Abstract
AIM: To clarify the characteristics of metabolite profiles in virus-related hepatocellular carcinoma (HCC) patients using serum metabolome analysis.
METHODS: The serum levels of low-molecular-weight metabolites in 68 patients with HCC were quantified using capillary electrophoresis chromatography and mass spectrometry. Thirty and 38 of the patients suffered from hepatitis B virus-related HCC (HCC-B) and hepatitis C virus-related HCC (HCC-C), respectively.
RESULTS: The main metabolites characteristic of HCC were those associated with glutathione metabolism, notably 13 γ-glutamyl peptides, which are by-products of glutathione induction. Two major profiles, i.e., concentration patterns, of metabolites were identified in HCC patients, and these were classified into two groups: an HCC-B group and an HCC-C group including some of the HCC-B cases. The receiver operating characteristic curve for the multiple logistic regression model discriminating HCC-B from HCC-C incorporating the concentrations of glutamic acid, methionine and γ-glutamyl-glycine-glycine showed a highly significant area under the curve value of 0.94 (95%CI: 0.89-1.0, P < 0.0001).
CONCLUSION: The serum levels of γ-glutamyl peptides, as well as their concentration patterns, contribute to the development of potential biomarkers for virus-related HCC. The difference in metabolite profiles between HCC-B and HCC-C may reflect the respective metabolic reactions that underlie the different pathogeneses of these two types of HCC.
Keywords: Metabolome, Hepatocellular carcinoma, γ-glutamyl peptides, Glutathione, Oxidative stress
Core tip: Serum metabolome analysis was applied to the patients of hepatocellular carcinoma (HCC) infected with hepatitis B virus (HBV) or hepatitis C virus (HCV) to clarify the characteristics of their metabolite profiles. This study demonstrated that the serum concentrations of γ-glutamyl peptides and their concentration patterns contribute to the development of potential biomarkers for virus-related HCC. The difference in metabolite profiles between the HCC patients infected with HBV and HCV may reflect the respective metabolic reactions that underlie the different pathogeneses of these two types of HCC.
INTRODUCTION
Chronic hepatitis due to hepatitis C virus (HCV) or hepatitis B virus (HBV) causes progressive liver inflammation, which may eventually lead to liver cirrhosis and hepatocellular carcinoma (HCC)[1,2]. The inflammatory process in hepatitis is closely associated with the oxidative stress induced by reactive oxygen species (ROS). Healthy individuals have their own protective mechanisms against oxidative stress, i.e., the induction of anti-oxidative substrates, such as glutathione, thioredoxin, vitamin A and vitamin E, or enzymes, such as superoxide dismutase, catalase and glutathione peroxidase, to remove ROS; however, these mechanisms are impaired in patients with chronic hepatitis B or C, and thus, the long-term exposure to oxidative stress during viral infection leads to progressive liver disease accompanied by a risk of HCC[3-9].
Metabolome analysis has been used to identify novel biomarkers for various liver diseases including HCC, based on the use of liquid chromatography and mass spectrometry (MS)[10,11] or nuclear magnetic resonance and MS[12-14]. In previous studies using capillary electrophoresis (CE) and MS[15,16], we showed that ophthalmate (γ-glutamyl-2-aminobutyrylglycine) was applicable as a biomarker of reduced glutathione depletion in mice with acetaminophen-induced hepatotoxicity. Furthermore, we have demonstrated that the serum levels of γ-glutamyl dipeptides (γ-Glu-X) were increased in the majority of patients with nine types of liver disease[17]. As γ-glutamyl dipeptides are synthesized via ligation of glutamine with various amino acids and amines by γ-glutamylcysteine synthetase, which is under feedback inhibition by glutathione, the level of γ-glutamyl dipeptides represents the degree of glutathione production. Therefore, γ-glutamyl dipeptides may represent key metabolites that reflect the extent of liver tissue injury due to oxidative stress.
In our previous study[17], we also evaluated the potential use of γ-glutamyl dipeptides for diagnosis of HCC, and found that multiple logistic regression (MLR) models employing four γ-glutamyl dipeptides (γ-Glu-Ala, γ-Glu-Citrulline, γ-Glu-Thr, and γ-Glu-Phe) were able to distinguish HCC from chronic hepatitis and cirrhosis. Furthermore, anti-viral therapy for chronic hepatitis C using pegylated interferon plus ribavirin, which is known to reduce the risk of HCC development[18-21], has been reported to improve the serum levels of γ-glutamyl dipeptides in treated patients[22]. Thus, γ-glutamyl dipeptides have the potential to become biomarkers for HCC, and monitoring of their serum levels may be useful for the prediction of HCC occurrence.
Although chronic HBV or HCV infection may lead to HCC as an end-stage liver disease, the inflammatory process during chronic viral infection, which finally results in HCC, might differ between HBV and HCV infections. In fact, a genomic study of gene expression in liver tissues chronically infected with HBV and HCV demonstrated that the genes expressed predominantly were those related to inflammation and the anti-inflammatory response, respectively[23]. It still remains unknown whether the serum metabolite profiles differ between patients with HBV-related HCC (HCC-B) and those with HCV-related HCC (HCC-C).
In the present study, we conducted a metabolome analysis of serum samples of HCC-B and HCC-C using CE-MS/MS. It was anticipated that this might lead to the development of a useful biomarker for virus-related HCC in clinical practice, and yield valuable information on differences in metabolite profiles between HCC-B and HCC-C.
