Abstract
The apple is a food rich in diverse classes of polyphenols (PP), among which the proanthocyanidins (PCs), which are primarily concentrated in the skin, are one of the most abundant. These compounds are of considerable interest for their possible positive health effects because of their antioxidant properties. However, depending on the classes of PP present (chemical composition) and their relative concentrations in the apple skin, their antioxidant effects vary and some of their components can even generate prooxidant effects. This work determined the chemical composition and antioxidant-prooxidant potential of a polyphenolic extract (PPE) and a proanthocyanidin-rich fraction (PRF) of apple skin, along with the contribution of their most abundant individual compounds, based on their copper chelating ability, ease in reducing peroxidase-generated free radicals and TEAC (Trolox-Equivalent Antioxidant Capacity) assay. For this purpose, chromatographic and colorimetric methods were used. The majority compounds identified in PPE were flavan-3-ols (44.58%), flavonols (42.89%) and dihydrochalcones (11.60%). In PRF, we detected monomers and oligomers from dimers to heptamers, which were composed of 97% (−)-epicatechin and 3% (+)-catechin. The antioxidant potential was notably higher in PRF than in PPE. The (−)-epicatechin monomer and the procyanidin B2 dimer showed more ease in reducing peroxidase-generated free radicals compared to other compounds of the apple skin, whereas phloridzin dihydrochalcone produced prooxidant effects.
Keywords: Food science, Food chemistry, Food analysis
1. Introduction
Apple fruits, especially the red varieties, have been recognized as a valuable source of phytochemicals, which are mainly concentrated in the skin. They not only affect the nutritional value, appearance, flavor, color and texture of foods in which they are present but also promote better health for consumers (Labarbe et al.,1999; Ou and Gu, 2014).
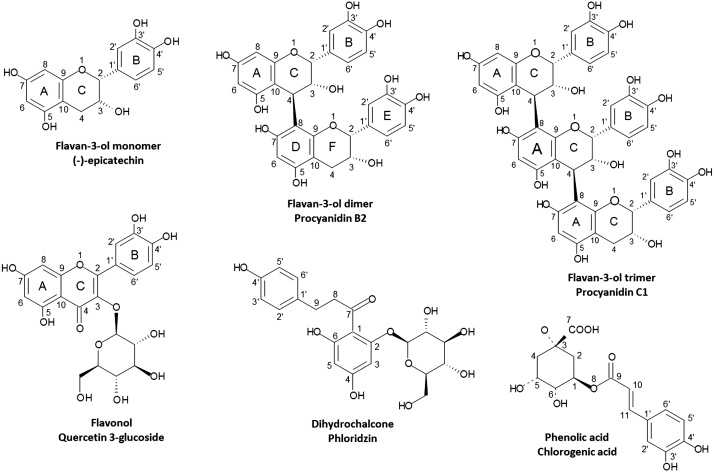
Several studies have reported that the main phytochemicals in apples belong to the group of the polyphenols (PP); they include flavan-3-ols, flavonols, dihydrochalcones, anthocyanidins and a smaller proportion of phenolic acids (Fig. 1) (Imeh and Khokhar, 2002; Tsao et al., 2003). Flavan-3-ols represent the most abundant class of PP in apple skin, approaching 30–90% of total PP present (Tsao et al., 2003; Jakobek et al., 2013). Within these compounds are grouped (+)-catechin and (−)-epicatechin in their monomeric, oligomeric and polymeric forms. The oligomers and polymers are called proanthocyanidins (PCs), which included both procyanidins and prodelphinidins (Hellström et al., 2007)
Fig. 1.
Molecular structure of the majority compounds in apple skin.
PP and PCs have a variety of chemical and biological properties such as the ability to inhibit free radicals and to chelate metals, both of which provide potential uses for the apple skin not only as a functional ingredient in other foods but also in the area of health and even bioremediation (i.e., in the complexation of metal contaminants) (Shoji et al., 2005; Casey, 2013; Laskow, 2013).
Some in vitro studies suggest that apple PP and PCs are among the most powerful natural antioxidants (Lu and Foo, 2000; Lotito and Frei, 2004). Nevertheless, because PP and PCs differ in structure and reactivity and are found in different combinations in apples because of factors such as variety, stage of maturity, growing region, weather conditions and preharvest and postharvest practices, their antioxidant properties and susceptibility to develop prooxidant effects vary. To explore the antioxidant-prooxidant potential of natural sources of PP and PCs such as apple skin, the first step is to isolate, identify, quantify and characterize such compounds. The study of the composition and antioxidant-prooxidant potential in tissues such as apple skin is complicated both because of the diversity of its constituent compounds and because there are very few commercial standards to identify and characterize PCs, which are higher-interest compounds because of their abundance and antioxidant potential. PCs have various degrees of polymerization (DP) and conformations and thus, their structures are complex and difficult to analyze.
Free radical formation can be initiated by the presence of enzymes catalyzing oxidation reactions (e.g., peroxidases) or metals (copper, iron), so the function of antioxidants should be directed to inhibit the action of enzymes, metals and free radicals already formed (Pietta, 2000). In this type of work, the methods used play an important role. To establish the chemical composition of plant tissue extracts, high-performance liquid chromatography (HPLC) equipped with both a UV-Visible diode array (UV-Vis-DAD) and a mass spectrometric (MS) detector is helpful; to study the antioxidant-prooxidant properties, spectroscopic methods such as UV-Visible (UV-Vis) coupled with stopped-flow are advisable.
The objective of this research was to determine the chemical composition and antioxidant-prooxidant potential of a PPE and a PRF of Red Delicious apple skin, employing HPLC-DAD-MS and UV–Vis spectroscopy coupled with stopped-flow methods. This research was justified in terms of the need to generate further information for a diversity of applications that the PP and specifically the PCs of apple skin can have as natural antioxidants.
2. Materials and methods
2.1. Materials
Procyanidin B1, procyanidin B2 and procyanidin C1 were acquired from Chromadex (Irvine, California, USA). (+)-catechin, (−)-epicatechin, quercetin glucoside, phloridzin, chlorogenic acid, horseradish peroxidase type I, hydrogen peroxide (H2O2), ABTS (2,2′-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid), potassium persulfate, potassium chloride (KCl), cupric sulfate pentahydrate (CuSO4.5H2O), hexamine, murexide, phloroglucinol, sodium carbonate, ascorbic acid, acetic acid, formic acid, ethanol, acetone, and methanol were purchased from Sigma-Aldrich (St. Louis MO, USA). Acetonitrile and hydrochloric acid were acquired from J.T. Baker (Edo. de Méx., México). Deionized water (18 MΩ•cm−1) was prepared with a Milli-Q system (Millipore, Bedford, MA, USA).
