Abstract
The hepatitis C virus (HCV) infected patients are prone to develop bone marrow or various tissue infiltrates with monoclonal B cells, monoclonal B lymphocytosis or different types of B cell non-Hodgkin’s lymphoma (BCNHL), of which the most common are splenic marginal zone BCNHL, diffuse large BCNHL and follicular lymphoma. The association between chronic HCV infection and non Hodgkin’s lymphoma has been observed especially in areas with high prevalence of this viral infection. Outside the limitations of some studies that have been conducted, there are also geographic, environmental, and genetic factors that contribute to the epidemiological differences. Various microenvironmental signals, such as cytokines, viral antigenic external stimulation of lymphocyte receptors by HCV antigens, and intercellular interactions contribute to B cell proliferation. HCV lymphotropism and chronic antigenic stimulation are involved in B-lymphocyte expansion, as mixted cryoglobulinemia or monoclonal gammopathy of undetermined significance, which can progress to BCNHL. HCV replication in B lymphocytes has oncogenic effect mediated by intracellular HCV proteins. It is also involved in an important induction of reactive oxygen species that can lead to permanent B lymphocyte damage, as DNA mutations, after binding to surface B-cell receptors. Post-transplant lymphoproliferative disorder could appear and it has a multiclonal potentiality that may develop into different types of lymphomas. The hematopoietic stem cell transplant made for lymphoma in HCV-infected patients can increase the risk of earlier progression to liver fibrosis and cirrhosis. HCV infected patients with indolent BCNHL who receive antiviral therapy can be potentially cured. Viral clearance was related to lymphoma response, fact that highlights the probable involvement of HCV in lymphomagenesis. Direct acting antiviral drugs could be a solution for the patients who did not tolerate or respond to interferon, as they seem to be safe and highly effective. The use of chemotherapy in combination with rituximab for the treatment of BCNHL in patients infected with HCV can produce liver dysfunction. The addition of immunotherapy with rituximab can increase the viral replication, and severe complications can occure especially in patients co-infected with hepatitis B virus or immune immunodeficiency virus, in those with hepatocarcinoma, cirrhosis, or liver cytolysis. But the final result of standard immunochemotherapy applied to diffuse large BCNHL patients with HCV infection is not notably worse than in those without this viral infection. The treatment of patients chronically infected with HCV and having BCNHL is complex and requires a multidisciplinary approach and the risk / benefit ratio of rituximab treatment must be evaluated especially in those with liver cytolysis.
Keywords: Chemotherapy, Cryoglobulinemia, Direct acting antiviral drugs, Hepatitis C virus, Hepatocytolysis, Interferon, Liver transplantation, Liver dysfunction, Non-Hodgkin’s lymphoma, Rituximab
Core tip: There are epidemiological observations on the association between hepatitis C virus (HCV) infection and non-Hodgkin's lymphoma. Various microenvironmental signals, such as cytokines, viral antigenic external stimulation of lymphocyte receptors by HCV antigens, and intercellular interactions contribute to B cell proliferation. HCV lymphotropism and chronic antigenic stimulation are involved in B-lymphocyte expansion, as mixted cryoglobulinemia or monoclonal gammopathy of undetermined significance, which can progress to B cell non-Hodgkin's lymphoma (BCNHL). HCV infected patients with indolent BCNHL who receive antiviral therapy can be potentially cured. Viral clearance was related to lymphoma response, fact that highlights the probable involvement of HCV in lymphomagenesis.
INTRODUCTION
The association between chronic hepatitis C virus (HCV) infection and some B cell non-Hodgkin’s lymphomas (BCNHL) has been discussed for a long time[1,2]. A higher incidence of these lymphomas has been found especially in countries where HCV prevalence is high (about 10%, according to a recent systematic review)[3]. Unfortunately, this connection is not well understood. But, as it happens in many situations as the scientific research progresses and offers useful information, a possible pathway explanation has been recently published: a mutated stereotypic IGHV4-59/IGHJ5-encodedB-cell receptors subset was found to be expressed in some BCNHL associated with HCV infection. These mutated receptors are high affinity monoreactive rheumatoid factors and emphasize the auto-antigen role of IgG in BCNHL pathway[4]. Given this news I decided to draw out a review on HCV-related BCNHL. For this purpose I have studied the articles published in PubMed since January 2013 until today.
The temporal relationship between HCV infection and non-Hodgkin’s lymphomas was analyzed in a large study conducted in Taiwan, after excluding patients infected with HCV who had cancers and infections with hepatitis B virus (HBV) or human immunodeficiency virus (HIV) at baseline. The follow-up of Asian patients infected with HCV established that their risk of developing a lymphoid neoplasm (and especially non-Hodgkin’s lymphomas) was 2 times higher than that of a group of HCV-uninfected patients[5].
EPIDEMIOLOGICAL DATA AND RISK FACTORS
The hepatic and extrahepatic manifestations of HCV are extremely varied as geographical distribution, fact which can be explained by a possible involvement of other environmental and/or genetic cofactors[6].
The epidemiological studies made in the last 20 years found an association between HCV infection and BCHNL[7]. Thus, Chronic Hepatitis Cohort Study followed a large group of patients with chronic HCV infection (12126 subjects) during 5 years and found the following values: the incidence of non-Hodgkin’s lymphoma was significantly higher [standardized rate ratios was 1.6 (1.2-2.1)], and age-adjusted mortality was also significantly higher than the general population[8].
Indolent non-Hodgkin’s lymphomas were more frequently associated with HCV infection[9]. Indeed, HCV infection was more frequently found in patients with marginal zone lymphomas, and especially in those with splenic type, compared to the control population, an argument for a possible viral involvement in lymphoma genesis[10]. A large meta-analysis which included 2440 patients with small lymphocytic lymphoma and chronic lymphocytic leukemia confirmed the association of these hemopathies with HCV infection[11]. But in a cohort of 524 Bulgarian patients with non-Hodgkin’s lymphoma, only 1.84% were HCV positive[9]. Patients with dual viral infection - HCV and HIV - are also more likely to develop marginal zone/lymphoplasmacytic BCNHL, compared to HIV only-infected patients[12].
The association between HCV infection and indolent BCNHL has been known for a long time, but this virus infection can also be associated with diffuse large BCNHL, especially in some geographical regions[13], so it is considered that marginal zone lymphomas and diffuse large BCNHL are the histological type commonly associated with this viral infection[14]. Indeed, the most frequent type of BCNHL found in 89 HCV infected patients was that with large cells (62%) in a study realized at MD Anderson Cancer Center during 7 years. Their liver disease was mostly mild (only 18% of patients had a Metavir stage ≥ 3), the most frequent genotype was 1 (62%), and viremia was detected in 90% of patients[2]. HCV infection was more frequently found in patients with splenic diffuse large BCNHL and splenic marginal zone BCNHL compared to patients with all types of lymphoma in Italy, while the prevalence of this virus was higher only in those with diffuse large BCNHL compared to the patients with all types of lymphoma, in Japan. Forty-four percent of patients with diffuse large BCNHL and 10% of those with splenic marginal zone BCNHL were HCV positive in a study conducted in Taiwan[15]. In ANRS HC-13 Lympho-C study which included 116 HCV infected patients with BCNHL, the most frequent hystological types were marginal zone lymphoma and diffuse large BCNHL (both present in 39% of patients)[16].