MATERIALS AND METHODS
Patients and sample collection
Sixty-eight patients who had been diagnosed for the first time as having HCC had chronic HBV or HCV infection, comprising 50 men and 18 women, with an age range of 41 to 87 years (68.6 ± 11.5 years, mean ± SD). HCC patients without chronic viral infection such as those having alcoholic liver injury, autoimmune liver disease or metabolic liver disease were excluded. Each patient’s serum was collected as early as possible following the date of HCC diagnosis; the mean date of serum collection was 33.8 d after the date of HCC diagnosis. Fifty-three (78%) of the serum samples were collected within 30 d after diagnosis. The samples were kept in clean tubes and stored at -80 °C. The metabolome analysis was carried out at the Institute for Advanced Biosciences, Keio University, Tsuruoka, Japan. This study was approved by the institutional ethics committee, and all patients gave their informed consent.
Serum metabolome analysis
Treatment of samples and quantification of metabolites: Following centrifugation for 10 min at 2000 × g, the serum samples were collected and stored at -80 °C until measurement. Prior to metabolome analysis, the samples (40 μL) were added to 400 μL of methanol containing internal standards, together with methionine sulfone (Wako, Osaka, Japan), D-camphor-10-sulfonic acid (Wako), and 2-(n-morpholino)ethanesulfonic acid (Dojindo, Kumamoto, Japan), each at 20 μmol/L. Then 120 μL of Milli-Q water (Millipore, Billerica, MA) and 400 μL of chloroform were added, and the solution was centrifuged at 10000 × g for 3 min at 4 °C. The 300 μL upper aqueous layer was centrifugally filtered through a Millipore 5-kDa cutoff filter to remove large molecules, and the filtrate was dried by centrifugal concentration for 2 h at 40 °C. Finally, it was dissolved in 20 μL Milli-Q water containing reference compounds (3-aminopyrrolidine and trimesate at 200 μmol/L each). The γ-glutamyl peptides, glucosamine, and reduced and oxidized forms of glutathione were quantified using CE-MS/MS. The other metabolites were quantified using capillary electrophoresis-time-of-flight mass spectrometry, following the procedures previously described[22] using different version of software; Agilent ChemStation software (B.04.03) and Agilent MassHunter (B.04.00 for anion and B02.01 for cation).
Profiling technique for serum metabolites using CE-MS/MS: The metabolites were separated in a fused silica capillary (50 μm i.d. × 100 cm) filled with 200 mmol/L ammonium acetate (pH 3.3) as the run buffer[15]. Prior to each run, the run buffer was injected for 8 min as preconditioning. A sample solution was injected at 50 mbar for 10 s (10 nL), and a voltage of 30 kV was applied. The capillary temperature and sample tray were set at 20 °C and below 5 °C, respectively. The mass spectrometer was set to run a multi-channel reaction, while monitoring in positive ion mode. The sheath liquid comprised 0.5 mmol/L ammonium acetate in methanol/water (50% v/v) delivered at 10 μL/min. The flow rate of heated dry nitrogen gas (heater temperature, 300 °C) was maintained at 10 L/min. The capillary voltage was set at 4 kV. The pressure and flow rate of the nebulizer gas were 7 psi and 10 μL/min, respectively. Exact mass data were acquired at a rate of 1.5 spectra/s. Mass values for precursor and productions, fragmenter voltage, and collision energy were optimized for the individual metabolites. AS the CE-MS/MS instrument, we used a G1600AX Agilent CE capillary electrophoresis system (Agilent), an Agilent 1100 series binary HPLC pump, a G1948B ion source, a G1607A Agilent CE-ESI-MS sprayer kit, and an Agilent 6410 series Triple Quad. Data were acquired with Agilent ChemStation software (A.09.03) and Agilent MassHunter (Data Acquisition for Triple Quad B.04.01). Metabolite identification was conducted by matching the m/z values and corrected migration times[24]. To quantify individual metabolites, commercially available standards were analyzed prior to sample analysis. The peak areas of all metabolites were divided by the peak area of the internal standard and compared with the standard compounds to calculate the concentrations. Raw data were processed using MasterHands[25].
Statistical analysis
The Mann-Whitney U-test, Wilcoxon matched-pairs signed rank test and Fisher exact test were used to assess the statistical significance of differences at a significance level of P < 0.05. Heat map visualization was performed to indicate the similarities of metabolites and samples. Hierarchical clustering was conducted by Pearson correlation, and only metabolites showing P < 0.05 (Mann-Whitney U-test) between HCC-B and HCC-C were used. The colors on the heat map were determined by the Z-score of the metabolite concentration. Receiver operating characteristic (ROC) curve analysis was used to assess the discrimination ability of individual metabolites. To assess the ability to discriminate HCC-C from HCC-B using multiple metabolites, we developed a MLR model, for which metabolites were selected by the forward and backward feature selection method using a threshold of P < 0.2 for addition and one of P > 0.2 for elimination of metabolites. To evaluate the generalization abilities of the developed model, we conducted cross-validation (CV) and bootstrap analysis. In this study, we repeated 200 runs with different random values for each of the 2-fold, 5-fold, and 10-fold CV analyses. Bootstrap analysis was conducted to obtain unbiased estimates of the developed model; 200 repetitions were generated via random selection of individuals allowing redundancy. We used JMP (version 10.0.2, SAS Institute, Cary, NC) for the development of the MLR, Weka (version 3.6.10, The University of Waikato, Hamilton, New Zealand) for CV and bootstrap analysis, Mev TM4 software (version 4.7.4, Dana-Farber Cancer Institute, Boston, MA) for clustering and heat map visualization, and GraphPad Prism (version 5.0.4, Intuitive Software, San Diego, CA) for ROC curve analysis and box-plot visualization.
RESULTS
Clinical characteristics of the patients
The characteristics of the HCC patients in this study are shown in Table 1. Thirty and thirty-eight of them had chronic HBV and HCV infection, respectively. Co-infection of HBV and HCV was not found in any of the subjects. Eighteen and 41 patients had AFP (6.2 ng/mL) and des-γ-carboxy prothrombin (DCP) (40 mAU/mL) levels lower than the reference value, respectively. The viral genotype was determined in all cases: genotype B in 14, genotype C in 15 and genotype D in 1 for the HBV genotype, and genotype 1b in 34 and genotype 2a in 4 for the HCV genotype. Higher proportions of men and younger patients were found in the HCC-B group than in the HCC-C group. There were no significant differences in the levels of serum albumin, total bilirubin, aspartate aminotransferase, alanine aminotransferase, fasting blood glucose, γ-glutamyl transferase, creatinine, platelet count, Fib-4 index, elevated AFP or DCP, between the two groups.
