Abstract
Liver transplantation has become the treatment of choice for acute or chronic liver disease. Because the liver acts as an innate immunity-dominant organ, there are immunological differences between the liver and other organs. The specific features of hepatic natural killer (NK), NKT and Kupffer cells and their role in the mechanism of liver transplant rejection, tolerance and hepatic ischemia-reperfusion injury are discussed in this review.
Keywords: Liver transplantation, Natural killer cells, Kupffer cells, Graft rejection, Ischemia-reperfusion injury
Core tip: Liver transplantation has become the treatment of choice for acute or chronic liver disease. There are immunological differences between the liver and other organs. The specific features of selected hepatic immune cells, such as natural killer (NK), NKT and Kupffer cells, and their role in the mechanism of liver transplant rejection, tolerance and hepatic ischemia-reperfusion injury are discussed in this review.
INTRODUCTION
Previous studies have intensively investigated immunological processes after liver transplantation. Ischemia-reperfusion injury and graft rejection are two major causes for poor outcomes following liver transplantation. Both processes are triggered and maintained by immune cells. The specific features of hepatic natural killer (NK), NKT and Kupffer cells and their role in the mechanism of liver transplant rejection and hepatic ischemia-reperfusion injury based on the current literature are discussed in this review.
LIVER TRANSPLANTATION AND IMMUNOLOGICAL PROCESSES
During the last 50 years liver transplantation has become the treatment of choice for acute or chronic liver disease[1]. The main indications for liver transplantations are primary liver tumors, chronic viral hepatitis, alcohol-related cirrhosis, chronic cholestatic liver disease, autoimmune hepatitis, vascular and metabolic disorders[2,3]. With an overall 5-year survival of approximately 70%, the life expectancy of liver transplant recipients is lower than the general population[4,5]. In addition to de novo malignancies, infections, cardiovascular or renal disease, ischemia-reperfusion-injury of the liver graft and graft rejection are important immunological processes responsible for long-term graft and patient survival after liver transplantation.
The liver acts as an innate immunity-dominant organ, therefore, hepatic immune cells provide the first line of defense against pathogens, infections or tumors[6]. In addition to NK cells, macrophages (Kupffer cells), NKT cells and γδT cells, there are a large number of innate immune cells within the liver[7,8]. In humans, NK cells are the most abundant lymphocyte population in the liver[9].
Two specific and immunologically important processes occur after liver transplantation: (1) donor liver-resident cells enter the blood flow of the recipient; and (2) recipient immune cells invade the donor graft. This phenomenon occurs early after transplantation[10-12]. It has been shown, that after liver transplantation, donor specific liver NK cells are detectable in the recipients’ circulation up to two weeks after liver transplantation[1,12]. The liver has been described as an immunotolerant organ[6,13]. This immunotolerance is believed to be responsible for the lower levels of immunosuppressive drugs needed and the lower rate of allograft rejection after liver transplantation compared to other solid organ transplantations[1,14]. This is reflected by the withdrawal of immunosuppression, in some cases, after liver transplantation, and the aim to wean patients from immunosuppressive drugs as soon as possible[1,15]. In addition, it has been shown that hepatic grafts might facilitate the acceptance or reverse the rejection of other transplanted grafts, e.g., heart or kidney after liver transplantation[16].
MECHANISM OF GRAFT REJECTION
Acute graft rejection is a combined response of the adaptive (cellular immunity) and humoral immune system (secreted antibodies by activated B cells) in combination with the innate immune system (phagocytosis). Furthermore, early organ rejection can be distinguished from late organ rejection. Wiesner et al[17] suggested the following risk factors: lower recipient age, cold ischemia duration longer than 15 h, donor age and fewer human leukocyte antigen (HLA)-DR matches. T cells were believed to be solely responsible for graft rejection. However, there is increasing evidence that other cells of the adaptive immune system, such as NK cells, are also responsible and interact with T cells during graft rejection[1,18,19]. In contrast to other solid organ transplantations, HLA cross-matching is not routinely performed prior to liver transplantation despite recent studies suggesting HLA markers, such as killer cell immunoglobulin-like receptors, influence the outcome of liver grafts[20-22]. To date, clinical experience, analysis of immunosuppressive drug levels, serum liver enzymes and histological assessment have been used as markers to diagnose graft rejection[23]. During acute graft rejection, mononuclear cells infiltrate the portal tract and the accumulation of activated lymphocytes leads to the secretion of chemokines and cytokines and subsequently, liver tissue injury[24]. Furthermore, bile duct injuries and venous endotheliitis are histological features for the diagnosis of graft rejection[25]. Although the exact chemotactic triggers are still under investigation, it is postulated that for NK cells, CCL3 leads to NK cell migration to the site of liver injury[26-28].
MECHANISMS OF HEPATIC ISCHEMIA-REPERFUSION INJURY
During organ donation and transplantation, the liver undergoes trauma due to cold and non-perfused storage, warm ischemia and finally, engraftment. During ischemia-reperfusion liver injury, one important issue is organ preservation, which is initially triggered by endothelial cell injury and causes an acute inflammatory response that involves Kupffer cells, hepatocytes and hepatic stellate cells[29]. Furthermore, cell death is caused by oxidative stress, which leads to increased microcirculatory disturbances, cell dysfunction and inflammation[30,31]. To avoid organ damage due to organ preservation, several modifications have been investigated, such as perfusion solutions[32-34], use of antioxidants[35], vasodilators[36,37], hydrogen gas[38], or ex-vivo liver perfusion systems[39-41]. Ischemia-reperfusion injury is crucial for initial and long-term organ function[42]. Hepatic ischemia-reperfusion injury is associated with an inflammatory response, which leads to liver tissue injury, the release of reactive oxygen species (ROS), the induction of adhesion molecules, the secretion of cytokines and the activation of leukocytes[43]. In addition, several immune cells, such as T cells, B cells, NK cells, NKT cells, and Kupffer cells, are involved in hepatic ischemia-reperfusion injury[44-51], which affect liver-specific cells, such as sinusoidal endothelial cells and hepatocytes[52]. During cell injury and necrosis, danger-associated molecular patterns (DAMP) and, subsequently, pathogen-associated molecular patterns (PAMP) are released and trigger an immune response[53,54]. Tissue ischemia leads to mitochondrial dysfunction, ATP depletion, and ionic changes within the cells, which promotes further cell damage and organ dysfunction (Figure 1)[52].
Figure 1.
