Abstract
AIM: To investigate the effects of dietary vitamin C and foods containing vitamin C on gastric cancer risk.
METHODS: Our study included 830 control subjects and 415 patients. Data regarding demographics, medical history, and lifestyle, including dietary and nutrient intake, were collected using reliable self-administered questionnaires. Dietary intake information was collected from the participants using a food frequency questionnaire that has been previously reported as reliable and valid. A rapid urease test and a histological evaluation were used to determine the presence of Helicobacter pylori (H. pylori) infection. Twenty-three vitamin C-contributing foods were selected, representing over 80% of the cumulative vitamin C contribution.
RESULTS: In analyses adjusted for first-degree family history of gastric cancer, education level, job, household income, smoking status, and regular exercise, an inverse association between vitamin C intake and gastric cancer risk was observed for the highest (≥ 120.67 mg/d) vs the lowest (< 80.14 mg/d) intake category [OR (95%CI): 0.64 (0.46-0.88)], with a significant trend across the three intake categories (P = 0.007). No protective effect of vitamin C was detected after stratification by gender. No effect of vitamin C intake on the gastric cancer incidence was found in either men or women infected with H. pylori. Vitamin C-contributing foods, including cabbage [0.45 (0.32-0.63), 0.50 (0.34-0.75), 0.45 (0.25-0.81)], strawberries [0.56 (0.40-0.78), 0.49 (0.32-0.74), 0.52 (0.29-0.93)], and bananas [0.40 (0.29-0.57), 0.41 (0.27-0.62), 0.34 (0.19-0.63)], were protective factors against the risk of gastric cancer based on the results of the overall adjusted analyses and the results for men and women, respectively.
CONCLUSION: A protective effect of vitamin C and vitamin C-contributing foods against gastric cancer was observed. Further studies using larger sample sizes are required to replicate our results.
Keywords: Vitamin C, Vitamin C-contributing foods, Helicobacter pylori, Gastric cancer, Korean population
Core tip: An increased intake of vitamin C and vitamin C-contributing foods, including vegetables and fruits, may protect individuals against the risk of gastric cancer. However, we have no sufficient evidence to support the hypothesis that vitamin C has protective effect against gastric cancer in individuals infected with Helicobacter pylori.
INTRODUCTION
Although the incidence and mortality rates of gastric cancer have decreased worldwide, stomach cancer remains the fifth most common cancer and the third leading cause of cancer death in both sexes worldwide[1]. Being the most common cancer among men in Korea, gastric cancer had 41.3 and 7.8 per 100000 persons in the estimated age-standardized incidence and mortality rates in 2015, respectively[2].
Dietary habits and nutrient intake play important roles in the prevention and etiology of gastric cancer[3]. According to the World Cancer Research Fund and the American Institute for Cancer Research report, increased consumption of non-starchy vegetables and fruits may decrease the risk of gastric cancer, whereas salt and salted foods may be the risk factors of gastric cancer. Additionally, a number of other foods may associate with gastric cancer. However, no specific constituent of these foods has yet been identified to explain these reported associations[3]. Being one of the most common antioxidants found in fruits and vegetables, vitamin C may have a chemopreventive effect[4]. Vitamin C protects cells from oxidative DNA damage, thereby blocking carcinogenesis[5]. Additionally, the protective effect of vitamin C is supported by many observational studies and meta-analyses[6-15]. However, some observational studies did not successfully demonstrate a significant association between vitamin C intake and gastric cancer[16-18]. To date, the association between vitamin C intake and gastric cancer risk has been inconsistent.
Helicobacter pylori (H. pylori) is classified as a cause of stomach cancer in a monograph from the International Agency for Research on Cancer (IARC)[19,20]. Epidemiological studies in humans have linked vitamin C deficiency to more severe H. pylori-associated gastritis and a higher risk of gastric cancer[21,22]. Furthermore, reduced vitamin C levels in the gastric juice and plasma in H. pylori-infected patients returned to normal levels after H. pylori eradication[7,22-24]. Therefore, H. pylori-induced gastric cancer may be prevented by an appropriate diet.
We performed a case-control study to investigate the effects of dietary vitamin C and vitamin C-contributing foods on gastric cancer risk.