2.2. Preparation of apple skin powder
The skin of Red Delicious apples cultivated in Chihuahua, México, was used in this work. A batch of 30 fruits was washed with distilled water; the skin was removed and scalded in boiling water for 30 s to inactivate the enzyme polyphenol oxidase. Subsequently, the batch was dried at 60 °C for 48 h to remove moisture in an Enviro Pack Micro-Pack Series Mp 1000 Oven (Enviro Pack, Clackamas, OR). Dry skin was reduced to a 40-mesh particle size (0.4 mm) using an Epices Krups GX4100 mill and stored at -40 °C (Gao and Liu, 2005).
2.3. Obtaining PPE from apple skin powder
The PPE was obtained from 1 g of apple skin powder with 25 mL of milli-Q water by sonication during 0.5 h in an ultrasonic bath (Branson 2210). The mixture was centrifuged in a refrigerated centrifuge Allegra 64R (Beckman Coulter Inc., CA, USA), for 15 min at 4 °C and 10,000 g. The supernatant was successively filtered through organza cloth and Whatman No. 1 and the filtrate was frozen at -40 °C until use (Xu and Chang, 2007).
2.4. Isolation of PRF from PPE
To isolate the PRF from the PPE, we used a 26 cm long, 2.8 cm internal diameter glass column packed with TSKgel Toyopearl HW-40EC (extra course grade, Tosoh). The 25 mL of PPE were loaded onto the column, after which we eluted 100 mL of milli-Q water. Next, the dihydrochalcones, anthocyanins and flavonol glycosides contained in the extract were eluted with 250 mL of 40% ethanol. Afterwards, the elution was continued with 100 mL of 60% acetone to obtain the PCs polymer fraction, which was rotoevaporated to dryness and reconstituted with 6 mL of milli-Q water. To recover the PCs oligomers and the flavan-3-ols monomers that could have been eluted in the 40% ethanol fraction, we proceeded to pass this fraction through a sep-pak C18 cartridge. The recovered solution and the 60% acetone fraction were mixed together and called PRF, which was lyophilized and stored at -40 °C until use (Yanagida et al., 1999).
2.5. Analysis of PPE and PRF by reversed-phase HPLC-DAD-ESI/MS
The identification of compounds in PPE and PRF was performed on an Agilent 1100 liquid chromatography system (Agilent Technologies, Santa Clara, CA) equipped with a UV–Vis diode array detector (UV-Vis-DAD) and a mass spectrometric detector with an ion trap and an electrospray ionization source (LC-DAD-ESI-MS-Ion Trap). The separation of compounds from PPE and PRF was achieved on a reversed phase column Microsorb-MV, 100-5C18 of 250 × 4.6 mm, acquired from Varian (Lake Forest, CA), operating at room temperature and a flow rate of 1 mL/min. The aliquot of injection was 200 μL of samples or standards previously filtered through PVDF 13 mm GD/X 0.2 μm pore size filters (Whatman, USA). The mobile phases consisted of water/formic acid (1% v/v) (phase A) and acetonitrile (phase B). The elution gradient was as follows: 0–2 min, the mobile phase composition was constant, 95% A and 5% B; 2–32 min, the mobile phase composition changed linearly, 83% A and 17% B; 32–50 min, the mobile phase composition changed linearly, 30% A and 70% B; 50–60 min, an isocratic elution was maintained, 30% A and 70% B; and 60–70 min, the mobile phase composition changed back to starting conditions, 95% A and 5% B (Karonen et al., 2004; Maiga et al., 2007). Electrospray ionization mass (ESI/MS/MS) spectrometry was operated in negative mode using nitrogen as a drying and nebulizing gas, a nebulizer with 50 psi pressure, a gas flow of 11 L min−1 and a temperature of 350 °C; the capillary voltage was set at 3500 V. Helium was used for fragmentation. The mass scan and mass spectra were measured in a range of 50–2200 m/z. The most abundant ion in the complete analysis was isolated and the production ion spectra were recorded.
2.6. Acid catalysis in presence of phloroglucinol
Acid catalysis was carried out employing 100 μL of a solution of 0.1 M hydrochloric acid in methanol containing 1 mg/mL PRF, 50 mg/mL phloroglucinol and 10 mg/mL ascorbic acid. The reaction mixture was homogenized and heated at 50 °C for 20 min, after which 500 μL of a 40 mM aqueous sodium acetate was added to stop the reaction (Kennedy and Jones, 2001; Esatbeyoglu et al., 2011). Depolymerization products were immediately analyzed following the previously described HPLC-UV methodology, employing standards of PB1 and PB2, whose composition is known.
2.7. Quantification of compounds in PPE and PRF of apple skin
To quantify the compounds of PPE, calibration curves of standards from 0.01 to 1 mg/mL were used: (−)-epicatechin for flavan-3-ols (280 nm), quercetin glucoside for flavonols (360 nm), phloridzin for dihydrochalcones (280 nm), cyanidin glucoside for anthocyanidins (520 nm) and chlorogenic acid for phenolic acids (320 nm). The quantification of the compounds in PRF was conducted from the products of the phloroglucinolysis, which it is valid because (−)-epicatechin, (+)-catechin and their phloroglucinol adducts have the same molar absorptivities (Kennedy and Jones, 2001). A calibration curve of (−)-epicatechin in a range of concentrations of 0.01–0.1 mg/mL with r2 = 0.9994 was employed. The average DP of PRF was calculated using the sum of the adducts and monomers of flavan-3-ols divided between the monomers of flavan-3-ols, with both amounts expressed in moles (Jakobek et al., 2013).