Table 1.
Clinical characteristics of the study subjects
| Hepatitis B-associated HCC (n = 30) | Hepatitis C-associated HCC (n = 38) | P value | |
| Sex (M/F) | 28/2 | 22/16 | < 0.011 |
| Age (yr) | 62.6 ± 10.9 | 73.4 ± 9.7 | < 0.012 |
| Albumin (g/dL) | 3.4 ± 0.6 | 3.4 ± 0.5 | NS2 |
| Total bilirubin (mg/dL) | 1.2 ± 0.6 | 1.4 ± 1.3 | NS2 |
| AST (U/L) | 75.6 ± 76.2 | 57.8 ± 31.6 | NS2 |
| ALT (U/L) | 54.3 ± 38.4 | 49.8 ± 38.3 | NS2 |
| Fasting blood glucose (mg/dL) | 104.5 ± 22.2 | 113.1 ± 36.0 | NS2 |
| γ-glutamyl transferase (U/L) | 77.0 ± 64.8 | 60.4 ± 58.1 | NS2 |
| Creatinine (mg/dL) | 0.73 ± 0.19 | 1.01 ± 1.53 | NS2 |
| Platelet count (× 103/μL) | 13.5 ± 8.4 | 11.7 ± 4.7 | NS2 |
| Fib-4 index | 5.7 ± 4.4 | 6.4 ± 3.9 | NS2 |
| AFP (ng/mL) ≤ 6.2 /> 6.2 | 10/20 | 8/30 | NS1 |
| DCP (mAU/mL) ≤ 40 /> 40 | 17/13 | 24/14 | NS1 |
| HBs antigen, positive | 30 | 0 | |
| HCV-antibody, positive | 0 | 38 | |
| HBV genotype (A/B/C/D) | 0/14/15/1 | NA | |
| HCV genotype (1b/2a/2b) | NA | 34/4/0 |
Fisher exact test;
Mann-Whitney U-test. Data were expressed as mean ± SD. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; NS: Not significant; NA: Not applicable; DCP: Des-γ-carboxy prothrombin; HCC: Hepatocellular carcinoma.
Serum metabolite profiles in virus-related HCC
The serum metabolite profiles of patients with HCC-B or HCC-C are shown as a heat map in Figure 1. Two major patterns of metabolite profiles were identified in cases of virus-related HCC. Cluster X on the horizontal axis of Figure 1 was a characteristic of only HCC-B cases. The other one, shown as cluster Y on the horizontal axis of Figure 1, was characteristic of HCC-C cases and some HCC-B cases. The concentrations of metabolites showed an almost inverse association between these two patterns. γ-Glutamyl peptides were identified as a major marker of HCC. The concentrations of nine γ-glutamyl dipeptides (γ-Glu-Trp, γ-Glu-Tyr, γ-Glu-Phe, γ-Glu-Val, γ-Glu-Leu, γ-Glu-Ile, γ-Glu-His, γ-Glu-Thr and γ-Glu-Glu), as well as those of their binding amino acids (Asp, Glu, Ile, Val, Leu, Thr, Ser, Gly and Ala), were greater in cluster X than in cluster Y, and are shown as cluster A on the vertical axis of Figure 1. On the other hand, the concentrations of four γ-glutamyl peptides, including two γ-glutamyl dipeptides and two γ-glutamyl tripeptides (γ-Glu-Gly-Gly, γ-Glu-Gln, γ-Glu-Asp-Gly and γ-Glu-Met), as well as those of their binding peptides and amino acids (Gly-Gly, Gln and Met), were greater in cluster Y than in cluster X, and are shown as cluster B on the vertical axis of Figure 1. In addition to the γ-glutamyl peptides, 1-methyladenosine, which is a modified nucleoside induced by the post-transcriptional methylation of adenosine by methyl-1-adenosine transferase, was also demonstrated to be a potential marker of HCC. The usefulness of 1-methyladenosine for a diagnostic marker of HCC was first reported by Chen et al[39], the current study replicated this importance and demonstrated that the concentration of 1-methyladenosine was increased in cluster Y.
Figure 1.
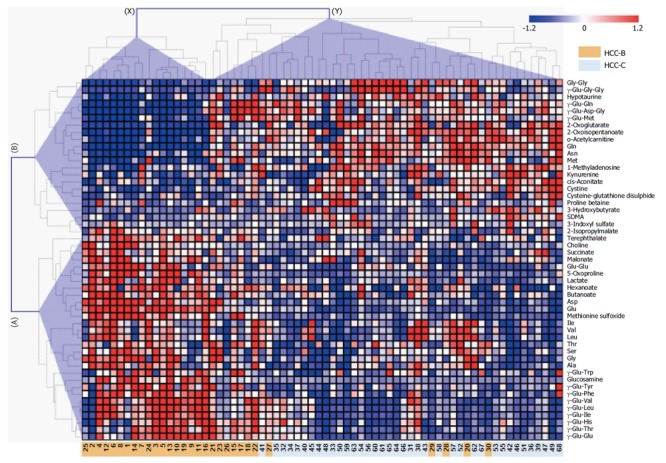
Heat map of quantified metabolites showing significant differences (P < 0.05; Mann-Whitney test) between the hepatitis B virus-related hepatocellular carcinoma and hepatitis C virus-related hepatocellular carcinoma groups. The colors on the heat map were determined by the Z-score, reflecting the relative concentrations. The red, blue, and white colors indicate relatively higher, lower and average concentrations, respectively. The numbers at the bottom indicate the case number where orange and light blue indicate HCC-B and HCC-C, respectively. The clusters of metabolites and individuals are labeled A-B on the vertical axis and X–Y on the horizontal axis, respectively. HCC-B: Hepatitis B virus-related HCC; HCC-C: Hepatitis C virus-related HCC; HCC: Hepatocellular carcinoma.