MATERIALS AND METHODS
Study population
This study is an expansion of two previously published case-control studies[25,26]. The control and case groups were obtained from the National Cancer Center Hospital in South Korea between March 2011 and December 2014. Individuals who were histologically confirmed as early gastric cancer patients within the preceding three months at the Center for Gastric Cancer were included in the case group. Early gastric carcinoma is an invasive carcinoma confined to the mucosa and/or submucosa, with or without lymph node metastases, irrespective of the tumor size[27]. Patients in the case group did not have diabetes mellitus, a history of cancer within the past five years, advanced gastric cancer, or severe systemic or mental disease, nor were they women who were pregnant or breastfeeding. We selected the control group from patients undergoing health-screening examinations at the Center for Cancer Prevention and Detection at the same hospital.
In total, 1727 participants were recruited, with 1227 in the control group and 500 in the case group; 1671 individuals provided data through a food frequency questionnaire (FFQ) and a self-administered questionnaire. Participants with a total energy intake of < 500 kcal or ≥ 4000 kcal (n = 15) were excluded because of the implausibility of the data. Of the 1656 individuals remaining, the control and case subjects were frequency-matched by age (within 5 years) and sex at a ratio of 2:1 (controls:cases). The final analysis consisted of 1245 participants, including 830 controls and 415 cases (men, 810; women, 435; Figure 1). Our study was approved by the Institutional Review Board of the National Cancer Center [IRB Number: NCCNCS-11-438]. We collected written informed consent from all participants.
Figure 1.
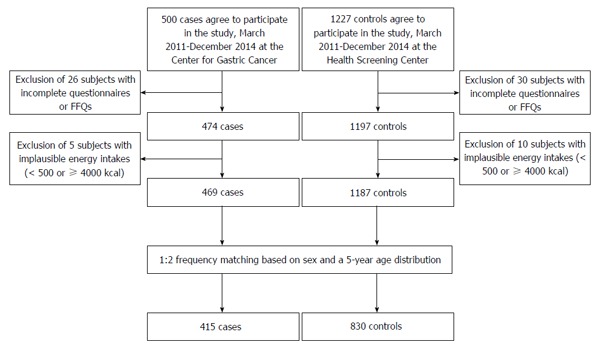
Flow diagram for included participants.
Data collection
The participants were asked to complete a self-administered questionnaire that included demographic, lifestyle, and medical history information. Dietary intake information was collected from the participants using the FFQ, which has been previously reported as a reliable and valid questionnaire[28]. The FFQ contains nine food consumption frequency categories (never or rarely, once a month, 2 or 3 times a month, once or twice a week, 3 or 4 times a week, 5 or 6 times a week, once a day, twice a day, and 3 times a day) and three portion size categories (small, medium, and large) for specific food items consumed within the past 12 mo. We used CAN-Pro 4.0 (Computer Aided Nutritional Analysis Program, The Korean Nutrition Society, Seoul, Korea) to calculate the average daily nutrient intake for each participant, and we summed the amounts of vitamin C obtained from various food groups to compute the vitamin C intake (mg/d). A rapid urease test and histological evaluation were used to assess H. pylori infection.
Statistical analysis
We used t tests and χ2 tests for continuous and categorical variables, respectively, to compare the characteristics of the control and case groups. We conducted a contribution analysis to select vitamin C-contributing foods, which were ranked by the percentage of the total vitamin C intake that they provide for the population as a whole. A total of 23 vitamin C-contributing foods were selected, representing over 80% of the cumulative contribution. To compare the difference in dietary vitamin C intake and vitamin C-contributing foods, consumption was adjusted for total energy intake using the linear residual regression method[29]. The intake levels of vitamin C and vitamin C-contributing foods were categorized into tertiles according to the distribution of the control group. The lowest tertile group was used as the reference group. The median values of each tertile category of the dietary vitamin C intake and vitamin C-contributing foods were used as a continuous variable to test for trends.
The association between dietary factors and gastric cancer risk was assessed using an analysis with logistic regression models adjusted for potential confounding variables, and the odds ratios (OR) and their 95% confidence intervals (CIs) were calculated. Multivariate models were adjusted for first-degree family history of gastric cancers (yes, no), education level (middle school or less, high school, and college or more), job (managers and professionals, clerical, sales and service, production workers and laborers, and not in the labor force), monthly household income (< 2000000 KRW, 2000000-4000000 KRW, ≥ 4000000 KRW), smoking status (nonsmoker, ex-smoker, and current smoker), regular exercise (yes, no), and H. pylori infection (positive, negative). SAS 9.3 software (SAS Institute., Cary, NC, United States) was used to perform the calculations, and a two-sided P value less than 0.05 was considered statistically significant.
