Abstract
The current study used a multifaceted approach to assess whether children with ASD have a distinctive diurnal rhythm of cortisol that differentiates them from typically developing (TD) peers and whether sub-groups of ASD children can be identified with unique diurnal profiles. Salivary cortisol was sampled at four time points during the day (waking, 30-min post-waking, afternoon, and evening) across three days in a sample of 36 children with autism spectrum disorder (ASD) and 27 typically developing (TD) peers. Between-group comparisons on both mean levels and featural components of diurnal cortisol indicated elevated evening cortisol and a dampened linear decline across the day in the ASD group. No differences were evident on the cortisol awakening response (CAR). Group-based trajectory modeling indicated that a subgroup (25%) of ASD children demonstrated an attenuated linear decline while the cortisol trajectory of the second subgroup was indistinguishable from that of the TD group. Intraclass correlations indicated that, when aggregated across days, cortisol measures were generally stable over the interval assessed. There were few significant relations between cortisol measures or sub-groups and measures of stress, temperament, and symptoms. Results encourage follow-up studies to investigate the functional significance, heterogeneity and longer-term stability of diurnal cortisol profiles in children with ASD.
Keywords: autism, diurnal cortisol, salivary cortisol, HPA-axis
Individuals with autism spectrum disorder (ASD) demonstrate profound impairments in social interaction, communication, and stereotypic behaviors (APA, 2013) that are often manifest as difficulty responding to changes in daily routines. These difficulties may be related to atypical functioning of the hypothalamic-pituitary-adrenal (HPA) axis. Indeed, it is well known that novelty, unpredictability, and change increase activation of the HPA axis and are associated with heightened levels of cortisol (e.g., Gunnar, Marvinney, Isensee, & Fisch, 1988; Levine, Coe, & Wiener, 1989). Because cortisol is crucial for homeostatic regulation and the ability to adapt to environmental challenges, it is important to assess whether children with ASD have a distinctive cortisol signature that differentiates them from typically developing (TD) peers.
The focus of the present study was the diurnal rhythm of cortisol secretion, the functional form of which is well characterized across individuals. Cortisol is highest in the morning upon waking, and an estimated 77% of people experience a sharp rise in cortisol 30-minutes post waking, referred to as the cortisol awakening response (CAR) (Pruessner et al., 1997; Wust et al., 2000). The CAR has been conceptualized as a preparatory phenomenon as individuals anticipate daily events and challenges that may occur throughout the course of the day (Fries et al., 2009). The CAR is followed by a steady decline of cortisol levels throughout the day until it reaches a nadir in the evening (Anders, 1982; Weitzman et al., 1971). Together, the CAR and subsequent decline are the two dominant featural components of the diurnal rhythm of cortisol secretion.
One study of children and adolescents with and without ASD found that there were no significant differences in the overall amount of daily cortisol secretion (Marinovic-Curin et al., 2008), thus suggesting the importance of studying more specific components of the diurnal cycle. Indeed, while afternoon levels of cortisol appear comparable between children with and without ASD, elevated evening cortisol levels in children with ASD have been reported (Corbett et al., 2008) that have been associated with measures of daily stress and sensory sensitivity (Corbett et al., 2009).
Other investigations have focused on the featural components of the diurnal cycle. To date, studies have yielded no differences between ASD and TD children in the CAR (Corbett and Schupp, 2014; Marinovic-Curin et al., 2008; Zinke et al., 2010) and mixed findings regarding differences in the slope of the peak-to-trough decline of cortisol throughout the day. Some studies have reported no between-group differences (Brosnan et al., 2009; Corbett et al., 2006; Kidd et al. 2012), while others have reported a dampened linear decline in the ASD group (Corbett et al., 2008; Corbett et al., 2009).
The present study was designed to provide a more complete and nuanced picture of cortisol differences between children with ASD and TD peers and, in the process, elucidate factors that might account for inconsistent results in previous studies. One source of variation in prior findings could be differences in the data-analytic procedures used to assess variations in the functional form of cortisol across the day. We used a more comprehensive approach than previous studies by including assessments of both changes in mean levels over time and of specific featural components of the cortisol rhythm (i.e., the CAR and linear decline in cortisol from morning through evening). In addition, to enhance sensitivity of measurement, we used the precise times at which cortisol was sampled as a predictor in our featural models.
Heterogeneity within the ASD group is another factor that might account for inconsistencies across studies. It is well-known that ASD is highly heterogeneous across multiple domains (for recent reviews, see, e.g., Jeste & Geschwind, 2014; Lenroot & Yeung, 2013). Previous studies have generally reported that groups of children with ASD have more variability in diurnal cortisol values (Corbett et al., 2006; Corbett et al., 2008; Hoshino et al., 1987; Richdale and Prior, 1992; Yamazaki et al., 1975) and parents of ASD patients demonstrate heterogeneity in daily cortisol profiles (Dykens & Lambert, 2013). To our knowledge, however, no prior studies have explicitly assessed whether sub-groups of ASD children can be identified based on distinct patterns of the diurnal rhythm of cortisol. We used group-based trajectory modeling (GBTM; Nagin, 2005; Jones & Nagin, 2007) to assess whether subgroups could be identified that displayed distinct trajectories of cortisol across the day. To understand potential causes of differences in cortisol measures between ASD and TD children and any ASD subgroups identified, we assessed the relation between cortisol and measures of daily stress, trait anxiety, and sensory sensitivity, as well as demographic and symptomatic features.
One limitation of previous studies on HPA functioning in ASD is the failure to assess psychometric properties of cortisol measures that may also account for differences across studies, or in the specific pattern of effects observed in a given study. There are several reasons why an examination of reliability, stability, and variability is important. First, if basal cortisol levels truly reflect individual differences in HPA functioning, it should demonstrate the temporal stability expected of an individual difference measure. Second, because increased measurement error tends to attenuate relations with external variables (e.g., Nunnally & Bernstein, 1994), patterns of significant and non-significant correlations and group differences could be linked to differences in the reliability of the different measures of diurnal cortisol that can be extracted. Third, it is important to assess whether prior evidence for greater variability of cortisol within the ASD group is due to greater variability between individuals (perhaps due to subgroup heterogeneity) or greater fluctuations on a within-subjects basis. For all these reasons, we additionally compared the TSD and AD groups on the stability and variability of cortisol measures.
In sum, the current study examined characteristics of diurnal cortisol variation in a sample of children with and without ASD by assessing mean differences at different times of day, featural components of the diurnal rhythm, subgroup heterogeneity in cortisol trajectories, and psychometric properties (i.e., stability and variability) of cortisol.
Method
Participants
The participants consisted of 63 unmedicated, healthy children between the ages of 7 and 16 years old, 36 with ASD (30 males, mean age=10.20, SD=1.96), and 27 TD controls (23 males, mean age=9.71, SD=1.54). Diagnoses were made in accordance with the Diagnostic and Statistical Manual (DSM-IV) criteria (APA, 2000) and were confirmed by a previous diagnosis by a psychologist, psychiatrist, or behavioral pediatrician with ASD expertise, clinical judgment at the time of participation (by BAC or another doctoral level psychologist experienced in the diagnosis of ASD), and the ADOS (Lord et al., 2000), administered by research-reliable personnel. Inclusion in the study required an estimated IQ of 70 or higher (see Table 1).
Table 1.
