Abstract
Presently, various studies had investigated the accuracy of autofluorescence in diagnosing oral squamous cell carcinoma (OSCC) and oral potentially malignant disorders (OPMD) with diverse conclusions. This study aimed to assess its accuracy for OSCC and OPMD and to investigate its applicability in general dental practice. After a comprehensive literature search, a meta-analysis was conducted to calculate the pooled diagnostic indexes of autofluorescence for premalignant lesions (PML) and malignant lesions (ML) of the oral cavity, lung, esophagus, stomach and colorectum and to compute indexes regarding the detection of OSCC aided by algorithms. Besides, a u test was performed. Twenty-four studies detecting OSCC and OPMD in 2761 lesions were included. This demonstrated that the overall accuracy of autofluorescence for OSCC and OPMD was superior to PML and ML of the lung, esophagus and stomach, slightly inferior to the colorectum. Additionally, the sensitivity and specificity for OSCC and OPMD were 0.89 and 0.8, respectively. Furthermore, the specificity could be remarkably improved by additional algorithms. With relatively high accuracy, autofluorescence could be potentially applied as an adjunct for early diagnosis of OSCC and OPMD. Moreover, approaches such as algorithms could enhance its specificity to ensure its efficacy in primary care.
The global incidence rate of oral cancer is 8.2 per 100,000 annually for males and 2.8 per 100,000 annually for females1. More than 90% of oral cancers are oral squamous cell carcinomas (OSCCs), which are one of the most common malignant tumors and are more prevalent in South-Central Asia and in Central and Eastern Europe1,2. Owing to the unapparent symptoms in early stages and delayed diagnosis3,4, OSCC tends to be detected at advanced stages with high mortality, accounting for approximately 300,000 cases of OSCC and 145,000 deaths in 201212. Despite treatment advancements, the 5-year survival rate for OSCC patients has remained poor over the past three decades5. However, if OSCC is diagnosed early (stage I-II) and effective treatment is administered, a 5-year survival rate of approximately 80% is obtainable compared with only 20% for those detected at advanced stages (stage III-IV)5. Therefore, early detection is crucial to help improve the survival rate of OSCC6.
Usually, patients initially present to general dental practice when they have oral discomfort. Thus, as frontline health workers, general dentists bear the responsibility for early screening of oral abnormalities6. Furthermore, dental practitioners play a vital role in making correct decisions about the lesions, by which unnecessary or delayed referrals could be avoided and the mortality of OSCC could be considerably reduced5,7,8. In most cases, OSCC is preceded by oral potentially malignant disorders (OPMD) such as oral leukoplakia and oral submucous fibrosis9,10, so the early detection and management of epithelial dysplasia in OPMD is an important preventative step against malignant transformation11; Moreover, since dysplastic and neoplastic lesions of the oral cavity are readily accessible and the mucosal changes are frequently visible, OSCC and dysplasia are both promising candidates for routine screening5. According to one large population-based study in India, periodic visual screening of the oral cavity has contributed to a reduction of 32% in mortality during a period of 9 years. Therefore, dentists are encouraged to commit to OSCC screening as a routine daily practice12.
The current guideline for detecting OSCC and OPMD recommends a conventional oral examination (COE), which involves visual examination and tactile palpation under white light11. A Cochrane review stated that insufficient evidence was suggested for the application of COE in OSCC screening programs within a low-risk population13; a systemic review also illustrated the ineffectiveness of COE in detecting dysplasia and OSCC14. One main limitation is the possible inability of general dentists to differentiate between benign and high-risk lesions, as early stage of advanced lesions may not present with typical features7. Apart from COE, tissue biopsy is only suggested for clinically suspicious lesions15. Although histological biopsy is recognized as the gold standard for the diagnosis of OSCC and dysplasia in OPMD, it is invasive, time-consuming and painful16.
Given the concerns about COE and biopsy, alternative methods should be developed for the early identification of OSCC and OPMD, particularly for the primary care5,17. As is reported, the wide application of exfoliative cytology in the early diagnosis of cervical cancer has decreased its mortality remarkably18. Hopefully, analogous early diagnostic tools with high accuracy could be applied to relieve the global disease burden of OSCC. Currently, various non-invasive diagnostic methods have emerged8. As a novel approach for early cancer diagnosis and a representative light-based detection system, autofluorescence is a non-invasive, convenient and real-time device16,19.
In an attempt to apply autofluorescence in the inspection of premalignant lesions (PML) and malignant lesions (ML), understanding the biological basis of autofluorescence is indisputably essential. In brief, the diagnostic potential of autofluorescence lies in the ability to probe alterations in tissue structure and metabolism that occur during malignant progression10. When normal tissue is illuminated by the excitation light of certain wavelength, some molecules in the tissue, called fluorophores, would absorb photons and emit lower energy photons that could be detected as fluorescence from the mucosal surface20. And the dominant fluorophores, responsible for autofluorescence signals, include reduced nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD) in the epithelium together with collagen matrix and elastin in the stroma20. However, in PML and ML, loss or weakening of autofluorescence would appear, mainly attributed to the breakdown of collagen matrix and elastin and the altered metabolism (decrease of NADH and FAD) during neoplastic progression10. Further, other metabolic or structural alterations in PML and ML are also associated with the loss or alteration of autofluorescence. Specifically, accumulated porphyrins might lead to an increased red fluorescence in addition to changes in the green one20; besides, massive heme formed by the porphyrins, and the structural changes (epithelial thickening, increased nuclear size, hyperchromatin and increased microvascularity) would contribute to the increased absorption and/or scattering of light, eventually reducing the detectable autofluorescence10,19,21.
Over the past three decades, the accuracy of autofluorescence has been widely evaluated in the diagnosis of OSCC, lung cancer (bronchiogenic carcinoma), esophageal cancer, stomach cancer, colorectal cancer and PML16,22,23,24,25, with PML representing dysplastic or neoplastic lesions21,26,27. After confirmation of its reliability, some autofluorescence technology such as LIFE has been used for routinely endoscopy28. However, inconsistent conclusions have been reached in various studies exploiting different autofluorescence technology.
In previous studies, Lane et al. had achieved relatively high diagnostic accuracy of autofluorescence when used a simple device for visualisation of oral-cavity fluorescence21, while Farah et al. obtained an opposite result aided by a representative of autofluorescence termed the Visually Enhanced Lesion Scope (VELScope; LED Dental, Vancouver, BC, Canada)29. Interestingly, Roblyer et al. developed an algorithm with autofluorescence to detect OSCC and OPMD with ideal sensitivity and specificity, implying the potential role of an additional algorithm for improving the accuracy of autofluorescence20. One systemic review on the use of autofluorescence to diagnose OPMD and OSCC has implied that it was more suitable for specialist clinics than for primary care30. In addition, a recent Cochrane review appraised the diagnostic accuracy of light-based detection for OPMD and OSCC, and its sensitivity and specificity were estimated as 0.91 and 0.58, respectively. They reported that there was a lack of evidence to support the replacement of histological biopsy by light-based detection combined with COE31. However, only 5 of those studies were about autofluorescence and their inclusion criteria was different from our study.
Thus far, although community dentists are in greater need of a screening tool for OSCC and OPMD, a limited number of studies have been performed to evaluate autofluorescence for routine screening based on the general population. Huff et al.32, as the first to report the efficacy of autofluorescence for screening in a community practice setting, showed that there was higher yield of mucosal abnormalities for VELscope than COE, and 83% of these abnormalities found by VELscope were epithelial dysplasia. Conversely, McNamara et al.33 concluded that COE was more valid than the VELscope findings since the FP of VELscope remain a concern in general practice. Nevertheless, several studies have demonstrated the effectiveness of autofluorescence in discerning OSCC or dysplastic lesions from clinically innocuous lesions that were missed by COE29,34. Moreover, aiming at overcoming the low specificity of autofluorescence, two studies have integrated autofluorescence into decision-making protocols for general dentists with encouraging outcome17,35.
As distinct conclusions are made by numerous studies about the accuracy of autofluorescence for OSCC and OPMD and its applicability in general practice, a more thorough study is needed. In this study, based on a meta-analysis and the u test method, by making a comparison with other common aero-digestive PMLs and MLs and calculating the pooled diagnostic indexes of autofluorescence for the 5 aforementioned parts, we attempted to evaluate the overall diagnostic accuracy of autofluorescence for OSCC and OPMD and discuss its applicability as an adjunct in general dental practice. Further, a contrast of using autofluorescence alone or with proper algorithms for OSCC and OPMD was performed to appraise the impact of additional algorithms. To the best of our knowledge, no study on this issue has been previously conducted.