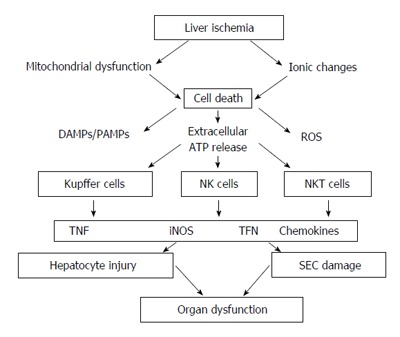
Simplified overview of the role of Kupffer cells, natural killer and natural killer T cells, including the humoral and cellular factors, involved in hepatocyte dysfunction and injury during hepatic ischemia-reperfusion injury. DAMPs: Damage associated molecular pattern; PAMPs: Pathogen-associated molecular pattern; ROS: Reactive oxygen species; TNF: Tumor necrosis factor; IFN: Interferon; iNOS: Inducible nitric oxide synthase; SEC: Sinusoidal endothelial cells.
HEPATIC NK CELLS
There is growing evidence that peripheral NK cells differ from hepatic NK cells with regard to function and differentiation; however, the exact mechanism of NK cell differentiation and maturation in the liver is not completely understood.
NK cells are the major lymphocyte population in the human liver and make up to 50% of the lymphocyte population. During liver disease, the number of NK cells in the liver changes possibly due to increased recruitment of NK cells to the liver[9,55]. A diverse range of receptors expressed on the surface of NK cells allows them to recognize and rapidly respond to damaged or stressed cells. Furthermore, NK cells coordinate early events in the innate immune response to injury by rapidly producing cytokines and controlling cytotoxic activity.
Human NK cells in the blood can be distinguished from other T cells by the absence of CD3 and the presence of CD56[56,57]. Furthermore, NK cells in the blood can be further differentiated into two major subsets: CD3-CD56dimCD16+CD27- (cytotoxic activity) and CD3-CD56brightCD16-CD27+ (cytokine producing)[6]. Bone marrow-derived NK precursor cells undergo a complex maturation process, which determines their function and the expression of chemokine receptors and adhesion molecules[6,58-60]. This determination is organ specific[6,61]. Because NK cells recirculate between different organs, the maturation process is dynamic and not stationary[58]. Adoptively transferred splenic NK cells change their phenotypic and functional markers after migrating to the liver, which suggests a modification of NK cells due to the hepatic microenvironment[62]. In contrast to peripheral NK cells, hepatic NK cells lack CD16[63,64], express higher numbers of granules, and express higher levels of TRAIL, perforin, and granzyme B[65].
NK cells can potentially lyse dividing hepatocytes and/or other immune cells within the liver that contribute to the cytokine and chemokine microenvironment during regeneration and liver injury[66,67]. NK cells actively eliminate susceptible targets through multiple, non-redundant mechanisms and recruit and amplify the inflammatory response[68]. Because NK cells are closely linked to other immune cells, they are associated with Kupffer cells in the liver sinusoids, which suggests a complex interaction between these two cell types that involves cytokine and chemokine secretion[69,70].
HEPATIC NATURAL KILLER T CELLS
Natural killer T (NKT) cells are a subset of regulatory T lymphocytes[71]. In contrast to NK cells, NKT cells are found less frequently in the liver[60], and their ultrastructure contains a low nuclear:cytoplasmic ratio and dense granules compared to NK cells[72]. Therefore, NKT cells are less mature and have only a few organelles and mitochondria and short profiles of the rough endoplasmic reticulum[72]. Compared to NK cells, the granules of NKT contain perforin, but are smaller in size and less frequently observed using electron microscopy[73,74]. Interestingly, NKT cells have comparable functions with T cells, and NK cells and are able to secrete large amounts of cytokines[72]. Similar to other immune cells, NKT cells are located within the liver sinusoids and are responsible for killing tumor cells, secretion of cytokines and elimination of toxins and pathogens[60,75]. In addition, activated NKT cells are important for inducing liver injury[76-78]. In contrast to NK cells, the number of NKT cells decreases during various experimental models, such as in leptin-deficient mice[79], bacterial liver injury[80], hepatotoxic liver injury[81,82], liver steatosis[83,84], and Concanavalin A-induced liver injury[85]. However, following liver transplantation[24], hepatic ischemia-reperfusion injury[43,44], liver resection[86-89] or stress[90], the number of hepatic NKT cells increase. This change in cell number has been postulated to be due to activation-induced cell death, loss of specific NKT cell surface markers[26,76,91,92], apoptosis[93] or sympathetic activation[89]. Flow cytometry analysis of hepatic NKT cells shows that they are mostly CD4-CD8- or CD4+CD8-[94] and express the NK cell receptor-CD161 and the invariant TCR-alpha chain[95]. NKT cells express IL-12 receptors and secret and produce perforin and interferon (IFN)[96] after stimulation, which are key mediators of cytotoxicity, inhibition of tumor angiogenesis and immune cell activation[97,98]. Furthermore, NKT cells produce anti-inflammatory and anti-tumorigenic cytokines such as IL-13 and IL-4[99-101].
KUPFFER CELLS
In 1876, von Kupffer first identified liver resident macrophages[102]. These macrophages are colocalized with sinusoidal endothelial cells, Ito cells, and pit cells in the hepatic sinusoids[103]. Kupffer cells are abundant in the liver and make up more than 50% of all resident macrophages in the human body and 15% of all hepatic cells[104,105]. Depending on their location within the liver, the function, morphology and number of Kupffer cells changes[103,106,107]. Interestingly, the intensity of immunohistochemical markers for Kupffer cells is heterogeneous. In general, the intensity of these markers decreases as the size of Kupffer cells decreases, which reflects a more immature phenotype that involves more scavenging and less inflammatory functions[103,107]. The main function of hepatic macrophages is to clear the portal circulation from foreign materials and pathogens using phagocytosis[103,108]. During this process, Kupffer cells release pro-inflammatory cytokines such as IL-1, IL-6, IL-12, IL-18, TNF and IFN[109].
SPECIFIC FUNCTION OF IMMUNE CELLS IN HEPATIC ISCHEMIA-REPERFUSION
Ischemia-reperfusion injury (IRI) significantly contributes to graft dysfunction after liver transplantation[110]. Ischemia during the early phase of IRI leads to cell necrosis, which is associated with a release of danger signals that activate innate immune cells through signaling of TLR4, RAGE and TLR9 on Kupffer cells and through signaling of the CD154-CD40 pathway on neutrophils and CD4 Th1 effector T cells[42]. This immune activation is further increased through the release of IFN from T cells, NKT and NK cells, which are stimulated by CD1d and CD39. Pro- and anti-inflammatory mediators further activate and recruit immune cells, which promotes or inhibits local inflammation[42].