Group Differences on Demographic and Background Variables
| Measure | ASD | TD | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | SD | Range | Mean | SD | Range | p-value | |
| Age | 10.20 | 1.96 | (7.66, 16.31) | 9.72 | 1.54 | (7.02, 12.85) | 0.269 |
| IQ | |||||||
| WASI Full Scale | 104.42 | 25.45 | (70, 149) | 120.45 | 13.97 | (96, 151) | 0.006 |
| Performance | 101.18 | 19.10 | (68, 140) | 113.10 | 14.34 | (85, 143) | 0.006 |
| Verbal | 101.18 | 24.07 | (55, 150) | 123.59 | 14.62 | (96, 147) | <0.001 |
| SSS | 112.32 | 23.91 | (58, 155) | 64.66 | 12.50 | (49, 93) | <0.001 |
| SSP | 121.46 | 17.69 | (89, 155) | 172.58 | 16.85 | (117, 190) | <0.001 |
|
| |||||||
| Waking Time | 7:10 AM | 1:15 | (4:13 AM, 10:56 AM) | 7:26 AM | 1:04 | (5:45 AM, 9:38 AM) | 0.414 |
|
| |||||||
| Bed Time | 9:20 PM | 0:49 | (7:30 PM, 11:10 PM) | 9:42 PM | 0:50 | (8:10 PM, 11:05 PM) | 0.086 |
Note. WASI=Wechsler Abbreviated Scale of Intelligence; SSS=Stress Survey Schedule; SSP=Short Sensory Profile; Waking Time and Bed Time values shown in HH:MM clock time. Waking Time = average of reported times for M1 cortisol sampling. Bed Time = average of reported times for E cortisol sampling + 30 min
The Vanderbilt University Institutional Review Board approved the study. Prior to participation in the study, parents provided informed written consent and participants provided verbal assent. Participants were recruited by IRB approved flyers and established recruitment systems (e.g., clinics, resource centers, support groups, school, and recreational facilities).
Measures
The diagnostic and parent report measures and salivary cortisol collection training were administered during one visit to the University.
Autism diagnostic observation schedule (ADOS)
Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000) is a semi-structured interview used to assess diagnostically characteristic behaviors of ASD. Test-retest reliability for the domains include social (.78), communication (.73), social communication (.82), and restricted, repetitive behavior (.59). Internal consistency for all domains and modules ranges from .47 to .94. (Lord et al., 2000)
Wechsler abbreviated scale of intelligence (WASI)
Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999) is a measure of general intelligence used to estimate intellectual functioning. Reported test-retest reliabilities range from .76 to .85 for each subtest, and are .95 for the full-scale estimated IQ (Wechsler, 1999).
Stress Survey Schedule (SSS)
Stress Survey Schedule (Groden et al., 2001) is a parent-report measure of stress designed for individuals with autism and other developmental disabilities. The measure consists of 60 daily stress-related items rated on a five-point Likert scale and includes eight dimensions of stress. Internal consistency correlations range from 0.70 to 0.87. Based on evidence indicating linkages between cortisol and uncertainty and change (e.g., Gunnar et al., 1988), we used both the SSS total score and scores on the Anticipation/Uncertainty and Changes and Threats subscales.
Short Sensory Profile (SSP)
Short Sensory Profile (Dunn, 1999) is a parent questionnaire related to sensory sensitivity across several domains. We used the SSP total score. Lower values indicate greater impairment.
Child Behavior Checklist (CBCL)
Child Behavior Checklist (Achenbach, 1992) is a parent-report measure that assesses behavioral and emotional problems in children. Eight lower-order and two higher-order (internalizing, externalizing) domains are extracted, as well as scores for DSM-IV diagnostic disorders. Across subscales, reported reliability coefficients range from .71 to .89. Given prior evidence linking cortisol in school-age children to internalizing problems (e.g., Granger, Weisz, Ikeda, McCracken, & Douglas, 1996), we focused on the anxious/depressed, withdrawn/depressed, and overall internalizing scales and the DSM-IV anxiety diagnostic scale.
State-Trait Anxiety Inventory for Children (STAI-C)
State-Trait Anxiety Inventory for Children (Spielberger & Edwards, 1973) consists of two 20-item self-report scales designed to measure anxiety in children. One form measures current (state) anxiety, and the other measures persistent (trait) anxiety. The measure has been validated in typically developing individuals with a Cronbach’s alpha of .91.
The CBCL and STAI-C were not administered to 9 children in the ASD group due to experimental constraints.
Cortisol Sampling Method
Following the diagnostic assessment, basal levels of salivary cortisol were collected from home over three (3) days at four (4) time points per day to obtain a representative aggregate of cortisol values to characterize the diurnal rhythm of each participant. For each of the three days, the sampling times were M1 (immediately upon waking), M2 (30-minutes post-waking), A (afternoon, approximately 3 PM), and E (evening, 30 min. before bedtime), thus resulting in 12 home samples in total. A well-established method (Corbett, Mendoza, Wegelin, Carmean, & Levine, 2008) was used, which included the provision of consistent collection materials and methods, controls for the intake and time of drinks, foods, and medications, and the use of standardized procedures and protocols. For assessment of the CAR, participants were instructed to take the first sample immediately upon waking. Then they were allowed to get out of bed and go about their typical morning routines but were not allowed to eat or brush their teeth before the 30 minute post-waking sample. Samples were collected by passive drool.
Cortisol Assay
The salivary cortisol assay was completed using a Coat-A-Count® radioimmunoassay kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA) that was modified to accommodate lower levels of cortisol in human saliva compared to plasma. All saliva samples were stored at −20°C, and thawed and centrifuged at 2558 g for 15 minutes to separate the aqueous component from mucins and other suspended particles in the sample. The coated tube from the kit was substituted with a glass tube into which 100μl of saliva, 100μl of cortisol antibody (courtesy of Wendell Nicholson, Vanderbilt University, Nashville, TN), and 100μl of 125I-cortisol were mixed. Following incubation at 4°C for 24 hours, 100μl of normal rat serum in 0.1% PO4//EDTA buffer (1:50) and precipitating reagent (PR81) were added. The mixture was centrifuged at 2558 g for 30 minutes, decanted, and counted. Serial dilution of samples indicated a linearity of 0.99. Interassay coefficient of variation was 10.4%. The cross-reactivity of cortisone with the cortisol antibody used is 2.6%. Given that normal human cortisone levels average 25 ng/ml and the fact that plasma cortisone is 16.2% free, this level of cross-reactivity implies that the contribution of cortisone to levels of cortisol can be estimated to be approximately .105 ng/ml. Most importantly, there is minimal variability in cortisone levels among healthy humans and levels do not appear to vary as cortisol secretion increases, even among patients with adrenocortical disorders (Morita, Isomura, Mune et al., 2004). Because cortisone levels are likely to be consistent between and within participants over time, they represent a constant offset that would not affect the between- and within-subjects comparisons that are the primary focus of the study.
Data Analysis
Because cortisol is positively skewed, values were log transformed (base 10) prior to analyses. All analyses were conducted using SAS/STAT software, Version 9.4 of the SAS System for Windows™ (Copyright © 2002–2012 SAS Institute Inc. SAS and all other SAS Institute Inc. products or service names are registered trademarks of SAS Institute Inc., Cary, NC, USA). Although the statistical procedures used accommodate missing data, proportions were small: 94.7% and 98.1% of the possible cortisol samples were non-missing for the ASD and TD groups, respectively. Zero-inflated negative binomial (ZINB) models indicated no significant group differences in numbers of missing observations (likelihood ratio χ2(1) = .66, p =.42).