Materials and Methods
Search strategy and study selection
Two reviewers (X. Luo and H. Xu) independently conducted a literature search using the electronic databases PubMed, Ovid Medline and Embase (updated to November 18, 2015). There was no language restriction, and we mainly focused on English literature. The search terms were listed below: “Autofluorescence” or “spectrometry, fluorecence” or “VELscope”and “Leucoplakia, Oral” or ”precancerous conditions” or “Mouth Neoplasms”, “Autofluorescence” or “spectrometry, fluorecence” and “precancerous conditions” or ”lung neoplasms” or ”stomach neoplasms” or ”esophageal neoplasms” or ”colorectal neoplasms”. The reference lists of all of the included studies were also searched for possible inclusion. The inclusion criteria used in the selection of literature for our study were as follows: (1) Adopting VELscope or other autofluorescence tools alone or with algorithms as diagnostic tools of OPMD and OSCC, and utilising autofluorescence tools alone or with algorithms for the diagnosis of PML and ML of the lung, esophagus, stomach, or colorectum; (2) lesions of the study population were clinically diagnosed as PML or suspicious ML of the oral cavity, lung, esophagus, stomach, or colorectum; (3) histological results are the gold standard for diagnosis, and positive histological findings should include mild to moderate to severe epithelial dysplasia or mucosa low-grade to high-grade neoplasia, carcinoma in situ, or a malignant tumor. Otherwise, these results should be defined as negative histological results; (4) only original clinical trials of human would be included; (5) providing sufficient data to construct a 2 × 2 table to calculate the sensitivity and specificity.
Data extraction and quality assessment
Data extraction was performed by the same reviewers ((X. Luo and H. Xu)) independently. Any discrepancies were resolved by discussion with a third author (Q. Chen). The following data were collected from each study: First author’s name, publication year, country, tumor type, sample size, true positive (TP), false positive (FP), false negative (FN), true negative (TN), sensitivity, and specificity. Initially, the standards for reporting diagnostic accuracy (STARD) is utilised to evaluate the methodological quality of the included studies36,37. Moreover, the quality assessment for studies of diagnostic accuracy (QUADAS-2) tool, as another powerful quality-assessing tool, was applied to evaluate the risk of bias and applicability concerns of these included studies in 4 key domains of patient selection, index test, reference standard, and flow and timing38,39.
Statistical analyses
The standard statistical methods, recommended for meta-analysis of diagnostic test evaluations, were used for assessing the accuracy of autofluorescence for detecting PML and ML of the oral cavity, lung, esophagus, stomach and colorectum and evaluating the accuracy of using autofluorescence alone or with algorithms for OSCC and OPMD. Upon TP, FP, FN, TN results derived from each original study, the following indexes regarding the diagnostic accuracy of autofluorescence for PML and ML of a certain area of the body were calculated for the according study: sensitivity (Sen), specificity (Spe), positive likelihood ratio (PLR), negative likelihood ratio (NLR) and diagnostic odds ratio (DOR)40. Then, aimed to calculate the pooled estimates of sensitivity and specificity, together with their 95% confidence intervals (CI), and present sensitivity and specificity more intuitively, the forest plots were plotted, each segment representing the effect size and confidence intervals of every study in the coordinate system40. Similarly, the pooled PLR, NLR and DOR were computed by depicting their forest plots. The threshold effect, usually arising when different cut-off points are applied to define a positive result of a diagnostic test among the included studies, was detected by calculating the Spearman’s correlation coefficient based on the aforementioned sensitivity and specificity, with p < 0.05 indicating existence of threshold effect40. If threshold effect is implied, Hierarchical summary receiver operating characteristic (HSROC) curves, based on sensitivity and specificity of autofluorescence for every site, could be depicted, with a hope to characterize the overall diagnostic performance of autofluorescence for each anatomical site by computing the area under the curve (AUC)41. Moreover, the wind rose, a frequently used chart in meteorology, was taken as a reference to reflect the overall diagnostic accuracy of autofluorescence among PML and ML of the oral cavity and the other 4 parts more intuitively, containing the 6 indexes (pooled Sen, Spe, PLR, NLR, DOR and AUC) calculated above. Besides, the maximum values of the 6 indexes were all standardized as 1 to allow a plain understanding of these results. Specifically, in terms of each diagnostic index except for NLR, farther distance from the center of the wind rose means higher accuracy, while it is just the opposite for NLR. To contrast a certain index of autofluorescence among PML and ML of the 5 body parts and to compare the diagnostic index of applying autofluorescence alone or with algorithms, a u test was administered42. Then, Bonferroni was adopted as a correction to reduce I type error, and the inspection level was 0.0125 and 0.05, respectively43.
The chi-squared-based Q test and the inconsistency index I2 were used to calculate the inter-study heterogeneity44,45. Heterogeneity among studies would be suggested when the Q test was significant (p < 0.05) or I2 > 50%; thereafter, the random-effect model (DerSimonian–Laird method) would be chosen to conduct the meta-analysis. Otherwise, the fixed-effect model would be selected44,45. Meta-regression was then administered to investigate the source of observed heterogeneity40.
Besides, Begg’s funnel plot and Egger’s test were used to test publication bias and each circle in the funnel plot means one study, with symmetrical inverted funnel in the chart representing none publication bias and asymmetrical one signifying publication bias46,47.
All of the analyses were performed using the following statistical software programs: STATA 11.0 (Statacorp, Texas, USA) and Revman 5.0 (The Cochrane Collaboration). All tests were two-sided, and p < 0.05 was considered statistically significant.
Results
Quality of reporting and study characteristics
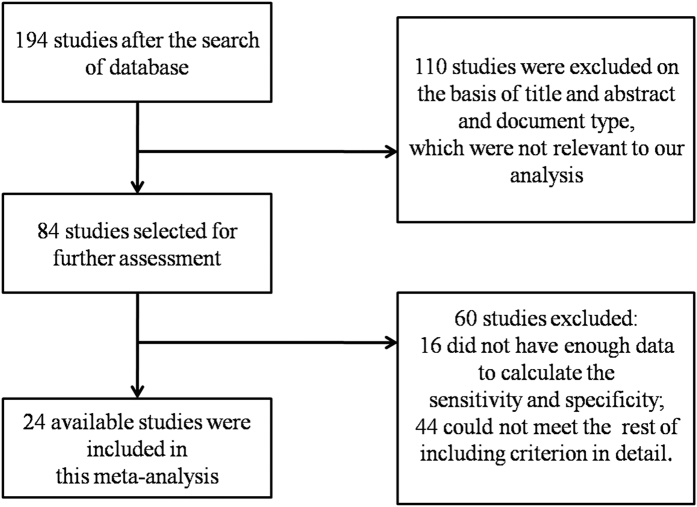
We found 194, 197, 61, 46, and 117 articles that applied autofluorescence to diagnose OSCC, lung cancer, esophageal cancer, stomach cancer and colorectal cancer, respectively, with an available PML. After identifying the titles and abstracts, a full-text assessment, and strict filtering according to the inclusion criteria, the corresponding numbers of included studies were 24,16,19,20,21,29,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66 25,22,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90 12,23,91,92,93,94,95,96,97,98,99,100,101 9,24,102,103,104,105,106,107,108,109 and 19,25,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127, respectively. The search and selection process were described as a flow diagram in Fig. 1 and Supplementary Fig. S1. A summary of the main characteristics of the included diagnostic studies was presented in Table 1 and Supplementary Table S1. All of the research that used autofluorescence for OSCC and OPMD were prospective studies, while for studies detecting the PML and ML of the lung, esophagus, stomach and colorectum, only 1, 1, 1, and 1 study was found to be a retrospective study90,99,105,125. Most of the studies treated autofluorescence loss or color change as a positive result, whereas 8, 5, 4, and 9 studies on the detection of PML and ML of the oral cavity, esophagus, stomach and colorectum, respectively, applied algorithms to discriminate positive or negative results.
Figure 1. Flow diagram shows the selection process of eligible articles that applying autofluorescence to diagnose OSCC and OPMD.
OSCC: oral squamous cell carcinoma; OPMD: oral potentially malignant disorders.