Comparison of serum metabolite levels between HCC-B and HCC-C
The serum levels of metabolites were compared between HCC-B and HCC-C. ROC curve analysis was conducted to calculate the area under the curve (AUC) values for all metabolites. The AUC values for individual metabolites showing significant (P < 0.05) ability to discriminate between HCC-B and HCC-C are visualized in Figure 2. These metabolites included 15 γ-glutamyl peptides (12 γ-glutamyl dipeptides and 3 γ-glutamyl tripeptides) and 12 amino acids. The 23 metabolites showed relatively high AUC values of > 0.75, and glutamic acid exhibited the highest value at 0.89 (95%CI: 0.79-0.98, P < 0.0001). Of these metabolites, methionine, γ-Glu-Gly-Gly and glutamic acid were selected for the MLR model using the feature selection procedure (Table 2). Serum concentrations of glutamic acid were significantly lower in HCC-C than in HCC-B, whereas those of methionine and γ-Glu-Gly-Gly were significantly higher in HCC-C than in HCC-B (glutamic acid: 475.4 ± 206.7 vs 182.6 ± 71.7, P < 0.0001; methionine: 37.1 ± 36.1 vs 52.6 ± 29.2, P = 0.016; γ-Glu-Gly-Gly: 0.035 ± 0.052 vs 0.109 ± 0.106, P = 0.001, HCC-B vs HCC-C, mean ± SD) (Figure 3A-C). The MLR model using these three metabolites showed significant ability to discriminate between HCC-B and HCC-C (probability of HCC %: 17.6 ± 31.3 vs 86.1 ± 14.2, P < 0.0001, HCC-B vs HCC-C, mean ± standard deviation) (Figure 3D). The AUC values of the three metabolites for discriminating HCC-C from HCC-B were 0.89 (95%CI: 0.79-0.98, P < 0.0001) for glutamic acid, 0.67 (95%CI: 0.53-0.81, P = 0.016) for methionine, and 0.73 (95%CI: 0.61-0.85, P = 0.001) for γ-Glu-Gly-Gly (Figure 4A-C). The ROC curve for the MLR model incorporating the levels of these three metabolites for discriminating HCC-C from HCC-B showed a high and significant AUC value (AUC = 0.94, 95%CI: 0.89-1.0, P < 0.0001) (Figure 4D). The model also showed high AUC values in each of the 200 repetitions of CV analysis, with a median of 0.91 (0.87-0.93, from minimum to maximum), 0.91 (0.85-0.94), and 0.91 (0.73-0.94), for 10-fold, 5-fold, and 2-fold CV, respectively. Bootstrap analysis also yielded high AUC values of 0.95 (0.86-1.0). The results of both analyses indicated the high generalization ability of the developed model.
Figure 2.
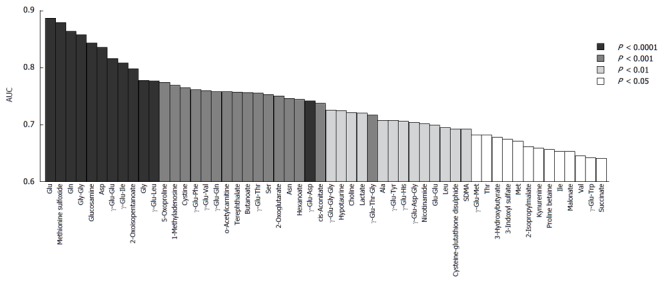
Area under the curve values of individual metabolites showing significant ability to discriminate (P < 0.05) between hepatitis B virus-related hepatocellular carcinoma and hepatitis C virus-related hepatocellular carcinoma by receiver operating characteristic analysis. The color corresponds to the level of statistical significance. HCC-B: Hepatitis B virus-related HCC; HCC-C: Hepatitis C virus-related HCC; HCC: Hepatocellular carcinoma.
Table 2.
Parameters of multiple logistic regression model
| Metabolite | Coefficient | 95%CI | Odds ratio | 95%CI | P value | ||
| (Intercept) | -8.01 | -14.0 | -4.14 | 1.00 × 10-3 | |||
| Met | 0.0521 | 0.018 | 0.0979 | 1.05 | 1.02 | 1.10 | 8.60 × 10-3 |
| γ-Glu-Gly-Gly | -17.6 | -35.7 | -5.31 | 2.23 × 10-8 | 3.18 × 10-16 | 4.93 × 10-3 | 1.97 × 10-2 |
| Glu | 0.0255 | 0.0135 | 0.0454 | 1.03 | 1.01 | 1.05 | 1.30 × 10-3 |
Met: Methionine; Glu: Glutamic acid; γ-Glu-Gly-Gly: γ-glutamyl-glycine-glycine.
Figure 3.