2.8. Determination of the copper chelating ability
To determine the Cu(II) chelation ability, samples were dissolved in 0.01 M hexamine/HCl buffer with the addition of 0.01 M KCl, pH 5.0, diluted in the range of 0.25–1.0 mg/mL. A solution of CuSO4.5H2O was prepared in the same buffer at a concentration of 0.25 mM. Sample solution (0.25 mL) was mixed with hexamine buffer (0.25 mL) and CuSO4.5H2O solution (0.5 mL); next, 0.05 mL of 1 mM murexide was added. The mixture was vortexed and allowed to stand for 10 min. Absorbance was recorded at 482 nm and 530 nm employing a Cary 60 Bio spectrophotometer (Varian Inc., Palo Alto, CA) and the ratio of these absorbances (A482/A530) was calculated. The amount of free copper in samples was determined, and the percentage of chelated copper was calculated as the difference from the total copper. Quantification of free copper content was made from a standard curve with concentrations of CuSO4.5H2O in the range of 0.025–0.125 mM (Karamać, 2009).
2.9. Determination of the ability to reduce the peroxidase-generated free radicals
For the simultaneous determination of antioxidant and prooxidant abilities of PPE, PRF and some of their constituent compounds, we measured the ability to reduce the peroxidase-generated ABTS•+ radical cation. The experiments were performed using a stopped-flow spectrometer, model SX20 (Applied Photophysycs Ltd, UK), equipped with a 20 μL cell (dead-time 1 ms) and two syringes of rapid mixing. Syringe 1 contained the peroxidase (0.8 units/mL in 0.1 M phosphate buffer, pH 7.20) and syringe 2 contained the ABTS (400 μg), H2O2 (400 μg) and the analyte (40 μg) prepared in the same buffer. The ABTS•+ formation was measured during 5 min by monitoring the absorbance at 734 nm (Chan et al., 2003).
2.10. TEAC assay
The TEAC assay was performed following the methodology of Pellegrini et al. (2003). A stable stock solution of ABTS•+ was produced using the reaction of a 7 mM aqueous solution of ABTS with 2.45 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 16 h before use. At the beginning of the analysis, a working solution was obtained by the dilution of the stock solution in ethanol (95% v/v), adjusted to an absorbance of 0.70 (±0.02) at 734 nm in the UV–Vis Cary 60 Bio spectrophotometer. Results were expressed as a TEAC (Trolox-Equivalent Antioxidant Capacity) value, which is defined as the millimoles (mmol) of Trolox having the same percentage of inhibition as one gram of dry weight (DW) of the sample. A standard curve of Trolox from 0.1 mg/mL to 0.7 mg/mL was used to quantify the samples.
2.11. Statistical analysis
All of the analyses were carried out in triplicate and because of the type of variables studied, the standard deviations were calculated.
3. Results and discussion
3.1. Identification of compounds in PPE and PRF of apple skin by reversed-phase HPLC-DAD-ESI/MS
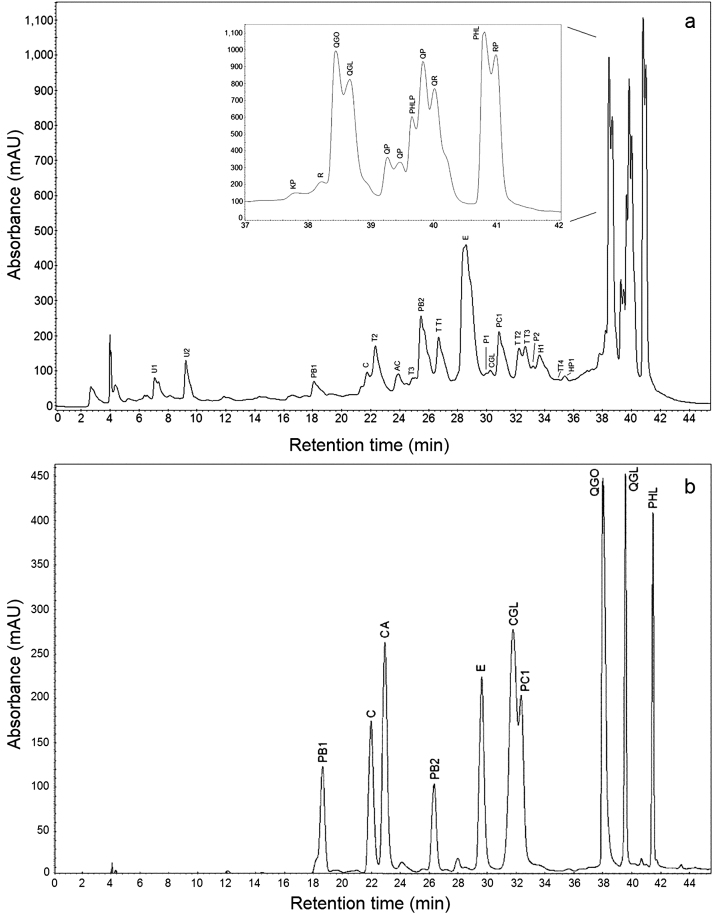
In the PPE, we identified compounds belonging to classes of flavan-3-ols, flavonols, dihydrochalcones, anthocyanidins and phenolic acids (Fig. 2a,b and Table 1).
Fig. 2.
Reversed-phase HPLC-DAD-ESI/MS chromatograms at 280 nm of the PPE of apple skin (a) and a mixture of standards [procyanidin B1 (PB1), (+)-catechin(C), chlorogenic acid (CA), procyanidin B2 (PB2), (−)-epicatechin (E), cyanidin glucoside (CGL), procyanidin C1 (PC1), quercetin galactoside (QGO), quercetin glucoside (QGL), Phloridzin (PHL)] (b).
Table 1.
Identification of compounds in PPE of Red Delicious apple skin by reversed phase HPLC-UV-Vis-DAD-ESI/MS.
