Abstract
Background
Numerous epidemiological studies have suggested that metal exposure may promote the atherosclerosis disorder in humans.
Objective
This study is carried out to assess the distribution, correlation and multivariate apportionment of cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), lead (Pb) and zinc (Zn) in the blood of atherosclerosis patients in comparison with healthy donors.
Method
The quantification of metals is done by atomic absorption spectrometry, after wet-acid digestion of the blood samples.
Results
Significantly higher concentrations of cadmium, chromium, copper, iron and manganese are found in the blood of atherosclerosis patients. The correlation study shows diverse relationships among the metals in blood of the patients and controls. Multivariate cluster analysis based on the metal levels in patients and controls reveals clearly separate grouping for the patients and healthy donors. Moreover, principal component analysis shows divergent grouping of the metals for the patients and healthy donors, which may be associated with the altered metabolism of the metals in atherosclerosis patients.
Conclusion
Overall, the distribution, correlation and multivariate apportionment of selected metals in atherosclerosis patients and healthy donors are significantly divergent. Hence, present findings suggest that the trace and redox metals accumulated in the body may pose a high risk for atherosclerosis development.
Keywords: Atherosclerosis, Clinical biochemistry, Medical science, Atomic spectroscopy
1. Introduction
Progressive urbanisation, industrialisation and poor food quality contributed to the rising burden of cardiovascular disease (CVD) in the developing world [1]. This is mainly the result of increasing prevalence of atherosclerosis; a hallmark of ischemia heart disease which occurs due to the accumulation of lipids/fibrous elements in arteries [2, 3]. Atherosclerosis development depends on the balance between pro-inflammatory stimuli, anti-inflammatory and antioxidant defence mechanisms [4, 5]. A critical event in early stages of atherosclerosis is the role of low-density lipoprotein oxidation by some trace metals in atherogenesis and the focal accumulation of lipid-laden foam cells derived from macrophages [6]. Major risk factors for atherosclerosis are hyperlipidemia, elemental imbalance, hypertension, age, cigarette smoking, obesity, genetic factors, low income and low levels of education in third world countries especially in Pakistan [1].
The significance of trace metals is clearly understood from the fact that slight changes in their concentration in human body trigger some kind of complications which later on develop into life threatening diseases [7]. It is well established that trace metals play vital role as activators or inhibitors of enzymatic reactions, by competing with other metals/proteins for binding sites, by influencing the permeability of cell membranes, or through other mechanisms [8]. Trace metals are recognized as essential mediators for the development and progression of cardiovascular disease, although there is no concrete evidence for the direct relationship between the metals and progression of the disease [9, 10]. Blood is one of the widely used specimens for biological metal research because of its natural significance and ease of sampling [7, 11, 12]. It is the medium of transport of trace metals and provides direct evidence about their concentrations and therefore, considered as a good indicator of the current body burden of the metals [13].
The present investigation is aimed at the measurement of Cd, Co, Cr, Cu, Fe, Mn, Pb and Zn concentrations in blood samples of atherosclerosis patients and healthy subjects. The objectives of this study are to evaluate the comparative distribution, mutual correlations and multivariate apportionment of selected metals in blood samples of the patients and healthy subjects. Information about the variations in metal levels in atherosclerosis patients with respect to the healthy donors might help to scrutinize the possible role of these metals in the initiation and development of the disease.
2. Materials and methods
2.1. Study population
A total of 90 atherosclerosis patients, aged between 30 and 62 years, were included in this study, on volunteer basis. Blood samples were collected from the patients admitted in Punjab Institute of Cardiology (PIC), Lahore, Pakistan. All the patients were newly diagnosed and were not taking any mineral supplement for last three months. Before the sample collection, protocol of the study was approved by the human ethical committee of the hospital (App. No. QAUC/2012/PIC-A53). The diagnosis of CVD in patients had previously been established by a specialist cardiologist, by performing and analysis of the angiograms using routine procedure before blood was taken for biochemical assays. The presence of 1 or more stenoses ≥50% in diameter of at least in one major coronary artery was considered as the evidence of significant CVD in the patient [14, 15]. The control subjects (n = 90) were also selected on volunteer basis from the same localities and matched with patients for gender, age, environment, food habits and socioeconomic status (Table 1). Before the sample collection, all subjects were briefed about the aim of the study and they agreed to precipitate on volunteer basis. A signed consent was obtained from each of them. A pro-forma was filled to record the information such as age, sex, ailment duration, food habits, smoking habits and occupation at the time of sample collection from the subjects of both categories.
