Abstract
The present study examined the hepatotoxicity and nephrotoxicity of morphine and illicit street heroin and their amelioration by a standardized methanolic extract of Bacopa monnieri (L.) (mBME) in rats. Morphine or street heroin was administered at a dose of 20 mg/kg for 14 and 21 days. mBME (40 mg/kg) or ascorbic acid (50 mg/kg) was administered two hours before morphine or street heroin. High performance liquid chromatography (HPLC) was used for the standardization of bacoside-A major components in mBME. The antioxidant potential of mBME was evaluated by 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. Administration of morphine and street heroin resulted in marked elevation of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and creatinine. Histopathological changes induced by morphine and street heroin after 14 days were of reversible nature while treatment for 21 days was associated with irreversible changes. Pretreatment with mBME or ascorbic acid restored the elevation of serum ALT, AST and creatinine and protected liver and kidneys from the toxicological influence of morphine and street heroin. HPLC analysis showed that mBME contained bacoside-A major components i.e. bacoside-A3 (37.5 μg/mg), bacopaside-II (4.62 μg/mg) and bacopasaponin-C (1.91 μg/mg). The EC50 for the DPPH free radical scavenging assay revealed that mBME possessed strong antioxidant potential. These results concluded that as compared to morphine, street heroin was associated with severe biochemical and histopathological changes in the liver and kidneys. Bacopa monnieri having strong antioxidant potential may provide a beneficial herbal remedy for the efficient management of opioid related hepatotoxicity and nephrotoxicity.
Keywords: Pharmacotherapy, Biochemical pharmacology, Toxicology, Systemic pharmacology, Environmental toxicology, Substance management
1. Introduction
Addiction is a chronic relapsing disease associated with compulsive drug use despite complex physiological and social causes and consequences (Cami and Farré, 2003). Street heroin addiction is one of the most serious and hazardous social and medical issue in the world and its use accounts for a substantial number of drug related illnesses, injuries and deaths despite preventative measures and treatment programs (Clausen et al., 2009; van den Brink and van Ree, 2003). Chronic heroin abuse produce various pathologic changes in the liver including vesicular degeneration, fatty changes, reduction of glycogen content in hepatocytes and vascular changes (Fazelipour et al., 2008). Heroin abusers are frequently found to have abnormal liver function tests and hepatic histology (Degenhardt et al., 2011). Renal interstitial scarring is a major component of heroin associated nephropathy (Dettmeyer et al., 2005). Focal glomerulosclerosis is the predominant glomerular lesion in patients with heroin addiction (Sameiro Faria et al., 2003). Heroin is often considered as a constant product, albeit with variation in the purity of the black market product or the contaminants that are found therein (John et al., 1997). Adulterants in street heroin have been associated with street heroin related mortality (Darke et al., 1999). Heroin is converted within minutes in the body to morphine through the intermediate 6-acetyl morphine (Drummer, 2004). Morphine has been associated with hepatotoxicity and nephrotoxicity (Christrup, 2008). Morphine induced hepatotoxicity is manifested in the form of aggregation or clusters of inflammatory cells with fibrosis of the portal area, proliferation of bile ducts and dilatation of the central vein (Bekheet, 2010). Renal tubular cells vacuolization, mononuclear cells infiltration in the interstitial spaces, focal necrosis and hemorrhage as well as an increase in blood urea nitrogen and creatinine can be regarded as evidence of morphine associated renal damage (Atici et al., 2005).
Management of heroin addiction has evolved with the development of various substitution therapies (Kosten and O'Connor, 2003). Herbal remedies have been used for a long time for the management and detoxification from drugs of addiction (Tang et al., 2006). Herbal medicine has shown promise in relieving abstinence symptoms and anxiety during heroin detoxification (Liu et al., 2009). Bacopa monnieri (Linn.) Pennell, a reputed nootropic plant (Russo and Borrelli, 2005) from family Scrophulariaceae, has been studied widely for its cognitive enhancing (Vollala et al., 2010), antidepressant (Sairam et al., 2002), antihypertensive (Kamkaew et al., 2011), anti-asthmatic (Dar and Channa, 1997), antiulcer (Sairam et al., 2001), analgesic (Abbas et al., 2011), neuroprotective (Limpeanchob et al., 2008), hepatoprotective (Sumathy et al., 2001) and nephroprotective properties (Sumathi and Devaraj, 2009). The major chemical constituents isolated from Bacopa monnieri are dammarane type triterpenoid saponins with jujubogenin and pseudojujubogenin as the aglycones including bacosides A1–A3, bacopasaponins A-G and bacopasides I-V (Deepak et al., 2005; Murthy et al., 2006). Bacoside-A is the major chemical entity responsible for Bacopa monnieri well known nootropic effect as well as other neuromodulatory (Calabrese et al., 2008; Morgan and Stevens, 2010), hepatoprotective and antioxidant activities (Sumathi and Nongbri, 2008). Bacopa monnieri inhibits pharmacological effects induced by morphine (Sumathi and Veluchamy, 2007) and is effective for the reduction of morphine associated withdrawal symptoms (Sumathi et al., 2002). The use of Bacopa monnieri as adjuvant therapy in the management of opioid tolerance may be beneficial (Rauf et al., 2011a).
The present study was aimed to find out the hepatotoxicity and nephrotoxicity associated with street heroin and to compare its severity with that of morphine using rat as an animal model. Moreover, the ameliorative effect of standardized Bacopa monnieri methanolic extract (mBME) on morphine and street heroin induced hepatotoxicity and nephrotoxicity was also investigated in comparison to that of ascorbic acid.
2. Materials and methods
2.1. Animals
Male Sprague Dawley rats, weighing 150–200 gm and maintained in a 12 h light/dark cycle at 22 ± 2 °C were used in the experiment. Food and water were provided ad libitum. The animals were transferred to grid floor cages to avoid suffocation during cataleptic episodes after dosing with morphine or street heroin. Experiments on animals were performed in compliance with the UK Animals (Scientific Procedures) Act 1986 and according to the rules and ethics set forth by the Ethical Committee of the Department of Pharmacy, University of Peshawar. Approval for the study was granted with the registration number: Pharm/EC/446.
2.2. Chemicals and standards
Morphine sulphate and street heroin (procured through legal channels from M/S Punjab Drug House laboratories, Lahore and Anti Narcotics Force, Peshawar, Pakistan respectively), HPLC grade acetonitrile and methanol (Sigma-Aldrich, Switzerland), HPLC grade phosphoric acid (Acros-Organic, Belgium), 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma-Aldrich, Germany), ascorbic acid (Sigma-Aldrich, Germany), butylated hydroxytoluene (BHT; Sigma-Aldrich, Germany), bacosides standards (bacoside-A3, bacopaside-II and bacopasaponin-C; gifted by Prof. Dr. Ikhlas A. Khan, National Center for Natural Products Research, Mississippi, USA).
2.3. Plant material
Bacopa monnieri whole plant was collected in April from Ramali stream near Quaid-e-Azam University, Islamabad. It was authenticated by Prof. Dr. Mohammad Ibrar (Pharmacognosist) of the Department of Botany, University of Peshawar and a specimen was deposited in the herbarium with a voucher number 20016 (PUP). The aerial parts were separated, shade dried and coarsely grinded. It was defatted with n-hexane and was further treated with acetone to remove chlorophyll type pigments. Extraction was done with methanol in Soxhlet apparatus and the extract was then filtered and concentrated under reduced pressure at 50 °C in a rotary evaporator. A semisolid mass (yield 6.5%) was obtained on drying the concentrated extract on a water bath at 50 °C.
2.4. Quantification of bacosides in Bacopa monnieri methanolic extract
High performance liquid chromatography (HPLC) system included double pumps (LC-20AT Shimadzu, Japan) with UV detector (SPD-20A Shimadzu, Japan) and column (Purospher C18, 250 mm × 4.6 mm × 4 μm particle size) was used for the quantification of bacosides in mBME. The method of Rauf (Rauf et al., 2011b) was followed with slight modifications for the determination of bacoside-A major components i.e. bacoside-A3, bacopasaponin-C and bacopaside-II. Briefly, 5 mg of mBME was mixed with 5 ml of HPLC grade methanol, centrifuged for 10 min at 3000 rpm, filtered through a 0.45 μm filter and the filtered solution was then injected into the HPLC system. Mobile phase was prepared by mixing 0.2% phosphoric acid and acetonitrile (62:38 v/v), sonicated for 15 min and filtered under vacuum through a 0.45 μm filter paper. With the system flow rate set at 0.6 ml/min and the wavelength of the detector at 205 nm, all the peaks in mBME were obtained within a runtime of 33 min. The peaks in mBME were confirmed by spiking the standards with samples.
2.5. Treatment groups
All drugs were dissolved in normal saline. mBME (Sumathi and Devaraj, 2009) or ascorbic acid (Zhang et al., 2004) were administered two hours before morphine or street heroin administration (Pacifici et al., 2000). Treatment was continued for 14 and 21 days. A total of 144 animals were randomly assigned to 18 groups (n = 8 rats per group). Half of those (i.e. 9 groups) were used for 14 days treatment and the other half were used for 21 days treatment. Animals received the following treatment, either for 14 or 21 days.
Group I: Control (Saline) (n = 8)
Group II: Morphine (20 mg/kg/day, i.p) (n = 8)
Group III: Street heroin (20 mg/kg/day, i.p) (n = 8)
Group IV: mBME (40 mg/kg/day, p.o) + Morphine (20 mg/kg/day, i.p) (n = 8)
Group V: mBME (40 mg/kg/day, p.o) + Street heroin (20 mg/kg/day, i.p) (n = 8)
Group VI: Ascorbic acid (50 mg/kg/day, i.p) + Morphine (20 mg/kg/day, i.p) (n = 8)
Group VII: Ascorbic acid (50 mg/kg/day, i.p) + Street heroin (20 mg/kg/day, i.p) (n = 8)
Group VIII: mBME (40 mg/kg/day, p.o) (n = 8)
Group IX: Ascorbic acid (50 mg/kg/day, i.p) (n = 8)
2.6. Biochemical analysis
After 14 and 21 days of treatment, blood samples were collected in tubes, allowed to clot and serum was separated by centrifugation (K240R, Centurion scientific, UK) at 3000 rpm for 15 min at 37 °C. The serum samples were stored at 4 °C till determination of biochemical parameters. Serum samples were assayed for alanine aminotransferase, aspartate aminotransferase (ALT, AST; GO F400CH, Chema Diagnostica, Italy) and creatinine (CR 0500CH, Chema Diagnostica, Italy).