Beside splenic marginal zone BCNHL, diffuse large BCNHL and follicular lymphoma, HCV chronically infected patients can develop a disseminated type of marginal zone lymphoma with different characters from splenic marginal zone lymphoma or a monoclonal B lymphocytosis and bone marrow or various tissue infiltrate with monoclonal B cells, without histology of lymphoma[17]. A higher risk for B-cell activating autoimmune conditions was found to be associated with all 3 subtypes of marginal zone BCNHL (nodal, extranodal and splenic), but HCV infection was a risk factor only for the extranodal subtype, vs the witnesses[18].
A large meta-analysis established that the metalworker occupation, the presence of hematologic neoplasias in the family history, and the patient-declared peptic ulcers are risk factors for the extranodal subtype of marginal zone BCNHL. On the contrary, a reduced risk for this subtype of lymphoma was present in teachers and those who drank any kind of alcohol[18].
The diffuse large BCNHL was associated with HCV infection, but also with B-cell activating autoimmune disorders, the presence of non-Hodgkin lymphoma in the family history, a body mass index at young adult age, any atopic disturbance, higher socioeconomic status, and higher sun exposure in free time, according to another large meta-analysis[19].
The patients with non-Hodgkin’s lymphoma have an 1.5 times higher risk for a second primary malignancy occurrence, most commonly for leukemia and myeloma. Liver cirrhosis and HCV infection were significant predictors for the appearance of such a new malignancy in a retrospective study made in Taiwan[20].
PATOPHYSIOLOGY NOTIONS
Some cases of non-Hodgkin’s lymphoma might be due to HCV infection, particularly in areas with high prevalence of this infection[1,2,21]. In the early-stage of diffuse large BCNHL HCV seroprevalence was high in a study conducted in Taiwan, fact which advocates for the involvement of HCV in lymphoma pathway[15].
A question remains unanswered: why didn’s some studies find any association between HCV infection and lymphoma. It is considered that the small number of patients, the short follow-up period analyzed and database limitations have influenced the results of these studies[21]. In addition, epidemiological studies used mainly anti-HCV antibody test, which has lower sensitivity compared to HCV-RNA detection, the most widely used test in order to detect the association between HCV and non-Hodgkin’s lymphoma[7].
Among lymphoproliferative diseases, BCNHL appears to be mostly associated with HCV infection[21]. It is known that HCV has both hepato- and lymphotropism and is involved in a polyoligoclonal B-lymphocyte expansion, followed sometimes by the occurence of mixed cryoglobulinemia (an immune-mediated disorder)[6]. Both chronic inflammation and alterations in immune function, are invloved in B cell lymphoproliferative disorders[21], as mixted cryoglobulinemia or monoclonal gammopathy of undetermined significance[22]. These may progress to BCNHL[6,21] (Figure 1). Therefore, it can be considered that the evolution of the malignant clone is the result of both HCV lymphotropism and chronic antigenic stimulation[23]. The presence of cirrhosis in HCV infected patients is a supplementary risk factor for monoclonal gammopathy of undetermined significance or BCNHL occurence, in agreement with a multivariate logistic regression analysis[22].
Figure 1.
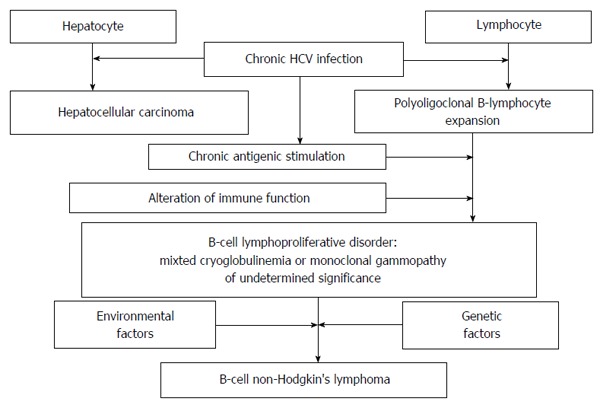
Main mechanisms of lymphomagenesis. HCV: Hepatitis C virus.
Regarding the histological subtypes of BCNHL, the rheumatoid factor was more frequently noticed in patients with marginal zone lymphoma, than in those with diffuse large B-cell (68% compared to 35%). Mixed cryoglobulinemia was also significantly more often found in the first mentioned subtype (74% vs 44%)[16]. There are arguments in favor and against the association of chronic HCV infection and Waldenström macroglobulinemia. The HCV potential to promote lymphoproliferation is supported by some authors, as in the case of an HCV-infected patient with cryoglobulinemia and clinical manifestations of hyperviscosity, where bone marrow biopsy estabilished the diagnosis[24]. In HCV-infected patients with cryoglobulinemia only memory B cells, not naïve cells, were found to be significantly activated compared to healthy subjects. Markers of these cell activation in patients with these associated pathology were CD71, CD86, and HLA-DR, and in those with advanced hepatic disease - CD86[25].
The association of BCNHL with HCV infection is not completely defined although epidemiological studies argued for its support[14].
The mechanism of lymphomagenesis should be considered. It is known that infectious, environmental, and genetic factors are involved in this multifactorial process[21]. As mentioned above, some laboratory and clinicoepidemiological studies have suggested the oncogenic potential of HCV[6]. It is believed that lymphomagenesis depends on chronic rather than cleared HCV infection[7].
Usually, the malignant cells coexist with the microenvironmental factors. At least at the beginning, lymphoma development depends on various microenvironmental signals, such as: cytokines, viral antigens, and intercellular interactions[14]. Continuous viral antigenic external stimulation of lymphocyte receptors by HCV seems to be of major importance for B cell proliferation[14,26]. The regression of BCNHL after HCV infection eradication with antiviral treatment is another argument for the virus involvement in BCNHL pathway[26].
It is known that HCV-associated lymphomas use a restricted immunoglobulin variable region gene repertoire, so that the lymphoma B-cell receptors expressed as soluble immunoglobulin Gs and membrane IgMs do not bind to the HCV antigens. It follows that the majority of lymphomas do not occur from B cells that are involved in viral clearance[27].
Another theory states that HCV replication in B lymphocytes has oncogenic effect mediated by intracellular HCV proteins[26].