Table 1.
Characteristics of the Subjects.
| Characteristics | Atherosclerosis Patients (n = 90) |
Healthy Donors (n = 90) |
|---|---|---|
| Age (yrs) | ||
| Range | 30–62 | 33–61 |
| Mean | 45.5 | 46.4 |
| Gender | ||
| Female | 45 (50%) | 45 (50%) |
| Male | 45 (50%) | 45 (50%) |
| Diet | ||
| Vegetarian | 63 (70%) | 63 (70%) |
| Non-vegetarian | 27 (30%) | 27 (30%) |
| Residence | ||
| Urban | 63 (70%) | 75 (83%) |
| Rural | 27 (30%) | 15 (17%) |
| Use of tobacco | ||
| No use | 48 (53%) | 48 (53%) |
| Use | 42 (47%) | 42 (47%) |
2.2. Sample collection and preparation
The blood samples were collected from antecubital vein with the help of pre-cleansed syringe by venipuncture method. The blood samples were immediately transferred to the evacuated polyethylene tubes. Every care was taken to reduce the possibility of contamination during the sample collection. This collection method was selected in present study because it dose not involve any kind of chemical containing tubes. Samples were kept in refrigerator at –15 °C until analyses were performed [16]. This method is free of any chemical contamination during the sample collection hence it was suitable for accomplishing the accurate results. Blood sample was transferred from storage tube to the digestion flask and accurately weighed. It was followed by addition of 10 mL HNO3 and left for 5 min; afterwards 10 mL HClO4 was added. The flasks were then closed with their screw caps and kept for 15 min at room temperature. This method previously was optimized by using different proportions of these acids, out of which 1:1 (v/v) of HNO3 and HClO4 was the best choice. The sample contents were then placed in a microwave oven at 500 watts for 5 min. The contents were then cooled down to room temperature. This heating and cooling process was repeated for couple of times more, so a total of 15 min were given for heating inside the microwave oven. At this point the samples were completely digested. A blank sample was also processed in the same manner along with each batch. The blank contained all the reagents in the same sequence and having the same steps except the sample was not present in it. The digested samples were transferred to the volumetric flasks (25 mL) and final volume was appropriately adjusted. This microwave digestion method has many advantages over other methods; it is relatively easy/simple, it involves least chance of contamination, it is environmental friendly and it is more accurate in the metal analysis [17].
2.3. Quantification of the metals
Quantitative analysis of Cd, Co, Cr, Cu, Fe, Mn, Pb and Zn was carried out on flame atomic absorption spectrophotometer (Shamidzu AA-670, Japan), with automatic background compensation and under optimum analytical conditions as shown in Table 2, which also enlists the limit of detection and limit of quantification of the metals. Three sub-samples of each sample were treated and run separately onto the spectrophotometer to pool the mean concentrations. Parallel routine check on the accuracy of quantified results was ensured through the use of standard reference material (NIST SRM 1598a), which showed very good recovery (Table 2). All reagents used were of ultrahigh purity (certified > 99.9%) procured from E-Merck, Germany. Working standard solutions were prepared by serial dilution of 1000 mg L−1 stock standard solutions, just before the analysis of the metals on the instrument.
Table 2.
Optimum analytical conditions for the metal analyses along with their limits of detection and quantification and certified Vs measured (± SD) concentrationsa (μg L−1) of the metals in standard reference material (NIST SRM 1598a).
| Metal | Wavelength (nm) | Slit width (nm) | Limit of Detection (mg L−1) | Limit of Quantification (mg L−1) | NIST SRM 1598a | ||
|---|---|---|---|---|---|---|---|
| Certified Level | Measured Level | Recovery (%) | |||||
| Cd | 228.8 | 0.3 | 0.004 | 0.013 | 0.048 | 0.046 ± 0.005 | 96 |
| Co | 240.7 | 0.2 | 0.005 | 0.016 | 1.24 | 1.21 ± 0.09 | 98 |
| Cr | 357.9 | 0.5 | 0.006 | 0.018 | 0.33 | 0.31 ± 0.08 | 94 |
| Cu | 324.8 | 0.5 | 0.004 | 0.013 | 1580 | 1560 ± 88 | 99 |
| Fe | 248.3 | 0.2 | 0.006 | 0.018 | 1680 | 1651 ± 95 | 98 |
| Mn | 279.5 | 0.4 | 0.003 | 0.010 | 1.78 | 1.73 ± 0.36 | 97 |
| Pb | 217.0 | 0.3 | 0.010 | 0.029 | – | – | – |
| Zn | 213.9 | 0.5 | 0.002 | 0.006 | 880 | 892 ± 31 | 101 |
Triplicate sub-samples.