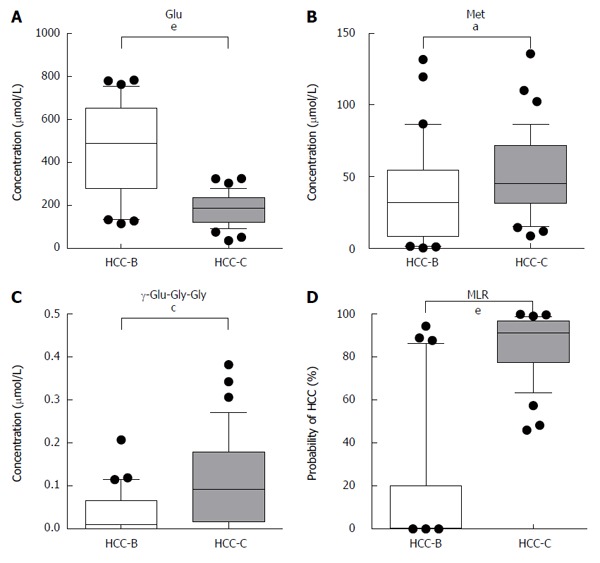
Serum concentrations of glutamic acid (A), methionine (B) and γ-glutamyl-glycine-glycine (C) in hepatitis B virus-related hepatocellular carcinoma and hepatitis C virus-related hepatocellular carcinoma. The multiple logistic regression model using these three metabolites demonstrated that they had a significant ability to discriminate between HCC-B and HCC-C (D). aP < 0.05, cP < 0.01, eP < 0.0001 between groups. Glu: Glutamic acid; Met: Methionine; γ-Glu-Gly-Gly: γ-glutamyl-glycine-glycine; MLR: Multiple logistic regression; HCC-B: Hepatitis B virus-related HCC; HCC-C: Hepatitis C virus-related HCC; HCC: Hepatocellular carcinoma.
Figure 4.
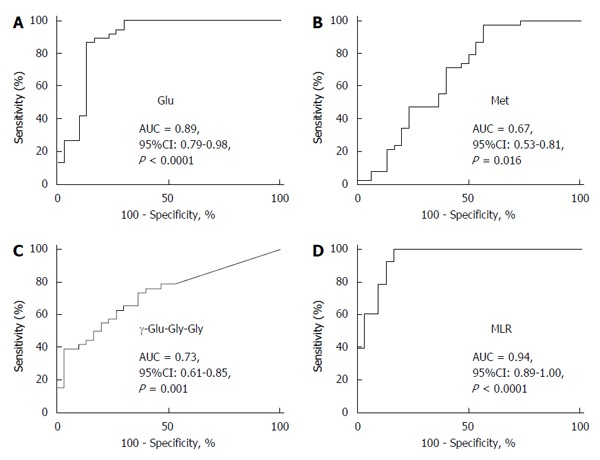
Area under the curve values for glutamic acid (A), methionine (B) and γ-glutamyl-glycine-glycine (C), and the multiple logistic regression model incorporating the concentrations of these three metabolites (D) for discriminating hepatitis B virus-related hepatocellular carcinoma from hepatitis C virus-related hepatocellular carcinoma by receiver operating characteristic analysis. Glu: Glutamic acid; Met: Methionine; γ-Glu-Gly-Gly: γ-glutamyl-glycine-glycine; MLR: Multiple logistic regression; AUC: Area under the curve; HCC-B: Hepatitis B virus-related HCC; HCC-C: Hepatitis C virus-related HCC; HCC: Hepatocellular carcinoma.
Correlation of serum concentrations between γ-glutamyl peptides and their binding amino acids
The serum concentrations of most γ-glutamyl peptides were correlated significantly with those of their binding amino acids (Table 3).
Table 3.
Correlation coefficients between amino acids and γ-glutamyl peptides
| Amino acid | γ-glutamyl peptides | Spearman R | 95%CI | P value (two-tailed) |
| Ile | γ-Glu-Ile | 0.4584 | 0.2401-0.6325 | < 0.0001 |
| Ala | γ-Glu-Ala | 0.04022 | -0.4898 | 0.7447 |
| Ser | γ-Glu-Ser | 0.2117 | -0.4698 | 0.0831 |
| Val | γ-Glu-Val | 0.4565 | 0.2379-0.6311 | < 0.0001 |
| Thr | γ-Glu-Thr | 0.3909 | 0.1610-0.5805 | 0.001 |
| Leu | γ-Glu-Leu | 0.4898 | 0.2779-0.6562 | < 0.0001 |
| Asn | γ-Glu-Asn | 0.4012 | 0.1729-0.5885 | 0.0007 |
| Lys | γ-Glu-Lys | 0.2909 | 0.04915-0.5004 | 0.0161 |
| Gln | γ-Glu-Gln | 0.6387 | 0.4665-0.7642 | < 0.0001 |
| Glu | γ-Glu-Glu | 0.8268 | 0.7294-0.8913 | < 0.0001 |
| His | γ-Glu-His | 0.2982 | 0.05714-0.5064 | 0.0135 |
| Phe | γ-Glu-Phe | 0.4886 | 0.2765-0.6554 | < 0.0001 |
| Arg | γ-Glu-Arg | 0.4258 | 0.2016-0.6076 | 0.0003 |
| Tyr | γ-Glu-Tyr | 0.6937 | 0.5404-0.8024 | < 0.0001 |
| Asp | γ-Glu-Asp | 0.5566 | 0.3606-0.7056 | < 0.0001 |
| Met | γ-Glu-Met | 0.5418 | 0.3420-0.6947 | < 0.0001 |
| Trp | γ-Glu-Trp | 0.3433 | 0.1070-0.5428 | 0.0042 |
| Gly | γ-Glu-Gly | 0.05704 | -0.489 | 0.6441 |
| Gly | γ-Glu-Gly-Gly | 0.006012 | -0.4905 | 0.9612 |
DISCUSSION
The progression of liver disease in patients with chronic HBV or HCV infection, which ultimately leads to HCC, is attributable to long-standing inflammation and fibrosis. As oxidative stress plays an important role in this clinical course[3-9], the metabolites accompanying production of oxidative stress would be likely candidates for peptide-based biomarker diagnosis of HCC[26,27]. From this viewpoint, we have focused on the metabolites associated with glutathione metabolism as indicators for monitoring of liver disease, because glutathione metabolism in liver cells plays a significant role in protecting them against ROS[15-17]. Specifically, the γ-glutamyl peptides are potential candidate biomarkers of liver disease because they are formed by the binding of glutamic acid to various amino acids through catalysis by γ-glutamylcysteine synthetase, and are produced as by-products of glutathione, which confers a protective effect against oxidative stress[17,28,29]. The γ-glutamyl cycle is activated by glutathione production in patients with liver diseases such as hepatitis, the glutathione being consumed to neutralize generated ROS, which, in turn, leads to activation of γ-glutamylcysteine synthetase and results in biosynthesis of glutathione together with γ-glutamyl peptides. Our previous metabolome study demonstrated that γ-glutamyl dipeptides can be useful indicators of liver diseases including HCC[17].