2.7. Measurement of body and organs weight
Body weight was measured daily throughout the treatment period before administration of drugs or saline. After 14 and 21 days of treatment, each rat was euthanized and its cranial, thoracic, abdominal and iliac cavities were dissected out and major organs including liver, adrenal glands, kidneys, spleen, brain, testis, thymus gland, heart and lungs were removed and weighted.
2.8. Histological evaluation
After 14 and 21 days of treatment, liver and kidneys were removed and fixed immediately in 10% neutral buffered formalin for 48 h. The tissues were dehydrated in graded ethanol solutions (50, 70, 80, 90, two changes each of 100%), cleared in two changes each of 100% xylene and were infiltrated and embedded in paraffin wax. Tissue blocks were sectioned at 4 μm through a rotary microtome (SLEE Mainz CUT 5062, Germany) and were stained with Harris hematoxylin and eosin (H & E) for microscopic observation (Labomed Lx400 with digital camera iVu 3100, USA). Histopathological changes were scored as none (–), mild (+), moderate (++), or severe (+++) damage (Shahid and Subhan, 2014).
2.9. In vitro antioxidant activity of Bacopa monnieri methanolic extract
The in vitro antioxidant activity of mBME was evaluated by DPPH free radical scavenging assay (Meena et al., 2012; Shahid and Subhan, 2014). Various doses (20–80 μl) of 4 mg/ml of mBME or standards in methanol were mixed with 2 ml of methanolic 0.1 mM DPPH free radical solution. Final volume of 3 ml was adjusted with methanol. The solutions were vortexed and incubated in dark at ambient temperature for 40 min. Absorbance was then measured at 517 nm using UV/Visible spectrophotometer (Lambda 25, PerkinElmer, USA). Ascorbic acid and butylated hydroxytoluene (BHT) were used as standards. Control was prepared by mixing 2 ml of 0.1 mM DPPH solution with 1 ml of methanol. The percent scavenging of DPPH free radicals was calculated as follows.
The absorbance of the control reaction was AI while the absorbance in the presence of sample was AII. The EC50 was calculated according to Lue and others (Lue et al., 2010). 2 ml of methanolic 0.1 mM DPPH free radical solution was added to 1 ml of different concentrations (1, 10, 30, 50, 100, 200, 400, 600, 800, 1000 μg/ml) of mBME or standards in methanol. The solutions were shaken thoroughly, incubated in dark at ambient temperature for 30 min and absorbance was measured at 517 nm. The antiradical power and stoichiometry was determined according to Mishra and coworkers (Mishra et al., 2012). The experiments were performed in triplicate.
2.10. Statistical analysis
The statistical significance of the differences between groups was tested by one way ANOVA followed by Tukey's multiple comparison post hoc test using GraphPad Prism 5 (GraphPad Software Inc. San Diego CA, USA).
3. Results
3.1. Contents of bacoside-A in Bacopa monnieri methanolic extract
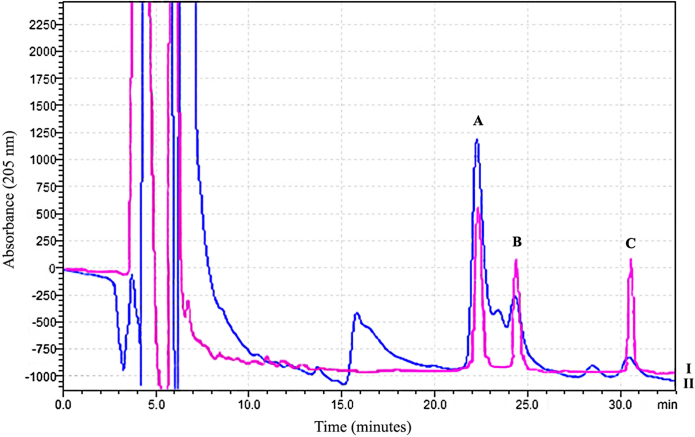
The contents of bacoside-A3, bacopaside-II and bacopasaponin-C present in mBME were 37.5 μg/mg, 4.62 μg/mg and 1.91 μg/mg respectively with total quantity of bacoside-A three major components were 44.03 μg/mg of mBME. The chromatographic analysis showed that bacoside-A3 was the major component of bacoside-A in mBME. The HPLC chromatogram of bacosides standards overlaid with mBME is shown in Fig. 1.
Fig. 1.
HPLC chromatogram of bacosides standards (I) overlaid with mBME (II) showing peaks of three major components i.e. bacoside-A3 (A), bacopaside-II (B) and bacopasaponin-C (C).
3.2. Effect of morphine, street heroin, mBME or ascorbic acid alone or in combination on serum ALT, AST and creatinine after 14 and 21 days
Table 1 shows the serum levels of ALT, AST and creatinine after 14 and 21 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination. In morphine alone treated rats (group II), ALT, AST and creatinine were significantly increased (P < 0.001) as compared to saline (group I) after 14 and 21 days. Similarly, in street heroin alone treated rats (group III), ALT (P < 0.001), AST (P < 0.001) and creatinine (P < 0.001 or P < 0.05) were significantly higher than saline (group I). Pretreatment with mBME before morphine (group IV) significantly restored the levels of ALT (P < 0.01), AST (P < 0.01) and creatinine (P < 0.001) as compared to morphine alone treated rats (group II) after 14 and 21 days. Similarly, pretreatment with ascorbic acid before morphine (group VI) significantly restored ALT (P < 0.001 or P < 0.01), AST (P < 0.001) and creatinine (P < 0.001) levels. Likewise, pretreatment with mBME (group V) or ascorbic acid (group VII) before street heroin significantly decreased (P < 0.001) the levels of ALT, AST and creatinine as compared to street heroin alone treated rats (group III) after 14 and 21 days.
Table 1.
Effect of morphine, street heroin, mBME or ascorbic acid alone or in combination on serum ALT, AST and creatinine after 14 and 21 days of treatment.
| Groups (Treatment) | ALT (U/L) |
AST (U/L) |
Creatinine (mg/dl) |
|||
|---|---|---|---|---|---|---|
| 14 Days | 21 Days | 14 Days | 21 Days | 14 Days | 21 Days | |
| Group I (Saline) |
31.10 ± 3.51 | 34.21 ± 3.70 | 30.17 ± 2.29 | 27.34 ± 1.90 | 0.901 ± 0.10 | 0.917 ± 0.05 |
| Group II (Morphine) |
59.04 ± 7.64### | 62.95 ± 5.68### | 55.17 ± 3.26### | 47.01 ± 3.12### | 1.462 ± 0.09### | 1.319 ± 0.10### |
| Group III (Street heroin) |
71.68 ± 5.75### | 75.06 ± 5.60### | 57.77 ± 4.73### | 55.61 ± 3.33### | 1.437 ± 0.07### | 1.208 ± 0.10# |
| Group IV (mBME + Morphine) |
32.09 ± 3.01** | 35.56 ± 3.55** | 34.38 ± 4.12** | 30.41 ± 2.65** | 0.594 ± 0.05*** | 0.660 ± 0.03*** |
| Group V (mBME + Street heroin) |
40.96 ± 2.18*** | 37.08 ± 2.84*** | 31.37 ± 3.81*** | 27.42 ± 2.24*** | 0.611 ± 0.06*** | 0.597 ± 0.04*** |
| Group VI (Ascorbic acid + Morphine) |
28.71 ± 2.90*** | 34.68 ± 3.39** | 29.86 ± 3.66*** | 26.16 ± 3.70*** | 0.645 ± 0.05*** | 0.751 ± 0.03*** |
| Group VII (Ascorbic acid + Street heroin) |
28.86 ± 4.77*** | 29.66 ± 3.76*** | 25.91 ± 3.44*** | 29.14 ± 3.16*** | 0.797 ± 0.04*** | 0.703 ± 0.06*** |
| Group VIII (mBME) |
32.89 ± 3.09 | 33.97 ± 6.15 | 32.98 ± 4.84 | 26.98 ± 3.27 | 0.712 ± 0.04 | 0.608 ± 0.03 |
| Group IX (Ascorbic acid) |
32.21 ± 3.28 | 22.76 ± 4.36 | 25.10 ± 2.75 | 22.97 ± 3.47 | 0.662 ± 0.04 | 0.584 ± 0.02 |
Values are expressed as mean ± SEM. #P < 0.05, ###P < 0.001 compared to group I. **P < 0.01, ***P < 0.001 compared to group II or III. ANOVA followed by Tukey's multiple comparison post hoc test. n = 8 rats per group.
3.3. Histopathological changes in liver and kidneys after 14 and 21 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination
In the liver, treatment with morphine for 14 days was associated with depletion of glycogen and accumulation of small number of fatty vacuoles in the cytoplasm of some hepatocytes. The sinusoids and central vein exhibited marked dilatation with ruptured endothelium (Fig. 2A1). After 21 days, there was an increase in the number of focal aggregations of lymphocytes with necrotic hepatocytes around the central vein. The portal area showed fibrosis and proliferation of bile ducts. The sinusoids remained dilated and were infiltrated with lymphocytes (Fig. 2B1). Similarly, treatment with street heroin for 14 days caused extensive hemorrhage and microvesicular steatosis throughout the hepatic lobule. The central vein showed congestion and the sinusoids were dilated which were infiltrated with large number of red blood cells (Fig. 3A1). After 21 days, large aggregations of ballooning degeneration, necrotic hepatocytes and apoptotic bodies were visible throughout the hepatic lobules. The sinusoids were markedly dilated and were infiltrated with large number of lymphocytes (Fig. 3B1).
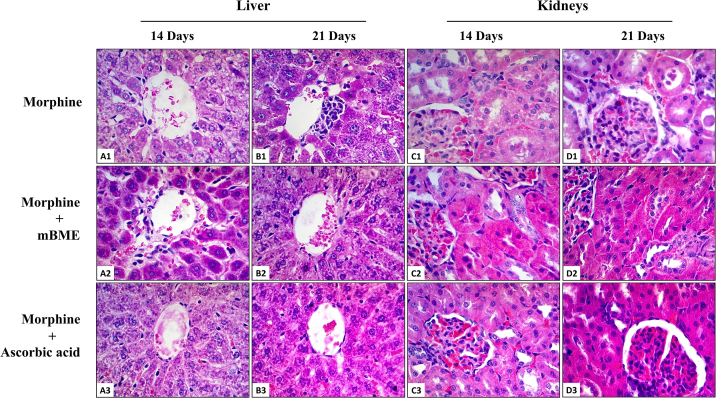
Fig. 2.
