Abstract
Background:
Few studies have examined whether change in cognition is linked to mortality. This study examined the relationship between cognitive trajectories in older age and risk of death.
Methods:
We studied community-dwelling, nondemented women aged 65+ (mean age = 71) enrolled in a prospective study of aging and followed up to 25 years. A modified Mini-Mental State Examination (mMMSE) and Trail Making Task Part B (TMTB) were administered at multiple visits during follow-up. We examined the association between cognitive trajectories (analyzed by quintiles) from baseline to age 80 (n = 7,477 for mMMSE and n = 6,503 for TMTB) and all-cause mortality after age 80 using Cox regression models, both unadjusted and adjusted for education, physical activity, alcohol, depression score, current smoking and history of hypertension and diabetes. Cause of death was determined from death certificates, classified as cardiovascular, cancer and other.
Results:
Women with greater rate of decline were older, less educated, less physically active, had higher depression score and were more likely to have a history of hypertension and diabetes (all p < .01). Participants with the greatest decline (quintile 1) had an increased risk of death (mMMSE hazard ratio [HR] = 1.28; TMTB HR = 1.43] and those with the least decline (quintile 5) had a decreased risk of death (mMMSE HR = 0.73; TMTB HR = 0.61) compared with intermediate decliners (quintiles 2–4). Cognitive trajectories were associated with cardiovascular mortality and other causes of death, but not cancer deaths.
Conclusions:
Our study suggests that greater decline in general cognition or executive function is associated with higher rates of mortality in oldest-old women.
Keywords: Cognitive trajectories, Global cognition, Executive function, All-cause mortality, Cardiovascular mortality, Oldest old
Cognition in aging, while greatly variable, generally declines starting in the 60s (1–3). Many studies report that cognitive decline increases with age, leading to larger declines in individuals in their 80s and beyond (1,3,4). Mortality also increases with age, with the oldest-old people (aged 80 and older) (5,6) having the highest mortality rate. Several studies report an association between clinically diagnosed dementia and mortality (7–9); even individuals with low-normal scores on cognitive tests have an increased risk of death compared with people with high-normal scores (10,11). A fairly substantial literature examines “terminal decline,” in which cognitive decline accelerates a few years before death (12–14), but only a small number of studies have investigated whether long-term cognitive trajectories are predictive of future mortality, particularly among oldest-old participants. Even less is known about whether maintained cognition over time may be protective (15–18). Furthermore, a comprehensive meta-analysis on the relationship between cognitive impairment and risk of death acknowledges that the cause of this association remains unclear (19). Although preliminary evidence from observational studies links cognitive decline to cardiovascular mortality (20,21), further examination of this association by cause of death could provide a better understanding of the mechanisms of this relationship.
The purpose of this study is to determine if there is a relationship between cognitive trajectories in older age and risk of death after age 80. We aimed to define long-term age-related trajectories of cognitive function with three hypotheses: (i) a poor trajectory in cognitive function will be associated with an increased risk of mortality, (ii) a favorable trajectory in cognitive function will be associated with a decreased risk of mortality, and (iii) extent of cognitive decline will be more strongly related to certain causes of death (cardiovascular disease) but not others (cancer).
Methods
Participants
All participants were enrolled in the Study of Osteoporotic Fracture (SOF) (22), a prospective study of aging and risk factors for osteoporotic fractures, involving 9,704 ambulatory, community-dwelling women aged 65 and older. Participants were initially recruited between September 1986 and October 1988 from population-based listings in four areas of the United States: Baltimore, Maryland; Minneapolis, Minnesota; the Monongahela Valley, near Pittsburgh, Pennsylvania; and Portland, Oregon. The San Francisco Coordinating Center, based at California Pacific Medical Center Research Institute, and the University of California, San Francisco coordinated the study. Black women were initially excluded because of their low incidence of osteoporotic fractures. Women who were unable to walk without assistance and those with bilateral hip replacements were also excluded. The baseline visit evaluated medical history, medication usage, and cognitive function. From this evaluation, we determined that all women were free of a diagnosis for dementia and were not taking any medication for dementia. All women provided written informed consent, and the institutional review boards at each site approved the study.