Effects of diagnosis on mean differences on cortisol levels
We tested for the main and interactive effects of Diagnosis (Dx), Day, and Time of Day (TOD) on mean cortisol levels using the marginal linear model for correlated response data instantiated in SAS PROC MIXED (Littell, Milliken, Stroup, Wolfinger, & Schabenberger, 2006; Verbeke & Molenberghs, 2009). Restricted maximum likelihood (REML) was used for estimation and general F statistics were used for tests of fixed effects. A Kronecker Product (KP) structure (Galecki, 1994) for the effects of time of day and day was imposed on the residual covariance matrix to model non-independence. To control for sleep-waking variables, we included reported waking time and bedtime as covariates in the model. Because participants were instructed to take the evening sample 30 min. before bedtime, we added 30 min. to the reported evening sample time to generate an estimate of bedtime. To replicate and extend prior findings indicating group differences in evening cortisol, we conducted Dx X Day simple effects analyses at each of the four times of day. A step-down Bonferroni approach (e.g., Westfall, Tobias, & Wolfinger, 2011) was used to control for multiplicity effects.
Effects of diagnosis on featural components of the diurnal cycle
To examine the temporal pattern of the diurnal rhythm, we implemented a piecewise linear mixed effects (LME) approach using REML estimation. The model included fixed effect terms denoting the CAR and the expected linear decline in cortisol from M2 through A to E. An additional goal was to maximize precision by using the exact sampling times reported by respondents. We used a random effects structure to model non-independence across days and time of day.
Heterogeneity within the ASD group
To assess heterogeneity of daily cortisol profiles within the ASD group, we used group-based trajectory modeling (GBTM) (Nagin, 1999; 2005) estimated via SAS PROC TRAJ (Jones, 2004; Jones, Nagin, & Roeder, 2001). This procedure is designed to uncover patterns of change across time (estimated as polynomials of a given degree) that differ across sub-groups but are common among members of a sub-group. For each participant we averaged cortisol values across days for each time of day. For a specified number of latent classes (e.g., two), PROC TRAJ then generated maximum likelihood (ML) estimates of regression coefficients for polynomial equations and of probabilities of group membership. We specified a normal distribution of residuals. Two types of model were specified. The first included all four times of day and the second separately assessed the CAR and the morning-to-evening decline. For the overall and diurnal decline assessments, we followed the general procedure recommended by Nagin (2005) by first estimating quadratic polynomial models specifying one through four classes. Then, after determining the number of classes associated with the best-fitting model, we assessed whether a more parsimonious linear model would provide better fit. To select the best-fitting model, we used the Bayesian Information Criterion (BIC; Raftery, 1995, Schwarz, 1978) and an estimate derived from the BIC values of the relative probability that a model in a given set is the correct one (Kaas & Wasserman, 1995). The BIC is designed to prevent overfitting by balancing model complexity against goodness of fit to the data.
Predictors of Cortisol
Correlational analyses assessed the relation between cortisol measures and scores on the SSS, SSP, ADOS, and CBC measures of interest. Given that most of the measures of primary interest pertain specifically to ASD and that ASD and TD groups had substantial differences on most of the other measures, our primary focus was on correlations computed within the ASD group. We used t tests to assess differences on these measures between ASD sub-groups formed by the GBTM models.
Stability and variability of cortisol levels
We computed intraclass correlations (ICCs) (e.g., Shrout & Fleiss, 1979, Strube & Newman, 2007) at each of the four daily times of assessment (M1, M2, A, E) to estimate the stability of cortisol across days for the ASD and TD groups. The ICC was defined according to the following formula:
where σu2 = variance of the random effects (denoting differences between subjects) and σe2 = variance of the residuals (denoting variability among the observations of a given subject). By applying the Spearman-Brown formula, we also generated ICCs that estimated the test-retest stability of cortisol values aggregated across the three days with a hypothetical three-day average assessed under identical conditions (e.g., Shrout & Fleiss, 1979). Below we denote the two types of ICCs as ICC 1 and ICC 3. In addition to the ICCs computed at each time of day, we computed ICCs for overall daily mean, the CAR, and the linear decline in cortisol from M2 to E.
To estimate ICCs and compare groups, we used a Bayesian approach (Spiegelhalter, 2001; Turner, Omar, & Thompson, 2001) implemented in SAS PROC MCMC. Vague prior distributions were specified for all parameters estimated (e.g., Spiegelhalter, 2001) and Markov Chain Monte Carlo (MCMC) simulation methods were used to generate a minimum of 10,000 samples from the posterior distribution of the parameters, after which we computed medians and highest posterior density (HPD) confidence intervals (e.g., Christensen, Johnson, Branscum, & Hanson, 2011). To compare the ICC values of the TD and ASD groups, we computed difference scores for each posterior sample.
Results
Preliminary Analyses
Two-sample t tests indicated the expected differences between the ASD and TD groups on IQ measures, although the ASD group scored in the average range on the WASI (see Table 1). The groups did not differ significantly in age (Table 1). The between-group differences on the SSS and SSP in Table 1 indicate that individuals with ASD have higher parent-reported ratings of daily stress and experience greater impairment due to sensory sensitivity. Group X Day mixed effects analyses indicated no significant effects on waking time (all ps > .15) or bedtime (all ps > .08) (see Table 1). We included mean values of both measures as covariates in the analyses of cortisol because they predicted the latter and are potentially linked to circadian rhythms.
Comparison of Mean Cortisol Levels
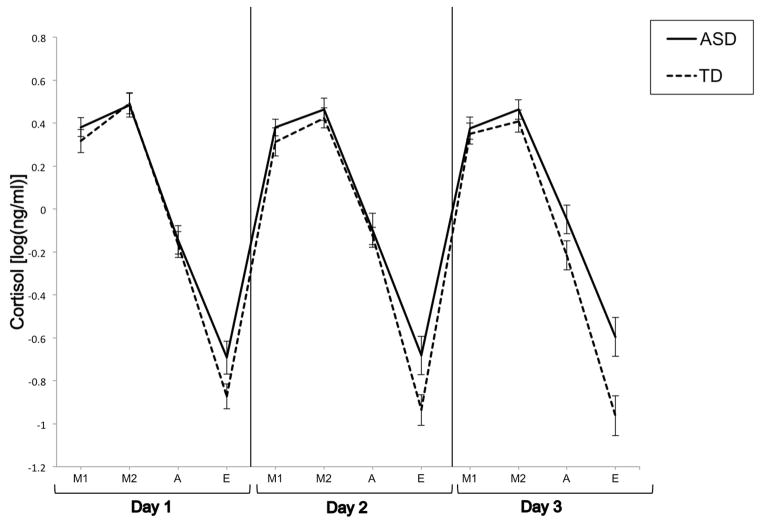
Figure 1 displays the predicted values on log cortisol yielded by the omnibus Dx (Diagnosis) X Day X TOD (Time of Day) ANCOVA, with waking time and bedtime serving as covariates (see Table 2 for both logged and unlogged ng/mL mean values). As shown, both the ASD and TD groups show a diurnal pattern of cortisol where cortisol is at a maximum in the morning and gradually declines throughout the afternoon until reaching a minimum in the evening. The omnibus ANOVA yielded a significant main effect of Dx (F(1, 128)= 4.78, p =0.031) that reflected higher levels of cortisol across days and times by the ASD relative to the TD group. Furthermore, the analysis also yielded a trend for the interaction between Dx X TOD (F(3, 111)=2.30, p =0.082). Planned step-down Bonferroni simple effects contrasts indicated that the ASD group had significantly higher evening cortisol levels (F(1, 122)=7.86, unadjusted p = 0.006, adjusted p =0.024) than the TD group. There were no significant differences between the two groups at the M1 (F(1,103) < 1, unadjusted p = .50), M2 (F(1,123)<1, unadjusted p =.76), and A (F(1,110)<1, unadjusted p =.40) times of day.
Figure 1.
Mean cortisol values for the ASD and TD groups across days and times of day. Values are adjusted for waking time and bedtime.
Table 2.