| Compounds | Rt (min) | Molecular ions (m/z) | Fragments (m/z) | UV-Vis(nm) |
|---|---|---|---|---|
| Flavan-3-ols Monomers | ||||
| (-)-Epicatechin (E) | 28.38 | 289 | - | 280 |
| (+)-Catechin (C) | 21.45 | 289 | - | 280 |
| Oligomers | ||||
| Procyanidin B1 (dimer) (PB1) | 18.33 | 577 | 407, 289 | 280 |
| Procyanidin B2 (dimer) (PB2) | 25.35 | 577 | 451,425, 407, 289 | 280 |
| Procyanidin C1 (trimer) (PC1) | 30.93 | 865 | 847, 739, 713, 695, 577, 451, 425, 407, 287 | 280 |
| Trimer 2 (T2) | 22.32 | 865 | 847, 713, 695, 577, 451, 425, 407, 289, 287 | 280 |
| Trimer 3 (T3) | 24.89 | 865 | 577, 451, 407, 289, 287 | 280 |
| Tetramer 1 (TT1) | 26.69 | 1153 | 983, 863, 739, 577, 575, 407, 289 | 280 |
| Tetramer 2 (TT2) | 32.23 | 1153 | 983, 865, 577, 407, 289 | 280 |
| Tetramer 3 (TT3) | 32.67 | 1153 | 983, 865, 577, 575, 451, 289, 287 | 280 |
| Tetramer 4 (TT4) | 35.39 | 1153 | 865, 863, 739, 577, 575, 425, 289 | 280 |
| Pentamer 1 (P1) | 29.99 | 1441 | 1315, 1153, 1151, 863, 575, 450, 289 | 280 |
| Pentamer 2 (P2) | 33.20 | 1441 | 1272, 1154, 1027, 865, 575, 407, 289, | 280 |
| Hexamer (H1) | 33.65 | 1729 | 1443, 863, 575, 289 | 280 |
| Heptamer (HP1) | 35.83 | 2017 | 1730, 1153, 863, 739, 575, 289 | 280 |
| Unknown 1 (U1) | 7.09 | 447 | 370, 285 | 236, 282 |
| Unknown 2 (U2) | 9.23 | 447 | 384, 285 | 236, 284 |
| Flavonols | ||||
| Kaempferol pentoside(KP) | 37.81 | 417 | 285, 341 | 266, 352 |
| Rutin (RU) | 38.21 | 609 | 301 | 352 |
| Quercetin galactoside (QGO) | 38.44 | 463 | 301 | 350 |
| Quercetin glucoside (QGL) | 38.67 | 463 | 301 | 352 |
| Quercetin pentoside (QP) | 39.27 | 433 | 301 | 352 |
| Quercetin pentoside (QP) | 39.83 | 433 | 301, 567 | 354 |
| Quercetin ramnoside (QR) | 40.01 | 447 | 300 | 350 |
| Ramnetin pentoside (RP) | 40.99 | 447 | 314 | 348 |
| Dihydrochalcones | ||||
| Phloretin pentoside (FLP) | 39.65 | 567 | 162, 273 | 238, 284 |
| Phloridzin (FL) | 40.81 | 435 | 167, 273 | 236, 284 |
| Phenolic acids | ||||
| Chlorogenic acid (AC) | 24.47 | 353 | 191, 225 | 240, 292 |
| Anthocyanidins | ||||
| Cyanidin glucoside (CGL) | 30.25 | 447 | 284 | 280, 516 |
Within the flavan-3-ols, we detected monomers and oligomers of PCs from dimers to heptamers. The main monomers were identified as (+)-catechin (C) and (−)-epicatechin (E) based on the signal of their deprotonated molecules at m/z 289 in negative ion mode and their retention times at 21.45 min and 28.38 min, respectively, compared with standards.
Two dimers of PCs were identified. Procyanidin B1 (PB1) and procyanidin B2 (PB2) showed the same ions and fragmentation pattern: a molecular ion [M−H]− at m/z 577 and a main fragment at m/z 289 from cleavage at the interflavanoid linkage; additional fragments at m/z 451, 425 and 407 were detected and are in complete agreement with analogous studies (Jakobek et al., 2013; Sun et al., 2007). Because of the similarity of the (−) ESI-MS/MS spectra of PB1 and PB2, the difference between these dimers was established from their retention times, which were 18.33 min and 25.35 min, respectively, compared to standards.
All trimers of PCs (PC1, T2, T3) showed the molecular ion [M−H]− at m/z 865 and main fragments at m/z 577 and 289 produced by the loss of one and two units. Additional fragments were found at m/z 847, 739, 713, 695, 451, 425, 407 and 287, in agreement with previous literature (Esatbeyoglu et al., 2011; Huang et al., 2013). Because of the substantial similarity of the (−) ESI-MS/MS spectra of the trimers detected, only one was unambiguously identified as procyanidin C1 (PC1) by its retention time at 30.93 min when compared to a standard.
The tetramers of PCs (TT1, TT2, TT3, TT4) gave the characteristic ion [M−H]− at m/z 1153, main fragments at m/z 865, 577 and 289, plus additional fragments at m/z 983, 863, 739, 575, 451, 425, and 407, which had already been reported in other papers (Jakobek et al., 2013; Huang et al., 2013).
The pentamers of PCs (P1, P2) produced a molecular ion [M−H]− at m/z 1441, main fragments at m/z 1153, 865, 863, 575 and 289; additional fragments at m/z 1315, 1272, 1151, 1027, 450 and 407 were also detected in similar studies (Jakobek et al., 2013).
The hexamer (H1) showed a ion [M−H]− at m/z 1729.6, main fragments at m/z 1443, 863, 575 and 289 and one additional fragment at m/z 407 in accordance with previously reported data (Jakobek et al., 2013).
Finally, the heptamer (HP1) gave a weak signal corresponding to its molecular ion [M−H]− at m/z 2017.9 and a double-charged ion at m/z 1008. The mass spectrometer employed in this work allowed us to perform scans in the range of 50–2200 m/z, so it detected the signal of the deprotonated molecule of the heptamer. Additionally, we visualized the main fragments at m/z 1730, 1153, 863, 575 and 289, plus an additional fragment at m/z 739.
As part of the flavonols, we identified several compounds, including kaempferol pentoside (KP), by its ion [M−H]− at m/z 417 and fragments at m/z 285 and 341. We also found rutin (RU), which had an ion [M−H]− at m/z 609 and a fragment at m/z 301. In the case of quercetin galactoside (QGO) and quercetin glucoside (QGL), both showed the same ion [M−H]− at m/z 463 and a fragment at m/z 301, so identification was made following the elution order based on their retention times of 38.44 and 38.67 min, respectively, when they were compared to their standards. Two isomers of quercetin pentoside (QP) were found and identified by their common ion [M−H]− at m/z 433 and fragments at m/z 301 and 567. A compound with [M−H]− at m/z 447 and a fragment at m/z 300 were identified as quercetin rhamnoside (QR). Finally, rhamnetin pentoside (RP) showed an ion [M−H]− at m/z 447 and a fragment at m/z 314.
Between the dihydrochalcones detected, we found phloretin pentoside (PHLP) and phloridzin (PHL), which were identified by their molecular ions [M−H]− at m/z 567 and m/z 435, respectively. Additionally, both showed the same fragments at m/z 162 and 273.