Histopathological evaluation of morphine induced hepatotoxicity and nephrotoxicity pretreated with mBME or ascorbic acid for 14 and 21 days (H & E; x400 original magnification) (n = 8 rats per group). (A1): Photomicrograph of a section of liver from a rat treated with morphine for 14 days showing dilatation of central vein and sinusoidal spaces, fatty accumulation, glycogen depletion and detachment of sinusoidal endothelial cells. (B1): Photomicrograph of a section of liver from a rat treated with morphine for 21 days showing central vein congestion, perivenular aggregation of lymphocytes, fatty accumulation, sinusoidal dilatation and infiltration of lymphocytes. Normal histology of central vein, hepatocytes and sinusoidal spaces were found in groups of rats treated with mBME (A2, B2) or ascorbic acid (A3, B3), two hours before administration of morphine for 14 and 21 days. (C1): Photomicrograph of a section of kidney from a rat treated with morphine for 14 days showing dilatation of renal tubules with cellular cast, proximal tubular epithelial cell vacuolization, increase amount of connective tissue in the glomerulus and interstitial fibrosis. (D1): Photomicrograph of a section of kidney from a rat treated with morphine for 21 days showing dilatation of renal tubules with cellular cast, interstitial fibrosis, congestion of glomerulus with red blood cells and an increase in the width of parietal layer of Bowman's capsule. Normal histology of renal corpuscle, proximal and distal convoluted tubules were found in groups of rats treated with mBME (C2, D2) or ascorbic acid (C3, D3), two hours before administration of morphine for 14 and 21 days.
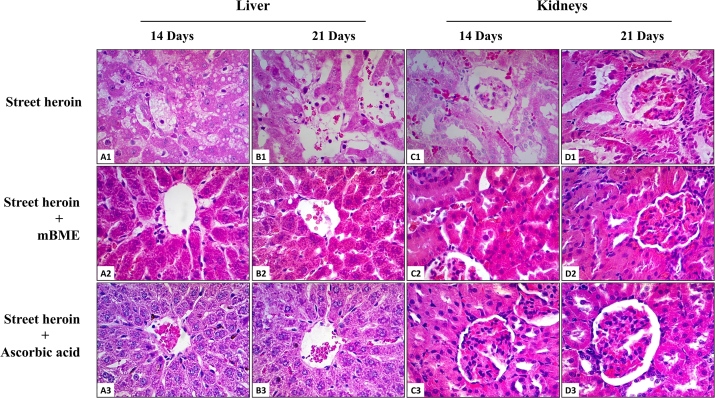
Fig. 3.
Histopathological evaluation of street heroin induced hepatotoxicity and nephrotoxicity pretreated with mBME or ascorbic acid for 14 and 21 days (H & E; x400 original magnification) (n = 8 rats per group). (A1): Photomicrograph of a section of liver from a rat treated with street heroin for 14 days showing central vein congestion, sinusoidal dilatation, fatty accumulation and infiltration of lymphocytes. (B1): Photomicrograph of a section of liver from a rat treated with street heroin for 21 days showing destruction of central vein, necrosis of hepatocytes, sinusoidal dilatation and infiltration of lymphocytes. Normal histology of central vein with intact endothelium, hepatocytes and sinusoidal spaces were found in groups of rats treated with mBME (A2, B2) or ascorbic acid (A3, B3), two hours before administration of street heroin for 14 and 21 days. (C1): Photomicrograph of a section of kidney from a rat treated with street heroin for 14 days showing glomerular atrophy, dilatation of renal tubules, vacuolization and necrosis of epithelial cells and infiltration of red blood cells in the interstitial spaces. (D1): Photomicrograph of a section of kidney from a rat treated with street heroin for 21 days showing glomerular atrophy with congestion and an increase amount of connective tissue, dilatation of proximal convoluted tubules, shredding of tubular epithelial cells with cellular cast and interstitial fibrosis. Normal histology of renal corpuscle, proximal and distal convoluted tubules were found in groups of rats treated with mBME (C2, D2) or ascorbic acid (C3, D3), two hours before administration of street heroin for 14 and 21 days.
In the kidneys, treatment with morphine for 14 days produced moderate dilatation of renal tubules having cellular casts in their lumen. The cuboidal epithelial cells contained small vacuoles in their cytoplasm (Fig. 2C1). After 21 days, there was extensive hemorrhage and interstitial fibrosis. The glomeruli were heavily congested with red blood cells and the parietal layer of the Bowman's capsule was thickened with increase amount of connective tissue in the renal corpuscle (Fig. 2D1). Similarly, treatment with street heroin for 14 days was associated with marked dilatation of renal tubules and vacuolization of their cuboidal epithelial cells. The brush border was destroyed and the interstitial spaces were heavily congested with red blood cells. Some glomeruli were atrophied while others showed disruption of visceral and parietal layers of Bowman's capsule (Fig. 3C1). After 21 days, the markedly dilated renal tubules showed exfoliation of necrotic cuboidal epithelial cells into the lumen of renal tubules. There was marked interstitial fibrosis and segmental glomerulosclerosis. The parietal layer of the Bowman's capsule showed thickening with increased amount of connective tissue in the renal corpuscle (Fig. 3D1).
In comparison to morphine, street heroin was associated with severe histopathological changes in the liver and kidneys. The severity of street heroin induced hepatotoxicity and nephrotoxicity as compared to that of morphine is scored in Table 2.
Table 2.
Effect of Bacopa monnieri methanolic extract (mBME) and ascorbic acid on the severity of morphine or street heroin induced hepatotoxicity and nephrotoxicity after 14 and 21 days of treatment.
| Organ | Histopathological findings | Morphine |
Street heroin |
mBME + Morphine |
mBME + Street heroin |
Ascorbic acid + Morphine |
Ascorbic acid + Street heroin |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 Days | 21 Days | 14 Days | 21 Days | 14 Days | 21 Days | 14 Days | 21 Days | 14 Days | 21 Days | 14 Days | 21 Days | ||
| Liver |
Glycogen depletion | +++ | + | + | + | – | – | – | – | – | – | – | – |
| Hemorrhage | – | + | +++ | + | – | – | – | – | – | – | – | – | |
| Congestion | ++ | ++ | +++ | +++ | + | – | – | – | – | – | – | + | |
| Sinusoidal dilatation | + | + | +++ | ++ | – | – | – | – | – | – | – | – | |
| Hydropic degeneration | – | – | – | +++ | – | – | – | – | – | – | – | – | |
| Cytolysis | – | + | – | +++ | – | – | – | – | – | – | – | – | |
| Granuloma formation | ++ | +++ | – | ++ | + | – | – | – | – | – | – | – | |
| Apoptotic body | – | + | – | ++ | – | – | – | – | – | – | – | – | |
| Perivenular necrosis | – | ++ | – | +++ | – | – | – | – | – | – | – | – | |
| Microvesicular steatosis | + | + | +++ | + | – | – | – | – | – | – | – | – | |
| Kidney | Tubular cell swelling | + | + | + | ++ | – | – | – | – | – | – | – | – |
| Interstitial inflammation | – | +++ | +++ | +++ | – | + | – | – | – | – | – | – | |
| Tubular dilatation | + | ++ | +++ | ++ | – | – | – | – | – | – | – | – | |
| Necrosis of epithelium | + | ++ | ++ | ++ | – | – | – | – | – | – | – | – | |
| Interstitial scarring | – | ++ | – | +++ | – | – | – | – | – | – | – | – | |
| Glomerular congestion | + | +++ | + | +++ | – | – | – | – | – | – | – | – | |
| Glomerular atrophy | – | – | + | + | – | – | – | – | – | – | – | – | |
| Tubular cast | + | +++ | ++ | +++ | + | – | – | – | – | – | – | – | |
| Focal glomerulosclerosis | – | – | ++ | +++ | – | – | + | + | – | – | + | + | |
(–) none; (+) mild; (++) moderate; (+++) severe.
Pretreatment with mBME (Figs. 2A2–D2, 3A2–D2) or ascorbic acid (Figs. 2A3–D3, 3A3–D3) for 14 and 21 days provided protection against morphine and street heroin induced histopathological changes in the liver and kidneys (Table 2). Moreover, animals treated with mBME or ascorbic acid alone showed no significant histopathological effects in both liver and kidneys.
3.4. Body weight after 14 and 21 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination
Table 3 shows the body weight gain after 14 and 21 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination. In morphine alone treated rats (group II) the gain in body weight was found to be significantly lower after 14 (P < 0.001) and 21 (P < 0.05) days as compared to saline (group I); however, there was less body weight reduction in rats treated with mBME two hours before morphine (group IV) after 14 (P < 0.01) and 21 (P < 0.05) days. In rats treated with ascorbic acid before morphine or street heroin, the body weight gain was significantly lower (P < 0.001) as compared to saline treated rats (group I). Similarly less reduction (P < 0.05) in body weight gain was found after 14 days of treatment with mBME two hours before street heroin administration (group V). Moreover, no significant change in body weight was observed in rats treated with street heroin alone (group III) after 14 and 21 days.
Table 3.