RESULTS
General characteristics
Table 1 shows the distribution of 830 control subjects and 415 patients with gastric cancer according to general characteristics. Gastric cancer patients who had a higher family history of gastric cancer (P = 0.001) and tended to have a lower education level (P < 0.001), lower levels of employment (P < 0.001) and household income (P < 0.001) reported using more tobacco (P < 0.001), performing less regular exercise (P < 0.001), and having a higher proportion of H. pylori infection (P < 0.001). Compared with the control group, both men and women in the case group had a lower education level, job, and household income, used more tobacco, performed less regular exercise, and had a higher proportion of H. pylori infection. In particular, the men in the case group had a higher percentage of family history of gastric cancer than the men in the control group (P = 0.002).
Table 1.
General characteristics of the study subjects
|
Total (n = 1245) |
Men (n = 810) |
Women (n= 435) |
|||||||
| Case (n = 415) | Control (n = 830) | P value1 | Case (n = 270) | Control (n = 540) | P value | Case (n = 145) | Control (n = 290) | P value | |
| Age (yr), mean ± SD | 53.8 ± 9.3 | 53.7 ± 9.0 | 0.892 | 54.9 ± 8.7 | 54.8 ± 8.4 | 0.905 | 51.7 ± 10.0 | 51.6 ± 9.8 | 0.942 |
| Body mass index (kg/m2) | |||||||||
| < 23 | 159 (38.3) | 314 (37.8) | 0.975 | 91 (33.7) | 161 (29.8) | 0.509 | 68 (46.9) | 153 (52.8) | 0.533 |
| 23-25 | 122 (29.4) | 249 (30.0) | 78 (28.9) | 170 (31.5) | 44 (30.3) | 79 (27.2) | |||
| ≥ 25 | 133 (32.1) | 266 (32.1) | 101 (37.4) | 209 (38.7) | 32 (22.1) | 57 (19.7) | |||
| First-degree family history of gastric cancer | |||||||||
| No | 332 (80.0) | 725 (87.4) | 0.001 | 209 (77.4) | 464 (85.9) | 0.002 | 123 (84.8) | 261 (90.0) | 0.114 |
| Yes | 82 (19.8) | 103 (12.4) | 60 (22.2) | 74 (13.7) | 22 (15.2) | 29 (10.0) | |||
| Marital status | |||||||||
| Married | 361 (87.0) | 716 (86.3) | 0.611 | 243 (90.0) | 478 (88.5) | 0.475 | 118 (81.4) | 238 (82.1) | 0.975 |
| Other | 52 (12.5) | 113 (13.6) | 26 (9.6) | 61 (11.3) | 26 (17.9) | 52 (17.9) | |||
| Education level | |||||||||
| Less than middle school | 142 (34.2) | 119 (14.3) | < 0.001 | 91 (33.7) | 71 (13.2) | < 0.001 | 51 (35.2) | 48 (16.6) | < 0.001 |
| High school | 174 (41.9) | 253 (30.5) | 112 (41.5) | 140 (25.9) | 62 (42.8) | 113 (39.0) | |||
| College or higher | 97 (23.4) | 426 (51.3) | 66 (24.4) | 301 (55.7) | 31 (21.4) | 125 (43.1) | |||
| Job | |||||||||
| Managers and professionals | 70 (16.9) | 156 (18.8) | 0.001 | 59 (21.9) | 117 (21.7) | 0.010 | 11 (7.6) | 39 (13.5) | 0.002 |
| Clerical, sales and service workers | 122 (29.4) | 266 (32.1) | 81 (30.0) | 203 (37.6) | 41 (28.3) | 63 (21.7) | |||
| Production workers, and laborers | 104 (25.1) | 128 (15.4) | 83 (30.7) | 111 (20.6) | 21 (14.5) | 17 (5.9) | |||