Another mechanism would be the one in which the HCV is involved in an important induction of a reactive oxygen species[28] and can lead to permanent B lymphocyte damage, as DNA mutations of tumor suppressor gene[26] TP53 and proto-oncogenes CTNNB1 and BCL6[29] and/or lower antigen response thresholds, after binding to surface B-cell receptors[21]. Still, a study made on 6 HCV-infected patients did not find any suspected mutation, so the authors concluded that HCV does not generally induce mutations in the genes involved in oncogenesis, as CTNNB1, TP53, and BCL6 in B lymphocytes[29]. Moreover, it was found that cytotoxic T-lymphocyte antigen 4 (CTLA-4) + 49 A/G polymorphism is associated with a higher risk of BCNHL occurence. HCV infection was more frequently present in subjects carriers of the mutant genotype + 49 A/G and - 318 C/T SNPs was more often found in patients with BCNHL and was a risk factor for BCNHL occurence[30].
Interleukin 28B gene polymorphisms also seems to be involved in lymphomagenesis. Thus, the IL-28B C/C genotype is distinguished biologically by a higher frequency of restriction of B cell response and its presence is correlated with a higher probability of cryoglobulinemic nephropathy and B cell malignant proliferations[31].
A deregulation of NF-κB, NOTCH, and BCR signaling pathway can be found to arise in the pathogenesis of splenic marginal zone lymphoma. But there is evidence that NOTCH pathway lesions are significantly more frequently found in HCV-infected patients with diffuse large BCNHL as compared to patients with the same type of lymphoma but uninfected with HCV. In addition, those who had a NOTCH pathway mutation had a significantly shorter 5-year overall survival vs the patients without lesions in NOTCH pathway (27% compared to 62%)[32]. Indeed, both canonical and alternative NF-κB signalling pathways were activated and miR-26b expression was down-regulated in a transgenic mice model that express the full-length HCV genome specifically in B cells and which developed an HCV-associated BCNHL[33].
An area of particular interest on lymphomagenesis in HCV-infected patients is the study of microRNA levels in lymphoma tissues. Thus, an increased expression level of miR-30b in 14 biopsies from HCV- and HBV-infected patients with indolent BCNHL was observed. An association between miR-29a, miR-29b, and miR-223, and the presence of HCV infection was found in patients with nodal marginal zone lymphoma[34]. Regarding the HCV-infected patients with diffuse large BCNHL, a set consisting of 52 miRNAs could be a signature for them. It should also be noted that miR-138-5p which had a decreased expression, and miR-511-5p, miR-147a, and miR-147b which had an increased expression serve as a negative prognostic factor in HCV-infected patients with diffuse large BCNHL[35]. Further research is needed in order to establish the role of microRNA over- or underexpression in BCNHL pathogenesis and their usefulness in a possible new classification of lymphomas.
The t(14;18) translocation has been found in B lymphocyte proliferation and it is considered to be associated with MALT lymphomas occurence in HCV-infected patients[36]. A more frequent telomeric 1p36.3 deletion and an increased expression of Ki-67 were found in BCNHL patients with HCV infection vs those without this infection. This is an argument for a possible virus involvement in cancers occurence at the 1p36.3 locus[37].
Oncogenic potential of HCV can lead to a concomitant induction of hepatocellular carcinoma and a BCNHL, as in a case of synchronous neoplasia found during the process of liver transplantation surgery[38].
INFLUENCE OF LIVER TRANSPLANTATION
Post-transplant lymphoproliferative disorder could appear and it has a multiclonal potentiality that may develop into different types of lymphomas[39].
In a large study which included 10010 Swedish patients with a solid organ transplant, 135 patients developed a lymphoma. The incidence rate of lymphomas was 159/100000 person-years. Forty-eight percent of them were negative for Epstein-Barr virus (EBV) infection and associated with HCV infection. HCV infection was found to be an independent negative prognostic factor for survival in these patients[40].
In such a patient who underwent liver transplantation for hepatitis C liver cirrhosis, developed a relapse of his hepatitis C 2 mo after the graft. An EBV-negative polymorphic B-cell and an EBV-negative monomorphic T-cell ALK-positive post-transplant lymphoproliferation occured after another 32 mo. They were treated with R-CHOP regimen followed by complete remission. However, the lymphoproliferation reccured and the patient died half a year after the first post-transplant lymphoproliferative diagnosis. Histopathological examination of the liver and other organs discovered a third type of lymphoproliferation: an EBV-negative monomorphic T-cell ALK-negative lymphoma[39]. Immunosuppression, but also relapsed HCV infection are involved in the pathogenesis of these post liver transplant lymphoproliferations. A primary hepatic diffuse large BCNHL was also histologically diagnosed in an HCV-infected patient who had undergone a partial hepatectomy. After he was treated by systemic chemotherapy, the patient developed a new liver tumor, which was also operated by partial hepatectomy and was a hepatocellular carcinoma[41].
The hematopoietic stem cell transplant made for lymphoma in HCV-infected patients can increase the risk of earlier progression to liver fibrosis and cirrhosis. At this point, it is unknown whether this risk is or is not dependent of prior treatment of lymphoma[42].
CLINICAL AND LABORATORY FINDINGS
The clinical manifestations of diffuse large BCNHL are not the same in HCV-positive or negative patients[13]. Indeed, it seems that liver involvement is greater and the number of affected nodal regions is higher in patients with diffuse large BCNHL with HCV/HBV infection as in those without such viral infections, according to the results of a study witch included 224 diffuse large BCNHL patients but of which only 9.3% were HCV/HBV-positive. Despite these differences, identically treated patients had similar responses and evolution in the study made by Rubio et al[43].
Sometimes, HCV-infected patients can develop BCNHL with various locations. In such a patient who had liver cirrhosis with double etiology (alcoholic, too) a primary follicular lymphoma appeared in the spleen was found when he underwent a splenectomy performed in order to reduce symptomatic pancytopenia[44]. Another rare location is on the skin. A localized lipoatrophy was reported to be a clinical manifestation of a marginal zone BCNHL[45]. A primary large BCNHL was also found in the body cavity of an HCV-infected patient[46] and a marginal zone lymphoma located on the right lacrimal gland associated to an HCV-infection was also reported[47].
As lymphoma patients are immunosuppressed, RNA detection techniques of HCV infection are more frequently requested compared to the other patients[42], in order to detect early this possible infection and to avoid a possible viral flare induced by chemotherapy.
Based on a multiparametric analysis, four serum parameters were identified in order to constitute a signature able to differentiate the HCV-infected patients with or without overt BCNHL, with a a sensitivity of 100% and a specificity of 90%: sCD27, C4 levels, sIL-2Rα, and gammaglobulins[48].
Mixed cryoglobulinemia (MC) was found in about a quarter of a lage cohort of patients with chronic HCV infection and three quarters of those with MC had also cryoglobulinemic syndrome. These patients presented BCNHL significantly more often than those without MC (15% as against 7.1%). If cryoglobulinemic syndrome had no impact on the overall survival, it would modify the natural history of these patients, as shown in a 15-years prospective cohort study[49].