Table 1. Summary of the main characteristics of studies that applying autofluorescence to detect OSCC and OPMD.
| First Author | Year | Country | Sample Size | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|
| Onizawa K48 | 1996 | Japan | 32 | 14 | 1 | 2 | 15 |
| Gillenwater A49 | 1998 | USA | 28 | 16 | 0 | 1 | 11 |
| Wang CY50 | 1999 | China | 32 | 13 | 1 | 3 | 15 |
| Onizawa K51 | 1999 | Japan | 124 | 75 | 2 | 5 | 42 |
| van Staveren HJ52 | 2000 | Netherlands | 28 | 19 | 0 | 3 | 6 |
| Wang CY53 | 2003 | China | 97 | 21 | 3 | 5 | 68 |
| Muller MG54 | 2003 | USA | 74 | 27 | 1 | 2 | 44 |
| Majumder SK55 | 2003 | India | 325 | 78 | 9 | 5 | 233 |
| Svistun E56 | 2004 | USA | 23 | 15 | 1 | 1 | 6 |
| Majumder SK57 | 2005 | India | 325 | 74 | 16 | 9 | 146 |
| Poh CF16 | 2006 | Canada | 122 | 52 | 4 | 1 | 65 |
| Lane PM21 | 2006 | Canada | 50 | 43 | 0 | 1 | 6 |
| Jayaprakash V58 | 2009 | USA | 249 | 123 | 40 | 47 | 39 |
| Roblyer D20 | 2009 | USA | 159 | 69 | 5 | 2 | 83 |
| Koch FP59 | 2010 | Germany | 78 | 31 | 37 | 2 | 8 |
| Mehrotra R60 | 2010 | India | 156 | 6 | 88 | 6 | 56 |
| Awan K.H.19 | 2011 | United Kingdom | 116 | 37 | 61 | 7 | 11 |
| Scheer M61 | 2011 | Germany | 64 | 12 | 10 | 0 | 42 |
| Matsumoto K62 | 2011 | Japan | 74 | 39 | 8 | 25 | 2 |
| Farah CS29 | 2012 | Australia | 118 | 8 | 34 | 19 | 57 |
| Hanken H63 | 2013 | Germany | 60 | 47 | 8 | 1 | 4 |
| Jayaprakash V64 | 2013 | USA | 255 | 164 | 59 | 20 | 12 |
| Piazza C65 | 2013 | Italy | 116 | 66 | 13 | 18 | 19 |
| Petruzzi M66 | 2014 | Italy | 56 | 21 | 11 | 9 | 15 |
TP: true positive; FP: false positive; FN: false negative; TN: true negative; OSCC: oral squamous cell carcinoma; OPMD: oral potentially malignant disorders.
As for the reporting quality of the diagnostic studies, the STARD scores of the corresponding 24, 25, 12, 9, and 19 studies were all ≥17 and were of relatively high quality. Aimed to further assess the quality of the diagnostic trials, the QUADAS-2 tool was used. Based on these studies, each of the 7 components was graded as “low risk of bias” (LR), “unclear risk of bias” (UR), “high risk of bias” (HR) and “low concern” (LC), “high concern” (HC), and “unclear concern” (UC). Among the 24 studies for OSCC and OPMD, HR results of patient selection were yielded in 8 studies, 6 of the 8 reports were associated with high-risk patients who were histologically diagnosed as OSCC16,21,50,53,57,60. In terms of the remaining 6 components for OSCC and OPMD, LR, UR, LC and UC were generally displayed. Overall, the qualities of these studies were shown in Table 2 and Supplementary Table S2.
Table 2. Summary of the methodological quality of the included studies that employing autofluorescence to identify OSCC and OPMD according to QUADAS-2 criteria.
| Studies | Risk of Bias |
Applicability Concerns |
|||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Onizawa K-1996 | UR | LR | LR | LR | LC | LC | LC |
| Gillenwater A-1998 | LR | LR | LR | LR | LC | LC | LC |
| Wang CY-1999 | HR | UR | LR | LR | UC | UC | LC |
| Onizawa K-1999 | UR | UR | HR | UR | LC | LC | LC |
| Van Staveren HJ-2000 | LR | LR | LR | LR | LC | LC | LC |
| Wang CY-2003 | LR | LR | LR | LR | LC | LC | LC |
| Muller MG-2003 | HR | LR | LR | HR | UC | UC | LC |
| Majumder SK-2003 | LR | LR | LR | LR | LC | LC | LC |
| Svistun E-2004 | UR | LR | LR | LR | LC | LC | LC |
| Majumder SK-2005 | LR | LR | LR | LR | LC | LC | LC |
| Poh CF-2006 | HR | LR | LR | LR | UC | LC | LC |
| Lane PM-2006 | HR | LR | LR | LR | UC | LC | LC |
| Jayaprakash V-2009 | HR | LR | LR | LR | UC | LC | LC |
| Roblyer D-2009 | LR | LR | LR | LR | LC | LC | LC |
| Koch FP-2010 | UR | UR | LR | LR | LC | LC | LC |
| Mehrotra R-2010 | HR | LR | LR | UR | UC | LC | LC |
| Awan K.H. -2011 | LR | UR | UR | HR | LC | LC | LC |
| Scheer M-2011 | HR | UR | LR | LR | UC | LC | LC |
| Matsumoto K-2011 | LR | LR | LR | LR | LC | LC | LC |
| Farah CS-2012 | HR | LR | LR | UR | UC | LC | LC |
| Hanken H-2013 | LR | LR | LR | LR | LC | LC | LC |
| Jayaprakash V-2013 | UR | UR | LR | LR | LC | LC | LC |
| Piazza C-2013 | UR | UR | LR | LR | LC | LC | LC |
| Petruzzi M-2014 | LR | LR | LR | UR | LC | LC | LC |
LR: low risk; HR: high risk; UR: unclear risk; LC: low concern; HC: high concern; UC: unclear concern; OSCC: oral squamous cell carcinoma; OPMD: oral potentially malignant disorders; QUADAS-2: quality assessment for studies of diagnostic accuracy.
Diagnostic accuracy of autofluorescence for OSCC and OPMD
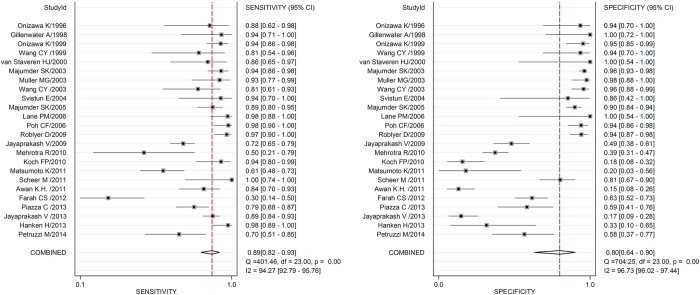
To assess the diagnostic accuracy of autofluorescence for OSCC, lung cancer, esophageal cancer, stomach cancer and colorectal cancer and their PML, Fig. 2 and Supplementary Fig. S2 showed the forest plots of the pooled sensitivity and specificity of the corresponding 24, 25, 12, 9, and 19 studies; moreover, the forest plots of sensitivity and specificity on the use of autofluorescence alone or with algorithms for OSCC and OPMD were also depicted in Supplementary Fig. S3 and S4, respectively. Analogously, the pooled PLR, NLR and DOR were achieved by the same methods.
Figure 2. Forest plots of sensitivity and specificity of studies that employing autofluorescence in the diagnosis of OSCC and OPMD.
The solid circles indicate estimates of sensitivity and specificity for each study, and the size of each solid circle represents the sample size of each study. The error bars are 95% confidence intervals. OSCC: oral squamous cell carcinoma; OPMD: oral potentially malignant disorders.
Comprehensive evaluation of diagnostic performance of autofluorescence for OSCC and OPMD
After computation of Spearman’s correlation coefficient upon the sensitivity and specificity of according studies, existence of threshold effect (all p < 0.05) was generally revealed in studies with regard to diagnosis of the 5 various body sites. Thus, the HSROC curves were depicted in Fig. 3 and Supplementary Fig. S5. As a descriptive index of the HSROC curve, AUC, pertaining to the overall diagnostic accuracy of autofluorescence for the various area of body, were computed based on the curves.
Figure 3. Hierarchical summary receiver operating characteristic curve (HSROC) of studies utilising autofluorescence for the detection of OSCC and OPMD.
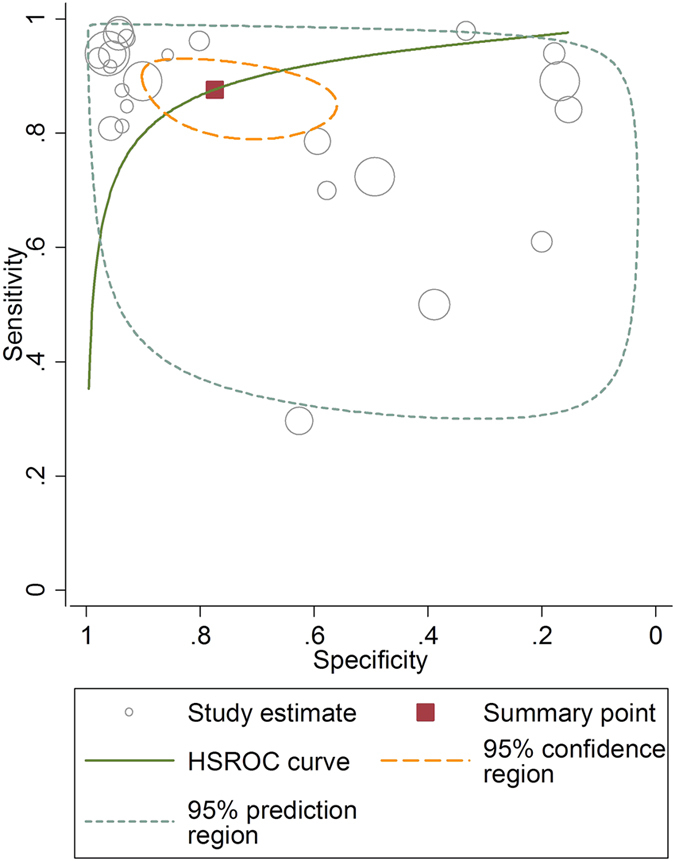
Each empty circle represents one study, and the size of every circle indicates the sample size of each study. OSCC: oral squamous cell carcinoma; OPMD: oral potentially malignant disorders.