In this study, high concentrations of γ-glutamyl peptides, including 11 γ-glutamyl dipeptides and 2 γ-glutamyl tripeptides, were demonstrated in patients with virus-related HCC. The concentrations of the amino acids that composed these γ-glutamyl peptides were also high, and were significantly correlated with those of their γ-glutamyl peptides. Metabolome analysis of the present set of HCC cases enabled us to separate them into two metabolite clusters: pattern X representing HCC-B, and pattern Y representing HCC-C and a proportion of HCC-B. Although both patterns included various γ-glutamyl peptides, the concentrations of metabolites were characteristic to each cluster pattern. In cluster pattern X that included only HCC-B, higher concentrations of metabolites related to glutathione metabolism, γ-glutamyl peptides and their binding amino acids, were notable. In cluster pattern Y including all the HCC-C and some of the HCC-B, higher concentrations of not only metabolites related to glutathione metabolism but also those associated with fatty acid metabolism and the anti-inflammatory response, such as o-acetylcarnitine and kynurenine, were evident. These findings suggest that the liver metabolism finally resulting in cancer through long-term viral infection might have differed between the two patterns.
The two major metabolite clusters shown by the heat map indicated that they were roughly divisible into two categories representing HCC-B and HCC-C. In fact, the concentrations of many metabolites differed significantly between HCC-B and HCC-C, and MLR analysis using three chosen metabolites - glutamic acid, methionine and γ-Glu-Gly-Gly - was able to discriminate cases of HCC-C from those of HCC-B with a high degree of significance (P < 0.0001). This difference in the profiles of metabolites may reflect the fact that the pathway of cancer occurrence may differ between HCC-B and HCC-C. It is well known that chronic HCV infection is accompanied by metabolic disorders such as lipid metabolic disorder[30,31] and/or glucose metabolic disorder[32-34], both of which are closely associated with occurrence of HCC, and such impairment of metabolism has been reported in the metabolome of HCV-infected cells[35]. Patients infected with HBV or HCV show impairments of the protective mechanisms against oxidative stress[3-9]. The life-cycle of the virus in liver cells differs between HBV and HCV[36]. Such pathological and virological differences between patients with HBV and HCV infection may influence the metabolic pathway that culminates in HCC as an end-stage liver disease, thus possibly resulting in different patterns of metabolite concentrations between HCC-B and HCC-C.
Other than γ-glutamyl peptides, 1-methyladenosine has also been demonstrated to be characteristic of HCC. The concentration of 1-methyladenosine in urine has been reported to be increased in patients with ovarian and breast cancer[37,38]. Chen et al[39] identified serum 1-methyladenosine as a characteristic metabolite of HCC for the first time by a liquid chromatography-MS-based metabolome study, and reported that elevation of its serum concentration was applicable as an additional diagnostic biomarker for HCC, particularly in combination with serum AFP. The increased serum concentration of 1-methyladenosine in HCC patients may reflect the hyperactive nucleoside modification associated with hyper methylation in response to long-term inflammation and oxidative DNA damage, which have often been found in patients with cancers of other digestive organs[40,41]. In the present study, 1-methyladenosine clearly discriminated HCC in cluster pattern Y, therefore, it is expected to become an indicator in this type of HCC. As the limitations of this study, many conditions that may affect the concentration and variation of metabolites in patient’s serum, such as the stage of tumor, the stage of fibrosis or inflammation in the liver, their received treatment and viral load, were not identical among the cases studied. Further studies are needed to validate the present findings in a larger cohort of patients.
In conclusion, the present study has shown that high concentrations of γ-glutamyl peptides, including 11 γ-glutamyl dipeptides and 2 γ-glutamyl tripeptides, were demonstrated in patients with virus-related HCC. Two major patterns of metabolite profiles identified were roughly divisible into two categories representing HCC-B and HCC-C. These findings contribute to the development of potential biomarkers for virus-related HCC. The difference in metabolite profiles between HCC-B and HCC-C may reflect the respective metabolic reactions that underlie the different pathogeneses of these two types of HCC.
COMMENTS
Background
Chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection is a major causes of hepatocellular carcinoma (HCC) worldwide. In this study, the authors conducted a metabolome analysis of serum samples of HBV-related HCC (HCC-B) and HCV-related HCC (HCC-C) using capillary electrophoresis chromatography and mass spectrometry, trying to explore a useful biomarker for virus-related HCC in clinical practice, and yield valuable information on differences in metabolite profiles between HCC-B and HCC-C.
Research frontiers
Metabolome analysis has been used to identify novel biomarkers for various liver diseases, based on the use of liquid chromatography and mass spectrometry or nuclear magnetic resonance and mass spectrometry. The presented study have demonstrated that serum levels of γ-glutamyl dipeptides can be useful indicators of liver diseases.
Innovations and breakthroughs
In this study, high concentrations of γ-glutamyl peptides, including 11 γ-glutamyl dipeptides and 2 γ-glutamyl tripeptides, were demonstrated in patients with virus-related HCC. Two major patterns of metabolite profiles identified were roughly divisible into two categories representing HCC-B and HCC-C.
Applications
The findings of this study contribute to the development of potential biomarkers for virus-related HCC, and they may reflect the respective metabolic reactions that underlie the different pathogeneses of these two types of HCC.