NK cells express tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), which is a potent inducer of hepatocyte cell death. In an experimental study, the effect of TRAIL expression on NK cells during hepatic IRI was investigated and showed that mice lacking TRAIL exhibited significantly higher liver injury, signs of necrosis, and neutrophil infiltration[47]. The adoptive transfer of NK cells into immunodeficient RAG2/common gamma null mice (lacking T, B and NK cells) revealed the specific role of NK cells during IRI and showed that the expression of TRAIL on NK cells is protective in a murine model of hepatic IRI[47]. In another study, the effect of CD39, an ectonucleotidase hydrolyzing extracellular nucleotides, on NK cells was investigated and revealed that NK cells have an important influence on the extent of hepatic IRI. This effect was based on the modulation in IFN secretion, which was regulated by pericellular ATP levels and purinergic responses[48]. Furthermore, it is postulated that liver resident NK cells are responsible for the innate immune response in the early phase of IRI through self/non-self-recognition[49].
There are two types of NKT cells that have opposing roles during IRI to promote or protect against liver injury[50]. During the early phase of IRI, NKT cells are promptly activated and release IFN[50]. This activation is mediated by the interaction of CD1d antigen-presenting molecules, which are expressed on antigen-presenting cells in the liver and on hepatocytes containing self or foreign glycolipid antigens[43,111]. NKT cells are then able to damage hepatocytes directly or through the secretion of IFN, which in turn activates Kupffer cells, neutrophils and hepatocytes[43,111]. Knockout models with reduced NKT activity result in significantly reduced IRI[43,44,111]. In addition, the recruitment of NK cells into the liver during IRI is dependent on the presence and activation of NKT cells[50].
Hepatic hypoxia electron microscopy analysis revealed morphological changes in Kupffer cells that reflected cell activation[112] and a release of cytokines and inflammatory mediators to attract neutrophils and produce reactive oxygen species[113,114]. This activation is triggered by endogenous damage-associated and/or pathogen-associated molecular pattern (DAMP/PAMP) molecules, which are generated during cellular stress or cellular injury[42]. During IRI, TLR4 on Kupffer cells is activated, which leads to hepatic injury[115]. Activation of TLR4 enhances TNF secretion probably through an antigen independent pathway[115,116] and is further associated with hepatocyte apoptosis[117,118], CD4+ T cell recruitment to the liver[119], and the release of endothelin-1, which results in circulatory disturbance and increased liver injury[120,121]. Activation of the complement system is present during IRI[122] and responsible for Kupffer cell-induced oxidant stress, the formation of reactive oxygen species and continuous neutrophil recruitment to the ischemic liver[123]. Furthermore, inducible nitric oxide synthase (iNOS), which is produced by Kupffer cells and neutrophils early during hepatic IRI, leads to reduced capillary perfusion, increased liver injury and mortality[124,125]. Activated Kupffer cells enhance alterations in hepatic microcirculation during IRI through the activation and production of oxygen free radicals[126], TNF, MIP-2 and keratinocyte chemoattractant chemokine, which leads to increased liver injury[127,128].
NK CELLS, NKT CELLS AND KUPFFER CELLS DURING GRAFT REJECTION AND TOLERANCE INDUCTION
It is postulated that the rejection of solid organ grafts is mainly mediated by allospecific T lymphocytes. These T lymphocytes recognize foreign MHC molecules that are located on donor tissue cells[18,19]. However, it has been shown, that the depletion of CD8+ T cells does not prevent graft rejection and an alternative pathway of organ rejection has been postulated[129,130]. Several studies using different experimental transplantation models have investigated the role of NK cells during graft rejection and demonstrated NK cell graft infiltration[131-135]. Additionally, it has been shown that recipient-derived NK cells are located in the liver graft and produce IFN after liver transplantation[24]. The depletion of NK cells or the decrease in IFN production leads to increased graft survival, therefore, NK cells are for graft rejection and survival[24]. IFN, an immunoregulatory cytokine that is one of the main cytokines secreted of NK cells, has been shown to be important during both allograft rejection[136-138] and tolerance induction[139]. Studies investigating immunosuppression withdrawal demonstrated that NK cells play a role in tolerance induction[140]. In addition, 13 genes that are highly expressed in NK cells, were found to be present in liver transplant recipients with graft tolerance, which further confirms that NK cells are involved in tolerance induction[141]. Although this conflicting role of NK cells is still not fully understood, it might explain why donor NK cells are responsible for tolerance and recipient NK cells are responsible for rejection[1]. In addition to cytokines, chemokines, such as CCL2, CCL3, CX3CL1 or CXCL10, attract and activate NK cells. Some of these chemokines are already present in the transplanted graft before NK cell infiltration is detectable[142]. Specific analysis of NK cells in the rejected liver graft revealed that these NK cells produce high amounts of cytokines, granzyme B and highly express FasL[135].
NKT cells are believed to be responsible for tolerance induction[71]. Because activated NKT cells release pro- and anti-inflammatory cytokines, they have different functions in immune response[143-145]. It has been further shown, that specific Vα14 NKT cells are responsible for the development of tolerance towards transplanted antigens[145].
As stated above, the main function of Kupffer cells is to kill and engulf microorganisms and pathogens, secrete cytokines and effect antigen presentation[146,147]. Additionally, it has been shown, that Kupffer cells are able to induce T cell apoptosis and therefore play an important role during graft tolerance[148]. After liver transplantation Kupffer cells act as antigen-presenting cells by increasing the expression of MHC class II[149,150] and identifying and interacting with recipient T cells migrating to the liver, which leads to T cell apoptosis through the Fas/FasL pathway[109]. In a study in rats, pretreatment of the recipients with Kupffer cells before liver transplantation lead to decreased liver injury, reduced cytokine levels and reduced apoptosis. The authors concluded that this lead to increased immune tolerance and improved graft survival[148]. As mentioned above, Kupffer cells secret varying amounts of cytokines, such as TNF, which in high levels can lead to hepatocyte apoptosis but in physiological levels is associated with a resistance of hepatocytes to apoptosis[151]. Therefore, further studies are necessary to elucidate the contrasting roles of Kupffer cells in the induction of immune tolerance following liver transplantation.
CONCLUSION
Several specific immune reactions that involve NK, NKT and Kupffer cells are responsible for the short- and long-term outcomes of liver transplantation. This review demonstrates that many immune cells and mediators as well as molecular signaling cascades participate in the process of liver transplantation tolerance. Despite intense research within the field of ischemia-reperfusion injury, there are still many pathophysiological and immunological mechanisms involved in tolerance induction and graft rejection that still need to be elucidated.