Mean Cortisol at Each Time of Day
| Cortisol Units | Group | M1 (SD) | M2 (SD) | A (SD) | E (SD) | Total (SD) |
|---|---|---|---|---|---|---|
| ng/mL | ASD | 2.86(1.20) | 3.58 (1.60) | 1.40 (1.73) | 0.47 (0.59) | 2.09 (0.85) |
| TD | 2.54(1.11) | 3.23 (1.46) | 0.83 (0.35) | 0.18 (0.14) | 1.69 (0.59) | |
|
| ||||||
| Log10 (ng/mL) | ASD | 0.38 (0.19) | 0.47 (0.24) | −0.10 (0.34) | −0.68 (0.45) | 0.03 (0.21) |
| TD | 0.33 (0.23) | 0.44 (0.22) | −0.17 (0.22) | −0.92 (0.31) | −0.08 (0.17) | |
Note. ASD n =36; TD n = 27; Cortisol values at teach time of day (M1=Immediately upon waking, M2=30 minutes post-waking, A= Afternoon, approximately 3pm; and E=Evening, 30 minutes before bedtime), averaged across three days. Total cortisol was calculated as the average of the four times of day.
Comparison of Featural Components
The piecewise mixed effects analysis with sleep-wake variables as covariates indicated significant main effects of CAR (F(1, 60.5)=14.92, p <0.001) and of the linear decline from M2 to E (F(1,76.7)=633.28, p<0.0001). This result serves to confirm that the two pieces are distinguishable and are major featural components of the diurnal cycle. Consistent with the visual representation in Figure 1, the analysis also revealed a marginally significant Group X linear decline interaction (F(1, 76.5)=3.84, p =0.054) due to the fact that the slope of the linear decline in the ASD group was attenuated relative to the TD group (BASD=−2.05, BTSD=−2.40). There was no significant Dx X CAR interaction (F(1, 60.4) < 1).
Group-based Trajectory Models within the ASD Group
A first set of group-based trajectory models (GBTMs) assessed diurnal variation across all four times of day (M1, M2, A, and E). We assessed the fit of quadratic models for numbers of groups varying from 1 (i.e., no distinct grouping) to 4. The BIC values and model probability indices indicated that the best-fitting model specified two groups, with estimated probabilities of group membership equal to 27% and 73 % of the ASD population respectively. Subsequent analyses showed that this model fit better than linear alternatives.
Examination of the predicted quadratic polynomial curves across the 4 times of day clearly indicated that the most pronounced differences between the two groups were in the decline in cortisol from M2 to A and E. The results of the featural GBTM analyses were consistent with this observation. An analysis of the CAR (linear due to the presence of only two time points) clearly indicated that the best-fitting model specified only one group. In contrast, the best-fitting quadratic and linear models for the decline across M2, A, and E both specified two groups (see Table 3). A comparison of the 2-group linear and quadratic models indicated superior fit for the linear model based on BIC and probability-correct values (Table 3). Several additional criteria (Nagin, 2005; Nagin & Odgers, 2010) indicated excellent fit of the two-group linear model, including posterior probabilities of group membership for individual participants that were quite high (group 1 mean p = .92, group 2 mean p = .97) and a close correspondence between the estimated population probabilities of group membership (.252 and .748 respectively) and the proportion of participants assigned to groups (.25 and .75 respectively). The two groups failed to differ in waking time (p > .45) or bedtime (p > .58).
Table 3.
BIC Values and Model Comparisons for Group-based Trajectory Models (Morning to Evening
| Polynomial | # Groups | BIC | BIC (n=106) | Probability Correct (within Polynomial) | Probability Correct (across Polynomials) |
|---|---|---|---|---|---|
| Linear | |||||
| 1 | −41.59 | −43.21 | .00 | .00 | |
| 2 | −33.37 | −36.61 | .99 | .87 | |
| 3 | −36.42 | −41.28 | .01 | .00 | |
| 4 | −37.65 | −44.13 | .00 | .00 | |
| Quadratic | |||||
| 1 | −41.85 | −44.01 | .00 | .00 | |
| 2 | −34.25 | −38.57 | .99 | .12 | |
| 3 | −37.96 | −44.44 | .00 | .00 | |
| 4 | −40.82 | −49.46 | .00 | .00 |
Decline) BIC =Bayesian Information Criterion = log(L)-0.5* k*log(N), where L = maximized log likelihood, k=# parameters in the model, and N=sample size. Higher values of the BIC indicate better fit. N=36 = number of participants, N=106 = total number of observations. The two BIC scores bracket the theoretically correct value (Nagin, 2005). Probability correct values were computed using the BIC values for n=106 and the formula , where BICmax= the best-fitting model in the set and J = the number of models in the set. Within polynomial probabilities are comparisons among the J=4 models within a given type (linear or quadratic). Across polynomial probabilities are comparisons among all J=8 models
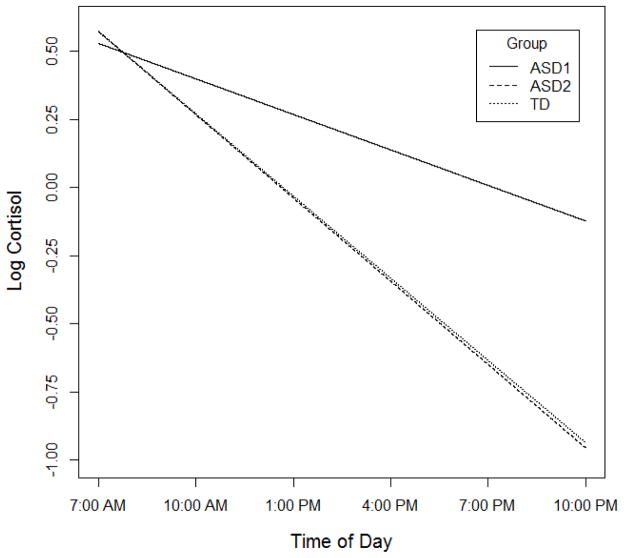
Focusing on the two-group linear model for the diurnal decline, we denote the smaller group (25% of participants) as ASD1 and the larger group (75%) as ASD2. Figure 2 shows the average predicted trajectories across the M2, A, and E time points for each of these two groups and also displays the predicted decline for the TD group as a whole based on a linear fit. As this figure makes clear, the ASD1 group displays an attenuated linear decline across time relative to the other two groups and the ASD2 and TD groups are virtually indistinguishable. Consistent with these observations, ANOVAs and subsequent comparisons indicated that cortisol values for the smaller ASD subgroup at both the A and E time points were lower than those of both other groups (all ANOVA and contrast ps < .0001). There were no differences or even trends when the larger ASD and TD groups were compared (both ps > .38). Similarly, a direct comparison of the linear decline indicated that the slope of the ASD1 group was attenuated relative to the slope of the other two groups (Group X Linear trend F(2,61) = 25.18, p < .0001, ASD1 vs ASD2 contrast t(61) = 6.70, p < .0001, ASD1 vs TD contrast t(61) = 6.58, p < .0001). The slope coefficients for the ASD2 and TD groups were almost identical (ASD B = .112, TD B = .109, t(61) < 1, p = .82. Results were unchanged when we included waking and bedtime as covariates in the analyses.
Figure 2.
Predicted linear decline across ASD1, ASD2, and TD groups.
Correlates of Cortisol Measures
Within the ASD group we computed correlations between age, the ADOS, the SSS, SSP, CBCL, and STAI and measures of cortisol at each time of day, the CAR, and the linear decline from A2 to E. Because scatterplots indicated a tendency toward outliers, robust percentage bend correlations (Wilcox, 1994) were used. The only statistically significant correlations indicated that higher scores on the CBCL anxious-depressed (r = −0.43, p =.03) and anxiety diagnostic scales (r= −0.39, p =.048) were associated with lower cortisol levels upon awakening. T tests assessing differences between the two ASD sub-groups failed to indicate significant differences on any measures (all ps > .15), as did multiple logistic regression analyses predicting group assignment from sets of interrelated variables (e.g., SSS sub-scales). We also tested for differences between those ASD participants who demonstrated a CAR greater than 0 and those who did not. Although there was a trend toward higher ADOS total scores among those who did not demonstrate a CAR (t(33)=1.77, p= .09), no other effects approached significance (all ps > .05).