The patients with BCNHL would have a shorter overall survival if they were HCV-infected and had 1p36.3 deletion found by FISH, vs those without HCV infection (37). Three risk factors which correlated with a bed prognosis in HCV-infected patients with diffuse large BCNHL were found in a multivariate analysis: an ECOG performance status ≥ 2, serum level of albumin < 3.5 g/dL, and HCV-RNA viremia > 1000 KIU/mL. A score which includes these three factors could be used to discriminate the patients with different overall and progression-free survival, independent of their treatment (with or without rituximab)[50].
THERAPEUTIC PARTICULARITIES
Classical antiviral therapy
The treatment of both HCV infection and lymphoma is a challenge for physicians. The main findings in this field are presented in Table 1. It is a pity that not all cancer patients can benefit from antiviral therapy[42]. In addition, we do not know which is the best course of treatment in BCNHL patients chronically infected with HCV, currently[51]. Although 53 patients of the study realized at MD Anderson Cancer Center were detected with HCV infection before the diagnosis of non-Hodgkin’s lymphoma (which was made later), almost half of them were not treated with antiviral medication, especially as they had mild liver disease at diagnosis[2]. As HCV infection can be involved in cancer occurence, including hepatocellular carcinoma and non-Hodgkin’s lymphoma[42], early antiviral treatment should also be indicated for a possible prevention of lymphoma occurrence[2].
Table 1.
Main therapeutical findings
| Study findings | Ref. |
| Antiviral treatment should be indicated in order to prevent lymphoma occurrence | [2] |
| Low-grade malignant lymphomas can respond to antiviral therapy | [26,51] |
| Non-Hodgkin’s lymphoma with high grade of malignancy need immuno-chemotherapy associated treatment | [7,15] |
| Antiviral treatment contributed to an improved outcome of HCV-infected patients with non-Hodgkin’s lymphoma | [16] |
| Antiviral treatment could be an alternative to chemo-immunotherapy in some cases | [23] |
| Splenic marginal zone lymphoma is most frequently associated with HCV infection and can evolve favorably after HCV eradication | [53] |
| HCV-infected patients with indolent BCNHL who receive antiviral therapy can be potentially cured | [13] |
| Forty-four of HCV-infected patients with indolent BCNHL obtained a complete remission and 33% a partial response of lymphoma after antiviral therapy used as first-line treatment | [55] |
| Viral clearance was related to lymphoma response | [55] |
| The clinical response of lymphoma is dependent on HCV-RNA eradication | [14] |
| The combined treatment with peginterferon and ribavirin proved to be useful for the treatment of BCNHL | [56] |
| Repeated plasmapheresis are needed, if hyperviscosity is present, followed by antiviral ± cytostatic therapy | [24] |
| The administration of direct antiviral agents is useful in onset of therapy of patients with marginal zone BCNHL who have no severe complications, and early in those with diffuse large BCNHL in order to prevent the potential liver damage induced by the use of immunochemotherapy and avoid BCNHL relapse | [56] |
| A chronic HCV-infected patient with splenic marginal zone lymphoma obtained rapid viral clearance and his lymphoma was cured with an interferon-free regimen based on NS3-NS4A inhibitor | [57] |
| A HCV-infected female patient with chronic lymphocytic leukaemia received telaprevir-based triple therapy followed by successful result, without chronic lymphocytic leukaemia progression | [58] |
| HCV-infected diffuse large BCNHL patients had a higher liver toxicity induced by immunochemotherapy and a higher delay of their chemotherapy application | [59] |
| Severe liver toxicity (grade 3-4) was significantly more frequently found in diffuse large BCNHL patients infected with HCV treated also by immunotherapy compared with those treated only by chemotherapy | [60] |
| The liver toxicity of grade 3-4 was significantly more frequently found in HCV-infected patients with diffuse large BCNHL treated with chemo-immunotherapy and the progression-free survival and overall survival were significantly shorter in comparison with those who received only chemotherapy | [61] |
| Fourteen percent of HCV-infected patients with diffuse large BCNHL who received an anthracycline-based chemotherapy (with rituximab in 255 of them) developed severe liver toxicity | [50] |
| A patient with diffuse large BCNHL and HCV infection developed a cholestatic hepatitis C after chemoimmunotherapy | [62] |
| The addition of immunotherapy with rituximab can increase the viral replication | [13] |
| The final result of standard immunochemotherapy applied to diffuse large BCNHL patients with HCV infection is not less good compared to those without this infection | [13] |
| A solution to avoid a severe liver toxicity in patients with compensated HCV induced liver cirrhosis and indolent BCNHL is the combination of bendamustine with rituximab | [63] |
HCV: Hepatitis C virus; BCNHL: B cell non-Hodgkin's lymphoma.
Low-grade malignant lymphomas can respond to antiviral therapy[26,51] if the disease is limited and do not require immediate cytoreductive drugs[52]. Those with high grade of malignancy need also immuno-chemotherapy associated treatment[7,51], despite the probable liver toxicity[7].
It was shown that antiviral treatment contributed to an improved outcome of HCV-infected patients with non-Hodgkin’s lymphoma[16] and, in some cases, could also be an alternative to chemo-immunotherapy[23]. Of the three types of marginal zone lymphoma (MALT, nodal and splenic), the last is most frequently associated with HCV infection and can evolve favorably after HCV eradication[53]. A rare association between BCNHL and mixed cryoglobulinemic endocapillary proliferative glomerulonephritis found sometimes in HCV-infected patients can have a favourable evolution under interferon: such a patient had a reduction of its clinical symptoms, proteinuria disappeared and HCV viremia decreased after one year of treatment[54].
HCV-infected patients with indolent BCNHL who receive antiviral therapy can be potentially cured[13]. Forty-four of HCV-infected patients with indolent BCNHL showed complete remission and 33% a partial response of lymphoma after antiviral therapy used as first-line treatment, in a large multicenter study[55]. As it is known that sustained virological responses to HCV antiviral treatment in cancer patients may be poorer as in those without cancer[42], it is very important to note that viral clearance was related to lymphoma response in this multicenter study[55]. One can speculate that the persistence of virus in malignant lymphocytes could constitute a reservoir that can contribute to hepatitis relapse. On the other hand, the clinical response of lymphoma is dependent on HCV-RNA eradication, fact that highlights the probable involvement of HCV in lymphomagenesis[14].
The combined treatment with peginterferon and ribavirin proved to be useful for the treatment of BCNHL[56]. The fact that antiviral treatment may be followed by complete remission of lymphoma is an argument for a possible involvement of chronic antigenic stimulation and HCV in BCNHL pathway[51].
If hyperviscosity is present, as in HCV-infected patients with IgM or IgG gammopathy, or Waldenström’s macroglobulinemia, repeated plasmapheresis are needed in order to fight against this syndrome, followed by antiviral ± cytostatic therapy[24].