Footnotes
Conflict-of-interest statement: All authors have no conflicts of interest or financial ties to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 24, 2016
First decision: May 12, 2016
Article in press: June 15, 2016
P- Reviewer: Topaloglu S S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Harmon C, Sanchez-Fueyo A, O’Farrelly C, Houlihan DD. Natural Killer Cells and Liver Transplantation: Orchestrators of Rejection or Tolerance? Am J Transplant. 2016;16:751–757. doi: 10.1111/ajt.13565. [DOI] [PubMed] [Google Scholar]
- 2.Holstege A. [Indications for liver transplantation in chronic liver diseases] Z Gastroenterol. 2002;40:891–902. doi: 10.1055/s-2002-35256. [DOI] [PubMed] [Google Scholar]
- 3.Prince MI, Hudson M. Liver transplantation for chronic liver disease: advances and controversies in an era of organ shortages. Postgrad Med J. 2002;78:135–141. doi: 10.1136/pmj.78.917.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Cailliez V, Majno P, Karam V, McMaster P, Caine RY, O’Grady J, Pichlmayr R, Neuhaus P, Otte JB, et al. Normalised intrinsic mortality risk in liver transplantation: European Liver Transplant Registry study. Lancet. 2000;356:621–627. doi: 10.1016/s0140-6736(00)02603-9. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR) J Hepatol. 2012;57:675–688. doi: 10.1016/j.jhep.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Sun H, Sun C, Tian Z, Xiao W. NK cells in immunotolerant organs. Cell Mol Immunol. 2013;10:202–212. doi: 10.1038/cmi.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kita H, Mackay IR, Van De Water J, Gershwin ME. The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology. 2001;120:1485–1501. doi: 10.1053/gast.2001.22441. [DOI] [PubMed] [Google Scholar]
- 8.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 9.Yamagiwa S, Kamimura H, Ichida T. Natural killer cell receptors and their ligands in liver diseases. Med Mol Morphol. 2009;42:1–8. doi: 10.1007/s00795-008-0434-7. [DOI] [PubMed] [Google Scholar]
- 10.Navarro F, Portalès P, Pageaux JP, Perrigault PF, Fabre JM, Domergue J, Clot J. Activated sub-populations of lymphocytes and natural killer cells in normal liver and liver grafts before transplantation. Liver. 1998;18:259–263. doi: 10.1111/j.1600-0676.1998.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 11.Heerwagen C, Schuster M, Bornscheurer A, Pape L, Kirchner E, Schlitt HJ, Luettig B, Westermann J. Rapid exchange of large numbers of donor- and host leukocytes after human liver transplantation. Transpl Int. 2001;14:240–247. doi: 10.1007/s001470100323. [DOI] [PubMed] [Google Scholar]
- 12.Moroso V, Metselaar HJ, Mancham S, Tilanus HW, Eissens D, van der Meer A, van der Laan LJ, Kuipers EJ, Joosten I, Kwekkeboom J. Liver grafts contain a unique subset of natural killer cells that are transferred into the recipient after liver transplantation. Liver Transpl. 2010;16:895–908. doi: 10.1002/lt.22080. [DOI] [PubMed] [Google Scholar]
- 13.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Demirkiran A, Kok A, Kwekkeboom J, Kusters JG, Metselaar HJ, Tilanus HW, van der Laan LJ. Low circulating regulatory T-cell levels after acute rejection in liver transplantation. Liver Transpl. 2006;12:277–284. doi: 10.1002/lt.20612. [DOI] [PubMed] [Google Scholar]
- 15.Lerut J, Sanchez-Fueyo A. An appraisal of tolerance in liver transplantation. Am J Transplant. 2006;6:1774–1780. doi: 10.1111/j.1600-6143.2006.01396.x. [DOI] [PubMed] [Google Scholar]
- 16.Carambia A, Herkel J. CD4 T cells in hepatic immune tolerance. J Autoimmun. 2010;34:23–28. doi: 10.1016/j.jaut.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Wiesner RH, Demetris AJ, Belle SH, Seaberg EC, Lake JR, Zetterman RK, Everhart J, Detre KM. Acute hepatic allograft rejection: incidence, risk factors, and impact on outcome. Hepatology. 1998;28:638–645. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 18.Krensky AM, Weiss A, Crabtree G, Davis MM, Parham P. T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med. 1990;322:510–517. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- 19.Sayegh MH, Turka LA. The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med. 1998;338:1813–1821. doi: 10.1056/NEJM199806183382506. [DOI] [PubMed] [Google Scholar]
- 20.Moya-Quiles MR, Muro M, Torío A, Sánchez-Bueno F, Miras M, Marín L, García-Alonso AM, Parrilla P, Dausset J, Alvarez-López MR. Human leukocyte antigen-C in short- and long-term liver graft acceptance. Liver Transpl. 2003;9:218–227. doi: 10.1053/jlts.2003.50043. [DOI] [PubMed] [Google Scholar]
- 21.Hanvesakul R, Spencer N, Cook M, Gunson B, Hathaway M, Brown R, Nightingale P, Cockwell P, Hubscher SG, Adams DH, et al. Donor HLA-C genotype has a profound impact on the clinical outcome following liver transplantation. Am J Transplant. 2008;8:1931–1941. doi: 10.1111/j.1600-6143.2008.02341.x. [DOI] [PubMed] [Google Scholar]
- 22.López-Alvarez MR, Gómez-Mateo J, Ruiz-Merino G, Campillo JA, Miras M, García-Alonso AM, Sánchez-Bueno F, Parrilla P, Alvarez-López MR, Minguela A. Analysis of KIR2D receptors on peripheral blood lymphocytes from liver graft recipients. Transpl Immunol. 2006;17:51–54. doi: 10.1016/j.trim.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Germani G, Rodriguez-Castro K, Russo FP, Senzolo M, Zanetto A, Ferrarese A, Burra P. Markers of acute rejection and graft acceptance in liver transplantation. World J Gastroenterol. 2015;21:1061–1068. doi: 10.3748/wjg.v21.i4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obara H, Nagasaki K, Hsieh CL, Ogura Y, Esquivel CO, Martinez OM, Krams SM. IFN-gamma, produced by NK cells that infiltrate liver allografts early after transplantation, links the innate and adaptive immune responses. Am J Transplant. 2005;5:2094–2103. doi: 10.1111/j.1600-6143.2005.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 26.Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241–247. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- 27.Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1alpha (MIP-1alpha)-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salazar-Mather TP, Hamilton TA, Biron CA. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Invest. 2000;105:985–993. doi: 10.1172/JCI9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 30.Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 2012;26:103–114. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menger MD, Vollmar B. Role of microcirculation in transplantation. Microcirculation. 2000;7:291–306. [PubMed] [Google Scholar]