Body weight gain (B.W.G) after 14 and 21 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination.
| Groups (Treatment) | Treatment for 14 days (gm) |
Treatment for 21 days (gm) |
||||
|---|---|---|---|---|---|---|
| Day 1 | Day 14 | B.W.G | Day 1 | Day 21 | B.W.G | |
| Group I (Saline) |
174.5 ± 5.94 | 215.2 ± 6.31 | 40.75 ± 3.03 | 180.7 ± 5.69 | 240.1 ± 7.84 | 59.37 ± 8.36 |
| Group II (Morphine) |
174.0 ± 5.13 | 191.8 ± 3.86 | 17.85 ± 3.40*** | 185.7 ± 3.15 | 217.5 ± 6.82 | 31.85 ± 4.67* |
| Group III (Street heroin) |
168.7 ± 5.52 | 205.7 ± 14.0 | 37.00 ± 9.00 | 179.5 ± 7.59 | 232.7 ± 5.46 | 53.25 ± 3.27 |
| Group IV (mBME + Morphine) |
162.7 ± 4.07 | 182.7 ± 4.62 | 20.00 ± 3.04** | 167.8 ± 4.20 | 202.0 ± 4.33 | 34.14 ± 3.29* |
| Group V (mBME + Street heroin) |
179.7 ± 6.12 | 202.2 ± 7.93 | 22.50 ± 2.32* | 176.0 ± 7.07 | 222.2 ± 9.83 | 46.25 ± 4.00 |
| Group VI (AA + Morphine) |
161.3 ± 1.40 | 165.3 ± 2.10 | 4.000 ± 2.17*** | 156.1 ± 3.44 | 171.5 ± 2.48 | 15.33 ± 2.47*** |
| Group VII (AA + Street heroin) |
159.2 ± 1.71 | 161.8 ± 1.98 | 2.600 ± 1.72*** | 161.4 ± 1.86 | 176.8 ± 4.95 | 15.40 ± 3.47*** |
| Group VIII (mBME) |
175.0 ± 4.60 | 209.1 ± 5.62 | 34.12 ± 3.51 | 182.0 ± 4.96 | 230.1 ± 8.24 | 52.62 ± 6.72 |
| Group IX (Ascorbic acid) |
165.5 ± 4.73 | 182.0 ± 6.94 | 16.50 ± 3.70** | 166.0 ± 8.08 | 214.0 ± 5.00 | 48.00 ± 4.50 |
Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared to saline treated group. ANOVA followed by Tukey's multiple comparison post hoc test. n = 8 rats per group.
3.5. Organs weight after 14 and 21 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination
Table 4 shows the weight of organs after 14 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination. In rats treated with ascorbic acid plus morphine (group VI) or street heroin (group VII), the weight of spleen was significantly decreased (P < 0.01) as compared to saline (group I). Similarly, the weight of thymus was significantly decreased in rats treated with street heroin alone (P < 0.01), mBME plus morphine (P < 0.01), ascorbic acid plus morphine (P < 0.001) and ascorbic acid plus street heroin (P < 0.05) as compared to saline treated rats (group I). Significant reduction in the weight of right kidney was observed after treatment with ascorbic acid plus morphine (P < 0.01) and ascorbic acid plus street heroin (P < 0.05) when compared to saline treatment (group I). Moreover, in rats treated with ascorbic acid plus morphine (group VI) and ascorbic acid plus street heroin (group VII), the weight of left kidney was found significantly lowered (P < 0.05) than saline (group I). Furthermore, the weight of left testis was significantly decreased (P < 0.05) in rats treated with ascorbic acid plus morphine (group VI) as compared to saline treated rats (group I).
Table 4.
Organs weight (gm) after 14 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination.
| Organs | Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | Group IX | |
|---|---|---|---|---|---|---|---|---|---|---|
| Liver |
7.256 ± 0.248 |
7.057 ± 0.343 |
6.713 ± 0.403 |
6.750 ± 0.579 |
6.219 ± 0.212 |
6.076 ± 0.254 |
5.520 ± 0.134 |
8.055 ± 0.586 |
8.434 ± 0.285 |
|
| Spleen |
0.593 ± 0.053 |
0.463 ± 0.027 |
0.526 ± 0.028 |
0.482 ± 0.022 |
0.521 ± 0.051 |
0.381 ± 0.013** |
0.358 ± 0.018** |
0.556 ± 0.022 |
0.551 ± 0.082 |
|
| Brain |
1.631 ± 0.036 |
1.576 ± 0.042 |
1.588 ± 0.035 |
1.620 ± 0.051 |
1.493 ± 0.101 |
1.453 ± 0.024 |
1.385 ± 0.042 |
1.618 ± 0.077 |
1.531 ± 0.088 |
|
| Thymus |
0.391 ± 0.031 |
0.336 ± 0.019 |
0.225 ± 0.023** |
0.261 ± 0.024** |
0.326 ± 0.023 |
0.222 ± 0.018*** |
0.275 ± 0.016* |
0.359 ± 0.030 |
0.374 ± 0.015 |
|
| Heart |
0.744 ± 0.040 |
0.717 ± 0.043 |
0.827 ± 0.062 |
0.677 ± 0.030 |
0.811 ± 0.069 |
0.587 ± 0.015 |
0.568 ± 0.035 |
0.807 ± 0.128 |
0.804 ± 0.062 |
|
| Adrenals |
Right |
0.027 ± 0.004 | 0.025 ± 0.004 | 0.042 ± 0.007 | 0.034 ± 0.004 | 0.030 ± 0.003 | 0.025 ± 0.001 | 0.027 ± 0.002 | 0.024 ± 0.002 | 0.033 ± 0.005 |
| Left | 0.025 ± 0.004 | 0.029 ± 0.004 | 0.043 ± 0.004 | 0.028 ± 0.004 | 0.044 ± 0.006 | 0.027 ± 0.002 | 0.024 ± 0.001 | 0.024 ± 0.007 | 0.036 ± 0.007 | |
| Kidneys |
Right |
0.769 ± 0.038 | 0.705 ± 0.035 | 0.732 ± 0.025 | 0.702 ± 0.022 | 0.734 ± 0.018 | 0.580 ± 0.013** | 0.578 ± 0.013* | 0.874 ± 0.041 | 0.889 ± 0.075 |
| Left | 0.722 ± 0.032 | 0.711 ± 0.044 | 0.691 ± 0.054 | 0.745 ± 0.022 | 0.752 ± 0.034 | 0.556 ± 0.011* | 0.554 ± 0.015* | 0.841 ± 0.034 | 0.867 ± 0.045 | |
| Testis |
Right |
1.995 ± 0.103 | 1.811 ± 0.032 | 2.137 ± 0.127 | 1.834 ± 0.054 | 2.011 ± 0.075 | 1.685 ± 0.055 | 1.653 ± 0.055 | 1.900 ± 0.118 | 2.098 ± 0.221 |
| Left | 2.057 ± 0.125 | 1.818 ± 0.049 | 2.009 ± 0.111 | 1.900 ± 0.062 | 2.095 ± 0.050 | 1.696 ± 0.045* | 1.717 ± 0.012 | 1.894 ± 0.047 | 1.930 ± 0.135 | |
| Lungs | Right |
0.831 ± 0.037 | 0.814 ± 0.081 | 0.924 ± 0.160 | 0.685 ± 0.045 | 0.986 ± 0.185 | 0.753 ± 0.066 | 0.767 ± 0.061 | 0.829 ± 0.040 | 0.978 ± 0.141 |
| Left |
0.414 ± 0.027 | 0.385 ± 0.037 | 0.481 ± 0.092 | 0.362 ± 0.037 | 0.481 ± 0.093 | 0.395 ± 0.046 | 0.364 ± 0.034 | 0.487 ± 0.063 | 0.514 ± 0.081 | |
Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared to saline treated group. ANOVA followed by Tukey's multiple comparison post hoc test. n = 8 rats per group. Group I: (Saline), Group II: (Morphine), Group III: (Street heroin), Group IV: (mBME + Morphine), Group V: (mBME + Street heroin), Group VI: (Ascorbic acid + Morphine), Group VII: (Ascorbic acid + Street heroin), Group VIII: (mBME), Group IX: (Ascorbic acid).
Table 5 shows the weight of organs after 21 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination. The weight of liver was significantly decreased as compared to saline (group I) in groups of rats treated with mBME plus morphine (P < 0.05), ascorbic acid plus morphine (P < 0.01) and ascorbic acid plus street heroin (P < 0.001). Similarly, the weight of brain was significantly decreased (P < 0.05) in rats treated with ascorbic acid plus street heroin (group VII) and ascorbic acid alone (group IX) as compared to saline treated rats (group I). Significant reduction (P < 0.01) in the weight of right kidney was observed after treatment with ascorbic acid plus morphine (group VI) and ascorbic acid plus street heroin (group VII) when compared to saline treatment (group I). Moreover, the weight of left kidney was significantly decreased in rats treated with ascorbic acid plus morphine (P < 0.05) and ascorbic acid plus street heroin (P < 0.01) as compared to saline (group I).
Table 5.
Organs weight (gm) after 21 days of treatment with morphine, street heroin, mBME or ascorbic acid alone or in combination.
