 1% DMSO) or verapamil (positive control) at either 40 μM or 200 μM were treated with MgATP as per the Pgp-GloTM assay protocol. Change in luminescence in relative light units (RLU) was calculated by subtracting the RLU of each test compound from the RLU of sodium orthovanadate (selective inhibitor of P-gp ATPase) to correct for P-gp independent ATP consumption. Change in luminescence is inversely proportional to ATP levels, which are negatively correlated with the activity of P-gp ATPase and therefore P-gp-mediated transport. Red line indicates basal activity. Data are presented as the mean ± SD (n = 3). **P < 0.01; *P < 0.05; ns = not significant.
3.3.2. P-gp ATPase assay
In order to further confirm that compounds 1-5 do not directly interact with P-gp, we measured the change in luminescence associated with P-gp ATPase hydrolysis. Compounds that do not directly interact with P-gp have no effect on basal ATPase activity; compounds that are substrates for transport by P-gp stimulate basal ATPase activity while inhibitors decrease basal ATPase activity. Verapamil is a substrate for P-gp transport and showed a significant increase in the change in luminescence (RLUNa3VO4–RLUverapamil) at 40 μM and 200 μM (P < 0.05 and P < 0.01, respectively) compared to basal activity (RLUNa3VO4–RLUuntreated), which represents verapamil-stimulated P-gp ATPase activity (Fig. 7). Compounds 1-5 did not show a significant change in luminescence compared to basal activity; therefore, N-alkylisatins do not interact with P-gp.
4. Discussion
MDR has become a major barrier to the clinical efficacy of a wide range of chemotherapeutic drugs, prompting the development of new strategies in cancer treatment [31, 32, 33, 34]. The N-alkylisatins are part of a continually growing, diverse family of potent cytotoxins which show activity in vitro against cancers that are often associated with clinical resistance. Further, investigations in vivo have identified 5,7-dibromo-N-(p-trifluoromethylbenzyl)isatin to be efficacious in an orthotopic human breast carcinoma xenograft mouse model [30] and highlights the importance of further investigations into this class of compounds in the context of MDR. In determining the ability of the N-alkylisatins to overcome MDR, a vinblastine resistant U937 cell line, previously shown to be sensitive to N-alkylisatins, was established as a model of clinically-developed, acquired chemoresistance due to continuous drug exposure. Vinblastine was chosen as it is a microtubule destabilizer and exhibits a similar mode of action to that of the N-alkylisatins [26]. It was also chosen for the potential to successfully create an MDR phenotype with broad cross-resistance, as cell lines resistant to the Vinca alkaloids have been shown to exhibit decreased sensitivity to a number of structurally unrelated small-molecule anticancer agents, such as colchicine and anthracyclines [35, 36, 37]. The mechanism of resistance is primarily due to over-expression of the efflux pump protein, P-gp [1, 38].
We found the most effective method for inducing vinblastine resistance in the U937 cell line was through continuous low dose exposure to 120 nM vinblastine, excluding a recovery period in drug-free media. A resistance index (RI) of 75 was observed for vinblastine and cross-resistance to structurally unrelated chemotherapeutics such as colchicine, paclitaxel and doxorubicin was clearly demonstrated (Fig. 3; Table 1). Marked upregulation of the ABCB1 gene and decreased uptake and retention of the P-gp substrate R123 was observed in the U937VbR cell line suggesting that P-gp mediated drug efflux plays a role in the chemoresistance profile observed in this new subline. Other mechanisms of resistance to microtubule-binding agents such as the Vinca alkaloids and taxanes include alterations to their microtubule targets. Mechanisms such as mutations in the binding motifs in α- or β-tubulin, or upregulation of alternative tubulin isotypes such as βIII tubulin have been shown to be important in driving paclitaxel and vincristine resistance in non-small-cell lung cancer however reasons for this are still poorly understood [39]. Cross-resistance of such mechanisms, however, appear to be limited to tubulin-binding agents only, suggesting that they are not the determinant of the MDR phenotype observed in response to continuous exposure to vinblastine in this study. This is supported by our cell morphology studies (Fig. 6) which indicates that there has been no change in the intracellular microtubule target of the isatins under the selection pressure of the microtubule disrupting agent vinblastine. Further, the breast cancer resistance protein (BCRP) [40], another ATP-binding cassette transporter capable of inducing drug resistance, does not confer resistance to the Vinca alkaloids, epipodophyllotoxins, paclitaxel, or cisplatin [41] suggesting that it is unlikely to be contributing to the MDR phenotype observed in our MDR subline. While our data is consistent with overexpression of ABCB1 as the major efflux mechanism responsible for the resistant phenotype observed in the U937VbR cell line in this study, the expression of other efflux pumps such as multidrug resistance associated-protein 1 (MRP1) should also be profiled to fully complete characterization.