- 32.Erhard J, Lange R, Scherer R, Kox WJ, Bretschneider HJ, Gebhard MM, Eigler FW. Comparison of histidine-tryptophan-ketoglutarate (HTK) solution versus University of Wisconsin (UW) solution for organ preservation in human liver transplantation. A prospective, randomized study. Transpl Int. 1994;7:177–181. doi: 10.1007/BF00327084. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Andujar R, Deusa S, Montalvá E, San Juan F, Moya A, Pareja E, DeJuan M, Berenguer M, Prieto M, Mir J. Comparative prospective study of two liver graft preservation solutions: University of Wisconsin and Celsior. Liver Transpl. 2009;15:1709–1717. doi: 10.1002/lt.21945. [DOI] [PubMed] [Google Scholar]
- 34.Schoening W, Ariyakhagorn V, Schubert T, Olschewski P, Andreou A, Neuhaus P, Pratschke J, Puhl G. Warm HTK donor pretreatment reduces liver injury during static cold storage in experimental rat liver transplantation. Hepatobiliary Pancreat Dis Int. 2015;14:596–602. doi: 10.1016/s1499-3872(15)60426-x. [DOI] [PubMed] [Google Scholar]
- 35.Yokota R, Fukai M, Shimamura T, Suzuki T, Watanabe Y, Nagashima K, Kishida A, Furukawa H, Hayashi T, Todo S. A novel hydroxyl radical scavenger, nicaraven, protects the liver from warm ischemia and reperfusion injury. Surgery. 2000;127:661–669. doi: 10.1067/msy.2000.105864. [DOI] [PubMed] [Google Scholar]
- 36.Schoening WN, Feige I, Schubert T, Olschewski P, Buescher N, Helbig M, Schmitz V, Neuhaus P, Pratschke J, Puhl G. Iloprost donor treatment reduces ischemia-reperfusion injury in an isolated extracorporeal pig liver perfusion model. Exp Clin Transplant. 2015;13:51–61. [PubMed] [Google Scholar]
- 37.Shimamura T, Zhu Y, Zhang S, Jin MB, Ishizaki N, Urakami A, Totsuka E, Kishida A, Lee R, Subbotin V, et al. Protective role of nitric oxide in ischemia and reperfusion injury of the liver. J Am Coll Surg. 1999;188:43–52. doi: 10.1016/s1072-7515(98)00259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada S, Wakayama K, Fukai M, Shimamura T, Ishikawa T, Fukumori D, Shibata M, Yamashita K, Kimura T, Todo S, et al. Hydrogen Gas Ameliorates Hepatic Reperfusion Injury After Prolonged Cold Preservation in Isolated Perfused Rat Liver. Artif Organs. 2016 doi: 10.1111/aor.12710. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 39.Banan B, Watson R, Xu M, Lin Y, Chapman W. Development of a normothermic extracorporeal liver perfusion system toward improving viability and function of human extended criteria donor livers. Liver Transpl. 2016;22:979–993. doi: 10.1002/lt.24451. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZB, Gao W, Shi Y, Liu L, Ma N, Chen J, Zhu ZJ. Protective role of normothermic machine perfusion during reduced-size liver transplantation in pigs. Liver Transpl. 2016;22:968–978. doi: 10.1002/lt.24453. [DOI] [PubMed] [Google Scholar]
- 41.Knaak JM, Spetzler VN, Goldaracena N, Louis KS, Selzner N, Selzner M. Technique of subnormothermic ex vivo liver perfusion for the storage, assessment, and repair of marginal liver grafts. J Vis Exp. 2014;(90):e51419. doi: 10.3791/51419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhai Y, Busuttil RW, Kupiec-Weglinski JW. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am J Transplant. 2011;11:1563–1569. doi: 10.1111/j.1600-6143.2011.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimamura K, Kawamura H, Nagura T, Kato T, Naito T, Kameyama H, Hatakeyama K, Abo T. Association of NKT cells and granulocytes with liver injury after reperfusion of the portal vein. Cell Immunol. 2005;234:31–38. doi: 10.1016/j.cellimm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 45.Le Moine O, Louis H, Demols A, Desalle F, Demoor F, Quertinmont E, Goldman M, Devière J. Cold liver ischemia-reperfusion injury critically depends on liver T cells and is improved by donor pretreatment with interleukin 10 in mice. Hepatology. 2000;31:1266–1274. doi: 10.1053/jhep.2000.7881. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto S, Sato Y, Shimizu T, Halder RC, Oya H, Bannai M, Suzuki K, Ishikawa H, Hatakeyama K, Abo T. Consistent infiltration of thymus-derived T cells into the parenchymal space of the liver in normal mice. Hepatology. 1999;30:705–713. doi: 10.1002/hep.510300331. [DOI] [PubMed] [Google Scholar]
- 47.Fahrner R, Trochsler M, Corazza N, Graubardt N, Keogh A, Candinas D, Brunner T, Stroka D, Beldi G. Tumor necrosis factor-related apoptosis-inducing ligand on NK cells protects from hepatic ischemia-reperfusion injury. Transplantation. 2014;97:1102–1109. doi: 10.1097/TP.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 48.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, Graubardt N, Rellstab A, Nowak M, Enjyoji K, Li X, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51:1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kimura S, Ozaki KS, Ueki S, Zhang M, Yokota S, Stolz DB, Geller DA, Murase N. Contribution of alloantigens to hepatic ischemia/reperfusion injury: Roles of natural killer cells and innate immune recognition of nonself. Liver Transpl. 2016;22:80–90. doi: 10.1002/lt.24330. [DOI] [PubMed] [Google Scholar]
- 50.Arrenberg P, Maricic I, Kumar V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology. 2011;140:646–655. doi: 10.1053/j.gastro.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, Afrazi A, Gandhi C, Tokita D, Geller DA, et al. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology. 2008;48:1608–1620. doi: 10.1002/hep.22482. [DOI] [PubMed] [Google Scholar]
- 52.Abu-Amara M, Yang SY, Tapuria N, Fuller B, Davidson B, Seifalian A. Liver ischemia/reperfusion injury: processes in inflammatory networks--a review. Liver Transpl. 2010;16:1016–1032. doi: 10.1002/lt.22117. [DOI] [PubMed] [Google Scholar]
- 53.Klune JR, Dhupar R, Cardinal J, Billiar TR, Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–73, Table of Contents. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krueger PD, Lassen MG, Qiao H, Hahn YS. Regulation of NK cell repertoire and function in the liver. Crit Rev Immunol. 2011;31:43–52. doi: 10.1615/critrevimmunol.v31.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inngjerdingen M, Kveberg L, Naper C, Vaage JT. Natural killer cell subsets in man and rodents. Tissue Antigens. 2011;78:81–88. doi: 10.1111/j.1399-0039.2011.01714.x. [DOI] [PubMed] [Google Scholar]