Afterwards, pooled estimates of the 5 indexes (Sen, Spe, PLR, NLR and DOR) and AUC of these studies regarding utilising autofluorescence in the detection of PML and ML of 5 body sites were shown in Table 3. Furthermore, the 6 indexes on the application of autofluorescence alone or with algorithms for OSCC and OPMD were also separately displayed in Table 4.
Table 3. Pooled estimates of diagnostic indexes regarding the accuracy of autofluorescence for the PML and ML of the oral cavity, lung, esophagus, stomach, and colorectum.
| Test | No. of studies | No. of samples | sensitivity | Pooled estimates (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| specificity | PLR | NLR | DOR | AUC | ||||
| Oral cavity | 24 | 2761 | 0.89 (0.82–0.93) | 0.80 (0.64–0.90) | 4.54 (2.28–9.04) | 0.14 (0.08–0.24) | 32.37 (10.47–100.12) | 0.92 (0.89–0.94) |
| Lung | 25 | 4384 | 0.89 (0.86–0.92) | 0.63 (0.52–0.73) | 2.43 (1.82–3.24) | 0.17 (0.13–0.22) | 14.52 (9.25–22.81) | 0.89 (0.86–0.91) |
| Esophagus | 12 | 2514 | 0.78 (0.57–0.91) | 0.77 (0.58–0.89) | 3.43 (1.81–6.50) | 0.28 (0.14–0.58) | 12.17 (4.32–34.27) | 0.85 (0.81–0.87) |
| Stomach | 9 | 2115 | 0.88 (0.76–0.94) | 0.73 (0.50–0.88) | 3.28 (1.52–7.08) | 0.17 (0.07–0.39) | 19.31 (4.49–83.05) | 0.89 (0.86–0.92) |
| Colorectum | 19 | 2904 | 0.91 (0.86–0.94) | 0.78 (0.64–0.88) | 4.15 (2.40–7.17) | 0.11 (0.07–0.19) | 36.27 (14.20–92.63) | 0.93 (0.91–0.95) |
PML: premalignant lesions; ML: malignant lesions; CI: confidence interval; PLR: positive likelihood ratio; NLR: negative likelihood ratio; DOR: diagnostic odd ratio; AUC: area under the curve.
Table 4. Pooled estimates of diagnostic indexes regarding the accuracy of using autofluorescence alone or with algorithms for detecting OSCC and OPMD.
| Test | No. of studies | No. of samples | sensitivity | Pooled estimates (95%CI) |
||||
|---|---|---|---|---|---|---|---|---|
| specificity | PLR | NLR | DOR | AUC | ||||
| (A) AF only | 16 | 1693 | 0.88 (0.77–0.94) | 0.62 (0.41–0.79) | 2.31 (1.31–4.09) | 0.20 (0.09–0.46) | 11.52 (3.10–42.87) | 0.85 (0.82–0.88) |
| (B) AF with algorithms | 8 | 1068 | 0.92 (0.88–0.94) | 0.95 (0.92–0.97) | 17.28 (10.96–27.22) | 0.09 (0.06–0.13) | 194.11 (94.27–399.71) | 0.98 (0.96–0.99) |
| u test of A and B (p value) | — | — | NS | * | * | NS | * | * |
OSCC: oral squamous cell carcinoma; OPMD: oral potentially malignant disorders; CI: confidence interval; PLR: positive likelihood ratio; NLR: negative likelihood ratio; DOR: diagnostic odd ratio; AUC: area under the curve; AF: autofluorescence;
NS: non-significant (p > 0.05); *p < 0.05.
To seek a comprehensive evaluation of these 6 indexes regarding the diagnostic value of autofluorescence for PML and ML of the 5 anatomical areas from another perspective, a wind rose picture derived from meteorology was depicted (Supplementary Fig. S6). It indicated that the AUC of PML and ML of the 5 areas were all relatively high, among which, the overall diagnostic accuracy of autofluorescence for OSCC and OPMD was slightly inferior to colorectal cancer along with its PML, but was superior to the PML and ML of the other 3 areas, whereas the values of the other 5 indexes varied in terms of PML and ML of diverse sites. Particularly, optimal specificity and PLR were suggested when autofluorescence was applied to detect OSCC and OPMD.
U test of 6 diagnostic indexes
A u test of the 6 indexes (pooled Sen, Spe, PLR, NLR, DOR and AUC) that implied the diagnostic accuracy of autofluorescence among PML and ML of the 5 areas was conducted, indicating that statistical significance only lies in the comparison of the AUC between detecting PML and ML of the oral cavity and esophagus. Subsequently, another u test result concluded that a significant difference could be observed in Spe, PLR, DOR and AUC (Table 4), suggesting that better accuracy for detecting OSCC and OPMD could be achieved with the use of algorithms rather than autofluorescence alone.
Heterogeneity test
Autofluorescence was used to diagnose PML and ML of 5 anatomic sites. Thus, we divided this study into 5 sections. The I2 of the 6 indexes including Sen, Spe, PLR, NLR, DOR and AUC of all 5 sections was greater than 50%, and all Q test results were p < 0.05, which implied a significant heterogeneity across the included studies for all 5 sections. The random effects model was chosen to calculate the 6 pooled indexes. A meta-regression analysis was performed to identify the possible cause of heterogeneity according to the following study characteristics: population characteristics, study design, sample size, threshold effect and lack of blinding. However, none of the above analysed factors were significantly different (all p > 0.05).
Evaluation of publication bias
The Begg’s funnel plot and Egger’s test were selected to explore publication bias of the 5 sections, and all results of the Egger’s test were p > 0.05, each funnel plot manifesting as symmetrical inverted funnel-shaped figure. Both analyses indicated insignificant publication bias (Fig. 4 and Supplementary Fig. S7).
Figure 4. Begg’s funnel plot for the evaluation of potential publication bias of studies that applying autofluorescence to diagnose OSCC and OPMD.
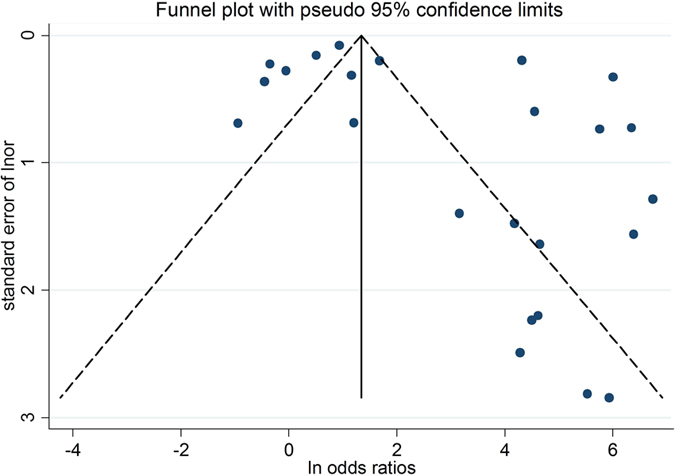
Each solid circle indicates one study; OSCC: oral squamous cell carcinoma; OPMD: oral potentially malignant disorders.
Discussion
Presently, early diagnosis is supposed to improve the outcome of OSCC by general dentists. While COE, as an available method, seems to be helpless particularly for inexperienced dental practitioners128. Thereby, adjunct diagnostic aids are desperately needed by primary care workers to facilitate the early detection of OSCC and dysplasia. Over the past three decades, the diagnostic performance of autofluorescence has been explored in OSCC and OPMD in several studies with conflicting results, also in 4 common aero-digestive lesions59,60,61. Therefore, our study was conducted to further investigate its overall diagnostic accuracy and to discuss its application stability in 5 anatomic parts in the hope of recommending it as a diagnostic aid in general dental practice. After calculating the pooled diagnostic indexes of autofluorescence for OSCC and OPMD along with the PML and ML of the lung, esophagus, stomach and colorectum, an overall good accuracy of autofluorescence for detecting OSCC and OPMD was indicated, with a pooled sensitivity and specificity of 0.89 and 0.80, respectively. In addition, the relatively preferable accuracy of autofluorescence for all of the 5 anatomical parts has implied its application stability and potential role in primary care. As our included studies were generally based on non-primary care settings, autofluorescence is presently more proper for specialists. However, based on our included studies, proper algorithms could be combined with autofluorescence to enhance its specificity for its promising use in primary care.
Aiming at initially evaluating the accuracy of autofluorescence for OSCC and OPMD, we calculated its pooled Sen, Spe, PLR, NLR and DOR. According to the forest plots, the pooled sensitivity of autofluorescence for detecting OSCC and OPMD is as high as 0.89, which is better than PML and ML of the esophagus and stomach, slightly worse than that of the colorectum and equal to that of lung, although no statistically significant difference was found. In addition, the pooled specificity of autofluorescence for detecting OSCC and OPMD was 0.8, although not high enough to reach statistical significance, it is optimal among the 5 areas. Besides, when the pooled sensitivity and specificity of using autofluorescence alone or with algorithms were separately computed, an encouraging result was indicated, presented as 0.88 and 0.62, 0.92 and 0.95, respectively.