Assessment of Cognitive Function
Two cognitive tests—a modified version of the Mini-Mental State Examination (mMMSE) and Trail Making Task Part B (TMTB)—were administered by trained staff. The mMMSE was administered at baseline and five follow-up visits (approximately 6, 8, 10, 15, and 20 years after baseline). TMTB was first administered approximately 2 years after baseline with four additional assessments on an average of 4, 8, 13, and 18 years later. The MMSE is a global measurement of cognition, with components for orientation, concentration, language, praxis, and immediate and delayed memory. Scores on the modified MMSE range from 0 to 26, with higher scores representing better cognitive functioning (23,24). The TMTB is a timed test that measures attention, sequencing, visual scanning and executive function (25). A faster time for completion (in seconds) represents better cognitive functioning (25).
Of the 9,704 women at baseline, 9,682 had baseline mMMSE and at least one additional mMMSE at subsequent visits; 8,422 completed an initial TMTB on an average of 2 years later and had at least one additional TMTB. Of the 9,682 women with mMMSE trajectories, 7,447 survived at least until age 80 and were active in SOF; 6,503 of the 8,422 with TMTB trajectory data survived at least until age 80 and were active study participants. The final analytic cohort was 7,447 and 6,503 for mMMSE and TMTB trajectory analyses, respectively. The MMSE and TMTB trajectory analysis groups are overlapping.
Ascertainment of Mortality
The methods for determining deaths have been previously published (26). Participants in SOF were contacted by postcard or telephone every 4–6 months since baseline to ascertain vital status. Information from designated proxy sources (eg, a family member or close friend) was utilized in the event the participant had died. After more than 25 years of follow-up, data on vital status are over 95% complete. Causes of death were confirmed by death certificates. The underlying cause of death was coded by a clinical epidemiologist using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and categorized as due to all causes, cardiovascular disease (CVD; ICD-9-CM codes 401 to <405, 410 to <415, 425, 428, 429.2, 430 to <439, 440 to <445, and 798), and cancer (ICD-9-CM codes 140–239). Those that were not classified as CVD or cancer-related deaths were considered due to “other causes.” Broad categories of specific causes of death were used due to the difficulty of assigning cause of death using death certificates, especially among older women. Participants were followed for an average of 10.0 years (mMMSE analysis) and 10.1 years (TMTB analysis) for deaths occurring after age 80 and before July 31, 2012.
Other Measurements
At the baseline clinic visit, participants provided information on age, education, and health behaviors including physical activity (total kilocalories per week burned in past year), alcohol use (number of drinks per week), and current smoking status. A selected medical history was obtained, including a history of physician diagnosis of diabetes and hypertension. The 15-item Geriatric Depression Scale was administered at Visit 2 (27). Scores range from 0 to 15, with higher scores indicating more symptoms of depression. During a physical exam, height was measured using a wall-mounted Harpenden stadiometer (Holtain Ltd, United Kingdom), and weight was assessed using a balance beam scale. Body mass index was derived as weight in kilograms divided by the square of the height in meters.
Statistical Analysis
Stage 1: Mixed Effects Models for Determination of Change in Cognitive Function
Cognitive function trajectories were calculated using random effects regression. Rate of change in cognitive function at each visit was determined for each woman using random effects regression models (PROC MIXED procedure in SAS 9.2, SAS Institute, Cary, NC). Random effects models account for between- and within-subject correlation between repeat measurements and allow for each woman to have a unique estimated intercept (baseline cognitive function) and estimated trajectory (change in cognitive function).
Change in cognitive function at each postbaseline visit was determined using all available cognitive function measurements from baseline through that visit. When participants were unable to perform cognitive testing at a visit, we used values of 421 seconds for TMTB (the maximum value) as per recommended protocol (28) and the 25th percentile value for mMMSE as there are no established cutoffs. Time was modeled as age at the time of cognitive function testing, centered to the mean age across all visits of 78.3 years for mMMSE and 78.7 years for TMTB. To accommodate steeper declines at older ages, the model included a quadratic term for age. Clinical center and education were included as fixed effects in the model, and trajectories were clinic specific. We used the fixed effects and random effects estimates from these models to calculate the average yearly change from baseline to each visit.
Characteristics of participants by trajectory of cognitive function (by quintile) were compared using analysis of variance for normally distributed continuous variables, the Kruskal–Wallis test for skewed continuous variables, and chi-square tests of homogeneity for categorical variables.