| Not in the labor force | 117 (28.2) | 277 (33.4) | 46 (17.0) | 106 (19.6) | 71 (49.0) | 171 (59.0) | |||
| Monthly household income2 | |||||||||
| < 200 | 133 (32.1) | 149 (18.0) | < 0.001 | 85 (31.5) | 85 (15.7) | < 0.001 | 48 (33.1) | 64 (22.1) | 0.016 |
| 200-400 | 148 (35.7) | 341 (41.1) | 106 (39.3) | 232 (43.0) | 42 (29.0) | 109 (37.6) | |||
| ≥ 400 | 96 (23.1) | 273 (32.9) | 55 (20.4) | 168 (31.1) | 41 (28.3) | 105 (36.2) | |||
| Alcohol consumption | |||||||||
| Non-drinker | 119 (28.7) | 236 (28.4) | 0.243 | 44 (16.3) | 89 (16.5) | 0.282 | 75 (51.7) | 147 (50.7) | 0.819 |
| Ex-drinker | 41 (9.9) | 60 (7.2) | 33 (12.2) | 47 (8.7) | 8 (5.5) | 13 (4.5) | |||
| Current drinker | 254 (61.2) | 534 (64.3) | 193 (71.5) | 404 (74.8) | 61 (42.1) | 130 (44.8) | |||
| Smoking status | |||||||||
| Non-smoker | 167 (40.2) | 384 (46.3) | < 0.001 | 39 (14.4) | 106 (19.6) | < 0.001 | 128 (88.3) | 278 (95.9) | 0.021 |
| Ex-smoker | 119 (28.7) | 284 (34.2) | 110 (40.7) | 277 (51.3) | 9 (6.2) | 7 (2.4) | |||
| Current-smoker | 128 (30.8) | 162 (19.5) | 121 (44.8) | 157 (29.1) | 7 (4.8) | 5 (1.7) | |||
| Regular exercise | |||||||||
| No | 268 (64.6) | 361 (43.5) | < 0.001 | 161 (59.6) | 234 (43.3) | < 0.001 | 107 (73.8) | 127 (43.8) | < 0.001 |
| Yes | 147 (35.4) | 466 (56.1) | 109 (40.4) | 303 (56.1) | 38 (26.2) | 163 (56.2) | |||
| H. pylori infection | |||||||||
| Negative | 33 (8.0) | 320 (38.6) | < 0.001 | 18 (6.7) | 187 (34.6) | < 0.001 | 15 (10.3) | 133 (45.9) | < 0.001 |
| Positive | 382 (92.1) | 486 (58.6) | 252 (93.3) | 333 (61.7) | 130 (89.7) | 153 (52.8) | |||
P values were calculated using the t test (for continuous variables) or χ2 test (for categorical variables);
Unit is 10000 won in Korean currency. Values are expressed as the mean ± SD (range) or n (%).
Vitamin C and vitamin C-contributing food consumption is described in Table 2. Lower vitamin C intake (P < 0.001), increased consumption of potatoes and starches (P = 0.013) and fruits (P < 0.001), and higher energy intake (P < 0.001) were found in the case group. In general, the case group consumed less cabbage (P < 0.001), lettuce (P = 0.004), mandarins (P < 0.001), strawberries (P < 0.001), orange juice (P < 0.001), watermelon (P = 0.004), apples (P < 0.001), and bananas (P < 0.001) than the control group. Compared with the control group, the men and women in the case group also consumed less vitamin C, cabbage, lettuce, fruits, strawberries, orange juice, watermelon, apples, and bananas. Some gender differences in vitamin C-contributing food consumption were found in both the case and control groups. The men in the case group consumed more energy (P < 0.001) and fewer starches (P = 0.005), potatoes (P = 0.035), sweet potatoes (P = 0.030), and green peppers (P = 0.044) than the control group. The women in the case group consumed fewer mandarins (P < 0.001) and persimmons (P = 0.004) than the women in the control group.
Table 2.