Four structurally diverse N-alkylisatin analogues (2-5) and the parent dibromoisatin molecule (1) were chosen for use in this study due to the N-alkylisatins' relatively high potency and synthetic availability. Furthermore, while P-gp and MRP are capable of extruding a wide variety of anticancer compounds, they can't remove them all, with structures containing the indole core featuring heavily in a growing number of anticancer and antibacterial compounds that are not substrates of, or can reverse drug efflux mechanisms [21, 42, 43]. The cytotoxic effect of compounds 1-5 was therefore evaluated in our in vitro MDR model system to determine their potential susceptibility to P-gp-mediated efflux. Two different parental cell lines were used; one derived from human histiocytic lymphoma and the other from human uterine sarcoma, as MDR screening is most often performed in model systems which employ cell lines derived from hematological malignancies while the use of cell lines derived from solid tumors is usually less popular [29]. The IC50 values obtained for all 5 isatin-based compounds against the U937VbR and MES-SA/Dx5 MDR sublines were not statistically different to those observed for the parental control cell lines. Further, the dose response profiles were virtually superimposable and not statistically different by 2 way ANOVA, which was in contrast to the dose response profiles of the commercial anticancer agents, suggesting that this class of molecule is not susceptible to P-gp-mediated efflux over concentrations ranging from 100 nM- 100 μM. Importantly, the growth properties of the resistant cell lines were identical to that of the parental cell lines (Supplementary Information Fig. S3) suggesting that differences in cell population doubling time (rate of mitosis) did not impact on our findings. Our results corroborate those reported by Hall et al. who describe isatin-β-thiosemicarbazones to be neither substrates nor inhibitors of P-gp, but which also act to reduce P-gp expression [21, 22]. While the effect of isatins 1-5 on the expression of P-gp was not interrogated in this study, experiments are underway to investigate the effect on ABCB1 expression in U937 and MES-SA cell lines after long-term, continuous culture with 5,7-dibromo-N-(p-hydroxymethylbenzyl) isatin (2). Preliminary findings suggest continuous exposure to increasing concentrations of compound 2 over a period of 4 months does not result in significant upregulation of ABCB1 and cells maintain drug sensitivity (Supplementary Information Fig. S4).
Research within our laboratory has demonstrated that N-benzylation of 5,7-dibromoisatin substantially increases cytotoxic activity in vitro by up to two orders of magnitude against a range of human cancer cell lines [25, 26]. Using pharmacophores, Hall et al. identified the aromatic/hydrophobic groups at the N4 position of the thiosemicarbazone, as well as the isatin moiety, to be important features for the maintenance of MDR-selective activity [21, 22]. In this study, structure activity relationship experiments found that alkylation at the N1 position increased cytotoxic activity as reported [25, 26], but had no effect on MDR-selective activity, confirming reliance on the isatin core. While the mechanisms by which the isatins overcome MDR have not yet been elucidated, we can hypothesize that may they include poor P-gp substrate affinity, ability to alter the biophysical properties of the plasma membrane or enter the cell via alternative drug uptake mechanisms.
5. Conclusions
The emergence of resistance to clinical cancer treatments poses a significant problem in the management and treatment of cancer. While combination therapies are showing promise, there is still a need to develop more efficacious therapeutics that are able to overcome resistance mechanisms employed by tumor cells. The potential novel interaction of the N-alkylisatins with tubulin, together with their high potency in vitro and ability to inhibit tumor growth in vivo, point to the importance of further investigations into these compounds.
Declarations
Author contribution statement
Kara L. Vine: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Lisa Belfiore: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Luke Jones; Elahe Minaei: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Julie M. Locke: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Samantha Wade: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Marie Ranson: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Kara L. Vine was supported by a University of Wollongong Vice Chancellor's Fellowship (2011-2015) and a Cure Cancer Australia Foundation Project grant (APP1045831).
Conflict of interest
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at :http://dx.doi.org/10.1016/j.heliyon.2015.e00060.