Stability of Cortisol
Table 4 shows ICC estimates and group differences for each time of day, the daily average across times of day, the CAR and the linear decline. Several notable patterns are evident. First, given that the ICCs indicate the proportion of between-subject variability that is consistent across days, all measures indicated a statistically significant individual differences component. Even the values indicating the stability of cortisol assessed on a single day (ICC1) generally indicated a high proportion of variance due to participant (i.e., ICC values range from .31 to .63) encompassing both specific times of day and featural components. Second, consistent with well-known results concerning the benefits of aggregation, the ICC3 values indicating the predicted consistency of cortisol averaged across three days typically represented a substantial improvement over the ICC1 values and, with a few exceptions approach the expected range (e.g., >= .70) for measures of individual differences in the area of personality and temperament. Although there were no significant group differences, there was a tendency for the TD group to demonstrate greater stability than the ASD group during the morning, with the reverse pattern in the afternoon and evening. The pattern of results remained unchanged when we adjusted morning ICCs for waking time and evening ICCs for bedtime.
Table 4.
Intraclass Correlations Assessing Stability of Cortisol Across Three Days
| TD (n=36) | ASD (n=27) | ICC Difference (ASD-TD) | ||||
|---|---|---|---|---|---|---|
| Measure | ICC1 (95% CI) | ICC3 (95% CI) | ICC1 (95% CI) | ICC3 (95% CI) | ICC1 (95% CI) | ICC3 (95% CI) |
| Morning 1 | .45 (−22, .66) | .71 (.49, .87) | .31 (.10, .52) | .57 (.29, .79) | −.14 (−.44, 17) | −.13 (−.49, .17) |
| Morning 2 | .63 (.43, .79) | .84 (.72, .93) | .41 (.20, .61) | .67 (.47, .85) | −.22 (−.49, .06) | −.16 (−.39, .07) |
| Afternoon | .32 (.07, .56) | .58 (.24, .83) | .41 (.20, .61) | .67 (.46, .84) | .09 (−.22, .41) | .09 (−.24, .51) |
| Evening | .44 (.21, .66) | .70 (.47, .87) | .56 (.35, .73) | .79 (64, .91) | .11 (−.19, .42) | .09 (−.16, .36) |
| Daily Mean | .62 (.40, .79) | .83 (.68, .93) | .56 (.36, .75) | .80 (.64, .91) | −.05 (−.34, .22) | −.03 (−.24, .16) |
| CAR (M1-M2) | .38 (.14, .61) | .64 (.38, .84) | .48 (.28, .68) | .73 (.55, .87) | .10 (−.22, .40) | .09 (−.20, .41) |
| Linear Decline (M2-E) | .33 (.10, .60) | .59 (.26, .83) | .57 (.35, .75) | .80 (.64, .91) | .24 (−.10, .54) | .20 (−.11, 55) |
Cortisol values are log-transformed. ICC1=Estimated intraclass correlation of cortisol assessed on a single day. ICC3 = Estimated intraclass correlation of cortisol aggregated across three days. ICC estimates are the medians of the Bayesian posterior distribution. CI= 95% Bayesian Highest Posterior Density (HPD) interval of the posterior distribution.
Between-group comparisons on the random and residual variance parameters for each measure indicated only isolated effects of small magnitude. The most notable difference was the significantly greater variability in the random variance for the ASD (estimated variance = .57) relative to the TD (estimated variance = .16) group on the measure of linear decline from M2 to E (median difference = .40, HPD = .02 to .96). This effect is consistent with the results of the GBTM analyses indicating heterogeneity within the ASD group in patterns of linear decline. When we computed ICCs for the linear decline within the subgroups defined by the GBTM analyses, we found lower values of the ICCs than for the ASD group as a whole (e.g., ICC3 = .36 for ASD1 and .64 for ASD2) that were largely due to the sharply reduced between-subjects random variance estimates within each ASD subgroup (variances = .10 for ASD1 and .20 for AD2). These effects are expected given that the goal of the GBTM analyses was to identify maximally homogeneous sub-groups.
Discussion
The current study used a multifaceted approach to examine between- and within-group differences in diurnal cortisol in children with and without ASD. We compared groups on mean cortisol at specific times of day and on featural components of the diurnal pattern. In addition, we assessed two important measurement issues: heterogeneity within the ASD group and the stability of individual differences in cortisol parameters.
Between- and Within-Group Differences in Diurnal Cortisol
The children with ASD demonstrated significantly higher cortisol levels overall than TD children. Although the absence of a significant Group X Time of Day interaction necessitates caution, the ASD group had significantly higher values in the evening, with no significant differences or even trends at the other three times. There were no group differences on the CAR but a marginally significant effect (p = .054) indicating that, relative to the TD group, children with ASD demonstrated an attenuated decline in cortisol from M2 to E than TD children. Such late-day effects were not confounded by sleep- and waking-time. The overall pattern of results is consistent with previous findings that ASD and TD samples differ in evening cortisol (Brosnan et al. 2009; Corbett & Schupp, 2014; Corbett et al., 2008; Nir et al., 1995) but fail to differ in the CAR and in morning (Brosnan et al., 2009; Corbett & Schupp, 2014; Marinovic-Curin et al., 2008) and afternoon (e.g., Corbett et al., 2008; Nir et al., 1995) levels. The present sample was comprised of high-functioning children and it is unclear the extent to which our findings and conclusions generalize to lower functioning children.
The group-based trajectory modeling analyses significantly extended previous results by indicating that only a subgroup of ASD participants (an estimated 25%) demonstrate a flatter diurnal profile than the TD group. An examination of the trajectories of both ASD subgroups and the TD group indicated marked differences between the smaller ASD subgroup and the other two groups and a diurnal pattern in the larger ASD subgroup that was strikingly similar to that of the TD group. An identical pattern was observed on analyses of cortisol levels at the afternoon and evening assessments. Given that the TD group was not included in the GBTM analysis, the marked similarity between the TD and ASD2 groups is especially notable and, indeed, was unanticipated. In a broader context, our findings are consistent with evidence that ASD is a highly heterogeneous disorder (e.g., Jeste & Geschwind, 2014; Lenroot & Yeung, 2013) which ostensibly extends to the diurnal cortisol profile.
While our GBTM analyses have potentially important implications, a few cautions are warranted. The study was not originally designed to assess sub-groups and, in that sense, the analyses were post-hoc. In addition, although the two-group models clearly had the best fit and the probabilities of group assignment were quite high, the sample size within the ASD group (n=36) was smaller than typical for an analysis designed to identify subgroups. The evidence that increases in sample size increase the probability that more subgroups will be identified (Sampson, Laub, & Eggleston, 2014) underscores the importance of replication and extension in a study with larger sample size. In addition, in the present study, no measures of ASD symptomatology, stress, and temperament discriminated the two groups. One reason for such null effects may be the small sample size within the ASD1 group, which consisted of only 9 individuals (that two of the measures were not administered to all ASD participants furthered lowered n’s for some comparisons). Finally, it is important to note that the groupings formed by GBTM and related statistical approaches (e.g., mixture modeling) do not necessarily correspond to taxonomically distinct entities (e.g., Bauer & Curran, 2003). We agree with Nagin and Odgers (2010), however, that even if GBTM analyses do not clearly uncover non-overlapping subtypes with unique etiological features, they make researchers aware of salient prototypes in the data that can be the basis for future study. In that spirit, we see the stark differences between the ASD1 group and both the ASD2 and TD groups as a clear sign that studying those ASD individuals with a flattened profile of diurnal variation is an important goal for future research -- whether or not such individuals differ more in degree than kind.