PLR and NLR are more clinically significant and practical for the measurement of diagnostic accuracy. In this study, the PLR and NLR values for detecting OSCC and OPMD were 4.54 and 0.14, respectively, suggesting that patients with OSCC or dysplastic lesions have about a 4.54-fold higher chance of being autofluorescence-positive compared to those without them, while the chance of having OSCC or dysplastic lesions in autofluorescence-negative patients is theoretically 14%. The PLR of the autofluorescence for detecting OSCC and OPMD is optimal, although it is not statistically significant. The NLR of autofluorescence for detecting OSCC and OPMD is better than PML and ML of the lung, esophagus and stomach, and slightly inferior to the colorectum, with no statistical significance indicated. DOR has combined sensitivity and specificity into a single number, with higher values represent better diagnostic performance129. In our study, the DOR for detecting OSCC and OPMD is 32.37 with good accuracy, slightly lower than PML and ML of the colorectum and greater than the other 3 areas. Additionally, a significant increase can be observed in the PLR, DOR of autofluorescence for identifying OSCC and OPMD assisted by proper algorithms, displayed as 17.28 and 194.11.
According to the AUC values of the HSROC curves, a better overall diagnostic accuracy of autofluorescence for PML and ML of the oral cavity and colorectum was revealed compared to the lung, esophagus and stomach, and a statistically significant difference was only indicated between the oral cavity and esophagus. Although the diagnostic accuracy of autofluorescence for OSCC and OPMD was slightly less than colorectal cancer and its PML, the AUC of the former was also greater than 0.9, which represents relatively good overall diagnostic accuracy. Further, the AUC of applying autofluorescence alone or with algorithms were individually estimated as 0.85 and 0.98 with statistical significance.
Aiming at understanding our results more vividly, the wind rose picture was delineated, revealing that the overall diagnostic accuracy of autofluorescence for OSCC and OPMD is superior to PML and ML of the lung, esophagus and stomach and is only inferior to the colorectum. In addition, the sensitivity and specificity for the 5 body parts fluctuated between 0.78 to 0.91 and 0.63–0.80, respectively. The stable and relatively high accuracy of autofluorescence across the five parts has confirmed its efficacy under diverse conditions, suggesting its potential role in primary care.
Across our included literatures, the sensitivity of autofluorescence for detecting OSCC and OPMD ranged from 30% to 100%; high sensitivity was indicated in the majority of studies except for low values in 3 studies29,60,62. Conversely, low specificity varying between 15.3% to 63% was implied in 10 of our included studies from 2009 to 201419,29,58,59,60,62,63,64,65,66. Other studies also reported low specificity by emphasizing its inability to discriminate benign lesions from malignant or premalignant mucosal conditions as well as a high rate of false positive results11,63,130. Meanwhile, Balevi et al. stated that adoption of autofluorescence as a cancer-screening device for general dentists was presently premature131. In addition, as all of our included studies regarding identifying OSCC and OPMD are based on non-primary care settings and 616,21,50,53,57,60 of these studies are on high-risk patients who were previously diagnosed as OSCC in the cancer clinics, the extrapolation of autofluorescence alone to the care remains a significant concern, and it is thought to be more valuable in the hands of specialists by most studies30,31,131. In specialist clinics, Poh et al. used it in the identification of tumor margins during the operation16. Kois et al. suggested that autofluorescence could assist in deciding the best biopsy area130. However, such a tool caters more to dental practitioners to uncover early OSCC and dysplasia in clinically innocuous lesions6.
Despite the fact that it seems unlikely to be widely applied in general dental practice mainly due to low specificity, fortunately, some available approaches could be combined with autofluorescence to facilitate its application within the primary care environment. Of the 24 studies regarding the use of autofluorescence for diagnosing OSCC and OPMD, 8 studies incorporated proper algorithms into the analysis of autofluorescence20,49,50,52,53,54,55,57, with a pooled sensitivity and specificity estimated to be 0.92 and 0.95, respectively. However, after separately calculating the diagnostic indexes of the remaining 16 studies, sensitivity and specificity were found to be 0.88 and 0.62, respectively. Thereby, the method of combining algorithms with autofluorescence could be generalized to low-risk groups to improve accuracy, and more similar studies in general dental settings are needed to warrant its efficacy.
Although inconsistent sensitivity and specificity of autofluorescence for OSCC and OPMD were presented in our included studies, primarily depending on both the different excitation and emission properties of the fluorophores and the diverse ability to discriminate PML and ML from normal regions based on the utilisation of various wavelength of excitation/emission light and algorithms, optimal accuracy were obtained in some of our included studies, either using autofluorescence alone or with proper algorithm. Thus, it indicates the potential of seeking a more suitable wavelength of excitation/emission light and algorithm, even individually designed for benign, dysplastic and malignant oral lesions, on the basis of these previous studies, to significantly enhance the accuracy of autofluorescence for detecting OSCC and OPMD. For instance, Pavlova I et al. proposed that in their study, excitation wavelengths in the UV range may improve the accurate diagnosis of PML and ML of oral cavity132.
Additionally, as the first to introduce autofluorescence into community dental practices, Laronde et al. devised a stepwise protocol, including patient history, visual screening examination, lesion risk assessment and direct fluorescence, to guide 18 general dentists to apply autofluorescence in their offices. Besides, a return for a 3-week reassessment was emphasized to reduce FP results on initial visits. Finally, it highlighted that combination of this protocol and autofluorescence could significantly help community clinicians in making wise decisions17. Subsequently, a similar prospective study was conducted by Bhatia et al. in general practice, in which a more detailed decision-making protocol was designed, including background information, COE, autofluorescence examination, combined examination, review appointment and referral appointment. Consequently, the combination of autofluorescence with COE achieved a dramatic increase in sensitivity and specificity compared to autofluorescence alone, as 73.9% and 97.9% rather than 64% and 54.3%, respectively. However, as it was only a single-center study, multi-center research on this protocol is needed to further verify its potential35.
Moreover, diascopic fluorescence has been shown to decrease the FP results caused by inflammation in previous studies29. Farah et al. found that complete blanching appeared in 10 dysplastic lesions and 1 OSCC, but the pressure and tool were both to blame29. In Bhatia’s study, they used diascopic fluorescence in general practice firstly and suggested that the back of a periodontal or sickle probe may be more proper for the test and that these lesions of partial blanching had the highest rate of referrals35.
The limitations of this study are as follows. First, failure to include letters to the editors and ongoing studies may cause publication bias. Second, as our included studies applying autofluorescence for the diagnosis of OSCC and OPMD were based on patients of superior clinics rather than primary care, overestimation of its diagnostic accuracy may be a concern. More well-designed clinical trials about its application in general practice are strongly encouraged to support its use by general dentists. Third, owing to the lack of necessary data in our included studies, we are unable to perform a subgroup analysis about whether factors such as age, race were the possible cause of heterogeneity. Lastly, with the advent of various non-invasive diagnostic tools, including chemiluminescence, oral CDx brush biopsy, toluidine blue and narrow band imaging (NBI) being used in oral clinics for OSCC and OPMD10, it may be necessary for us to compare the accuracy of autofluorescence with those approaches in the future, significant to help select more efficacious early diagnostic tool for OSCC and OPMD.
In conclusion, autofluorescence is a promising non-invasive tool with relatively high accuracy for the early diagnosis of OSCC and OPMD. It also presents with good application stability for detecting lesions of 5 anatomical parts. In light of its medium specificity when used alone, it is more reliable to serve as an adjunct in the hands of oral specialists. However, in an attempt to facilitate its use in primary care to improve the survival rates of OSCC, some promising approaches could be adopted, including its use in combination with proper algorithms.
Additional Information
How to cite this article: Luo, X. et al. Accuracy of autofluorescence in diagnosing oral squamous cell carcinoma and oral potentially malignant disorders: a comparative study with aero-digestive lesions. Sci. Rep. 6, 29943; doi: 10.1038/srep29943 (2016).
Supplementary Material
Acknowledgments
We thank all of the authors of the original studies included in our study. This study was supported by grants from Nonprofit Industry Research Specific Fund of National Health and Family Planning Commission of China (No. 201502018) and the National Natural Science Foundations of China (No. 81321002).
Footnotes
Author Contributions Conception and design: X.L., H.X. and Q.C. Development of methodology: M.H., Q.H., H.W. and C.S. Acquisition of data: X.L. and H.X. Analysis and interpretation of data: X.L., H.X., J.L., L.J., Y.Z. and H.D. Writing, review and/or revision of the manuscript: X.L., H.X., X.F., Q.C. and X.Z. Administrative, technical, or material support: M.H., Q.H., H.W., C.S. and X.Z. Study supervision: X.F. and Q.C.