| Organs | Group I | Group II | Group III | Group IV | Group V | Group VI | Group VII | Group VIII | Group IX | |
|---|---|---|---|---|---|---|---|---|---|---|
| Liver |
9.383 ± 0.816 |
7.446 ± 0.262 |
7.720 ± 0.492 |
7.044 ± 0.620* |
8.039 ± 0.436 |
6.529 ± 0.170** |
5.789 ± 0.215*** |
9.241 ± 0.209 |
7.222 ± 0.030 |
|
| Spleen |
0.502 ± 0.061 |
0.487 ± 0.020 |
0.532 ± 0.019 |
0.496 ± 0.032 |
0.542 ± 0.037 |
0.408 ± 0.016 |
0.403 ± 0.018 |
0.536 ± 0.021 |
0.552 ± 0.031 |
|
| Brain |
1.664 ±0.070 |
1.587 ± 0.044 |
1.653 ± 0.052 |
1.578 ± 0.026 |
1.677 ± 0.050 |
1.484 ± 0.030 |
1.415 ± 0.038* |
1.708 ± 0.041 |
1.369 ± 0.051* |
|
| Thymus |
0.335 ± 0.021 |
0.324 ± 0.024 |
0.233 ± 0.023 |
0.266 ± 0.022 |
0.278 ± 0.053 |
0.287 ± 0.017 |
0.253 ± 0.026 |
0.273 ± 0.024 |
0.337 ± 0.011 |
|
| Heart |
0.847 ± 0.098 |
0.783 ± 0.048 |
0.756 ± 0.067 |
0.733 ± 0.053 |
0.836 ± 0.102 |
0.631 ± 0.018 |
0.611 ± 0.027 |
0.747 ± 0.016 |
0.812 ± 0.078 |
|
| Adrenals |
Right |
0.027 ± 0.005 | 0.029 ± 0.002 | 0.026 ± 0.000 | 0.030 ± 0.002 | 0.022 ± 0.001 | 0.026 ± 0.003 | 0.027 ± 0.001 | 0.034 ± 0.004 | 0.034 ± 0.003 |
| Left | 0.029 ± 0.006 | 0.030 ± 0.003 | 0.028 ± 0.004 | 0.024 ± 0.003 | 0.027 ± 0.003 | 0.028 ± 0.002 | 0.024 ± 0.002 | 0.031 ± 0.003 | 0.037 ± 0.005 | |
| Kidneys |
Right |
0.858 ± 0.068 | 0.733 ± 0.031 | 0.757 ± 0.040 | 0.746 ± 0.037 | 0.799 ± 0.028 | 0.636 ± 0.017** | 0.597 ± 0.027** | 0.862 ± 0.011 | 0.735 ± 0.011 |
| Left | 0.835 ± 0.067 | 0.768 ± 0.030 | 0.776 ± 0.026 | 0.746 ± 0.022 | 0.811 ± 0.040 | 0.651 ± 0.014* | 0.617 ± 0.030** | 0.840 ± 0.021 | 0.724 ± 0.014 | |
| Testis |
Right |
1.852 ± 0.113 | 1.876 ± 0.027 | 1.848 ± 0.058 | 1.870 ± 0.096 | 2.151 ± 0.165 | 1.810 ± 0.052 | 1.747 ± 0.062 | 1.891 ± 0.090 | 1.937 ± 0.077 |
| Left | 1.905 ± 0.100 | 1.882 ± 0.057 | 1.953 ± 0.040 | 1.913 ± 0.101 | 2.127 ± 0.107 | 1.882 ± 0.059 | 1.726 ± 0.041 | 1.907 ± 0.086 | 1.903 ± 0.063 | |
| Lungs |
Right |
0.856 ± 0.044 | 0.803 ± 0.086 | 0.776 ± 0.043 | 0.687 ± 0.031 | 0.905 ± 0.016 | 0.754 ± 0.045 | 0.623 ± 0.070 | 0.793 ± 0.030 | 0.774 ± 0.088 |
| Left |
0.396 ± 0.018 | 0.383 ± 0.037 | 0.412 ± 0.071 | 0.361 ± 0.026 | 0.486 ± 0.089 | 0.349 ± 0.019 | 0.314 ± 0.043 | 0.402 ± 0.023 | 0.347 ± 0.011 | |
Values are expressed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 compared to saline treated group. ANOVA followed by Tukey's multiple comparison post hoc test. n = 8 rats per group. Group I: (Saline), Group II: (Morphine), Group III: (Street heroin), Group IV: (mBME + Morphine), Group V: (mBME + Street heroin), Group VI: (Ascorbic acid + Morphine), Group VII: (Ascorbic acid + Street heroin), Group VIII: (mBME), Group IX: (Ascorbic acid).
3.6. Antioxidant activity of Bacopa monnieri methanolic extract
The maximum inhibition of DPPH free radical scavenging activity by mBME was 95.26% while those of ascorbic acid and BHT were 97.25% and 94.60% respectively. The DPPH free radicals scavenging activity decreased in the following rank order: Ascorbic acid > mBME > BHT. The EC50 of mBME was 36.55 μg/ml while those of ascorbic acid and BHT were 29.50 μg/ml and 34.33 μg/ml respectively. The EC50 for the DPPH free radical scavenging effect was in the order of: Ascorbic acid < BHT < mBME. The antiradical power of mBME was 0.0273 while those of ascorbic acid and BHT were 0.033 and 0.029 respectively. Similarly, the stoichiometry of mBME was 73.09 while those of ascorbic acid and BHT were 59.00 and 68.65 respectively. The percent inhibition, EC50, antiradical power and stoichiometry values of mBME and standards are shown in Table 6.
Table 6.
Percent inhibition, EC50, antiradical power and stoichiometry of mBME or standards (ascorbic acid and BHT).
| Parameter | Ascorbic acid | BHT | mBME | |
|---|---|---|---|---|
| Percent inhibition |
20 μl | 96.91 ± 0.310 | 94.60 ± 0.721 | 55.83 ± 2.398 |
| 40 μl | 97.11 ± 0.293 | 93.29 ± 0.880 | 87.26 ± 1.782 | |
| 60 μl | 97.24 ± 0.406 | 93.44 ± 0.778 | 94.90 ± 1.408 | |
| 80 μl | 97.25 ± 0.285 | 93.10 ± 0.706 | 95.26 ± 1.644 | |
| EC50 (μg/ml) |
29.50 ± 0.2485 |
34.33 ± 1.0080 |
36.55 ± 0.2411 |
|
| Antiradical power |
0.033 ± 0.0002 |
0.029 ± 0.0008 |
0.027 ± 0.0001 |
|
| Stoichiometry |
59.00 ± 0.4969 |
68.65 ± 2.0151 |
73.09 ± 0.4822 |
|
Results are mean ± SD of three separate experiments.
4. Discussion
Heroin overdose is a major cause of morbidity and premature death among heroin abusers (Darke and Hall, 2003). Street heroin is illicitly synthesized from morphine and depending on its origin or method of synthesis; it may contains different quantities of heroin and other components (Cunha-Oliveira et al., 2007). The street heroin found in Southwest Asia is a cruder, brown powder containing different quantities of heroin and adulterants (Ciccarone, 2009). Analysis of the same street heroin specimen in the previous study of our laboratory showed that in addition to diacetylmorphine content, the sample also contained different quantities of other adulterants including caffeine (8.4%), phenobarbitone (12.7%), 6-acetyl codeine (5.3%), 6-acetyl morphine (10.9%) and noscapine (15.8%) (Subhan et al., 2009). The toxic effects of street heroin may be either due to its heroin content or combination of different components (Cunha-Oliveira et al., 2007; Kinoshita et al., 2002). This study is the first to highlight the severity of hepatotoxicity and nephrotoxicity associated with street heroin in comparison to that of morphine using rat as an animal model. Our study showed that street heroin induced severe biochemical and histopathological changes in the liver and kidneys when compared to morphine, which might be attributed to the presence of adulterants or due to the synergistic effects of its components. Adulterants in street heroin play a significant role in the pathogenic mechanism of death (Barbera et al., 2012). The acetylcodeine component present in street heroin is considered a significant contributor to the toxicological effects of illicitly manufactured heroin (O'Neal et al., 2001).
In the present study, treatment with morphine or street heroin caused elevation of serum ALT, AST and creatinine and produced moderate to severe histopathological changes in the liver and kidneys after 14 and 21 days. These results are in agreement with the previous studies on heroin (Junhua et al., 2008; Yang et al., 2006) and morphine (Atici et al., 2005; Bekheet, 2010). A significant increase in the levels of ALT (James et al., 1982; Zhang et al., 2004), AST, blood urea nitrogen, creatinine (Atici et al., 2005) and lower hepatic glutathione (Payabvash et al., 2006) have been observed with morphine. During short term treatment with morphine, the liver exhibits remarkable central vein dilatation with infiltration of inflammatory cells (Bekheet, 2010) whereas long term treatment was associated with sinusoidal dilatation and congestion, hydropic degeneration (ballooning) in perivenular region with necrosis, hemorrhage, focal microvesicular steatosis (Atici et al., 2005) and induction of apoptosis (Payabvash et al., 2006). In kidneys, morphine causes tubular cells vacuolization, interstitial mononuclear cell infiltration with focal necrosis and hemorrhage (Atici et al., 2005). Similarly chronic heroin use was related with significant increase in the levels of ALT (Junhua et al., 2008), LDH, lipid peroxides (Panchenko et al., 1999) and creatinine (Yang et al., 2006). Elevated serum transaminases, as well as depletion of hepatic glutathione, are important biochemical consequences of liver injury after morphine, cocaine and heroin administration (De Araújo et al., 1992). In liver, street heroin exert a significant effect on the size of hepatocytes (Fazelipour and Tootian, 2008) with hepatic congestion and slight to moderate fatty vacuoles in hepatocytes (Dettmeyer et al., 2009), infiltration of polymorphonuclear and lymphomonocytes in the sinusoidal lumen, sinusoidal dilatation, centrilobular perisinusoidal fibrosis and hepatic lesions (de Araújo et al., 1990), cirrhosis, necrosis (Darke et al., 2010) and apoptosis of hepatocytes (Fecho and Lysle, 2000). Heroin associated nephropathy is manifested as membranoproliferative glomerulonephritis with thickened glomerular capillary walls and marked endocapillary hypercellularity, frequently with a lobular pattern (Sameiro Faria et al., 2003), interstitial nephritis and renal interstitial scarring (Singhal et al., 1998).
In this study, pretreatment with standardized mBME restored opioid induced elevation of serum ALT, AST and creatinine and provided protection against histopathological changes in the liver and kidneys after 14 and 21 days. The hepato- and nephro-protective effect of mBME at the same dose (40 mg/kg) has been reported by Sumathi and Devaraj (Sumathi and Devaraj, 2009) against morphine. The HPLC analysis of mBME in our study revealed higher contents of bacoside-A components. Bacopa monnieri obtained from different sources exhibit variation in the contents of bacoside-A (Deepak et al., 2005). Bacoside-A is considered as part of major saponins along with bacopaside I and constituted more than 96% w/w of the total saponins of Bacopa monnieri (Deepak and Amit, 2013). Bacopa monnieri is a potent natural scavenger of free radicals (Bhattacharya et al., 2000; Russo et al., 2003). DPPH antioxidant assay is commonly used for the determination of free radical scavenging property of pure and natural compounds (Mishra et al., 2012). The strength of antioxidants present in an extract is determined by EC50, antiradical power and stoichiometry values, with low EC50 and stoichiometry values and high antiradical power indicate strong antioxidant activity (Loo et al., 2007). In this study, mBME exhibited a concentration dependant increase of percent scavenging of DPPH free radicals and is in accordance with the previous study (Shahid and Subhan, 2014). Bacopa monnieri is a strong natural antioxidant (Anand et al., 2011) and is an effective scavenger of DPPH free radicals (Srivastava et al., 2012), peroxynitrites (Alam et al., 2010), hydrogen peroxide (Shinde et al., 2011), nitric oxide (Alam et al., 2012) and superoxide radicals (Ghosh et al., 2007). Bacoside-A is responsible for the pharmacological effects of Bacopa monnieri (Sudharani, 2011) and is due to its strong free radical scavenging capacity (Anbarasi et al., 2005).