2.4. Statistical analyses
Mean, geometric mean (GM), median, range, standard deviation (SD) and skewness were computed along with correlation analysis and multivariate statistics in terms of principal component analysis (PCA) and cluster analysis (CA) were also carried out [17].
3. Results and discussion
3.1. Characteristics of the subjects
The demographic characteristics of the atherosclerosis patients and healthy subjects/controls are given in Table 1. Gender characteristics of the subjects of both categories were closely matched (50% each). Majority of them (70%) were vegetarians. Seventy percent of the patient group and 83% of the control group were from urban areas. About half of the patients and controls (53%) were non-smokers.
3.2. Distribution of selected metals
Most metals exhibit broad ranges of concentrations in both donor-groups (Table 3). Highest mean levels in blood samples of the patients are found for Fe (420 μg g−1), followed by Zn (4.24 μg g−1), Pb (3.73 μg g−1), Co (1.41 μg g−1), Mn (1.31 μg g−1), Cr (1.31 μg g−1), Cu (1.21 μg g−1) and Cd (0.70 μg g−1). On the average basis, the decreasing trend of the metal levels in the blood of atherosclerosis patients reveal following order: Fe > Zn > Pb > Co > Mn > Cr > Cu > Cd. Some of the metals exhibit appreciable randomness in their distribution pattern as manifested by dissimilar mean, geometric mean and median levels, as well as relatively higher SD and skewness values. In case of healthy subjects, higher average concentration is noted for Fe (350 μg g−1), followed by Pb (4.94 μg g−1), Zn (4.84 μg g−1), Co (1.41 μg g−1) and Cu (1.01 μg g−1), while Cr, Cd and Mn are measured at the lowest concentrations. Most of the metals exhibit random distribution in blood of healthy donors as indicated by higher SD and skewness values; the dispersion in the levels is comparatively lower than the patients. The metals in the blood of healthy subjects show the following order in their mean concentrations: Fe > Pb > Zn > Co > Cu > Cr > Cd > Mn (Table 3). Relatively higher metal levels in the blood of both donor groups may be attributed to their abode, food habits and tobacco use. Most of the subjects are residing in urban areas where they are exposed to relatively higher pollutant levels which may partially be attributed to diet/vegetables. Almost half (47%) of the participants are using tobacco which is well known contributor for Cd.
Table 3.
Statistical distribution parameters for the metals concentrations (μg g−1) in blood of atherosclerosis patients and healthy donors.
| Atherosclerosis Patients | Healthy Donors |
p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mean | GMa | Median | SDb | Skew | Range | Mean | GMa | Median | SDb | Skew | ||
| Cd | 0.02–1.91 | 0.70 | 0.54 | 0.71 | 0.52 | 0.71 | 0.08–1.11 | 0.50 | 0.42 | 0.52 | 0.33 | 0.44 | <0.01 |
| Co | 0.22–3.40 | 1.41 | 1.04 | 1.31 | 0.91 | 0.64 | 0.12–3.02 | 1.41 | 1.23 | 1.42 | 0.72 | 0.43 | NSc |
| Cr | 0.14–4.21 | 1.31 | 0.91 | 1.11 | 1.04 | 1.21 | 0.04–2.24 | 1.01 | 0.71 | 0.91 | 0.64 | 0.52 | <0.01 |
| Cu | 0.20–2.43 | 1.21 | 1.02 | 1.20 | 0.52 | 0.52 | 0.14–2.21 | 1.01 | 0.93 | 1.03 | 0.44 | 0.82 | <0.05 |
| Fe | 230–700 | 420 | 403 | 390 | 122 | 0.73 | 140–690 | 350 | 330 | 340 | 116 | 0.74 | <0.05 |
| Mn | 0.03–6.50 | 1.31 | 0.62 | 0.51 | 1.73 | 1.61 | 0.01–1.32 | 0.40 | 0.32 | 0.31 | 0.33 | 1.21 | <0.001 |
| Pb | 0.13–13.8 | 3.73 | 1.61 | 1.11 | 4.11 | 1.14 | 0.34–15.1 | 4.94 | 2.91 | 2.73 | 4.41 | 0.92 | NSc |
| Zn | 0.81–8.51 | 4.24 | 3.74 | 3.73 | 2.02 | 0.44 | 0.72–12.3 | 4.84 | 4.23 | 4.32 | 2.41 | 0.94 | NSc |
GM – geometric mean.