Diurnal Rhythm of Cortisol in ASD
It is presently unclear why some ASD children demonstrate a flatter diurnal rhythm of cortisol when compared to TD children or other ASD children. One obvious hypothesis is that this pattern reflects the cumulative effects of stress. Exposure to chronic stress is associated with significantly higher concentrations of afternoon and evening cortisol, a flatter diurnal rhythm, and a higher daily volume of output (Miller, Chen, & Zhou, 2007). In the present study, these outcomes were all characteristics of the ASD1 sub-group (see Figure 2) and of the ASD group as a whole when compared to the TD group. Corbett et al. (2009) found an association between evening cortisol and parent-reported measures of daily stress and sensory sensitivity among children with ASD. In the present study, however, these measures were not significantly correlated with any cortisol measures among the ASD group as a whole and were not linked to differences between the two ASD subgroups. Disparities in subject characteristics (autistic disorder vs. ASD participants) and in experimental design (Taylor & Corbett, 2014), as well as sampling error may all have contributed to these differing results. The measure of stress was also administered on only one occasion in the present study. An important goal for future studies is the use of measures that validly assess daily stress and its relation to cortisol in ASD children.
An additional goal is to clarify what components of stress might be linked to elevated cortisol and heterogeneity in diurnal cortisol profiles among ASD children. As they age, ASD children become especially susceptible to social-evaluative and interpersonal anxiety (Bellini, 2006) and this pattern is linked to heightened cortisol responses during social interactions with TD children (Schupp, Simon, & Corbett, 2013). It is important to assess whether children in the ASD1 sub-group are more socially anxious and/or susceptible to perceived stigmatization (e.g., Hebron & Humphrey, 2014; Chi et al. 2015). Late-day elevations in cortisol may also reflect an inability to disengage from the various demands or stressors that accumulate throughout the day (e.g., Corbett et al, 2009; Sapolsky et al., 1986).
It is also important to assess the underlying neurobiological mechanisms for heightened cortisol levels and a flatter slope of cortisol across the day. Although most theories of HPA activation linked to individual differences explicitly or implicitly posit heightened CNS drive as the critical mechanism (e.g., Sapolsky et al., 1986), more peripheral mechanisms involving metabolism and clearance cannot be ruled out. Identification of those components of the HPA axis requires challenge studies (e.g., Heuser, Yassouridis, & Holsboer, 1994) and specific assessments of cortisol metabolism (e.g., Boonen, Vervenne, Meersseman, et all., 2013). The fact that some biological mothers of children with ASD also demonstrate a blunted trajectory of diurnal cortisol (Dykens & Lambert, 2013; Seltzer, Floyd, Song, Greenberg, & Hong, 2011) suggests the possibility of intergenerational transmission. In the present study, however, the ASD participants with a flatter slope had higher levels of cortisol during the afternoon and evening than other groups, while mothers of ASD children with a blunted trajectory have tended to have overall lower levels of cortisol in prior studies.
It is also important to examine the functional significance and long-term consequences of the flatter slope of diurnal cortisol and elevated evening values demonstrated by some ASD children. Reduced cortisol in the evening may contribute to rest and sleep. Sleep problems are common in ASD (e.g., Reynolds and Malow, 2011) and pre-sleep arousal is associated with greater sleep disturbance and psychopathology in youth with ASD (Richdale, Baker, Short & Gradisar, 2014). Possibly, those children with a flatter diurnal profile are at heightened risk for future affective and anxiety disorders and arousal dysregulation. In addition, a flatter slope of cortisol and elevations in evening cortisol predict negative health outcomes among adult populations (e.g., Kumari, Shipley, Stafford, & Kivimaki, 2011; Sephton et al., 2000; 2013). These results suggest that the subgroup of ASD children who demonstrated this pattern may be at risk for negative health outcomes. Although there is some variability within each category, the cortisol levels observed in these studies with adults and in the present study with children are typically much lower than the average levels and clinical cut-offs for disorders characterized by hyper-cortisolism such as Cushing’s Disease (compare, for example, Hackett et al., 2014, and Sephton et al, 2013, to Deutschbein, Broecker-Preuss, Flitsch et al., 2012, Papanicolaou, Mullen, Kyrou, & Nieman, 2002, and Raff, Raff, & Findling, 1998). This observation indicates that a flatter diurnal slope of cortisol may have long-term effects on health outcomes at levels appreciably lower than the clinical cutoffs commonly used by endocrinologists to diagnose hypercortisolemic states. In other words, the precise levels of cortisol that have measurable effects on outcomes appear conditional upon both the specific measure of cortisol derived (e.g., diurnal slope vs overall level) and the specific outcome of interest. The evidence that the HPA axis is centrally involved in the regulation of many essential bodily processes (Herman & Cullinan, 1997) may account for why features of cortisol that are different but not clinically discrepant from the normal range may have long-term negative effects.
Stability and Variability of Cortisol
For both the ASD and TD groups, all ICC measures that we derived indicated a significant proportion of variance attributable to differences among participants that were stable over the time interval assessed. Indeed, the ICC3 values that we obtained indicate that aggregation of measures across a three-day interval yields measures with stability estimates that are acceptable from a psychometric perspective. Although we observed a trend indicating higher stability on morning assessments for the TD and on evening assessments for the ASD group, there were no significant group differences on any ICC measures.
For both the TD and ASD groups, evening cortisol measures were the only ones that attained ICC3 values greater than or equal to .70 among both children with ASD and TD children. This observation suggests that the between-group mean differences on evening cortisol observed in previous studies may be due to dispositional, biological and/or environmental factors that are themselves stable. We should note, however, that the ICC3 values for the earlier times of day are sufficiently high to render it unlikely that differences in the precision of measurement are a major factor accounting for the absence of significant differences between groups on these measures. In a similar vein, while groups marginally differed in the linear decline but not in the CAR, the ICC values for these two featural components differed only slightly, with no systematic trends evident.
Our stability analyses merit two cautions. First, while the ICC1 results indicate that a notable proportion of the variance in cortisol assessed on a given day is due to within-subjects homogeneity, a substantial proportion of variance – in some cases the major proportion – appears due to the combination of day-specific factors and/or random error. Second, cortisol was assessed only during three consecutive days. Conclusions yielded by this narrow window of assessment may not adequately generalize to wider time intervals (e.g., 6–24 months). The results of prior studies using normative samples have indicated a notable decline in the strength of more stable, trait-like components of variance as time intervals increase (e.g., Ross, Murphy, Adam, Chen, & Miller, 2014; Shirtcliff, Allison, Armstrong, Slattery, Kalin, & Essex, 2012).
Finally, there was a notable reduction in the within-group variability of the linear decline when random variance parameters were estimated within the ASD trajectory sub-groups. This result is consistent with the goal of the GBTM analyses to identify groups that are maximally homogeneous. It also suggests that the presence of distinct prototypes may account for the greater variability among ASD children found in previous studies (e.g., Corbett et. al., 2006, 2008).
Summary
This study aimed to provide a comprehensive assessment of the between- and within-group differences in the temporal patterning of the diurnal cortisol rhythm in children with and without ASD. Our findings indicate that children with ASD have elevated evening cortisol levels compared to their TD peers, and that children with ASD also exhibit a dampened linear decline of cortisol throughout the day. Subsequent analyses indicated that this latter pattern is characteristic of a subgroup of ASD children. Cortisol aggregated across the three-day sampling interval had acceptable psychometric properties in both groups. Follow-up studies are warranted to investigate heterogeneity and functional significance of the diurnal cortisol profiles in children with ASD and the long-term stability of cortisol.