Direct acting antiviral therapy
Direct acting antiviral drugs could be a solution for the patients who did not tolerate or respond to interferon, as they are safe and highly effective, according to recent findings[51,57]. Indeed, five cases of BCNHL patients infected with HCV obtatined sustained virological response after direct anti-viral agents therapy, given alone, together with rituximab or followed by chemotherapy. Four of them achived complete remission of BCNHL 6 mo after the treatment ended. These results suggest the administration of direct antiviral agents in onset of therapy of patients with marginal zone BCNHL who have no severe complications, and early in those with diffuse large BCNHL in order to prevent the potential liver damage induced by the use of chemotherapy in combination with rituximab and avoid BCNHL relapse[56]. A chronic HCV-infected patient with splenic marginal zone lymphoma obtained rapid viral clearance and his lymphoma was cured with an interferon-free regimen based on NS3-NS4A inhibitor, which consisted in a 16 wk administration of deleobuvir, faldaprevir, and ribavirin. Such therapeutic results, achieved even with interferon free regimens highlight the pathogenetic role of the virus in the development of lymphoma and also suggest that the effectiveness of interferon therapy of lymphoma is especially due to its antiviral and less antiproliferative effect[57].
The case of a patient who obtained a haematological response after peginterferon plus ribavirin was also published. Still, a virological relapse was noted at week 24, for which she received telaprevir-based triple therapy, followed by successful result without chronic lymphocytic leukaemia progression[58].
We hope that the era of interferon-free regimen will also bring clarifications on the importance of the lymphoid reservoir in HCV removal[23].
Rituximab-based chemotherapy
There is a debate on the safety of rituximab-based chemotherapy used for the treatment of diffuse large BCNHL. Twenty-nine HCV-infected patients with this type of lymphoma were compared with 139 patients without HCV infection but with the same type of lymphoma. HCV-infected patients had a higher liver toxicity induced by immunochemotherapy (manifested in particular by an increase of AST and total bilirubin) and a higher delay of their chemotherapy application, without affecting survival, during a median follow-up of 3 years[59]. In another study, 200 diffuse large BCNHL patients infected with HCV were treated with chemotherapy combined with rituximab, vs 80 patients with the same two diseases who received only chemotherapy. There were no significant differences on median progression-free survival or median overall survival, but severe liver toxicity (grade 3-4) was significantly more frequently found in those treated also by immunotherapy compared with those treated only by chemotherapy (26.5% vs 13.75%). A quarter of patients who received rituximab could not complete the therapy due to liver toxicity or their progressive disease. A risk factor predictive for severe liver toxicity was the presence of liver dysfunction before the treatment[60]. The results were worse in another study, which included 137 HCV-infected patients with diffuse large BCNHL treated with CHOP ± rituximab regimen. The liver toxicity of grade 3-4 was significantly more frequently found in those treated with chemo-immunotherapy (28% vs 18%), while the progression-free survival and overall survival were significantly shorter in this group of patients in comparison with those who received only chemotherapy[61]. In a larger study, made on 535 HCV-infected patients with diffuse large BCNHL who received an anthracycline-based chemotherapy (with rituximab in 255 of them), 14% of patients developed severe liver toxicity, but, in this study, rituximab did not contribute to an increased severe liver toxicity. Overall survival and progression-free survival at 3 years were 71% and, respectively, 55%[50].
Therefore, the use of chemotherapy in combination with rituximab (an anti-CD20 monoclonal antibody) for the treatment of BCNHL in patients infected with HCV can produce liver dysfunction (as adverse effect), like the chemotherapy applied for Hodgkin’s lymphoma cure. A rare case of cholestatic hepatitis C was also published; it occured in a patient with diffuse large BCNHL and HCV infection[62]. The addition of immunotherapy with rituximab can increase the viral replication, but severe complications can occure especially in patients co-infected with HBV or immune immunodeficiency virus, in those with hepatocarcinoma, cirrhosis, or liver cytolysis (an increase of transaminases of grade > 2). There is not necessarily a direct association between the level of HCV viremia and the liver lesions[13]. The final result of standard immunochemotherapy applied to diffuse large BCNHL patients with HCV infection is not less good compared to those without this viral infection[13]. A solution to avoid severe liver toxicity in patients with compensated HCV induced liver cirrhosis and indolent BCNHL is the combination of bendamustine with rituximab[63].
CONCLUSION
There are strong arguments on the association between chronic HCV infection and BCNHL.
HCV lymphotropism and chronic antigenic stimulation are involved in B-lymphocyte expansion, as mixted cryoglobulinemia or monoclonal gammopathy of undetermined significance, which can progress to BCNHL[6,21].
Classical or direct acting antiviral therapy can help cure HCV-infected patients with indolent BCNHL. This also highlights the probable involvement of HCV in lymphomagenesis[14].
The use of chemotherapy in combination with rituximab for the treatment of BCNHL in HCV-infected patients can produce liver dysfunction.
The treatment of patients chronically infected with HCV and having BCNHL is complex and requires a multidisciplinary approach: a hematologist and a hepatologist should also be invited to participate. A careful monitoring of hepatic function is necessary[13,51].
What is the best conduct in front of BCNHL patients (and especially of those with large cells) with liver cytolysis, what is the risk/benefit ratio of rituximab treatment, and what are the conditions in which we need to start or stop the immuno-chemotherapy are topics to which we expect future answers from the scientific research[13].