SD –standard deviation.
NS – non-significant.
Most of the metals show relatively large spread in the blood of atherosclerosis patients except Fe which reveals narrow distribution. In case of controls, most of the metals exhibit comparatively narrow distribution then the patients with the exception of Zn, thus indicating more consistent concentrations and relatively lower variations in the blood of controls. Moderately symmetric distributions are observed for Cd and Zn in the blood of healthy subjects.
3.3. Comparison of average metal levels in the patients and healthy subjects
Average metal levels in blood samples of the patients and healthy donors are compared to find out the significant differences by applying t-test (Table 3). Mean concentrations of Mn (p < 0.001), Cd (p < 0.01), Cr (p < 0.01), Fe (p < 0.05) and Cu (p < 0.05) are found to be significantly higher in blood samples of atherosclerosis patients than healthy donors. However, in case of controls the average blood levels of Pb and Zn are observed to be slightly higher than the patients but the differences are statistically insignificant. Average concentration of Co is comparable in blood of the two donor groups. Significantly elevated levels of Mn, Cd, Cr, Fe and Cu in the blood of atherosclerosis patients indicate considerable association of these metals with the disease.
3.4. Correlation study
Significant positive correlations in blood of the patients are noted between Pb–Fe (r = 0.51), Fe–Zn (r = 0.33) and Cr–Pb (r = 0.33) as shown in Table 4. Some of the metals reveal inverse relationships manifested by significantly negative correlations; Cd–Pb (r = -0.47) and Co–Pb (r = -0.31), which indicate the depletion or enrichment of specific metals at the cost of others. The correlation study shows mutual relationships among the trace and essential metals; Pb and Cr with Fe and Zn which indicate their significant association with the onset and/or progress of the disease. Apparently positive correlation of the trace with essential metals evidences a build-up of the trace metals in the blood of the patients. In case of healthy subjects, strong positive correlations are noted between Fe–Zn (r = 0.64), Mn–Pb (r = 0.59), Zn–Cu (r = 0.53) and Mn–Cd (r = 0.50). Some other significant correlations are also observed in blood of the controls; Pb–Cr and Pb–Cd. Consequently, most of the essential metals reveal strong mutual correlations in the blood of the controls. The demographic characteristics of the patients and controls mostly remained comparable (Table 1); hence the mutual relationships among the metals are not affected by these factors. Overall, correlation pattern of the metals in blood of controls remain noticeably different compared to the atherosclerosis patients, which may be attributed to the disproportions of the nutrients and trace metals in the patients.
Table 4.
Correlation coefficient (r)a matrix of the metals in blood of atherosclerosis patients (below the diagonal) and healthy donors (above the diagonal).
| Cd | Co | Cr | Cu | Fe | Mn | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|
| Cd | 1 | 0.24 | 0.01 | 0.13 | 0.02 | 0.50 | 0.37 | -0.12 |
| Co | 0.22 | 1 | 0.20 | 0.27 | 0.17 | -0.06 | -0.07 | -0.02 |
| Cr | -0.17 | -0.07 | 1 | 0.29 | 0.27 | 0.14 | 0.37 | 0.14 |
| Cu | 0.19 | -0.08 | -0.17 | 1 | 0.45 | -0.15 | -0.14 | 0.53 |
| Fe | -0.14 | -0.22 | 0.07 | 0.23 | 1 | 0.14 | 0.23 | 0.64 |
| Mn | 0.13 | 0.12 | -0.09 | 0.18 | 0.20 | 1 | 0.59 | -0.22 |
| Pb | -0.47 | -0.31 | 0.33 | 0.19 | 0.51 | -0.12 | 1 | -0.11 |
| Zn | 0.23 | 0.16 | -0.07 | 0.17 | 0.33 | 0.02 | -0.10 | 1 |
Bold r-values are significant at p ≤ 0.01.