The only phenolic acid identified was chlorogenic acid (CA) by its characteristic ion [M−H]− at m/z 353 and fragments at m/z 191 and 225, along with its retention time at 24.47 min, when compared to a standard.
Cyanidin glucoside (CGL) was detected only among the anthocyanidins by its distinctive ion [M−H]− at m/z 447 and a fragment at m/z 284, in addition to its retention time at 30.25 min when compared to a standard.
In PRF, we identified the same monomers of flavan-3-ols and oligomers of PCs (dimers to heptamers), which were found in PPE but in different proportions.
There are no commercial standards for the direct characterization of all of the oligomers and polymers of PCs because of the large number of isomers that they can form. For this reason, to assist in the characterization and quantification of PCs, it is common to resort to depolymerization reactions by acid catalysis in the presence of a nucleophilic reactive such as phloroglucinol.
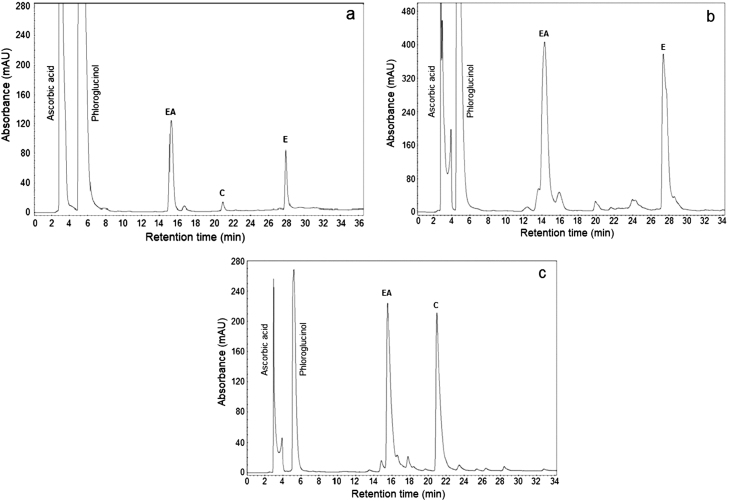
To monitor the depolymerization products of a sample and correctly characterize the PCs present, it is convenient to work first with the available standards. From this perspective, we employed standards of PB1 [(−)-epicatechin-(4β→8)-(+)-catechin] and PB2 [(−)-epicatechin-(4β→8)-(−)-epicatechin]. As displayed in Fig. 3a,b,c, PRF showed a similar behavior to standards, giving the following products of phloroglucinolysis: one (−)-epicatechin adduct (EA) from extension units and the release of terminal units as (+)-catechin (C) and (−)-epicatechin (E). The formation of the EA indicates that the extension units of the dimers, trimers, tetramers, pentamers, hexamer and heptamer detected in PRF were formed of E in the same manner as PB1 and PB2 standards. Conversely, the release of the monomers of flavan-3-ol E and C suggest that these represent the terminal units.
Fig. 3.
Reversed-phase HPLC-UV chromatograms at 280 nm of the products formed in the phloroglucinolysis reaction of the PRF of apple skin (a), and the standards of PB2 (b) and PB1 (c) at 50 °C for 20 min.
3.2. Quantification of compounds in PPE and PRF of apple skin
The total content of PP in PPE is shown in Table 2 as 1635.60 mg/100 g of dry weight (DW), of which 729.20 mg/100 g DW corresponded to flavan-3-ols, 701.53 mg/100 g DW corresponded to flavonols, 189.70 mg/100 g DW corresponded to dihydrochalcones, 11.50 mg/100 g DW corresponded to phenolic acids and 3.64 mg/100 g DW corresponded to anthocyanidins. The flavan-3-ols composed of monomers and oligomers of PCs represented 44.58% of total PPE content.
Table 2.
Quantification of compounds in PPE of Red Delicious apple skin.
| Compounds | mg/ 100 g DW | % |
|---|---|---|
| Flavan-3-ols: | 729.20 | 44.58 |
| (-)-Epicatechin (monomer) | 250.21 | 15.30 |
| (+)-Catechin (monomer) | 15.59 | 0.95 |
| Procyanidin B1 (dimer) | 16.14 | 0.99 |
| Procyanidin B2 (dimer) | 93.54 | 5.72 |
| Procyanidin C1 (trimer) | 64.75 | 3.96 |
| Trimer 2 | 57.36 | 3.51 |
| Trimer 3 | 6.71 | 0.41 |
| Tetramer 1 | 63.51 | 3.88 |
| Tetramer 2 | 27.68 | 1.69 |
| Tetramer 3 | 31.92 | 1.95 |
| Tetramer 4 | 1.38 | 0.08 |
| Pentamer 1 | 5.15 | 0.31 |
| Pentamer 2 | 8.77 | 0.54 |
| Hexamer | 38.26 | 2.34 |
| Heptamer | 3.18 | 0.19 |
| Unknown 1 | 22.02 | 1.35 |
| Unknown 2 | 23.02 | 1.40 |
| Flavonols | 701.53 | 42.89 |
| Kaempferol pentoside | 0.47 | 0.028 |
| Rutin | 0.23 | 0.014 |
| Quercetin-galactoside | 147.07 | 8.99 |
| Quercetin-glucoside | 159.41 | 9.75 |
| Quercetin-pentoside | 9.37 | 0.57 |
| Quercetin-pentoside | 128.58 | 7.86 |
| Quercetin-ramnoside | 139.06 | 8.50 |
| Ramnetin-pentoside | 117.34 | 7.17 |
| Dihydrochalcones | 189.70 | 11.60 |
| Phloridzin-pentoside | 54.74 | 3.35 |
| Phloridzin | 134.96 | 8.25 |
| Phenolic acids: Chlorogenic acid | 11.50 | 0.70 |
| Anthocyanidins: Cyanidin glucoside | 3.64 | 0.22 |
| Total PP | 1635.60 | 100.00 |
By isolating PRF from PPE, it was possible to obtain 366.25 mg/100 g DW of the available 729.20 mg/100 g DW of flavan-3-ols. As can be appreciated in Table 3, PRF (366.25 mg/100 g DW) was composed of 265.55 mg/100 g DW of oligomers from dimers to heptamers (72.50%) and 100.70 mg/100 g DW of monomers (27.5%). The predominant oligomers in PRF were dimers, which totaled 105.99 mg/100 g DW (28.94%), followed by trimers with a total of 61.87 mg/100 g DW (16.89%) and tetramers with 59.93 mg/100 g DW (16.36%). The most abundant individual compounds were the dimer PB2 and the trimer PC1.