No additional information is available for this paper.
Acknowledgements
We are grateful to Emeritus Professor John Bremner (School of Chemistry, University of Wollongong) for the review of and comments on this manuscript and Dr Danielle Skropeta/ Dr Lidia Matesic (School of Chemistry, University of Wollongong) for providing compound 5.
Supplementary data
The following are Supplementary data to this article:
References
- 1.Szakács G., Paterson J.K., Ludwig J.A., Booth-Genthe C., Gottesman M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006;5(3):219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 2.Szakács G., Hall M.D., Gottesman M.M., Boumendjel A., Kachadourian R., Day B.J., Baubichon-Cortay H., Di Pietro A. Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chem. Rev. 2014;114(11):5753–5774. doi: 10.1021/cr4006236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binkhathlan Z., Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr. Cancer Drug Targets. 2013;13(3):326–346. doi: 10.2174/15680096113139990076. [DOI] [PubMed] [Google Scholar]
- 4.Tsuruo T., Lida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981;41(5):1967–1972. [PubMed] [Google Scholar]
- 5.Lamendola D.E., Duan Z., Yusuf R.Z., Seiden M.V. Molecular description of evolving paclitaxel resistance in the SKOV-3 human ovarian carcinoma cell line. Cancer Res. 2003;63(9):2200–2205. [PubMed] [Google Scholar]
- 6.Yusuf R.Z., Duan Z., Lamendola D.E., Penson R.T., Seiden M.V. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr. Cancer Drug Targets. 2003;3(1):1–19. doi: 10.2174/1568009033333754. [DOI] [PubMed] [Google Scholar]
- 7.Marbeuf-Gueye C., Broxterman H.J., Dubru F., Priebe W., Garnier-Suillerot A. Kinetics of anthracycline efflux from multidrug resistance protein-expressing cancer cells compared with P-glycoprotein-expressing cancer cells. Mol. Pharmacol. 1998;53(1):141–147. doi: 10.1124/mol.53.1.141. [DOI] [PubMed] [Google Scholar]
- 8.Lalloo A.K., Luo F.R., Guo A., Paranjpe P.V., Lee S.H., Vyas V., Rubin E., Sinko P.J. Membrane transport of camptothecin: facilitation by human P-glycoprotein (ABCB1) and multidrug resistance protein 2 (ABCC2) BMC Med. 2004;2:16. doi: 10.1186/1741-7015-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Widmer N., Colombo S., Buclin T., Decosterd L.A. Functional consequence of MDR1 expression on imatinib intracellular concentrations. Blood. 2003;102(3):1142. doi: 10.1182/blood-2003-03-0993. [DOI] [PubMed] [Google Scholar]
- 10.Mahon F.X., Belloc F., Lagarde V., Chollet C., Moreau-Gaudry F., Reiffers J., Goldman J.M., Melo J.V. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101(6):2368–2373. doi: 10.1182/blood.V101.6.2368. [DOI] [PubMed] [Google Scholar]
- 11.Lagas J.S., van Waterschoot R.A., van Tilburg V.A., Hillebrand M.J., Lankheet N., Rosing H., Beijnen J.H., Schinkel A.H. Brain accumulation of dasatinib is restricted by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and can be enhanced by elacridar treatment. Clini. Cancer Res. 2009;15(7):2344–2351. doi: 10.1158/1078-0432.CCR-08-2253. [DOI] [PubMed] [Google Scholar]
- 12.Chu C., Abbara C., Noël-Hudson M.S., Thomas-Bourgneuf L., Gonin P., Farinotti R., Bonhomme-Faivre L. Disposition of everolimus in mdr1a-/1b- mice and after a pre-treatment of lapatinib in Swiss mice. Biochem. Pharmacol. 2009;77(10):1629–1634. doi: 10.1016/j.bcp.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Amiri-Kordestani L., Basseville A., Kurdziel K., Fojo A.T., Bates S.E. Targeting MDR in breast and lung cancer: discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist. Updates. 2012;15(1–2):50–61. doi: 10.1016/j.drup.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volm M., Efferth T. Resistance to Targeted ABC Transporters in Cancer. Springer; Switzerland: 2015. Role of P-Glycoprotein for Resistance of Tumors to Anticancer Drugs: From Bench to Bedside; pp. 1–26. [Google Scholar]