3.5. Multivariate analyses
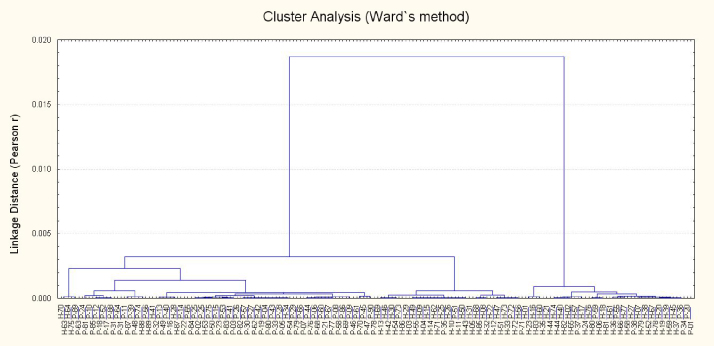
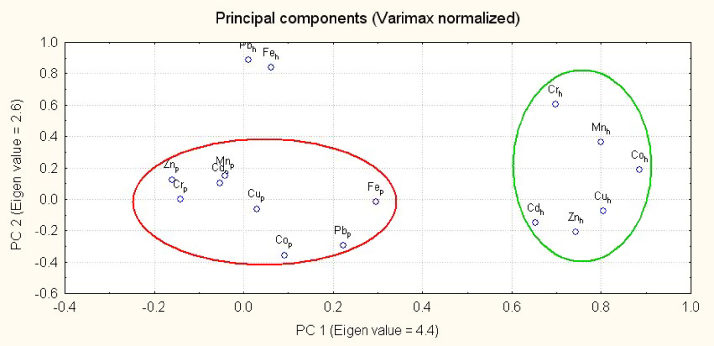
Multivariate analysis of the data using principal component and cluster analysis is another important aspect of the present study [18]. Cluster analysis of the subjects based on the metals levels in patients and controls reveal clearly separate grouping for the patients and healthy donors (Fig. 1) thus indicating diverse apportionment which may be applied for clinical and prognostic studies. Therefore, multivariate apportionment of the metals in the patients and controls are further applied to explore the diagnostic significance. PCA of metals in blood of the patients and healthy donors, extracted by using varimax normalized rotation on the data-set is shown in Fig. 2, as graphical form between PC1 (eigen value = 4.4) and PC 2 (eigen value = 2.6) which contribute 27.4 and 16.1% of the total variance, respectively. Interestingly, the metals constitute two distinctly different groups; one for the patients and other (except Fe and Pb) for healthy donors. Therefore, PCA shows diverse associations among the metals in atherosclerosis patients and healthy subjects which may be attributed to the altered metabolism of the metals in the patients. Among the metals in healthy donors, Fe and Pb show different behaviour which may be due to the anthropogenic impact of these metals as most of the healthy subjects (83%) are residing in urban areas where these metals are mainly associated with the anthropogenic activities. Additionally, significant numbers of controls are using tobacco on continuous basis (47%) and most of them were vegetarians (70%); the elevated metal levels may be attributed to their intake through food chain. Thus, PCA point out imbalance of Fe and Pb in healthy donors. Overall, PCA evidence noteworthy disproportions in the multivariate apportionment of the metals in blood of atherosclerosis patients compared with healthy subjects.
Fig. 1.
Cluster analyses of the subjects (atherosclerosis patients (P) and healthy donors (H)) based on metal levels in their blood.
Fig. 2.
Principal component analysis of the metals in blood of atherosclerosis patients (p) and healthy donors (h).
Several epidemiological studies have shown that some metals may be associated with the progression and development of CVD [6, 11, 19, 21]. Generally, high blood levels of Cd are associated with the initiation of atherosclerosis, which may be due to the hypertensive effects of Cd and its ability to cause endothelial cell damage. The role of Cd in development of atherosclerosis starts after its inhalation or ingestion and followed by its transfer as a free ion or protein-bound (attached to albumin or metallothioneins) via bloodstream to the different organs. Usually, Cd is taken up by cells of the target organs (e.g., aortic vessel wall) where it accelerates the formation of plaque in lumen of the artery. That's why Cd is reported to be an independent risk factor for early atherosclerotic vessel wall thickening in newly diagnosed patients [11, 19]. In present study Cd is reported noticeably higher in blood of patients than healthy donors (Table 3).