References
- Parkin D. M., Bray F., Ferlay J. & Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 55, 74–108 (2005). [DOI] [PubMed] [Google Scholar]
- Ferlay J. et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136, E359–86 (2015). [DOI] [PubMed] [Google Scholar]
- Grant E., Silver K., Bauld L., Day R. & Warnakulasuriya S. The experiences of young oral cancer patients in Scotland: symptom recognition and delays in seeking professional help. Br Dent J 208, 465–71 (2010). [DOI] [PubMed] [Google Scholar]
- Cleveland J. L. & Thornton-Evans G. Total diagnostic delay in oral cancer may be related to advanced disease stage at diagnosis. J Evid Based Dent Pract 12, 84–6 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh C. F. Heads up!–A call for dentists to screen for oral cancer. J Can Dent Assoc 72, 413–6 (2006). [PubMed] [Google Scholar]
- Rhodus N. L. Oral cancer and precancer: improving outcomes. Compendium of continuing education in dentistry 30, 486–8 (2009). [PubMed] [Google Scholar]
- Lingen M. W., Kalmar J. R., Karrison T. & Speight P. M. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol 44, 10–22 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully C., Bagan J. V., Hopper C. & Epstein J. B. Oral cancer: current and future diagnostic techniques. Am J Dent 21, 199–209 (2008). [PubMed] [Google Scholar]
- Warnakulasuriya S., Johnson N. W. & van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 36, 575–80 (2007). [DOI] [PubMed] [Google Scholar]
- Messadi D. V. Diagnostic aids for detection of oral precancerous conditions. Int J Oral Sci 5, 59–65 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna H. M., Rattay T., Smith J. & McConkey C. C. Treatment and follow-up of oral dysplasia-a systematic review and meta-analysis. Head Neck 31, 1600–9 (2009). [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R. et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet (London, England) 365, 1927–33 (2005). [DOI] [PubMed] [Google Scholar]
- Brocklehurst P. et al. Screening programmes for the early detection and prevention of oral cancer. Cochrane Database Syst Rev 11, CD004150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. B., Guneri P., Boyacioglu H. & Abt E. The limitations of the clinical oral examination in detecting dysplastic oral lesions and oral squamous cell carcinoma. Journal of the American Dental Association (1939) 143, 1332–42 (2012). [DOI] [PubMed] [Google Scholar]
- Natarajan E. & Eisenberg E. Contemporary concepts in the diagnosis of oral cancer and precancer. Dent Clin North Am 55, 63–88 (2011). [DOI] [PubMed] [Google Scholar]
- Poh C. F. et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res 12, 6716–22 (2006). [DOI] [PubMed] [Google Scholar]
- Laronde D. M. et al. Influence of fluorescence on screening decisions for oral mucosal lesions in community dental practices. J Oral Pathol Med 43, 7–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. J. et al. IR microspectroscopy: potential applications in cervical cancer screening. Cancer Lett 246, 1–11 (2007). [DOI] [PubMed] [Google Scholar]
- Awan K. H., Morgan P. R. & Warnakulasuriya S. Evaluation of an autofluorescence based imaging system (VELscope) in the detection of oral potentially malignant disorders and benign keratoses. Oral Oncology 47, 274–7 (2011). [DOI] [PubMed] [Google Scholar]
- Roblyer D. et al. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer prevention research (Philadelphia, Pa.) 2, 423–31 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P. M. et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt 11, 024006 (2006). [DOI] [PubMed] [Google Scholar]
- Divisi D., Di T. S., De Vico A. & Crisci R. Early diagnosis of lung cancer using a SAFE-3000 autofluorescence bronchoscopy. Interact Cardiovasc Thorac Surg 11, 740–4 (2010). [DOI] [PubMed] [Google Scholar]
- Mayinger B. et al. Light-induced autofluorescence spectroscopy for the endoscopic detection of esophageal cancer. Gastrointest Endosc 54, 195–201 (2001). [DOI] [PubMed] [Google Scholar]
- Kato M., Kaise M., Yonezawa J., Yoshida Y. & Tajiri H. Autofluorescence endoscopy versus conventional white light endoscopy for the detection of superficial gastric neoplasia: a prospective comparative study. Endoscopy 39, 937–41 (2007). [DOI] [PubMed] [Google Scholar]
- Mayinger B. et al. Endoscopic light-induced autofluorescence spectroscopy for the diagnosis of colorectal cancer and adenoma. J Photochem Photobiol B 70, 13–20 (2003). [DOI] [PubMed] [Google Scholar]
- Lam S. et al. Localization of bronchial intraepithelial neoplastic lesions by fluorescence bronchoscopy. Chest 113, 696–702 (1998). [DOI] [PubMed] [Google Scholar]
- Schlemper R. J. et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut, 47, 251–5 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana S., Kaneko M. & Mizuno H. Endoscopic diagnostic system using autofluorescence. Diagn Ther Endosc 5, 59–63 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah C. S., McIntosh L., Georgiou A. & McCullough M. J. Efficacy of tissue autofluorescence imaging (VELScope) in the visualization of oral mucosal lesions. Head Neck 34, 856–62 (2012). [DOI] [PubMed] [Google Scholar]
- Rashid A. & Warnakulasuriya S. The use of light-based (optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med 44, 307–28 (2014). [DOI] [PubMed] [Google Scholar]
- Macey R. et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev 5, CD010276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff K., Stark P. C. & Solomon L. W. Sensitivity of direct tissue fluorescence visualization in screening for oral premalignant lesions in general practice. Gen Dent. 57, 34–8 (2009). [PubMed] [Google Scholar]
- McNamara K. K., Martin B. D., Evans E. W. & Kalmar J. R. The role of direct visual fluorescent examination (VELscope) in routine screening for potentially malignant oral mucosal lesions. Oral Surg Oral Med Oral Pathol Oral Radiol 114, 636–43 (2012). [DOI] [PubMed] [Google Scholar]
- Marzouki H. Z. et al. Use of fluorescent light in detecting malignant and premalignant lesions in the oral cavity: a prospective, single-blind study. J Otolaryngol Head Neck Surg 41, 164–8 (2012). [PubMed] [Google Scholar]
- Bhatia N., Matias M. A. & Farah C. S. Assessment of a decision making protocol to improve the efficacy of VELscope in general dental practice: a prospective evaluation. Oral Oncol 50, 1012–9 (2014). [DOI] [PubMed] [Google Scholar]
- Bossuyt P. M. et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem 49, 1–6 (2003). [DOI] [PubMed] [Google Scholar]
- Bossuyt P. M. et al. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 138, W1–12 (2003). [DOI] [PubMed] [Google Scholar]
- Whiting P., Rutjes A. W., Reitsma J. B., Bossuyt P. M. & Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3, 25 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting P. F. et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155, 529–36 (2011). [DOI] [PubMed] [Google Scholar]
- Zamora J., Abraira V., Muriel A., Khan K. & Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 6, 31 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig L., Macaskill P., Glasziou P. & Fahey M. Meta-analytic methods for diagnostic test accuracy. J Clin Epidemiol 48, 119–30 (1995). [DOI] [PubMed] [Google Scholar]
- Ruxton G. D. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behav Ecol 17, 688–690 (2006). [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75, 800–802 (1988). [Google Scholar]
- Deeks J. J., Altman D. G. & Bradburn M. J. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis in Systematic Reviews in Health Care: Meta-Analysis in Context 2nd edn(eds Egger M. et al.) 285–312 (BMJ Publishing Group, 2008). [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–58 (2002). [DOI] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey S. G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 315, 629–34 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizawa K., Saginoya H., Furuya Y. & Yoshida H. Fluorescence photography as a diagnostic method for oral cancer. Cancer Lett 108, 61–6 (1996). [DOI] [PubMed] [Google Scholar]
- Gillenwater A. et al. Noninvasive diagnosis of oral neoplasia based on fluorescence spectroscopy and native tissue autofluorescence. Archives of otolaryngology–head & neck surgery 124, 1251–8 (1998). [DOI] [PubMed] [Google Scholar]
- Wang C. Y. et al. Diagnosis of oral cancer by light-induced autofluorescence spectroscopy using double excitation wavelengths. Oral Oncol 35, 144–50 (1999). [DOI] [PubMed] [Google Scholar]