Stage 2: Risk of Mortality by Change in Cognitive Function
Based on studies indicating that the rate of mortality changes after age 80 (6), we examined the association between trajectory of cognitive function and subsequent risk of mortality among women 80 years and older. For each woman, we used the cognitive trajectory up to age 80. Relative risk of mortality after age 80 was estimated as hazard ratios (HRs) and 95% confidence intervals (CIs) using Cox proportional hazards models. For analyses for specific causes of death, women who died from a cause other than the cause of interest were excluded (not censored) from that particular analysis. The counting process style of input was used to identify the at-risk interval for each woman, using age at the visit closest to her 80th birthday and age at death or censoring as the time interval endpoints (29). We compared the HR of mortality of the worst (greatest decline in mMMSE score, highest increase in TMTB time) and best (least decline in mMMSE score, least increase in TMTB time) quintiles to the middle three quintiles.
For both trajectory predictors, three sets of Cox models were run: (i) the unadjusted model that included baseline age and the interaction between baseline age and the predictor; (ii) a minimally adjusted model, which also included education, physical activity, alcohol use, and Geriatric Depression Scale score; (iii) a multivariate model, the minimally adjusted model plus current smoking, and history of hypertension and diabetes.
Results
The women were followed for nearly 25 years and cognitive trajectories from baseline to age 80 were calculated for each woman. The mean mMMSE score at baseline was 24.8 (SD = 1.6) and mean TMTB score was 130 seconds (SD = 65 seconds). Among SOF participants, the average follow-up for mMMSE trajectory (n = 7,447) was 7.3 years (SD = 4.4 years) while the average time for TMTB trajectory (n = 6,503) was 5.7 years (SD = 3.5 years). For analysis, the trajectories for each test were divided into quintiles of performance. The worst quintile of mMMSE performers declined more than 0.14 points per year, the middle performers (quintiles 2–4) declined between 0.04 and 0.14 points per year, and the best quintile performers declined no more than 0.04 points per year. The worst quintile of performers on TMTB had an increased completion time of 7.8 seconds or more per year, the middle performers (quintiles 2–4) increased 3.5 to 7.8 seconds per year, and the best performers increased less than 3.5 seconds per year. After age 80, women were followed for an average of 10 years to assess mortality. During follow-up, 4,451 (59.8%) of the 7,447 mMMSE women died, and 3,758 (57.8%) of the 6,503 TMTB women died. The average age at death of women in the MMSE analysis was 87.6 years (range 80–103). Similar ages were seen in the TMTB analysis group (average = 87.5 years, range 80–101).
Baseline characteristics across trajectory of mMMSE and TMTB by quintile are shown in Tables 1 and 2. For both measures, women in the worst performing quintiles were significantly (p < .001) older, had fewer years of education, lower physical activity, and higher scores on the Geriatric Depression Scale. Women in the worst quintiles were also more likely to report a history of hypertension (p < .01) or diabetes (p < .001). The worst TMTB performers were more likely to smoke but had lower alcohol consumption (p < .001), whereas the worst MMSE performers had the lowest body mass index (p < .05).
Table 1.
Baseline Characteristics of Participants, by mMMSE Trajectory Quintile (n = 7,447)
| Characteristic Mean ± SD or N (%) | Worst Performers | Middle Performers | Best Performers | p Value |
|---|---|---|---|---|
| Quintile 1 | Quintiles 2–4 | Quintile 5 | ||
| <−0.14 pt/y | −0.14 to −0.04 pt/y | ≥−0.04 pt/y | ||
| (N = 1,515) | (N = 4289) | (N = 1643) | ||
| Age (y) | 71.5±4.1 | 71.6±4.2 | 69.0±3.5 | <.001 |
| Education (y) | 12.5±3.2 | 12.8±2.8 | 12.4±2.4 | <.001 |
| Current smoker | 132 (8.7%) | 353 (8.3%) | 138 (8.4%) | .84 |
| Alcohol (drinks/wk) | 1.8±3.8 | 2.0±4.2 | 1.9±3.8 | .10 |
| Physical activity (kcal/wk) | 1566.2±1619.7 | 1676.4±1674.82 | 1780.5±1613.6 | <.001 |
| Body mass index (kg/m2) | 26.3±4.3 | 26.5±4.6 | 26.7±4.6 | .02 |
| Diabetes | 113 (7.5%) | 266 (6.2%) | 53 (3.2%) | <.001 |
| Hypertension | 574 (37.9%) | 1601 (37.3%) | 541 (32.9%) | .003 |
| Geriatric Depression Scale score | 1.8±2.4 | 1.6±2.1 | 1.2±1.9 | <.001 |
mMMSE = modified Mini-Mental State Examination.
Table 2.