Table 3.
Quantification of compounds in PRF of Red Delicious apple skin.
| Compounds | mg/100 g DW | % |
|---|---|---|
| (−)-Epicatechin | 93.85 | 25.63 |
| (+)-Catechin | 6.85 | 1.87 |
| Total monomers | 100.70 | 27.50 |
| Procyanidin B1 | 19.21 | 5.24 |
| Procyanidin B2 | 86.78 | 23.69 |
| Procyanidin C1 | 43.32 | 11.83 |
| Trimer 2 | 9.82 | 2.68 |
| Trimer 3 | 8.73 | 2.38 |
| Tetramer 1 | 21.37 | 5.84 |
| Tetramer 2 | 26.32 | 7.19 |
| Tetramer 3 | 10.34 | 2.82 |
| Tetramer 4 | 1.90 | 0.52 |
| Pentamer 1 | 6.10 | 1.66 |
| Pentamer 2 | 7.33 | 2.00 |
| Hexamer | 22.57 | 6.16 |
| Heptamer | 1.76 | 0.48 |
| Total Oligomers | 265.55 | 72.50 |
| Total PRF | 366.25 | 100.00 |
The analysis of phloroglucinolysis revealed that PRF had an average DP of 4.12. It was also found that the oligomers were composed of 76% E as the extension unit and 21% E and 3% C as terminal units; in other words, 97% of E and 3% of C. These results are consistent with Gu et al. (2003), who reported percentages very similar to ours of 93% and 7%, respectively, for apple PCs.
3.3. Copper chelating ability
Some metals such as iron (Fe) and copper (Cu) play a dual role. On the one hand, they act as cofactors of enzymes that function as antioxidant defenses, including the catalase and superoxide dismutase; on the other hand, they act as catalysts in oxidation reactions promoting the formation of free radicals and prooxidant effects. Antioxidants can prevent adverse reactions of metals by chelation. In this mechanism, the antioxidant molecule that acts as a ligand donates its atoms with unshared electron pairs (oxygens) to the empty or partially filled outer layers (d and f orbitals) of metals, forming a complex that provides stability through changes in oxidation states, e.g., Fe (III)/Fe(II) and Cu (II)/Cu(I). It has been reported that the flavan-3-ols have a stronger affinity to form complexes with Cu(II) compared to Fe(II); therefore, the Cu(II) chelating ability of PPE and PRF was measured (Fernández et al., 2002).
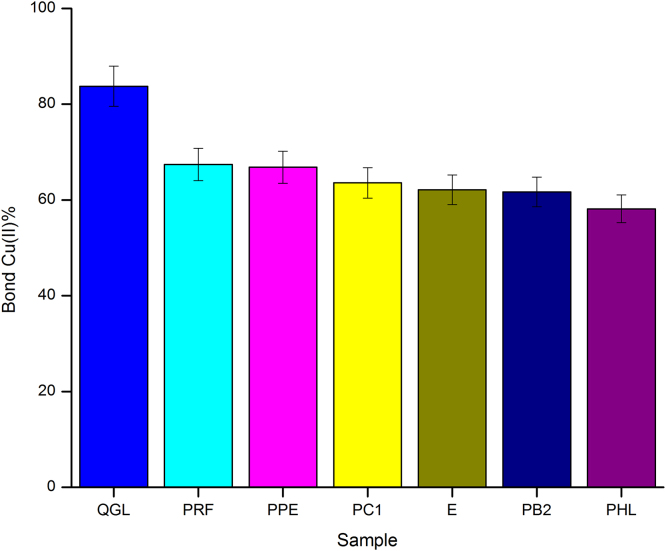
As displayed in Fig. 4, PRF had a slightly greater ability than PPE to chelate Cu(II) as they reached a chelation percentage of 67.40% and 66.83%, respectively, at a concentration of 0.5 mg/mL. With respect to the potential contribution of each compound present in PPE and PRF, measured by some representative standards of the various classes identified, we found that the QGL flavonol showed the highest percentage of chelation (83.75%), followed by the flavan-3-ols such as PC1 (63.57%), E (62.11%), PB2 (61.68%) and the dihydrochalcone PHL (58.14%). This result corroborates that the antioxidant properties were derived from the combined effect of the structure and concentration of the compounds that constitute the plant extracts. The relationship of the DP of PCs and their Cu(II) chelating ability is unclear because the values were very close from monomer to trimer. In addition, the monomer (E) had a value slightly larger than the dimer (PB2). In several PP, it has been demonstrated that chelating ability is favored by at least one deprotonated ligand and increases with the number of ligands available for sequestering metals (Hotta et al., 2001). In the case of PCs, although there are a higher number of hydroxyl groups, it is likely that their conformation determines whether or not they are available, thus affecting their complexation with metals.
Fig. 4.
Behavior of PPE, PRF and the majority compounds of apple skin during the Cu(II) chelation.
3.4. Ability to reduce the peroxidase-generated free radicals
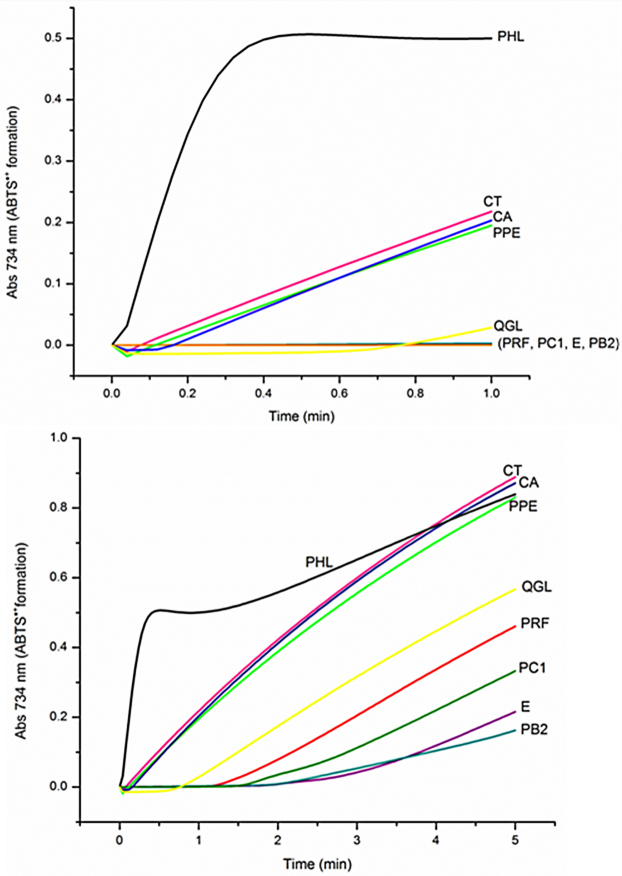
In previous works, it has shown that some PP can undergo a peroxidase-catalyzed bioactivation to form reactive phenoxyl radicals, which co-oxidize biomolecules contributing to the formation of free radicals and therefore generate prooxidant effects. It is considered that such effects of peroxidases are likely to occur in vivo. Peroxidases catalyze the simultaneous oxidation of two substrates, using H2O2 as an electron acceptor. The ABTS•+ radical cation is a stable chromophore that absorbs strongly at 734 nm. Because ABTS is a peroxidase substrate, the inhibition or stimulation of the formation of the ABTS•+ by various chemicals in the presence of H2O2 has been used to rank the antioxidant and prooxidant activities of a variety of compounds, including some PP (Chan et al., 2003). For the above reasons, this study determined the ability of PPE and PRF of apple skin, along with their most abundant components, to reduce or co-oxidize the peroxidase-generated ABTS•+ radical cation.