Despite the metabolic essentiality of Cr, its deficiency may cause atherosclerosis because Cr is an important component of the lipid and protein metabolism in human body. It has been reported that cholesterol especially low-density lipoprotein (LDL) decrease significantly when subjects were ingesting chromium picolinate. Moreover, animal studies especially on rabbit support the role of chromium picolinate on lipoprotein metabolism [20]. Many past studies examine the oxidation of LDL via reactive oxygen species (ROS), which later on induce the atherosclerosis [6].
Copper is also an essential metal that plays a critical role in haemoglobin synthesis, immune functions and as a cofactor for Cu/Zn superoxide dismutase and ceruloplasmin in a define range. High blood Cu level is thought to be an independent risk factor for CVD especially myocardial infarction and atherosclerosis [21]. Chronic inflammation, as indicated by increased levels of serum C-reactive protein, is associated with elevated levels of Cu. Due to the redox active nature of Cu, it catalyzes the production of highly reactive oxygen species which have the potential to cause oxidative damage to proteins, DNA, lipids and other molecules. As a result, Cu overload induces the tissue injuries, which may lead to diseases or affect the progression of diseases [22, 23]. Present study show the higher blood Cu level in patients (Table 3), which may further strengthen the above statements about cardio-toxic effect of Cu.
Though Mn is an essential metal for health in trace amount but at higher level it shows toxic effects. It is very important component of enzymatic antioxidants which is the defence part of our body. In some past studies, Mn contents of the heart and aorta of atherosclerotic subjects were lower and plasma levels were higher than the healthy controls. The rise was so rapid and specific that it may be used as a diagnostic indicator of myocardial infarction [22]. In the present study, Mn concentration was higher in the blood of atherosclerosis patients compared to the controls (Table 3) which supported the above mentioned hypothesis.
Iron is an important part of haemoglobin and various enzymes in human body but higher concentration of free iron is involved in oxidative stress. Its overload leads to free radical damage by the Fenton reaction. This reaction occurs when Fe2+ reacts with hydrogen peroxide to generate hydroxyl radicals/ions and highly reactive intermediates, causing oxidative stress in the cell. These reactive oxygen species are involved in the peroxidation of lipoproteins and in consequence produce the oxidized LDL, which along with other factors lead towards the development of atherosclerosis [24, 25]. In the present study, Fe levels tend to be higher in atherosclerosis patients (Table 3).
Lead (Pb) is one of the toxic metals that directly interrupts the activity of enzymes, competitively inhibits absorption of important trace minerals and deactivates antioxidant pools [26]. The cardiovascular effects of Pb are associated with increased blood pressure and hypertension. Several animal studies supported the fact that Pb promotes the ROS generation which later on induce the hypertension [27]. Studies in general populations have identified a positive association of Pb exposure with CVD (e.g., coronary artery disease, stroke, and peripheral arterial disease) [22, 28]. In the present study, Fe and Pb show a significant positive correlation in case of the patients (Table 4). This positive correlation indicates that these metals side by side play a crucial role in the development of oxidative stress.
In the present study, Cu and Zn are antagonistic to each other; Cu concentration is higher in blood samples of the patients, while, Zn concentration is lower. Epidemiological relevance between Zn status and CVD incidence is rare; however protective effects of Zn on the risk of CVD are found in some published data [29]. In one of the animal study, developing of atherosclerotic plaques in cholesterol-fed rabbits are enriched with Fe but depleted with Zn, which may have an antiatherogenic effect by decreasing Fe levels in the lesion, possibly leading to inhibition of iron-catalyzed free radical reactions which express the antioxidant and anti-inflammatory properties of Zn [30].
4. Conclusions
In conclusion, the present study evidences significant disparities in distribution of the metals in blood of the patients in comparison with the controls. Average concentrations of Cu, Fe, Cr, Cd and Mn are significantly higher in the blood of atherosclerosis patients which represents the change in body metal homeostasis. These metal imbalances may be a triggering factor for the development of CVD. The correlation study exhibits appreciably diverse associations of the metals in blood samples of the two groups. Moreover, positive correlation of the trace with essential metals evidences a build-up of the trace metals in the blood of the patients. Multivariate CA of the subjects based on the metals levels in patients and controls reveal clearly separate grouping for the patients and healthy donors, while multivariate PCA reveal significantly dissimilar grouping of the metals in the patients and controls; thus highlighting the significant role of trace metals in atherosclerosis in human. The results of this study provided guideline to the other researchers investigating the role of trace and toxic metals in the disease. However, more studies including other essential and toxic metals in different biological samples should be conducted to explore the potential relationship between elemental imbalance and the development of CVD. In addition, future studies should take account of other CVD risk factors, such as, hyperlipidemia, hypertension and obesity along with metal imbalances in order to understand the clearer picture of atherosclerosis mechanism.