Baseline Characteristics of Participants, by Quintile of TMTB Completion Time (n = 6,503)
| Characteristic Mean ± SD or N (%) | Worst Performers | Middle Performers | Best Performers | p Value |
|---|---|---|---|---|
| Quintile 1 | Quintiles 2–4 | Quintile 5 | ||
| ≥7.8s/y | 3.5–7.8s/y | <3.5s/y | ||
| (N = 1,288) | (N = 3,816) | (N = 1,399) | ||
| Age (y) | 73.2±3.3 | 73.3±3.7 | 69.6±1.9 | <.001 |
| Education (y) | 12.4±2.9 | 12.9±2.8 | 12.6±2.4 | <.001 |
| Current smoker | 116 (9.2%) | 263 (7.0%) | 71 (5.2%) | <.001 |
| Alcohol (drinks/wk) | 1.8±3.8 | 2.0±4.0 | 2.1±4.0 | <.001 |
| Physical activity (kcal/wk) | 1607.4±1726.3 | 1701.4±1632.8 | 1922.4±1728.2 | <.001 |
| Body mass index (kg/m2) | 26.4±4.5 | 26.2±4.6 | 26.5±4.5 | .24 |
| Diabetes | 96 (7.5%) | 201 (5.3%) | 52 (3.7%) | <.001 |
| Hypertension | 523 (40.6%) | 1392 (36.5%) | 381 (27.2%) | <.001 |
| Geriatric Depression Scale score | 2.0±2.4 | 1.6±2.1 | 1.1±1.7 | <.001 |
Note: TMTB = Trail Making Task Part B.
We examined the association between cognitive trajectories from baseline to age 80 and all-cause mortality after age 80 (Table 3). In the fully-adjusted model (adjusted for education, physical activity, alcohol, Geriatric Depression Scale score, smoking, history of hypertension, and diabetes), the worst mMMSE performers had a 1.3-fold higher risk of mortality (HR = 1.28, 95% CI = 1.17–1.41), whereas the best performers had a 27% reduced risk of mortality (HR = 0.73, 95% CI = 0.67–0.80). In the fully-adjusted model, the worst TMTB performers had a 1.4-fold higher risk of death (HR = 1.43, 95% CI = 1.31–1.57), whereas the best performers had a 39% reduced risk of death (HR = 0.61, 95% CI = 0.53–0.70).
Table 3.
Hazard Ratios (95% CI) for the Association Between Quadratic Trajectories and Risk of All-Cause Mortality*
| Worst Performers (Quintile 1) | Middle Performers (Quintiles 2–4) | Best Performers (Quintile 5) | ||
|---|---|---|---|---|
| MMSE | Age-adjusted | 1.27 (1.17–1.38) | 1.00 (ref) | 0.71 (0.66–0.77) |
| Minimally adjusted† | 1.29 (1.17–1.41) | 1.00 (ref) | 0.73 (0.67–0.79) | |
| Multivariate adjusted‡ | 1.28 (1.17–1.41) | 1.00 (ref) | 0.73 (0.67–0.80) | |
| TMTB | Age-adjusted | 1.52 (1.40–1.66) | 1.00 (ref) | 0.60 (0.52–0.68) |
| Minimally adjusted† | 1.46 (1.33–1.60) | 1.00 (ref) | 0.60 (0.52–0.69) | |
| Multivariate adjusted‡ | 1.43 (1.31–1.57) | 1.00 (ref) | 0.61 (0.53–0.70) |
Notes: MMSE = Mini-Mental State Examination; TMTB = Trail Making Task Part B.
*Trajectories from Baseline to age 80, predicting mortality after age 80.
†Adjusted for age, education, physical activity, alcohol use, and Geriatric Depression Scale score.
‡Additionally adjusted for current smoking, history of hypertension, and history of diabetes.