Footnotes
Conflict-of-interest statement: The author has no conflict of interest associated with this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 26, 2016
First decision: May 12, 2016
Article in press: June 15, 2016
P- Reviewer: Scicchitano P S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Gill K, Ghazinian H, Manch R, Gish R. Hepatitis C virus as a systemic disease: reaching beyond the liver. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26660706. [DOI] [PMC free article] [PubMed]
- 2.Torres HA, Mahale P. Most patients with HCV-associated lymphoma present with mild liver disease: a call to revise antiviral treatment prioritization. Liver Int. 2015;35:1661–1664. doi: 10.1111/liv.12825. [DOI] [PubMed] [Google Scholar]
- 3.Fiorino S, Bacchi-Reggiani L, de Biase D, Fornelli A, Masetti M, Tura A, Grizzi F, Zanello M, Mastrangelo L, Lombardi R, et al. Possible association between hepatitis C virus and malignancies different from hepatocellular carcinoma: A systematic review. World J Gastroenterol. 2015;21:12896–12953. doi: 10.3748/wjg.v21.i45.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bende RJ, Janssen J, Wormhoudt TA, Wagner K, Guikema JE, van Noesel CJ. Identification of a novel stereotypic IGHV4-59/IGHJ5-encoded B-cell receptor subset expressed by various B-cell lymphomas with high affinity rheumatoid factor activity. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=Identification of a novel stereotypic IGHV4-59/IGHJ5-encoded B-cell receptor subset expressed by various B-cell lymphomas with high affinity rheumatoid factor activity. [DOI] [PMC free article] [PubMed]
- 5.Su TH, Liu CJ, Tseng TC, Chou SW, Liu CH, Yang HC, Wu SJ, Chen PJ, Chen DS, Chen CL, et al. Hepatitis C viral infection increases the risk of lymphoid-neoplasms: A population-based cohort study. Hepatology. 2016;63:721–730. doi: 10.1002/hep.28387. [DOI] [PubMed] [Google Scholar]
- 6.Zignego AL, Gragnani L, Piluso A, Sebastiani M, Giuggioli D, Fallahi P, Antonelli A, Ferri C. Virus-driven autoimmunity and lymphoproliferation: the example of HCV infection. Expert Rev Clin Immunol. 2015;11:15–31. doi: 10.1586/1744666X.2015.997214. [DOI] [PubMed] [Google Scholar]
- 7.Paydas S. Hepatitis C virus and lymphoma. Crit Rev Oncol Hematol. 2015;93:246–256. doi: 10.1016/j.critrevonc.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Allison RD, Tong X, Moorman AC, Ly KN, Rupp L, Xu F, Gordon SC, Holmberg SD; Chronic Hepatitis Cohort Study (CHeCS) Investigators. Increased incidence of cancer and cancer-related mortality among persons with chronic hepatitis C infection, 2006-2010. J Hepatol. 2015;63:822–828. doi: 10.1016/j.jhep.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grudeva-Popova J, Nenova I, Mateva N, Ananoshtev N, Popov V, Atanasova M. Non-Hodgkin lymphomas and carrier state of viral hepatitis B and C. J BUON. 2013;18:239–244. [PubMed] [Google Scholar]
- 10.Vannata B, Stathis A, Zucca E. Management of the marginal zone lymphomas. Cancer Treat Res. 2015;165:227–249. doi: 10.1007/978-3-319-13150-4_9. [DOI] [PubMed] [Google Scholar]
- 11.Slager SL, Benavente Y, Blair A, Vermeulen R, Cerhan JR, Costantini AS, Monnereau A, Nieters A, Clavel J, Call TG, et al. Medical history, lifestyle, family history, and occupational risk factors for chronic lymphocytic leukemia/small lymphocytic lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014:41–51. doi: 10.1093/jncimonographs/lgu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terrier B, Costagliola D, Prevot S, Chavez H, Missy P, Rince P, Costello R, Escaut L, Gabarre J, Joly B, et al. Characteristics of B-cell lymphomas in HIV/HCV-coinfected patients during the combined antiretroviral therapy era: an ANRS CO16 LYMPHOVIR cohort study. J Acquir Immune Defic Syndr. 2013;63:249–253. doi: 10.1097/QAI.0b013e31828a77f0. [DOI] [PubMed] [Google Scholar]
- 13.Visco C, Finotto S. Hepatitis C virus and diffuse large B-cell lymphoma: Pathogenesis, behavior and treatment. World J Gastroenterol. 2014;20:11054–11061. doi: 10.3748/wjg.v20.i32.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone A, Gloghini A. Relationships between lymphomas linked to hepatitis C virus infection and their microenvironment. World J Gastroenterol. 2013;19:7874–7879. doi: 10.3748/wjg.v19.i44.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu SC, Lin CW. Early-stage splenic diffuse large B-cell lymphoma is highly associated with hepatitis C virus infection. Kaohsiung J Med Sci. 2013;29:150–156. doi: 10.1016/j.kjms.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michot JM, Canioni D, Driss H, Alric L, Cacoub P, Suarez F, Sibon D, Thieblemont C, Dupuis J, Terrier B, et al. Antiviral therapy is associated with a better survival in patients with hepatitis C virus and B-cell non-Hodgkin lymphomas, ANRS HC-13 lympho-C study. Am J Hematol. 2015;90:197–203. doi: 10.1002/ajh.23889. [DOI] [PubMed] [Google Scholar]
- 17.Mollejo M, Menárguez J, Guisado-Vasco P, Bento L, Algara P, Montes-Moreno S, Rodriguez-Pinilla MS, Cruz MA, Casado F, Montalbán C, et al. Hepatitis C virus-related lymphoproliferative disorders encompass a broader clinical and morphological spectrum than previously recognized: a clinicopathological study. Mod Pathol. 2014;27:281–293. doi: 10.1038/modpathol.2013.120. [DOI] [PubMed] [Google Scholar]
- 18.Bracci PM, Benavente Y, Turner JJ, Paltiel O, Slager SL, Vajdic CM, Norman AD, Cerhan JR, Chiu BC, Becker N, et al. Medical history, lifestyle, family history, and occupational risk factors for marginal zone lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014:52–65. doi: 10.1093/jncimonographs/lgu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerhan JR, Kricker A, Paltiel O, Flowers CR, Wang SS, Monnereau A, Blair A, Dal Maso L, Kane EV, Nieters A, et al. Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr. 2014;2014:15–25. doi: 10.1093/jncimonographs/lgu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chien SH, Liu CJ, Hong YC, Teng CJ, Hu YW, Ku FC, Yeh CM, Chiou TJ, Gau JP, Tzeng CH. Development of second primary malignancy in patients with non-Hodgkin lymphoma: a nationwide population-based study. J Cancer Res Clin Oncol. 2015;141:1995–2004. doi: 10.1007/s00432-015-1979-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury T, Chen S, Adar T, Jacob EO, Mizrahi M. Hepatitis C infection and lymphoproliferative disease: accidental comorbidities? World J Gastroenterol. 2014;20:16197–16202. doi: 10.3748/wjg.v20.i43.16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caviglia GP, Sciacca C, Abate ML, Olivero A, Rosso C, Touscoz GA, Ciancio A, Rizzetto M, Smedile A. Chronic hepatitis C virus infection and lymphoproliferative disorders: mixed cryoglobulinemia syndrome, monoclonal gammopathy of undetermined significance, and B-cell non-Hodgkin lymphoma. J Gastroenterol Hepatol. 2015;30:742–747. doi: 10.1111/jgh.12837. [DOI] [PubMed] [Google Scholar]