- 15.Goldstein L.J., Galski H., Fojo A., Willingham M., Lai S.L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G.M., Lieber M., Cossman J., Gottesman M.M., Pastan I. Expression of a multidrug resistance gene in human cancers. J. Natl. Cancer Inst. 1989;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- 16.Raderer M., Scheithauer W. Clinical trials of agents that reverse multidrug resistance. A literature review. Cancer. 1993;72(12):3553–3563. doi: 10.1002/1097-0142(19931215)72:12<3553::aid-cncr2820721203>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Pennock G.D., Dalton W.S., Roeske W.R., Appleton C.P., Mosley K., Plezia P., Miller T.P., Salmon S.E. Systemic toxic effects associated with high-dose verapamil infusion and chemotherapy administration. J. Natl. Cancer Inst. 1991;83(2):105–110. doi: 10.1093/jnci/83.2.105. [DOI] [PubMed] [Google Scholar]
- 18.List A.F., Spier C., Greer J., Wolff S., Hutter J., Dorr R., Salmon S., Futscher B., Baier M., Dalton W. Phase I/II trial of cyclosporine as a chemotherapy-resistance modifier in acute leukemia. J. Clin. Oncol. 1993;11(9):1652–1660. doi: 10.1200/JCO.1993.11.9.1652. [DOI] [PubMed] [Google Scholar]
- 19.Friedenberg W.R., Rue M., Blood E.A., Dalton W.S., Shustik C., Larson R.A., Sonneveld P., Greipp P.R. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): a trial of the Eastern Cooperative Oncology Group. Cancer. 2006;106(4):830–838. doi: 10.1002/cncr.21666. [DOI] [PubMed] [Google Scholar]
- 20.Nobili S., Landini I., Mazzei T., Mini E. Overcoming tumor multidrug resistance using drugs able to evade P-glycoprotein or to exploit its expression. Med. Res. Rev. 2012;32(6):1220–1262. doi: 10.1002/med.20239. [DOI] [PubMed] [Google Scholar]
- 21.Hall M.D., Brimacombe K.R., Varonka M.S., Pluchino K.M., Monda J.K., Li J., Walsh M.J., Boxer M.B., Warren T.H., Fales H.M., Gottesman M.M. Synthesis and structure-activity evaluation of isatin-beta-thiosemicarbazones with improved selective activity toward multidrug-resistant cells expressing P-glycoprotein. J. Med. Chem. 2011;54(16):5878–5889. doi: 10.1021/jm2006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall M.D., Salam N.K., Hellawell J.L., Fales H.M., Kensler C.B., Ludwig J.A., Szakács G., Hibbs D.E., Gottesman M.M. Synthesis, activity, and pharmacophore development for isatin-beta-thiosemicarbazones with selective activity toward multidrug-resistant cells. J. Med. Chem. 2009;52(10):3191–3204. doi: 10.1021/jm800861c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Türk D., Hall M.D., Chu B.F., Ludwig J.A., Fales H.M., Gottesman M.M., Szakács G. Identification of compounds selectively killing multidrug-resistant cancer cells. Cancer Res. 2009;69(21):8293–8301. doi: 10.1158/0008-5472.CAN-09-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig J.A., Szakács G., Martin S.E., Chu B.F., Cardarelli C., Sauna Z.E., Caplen N.J., Fales H.M., Ambudkar S.V., Weinstein J.N., Gottesman M.M. Selective toxicity of NSC73306 in MDR1-positive cells as a new strategy to circumvent multidrug resistance in cancer. Cancer Res. 2006;66(9):4808–4815. doi: 10.1158/0008-5472.CAN-05-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matesic L., Locke J.M., Bremner J.B., Pyne S.G., Skropeta D., Ranson M., Vine K.L. N-phenethyl and N-naphthylmethyl isatins and analogues as in vitro cytotoxic agents. Bioorg. Med. Chem. 2008;16(6):3118–3124. doi: 10.1016/j.bmc.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Vine K.L., Locke J.M., Ranson M., Pyne S.G., Bremner J.B. An investigation into the cytotoxicity and mode of action of some novel N-alkyl-substituted isatins. J. Med. Chem. 2007;50(21):5109–5117. doi: 10.1021/jm0704189. [DOI] [PubMed] [Google Scholar]
- 27.Vine K.L., Matesic L., Locke J.M., Ranson M., Skropeta D. Cytotoxic and anticancer activities of isatin and its derivatives: a comprehensive review from 2000-2008. Anti-cancer Agents Med. Chem. 2009;9(4):397–414. doi: 10.2174/1871520610909040397. [DOI] [PubMed] [Google Scholar]