- 57.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grégoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 60.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gotthardt D, Prchal-Murphy M, Seillet C, Glasner A, Mandelboim O, Carotta S, Sexl V, Putz EM. NK cell development in bone marrow and liver: site matters. Genes Immun. 2014;15:584–587. doi: 10.1038/gene.2014.55. [DOI] [PubMed] [Google Scholar]
- 62.Lassen MG, Lukens JR, Dolina JS, Brown MG, Hahn YS. Intrahepatic IL-10 maintains NKG2A+Ly49- liver NK cells in a functionally hyporesponsive state. J Immunol. 2010;184:2693–2701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hata K, Zhang XR, Iwatsuki S, Van Thiel DH, Herberman RB, Whiteside TL. Isolation, phenotyping, and functional analysis of lymphocytes from human liver. Clin Immunol Immunopathol. 1990;56:401–419. doi: 10.1016/0090-1229(90)90160-r. [DOI] [PubMed] [Google Scholar]
- 64.Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, Nolan N, Hegarty J, O’Farrelly C. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 65.Ishiyama K, Ohdan H, Ohira M, Mitsuta H, Arihiro K, Asahara T. Difference in cytotoxicity against hepatocellular carcinoma between liver and periphery natural killer cells in humans. Hepatology. 2006;43:362–372. doi: 10.1002/hep.21035. [DOI] [PubMed] [Google Scholar]
- 66.Campbell JS, Prichard L, Schaper F, Schmitz J, Stephenson-Famy A, Rosenfeld ME, Argast GM, Heinrich PC, Fausto N. Expression of suppressors of cytokine signaling during liver regeneration. J Clin Invest. 2001;107:1285–1292. doi: 10.1172/JCI11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol. 2006;24:257–286. doi: 10.1146/annurev.immunol.24.021605.090700. [DOI] [PubMed] [Google Scholar]
- 69.Fausto N. Liver regeneration. J Hepatol. 2000;32:19–31. doi: 10.1016/s0168-8278(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 70.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 71.Margalit M, Ilan Y. Induction of immune tolerance: a role for Natural killer T lymphocytes? Liver Int. 2005;25:501–504. doi: 10.1111/j.1478-3231.2005.01147.x. [DOI] [PubMed] [Google Scholar]
- 72.van der Vliet HJ, Pinedo HM, von Blomberg BM, van den Eertwegh AJ, Scheper RJ, Giaccone G. Natural Killer T cells. Lancet Oncol. 2002;3:574. doi: 10.1016/s1470-2045(02)00850-1. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanage T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–2983. [PubMed] [Google Scholar]
- 74.Fujikura S, Watanabe H, Abo T, Kaneda K. Ultrastructural comparison of CD3-intermediate positive T cells and natural killer cells from the liver of mice. In: Wisse E, Knook DL, Wake K, editors. Cells of the hepatic sinusoid, vol. 5. Leiden: Kupffer Cell Foundation; 1995. pp. 156–157. [Google Scholar]
- 75.Miyagi T, Takehara T, Tatsumi T, Kanto T, Suzuki T, Jinushi M, Sugimoto Y, Sasaki Y, Hori M, Hayashi N. CD1d-mediated stimulation of natural killer T cells selectively activates hepatic natural killer cells to eliminate experimentally disseminated hepatoma cells in murine liver. Int J Cancer. 2003;106:81–89. doi: 10.1002/ijc.11163. [DOI] [PubMed] [Google Scholar]
- 76.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, Yegutkin GG, Candinas D, Exley M, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito T, Okumura A, Watanabe H, Asano M, Ishida-Okawara A, Sakagami J, Sudo K, Hatano-Yokoe Y, Bezbradica JS, Joyce S, et al. Increase in hepatic NKT cells in leukocyte cell-derived chemotaxin 2-deficient mice contributes to severe concanavalin A-induced hepatitis. J Immunol. 2004;173:579–585. doi: 10.4049/jimmunol.173.1.579. [DOI] [PubMed] [Google Scholar]
- 78.Huang W, Dong Z, Wei H, Ding C, Sun R, Tian Z. Selective elimination of hepatic natural killer T cells with concanavalin A improves liver regeneration in mice. Liver Int. 2006;26:339–345. doi: 10.1111/j.1478-3231.2005.01221.x. [DOI] [PubMed] [Google Scholar]
- 79.Li Z, Oben JA, Yang S, Lin H, Stafford EA, Soloski MJ, Thomas SA, Diehl AM. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434–441. doi: 10.1002/hep.20320. [DOI] [PubMed] [Google Scholar]
- 80.Kim S, Lalani S, Parekh VV, Vincent TL, Wu L, Van Kaer L. Impact of bacteria on the phenotype, functions, and therapeutic activities of invariant NKT cells in mice. J Clin Invest. 2008;118:2301–2315. doi: 10.1172/JCI33071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawachi Y, Arai K, Moroda T, Kawamura T, Umezu H, Naito M, Ohtsuka K, Hasegawa K, Takahashi-Iwanaga H, Iwanaga T. Supportive cellular elements for hepatic T cell differentiation: T cells expressing intermediate levels of the T cell receptor are cytotoxic against syngeneic hepatoma, and are lost after hepatocyte damage. Eur J Immunol. 1995;25:3452–3459. doi: 10.1002/eji.1830251237. [DOI] [PubMed] [Google Scholar]
- 82.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miyazaki Y, Iwabuchi K, Iwata D, Miyazaki A, Kon Y, Niino M, Kikuchi S, Yanagawa Y, Kaer LV, Sasaki H, et al. Effect of high fat diet on NKT cell function and NKT cell-mediated regulation of Th1 responses. Scand J Immunol. 2008;67:230–237. doi: 10.1111/j.1365-3083.2007.02062.x. [DOI] [PubMed] [Google Scholar]
- 85.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma) Gastroenterology. 2004;127:1525–1539. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 87.Kato T, Sato Y, Takahashi S, Kawamura H, Hatakeyama K, Abo T. Involvement of natural killer T cells and granulocytes in the inflammation induced by partial hepatectomy. J Hepatol. 2004;40:285–290. doi: 10.1016/j.jhep.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 88.Ito H, Ando K, Nakayama T, Taniguchi M, Ezaki T, Saito K, Takemura M, Sekikawa K, Imawari M, Seishima M, et al. Role of Valpha 14 NKT cells in the development of impaired liver regeneration in vivo. Hepatology. 2003;38:1116–1124. doi: 10.1053/jhep.2003.50471. [DOI] [PubMed] [Google Scholar]
- 89.Minagawa M, Oya H, Yamamoto S, Shimizu T, Bannai M, Kawamura H, Hatakeyama K, Abo T. Intensive expansion of natural killer T cells in the early phase of hepatocyte regeneration after partial hepatectomy in mice and its association with sympathetic nerve activation. Hepatology. 2000;31:907–915. doi: 10.1053/he.2000.5850. [DOI] [PubMed] [Google Scholar]
- 90.Yang L, Jhaveri R, Huang J, Qi Y, Diehl AM. Endoplasmic reticulum stress, hepatocyte CD1d and NKT cell abnormalities in murine fatty livers. Lab Invest. 2007;87:927–937. doi: 10.1038/labinvest.3700603. [DOI] [PubMed] [Google Scholar]