In the present study, pretreatment with mBME provided protection against morphine induced reduction in body weight at least for 14 days as well as protected the major internal organs. Addictive drugs when interact with brain systems affect physiological stimuli such as water, food and social interaction, that are critical for survival (Cunha-Oliveira et al., 2008). The protective effect of Bacopa monnieri against opioid induced body weight loss might be due to its adaptogenic effect, mediated by hypothalamic pituitary axis. The dorsomedial nucleus of hypothalamus plays an important role in the homeostatic control of ingestive behavior and body weight regulation (Bellinger and Bernardis, 2002). Bacopa monnieri relieves both acute and chronic stress by attenuating systemic hypothalamic pituitary axis response and reversed the changes in ulcer index, adrenal gland weight, creatine kinase and AST (Rai et al., 2003). Moreover, the antistress effect of Bacopa monnieri is also mediated by modulating the expression of Hsp70 and the activity of P450s and superoxide dismutase, the enzymes known to be involved in the production and scavenging of reactive oxygen species in different regions of the brain (Chowdhuri et al., 2002).
Reactive oxygen species play an intimate role in addiction by aiding in propagation of signals with regard to ion transport, neuromodulation and transcription processes (Kovacic, 2005). Heroin addicts are frequently presented with an imbalance between oxidation and antioxidation and are more susceptible to injuries induced by nitric oxide and other free radicals (Zhou et al., 2001). Heroin induced hepatotoxicity has been associated with oxidative damage to proteins, DNA and lipids (Junhua et al., 2008; Pan et al., 2005). Similarly, chronic use of morphine causes marked inhibition of antioxidant enzymes in the liver (Zhang et al., 2004) which provide favorable conditions for H2O2 toxicity and triggered lipid peroxidation (Miskevich et al., 2007) leading to apoptosis of hepatocytes (Payabvash et al., 2006), increased accumulation of lipids in hepatocytes, deposition of collagen like fibrous material, reduction in the number of endothelial cell fenestrations (Bekheet, 2010) and opening of KATP channels (Afify et al., 2013). In the kidneys, morphine enhances mesengial cell formation of superoxide that is mediated through opioid receptors (Singhal et al., 1994). It has been investigated that injuries to the bodies of heroin addicts can be prevented by exogenous antioxidants which are able to reduce oxidative stress (Pan et al., 2005). In this regard, the hepato- and nephro-protective effect of Bacopa monnieri seen in this study might be attributed to its strong antioxidant capacity which is due to the presence of bacosides components. Accordingly, pretreatment with the standard antioxidant, ascorbic acid also restored the morphine and street heroin induced elevated levels of ALT, AST and creatinine and provided protection against histopathological changes in the liver and kidneys. Ascorbic acid abates the oxidative damage of DNA, protein and lipid as well as normalizing the plasma alanine aminotransferase activity induced by morphine (Zhang et al., 2004). Moreover, ascorbic acid reduces heroin induced oxidative stress in different tissues by ameliorating the oxidative damages of protein and lipids as well as increasing the total antioxidant capacity (Pan et al., 2005). From these effects, it can be argued that opioid induced hepato- and nephro-toxicity was associated with oxidative stress and Bacopa monnieri due to its strong antioxidant potential reduced this oxidative stress resulting in the amelioration of morphine and street heroin induced hepatotoxicity and nephrotoxicity. Furthermore, heroin abuse increases the activities of glutathione-S-transferase, selenium independent glutathione peroxidase and decreases the level of glutathione therefore mediates oxidative stress in parietal, occipital, frontal and temporal cortex, hippocampus, brain stem and white matter of the brain (Gutowicz et al., 2011). Bacopa monnieri by virtue of antioxidant effect of its major component, bacoside-A inhibits morphine induced brain oxidative stress by improving the activity of ATPases and maintaining the sodium, potassium, calcium and magnesium ionic equilibrium (Sumathi et al., 2011) as well as normalizing the activities of isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, succinate dehydrogenase, malate dehydrogenase, NADPH dehydrogenase and cytochrome c oxidase (Sumathy et al., 2001). Therefore, blocking of oxidative stress by natural antioxidants such as Bacopa monnieri may be useful in the development of new therapy for opiate abusers. However, the protective effect of Bacopa monnieri mediated by its antioxidant property against morphine or street heroin induced hepatotoxicity and nephrotoxicity needs further investigation for direct evidence linking reactive oxygen species with oxidative/redox stress injury by assay of antioxidant enzymes, malondialdehyde or protein markers.
The hepato- and nephroprotective effect mediated through strong antioxidant potential of Bacopa monnieri might be one of the mechanism and additional mechanisms responsible for the reduction in opioid induced toxicity cannot be ignored as Bacopa monnieri has been reported to reduce morphine induced hyperactivity by decreasing dopamine and serotonin levels in the striatum (Rauf et al., 2011b), morphine withdrawal physical symptoms (Sumathi et al., 2002) and morphine withdrawal depression (Rauf et al., 2013). Moreover, Bacopa monnieri decreases the release of tumor necrosis factor-α and interleukin-6 from mononuclear cells (Viji and Helen, 2011), inhibits the activities of cyclooxygenase-2, lipooxygenase-5 and lipooxygenase-15 (Viji and Helen, 2008), prostaglandin-E2 production (Channa et al., 2006), and stabilizes the lysosomal membranes (Jain et al., 1994) and mast cells (Samiulla et al., 2001). Bacopa monnieri besides preserving the mitochondrial membrane potential, also maintains the mitochondrial complex-I activity with activation of nuclear factor erythroid-2 related factor-2 pathway by modulating Keap1 expression and phosphorylation of Akt promotes its role in cell survival (Singh et al., 2012). Furthermore, Bacopa monnieri inhibits CYP2C19, CYP2C9, CYP1A2, and CYP3A4 enzymes (Ramasamy et al., 2014) which are involved in the opioid metabolism (Holmquist, 2009).
5. Conclusion
As compared to morphine, treatment with street heroin was associated with elevated levels of serum ALT, AST and creatinine and produced severe histopathological changes in the liver and kidneys after 14 and 21 days. The exaggerated hepatotoxicity and nephrotoxicity might be due to the synergistic effects of large number of adulterants and diacetylmorphine content in the street heroin specimen. Pretreatment with Bacopa monnieri or ascorbic acid restored the elevation of serum ALT, AST and creatinine and protected liver and kidneys from the toxicological influence of morphine and street heroin. Bacopa monnieri due to its content of bacoside-A which possessed strong antioxidant potential, may provide a beneficial herbal remedy for the management of opioid related hepatotoxicity and nephrotoxicity.
Declarations
Author contribution statement
Muhammad Shahid: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Fazal Subhan: Conceived and designed the experiments; Analyzed and interpreted the data.
Ihsan Ullah, Gowhar Ali: Contributed reagents, materials, analysis tools or data.
Javaid Alam, Rehmat Shah: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
The authors received no funding from an external source.
Conflict of interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are thankful to the Anti Narcotics Force, Peshawar branch and M/S Punjab Drug House laboratories, Pakistan for the supply of street heroin specimen and morphine respectively under strict control to Prof. Dr. Fazal Subhan, who is an opioid neuropharmacologist and has been legally authorized to obtain and conduct research on street heroin (No.1154ANF/PS/PR/2003) and morphine (No.9-2/U.Peshawar/06-Policy-I, No.F.7-3/2009-I&E) by the Anti Narcotics Force, Ministry of Narcotics Control and Ministry of Health, Government of Pakistan. We are grateful to Professor Dr. Ikhlas Khan, the National Center for Natural Products Research, Mississippi, USA for the gift of HPLC standards of bacosides.
Contributor Information
Muhammad Shahid, Email: shahidsalim_2002@hotmail.com.
Fazal Subhan, Email: fazal_subhan@upesh.edu.pk.