- Onizawa K., Saginoya H., Furuya Y., Yoshida H. & Fukuda H. Usefulness of fluorescence photography for diagnosis of oral cancer. Int J Oral Maxillofac Surg 28, 206–10 (1999). [PubMed] [Google Scholar]
- van Staveren H. J. et al. Classification of clinical autofluorescence spectra of oral leukoplakia using an artificial neural network: a pilot study. Oral Oncol 36, 286–93 (2000). [DOI] [PubMed] [Google Scholar]
- Wang C. Y., Tsai T., Chen H. M., Chen C. T. & Chiang C. P. PLS-ANN based classification model for oral submucous fibrosis and oral carcinogenesis. Lasers Surg Med 32, 318–26 (2003). [DOI] [PubMed] [Google Scholar]
- Muller M. G. et al. Spectroscopic detection and evaluation of morphologic and biochemical changes in early human oral carcinoma. Cancer 97, 1681–92 (2003). [DOI] [PubMed] [Google Scholar]
- Majumder S. K., Ghosh N., Kataria S. & Gupta P. K. Nonlinear pattern recognition for laser-induced fluorescence diagnosis of cancer. Lasers Surg Med 33, 48–56 (2003). [DOI] [PubMed] [Google Scholar]
- Svistun E. et al. Vision enhancement system for detection of oral cavity neoplasia based on autofluorescence. Head Neck 26, 205–15 (2004). [DOI] [PubMed] [Google Scholar]
- Majumder S. K., Ghosh N. & Gupta P. K. Relevance vector machine for optical diagnosis of cancer. Lasers Surg Med 36, 323–33 (2005). [DOI] [PubMed] [Google Scholar]
- Jayaprakash V. et al. Autofluorescence-guided surveillance for oral cancer. Cancer prevention research (Philadelphia, Pa.) 2, 966–74 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F. P., Kaemmerer P. W., Biesterfeld S., Kunkel M. & Wagner W. Effectiveness of autofluorescence to identify suspicious oral lesions–a prospective, blinded clinical trial. Clin Oral Investig 15, 975–82 (2011). [DOI] [PubMed] [Google Scholar]
- Mehrotra R. et al. A cross-sectional study evaluating chemiluminescence and autofluorescence in the detection of clinically innocuous precancerous and cancerous oral lesions. Journal of the American Dental Association (1939) 141, 151–6 (2010). [DOI] [PubMed] [Google Scholar]
- Scheer M. et al. Autofluorescence imaging of potentially malignant mucosa lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111, 568–77 (2011). [DOI] [PubMed] [Google Scholar]
- Matsumoto K. Detection of potentially malignant and malignant lesions of oral cavity using autofluorescence visualization device. The Journal of the Stomatological Society,Japan 78, 73–80 (2011). [PubMed] [Google Scholar]
- Hanken H. et al. The detection of oral pre- malignant lesions with an autofluorescence based imaging system (VELscope)-a single blinded clinical evaluation. Head Face Med 9, 23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaprakash V. et al. Autofluorescence visualization for detecting potentially malignant white oral mucosal lesions. Oral Oncology 49, S50 (2013). [Google Scholar]
- Piazza C., Del Bon F., Mangili S., Grazioli P. & Nicolai P. Optical biopsy by narrow band imaging and autofluorescence of oral and oropharyngeal erythro-leukoplakias. Oral Oncology 49, S49–50 (2013). [Google Scholar]
- Petruzzi M. et al. Evaluation of autofluorescence and toluidine blue in the differentiation of oral dysplastic and neoplastic lesions from non dysplastic and neoplastic lesions: a cross-sectional study. J Biomed Opt 19, 76003 (2014). [DOI] [PubMed] [Google Scholar]
- Yokomise H. et al. Clinical experience with lung-imaging fluorescence endoscope (LIFE) in patients with lung cancer. J Bronchol 4, 205–8 (1997). [Google Scholar]
- Vermylen P. et al. Detection of bronchial preneoplastic lesions and early lung cancer with fluorescence bronchoscopy: a study about its ambulatory feasibility under local anaesthesis. Lung cancer (Amsterdam, Netherlands) 25, 161–8 (1999). [DOI] [PubMed] [Google Scholar]
- Masatoshi K. et al. Early Detection of Bronchial Lesions Using System of Autofluorescence Endoscopy (SAFE) 1000. Diagn Ther Endosc 5, 99–104 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda N. et al. Early detection of bronchial lesions using lung imaging fluorescence endoscope. Diagn Ther Endosc 5, 85–90 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath T. et al. Detection of Bronchial Neoplasia in Uranium Miners by Autofluorescence Endoscopy (SAFE-IO00). Diagnostic and Therapeutic Endoscopy 5, 91–8 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K. et al. Fluorescence bronchoscopy in the detection of preinvasive bronchial lesions in patients with sputum cytology suspicious or positive for malignancy. Lung cancer (Amsterdam, Netherlands) 32, 19–25 (2001). [DOI] [PubMed] [Google Scholar]
- Ikeda N. et al. Histopathological evaluation of fluorescence bronchoscopy using resected lungs in cases of lung cancer. Lung cancer (Amsterdam, Netherlands) 41, 303–9 (2003). [DOI] [PubMed] [Google Scholar]
- Furukawa K. et al. Is autofluorescence bronchoscopy needed to diagnose early bronchogenic carcinoma? Pro: Autofluorescence bronchoscopy. J Bronchol 10, 64–9 (2003). [Google Scholar]
- Chhajed P. N. et al. A comparison of video and autofluorescence bronchoscopy in patients at high risk of lung cancer. The European respiratory journal 25, 951–5 (2005). [DOI] [PubMed] [Google Scholar]
- Chiyo M. et al. Effective detection of bronchial preinvasive lesions by a new autofluorescence imaging bronchovideoscope system. Lung cancer (Amsterdam, Netherlands) 48, 307–13 (2005). [DOI] [PubMed] [Google Scholar]
- Lang S. M., Ebelt K., Hautmann H., Stratakis D. F. & Huber R. M. Detection of cancerous endobronchial lesions by autofluorescence bronchoscopy combined with mutation analysis of p53. Eur J Med Res 10, 273–7 (2005). [PubMed] [Google Scholar]
- Fuso L. et al. Autofluorescence bronchoscopy to identify pre-cancerous bronchial lesions. Monaldi archives for chest disease 63, 124–8 (2005). [DOI] [PubMed] [Google Scholar]
- Jang T. W., Oak C. H., Chun B. K. & Jung M. H. Detection of pre-invasive endobronchial tumors with D-light/autofluorescence system. J Korean Med Sci 21, 242–6 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard M. P. et al. Optical spectroscopy for the classification of malignant lesions of the bronchial tree. Chest 129, 995–1001 (2006). [DOI] [PubMed] [Google Scholar]
- Ikeda N. et al. Early detection of bronchial lesions using newly developed videoendoscopy-based autofluorescence bronchoscopy. Lung cancer (Amsterdam, Netherlands) 52, 21–7 (2006). [DOI] [PubMed] [Google Scholar]
- Lam B. et al. The clinical value of autofluorescence bronchoscopy for the diagnosis of lung cancer. The European respiratory journal 28, 915–9 (2006). [DOI] [PubMed] [Google Scholar]
- Ueno K. et al. Clinical experience with autofluorescence imaging system in patients with lung cancers and precancerous lesions. Respiration 74, 304–8 (2007). [DOI] [PubMed] [Google Scholar]
- Nakanishi K. et al. Color auto-fluorescence from cancer lesions: improved detection of central type lung cancer. Lung cancer (Amsterdam, Netherlands) 58, 214–9 (2007). [DOI] [PubMed] [Google Scholar]
- Zaric B. et al. Autofluorescence videobronchoscopy (AFI) for the assessment of tumor extension in lung cancer. Technol Cancer Res Treat 8, 79–84 (2009). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Comparison of the autofluorescence bronchoscope and the white light bronchoscope in airway examination. Chin J Cancer 29, 1018–22 (2010). [DOI] [PubMed] [Google Scholar]
- Cetti E. J., Nicholson A. G., Singh S., Wells A. U. & Shah P. L. An evaluation of a videobronchoscopy-based autofluorescence system in lung cancer. The European respiratory journal 35, 1185–7 (2010). [DOI] [PubMed] [Google Scholar]
- Zaric B. et al. Autofluorescence imaging videobronchoscopy improves assessment of tumor margins and affects therapeutic strategy in central lung cancer. Jpn J Clin Oncol 40, 139–45 (2010). [DOI] [PubMed] [Google Scholar]
- Zaric B. et al. Combination of narrow band imaging (NBI) and autofluorescence imaging (AFI) videobronchoscopy in endoscopic assessment of lung cancer extension. Medical oncology (Northwood, London, England) 29, 1638–42 (2012). [DOI] [PubMed] [Google Scholar]
- Zhu L. Y., Xu Y. J., Liang D. & Chen P. The clinical value of autofluorescence bronchoscopy for the diagnosis of lung cancer. Chinese journal of tuberculosis and respiratory diseases 35, 419–22 (2012). [PubMed] [Google Scholar]
- Panjehpour M. et al. Endoscopic fluorescence detection of high-grade dysplasia in Barrett’s esophagus. Gastroenterology 111, 93–101 (1996). [DOI] [PubMed] [Google Scholar]