Comparison of intakes of vitamin C and vitamin C contributing foods1
| Food (g/d) (mean ± SD) |
Total (n = 1245) |
Men (n = 810) |
Women (n = 435) |
||||||
| Case (n = 415) | Control (n = 830) | P value2 | Case (n = 270) | Control (n = 540) | P value | Case (n = 145) | Control (n = 290) | P value | |
| Energy (Kcal/d) | 1924.1 ± 612.9 | 1713.6 ± 545.5 | < 0.001 | 2038.5 ± 634.8 | 1760.6 ± 541.5 | < 0.001 | 1711.1 ± 507.0 | 1626.0 ± 543.1 | 0.116 |
| Vitamin C (mg/d) | 96.1 ± 50.5 | 108.4 ± 56.1 | < 0.001 | 89.0 ± 45.3 | 97.1 ± 44.2 | 0.014 | 109.3 ± 56.7 | 129.5 ± 68.5 | 0.001 |
| Potatoes and starches | 39.3 ± 38.1 | 45.5 ± 45.4 | 0.013 | 32.8 ± 34.6 | 40.3 ± 36.5 | 0.005 | 51.5 ± 41.2 | 55.1 ± 57.4 | 0.456 |
| Potatoes | 32.3 ± 34.0 | 35.0 ± 35.0 | 0.209 | 27.7 ± 32.2 | 32.7 ± 30.3 | 0.035 | 41.0 ± 35.7 | 39.1 ± 42.1 | 0.635 |
| Sweet potatoes | 24.8 ± 210.0 | 42.3 ± 234.4 | 0.183 | 8.2 ± 41.9 | 15.1 ± 47.1 | 0.030 | 55.6 ± 349.4 | 92.8 ± 386.8 | 0.329 |
| Vegetables | 327.4 ± 185.0 | 328.2 ± 166.2 | 0.947 | 318.4 ± 177.5 | 320.5 ± 157.4 | 0.873 | 344.3 ± 197.7 | 342.5 ± 180.8 | 0.926 |
| Korean cabbage kimchi | 99.3 ± 71.0 | 96.1 ± 69.3 | 0.450 | 94.9 ± 66.6 | 97.3 ± 69.0 | 0.639 | 107.4 ± 78.1 | 93.8 ± 70.0 | 0.068 |
| Green pepper | 7.3 ± 11.0 | 7.9 ± 10.6 | 0.347 | 6.4 ± 6.8 | 7.5 ± 9.5 | 0.044 | 9.0 ± 16.0 | 8.6 ± 12.5 | 0.769 |
| Radish | 20.3 ± 17.5 | 21.2 ± 18.3 | 0.375 | 20.3 ± 16.8 | 21.5 ± 17.5 | 0.348 | 20.3 ± 18.8 | 20.8 ± 19.6 | 0.801 |
| Spinach | 9.9 ± 21.6 | 10.7 ± 22.8 | 0.572 | 8.0 ± 18.5 | 9.1 ± 17.3 | 0.380 | 13.6 ± 26.2 | 13.6 ± 30.4 | 0.994 |
| Radish kimchi | 27.1 ± 80.2 | 28.5 ± 58.9 | 0.765 | 20.7 ± 40.1 | 26.8 ± 52.3 | 0.065 | 39.1 ± 123.6 | 31.5 ± 69.6 | 0.491 |
| Cabbage | 6.6 ± 13.9 | 13.4 ± 27.1 | < 0.001 | 4.6 ± 10.3 | 9.7 ± 20.0 | < 0.001 | 10.3 ± 18.3 | 20.4 ± 35.8 | < 0.001 |
| Chonggak kimchi | 15.7 ± 44.9 | 16.5 ± 33.6 | 0.769 | 12.1 ± 23.1 | 15.6 ± 29.9 | 0.072 | 22.4 ± 68.7 | 18.1 ± 39.4 | 0.486 |
| Zucchini | 18.0 ± 22.1 | 17.5 ± 20.4 | 0.711 | 16.1 ± 20.3 | 15.4 ± 18.3 | 0.650 | 21.6 ± 24.9 | 21.5 ± 23.2 | 0.956 |
| Chinese cabbage | 22.3 ± 118.0 | 32.7 ± 142.8 | 0.172 | 20.7 ± 132.2 | 34.0 ± 164.8 | 0.217 | 25.1 ± 85.7 | 30.3 ± 88.5 | 0.564 |
| Lettuce | 7.2 ± 10.1 | 9.3 ± 15.5 | 0.004 | 6.1 ± 8.8 | 7.6 ± 10.7 | 0.039 | 9.1 ± 11.9 | 12.4 ± 21.4 | 0.040 |