References
- Abbas M., Subhan F., Mohani N., Rauf K., Ali G., Khan M. The involvement of opioidergic mechanisms in the activity of Bacopa monnieri extract and its toxicological studies. Afr. J. Pharm. Pharmacol. 2011;5:1120–1124. [Google Scholar]
- Afify E.A., Khedr M.M., Omar A.G., Nasser S.A. The involvement of K(ATP) channels in morphine-induced antinociception and hepatic oxidative stress in acute and inflammatory pain in rats. Fundam. Clin. Pharmacol. 2013;27(6):623–631. doi: 10.1111/fcp.12004. [DOI] [PubMed] [Google Scholar]
- Alam M., Hossain M., Asadujjaman M., Islam M., Mazumder M., Haque M. Peroxynitrite scavenging and toxicity potentail of different fractions of the ariel parts of Bacopa monniera Linn. Int. J. Pharm. Sci. Res. 2010;1:78–83. [Google Scholar]
- Alam M.N., Wahed T.B., Sultana F., Ahmed J., Hasan M. In vitro antioxidant potential of the methanolic extract of Bacopa monnieri L. Turk. J. Pharm. Sci. 2012;9:285–292. [Google Scholar]
- Anand T., Naika M., Swamy M., Khanum F. Antioxidant and DNA damage preventive properties of Bacopa monniera (L) Wettst. Free Rad. Antiox. 2011;1:84–90. [Google Scholar]
- Anbarasi K., Sabitha K.E., Devi C.S. Lactate dehydrogenase isoenzyme patterns upon chronic exposure to cigarette smoke: Protective effect of bacoside A. Environ. Toxicol. Pharmacol. 2005;20:345–350. doi: 10.1016/j.etap.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Atici S., Cinel I., Cinel L., Doruk N., Eskandari G., Oral U. Liver and kidney toxicity in chronic use of opioids: An experimental long term treatment model. J. Biosci. 2005;30:245–252. doi: 10.1007/BF02703705. [DOI] [PubMed] [Google Scholar]
- Barbera N., Busardò F.P., Indorato F., Romano G. The pathogenetic role of adulterants in 5 cases of drug addicts with a fatal outcome. Forensic Sci. Int. 2012;227:74–76. doi: 10.1016/j.forsciint.2012.08.041. [DOI] [PubMed] [Google Scholar]
- Bekheet S.H.M. Morphine sulphate induced histopathological and histochemical changes in the rat liver. Tissue Cell. 2010;42:266–272. doi: 10.1016/j.tice.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bellinger L.L., Bernardis L.L. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: Lessons learned from lesioning studies. Physiol. Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S., Bhattacharya A., Kumar A., Ghosal S. Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother. Res. 2000;14:174–179. doi: 10.1002/(sici)1099-1573(200005)14:3<174::aid-ptr624>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Calabrese C., Gregory W.L., Leo M., Kraemer D., Bone K., Oken B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: A randomized, double-blind, placebo-controlled trial. J. Altern. Complement. Med. 2008;14:707–713. doi: 10.1089/acm.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cami J., Farré M. Drug addiction. N. Engl. J. Med. 2003;349:975–986. doi: 10.1056/NEJMra023160. [DOI] [PubMed] [Google Scholar]
- Channa S., Dar A., Anjum S., Yaqoob M. Anti-inflammatory activity of Bacopa monniera in rodents. J. Ethnopharmacol. 2006;104:286–289. doi: 10.1016/j.jep.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Chowdhuri D.K., Parmar D., Kakkar P., Shukla R., Seth P., Srimal R. Antistress effects of bacosides of Bacopa monnieri: Modulation of Hsp70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Phytother. Res. 2002;16:639–645. doi: 10.1002/ptr.1023. [DOI] [PubMed] [Google Scholar]
- Christrup L. Morphine metabolites. Acta Anaesthesiol. Scand. 2008;41:116–122. doi: 10.1111/j.1399-6576.1997.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Ciccarone D. Heroin in brown, black and white: Structural factors and medical consequences in the US heroin market. Int. J. Drug Policy. 2009;20:277–282. doi: 10.1016/j.drugpo.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T., Waal H., Thoresen M., Gossop M. Mortality among opiate users: Opioid maintenance therapy, age and causes of death. Addiction. 2009;104:1356–1362. doi: 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T., Rego A.C., Garrido J., Borges F., Macedo T., Oliveira C.R. Street heroin induces mitochondrial dysfunction and apoptosis in rat cortical neurons. J. Neurochem. 2007;101:543–554. doi: 10.1111/j.1471-4159.2006.04406.x. [DOI] [PubMed] [Google Scholar]
- Cunha-Oliveira T., Rego A.C., Oliveira C.R. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain. Res. Rev. 2008;58:192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Dar A., Channa S. Relaxant effect of ethanol extract of Bacopa monniera on trachea, pulmonary artery and aorta from rabbit and guinea pig. Phytother. Res. 1997;11:323–325. [Google Scholar]
- Darke S., Duflou J., Torok M. The comparative toxicology and major organ pathology of fatal methadone and heroin toxicity cases. Drug Alcohol Depend. 2010;106:1–6. doi: 10.1016/j.drugalcdep.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Darke S., Hall W. Heroin overdose: Research and evidence-based intervention. J. Urban Health. 2003;80:189–200. doi: 10.1093/jurban/jtg022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S., Hall W., Weatherburn D., Lind B. Fluctuations in heroin purity and the incidence of fatal heroin overdose. Drug Alcohol Depend. 1999;54:155–161. doi: 10.1016/s0376-8716(98)00159-8. [DOI] [PubMed] [Google Scholar]
- De Araújo M.S.T., Gérard F., Chossegros P., Guerret S., Grimaud J.A. Lack of hepatocyte involvement in the genesis of the sinusoidal dilatation related to heroin addiction: A morphometric study. Virchows Arch. 1992;420:149–153. doi: 10.1007/BF02358806. [DOI] [PubMed] [Google Scholar]
- de Araújo M.S.T., Gerard F., Chossegros P., Porto L.C., Barlet P., Grimaud J.A. Vascular hepatotoxicity related to heroin addiction. Virchows Arch. 1990;417:497–503. doi: 10.1007/BF01625730. [DOI] [PubMed] [Google Scholar]
- Deepak M., Amit A. ‘Bacoside B’ - the need remains for establishing identity. Fitoterapia. 2013;87:7–10. doi: 10.1016/j.fitote.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Deepak M., Sangli G., Arun P., Amit A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem. Anal. 2005;16:24–29. doi: 10.1002/pca.805. [DOI] [PubMed] [Google Scholar]
- Degenhardt L., Bucello C., Mathers B., Briegleb C., Ali H., Hickman M., McLaren J. Mortality among regular or dependent users of heroin and other opioids: A systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- Dettmeyer R., Friedrich K., Schmidt P., Madea B. Heroin-associated myocardial damages-Conventional and immunohistochemical investigations. Forensic Sci. Int. 2009;187:42–46. doi: 10.1016/j.forsciint.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Dettmeyer R.B., Preuß J., Wollersen H., Madea B. Heroin-associated nephropathy. Expert Opin. Drug Saf. 2005;4:19–28. doi: 10.1517/14740338.4.1.19. [DOI] [PubMed] [Google Scholar]
- Drummer O.H. Postmortem toxicology of drugs of abuse. Forensic Sci. Int. 2004;142:101–113. doi: 10.1016/j.forsciint.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Fazelipour S., Kiaei S., Tootian Z., Dashtnavard H. Histomorphometric study of hepatocytes of mice after using heroin. Int. J. Pharmacol. 2008;4:496–499. [Google Scholar]
- Fazelipour S., Tootian Z. The study of histomorphometric changes of the liver tissue of mouse due to using heroin. Toxicol. Lett. 2008;180:496–499. [Google Scholar]
- Fecho K., Lysle D.T. Heroin-induced alterations in leukocyte numbers and apoptosis in the rat spleen. Cell. Immunol. 2000;202:113–123. doi: 10.1006/cimm.2000.1653. [DOI] [PubMed] [Google Scholar]
- Ghosh T., Kumar Maity T., Das M., Bose A., Kumar Dash D. In vitro: antioxidant and hepatoprotective activity of ethanolic extract of Bacopa monnieri Linn aerial parts. Iran. J. Pharmacol. Ther. 2007;6:77–85. [Google Scholar]
- Gutowicz M., Kaźmierczak B., Barańczyk-Kuźma A. The influence of heroin abuse on glutathione-dependent enzymes in human brain. Drug Alcohol Depend. 2011;113:8–12. doi: 10.1016/j.drugalcdep.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Holmquist G.L. Opioid metabolism and effects of cytochrome P450. Pain Med. 2009;10:S20–S29. [Google Scholar]
- Jain P., Khanna N., Trehan N., Pendse V., Godhwani J. Antiinflammatory effects of an Ayurvedic preparation, Brahmi Rasayan, in rodents. Indian J. Exp. Biol. 1994;32:633–636. [PubMed] [Google Scholar]
- James R.C., Goodman D.R., Harbison R.D. Hepatic glutathione and hepatotoxicity: changes induced by selected narcotics. J. Pharmacol. Exp. Ther. 1982;221:708–714. [PubMed] [Google Scholar]
- John S., Griffiths P., Michael G. Heroin in the United Kingdom: Different forms, different origins, and the relationship to different routes of administration. Drug Alcohol Rev. 1997;16:329–337. doi: 10.1080/09595239700186711. [DOI] [PubMed] [Google Scholar]
- Junhua L., Zhenhua W., Bao J., Qiusheng Z. Heroin-induced hepatotoxicity: Involved oxidative stress, Bioinformatics and Biomedical Engineering. The 2nd International Conference IEEE. 2008:1112–1118. [Google Scholar]
- Kamkaew N., Scholfield C.N., Ingkaninan K., Maneesai P., Parkington H.C., Tare M., Chootip K. Bacopa monnieri: and its constituents is hypotensive in anaesthetised rats and vasodilator in various artery types. J. Ethnopharmacol. 2011;137:790–795. doi: 10.1016/j.jep.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Kinoshita A., Wanibuchi H., Imaoka S., Ogawa M., Masuda C., Morimura K., Funae Y., Fukushima S. Formation of 8-hydroxydeoxyguanosine and cell-cycle arrest in the rat liver via generation of oxidative stress by phenobarbital: Association with expression profiles of p21WAF1/Cip1, cyclin D1 and Ogg1. Carcinogenesis. 2002;23:341–349. doi: 10.1093/carcin/23.2.341. [DOI] [PubMed] [Google Scholar]
- Kosten T.R., O'Connor P.G. Management of drug and alcohol withdrawal. N. Engl. J. Med. 2003;348:1786–1795. doi: 10.1056/NEJMra020617. [DOI] [PubMed] [Google Scholar]
- Kovacic P. Unifying mechanism for addiction and toxicity of abused drugs with application to dopamine and glutamate mediators: Electron transfer and reactive oxygen species. Med. Hypotheses. 2005;65:90–96. doi: 10.1016/j.mehy.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Limpeanchob N., Jaipan S., Rattanakaruna S., Phrompittayarat W., Ingkaninan K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 2008;120:112–117. doi: 10.1016/j.jep.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Liu T.-t., Shi J., Epstein D.H., Bao Y.-P., Lu L. A meta-analysis of Chinese Herbal Medicine in treatment of managed withdrawal from heroin. Cell. Mol. Neurobiol. 2009;29:17–25. doi: 10.1007/s10571-008-9290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo A., Jain K., Darah I. Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant. Rhizophora apiculata. Food Chem. 2007;104:300–307. [Google Scholar]