Peer-review
Serum metabolome profiles characterized by patients with hepatocellular carcinoma associated with hepatitis B and C. This is an interesting study. These researches tried to investigate the serum metabolome profiles of patients with hepatocellular carcinoma associated with hepatitis B and C.
Footnotes
Supported by the Japan Agency for Medical Research and Development; and Yamagata Prefectural Government and City of Tsuruoka.
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of Yamagata University School of Medicine and Keio University Institute for Advanced Biosciences.
Conflict-of-interest statement: The authors have no conflict of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: April 7, 2016
First decision: May 12, 2016
Article in press: June 15, 2016
P- Reviewer: Mendez-Sanchez N, Nagahara H S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Di Bisceglie AM, Goodman ZD, Ishak KG, Hoofnagle JH, Melpolder JJ, Alter HJ. Long-term clinical and histopathological follow-up of chronic posttransfusion hepatitis. Hepatology. 1991;14:969–974. doi: 10.1016/0270-9139(91)90113-a. [DOI] [PubMed] [Google Scholar]
- 2.Kiyosawa K, Sodeyama T, Tanaka E, Gibo Y, Yoshizawa K, Nakano Y, Furuta S, Akahane Y, Nishioka K, Purcell RH. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: analysis by detection of antibody to hepatitis C virus. Hepatology. 1990;12:671–675. doi: 10.1002/hep.1840120409. [DOI] [PubMed] [Google Scholar]
- 3.Tasdelen Fisgin N, Aydin BK, Sarikaya H, Tanyel E, Esen S, Sunbul M, Leblebicioglu H. Oxidative stress and antioxidant defense in patients with chronic hepatitis B. Clin Lab. 2012;58:273–280. [PubMed] [Google Scholar]
- 4.Li T, Zhao XP, Wang LY, Gao S, Zhao J, Fan YC, Wang K. Glutathione S-transferase P1 correlated with oxidative stress in hepatocellular carcinoma. Int J Med Sci. 2013;10:683–690. doi: 10.7150/ijms.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi J. Oxidative stress, endogenous antioxidants, alcohol, and hepatitis C: pathogenic interactions and therapeutic considerations. Free Radic Biol Med. 2012;52:1135–1150. doi: 10.1016/j.freeradbiomed.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Fujinaga H, Tsutsumi T, Yotsuyanagi H, Moriya K, Koike K. Hepatocarcinogenesis in hepatitis C: HCV shrewdly exacerbates oxidative stress by modulating both production and scavenging of reactive oxygen species. Oncology. 2011;81 Suppl 1:11–17. doi: 10.1159/000333253. [DOI] [PubMed] [Google Scholar]
- 7.Cardin R, Piciocchi M, Sinigaglia A, Lavezzo E, Bortolami M, Kotsafti A, Cillo U, Zanus G, Mescoli C, Rugge M, et al. Oxidative DNA damage correlates with cell immortalization and mir-92 expression in hepatocellular carcinoma. BMC Cancer. 2012;12:177. doi: 10.1186/1471-2407-12-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukiyama-Kohara K. Role of oxidative stress in hepatocarcinogenesis induced by hepatitis C virus. Int J Mol Sci. 2012;13:15271–15278. doi: 10.3390/ijms131115271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paracha UZ, Fatima K, Alqahtani M, Chaudhary A, Abuzenadah A, Damanhouri G, Qadri I. Oxidative stress and hepatitis C virus. Virol J. 2013;10:251. doi: 10.1186/1743-422X-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao JF, Varghese RS, Zhou B, Nezami Ranjbar MR, Zhao Y, Tsai TH, Di Poto C, Wang J, Goerlitz D, Luo Y, et al. LC-MS based serum metabolomics for identification of hepatocellular carcinoma biomarkers in Egyptian cohort. J Proteome Res. 2012;11:5914–5923. doi: 10.1021/pr300673x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang B, Chen D, Chen Y, Hu Z, Cao M, Xie Q, Chen Y, Xu J, Zheng S, Li L. Metabonomic profiles discriminate hepatocellular carcinoma from liver cirrhosis by ultraperformance liquid chromatography-mass spectrometry. J Proteome Res. 2012;11:1217–1227. doi: 10.1021/pr2009252. [DOI] [PubMed] [Google Scholar]
- 12.Gao H, Lu Q, Liu X, Cong H, Zhao L, Wang H, Lin D. Application of 1H NMR-based metabonomics in the study of metabolic profiling of human hepatocellular carcinoma and liver cirrhosis. Cancer Sci. 2009;100:782–785. doi: 10.1111/j.1349-7006.2009.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahon P, Amathieu R, Triba MN, Bouchemal N, Nault JC, Ziol M, Seror O, Dhonneur G, Trinchet JC, Beaugrand M, et al. Identification of serum proton NMR metabolomic fingerprints associated with hepatocellular carcinoma in patients with alcoholic cirrhosis. Clin Cancer Res. 2012;18:6714–6722. doi: 10.1158/1078-0432.CCR-12-1099. [DOI] [PubMed] [Google Scholar]
- 14.Dumas ME, Kinross J, Nicholson JK. Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology. 2014;146:46–62. doi: 10.1053/j.gastro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Soga T, Baran R, Suematsu M, Ueno Y, Ikeda S, Sakurakawa T, Kakazu Y, Ishikawa T, Robert M, Nishioka T, et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281:16768–16776. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 16.Shintani T, Iwabuchi T, Soga T, Kato Y, Yamamoto T, Takano N, Hishiki T, Ueno Y, Ikeda S, Sakuragawa T, et al. Cystathionine beta-synthase as a carbon monoxide-sensitive regulator of bile excretion. Hepatology. 2009;49:141–150. doi: 10.1002/hep.22604. [DOI] [PubMed] [Google Scholar]