- 23.Sulyok M, Makara M, Újhelyi E, Vályi-Nagy I. Non-Hodgkin lymphoma and hepatitis C: where we are and what next? Pathol Oncol Res. 2015;21:1–7. doi: 10.1007/s12253-014-9845-z. [DOI] [PubMed] [Google Scholar]
- 24.Nipp R, Mitchell A, Pishko A, Metjian A. Waldenström macroglobulinemia in hepatitis C: case report and review of the current literature. Case Rep Oncol Med. 2014;2014:165670. doi: 10.1155/2014/165670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santer DM, Ma MM, Hockman D, Landi A, Tyrrell DL, Houghton M. Enhanced activation of memory, but not naïve, B cells in chronic hepatitis C virus-infected patients with cryoglobulinemia and advanced liver fibrosis. PLoS One. 2013;8:e68308. doi: 10.1371/journal.pone.0068308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peveling-Oberhag J, Arcaini L, Hansmann ML, Zeuzem S. Hepatitis C-associated B-cell non-Hodgkin lymphomas. Epidemiology, molecular signature and clinical management. J Hepatol. 2013;59:169–177. doi: 10.1016/j.jhep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Ng PP, Kuo CC, Wang S, Einav S, Arcaini L, Paulli M, Portlock CS, Marcotrigiano J, Tarr A, Ball J, et al. B-cell receptors expressed by lymphomas of hepatitis C virus (HCV)-infected patients rarely react with the viral proteins. Blood. 2014;123:1512–1515. doi: 10.1182/blood-2013-10-532895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov AV, Bartosch B, Smirnova OA, Isaguliants MG, Kochetkov SN. HCV and oxidative stress in the liver. Viruses. 2013;5:439–469. doi: 10.3390/v5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucci FA, Broering R, Johansson P, Schlaak JF, Küppers R. B cells in chronically hepatitis C virus-infected individuals lack a virus-induced mutation signature in the TP53, CTNNB1, and BCL6 genes. J Virol. 2013;87:2956–2962. doi: 10.1128/JVI.03081-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khorshied MM, Gouda HM, Khorshid OM. Association of cytotoxic T-lymphocyte antigen 4 genetic polymorphism, hepatitis C viral infection and B-cell non-Hodgkin lymphoma: an Egyptian study. Leuk Lymphoma. 2014;55:1061–1066. doi: 10.3109/10428194.2013.820294. [DOI] [PubMed] [Google Scholar]
- 31.Sansonno D, Russi S, Serviddio G, Conteduca V, D’Andrea G, Sansonno L, Pavone F, Lauletta G, Mariggiò MA, Dammacco F. Interleukin 28B gene polymorphisms in hepatitis C virus-related cryoglobulinemic vasculitis. J Rheumatol. 2014;41:91–98. doi: 10.3899/jrheum.130527. [DOI] [PubMed] [Google Scholar]
- 32.Arcaini L, Rossi D, Lucioni M, Nicola M, Bruscaggin A, Fiaccadori V, Riboni R, Ramponi A, Ferretti VV, Cresta S, et al. The NOTCH pathway is recurrently mutated in diffuse large B-cell lymphoma associated with hepatitis C virus infection. Haematologica. 2015;100:246–252. doi: 10.3324/haematol.2014.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasama Y, Mizukami T, Kusunoki H, Peveling-Oberhag J, Nishito Y, Ozawa M, Kohara M, Mizuochi T, Tsukiyama-Kohara K. B-cell-intrinsic hepatitis C virus expression leads to B-cell-lymphomagenesis and induction of NF-κB signalling. PLoS One. 2014;9:e91373. doi: 10.1371/journal.pone.0091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruni R, Marcantonio C, Pulsoni A, Tataseo P, De Angelis F, Spada E, Marcucci F, Panfilio S, Bianco P, Riminucci M, et al. microRNA levels in paraffin-embedded indolent B-cell non-Hodgkin lymphoma tissues from patients chronically infected with hepatitis B or C virus. BMC Infect Dis. 2014;14 Suppl 5:S6. doi: 10.1186/1471-2334-14-S5-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Augello C, Gianelli U, Savi F, Moro A, Bonoldi E, Gambacorta M, Vaira V, Baldini L, Bosari S. MicroRNA as potential biomarker in HCV-associated diffuse large B-cell lymphoma. J Clin Pathol. 2014;67:697–701. doi: 10.1136/jclinpath-2014-202352. [DOI] [PubMed] [Google Scholar]
- 36.Rapisarda V, Marconi A, Candido S, Nicolosi D, Salmeri M, Gangemi P, Proietti L, Spandidos DA, Bracci M, Fenga C, et al. A tailored health surveillance program unveils a case of MALT lymphoma in an HCV-positive health-care worker. Oncol Lett. 2013;5:651–654. doi: 10.3892/ol.2012.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosad E, Said Abd El-Rahman Allam M, Moustafa HM, Mohammed AE, El kebeer AM, Abdel-Moneim SS. Telomeric 1p36.3 deletion and Ki-67 expression in B-Non-Hodgkin’s Lymphoma patients associated with chronic hepatitis C virus infection. J Viral Hepat. 2014;21:950–955. doi: 10.1111/jvh.12304. [DOI] [PubMed] [Google Scholar]
- 38.Lee SI, Heo NY, Park SH, Joo YD, Kim IH, Park JI, Kim JY, Kim SH, Shim HK. [Synchronous hepatocellular carcinoma and B-cell non-Hodgkin’s lymphoma in chronic hepatitis C patient] Korean J Gastroenterol. 2014;64:168–172. doi: 10.4166/kjg.2014.64.3.168. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi M, Asano N, Fukushima M, Honda T. Three different histological subtypes of Epstein-Barr virus-negative post-transplant lymphoproliferative disorder in a patient with hepatitis C infection. Int J Hematol. 2014;100:307–311. doi: 10.1007/s12185-014-1599-6. [DOI] [PubMed] [Google Scholar]
- 40.Kinch A, Baecklund E, Backlin C, Ekman T, Molin D, Tufveson G, Fernberg P, Sundström C, Pauksens K, Enblad G. A population-based study of 135 lymphomas after solid organ transplantation: The role of Epstein-Barr virus, hepatitis C and diffuse large B-cell lymphoma subtype in clinical presentation and survival. Acta Oncol. 2014;53:669–679. doi: 10.3109/0284186X.2013.844853. [DOI] [PubMed] [Google Scholar]
- 41.Tajiri H, Sugimachi K, Kinjo N, Ikebe M, Tanaka J, Tanaka K, Tsukamoto S, Mii S, Kajiwara E, Shimokama T, et al. Repeat hepatectomies for hepatic malignant lymphoma and hepatocellular carcinoma associated with chronic hepatitis C: report of a case. Surg Today. 2014;44:188–191. doi: 10.1007/s00595-013-0502-z. [DOI] [PubMed] [Google Scholar]