Highlights.
The current study used a multifaceted approach to assess whether children with ASD have a distinctive diurnal rhythm of cortisol that differentiates them from typically developing (TD) peers and whether sub-groups of ASD children can be identified with unique diurnal profiles.
Salivary cortisol was sampled at four time points during the day (waking, 30-min post-waking, afternoon, and evening) across three days in a sample of 36 children with autism spectrum disorder (ASD) and 27 typically developing (TD) peers.
Between-group comparisons on both mean levels and featural components of diurnal cortisol indicated elevated evening cortisol and a dampened linear decline across the day in the ASD group. No differences were evident on the cortisol awakening response (CAR).
Group-based trajectory modeling indicated that a subgroup (25%) of ASD children demonstrated an attenuated linear decline while the cortisol trajectory of the second subgroup was indistinguishable from that of the TD group.
Intraclass correlations indicated that, when aggregated across days, cortisol measures were generally stable over the interval assessed.
There were few significant relations between cortisol measures or sub-groups and measures of stress, temperament, and symptoms.
Results encourage follow-up studies to investigate the functional significance, heterogeneity and longer-term stability of diurnal cortisol profiles in children with ASD.
Acknowledgments
This work was supported by NIMH grant R01 MH085717 (Corbett), EKS NICHD Grant U54 HD083211, and NICHD grant P30 HD1505 awarded to the Vanderbilt Kennedy Center.
Funding
Neither funding source had involvement in the study design, collection, analysis or interpretation of data, the writing of the report, or the decision to submit the article for publication.
Footnotes
Conflicts of interest
None
Contributors
Andrew Tomarken: I contributed to the data analysis and the preparation of the article
Gloria Han: I contributed to the data analysis and the preparation of the article
Blythe Corbett: I designed the study, oversaw data collection, and contributed to the preparation of the article.
All authors have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Manual for the Child Behavior Checklist/2–3 and 1992 Profile. University of Vermont Department of Psychiatry; 1992. [Google Scholar]
- Anders TF. Biological rhythms in development. Psychosomatic Medicine. 1982;44(1):61–72. doi: 10.1097/00006842-198203000-00008. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, D.C: American Psychiatric Association; 2013. (DSM-5) [Google Scholar]
- Arrandale V, Koehoorn M, MacNab Y, Kennedy SM. How to use SAS Proc Traj and SAS Proc Glimmix in respiratory epidemiology. School of Occupational and Environmental Hygiene, University of British Columbia; 2006. [Google Scholar]
- Bauer DJ, Curran PJ. Distributional assumptions of growth mixture models: Implications for overextraction of latent trajectory classes. Psychological Methods. 2003;8:338–363. doi: 10.1037/1082-989X.8.3.338. [DOI] [PubMed] [Google Scholar]
- Bellini S. The development of social anxiety in adolescents with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2006;21(3):138–145. [Google Scholar]
- Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, Van den Berghe G. Reduced cortisol metabolism in critical illness. New England Journal of Medicine. 2013;386:1477–1488. doi: 10.1056/NEJMoa1214969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan M, Turner-Cobb J, Munro-Naan Z, Jessop D. Absence of a normal cortisol awakening response (CAR) in adolescent males with Asperger syndrome (AS) Psychoneuroendocrinology. 2009;34(7):1095–1100. doi: 10.1016/j.psyneuen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Chi P, Slatcher RB, Li X, Zhao J, Zhao G, Ren X, Zhu J, Stanton B. Perceived stigmatization, resilience, and diurnal cortisol rhythm among children of parents living with HIV. Psychological Science. 2015;26:843–852. doi: 10.1177/0956797615572904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R, Johnson W, Branscum A, Hanson TE. Bayesian ideas and data analysis: An introduction for scientists and statisticians. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- Corbett BA, Mendoza S, Abdullah M, Wegelin JA, Levine S. Cortisol circadian rhythms and response to stress in children with autism. Psychoneuroendocrinology. 2006;31(1):59–68. doi: 10.1016/j.psyneuen.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Mendoza S, Wegelin JA, Carmean V, Levine S. Variable cortisol circadian rhythms in children with autism and anticipatory stress. Journal of Psychiatry and Neuroscience. 2008;33(3):227–234. [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW. The cortisol awakening response (CAR) in male children with autism spectrum disorder. Hormones and Behavior. 2014;65(4):345–350. doi: 10.1016/j.yhbeh.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Levine S, Mendoza S. Comparing cortisol, stress and sensory sensitivity in children with autism. Autism Research. 2009;2:32–39. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutschbein T, Broecke-Preuss M, Flitsch J, Jaegar A, Althoff R, Walz MK, Mann K, Petersenn S. Salivary cortisol as a diagnostic tool for Cushing’s syndrome and adrenal insufficiency: Improved screening by an automatic immunoassay. European Journal of Endocrinology. 2012;166:613–618. doi: 10.1530/EJE-11-0945. [DOI] [PubMed] [Google Scholar]
- Do DP, Diez Roux AV, Hajat A, Auchincloss AH, Merkin SS, Ranjit N, Shea S, Seeman T. Circadian rhythm of cortisol and neighborhood characteristics in a population-based sample: The multi-ethnic study of atherosclerosis. Health Place. 2011;17:625–632. doi: 10.1016/j.healthplace.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. Short Sensory Profile. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Dykens EM, Lambert W. Trajectories of diurnal cortisol in mothers of children with autism and other developmental disabilities: Relations to health and mental health. Journal of Autism and Developmental Disorders. 2013;43:2426–2434. doi: 10.1007/s10803-013-1791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72(1):67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Galecki AT. General class of correlation structures for two or more repeated factors in longitudinal data analysis. Communications in Statistics-Theory and Methods. 1994;23(1):3105–3119. [Google Scholar]
- Gotham K, Bishop SL, Hus V, Huerta M, Lund S, Buja A, Lord C. Exploring the relationship between anxiety and insistence on sameness in autism spectrum disorders. Autism Research. 2013;6(1):33–41. doi: 10.1002/aur.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D, Weisz J, Ikeda J, McCracken S, Douglas P. Reciprocal influences among adrenocortical activation, psychosocial processes, and the behavioral adjustment of clinic-referred children. Child Development. 1996;67:3250–3262. [PubMed] [Google Scholar]
- Groden J, Diller A, Bausman M, Velicer W, Norman G, Cautela J. The development of a Stress Survey Schedule for persons with autism and other developmental disabilities. Journal of Autism and Developmental Disorders. 2001;31(1):207–217. doi: 10.1023/a:1010755300436. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Marvinney D, Isensee J, Fisch RO. Coping with uncertainty: New models of the relation between hormonal, behavioral, and cognitive processes. In: Palermo D, editor. Coping with uncertainty: Biological, behavioral, and developmental perspectives. Hillsdale, NJ: Erlbaum; 1988. pp. 101–130. [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Steptoe A, Kumari M. Association of diurnal patterns in salivary cortisol with Type 2 Diabetes in the Whitehall II study. Journal of Clinical Endocrinology and Metabolism. 2014;99:4625–4631. doi: 10.1210/jc.2014-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebron J, Humphrey N. Exposure to bullying among students with autism spectrum conditions: A multi-informant analysis of risk and protective factors. Autism. 2014;18:618–630. doi: 10.1177/1362361313495965. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Heuser I, Yassouridis A, Holsboer R. The combined dexamethasone/CRH test: A refined laboratory test for psychiatric disorders. Journal of Psychiatric Research. 1994;28:341–356. doi: 10.1016/0022-3956(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hill SD, Wagner EA, Shedlarski JG, Jr, Sears SP. Diurnal cortisol and temperature variation of normal and autistic children. Developmental Psychobiology. 1977;10(6):579–583. doi: 10.1002/dev.420100612. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yokoyama F, Watanabe M, Murata S, Kaneko M, Kumashiro H. The diurnal variation and response to dexamethasone suppression test of saliva cortisol level in autistic children. Japanese Journal of Psychiatry & Neurology. 1987;41(1):227–235. doi: 10.1111/j.1440-1819.1987.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Jeste SS, Geschwind DH. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nature Reviews Neurology. 2014;10:74–81. doi: 10.1038/nrneurol.2013.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin DS. Advances in group-based trajectory modeling and an SAS procedure for estimating them. Sociological Methods and Research. 2007;35:542–571. [Google Scholar]
- Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Kaas RE, Wasserman L. A reference Bayesian test for nested hypotheses and its relationship to the Schwarz Criterion. Journal of the American Statistical Association. 1995;90:928–934. [Google Scholar]
- Kidd SA, Corbett BA, Granger DA, Boyce WT, Anders TF, Tager IB. Daytime secretion of salivary cortisol and alpha-amylase in preschool-aged children with autism and typically developing children. Journal of Autism and Developmental Disorders. 2012;42(12):2648–2658. doi: 10.1007/s10803-012-1522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol and all-cause and cardiovascular mortality: Findings from the Whitehall II study. Journal of Endocrinology and Metabolism. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert PC, Sutton AJ, Burton PR, Abrams KR, Jones DR. How vague is vague? A simulation study of the impact of the use of vague prior distributions in MCMC using WinBUGS. Statistics in Medicine. 2005;24:2401–2428. doi: 10.1002/sim.2112. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Yeung PK. Heterogeneity within autism spectrum disorders: What have we learned from neuroimaging studies? Frontiers in Human Neuroscience. 2013;7:Article 733. doi: 10.3389/fnhum.2013.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene S, Coe C, Wiener SG. Psychoneuroendocrinology of stress: A psychobiological perspective. In: Brush FR, Levine S, editors. Psychoneuroendocrinology. New York: Academic Press; 1989. pp. 341–377. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2. SAS Institute Inc; Cary NC: 2006. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Marinovic-Curin J, Marinovic-Terzic I, Bujas-Petkovic Z. Slower cortisol response during ACTH stimulation test in autistic children. European Child & Adolescent Psychiatry. 2008;17(1):39–43. doi: 10.1007/s00787-007-0632-1. [DOI] [PubMed] [Google Scholar]
- Michaud K, Matheson K, Kelly O, Anisman H. Impact of stressors in a natural context on release of cortisol in healthy adult humans: A meta-analysis. Stress. 2008;11:177–197. doi: 10.1080/10253890701727874. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenal axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Morita H, Isomura Y, Mune T, Daido H, Takami R, Yamakita N, Ishizuki T, Takeda N, Yasuda K, Gomez-Sanchez CE. Plasma cortisol and cortisone concentrations in normal subjects and patients with adrenocortical disorders. Metabolism. 2004;53:89–94. doi: 10.1016/j.metabol.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Nagin D. Group-based Modeling of Development. Cambridge, MA: Harvard University Press; 2005. [Google Scholar]
- Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annual Review of Clinical Psychology. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. Journal of Autism and Developmental Disorders. 1995;25:641–654. doi: 10.1007/BF02178193. [DOI] [PubMed] [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric Theory. 3. New York: McGraw-Hill; 1994. [Google Scholar]
- Papanicolaou DA, Mullen N, Kyrou I, Nieman LK. Nighttime salivary cortisol: A useful test for the diagnosis of Cushing’s syndrome. Journal of Clinical Endocrinology and Metabolism. 2002;87:4515–4521. doi: 10.1210/jc.2002-020534. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. Journal of Clinical Endocrinology and Metabolism. 1998;83:2681–2686. doi: 10.1210/jcem.83.8.4936. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–164. [Google Scholar]
- Reynolds AM, Malow BA. Sleep and autism spectrum disorders. Pediatric Clinics of North America. 2011;58(3):685–698. doi: 10.1016/j.pcl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Prior MR. Urinary cortisol circadian rhythm in a group of high-functioning children with autism. J Autism Dev Disord. 1992;22(3):433–447. doi: 10.1007/BF01048245. [DOI] [PubMed] [Google Scholar]
- Richdale AL, Baker E, Short M, Gradisar M. The role of insomnia, pre-sleep arousal and psychopathology symptoms in daytime impairment in adolescents with high-functioning autism spectrum disorder. Sleep medicine. 2014;15(9):1082–1088. doi: 10.1016/j.sleep.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Ross KM, Murphy MLM, Adam ED, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson RJ, Laub JH, Eggleston EP. On the robustness and validity of groups (response to Daniel Nagin) Journal of Quantitative Criminology. 2004;20:37–42. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. The Neuroendocrinology of Stress and Aging: The Glucocorticoid Cascade Hypothesis. Endocrine reviews. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- Schupp CW, Simon D, Corbett BA. Cortisol responsivity differences in children with autism spectrum disorders during free and cooperative play. Journal of Autism and Developmental Disorders. 2013;43:2405–2417. doi: 10.1007/s10803-013-1790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating dimensions of a model. Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Seltzer BM, Greenberg JS, Hong J, Smith LE, Almeida DM, Coe C, Stawski RS. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. Journal of Autism and Developmental Disorders. 2010;40:457–469. doi: 10.1007/s10803-009-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain, Behavior, and Immunity. 2013;30:S163–S170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Developmental Psychobiology. 2012;54:493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86(1):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ. Bayesian methods for cluster randomized trials with continous responses. Statistics in Medicine. 2001;20:435–452. doi: 10.1002/1097-0258(20010215)20:3<435::aid-sim804>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Edwards CD. State-Trait Inventory for Children: STAIC: How I Feel Questionnaire: Professional Manual. Mind Garden; 1973. [Google Scholar]
- Strube MJ, Newman LC. Psychometrics. In: Cacioppo JT, Tassinary LG, Berntsen G, editors. Handbook of Psychophysiology. Vol. 3. New York: Cambridge University Press; 2007. pp. 789–811. [Google Scholar]
- Taylor JL, Corbett BA. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology. 2014;49:207–228. doi: 10.1016/j.psyneuen.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RM, Omar RZ, Thompson SG. Constructing intervals for the intraclass correlation coefficient using Bayesian modelling, and application in cluster randomized trials. Statistics in Medicine. 2006;25:1443–1456. doi: 10.1002/sim.2304. [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. 3. Springer Series in Statistics; New York: 2009. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. Jounral of Clinical Endocrinology Metabolism. 1971;33(1):14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Westfall PH, Tobias RD, Wolfinger RD. Multiple comparisons and multiple tests using SAS. SAS Institute; 2011. [Google Scholar]
- Wilcox RR. The percentage bend correlation coefficient. Psychometrika. 1994;59:601–616. [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2(7):79–88. [PubMed] [Google Scholar]
- Yamazaki K, Saito Y, Okada F, Fujieda T, Yamashita I. An application of neuroendocrinalogical studies in autistic children and Heller’s syndrome. Journal of Autism and Childhood Schizophrenia. 1975;5(4):323–332. doi: 10.1007/BF01540679. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with post-traumatic stress disorder. American Journal of Psychiatry. 1995;152:982–986. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- Zinke K, Fries E, Kliegel M, Kirschbaum C, Dettenborn L. Children with high-functioning autism show a normal cortisol awakening response (CAR) Psychoneuroendocrinology. 2010;35(10):1578–1582. doi: 10.1016/j.psyneuen.2010.03.009. [DOI] [PubMed] [Google Scholar]