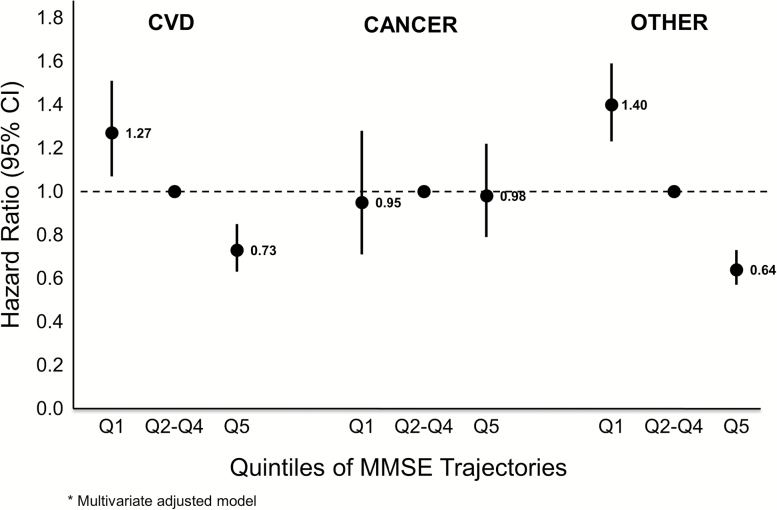
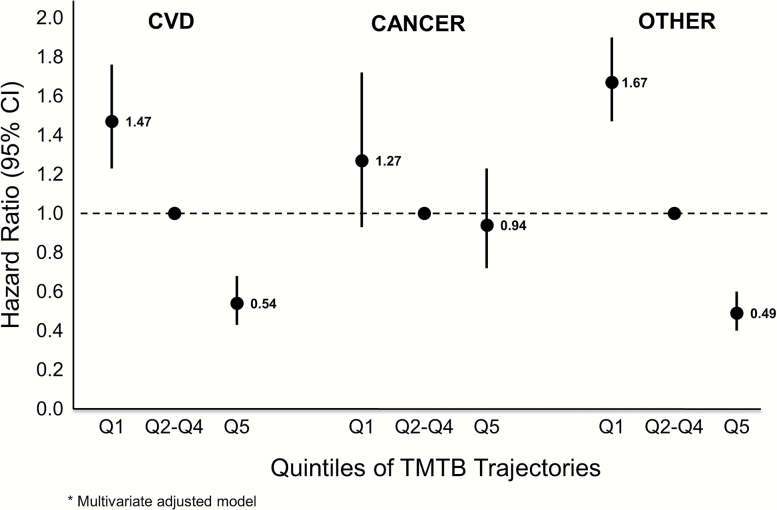
We separated the all-cause mortality outcome into three different causes of death: CVD, cancer, and other. Of the 4,451 women in the mMMSE analysis that died, 34.7% died from CVD, 14.9% died of cancer, and 50.4% died of other causes. The results showed that while CVD mortality and other causes of death were related to cognitive trajectories, cancer deaths were not (Figures 1 and 2). After multivariate adjustment, the best mMMSE performers had a decreased risk of CVD death (HR = 0.73, 95% CI = 0.63–0.85) and other causes of death (HR = 0.64, 95% CI = 0.57–0.73), whereas the worst mMMSE performers had an increased risk of CVD death (HR = 1.27, 95% CI = 1.07–1.51) and other deaths (HR = 1.40, 95% CI = 1.23–1.59) compared to those with intermediate decline (quintiles 2–4). TMTB trajectories had the same pattern with slightly stronger HRs for both CVD and other mortality. Results for the unadjusted and minimally adjusted models were very similar to those for the multivariate-adjusted models (data not shown).
Figure 1.
Risk of mortality cause by quintile of MMSE trajectories; multivariate adjusted model. MMSE = Mini-Mental State Examination.
Figure 2.
Risk of mortality cause by quintile of TMTB trajectories; multivariate-adjusted model. TMTB = Trail Making Task Part B.
Discussion
This study among older women, nondemented at baseline, examined the relationship between long-term cognitive trajectories before age 80 and subsequent mortality after age 80. We found that women in the quintile with the most decline on the mMMSE, a test of general cognition, had a 28% increased risk of mortality, whereas those in the quintile with the least decline had a 27% decreased risk of mortality. For TMTB, a test of executive function, the results were even more striking. Women in the worst performing quintile had a 43% increased risk of mortality, whereas women in the best performing quintile had a 39% decreased risk of mortality. These results show that long-term cognitive trajectories are informative in predicting future mortality even among oldest-old women. Separating all-cause mortality into specific causes of death revealed that the cognitive trajectories were only predictive for CVD mortality and other causes of death, but not for cancer mortality.