- Bourg-Heckly G. et al. Endoscopic ultraviolet-induced autofluorescence spectroscopy of the esophagus: tissue characterization and potential for early cancer diagnosis. Endoscopy 32, 756–65 (2000). [DOI] [PubMed] [Google Scholar]
- Egger K. et al. Biopsy surveillance is still necessary in patients with Barrett’s oesophagus despite new endoscopic imaging techniques. Gut 52, 18–23 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara M. A. et al. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus. Gastrointest Endosc 61, 679–85 (2005). [DOI] [PubMed] [Google Scholar]
- Borovicka J. et al. Autofluorescence endoscopy in surveillance of Barrett’s esophagus: a multicenter randomized trial on diagnostic efficacy. Endoscopy 38, 867–72 (2006). [DOI] [PubMed] [Google Scholar]
- Kara M. A., Peters F. P., Fockens P., ten K. F. J. & Bergman J. J. Endoscopic video-autofluorescence imaging followed by narrow band imaging for detecting early neoplasia in Barrett’s esophagus. Gastrointest Endosc 64, 176–85 (2006). [DOI] [PubMed] [Google Scholar]
- Curvers W. L. et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut 57, 167–72 (2008). [DOI] [PubMed] [Google Scholar]
- Stefanova M. Z. M., Zavada F. S. S., Tuckova I. H. P. & Martinek J. No benefit of autofluorescence in a diagnosis of low grade intraepitelial neoplasia in patients with Barrett’s oesophagus. Ceska a Slovenska Gastroenterologie a Hepatologie 65, 249–54 (2011). [Google Scholar]
- Sieron-Stoltny K. et al. Autofluorescence endoscopy with “real-time” digital image processing in differential diagnostics of selected benign and malignant lesions in the oesophagus. Photodiagnosis Photodyn Ther 9, 5–10 (2012). [DOI] [PubMed] [Google Scholar]
- Ishihara R. et al. Autofluorescence imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia: a phase II study. J Gastroenterol Hepatol 27, 86–90 (2012). [DOI] [PubMed] [Google Scholar]
- Holz J. A. et al. Optimized endoscopic autofluorescence spectroscopy for the identification of premalignant lesions in Barrett’s oesophagus. Eur J Gastroenterol Hepatol 25, 1442–9 (2013). [DOI] [PubMed] [Google Scholar]
- Chen W., He B. & Wei G. Ultraviolet laser-induced fluorescence spectroscopy diagnosis of human stomach malignant tissues. Lasers in Medical Science 13, 209–13 (1998). [Google Scholar]
- Kobayashi M. et al. Detection of early gastric cancer by a real-time autofluorescence imaging system. Cancer Lett 165, 155–9 (2001). [DOI] [PubMed] [Google Scholar]
- Mayinger B. et al. Evaluation of in vivo endoscopic autofluorescence spectroscopy in gastric cancer. Gastrointest Endosc 59, 191–8 (2004). [DOI] [PubMed] [Google Scholar]
- Ohkawa A. et al. Diagnostic performance of light-induced fluorescence endoscopy for gastric neoplasms. Endoscopy 36, 515–21 (2004). [DOI] [PubMed] [Google Scholar]
- Shi X. F. et al. Applying patial least-squares discriminant analysis on autofluorescence spectra to identify gastric cancer. Guang pu xue yu guang pu fen xi 26, 295–8 (2006). [PubMed] [Google Scholar]
- Kato M. et al. Trimodal imaging endoscopy may improve diagnostic accuracy of early gastric neoplasia: a feasibility study. Gastrointest Endosc 70, 899–906 (2009). [DOI] [PubMed] [Google Scholar]
- Bergholt M. S. et al. Combining near-infrared-excited autofluorescence and Raman spectroscopy improves in vivo diagnosis of gastric cancer. Biosens Bioelectron 26, 4104–10 (2011). [DOI] [PubMed] [Google Scholar]
- Krauss E. et al. Normalized autofluorescence imaging diagnostics in upper GI tract: a new method to improve specificity in neoplasia detection. Int J Clin Exp Pathol 5, 956–64 (2012). [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Lin J. K., Chen B. F. & Chiang H. K. Autofluorescence spectroscopic differentiation between normal and cancerous colorectal tissues by means of a two-peak ratio algorithm. J Formos Med Assoc 98, 837–43 (1999). [PubMed] [Google Scholar]
- Eker C. et al. Clinical spectral characterisation of colonic mucosal lesions using autofluorescence and delta aminolevulinic acid sensitisation. Gut 44, 511–8 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S. C., Hsiao T. C., Lin J. K., Wang C. Y. & Chiang H. K. Comparison of the performance of linear multivariate analysis methods for normal and dyplasia tissues differentiation using autofluorescence spectroscopy. IEEE Trans Biomed Eng 53, 2265–73 (2006). [DOI] [PubMed] [Google Scholar]
- Mashiko T. I. H. & Saito S. Novel autofluorescence imaging system is useful for detecting colorectal neoplastic lesions. Tokyo Jikeikai Medical Journal 122, 143–53 (2007). [Google Scholar]
- van den Broek F. J. et al. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut 57, 1083–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boparai K. S., van den Broek F. J., van Eeden S., Fockens P. & Dekker E. Hyperplastic polyposis syndrome: a pilot study for the differentiation of polyps by using high-resolution endoscopy, autofluorescence imaging, and narrow-band imaging. Gastrointest Endosc 70, 947–55 (2009). [DOI] [PubMed] [Google Scholar]
- Kamath S. D. & Mahato K. K. Principal component analysis (PCA)-based k-nearest neighbor (k-NN) analysis of colonic mucosal tissue fluorescence spectra. Photomed Laser Surg 27, 659–68 (2009). [DOI] [PubMed] [Google Scholar]
- van den Broek F. J. et al. Clinical evaluation of endoscopic trimodal imaging for the detection and differentiation of colonic polyps. Clin Gastroenterol Hepatol 7, 288–95 (2009). [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Nakamura S., Moriyama T., Hirahashi M. & Iida M. Autofluorescence imaging colonoscopy for the detection of dysplastic lesions in ulcerative colitis: a pilot study. Colorectal Dis 12, e291–7 (2010). [DOI] [PubMed] [Google Scholar]
- Ramsoekh D. et al. A back-to-back comparison of white light video endoscopy with autofluorescence endoscopy for adenoma detection in high-risk subjects. Gut 59, 785–93 (2010). [DOI] [PubMed] [Google Scholar]
- Arita K. et al. Quantitative analysis of colorectal mucosal lesions by autofluorescence endoscopy: discrimination of carcinomas from other lesions. Oncol Rep 26, 43–8 (2011). [DOI] [PubMed] [Google Scholar]
- Shao X., Zheng W. & Huang Z. In vivo diagnosis of colonic precancer and cancer using near-infrared autofluorescence spectroscopy and biochemical modeling. J Biomed Opt 16, 67005 (2011). [DOI] [PubMed] [Google Scholar]
- Kuiper T. et al. Endoscopic trimodal imaging detects colonic neoplasia as well as standard video endoscopy. Gastroenterology 140, 1887–94 (2011). [DOI] [PubMed] [Google Scholar]
- Sato R. et al. The diagnostic accuracy of high-resolution endoscopy, autofluorescence imaging and narrow-band imaging for differentially diagnosing colon adenoma. Endoscopy 43, 862–8 (2011). [DOI] [PubMed] [Google Scholar]
- Ignjatovic A. et al. What is the most reliable imaging modality for small colonic polyp characterization? Study of white-light, autofluorescence, and narrow-band imaging. Endoscopy 43, 94–9 (2011). [DOI] [PubMed] [Google Scholar]
- Odagaki T. et al. What is the accuracy of autofluorescence imaging in identifying non-polypoid colorectal neoplastic lesions when reviewed by trainees? A pilot study. Dig Endosc 25, 428–33 (2013). [DOI] [PubMed] [Google Scholar]
- Aihara H. et al. Computer-aided diagnosis of neoplastic colorectal lesions using ‘real-time’ numerical color analysis during autofluorescence endoscopy. Eur J Gastroenterol Hepatol 25, 488–94 (2013). [DOI] [PubMed] [Google Scholar]
- Liu L. et al. Pattern recognition of multiple excitation autofluorescence spectra for colon tissue classification. Photodiagnosis Photodyn Ther 10, 111–9 (2013). [DOI] [PubMed] [Google Scholar]
- Thomson P. J. Field change and oral cancer: new evidence for widespread carcinogenesis. Int J Oral Maxillofac Surg 31, 262–6 (2002). [DOI] [PubMed] [Google Scholar]
- Glas A. S., Lijmer J. G., Prins M. H., Bonsel G. J. & Bossuyt P. M. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 56, 1129–35 (2003). [DOI] [PubMed] [Google Scholar]
- Kois J. C. & Truelove E. Detecting oral cancer: a new technique and case reports. Dent Today 25, 94–7 (2006). [PubMed] [Google Scholar]
- Balevi B. Evidence-based decision making: should the general dentist adopt the use of the VELscope for routine screening for oral cancer. J Can Dent Assoc 73, 603–6 (2007). [PubMed] [Google Scholar]
- Pavlova I., Williams M., El-Naggar A., Richards-Kortum R. & Gillenwater A. Understanding the biological basis of autofluorescence imaging for oral cancer detection: high-resolution fluorescence microscopy in viable tissue. Clin Cancer Res 14, 2396–2404 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.