In Fig. 5a,b we clearly observe that the differences between PRF, PPE and some of their constituting compounds began to be noticeable from the first 0.30 min of their reaction with peroxidase. At that time, the PRF completely inhibited the formation of ABTS•+ and continued to do so during the first 1.2 min, whereas in the PPE an initial rate of formation of ABTS•+ generated by peroxidase of 0.228 Abs734/min was observed, which increased with time but never did exceed that of the control (0.242 Abs734/min). This result indicates that although both PPE and PRF exerted antioxidant effects, PRF was much more effective.
Fig. 5.
Ability of the PPE, PRF and some of their majority components to reduce the ABTS•+ generated by peroxidase during 1 min (a) and 5 min (b) of reaction. PHL= Phloridzin, CA= chlorogenic acid, QGL= quercetin glucoside, PC1= procyanidin C1, E= (−)-epicatechin, PB2= procyanidin B2.
The compounds that constitute PRF, including E, PB2 and PC1, inhibited the formation of ABTS•+ at 2.0, 1.9 and 1.5 min, respectively. Next, the production of ABTS•+ generated by peroxidase began, although it continued at a very low rate during the 5 min in which the reaction was monitored. In presence of either PB2 or E, peroxidase generated the lowest ABTS•+ formation rates of all of the compounds tested. Unlike the QGL, the CA and PHL detected in PPE presented a very different behavior. In presence of CA, the ABTS•+ formation rate was very close to the control, i.e., 0.237 Abs734/min, whereas QGL inhibited the formation of ABTS•+ during 0.6 min and subsequently, an ABTS•+ formation rate of 0.125 Abs734/min was produced by peroxidase. This result suggests that QGL had higher antioxidant capacity than CA. With respect to PHL, a prooxidant effect was found based on an initial rate of formation of ABTS•+ of 1.724 Abs734/min, which is significantly higher than the control. The prooxidant effects remained until 3.8 min after starting the reaction with peroxidase.
The prooxidant effects promoted by enzymes in the absence of transition metals are associated with the presence of one phenol group on the aromatic ring, as with PHL, because the phenoxyl radicals that are derived from there tend to be very unstable. Unlike in the PP, which has catechol moiety, prooxidant effects in the absence of transition metals are not generated (Chan et al., 2003). The different behavior observed in E, PB2, PC1, CA, QGL and PHL explains why PRF had greater ease in reducing the peroxidase-generated ABTS•+ compared to PPE.
3.5. Ability to inhibit free radicals by TEAC assay
Through this assay, in which only the antioxidant ability can be determined without considering the prooxidant effects, PRF showed values significantly higher than PPE, 4.055 and 0.173 TEAC (mmol Trolox/g DW). This result indicates that the PRF had an antioxidant ability 23 times higher than the PPE, reinforcing the results found in the presence of peroxidase. Nevertheless, both PRF and PPE showed a greater ability to inhibit the ABTS•+ radical cation compared both to the red variety of apple “envy” (0.013 mmol Trolox/g DW) (Quang et al., 2014), but lower ability than the red variety of apple “idared” (5.65 mmol Trolox/g DW) (Bonarska-Kujawa and Sarapuk, 2012). This result highlights the importance of the chemical composition on the antioxidant potential of the phenolic extracts from different red apple varieties.
4. Conclusions
The majority compounds identified in PPE were the flavan-3-ols (44.58%), flavonols (42.89%) and dihydrochalcones (11.60%). In PRF, we detected monomers and oligomers from dimers to heptamers, which were composed of 97% (−)-epicatechin and 3% (+)-catechin. The average DP of PRF was 4.12. The antioxidant potential determined through the Cu(II) chelating ability, along with the ease in reducing the peroxidase-generated ABTS•+ radical cation and the TEAC assay, was notably higher in PRF than in PPE. The results suggest that monomers and dimers of PCs could be more effective than trimers of PCs and other PP of apple skin in the reaction catalyzed by peroxidase because in the presence of either PB2 or E, peroxidase generated the lowest rates of ABTS•+ formation of all compounds tested. Instead, the prooxidant potential of PP of apple skin may originate from the dihydrochalcone PHL.
Declarations
Author contribution statement
Ana M. Mendoza-Wilson: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sergio Castro-Arredondo: Performed the experiments; Analyzed and interpreted the data.
Angélica Espinosa-Plascencia, Refugio Robles-Burgueño: Performed the experiments; Contributed reagents, materials, analysis tools or data.