- 17.Soga T, Sugimoto M, Honma M, Mori M, Igarashi K, Kashikura K, Ikeda S, Hirayama A, Yamamoto T, Yoshida H, et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J Hepatol. 2011;55:896–905. doi: 10.1016/j.jhep.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Imai Y, Kawata S, Tamura S, Yabuuchi I, Noda S, Inada M, Maeda Y, Shirai Y, Fukuzaki T, Kaji I, et al. Relation of interferon therapy and hepatocellular carcinoma in patients with chronic hepatitis C. Osaka Hepatocellular Carcinoma Prevention Study Group. Ann Intern Med. 1998;129:94–99. doi: 10.7326/0003-4819-129-2-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 19.Omata M, Yoshida H, Shiratori Y. Prevention of hepatocellular carcinoma and its recurrence in chronic hepatitis C patients by interferon therapy. Clin Gastroenterol Hepatol. 2005;3:S141–S143. doi: 10.1016/s1542-3565(05)00713-5. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe S, Enomoto N, Koike K, Izumi N, Takikawa H, Hashimoto E, Moriyasu F, Kumada H, Imawari M. Cancer preventive effect of pegylated interferon α-2b plus ribavirin in a real-life clinical setting in Japan: PERFECT interim analysis. Hepatol Res. 2011;41:955–964. doi: 10.1111/j.1872-034X.2011.00847.x. [DOI] [PubMed] [Google Scholar]
- 21.Qu LS, Chen H, Kuai XL, Xu ZF, Jin F, Zhou GX. Effects of interferon therapy on development of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis: A meta-analysis of randomized controlled trials. Hepatol Res. 2012;42:782–789. doi: 10.1111/j.1872-034X.2012.00984.x. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, Sugimoto M, Igarashi K, Saito K, Shao L, Katsumi T, Tomita K, Sato C, Okumoto K, Nishise Y, et al. Dynamics of serum metabolites in patients with chronic hepatitis C receiving pegylated interferon plus ribavirin: a metabolomics analysis. Metabolism. 2013;62:1577–1586. doi: 10.1016/j.metabol.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Honda M, Kaneko S, Kawai H, Shirota Y, Kobayashi K. Differential gene expression between chronic hepatitis B and C hepatic lesion. Gastroenterology. 2001;120:955–966. doi: 10.1053/gast.2001.22468. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto M, Kawakami M, Robert M, Soga T, Tomita M. Bioinformatics Tools for Mass Spectroscopy-Based Metabolomic Data Processing and Analysis. Curr Bioinform. 2012;7:96–108. doi: 10.2174/157489312799304431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010;6:78–95. doi: 10.1007/s11306-009-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marra M, Sordelli IM, Lombardi A, Lamberti M, Tarantino L, Giudice A, Stiuso P, Abbruzzese A, Sperlongano R, Accardo M, et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Transl Med. 2011;9:171. doi: 10.1186/1479-5876-9-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Q, Tan Y, Yin P, Ye G, Gao P, Lu X, Wang H, Xu G. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res. 2013;73:4992–5002. doi: 10.1158/0008-5472.CAN-13-0308. [DOI] [PubMed] [Google Scholar]
- 28.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 29.Zalups RK, Lash LH. Depletion of glutathione in the kidney and the renal disposition of administered inorganic mercury. Drug Metab Dispos. 1997;25:516–523. [PubMed] [Google Scholar]
- 30.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 31.Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S, et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036–3043. doi: 10.1002/cncr.11427. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499–1508. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arase Y, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Kawamura Y, Yatsuji H, Sezaki H, Hosaka T, Hirakawa M, et al. Sustained virological response reduces incidence of onset of type 2 diabetes in chronic hepatitis C. Hepatology. 2009;49:739–744. doi: 10.1002/hep.22703. [DOI] [PubMed] [Google Scholar]
- 34.Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, Kobayashi M, Sezaki H, Saito S, Hosaka T, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964–973. doi: 10.1002/hep.26087. [DOI] [PubMed] [Google Scholar]
- 35.Roe B, Kensicki E, Mohney R, Hall WW. Metabolomic profile of hepatitis C virus-infected hepatocytes. PLoS One. 2011;6:e23641. doi: 10.1371/journal.pone.0023641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumert TF, Meredith L, Ni Y, Felmlee DJ, McKeating JA, Urban S. Entry of hepatitis B and C viruses - recent progress and future impact. Curr Opin Virol. 2014;4:58–65. doi: 10.1016/j.coviro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Cho SH, Choi MH, Lee WY, Chung BC. Evaluation of urinary nucleosides in breast cancer patients before and after tumor removal. Clin Biochem. 2009;42:540–543. doi: 10.1016/j.clinbiochem.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 38.Woo HM, Kim KM, Choi MH, Jung BH, Lee J, Kong G, Nam SJ, Kim S, Bai SW, Chung BC. Mass spectrometry based metabolomic approaches in urinary biomarker study of women’s cancers. Clin Chim Acta. 2009;400:63–69. doi: 10.1016/j.cca.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Chen F, Xue J, Zhou L, Wu S, Chen Z. Identification of serum biomarkers of hepatocarcinoma through liquid chromatography/mass spectrometry-based metabonomic method. Anal Bioanal Chem. 2011;401:1899–1904. doi: 10.1007/s00216-011-5245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honda T, Tamura G, Waki T, Kawata S, Nishizuka S, Motoyama T. Promoter hypermethylation of the Chfr gene in neoplastic and non-neoplastic gastric epithelia. Br J Cancer. 2004;90:2013–2016. doi: 10.1038/sj.bjc.6601849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tozawa T, Tamura G, Honda T, Nawata S, Kimura W, Makino N, Kawata S, Sugai T, Suto T, Motoyama T. Promoter hypermethylation of DAP-kinase is associated with poor survival in primary biliary tract carcinoma patients. Cancer Sci. 2004;95:736–740. doi: 10.1111/j.1349-7006.2004.tb03254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