| Onion | 14.5 ± 8.7 | 15.0 ± 9.2 | 0.375 | 13.4 ± 7.6 | 14.3 ± 8.6 | 0.166 | 16.4 ± 10.2 | 16.3 ± 10.2 | 0.887 |
| Mustard leaf kimchi | 10.5 ± 61.5 | 14.2 ± 118.4 | 0.469 | 8.1 ± 62.4 | 8.2 ± 58.9 | 0.990 | 14.9 ± 59.8 | 25.4 ± 183.1 | 0.377 |
| Green onion | 4.8 ± 3.0 | 5.0 ± 3.3 | 0.410 | 4.9 ± 3.01 | 5.1 ± 3.3 | 0.410 | 4.7 ± 2.9 | 4.8 ± 3.5 | 0.811 |
| Fruits | 136.0 ± 165.8 | 191.8 ± 209.1 | < 0.001 | 115.5 ± 149.4 | 152.0 ± 163.5 | 0.002 | 174.1 ± 187.4 | 266.0 ± 259.0 | < 0.001 |
| Mandarins | 16.0 ± 26.3 | 23.2 ± 44.9 | < 0.001 | 12.9 ± 22.6 | 14.6 ± 23.4 | 0.319 | 21.8 ± 31.5 | 39.2 ± 66.0 | < 0.001 |
| Strawberries | 5.2 ± 8.7 | 8.8 ± 15.8 | < 0.001 | 4.3 ± 7.7 | 6.7 ± 11.2 | < 0.001 | 7.0 ± 10.0 | 12.6 ± 21.4 | < 0.001 |
| Orange juice | 8.9 ± 22.2 | 20.8 ± 56.1 | < 0.001 | 6.4 ± 14.2 | 16.9 ± 54.8 | < 0.001 | 13.6 ± 31.6 | 28.0 ± 57.8 | 0.001 |
| Watermelon | 13.4 ± 21.7 | 21.0 ± 69.4 | 0.004 | 11.3 ± 21.2 | 15.3 ± 36.5 | 0.050 | 17.5 ± 22.2 | 31.8 ± 105.6 | 0.028 |
| Apples | 30.4 ± 57.1 | 52.3 ± 89.7 | < 0.001 | 23.8 ± 49.1 | 42.7 ± 77.3 | < 0.001 | 42.7 ± 68.1 | 70.2 ± 107.0 | 0.001 |
| Persimmons | 17.4 ± 110.5 | 20.3 ± 57.6 | 0.617 | 17.2 ± 133.6 | 12.9 ± 43.3 | 0.603 | 17.8 ± 41.9 | 34.1 ± 75.5 | 0.004 |
| Bananas | 10.5 ± 26.5 | 20.3 ± 40.9 | < 0.001 | 7.3 ± 16.0 | 15.2 ± 28.9 | < 0.001 | 16.4 ± 38.5 | 29.8 ± 55.8 | 0.004 |
| Citrus tea | 23.6 ± 142.9 | 58.5 ± 729.7 | 0.184 | 11.8 ± 62.8 | 18.1 ± 67.4 | 0.201 | 45.5 ± 225.0 | 133.7 ± 1228.9 | 0.237 |
1Adjusted for total energy intake using the residuals method;
P values were calculated with the t test.
Vitamin C intake and the risk of gastric cancer
Table 3 reports the ORs and corresponding 95%CIs for vitamin C intake. Vitamin C intake exhibited was negatively associated with gastric cancer in both the unadjusted model [OR (95%CI): 0.53 (0.40-0.71), P for trend < 0.001] and the adjusted model (family history of gastric cancer, education level, job, household income, smoking status, and regular exercise; 0.64 (0.46-0.88), P for trend = 0.007. However, the association was marginally significant after an additional adjustment for H. pylori status [0.71 (0.50-1.00), P for trend = 0.052]. No protective effect of vitamin C was observed in either gender as a result of the adjusted model.
Table 3.