Declarations
Author contribution statement
Munir H. Shah: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Asim Ilyas: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Higher Education Commission, Government of Pakistan.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to the administration of Punjab Institute of Cardiology, Lahore, Pakistan for their invaluable help during the sample collection. Technical and financial help by Quaid-i-Azam University, Islamabad, Pakistan to execute this project is also acknowledged.
References
- 1.Khan M.S., Jafary F.H., Jafar T.H., Faruqui A.M., Rasool S.I., Hatcher J., Chaturvedi N. Knowledge of modifiable risk factors of heart disease among patients with acute myocardial infarction in Karachi, Pakistan: a cross sectional study. BMC Cardiovasc. Disord. 2006;6 doi: 10.1186/1471-2261-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lusis A.J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maier J.A.M. Endothelial cells and magnesium: implications in atherosclerosis. Clin. Sci. 2012;122:397–407. doi: 10.1042/CS20110506. [DOI] [PubMed] [Google Scholar]
- 4.Hansson G.K. Mechanisms of disease inflammation, atherosclerosis, and coronary artery disease. New Engl. J. Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 5.Stoker R., Keaney J.F. New insights on oxidative stress in the artery wall. J. Thromb. Haemost. 2005;3:1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 6.Jessup W., Kritharides L., Stocker R. Lipid oxidation in atherogenesis: an overview. Biochem. Soc. T. 2004;32:134–138. doi: 10.1042/bst0320134. [DOI] [PubMed] [Google Scholar]
- 7.Pasha Q., Malik S.A., Shaheen N., Shah M.H. Investigation of trace metals in the blood plasma and scalp hair of gastrointestinal cancer patients in comparison with controls. Clin. Chim. Acta. 2010;411:531–539. doi: 10.1016/j.cca.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Feinendegen L.E., Kasperek K. Medical aspects of trace element research. In: Bratter P., Schramel P., editors. Trace element analytical chemistry in medicine and biology. Walter de Gruyter; Berlin: 1980. [Google Scholar]
- 9.Giacconi R., Caruso C., Malavolta M., Lio D., Balistreri C.R., Scola L., Candore G., Muti E., Mocchegiani E. Pro-inflammatory genetic background and zinc status in old atherosclerotic subjects. Ageing. Res. Rev. 2008;7:306–318. doi: 10.1016/j.arr.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Ilyas A., Ahmad H., Shah M.H. Comparative study of elemental concentrations in the scalp hair and nails of myocardial infarction patients versus controls from Pakistan. Biol. Trace Elem. Res. 2015;166:123–135. doi: 10.1007/s12011-015-0259-x. [DOI] [PubMed] [Google Scholar]
- 11.Ari E., Kaya Y., Demir H., Asicioglu E., Keskin S. The correlation of serum trace elements and heavy metals with carotid artery atherosclerosis in maintenance hemodialysis patients. Biol. Trace Elem. Res. 2011;144:351–359. doi: 10.1007/s12011-011-9103-0. [DOI] [PubMed] [Google Scholar]
- 12.Heitland P., Koster H.D. Biomonitoring of 37 trace elements in the blood samples from inhabitants of northern Germany by ICP-MS. J. Trace Elem. Med. Biol. 2006;20:253–262. doi: 10.1016/j.jtemb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Arif S., Iqbal J., Shaheen N., Shah M.H. Comparative evaluation of trace elements in the blood of chronic bronchitis patients and healthy donors. Trace Elem. Electroly. 2013;30:122–129. [Google Scholar]
- 14.Afridi H.I., Kazi T.G., Kazi N., Kandhro G.A., Baig J.A., Shah A.Q., Jamali M.K., Arain M.B. Evaluation of toxic elements in scalp hair samples of myocardial infarction patients at different stages as related to controls. Biol. Trace Elem. Res. 2010;134:1–12. doi: 10.1007/s12011-009-8450-6. [DOI] [PubMed] [Google Scholar]