- 91.Wilson MT, Johansson C, Olivares-Villagómez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, Van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci USA. 2003;100:10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hobbs JA, Cho S, Roberts TJ, Sriram V, Zhang J, Xu M, Brutkiewicz RR. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001;75:10746–10754. doi: 10.1128/JVI.75.22.10746-10754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayakawa Y, Takeda K, Yagita H, Kakuta S, Iwakura Y, Van Kaer L, Saiki I, Okumura K. Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur J Immunol. 2001;31:1720–1727. [PubMed] [Google Scholar]
- 94.Abo T. Extrathymic pathways of T cell differentiation. Arch Immunol Ther Exp (Warsz) 2001;49:81–90. [PubMed] [Google Scholar]
- 95.Emoto M, Kaufmann SH. Liver NKT cells: an account of heterogeneity. Trends Immunol. 2003;24:364–369. doi: 10.1016/s1471-4906(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 96.Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-gamma upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- 97.Nakatani K, Kaneda K, Seki S, Nakajima Y. Pit cells as liver-associated natural killer cells: morphology and function. Med Electron Microsc. 2004;37:29–36. doi: 10.1007/s00795-003-0229-9. [DOI] [PubMed] [Google Scholar]
- 98.Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002;14:165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- 99.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 100.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 101.Burdin N, Brossay L, Kronenberg M. Immunization with alpha-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 102.Kupffer CV. Ueber Sternzellen der Leber. Arch Mikrosk Anat. 1876;12:353–358. [Google Scholar]
- 103.Naito M, Hasegawa G, Ebe Y, Yamamoto T. Differentiation and function of Kupffer cells. Med Electron Microsc. 2004;37:16–28. doi: 10.1007/s00795-003-0228-x. [DOI] [PubMed] [Google Scholar]
- 104.Wisse E. Kupffer cell reactions in rat liver under various conditions as observed in the electron microscope. J Ultrastruct Res. 1974;46:499–520. doi: 10.1016/s0022-5320(74)90070-7. [DOI] [PubMed] [Google Scholar]
- 105.Wisse E. Observations on the fine structure and peroxidase cytochemistry of normal rat liver Kupffer cells. J Ultrastruct Res. 1974;46:393–426. doi: 10.1016/s0022-5320(74)90064-1. [DOI] [PubMed] [Google Scholar]
- 106.Bouwens L, De Bleser P, Vanderkerken K, Geerts B, Wisse E. Liver cell heterogeneity: functions of non-parenchymal cells. Enzyme. 1992;46:155–168. doi: 10.1159/000468782. [DOI] [PubMed] [Google Scholar]
- 107.Sleyster EC, Knook DL. Relation between localization and function of rat liver Kupffer cells. Lab Invest. 1982;47:484–490. [PubMed] [Google Scholar]
- 108.Benacerraf B, Sebestyen MM, Schlossman S. A quantitative study of the kinetics of blood clearance of P32-labelled Escherichia coli and Staphylococci by the reticuloendothelial system. J Exp Med. 1959;110:27–48. doi: 10.1084/jem.110.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karimi MH, Geramizadeh B, Malek-Hosseini SA. Tolerance Induction in Liver. Int J Organ Transplant Med. 2015;6:45–54. [PMC free article] [PubMed] [Google Scholar]
- 110.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury--a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- 111.Kuboki S, Sakai N, Tschöp J, Edwards MJ, Lentsch AB, Caldwell CC. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1054–G1059. doi: 10.1152/ajpgi.90464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bzeizi KI, Jalan R, Plevris JN, Hayes PC. Primary graft dysfunction after liver transplantation: from pathogenesis to prevention. Liver Transpl Surg. 1997;3:137–148. doi: 10.1002/lt.500030206. [DOI] [PubMed] [Google Scholar]
- 113.Hisama N, Yamaguchi Y, Miyanari N, Ichiguchi O, Goto M, Mori K, Ogawa M. Ischemia-reperfusion injury: the role of Kupffer cells in the production of cytokine-induced neutrophil chemoattractant, a member of the interleukin-8 family. Transplant Proc. 1995;27:1604–1606. [PubMed] [Google Scholar]
- 114.Kobayashi S, Clemens MG. Kupffer cell exacerbation of hepatocyte hypoxia/reoxygenation injury. Circ Shock. 1992;37:245–252. [PubMed] [Google Scholar]
- 115.Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, Busuttil RW, Kupiec-Weglinski JW. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435–1443. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 116.Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, Kupiec-Weglinski JW. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 117.Rüdiger HA, Clavien PA. Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology. 2002;122:202–210. doi: 10.1053/gast.2002.30304. [DOI] [PubMed] [Google Scholar]
- 118.Tian Y, Jochum W, Georgiev P, Moritz W, Graf R, Clavien PA. Kupffer cell-dependent TNF-alpha signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. Proc Natl Acad Sci USA. 2006;103:4598–4603. doi: 10.1073/pnas.0600499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia-reperfusion. Transplantation. 2008;86:710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 120.Frankenberg MV, Weimann J, Fritz S, Fiedler J, Mehrabi A, Büchler MW, Kraus TW. Gadolinium chloride-induced improvement of postischemic hepatic perfusion after warm ischemia is associated with reduced hepatic endothelin secretion. Transpl Int. 2005;18:429–436. doi: 10.1111/j.1432-2277.2004.00058.x. [DOI] [PubMed] [Google Scholar]
- 121.Uhlmann D, Gaebel G, Armann B, Ludwig S, Hess J, Pietsch UC, Fiedler M, Tannapfel A, Hauss J, Witzigmann H. Attenuation of proinflammatory gene expression and microcirculatory disturbances by endothelin A receptor blockade after orthotopic liver transplantation in pigs. Surgery. 2006;139:61–72. doi: 10.1016/j.surg.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 122.Diepenhorst GM, van Gulik TM, Hack CE. Complement-mediated ischemia-reperfusion injury: lessons learned from animal and clinical studies. Ann Surg. 2009;249:889–899. doi: 10.1097/SLA.0b013e3181a38f45. [DOI] [PubMed] [Google Scholar]
- 123.Datta G, Fuller BJ, Davidson BR. Molecular mechanisms of liver ischemia reperfusion injury: insights from transgenic knockout models. World J Gastroenterol. 2013;19:1683–1698. doi: 10.3748/wjg.v19.i11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kimura H, Katsuramaki T, Isobe M, Nagayama M, Meguro M, Kukita K, Nui A, Hirata K. Role of inducible nitric oxide synthase in pig liver transplantation. J Surg Res. 2003;111:28–37. doi: 10.1016/s0022-4804(03)00036-2. [DOI] [PubMed] [Google Scholar]