There is robust evidence linking prevalent cognitive impairment and dementia to increased risk of death; low performance on tests of general cognition (10,30), processing speed (11), and memory (31) have also been related to future mortality. Our findings are consistent with a small number of previous reports suggesting an association between cognitive change and mortality. Two early studies with smaller sample sizes documented an association between cognitive change and risk of all-cause mortality (15,16). However, participant ages ranged from young adulthood to late-life, change in cognition was assessed at only two time points, and there was minimal adjustment for comorbidities and lifestyle risk factors. Two more recent studies examined the relationship between long-term cognitive trajectories and mortality in much larger, population-based cohorts. In a cohort of older adults followed for nearly 20 years, Rajan et al. found that greater decline on a composite measure of global cognitive function was associated with increased all-cause mortality (17). In a latent class analysis, which considered cognitive lifestyle variables but did not account for comorbidities, Marioni et al. found that compared with older adults with the least decline on global cognition over 20 years, both fast and slow decliners had higher mortality risk (18). Similarly, our study found that cognitive decline in general cognition is associated with a higher mortality risk, but we were also able to adjust for key confounders including lifestyle risk factors and comorbidities such as depressive symptoms, hypertension, and diabetes. We also report that in addition to change in global cognition, decline on executive function was predictive of future mortality in oldest-old participants. Furthermore, unlike these previous studies, our study described associations with differential causes of death.
Our findings of an association between cognitive trajectories and both all-cause and cardiovascular mortality but not cancer mortality parallel results from a smaller investigation of older adults (20). This prior study also adjusted for demographics, lifestyle variables, depression, and anxiety but did not consider cardiovascular comorbidities. The relationship between cognition and cardiovascular death is interesting and requires further study. It is likely that, in addition to joint genetic factors, multiple shared pathways including endothelial dysfunction, oxidative stress, and inflammatory processes promote vascular damage and link cognitive impairment to cardiovascular mortality (32).
Our study additionally found a stronger association between executive function and mortality (both all-cause and cardiovascular) than general cognition and mortality. There are several potential mechanisms that could contribute to this finding. Executive function compared with general cognition may be more reflective of systemic vascular disorders (eg, hypertension and diabetes) (33) and as a result may also be more highly associated with mortality even after adjusting for comorbidities. In addition, declines in executive function could be an indicator of cerebrovascular damage. This hypothesis is supported by data from MRI studies that suggest that there is a significant association between vascular brain damage and increased risk of mortality (34–36). Finally, executive function could also be a more sensitive marker of poor functional outcomes, which are also associated with mortality (37,38).
Studies of terminal decline that concentrate on cognitive decline in the more immediate period prior to death (generally a few years) generally report a steeper cognitive slope prior to death (12–14), which may be partially explained by participants with pre-clinical dementia, given the relatively short follow-up. The association we found with cognitive trajectories and other causes of death may be similar with some portion of the association likely attributable to dementia.
The large sample size in our study also allowed us to examine favorable cognitive trajectories in addition to poor ones. A previous study in the current cohort found that maintaining cognition in old age was associated with fewer medical comorbidities, better daily functioning, and healthy behaviors compared with those who decline (39). One recent study in slightly younger participants found that maintaining cognition may reduce the risk of disability and death (40). The data from our study gives further support to the importance of healthy cognitive aging, particularly executive function, for lowering risk of adverse health outcomes. While knowing that current impairment can increase risk of death is important, examining cognitive trajectories, particularly in cognitively normal people, might allow for health care planning and life choices on a policy and individual level. Our results also suggest that existing cardiovascular indices that predict mortality might be enhanced when combined with an evaluation of executive function.
A major strength of the current study is that the women were assessed and followed for nearly 25 years. Additionally, performance on two different tests was assessed up to five times during the study. Close communication between the study and informant and ascertainment of death certificates allowed us to examine mortality on the majority of the sample and to know the date of death, giving better precision and power to the analyses. However, this study also has some limitations. We used broad categories of cause-specific mortality based on death certificate data, and more nuanced categorizations of mortality may need further investigation. We assessed executive function with the TMTB, but without measures of attention and processing speed, we were unable to separate relevant components of executive function in the prediction of mortality. Another important limitation is that this study only included white women, so generalizing to men or more diverse populations may be difficult. Many of the demographic and lifestyle variables were significantly related to the cognitive test scores. Although we adjusted for them, it is possible that residual confounding remains and could further attenuate these associations, particularly as only baseline values were used. Because SOF study participants were followed for over 20 years, some women were lost to follow-up during the study. Among women who were included in the analysis, mean baseline mMMSE score was 24.8 (SD = 1.6), whereas mean baseline mMMSE score among the women who were not included was 24.4 (SD = 2.0). It is probable that women who were healthier remained in the study and those with onset of cognitive difficulties may have been more likely to drop out; therefore, a steeper decline may have been found among those who were lost to follow-up. However, this bias would likely be toward the null.