- 15.Marroquin O.C., Kip K.E., Kelley D.E., Johnson B.D., Shaw L.J., Merz C.N.B., Sharaf B.L., Pepine C.J., Sopko G., Reis S.E. Metabolic syndrome modifies the cardiovascular risk associated with angiographic coronary artery disease in women a report from the women's ischemia syndrome evaluation. Circulation. 2004;109:714–721. doi: 10.1161/01.CIR.0000115517.26897.A7. [DOI] [PubMed] [Google Scholar]
- 16.Aitio A., Jarvisalo J. Sampling and sample storage. In: Herber R.F.M., Stoeppler M., editors. Trace element analysis in biological samples. Elsevier; Amsterdam: 1994. [Google Scholar]
- 17.Pasha Q., Malik S.A., Shah M.H. Statistical analysis of trace metals in the plasma of cancer patients versus controls. J. Hazard. Mater. 2008;153:1215–1221. doi: 10.1016/j.jhazmat.2007.09.115. [DOI] [PubMed] [Google Scholar]
- 18.Zhai H., Chen X., Hu Z. Study on the relationship between intake of trace elements and breast cancer mortality with chemometric methods. Comput. Biol. Chem. 2003;27:581–586. doi: 10.1016/s1476-9271(03)00049-5. [DOI] [PubMed] [Google Scholar]
- 19.Messner B., Knoflach M., Seubert A., Ritsch A., Pfaller K., Henderson B., Shen Y.H., Zeller I., Willeit J., Laufer G., Wick G., Kiechl S., Bernhard D. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler. Thromb. Vasc. Biol. 2009;29:1392–1398. doi: 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- 20.Press R.I., Geller J., Evans G.W. The effect of chromium picolinate on serum cholesterol and apolipoprotein fractions in human subjects. Western J. Med. 1990;152:41–45. [PMC free article] [PubMed] [Google Scholar]
- 21.Kinsman G.D., Howard A.N., Stone D.L., Mullins P.A. Studies in copper status and atherosclerosis. Biochem. Soc. T. 1990;18:1186–1188. doi: 10.1042/bst0181186. [DOI] [PubMed] [Google Scholar]
- 22.Cebi A., Kaya Y., Gungor H., Demir H., Yoruk I.H., Soylemez N., Gunes Y., Tuncer M. Trace elements, heavy metals and vitamin levels in patients with coronary artery disease. Int. J. Med. Sci. 2011;8:456–460. doi: 10.7150/ijms.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang Y.J. Copper and homocysteine in cardiovascular diseases. Pharmacol. Therapeut. 2011;129:321–331. doi: 10.1016/j.pharmthera.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 24.de Valk B., Marx J.J.M. Iron, atherosclerosis, and ischemic heart disease. Arch. Intern. Med. 1999;159:1542–1548. doi: 10.1001/archinte.159.14.1542. [DOI] [PubMed] [Google Scholar]
- 25.Leiva E., Mujica V., Sepúlveda P., Guzmán L., Núñez S., Orrego R., Palomo I., Andrews M., Arredondo M.A. High levels of iron status and oxidative stress in patients with metabolic syndrome. Biol. Trace Elem. Res. 2013;151:1–8. doi: 10.1007/s12011-012-9525-3. [DOI] [PubMed] [Google Scholar]
- 26.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev. 2006;11:114–127. [PubMed] [Google Scholar]
- 27.Vaziri N.D. Mechanisms of lead-induced hypertension and cardiovascular disease. Am. J. Physiol. - Heart Circ. Physiol. 2008;295:454–465. doi: 10.1152/ajpheart.00158.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navas-Acien A., Guallar E., Silbergeld E.K., Rothenberg S.J. Lead exposure and cardiovascular disease - a systematic review. Environ. Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beattie J.H., Kwun I.S. Is zinc deficiency a risk factor for atherosclerosis. Brit. J. Nutr. 2004;91:177–181. doi: 10.1079/BJN20031072. [DOI] [PubMed] [Google Scholar]
- 30.Ren M., Rajendran R., Ning P., Huat B.T.K., Nam O.C., Watt F., Jenner A., Halliwell B. Zinc supplementation decreases the development of atherosclerosis in rabbits. Free Radic. Biol. Med. 2006;41:222–225. doi: 10.1016/j.freeradbiomed.2006.03.017. [DOI] [PubMed] [Google Scholar]