- Lue B.-M., Nielsen N.S., Jacobsen C., Hellgren L., Guo Z., Xu X. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem. 2010;123:221–230. [Google Scholar]
- Meena H., Pandey H.K., Pandey P., Arya M.C., Ahmed Z. Evaluation of antioxidant activity of two important memory enhancing medicinal plants Bacopa monnieri and Centella asiatica. Indian J. Pharmacol. 2012;44:114–117. doi: 10.4103/0253-7613.91880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra K., Ojha H., Chaudhury N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012;130:1036–1043. [Google Scholar]
- Miskevich D., Petushok N., Lelevich V., Lelevich S., Borodinsky A. Effect of chronic morphine treatment on time-course of free radical processes. Biomedical Chem. 2007;1:245–248. [Google Scholar]
- Morgan A., Stevens J. Does Bacopa monnieri improve memory performance in older persons? Results of a randomized, placebo-controlled, double-blind trial. J. Altern. Complement. Med. 2010;16:753–759. doi: 10.1089/acm.2009.0342. [DOI] [PubMed] [Google Scholar]
- Murthy P.B.S., Raju V.R., Ramakrisana T., Chakravarthy M.S., Kumar K.V., Kannababu S., Subbaraju G.V. Estimation of twelve bacopa saponins in Bacopa monnieri extracts and formulations by high-performance liquid chromatography. Chem. Pharm. Bull. 2006;54:907–911. doi: 10.1248/cpb.54.907. [DOI] [PubMed] [Google Scholar]
- O'Neal C.L., Poklis A., Lichtman A.H. Acetylcodeine, an impurity of illicitly manufactured heroin, elicits convulsions, antinociception, and locomotor stimulation in mice. Drug Alcohol Depend. 2001;65:37–43. doi: 10.1016/s0376-8716(01)00145-4. [DOI] [PubMed] [Google Scholar]
- Pacifici R., Di Carlo S., Bacosi A., Pichini S., Zuccaro P. Pharmacokinetics and cytokine production in heroin and morphine-treated mice. Int. J. Immunopharmacol. 2000;22:603–614. doi: 10.1016/s0192-0561(00)00023-0. [DOI] [PubMed] [Google Scholar]
- Pan J., Zhang Q., Zhang Y., Ouyang Z., Zheng Q., Zheng R. Oxidative stress in heroin administered mice and natural antioxidants protection. Life Sci. 2005;77:183–193. doi: 10.1016/j.lfs.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Panchenko L., Pirozhkov S., Nadezhdin A., Baronets V.I., Usmanova N. Lipid peroxidation, peroxyl radical-scavenging system of plasma and liver and heart pathology in adolescence heroin users. Vopr. Med. Khim. 1999;45:501–506. [PubMed] [Google Scholar]
- Payabvash S., Beheshtian A., Salmasi A.H., Kiumehr S., Ghahremani M.H., Tavangar S.M., Sabzevari O., Dehpour A.R. Chronic morphine treatment induces oxidant and apoptotic damage in the mice liver. Life Sci. 2006;79:972–980. doi: 10.1016/j.lfs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Rai D., Bhatia G., Palit G., Pal R., Singh S., Singh H.K. Adaptogenic effect of Bacopa monniera (Brahmi) Pharmacol. Biochem. Behav. 2003;75:823–830. doi: 10.1016/s0091-3057(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Ramasamy S., Kiew L.V., Chung L.Y. Inhibition of human cytochrome P450 enzymes by Bacopa monnieri standardized extract and constituents. Molecules. 2014;19:2588–2601. doi: 10.3390/molecules19022588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf K., Subhan F., Abbas M., Ali S.M., Ali G., Ashfaq M., Abbas G. Inhibitory effect of bacopasides on spontaneous morphine withdrawal induced depression in mice. Phytother. Res. 2013;28:937–939. doi: 10.1002/ptr.5081. [DOI] [PubMed] [Google Scholar]
- Rauf K., Subhan F., Abbas M., Badshah A., Ullah I., Ullah S. Effect of bacopasides on acquisition and expression of morphine tolerance. Phytomedicine. 2011;18:836–842. doi: 10.1016/j.phymed.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Rauf K., Subhan F., Sewell R.D.E. A bacoside containing Bacopa monnieri extract reduces both morphine hyperactivity plus the elevated striatal dopamine and serotonin turnover. Phytother. Res. 2011;26:758–763. doi: 10.1002/ptr.3631. [DOI] [PubMed] [Google Scholar]
- Russo A., Borrelli F. Bacopa monniera, a reputed nootropic plant: An overview. Phytomedicine. 2005;12:305–317. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Russo A., Izzo A.A., Borrelli F., Renis M., Vanella A. Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother. Res. 2003;17:870–875. doi: 10.1002/ptr.1061. [DOI] [PubMed] [Google Scholar]
- Sairam K., Dorababu M., Goel R., Bhattacharya S. Antidepressant activity of standardized extract of Bacopa monniera in experimental models of depression in rats. Phytomedicine. 2002;9:207–211. doi: 10.1078/0944-7113-00116. [DOI] [PubMed] [Google Scholar]
- Sairam K., Rao C.V., Babu M.D., Goel R. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine. 2001;8:423–430. doi: 10.1078/S0944-7113(04)70060-4. [DOI] [PubMed] [Google Scholar]
- Sameiro Faria M. Do, Sampaio S., Faria V., Carvalho E. Nephropathy associated with heroin abuse in Caucasian patients. Nephrol. Dial. Transplant. 2003;18:2308–2313. doi: 10.1093/ndt/gfg369. [DOI] [PubMed] [Google Scholar]
- Samiulla D., Prashanth D., Amit A. Mast cell stabilising activity of Bacopa monnieri. Fitoterapia. 2001;72:284–285. doi: 10.1016/s0367-326x(00)00309-9. [DOI] [PubMed] [Google Scholar]
- Shahid M., Subhan F. Protective effect of Bacopa monniera methanol extract against carbon tetrachloride induced hepatotoxicity and nephrotoxicity. Pharmacologyonline. 2014;2:18–28. [Google Scholar]
- Shinde R., Deshmukh S., Vitekari H., Kamble P. Effect of Bacoside extract on H2O2 stressed lymphocytes. Int. J. Pharm. Biol. Sci. 2011;1:567–571. [Google Scholar]
- Singh M., Murthy V., Ramassamy C. Standardized extracts of Bacopa monniera protect against MPP+- and paraquat induced toxicity by modulating mitochondrial activities, proteasomal functions, and redox pathways. Toxicol. Sci. 2012;125:219–232. doi: 10.1093/toxsci/kfr255. [DOI] [PubMed] [Google Scholar]
- Singhal P.C., Pamarthi M., Shah R., Chandra D., Gibbons N. Morphine stimulates superoxide formation by glomerular mesangial cells. Inflammation. 1994;18:293–299. doi: 10.1007/BF01534270. [DOI] [PubMed] [Google Scholar]
- Singhal P.C., Sharma P., Sanwal V., Prasad A., Kapasi A., Ranjan R., Franki N., Reddy K., Gibbons N. Morphine modulates proliferation of kidney fibroblasts. Kidney Int. 1998;53:350–357. doi: 10.1046/j.1523-1755.1998.00758.x. [DOI] [PubMed] [Google Scholar]
- Srivastava P., Raut H.N., Puntambekar H.M., Desai A.C. Stability studies of crude plant material of Bacopa monnieri and quantitative determination of bacopaside I and bacoside A by HPLC. Phytochem. Anal. 2012;23:502–507. doi: 10.1002/pca.2347. [DOI] [PubMed] [Google Scholar]
- Subhan F., Khan N., Sewell R.D.E. Adulterant profile of illicit street heroin and reduction of its precipitated physical dependence withdrawal syndrome by extracts of St John's wort (Hypericum perforatum) Phytother. Res. 2009;23:564–571. doi: 10.1002/ptr.2692. [DOI] [PubMed] [Google Scholar]
- Sudharani D. Pharmacological profiles of Bacopa monnieri: A review. Int. J. Pharmacy. 2011;1:15–23. [Google Scholar]
- Sumathi T., Devaraj S. Effect of Bacopa monniera on liver and kidney toxicity in chronic use of opioids. Phytomedicine. 2009;16:897–903. doi: 10.1016/j.phymed.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Sumathi T., Nathiya V., Sakthikumar M. Protective effect of bacoside-A against morphine-induced oxidative stress in rats. Indian J. Pharm. Sci. 2011;73:409–415. doi: 10.4103/0250-474X.95624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumathi T., Nayeem M., Balakrishna K., Veluchamy G., Devaraj S.N. Alcoholic extract of Bacopa monniera reduces the in vitro effects of morphine withdrawal in guinea-pig ileum. J. Ethnopharmacol. 2002;82:75–81. doi: 10.1016/s0378-8741(02)00139-3. [DOI] [PubMed] [Google Scholar]
- Sumathi T., Nongbri A. Hepatoprotective effect of Bacoside-A, a major constituent of Bacopa monniera Linn. Phytomedicine. 2008;15:901–905. doi: 10.1016/j.phymed.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Sumathi T., Veluchamy G. Inhibitory effect of Bacopa monniera on morphine induced pharmacological effects in mice. Nat. Prod. Sci. 2007;13:46–53. [Google Scholar]
- Sumathy T., Subramanian S., Govindasamy S., Balakrishna K., Veluchamy G. Protective role of Bacopa monniera on morphine induced hepatotoxicity in rats. Phytother. Res. 2001;15:643–645. doi: 10.1002/ptr.1007. [DOI] [PubMed] [Google Scholar]
- Tang Y. l., Zhao D., Zhao C., Cubells J.F. Opiate addiction in China: Current situation and treatments. Addiction. 2006;101:657–665. doi: 10.1111/j.1360-0443.2006.01367.x. [DOI] [PubMed] [Google Scholar]
- van den Brink W., van Ree J.M. Pharmacological treatments for heroin and cocaine addiction. Eur. Neuropsychopharmacol. 2003;13:476–487. doi: 10.1016/j.euroneuro.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Viji V., Helen A. Inhibition of lipoxygenases and cyclooxygenase-2 enzymes by extracts isolated from Bacopa monniera (L.) Wettst. J. Ethnopharmacol. 2008;118:305–311. doi: 10.1016/j.jep.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Viji V., Helen A. Inhibition of pro-inflammatory mediators: Role of Bacopa monniera (L.) Wettst. Inflammopharmacology. 2011;19:283–291. doi: 10.1007/s10787-010-0046-4. [DOI] [PubMed] [Google Scholar]
- Vollala V.R., Upadhya S., Nayak S. Effect of Bacopa monniera Linn. (brahmi) extract on learning and memory in rats: A behavioral study. J. Vet. Behav. 2010;5:69–74. [Google Scholar]
- Yang Y., Zhang J., Sun C., Yu H., Li Q., Hong M. Heroin affects purine nucleotides catabolism in rats in vivo. Life Sci. 2006;78:1413–1418. doi: 10.1016/j.lfs.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y.T., Zheng Q.S., Pan J., Zheng R.L. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin. Pharmacol. Toxicol. 2004;95:53–58. doi: 10.1111/j.1742-7843.2004.950202.x. [DOI] [PubMed] [Google Scholar]
- Zhou J., Si P., Ruan Z., Ma S., Yuan H., Peng F., Sun L., Ding D., Xu S. Primary studies on heroin abuse and injury induced by oxidation and lipoperoxidation. Chin. Med. J. 2001;114:297–302. [PubMed] [Google Scholar]