- 125.Meguro M, Katsuramaki T, Kimura H, Isobe M, Nagayama M, Kukita K, Nui A, Hirata K. Apoptosis and necrosis after warm ischemia-reperfusion injury of the pig liver and their inhibition by ONO-1714. Transplantation. 2003;75:703–710. doi: 10.1097/01.TP.0000053400.42842.5C. [DOI] [PubMed] [Google Scholar]
- 126.Brass CA, Roberts TG. Hepatic free radical production after cold storage: Kupffer cell-dependent and -independent mechanisms in rats. Gastroenterology. 1995;108:1167–1175. doi: 10.1016/0016-5085(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 127.Schauer RJ, Bilzer M, Kalmuk S, Gerbes AL, Leiderer R, Schildberg FW, Messmer K. Microcirculatory failure after rat liver transplantation is related to Kupffer cell-derived oxidant stress but not involved in early graft dysfunction. Transplantation. 2001;72:1692–1699. doi: 10.1097/00007890-200111270-00022. [DOI] [PubMed] [Google Scholar]
- 128.Mosher B, Dean R, Harkema J, Remick D, Palma J, Crockett E. Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J Surg Res. 2001;99:201–210. doi: 10.1006/jsre.2001.6217. [DOI] [PubMed] [Google Scholar]
- 129.Ogura Y, Martinez OM, Villanueva JC, Tait JF, Strauss HW, Higgins JP, Tanaka K, Esquivel CO, Blankenberg FG, Krams SM. Apoptosis and allograft rejection in the absence of CD8+ T cells. Transplantation. 2001;71:1827–1834. doi: 10.1097/00007890-200106270-00020. [DOI] [PubMed] [Google Scholar]
- 130.Krams SM, Hayashi M, Fox CK, Villanueva JC, Whitmer KJ, Burns W, Esquivel CO, Martinez OM. CD8+ cells are not necessary for allograft rejection or the induction of apoptosis in an experimental model of small intestinal transplantation. J Immunol. 1998;160:3673–3680. [PubMed] [Google Scholar]
- 131.Maier S, Tertilt C, Chambron N, Gerauer K, Hüser N, Heidecke CD, Pfeffer K. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28-/- mice. Nat Med. 2001;7:557–562. doi: 10.1038/87880. [DOI] [PubMed] [Google Scholar]
- 132.Kondo T, Morita K, Watarai Y, Auerbach MB, Taub DD, Novick AC, Toma H, Fairchild RL. Early increased chemokine expression and production in murine allogeneic skin grafts is mediated by natural killer cells. Transplantation. 2000;69:969–977. doi: 10.1097/00007890-200003150-00051. [DOI] [PubMed] [Google Scholar]
- 133.Petersson E, Ostraat O, Ekberg H, Hansson J, Simanaitis M, Brodin T, Dohlsten M, Hedlund G. Allogeneic heart transplantation activates alloreactive NK cells. Cell Immunol. 1997;175:25–32. doi: 10.1006/cimm.1996.1031. [DOI] [PubMed] [Google Scholar]
- 134.Fuggle SV. Immunophenotypic analysis of leukocyte infiltration in the renal transplant. Immunol Lett. 1991;29:143–146. doi: 10.1016/0165-2478(91)90216-w. [DOI] [PubMed] [Google Scholar]
- 135.Hsieh CL, Ogura Y, Obara H, Ali UA, Rodriguez GM, Nepomuceno RR, Martinez OM, Krams SM. Identification, cloning, and characterization of a novel rat natural killer receptor, RNKP30: a molecule expressed in liver allografts. Transplantation. 2004;77:121–128. doi: 10.1097/01.TP.0000110423.27977.6F. [DOI] [PubMed] [Google Scholar]
- 136.Yamaguchi Y, Matsumura F, Liang J, Akizuki E, Matsuda T, Okabe K, Ohshiro H, Ishihara K, Yamada S, Mori K, et al. Reduced interleukin-12, interleukin-18, and interferon-gamma production with prolonged rat hepatic allograft survival after donor-specific blood transfusion. Dig Dis Sci. 2000;45:2429–2435. doi: 10.1023/a:1005659529472. [DOI] [PubMed] [Google Scholar]
- 137.Stinn JL, Taylor MK, Becker G, Nagano H, Hasegawa S, Furakawa Y, Shimizu K, Libby P, Mitchell RN. Interferon-gamma-secreting T-cell populations in rejecting murine cardiac allografts: assessment by flow cytometry. Am J Pathol. 1998;153:1383–1392. doi: 10.1016/s0002-9440(10)65725-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hricik DE, Rodriguez V, Riley J, Bryan K, Tary-Lehmann M, Greenspan N, Dejelo C, Schulak JA, Heeger PS. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am J Transplant. 2003;3:878–884. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 139.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, Larsen CP, Pearson TC, Lakkis FG. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 140.Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, Benítez C, Pons JA, Parrilla P, Ramírez P, et al. Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118:2845–2857. doi: 10.1172/JCI35342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li L, Wozniak LJ, Rodder S, Heish S, Talisetti A, Wang Q, Esquivel C, Cox K, Chen R, McDiarmid SV, et al. A common peripheral blood gene set for diagnosis of operational tolerance in pediatric and adult liver transplantation. Am J Transplant. 2012;12:1218–1228. doi: 10.1111/j.1600-6143.2011.03928.x. [DOI] [PubMed] [Google Scholar]
- 142.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- 143.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 144.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 145.Ikehara Y, Yasunami Y, Kodama S, Maki T, Nakano M, Nakayama T, Taniguchi M, Ikeda S. CD4(+) Valpha14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest. 2000;105:1761–1767. doi: 10.1172/JCI8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Müller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 147.Huang HF, Zeng Z, Chen MQ. Roles of Kupffer cells in liver transplantation. Hepatogastroenterology. 2012;59:1251–1257. doi: 10.5754/hge12046. [DOI] [PubMed] [Google Scholar]
- 148.Chen GS, Qi HZ. Effect of Kupffer cells on immune tolerance in liver transplantation. Asian Pac J Trop Med. 2012;5:970–972. doi: 10.1016/S1995-7645(12)60184-9. [DOI] [PubMed] [Google Scholar]
- 149.Rogoff TM, Lipsky PE. Role of the Kupffer cells in local and systemic immune responses. Gastroenterology. 1981;80:854–860. [PubMed] [Google Scholar]
- 150.Gouw AS, Houthoff HJ, Huitema S, Beelen JM, Gips CH, Poppema S. Expression of major histocompatibility complex antigens and replacement of donor cells by recipient ones in human liver grafts. Transplantation. 1987;43:291–296. doi: 10.1097/00007890-198702000-00025. [DOI] [PubMed] [Google Scholar]
- 151.Sass G, Shembade ND, Haimerl F, Lamoureux N, Hashemolhosseini S, Tannapfel A, Tiegs G. TNF pretreatment interferes with mitochondrial apoptosis in the mouse liver by A20-mediated down-regulation of Bax. J Immunol. 2007;179:7042–7049. doi: 10.4049/jimmunol.179.10.7042. [DOI] [PubMed] [Google Scholar]