René R. Balandrán-Quintana, María del Carmen Bermúdez-Almada: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT) México (CB2012-183739).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Bonarska-Kujawa J., Sarapuk K.B. Antioxidant Activity of Extracts from Apple, Chokeberry and Strawberry. Pol. J. Food Nutr. Sci. 2012;62(4):229–234. [Google Scholar]
- Casey T. Bioremediation gets a new best friend: apple peels. 2013 http://cleantechnica.com/2013/07/24/bioremediation-gets-a-new-best-friend-applepeels/?utm_source=feedburner&utm_medium=feed&utm_campaign=Feed:+IM-cleantechnica+(CleanTechnica) (accessed December 3, 2015) [Google Scholar]
- Chan T.S., Galati G., Pannala A.S., Rice-Evans C., O'Brien P.J. Simultaneous detection of the antioxidant and pro-oxidant activity of dietary polyphenolics in a peroxidase system. Free Radic. Res. 2003;37(7):787–794. doi: 10.1080/1071576031000094899. [DOI] [PubMed] [Google Scholar]
- Esatbeyoglu T., Jaschok-Kentner B., Wray V., Winterhalter P. Structure elucidation of procyanidin oligomers by low-temperature 1H NMR spectroscopy. J. Agr. Food Chem. 2011;59(1):62–69. doi: 10.1021/jf1032334. [DOI] [PubMed] [Google Scholar]
- Fernández M.T., Mira M.L., Florêncio M.H., Jennings K.R. Iron and copper chelation by flavonoids: an electrospray mass spectrometry study. J. Inorg. Biochem. 2002;92(2):105–111. doi: 10.1016/s0162-0134(02)00511-1. [DOI] [PubMed] [Google Scholar]
- Gao M., Liu C.Z. Comparison of techniques for the extraction of flavonoids from cultured cells of Saussurea medusa Maxim. World J. Microb. Biot. 2005;21(8):1461–1463. [Google Scholar]
- Gu L., Kelm M.A., Hammerstone J.F., Beecher G., Holden J., Haytowitz D., Prior R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agr. Food Chem. 2003;51(25):7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- Hellström J., Sinkkonen J., Karonen M., Mattila P. Isolation and structure elucidation of procyanidin oligomers from saskatoon berries (Amelanchier alnifolia) J. Agr. Food Chem. 2007;55(1):157–164. doi: 10.1021/jf062441t. [DOI] [PubMed] [Google Scholar]
- Hotta H., Sakamoto H., Nagano S., Osakai T., Tsujino Y. Unusually large numbers of electrons for the oxidation of polyphenolic antioxidants. Biochim. Biophys. Acta. 2001;1526(2):159–167. doi: 10.1016/s0304-4165(01)00123-4. [DOI] [PubMed] [Google Scholar]
- Huang Y., Chen L., Feng L., Guo F., Li Y. Characterization of total phenolic ponstituents from the stems of Spatholobus suberectus using LC-DAD-MSn and their inhibitory effect on human neutrophil elastase activity. Molecules. 2013;18(7):7549–7556. doi: 10.3390/molecules18077549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeh U., Khokhar S. Distribution of conjugated and free phenols in fruits: antioxidant activity and cultivar variations. J. Agr. Food Chem. 2002;50(22):6301–6306. doi: 10.1021/jf020342j. [DOI] [PubMed] [Google Scholar]
- Jakobek L., García-Villalba R., Tomás-Barberán F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Comp. Anal. 2013;31:199–211. [Google Scholar]
- Karamać M. Chelation of Cu(II), Zn(II), and Fe(II) by tannin constituents of selected edible nuts. Int. J. Mol. Sci. 2009;10:5485–5497. doi: 10.3390/ijms10125485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karonen M., Loponen J., Ossipov V., Pihlaja K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase highperformance liquid chromatography-electrospray ionization mass spectrometry. Anal. Chim. Acta. 2004;522(1):105–112. [Google Scholar]
- Kennedy J.A., Jones G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001;49(4):1740–1746. doi: 10.1021/jf001030o. [DOI] [PubMed] [Google Scholar]
- Labarbe B., Cheynier V., Brossaud F. Quantitative ractionation of grape proanthocyanidins according to their degree of polymerization. J. Agric. Food Chem. 1999;47(7):2719–2723. doi: 10.1021/jf990029q. [DOI] [PubMed] [Google Scholar]
- Laskow S. Tomato and apple peels can help purify water. 2013 http://grist.org/list/tomato-and-apple-peels-can-help-purify-water/ 2013/07/24 (accessed December 3, 2015) [Google Scholar]
- Lotito S.B., Frei B. Relevance of apple polyphenols as antioxidants in human plasma: contrasting in vitro and in vivo effects. Free Radical Bio. Med. 2004;36(2):201–211. doi: 10.1016/j.freeradbiomed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lu Y., Foo L. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000;68(1):81–85. [Google Scholar]
- Maiga A., Malterud K.E., Mathisen G.H., Paulsen R.E., Thomas-Oates J., Bergstrom E., Reubsaet L., Diallo D., Paulsen B.S. Cell protective antioxidants from the root bark of Lannea velutina A. Rich., a Malian medicinal plant. J. Med. Plants Res. 2007;1(4):66–79. [Google Scholar]
- Ou K., Gu L. Absorption and metabolism of proanthocyanidins. J. Func. Foods. 2014;7:43–53. [Google Scholar]
- Pellegrini N., Serafini M., Colombi B., Del Rio D., Salvatore S., Bianchi M., Brighenti M. Total Antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003;133(9):2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- Pietta P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Quang P.S., Le T.T.T., Le V.V.M. Optimization of ultrasonic treatment of apple (Malus domestica) mash in the extraction of juice with high antioxidant content. Journal of Engineering. 2014;4(12):18–21. [Google Scholar]
- Shoji T., Masumoto S., Moriichi N., Kobori M., Kanda T., Shinmoto H., Tsushida T. Procyanidin trimers to pentamers fractionated from apple inhibit melanogenesis in B16 mouse melanoma cells. J. Agr. Food Chem. 2005;53(15):6105–6111. doi: 10.1021/jf050418m. [DOI] [PubMed] [Google Scholar]
- Sun J., Liang F., Bin Y., Li P., Duan C.H. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules. 2007;12(3):679–693. doi: 10.3390/12030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao R., Yang R., Young J.C., Zhu H. Polyphenolic profiles in eight apple cultivars using high-performance liquid chromatography (HPLC) J. Agr. Food Chem. 2003;51(21):6347–6353. doi: 10.1021/jf0346298. [DOI] [PubMed] [Google Scholar]
- Xu B., Chang S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007;72(2):S159–S166. doi: 10.1111/j.1750-3841.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Yanagida A., Kanda T., Shoji T., Ohnish M., Nagata T. Fractionation of apple procyanidins by size-exclusion chromatography. J. Chromatogr. A. 1999;855(1):181–190. doi: 10.1016/s0021-9673(99)00684-6. [DOI] [PubMed] [Google Scholar]