Despite these limitations, our study has shown that long-term cognitive trajectories, both in general cognition and, particularly, executive function, are predictive of future mortality in the oldest-old. Importantly, we showed that while poor trajectories are associated with increased mortality, good trajectories appear to be associated with decreased mortality. These findings demonstrate the need for more studies that examine the mechanisms of this association and explore the potential of cognitive function as a modifiable risk factor for healthy aging.
Funding
The Study of Osteoporotic Fractures is supported by National Institutes of Health funding. The National Institute on Aging provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.
Conflict of Interest
Dr. Yaffe is an Associate Editor of the Journal of Gerontology: Medical Sciences. None of the other authors report any declarations of interest.
References
- 1. Rabbitt P, Diggle P, Smith D, Holland F, Mc Innes L. Identifying and separating the effects of practice and of cognitive ageing during a large longitudinal study of elderly community residents. Neuropsychologia. 2001;39:532–543. doi:10.1016/S0028-3932(00)00099-3 [DOI] [PubMed] [Google Scholar]
- 2. Royall DR, Palmer R, Chiodo LK, Polk MJ. Normal rates of cognitive change in successful aging: The freedom house study. J Int Neuropsychol Soc. 2005;11:899–909. doi:10.1017/S135561770505109X [DOI] [PubMed] [Google Scholar]
- 3. Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. doi:10.1037/0882-7974.17.2.179 [PubMed] [Google Scholar]
- 4. Park HL, O’Connell JE, Thomson RG. A systematic review of cognitive decline in the general elderly population. Int J Geriatr Psychiatry. 2003;18:1121–1134. doi:10.1002/gps.1023 [DOI] [PubMed] [Google Scholar]
- 5. United Nations Population Division. World Population Prospects: The 2004 Revision Population Database. New York: United Nations; 2004. Retrieved from http://www.un.org/esa/population/publications/WPP2004/WPP2004_Vol3_Final/WPP2004_Analytical_Report.pdf, 2005. [Google Scholar]
- 6. Horiuchi S, Wilmoth JR. Deceleration in the age pattern of mortality at older ages. Demography. 1998;35:391–412. doi:10.2307/3004009 [PubMed] [Google Scholar]
- 7. Molsa PK, Marttila RJ, Rinne UK. Survival and cause of death in Alzheimer’s disease and multi-infarct dementia. Acta Neurol Scand. 1986;74:103–107. doi:10.1111/j.1600-0404.1986.tb04634.x [DOI] [PubMed] [Google Scholar]
- 8. Barclay LL, Zemcov A, Blass JP, Sansone J. Survival in Alzheimer’s disease and vascular dementias. Neurology. 1985;35:834–840. doi:10.1212/WNL.35.6.834 [DOI] [PubMed] [Google Scholar]
- 9. Martin DC, Miller JK, Kapoor W, Arena VC, Boller F. A controlled study of survival with dementia. Arch Neurol. 1987;44:1122–1126. doi:10.1001/archneur.1987.00520230012006 [DOI] [PubMed] [Google Scholar]
- 10. Gussekloo J, Westendorp RG, Remarque EJ, Lagaay AM, Heeren TJ, Knook DL. Impact of mild cognitive impairment on survival in very elderly people: Cohort study. BMJ. 1997;315:1053–1054. doi:10.1136/bmj.315.7115.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swan GE, Carmelli D, LaRue A. Performance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol. 1995;141:32–40. [DOI] [PubMed] [Google Scholar]
- 12. Bosworth HB, Siegler IC. Terminal change in cognitive function: An updated review of longitudinal studies. Exp Aging Res. 2002;28:299–315. doi:10.1080/03610730290080344 [DOI] [PubMed] [Google Scholar]
- 13. Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: Accelerated loss of cognition in the last years of life. Psychosom Med. 2007;69:131–137. doi:10.1097/PSY.0b013e31803130ae [DOI] [PubMed] [Google Scholar]
- 14. Wilson RS, Segawa E, Hizel LP, Boyle PA, Bennett DA. Terminal dedifferentiation of cognitive abilities. Neurology. 2012;78:1116–1122. doi:10.1212/WNL.0b013e31824f7ff2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deeg DJ, Hofman A, van Zonneveld RJ. The association between change in cognitive function and longevity in Dutch elderly. Am J Epidemiol. 1990;132:973–982. [DOI] [PubMed] [Google Scholar]
- 16. Bosworth HB, Schaie KW, Willis SL. Cognitive and sociodemographic risk factors for mortality in the Seattle Longitudinal Study. J Gerontol B Psychol Sci Soc Sci. 1999;54:P273-282. doi:10.1093/geronb/54B.5.P273 [DOI] [PubMed] [Google Scholar]