- 28.Harker W.G., Sikic B.I. Multidrug (pleiotropic) resistance in doxorubicin-selected variants of the human sarcoma cell line MES-SA. Cancer Res. 1985;45(9):4091–4096. [PubMed] [Google Scholar]
- 29.Wesolowska O., Paprocka M., Kozlak J., Motohashi N., Dus D., Michalak K. Human sarcoma cell lines MES-SA and MES-SA/Dx5 as a model for multidrug resistance modulators screening. Anti-cancer Res. 2005;25(1A):383–389. [PubMed] [Google Scholar]
- 30.Vine K.L., Indira Chandran V., Locke J.M., Matesic L., Lee J., Skropeta D., Bremner J.B., Ranson M. Targeting urokinase and the transferrin receptor with novel, anti-mitotic N-alkylisatin cytotoxin conjugates causes selective cancer cell death and reduces tumor growth. Curr. Cancer Drug Targets. 2012;12(1):64–73. doi: 10.2174/156800912798888983. [DOI] [PubMed] [Google Scholar]
- 31.Kunjachan S., Rychlik B., Storm G., Kiessling F., Lammers T. Multidrug resistance: Physiological principles and nanomedical solutions. Adv. Drug Deliv. Rev. 2013;65(13–14):1852–1865. doi: 10.1016/j.addr.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metzger-Filho O., Moulin C., de Azambuja E., Ahmad A. Larotaxel: broadening the road with new taxanes. Expert Opin. Investig. Drugs. 2009;18(8):1183–1189. doi: 10.1517/13543780903119167. [DOI] [PubMed] [Google Scholar]
- 33.Lee J.J., Swain S.M. Development of novel chemotherapeutic agents to evade the mechanisms of multidrug resistance (MDR) Semin. Oncol. 2005;32(6 Suppl 7):S22–S26. doi: 10.1053/j.seminoncol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Wu C.P., Calcagno A.M., Ambudkar S.V. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr. Mol. Pharmacol. 2008;1(2):93–105. doi: 10.2174/1874467210801020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemontt J.F., Azzaria M., Gros P. Increased mdr gene expression and decreased drug accumulation in multidrug-resistant human melanoma cells. Cancer Res. 1988;48(22):6348–6353. [PubMed] [Google Scholar]
- 36.Sehested M., Jensen P.B., Skovsgaard T., Bindslev N., Demant E.J., Friche E., Vindeløv L. Inhibition of vincristine binding to plasma membrane vesicles from daunorubicin-resistant Ehrlich ascites cells by multidrug resistance modulators. Br. J. Cancer. 1989;60(6):809–814. doi: 10.1038/bjc.1989.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friche E., Skovsgaard T., Nissen N.I. Anthracycline resistance. Acta Oncol. 1989;28(6):877–881. doi: 10.3109/02841868909092324. [DOI] [PubMed] [Google Scholar]
- 38.Chen G.K., Durán G.E., Mangili A., Beketic-Oreskovic L., Sikic B.I. MDR 1 activation is the predominant resistance mechanism selected by vinblastine in MES-SA cells. Br. J. Cancer. 2000;83(7):892–898. doi: 10.1054/bjoc.2000.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer. 2010;10(3):194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 40.Borges-Walmsley M.I., McKeegan K.S., Walmsley A.R. Structure and function of efflux pumps that confer resistance to drugs. Biochem. J. 2003;376(Pt 2):313–338. doi: 10.1042/BJ20020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen J.D., Schinkel A.H. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2) Mol. Cancer Ther. 2002;1(6):427–434. [PubMed] [Google Scholar]
- 42.Wink M., Ashour M.L., El-Readi M.Z. Secondary Metabolites from Plants Inhibiting ABC Transporters and Reversing Resistance of Cancer Cells and Microbes to Cytotoxic and Antimicrobial Agents. Front. Microbiol. 2012;3:130. doi: 10.3389/fmicb.2012.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto T., Fujii R., Sugita M., Sumizawa T., Sakai S., Takahashi T., Sueda N., Furukawa T., Akiyama S., Nagata Y. Effect of newly synthesized indole derivatives on multidrug resistance in human cancer cells. Anti-cancer Drug Des. 1994;9(3):251–261. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.