- 42.Borchardt RA, Torres HA. Challenges in managing hepatitis C virus infection in cancer patients. World J Gastroenterol. 2014;20:2771–2776. doi: 10.3748/wjg.v20.i11.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubio J, Franco F, Sánchez A, Cantos B, Méndez M, Calvo V, Maximiano C, Perez D, Millán I, Sánchez-Beato M, et al. Does the presence of hepatitis virus B and C influence the evolution of diffuse large B-cell lymphoma? Leuk Lymphoma. 2015;56:1686–1690. doi: 10.3109/10428194.2014.963576. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda I, Okada M, Inoue T, Tokugawa T, Ogawa H, Hirota S. Primary follicular lymphoma of the spleen incidentally found in a patient with alcohol- and hepatitis C-related liver cirrhosis. Int J Clin Exp Pathol. 2014;7:4484–4488. [PMC free article] [PubMed] [Google Scholar]
- 45.Beck K, Paul J, Sawardekar S, Harvey V. Secondary cutaneous marginal zone B-cell lymphoma presenting as lipoatrophy in a patient with hepatitis C. J Dermatol Case Rep. 2014;8:46–49. doi: 10.3315/jdcr.2014.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai H, Cherian R, Mathur S. Primary body cavity-based large B-cell lymphoma in an HIV and HHV-8 negative, HCV positive patient: a case report and literature review. Lab Med. 2014;45:136–140. doi: 10.1309/lmicz683orlrjrjo. [DOI] [PubMed] [Google Scholar]
- 47.Coskun A, Yukselen O, Yukselen V, Karaoglu AO. Lacrimal gland marginal zone lymphoma: regression after treatment of chronic hepatitis C virus infection: case report and review of the literature. Intern Med. 2013;52:2615–2618. doi: 10.2169/internalmedicine.52.0450. [DOI] [PubMed] [Google Scholar]
- 48.Terrier B, Chaara W, Dufat L, Geri G, Rosenzwajg M, Musset L, Sène D, Saadoun D, Six A, Klatzmann D, et al. Serum biomarker signature identifies patients with B-cell non-Hodgkin lymphoma associated with cryoglobulinemia vasculitis in chronic HCV infection. Autoimmun Rev. 2014;13:319–326. doi: 10.1016/j.autrev.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F, Sansonno D. Impact of Cryoglobulinemic Syndrome on the Outcome of Chronic Hepatitis C Virus Infection: A 15-Year Prospective Study. Medicine (Baltimore) 2013 doi: 10.1097/MD.0b013e31829d2abc. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merli M, Visco C, Spina M, Luminari S, Ferretti VV, Gotti M, Rattotti S, Fiaccadori V, Rusconi C, Targhetta C, et al. Outcome prediction of diffuse large B-cell lymphomas associated with hepatitis C virus infection: a study on behalf of the Fondazione Italiana Linfomi. Haematologica. 2014;99:489–496. doi: 10.3324/haematol.2013.094318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tasleem S, Sood GK. Hepatitis C Associated B-cell Non-Hodgkin Lymphoma: Clinical Features and the Role of Antiviral Therapy. J Clin Transl Hepatol. 2015;3:134–139. doi: 10.14218/JCTH.2015.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foster LH, Portell CA. The role of infectious agents, antibiotics, and antiviral therapy in the treatment of extranodal marginal zone lymphoma and other low-grade lymphomas. Curr Treat Options Oncol. 2015;16:28. doi: 10.1007/s11864-015-0344-6. [DOI] [PubMed] [Google Scholar]
- 53.Bonnet C, Lejeune M, Van Kemseke C, Bron D, Beguin Y. Current management of marginal zone lymphomas. Rev Med Suisse. 2015;11:1549–1556. [PubMed] [Google Scholar]
- 54.Zhao LJ, Chen F, Li JG, Yin R, Zhang XH, Huang SM, Liu F. Hepatitis C virus-related mixed cryoglobulinemic endocapillary proliferative glomerulonephritis and B-cell non-Hodgkin lymphoma: a case report and literature review. Eur Rev Med Pharmacol Sci. 2015;19:3050–3055. [PubMed] [Google Scholar]
- 55.Arcaini L, Vallisa D, Rattotti S, Ferretti VV, Ferreri AJ, Bernuzzi P, Merli M, Varettoni M, Chiappella A, Ambrosetti A, et al. Antiviral treatment in patients with indolent B-cell lymphomas associated with HCV infection: a study of the Fondazione Italiana Linfomi. Ann Oncol. 2014;25:1404–1410. doi: 10.1093/annonc/mdu166. [DOI] [PubMed] [Google Scholar]
- 56.Carrier P, Jaccard A, Jacques J, Tabouret T, Debette-Gratien M, Abraham J, Mesturoux L, Marquet P, Alain S, Sautereau D, et al. HCV-associated B-cell non-Hodgkin lymphomas and new direct antiviral agents. Liver Int. 2015;35:2222–2227. doi: 10.1111/liv.12897. [DOI] [PubMed] [Google Scholar]
- 57.Rossotti R, Travi G, Pazzi A, Baiguera C, Morra E, Puoti M. Rapid clearance of HCV-related splenic marginal zone lymphoma under an interferon-free, NS3/NS4A inhibitor-based treatment. A case report. J Hepatol. 2015;62:234–237. doi: 10.1016/j.jhep.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 58.Christensen S, Gillessen A. Response to peginterferon plus ribavirin and subsequent retreatment with telaprevir-based triple therapy in a patient with chronic lymphocytic leukaemia and chronic HCV genotype 1b infection. Infect Agent Cancer. 2014;9:10. doi: 10.1186/1750-9378-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen TT, Chiu CF, Yang TY, Lin CC, Sargeant AM, Yeh SP, Liao YM, Bai LY. Hepatitis C infection is associated with hepatic toxicity but does not compromise the survival of patients with diffuse large B cell lymphoma treated with rituximab-based chemotherapy. Leuk Res. 2015;39:151–156. doi: 10.1016/j.leukres.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Salah-Eldin MA, Ebrahim MA, El-Sadda W. Clinical outcome of HCV-positive patients with diffuse large B-cell lymphoma treated with rituximab-based chemotherapy. Ann Hematol. 2014;93:1903–1911. doi: 10.1007/s00277-014-2138-5. [DOI] [PubMed] [Google Scholar]
- 61.Zaky AH, Bakry R, El-sayed MI, Elwanis MA, Nabih O. Impact of treatment-related toxicity on outcome of HCV-positive diffuse large B-cell lymphoma in rituximab era. Hematology. 2014;19:412–416. doi: 10.1179/1607845413Y.0000000147. [DOI] [PubMed] [Google Scholar]
- 62.Pellicelli AM, D’Ambrosio C, Dessanti ML, Villani R, Fondacaro L, Miglioresi L, Grillo LR, Andreoli A. Cholestatic hepatitis C after chemotherapy containing rituximab in diffuse large B cell lymphoma. Ann Hepatol. 2015;14:756–761. [PubMed] [Google Scholar]
- 63.Parker SM, Hyder MA, Fesler MJ. Bendamustine and rituximab for indolent B-cell non-hodgkin lymphoma in patients with compensated hepatitis C cirrhosis: a case series. Clin Lymphoma Myeloma Leuk. 2013;13:e15–e17. doi: 10.1016/j.clml.2013.07.002. [DOI] [PubMed] [Google Scholar]