- 17. Rajan KB, Aggarwal NT, Wilson RS, Everson-Rose SA, Evans DA. Association of cognitive functioning, incident stroke, and mortality in older adults. Stroke. 2014;45:2563–2567. doi:10.1161/STROKEAHA.114.005143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marioni RE, Proust-Lima C, Amieva H, et al. Cognitive lifestyle jointly predicts longitudinal cognitive decline and mortality risk. Eur J Epidemiol. 2014;29:211–219. doi:10.1007/s10654-014-9881-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: A systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–761. doi:10.1002/gps.397 [DOI] [PubMed] [Google Scholar]
- 20. Batterham PJ, Mackinnon AJ, Christensen H. The association between change in cognitive ability and cause-specific mortality in a community sample of older adults. Psychology and Aging. 2012;27:229–236. doi:10.1037/a0024517 [DOI] [PubMed] [Google Scholar]
- 21. Shipley BA, Der G, Taylor MD, Deary IJ. Association between mortality and cognitive change over 7 years in a large representative sample of UK residents. Psychosom Med. 2007;69:640–650. doi:10.1097/PSY.0b013e31814c3e7c [DOI] [PubMed] [Google Scholar]
- 22. Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi:10.1056/NEJM199503233321202 [DOI] [PubMed] [Google Scholar]
- 23. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 24. Yaffe K, Grady D, Pressman A, Cummings S. Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community women. J Am Geriatr Soc. 1998;46:816–821. doi:10.1111/j.1532-5415.1998.tb02713.x [DOI] [PubMed] [Google Scholar]
- 25. Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological battery. Tucson, AZ: Neuropsychological Press; 1985. [Google Scholar]
- 26. Cauley JA, Seeley DG, Browner WS, et al. Estrogen replacement therapy and mortality among older women. The study of osteoporotic fractures. Arch Intern Med. 1997;157:2181–2187. doi:10.1001/archinte.1997.00440400031004 [PubMed] [Google Scholar]
- 27. Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 28. Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. New York: Oxford University Press; 2006. doi:10.1016/j.acn.2006.09.002 [Google Scholar]
- 29. Anderson PK, Gill RD. Cox’s regression model counting process: A large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 30. Korten AE, Jorm AF, Jiao Z, et al. Health, cognitive, and psychosocial factors as predictors of mortality in an elderly community sample. J Epidemiol Community Health. 1999;53:83–88. doi:10.1136/jech.53.2.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shipley BA, Der G, Taylor MD, Deary IJ. Cognition and all-cause mortality across the entire adult age range: Health and lifestyle survey. Psychosom Med. 2006;68:17–24. doi:10.1097/01.psy.0000195867.66643.0f [DOI] [PubMed] [Google Scholar]
- 32. Jefferson AL, Thompson M. Cardiovascular disease and cognitive aging. In: Yaffe K, ed. Chronic Medical Disease and Cognitive Aging: Toward a Healthy Body and Brain. New York: Oxford Press; 2013:41–66. doi:10.1002/ajhb.22788 [Google Scholar]
- 33. Rostamian S, van Buchem MA, Westendorp RGJ, et al. Executive function, but not memory, associates with incident coronary heart disease and stroke. Neurology. 2015;85:783–789. doi:10.1212/WNL.0000000000001895 [DOI] [PubMed] [Google Scholar]
- 34. Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The Framingham Offspring Study. Stroke. 2010;41:600–606. doi:10.1161/STROKEAHA.109.570044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henneman WJP, Sluimer JD, Cordonnier C, et al. MRI biomarkers of vascular damage and atrophy predicting mortality in a memory clinic population. Stroke. 2009;40:492–498. doi:10.1161/STROKEAHA.108.516286 [DOI] [PubMed] [Google Scholar]
- 36. Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi:10.1016/j.neurobiolaging.2006.06.010 [DOI] [PubMed] [Google Scholar]
- 37. Royall DR, Lauterbach EC, Kaufer D, et al. The cognitive correlates of functional status: A review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19:249–265. [DOI] [PubMed] [Google Scholar]
- 38. Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barnes DE, Cauley JA, Lui LY, et al. Women who maintain optimal cognitive function into old age. J Am Geriatr Soc. 2007;55:259–264. doi:10.1111/j.1532-5415.2007.01040.x [DOI] [PubMed] [Google Scholar]
- 40. Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58:889–894. doi:10.1111/j.1532-5415.2010.02818.x [DOI] [PMC free article] [PubMed] [Google Scholar]