ORs and 95%CIs of gastric cancer by tertiles of dietary vitamin C
| Range (mg/d) | No. of controls/cases | Model I OR (95%CI) | Model II OR (95%CI) | Model III OR (95%CI) | |
| Total (n = 1245) | |||||
| T1 | < 80.14 | 276/186 | 1 | 1 | 1 |
| T2 | 80.14-120.67 | 277/130 | 0.70 (0.53-0.92) | 0.81 (0.59-1.10) | 0.81 (0.58-1.12) |
| T3 | ≥ 120.67 | 277/99 | 0.53 (0.40-0.71) | 0.64 (0.46-0.88) | 0.71 (0.50-1.00) |
| P for trend1 | < 0.001 | 0.007 | 0.052 | ||
| Men (n = 810) | |||||
| T1 | < 73.18 | 180/107 | 1 | 1 | 1 |
| T2 | 73.18-110.59 | 180/93 | 0.87 (0.62-1.23) | 1.11 (0.75-1.64) | 1.07 (0.70-1.61) |
| T3 | ≥ 110.59 | 180/70 | 0.65 (0.45-0.94) | 0.78 (0.52-1.18) | 0.91 (0.59-1.41) |
| P for trend | 0.022 | 0.229 | 0.659 | ||
| Women (n = 435) | |||||
| T1 | < 91.70 | 96/69 | 1 | 1 | 1 |
| T2 | 91.70-139.52 | 97/45 | 0.65 (0.40-1.03) | 0.81 (0.48-1.36) | 0.85 (0.49-1.48) |
| T3 | ≥ 139.52 | 97/31 | 0.45 (0.27-0.74) | 0.57 (0.32-1.00) | 0.61 (0.34-1.12) |
| P for trend | 0.002 | 0.051 | 0.109 | ||
Trends were calculated using the median intake for each dietary vitamin C category as a continuous variable: Model I: Unadjusted; Model II: Adjusted by first-degree family history of gastric cancer, education level, job, household income, smoking status, regular exercise; Model III: Additionally adjusted for H. pylori infection.
The results were stratified by H. pylori status and sex in the present study. In the crude model, vitamin C intake was a protective factor against gastric cancer for participants infected with H. pylori [0.62 (0.45-0.87), P for trend = 0.006]. However, the association was weakened after an adjustment for confounding factors [0.74 (0.51-1.08), P for trend = 0.116]. No effect of vitamin C intake on the gastric cancer incidence was observed for both either men or women infected with H. pylori (data not shown).
Vitamin C - contributing food consumption and the risk of gastric cancer
Table 4 shows the association between vitamin C-contributing food consumption and the gastric cancer risk. Overall, the consumption of total fruit [0.57 (0.41-0.81)], sweet potatoes [0.62 (0.44-0.87)], cabbage [0.45 (0.32-0.63)], Chinese cabbage [0.58 (0.41-0.81)], lettuce [0.67 (0.49-0.93)], strawberries [0.56 (0.40-0.78)], orange juice [0.61 (0.44-0.85)], watermelon [0.69 (0.50-0.95)], apples [0.60 (0.43-0.85)], persimmons [0.56 (0.40-0.78)], and bananas [0.40 (0.29-0.57)] protects against gastric cancer based on the results of the adjusted model. Inverse associations between cabbage, strawberry, and banana consumption and gastric cancer risk were also observed for both men and women. Some different protective factors were found between genders. Starches, potatoes, sweet potatoes, spinach, Chinese cabbage, lettuce, orange juice, and apples decreased the risk of gastric cancer in men, and fruits and persimmons decreased the risk of gastric cancer in women. In particular, zucchini consumption increased the gastric cancer risk in women [1.87 (1.06-3.28)].
Table 4.
ORs and 95%CIs of gastric cancer by the highest tertile of vitamin C contributing food consumption
| Total (n = 1245) | P for trend2 | Men (n = 810) | P for trend | Women (n = 435) | P for trend | |
| Potatoes and starches | ||||||
| Model I OR1 (95%CI) | 0.74 (0.54-1.59) | 0.020 | 0.55 (0.37-0.82) | 0.001 | 0.97 (0.60-1.57) | 0.996 |
| Model II OR (95%CI) | 0.72 (0.52-1.01) | 0.028 | 0.55 (0.36-0.85) | 0.003 | 0.94 (0.55-1.60) | 0.889 |
| Model III OR (95%CI) | 0.85 (0.59-1.21) | 0.277 | 0.65 (0.41-1.03) | 0.042 | 1.01 (0.57-1.79) | 0.891 |
| Total vegetable consumption | ||||||
| Model I OR (95%CI) | 0.87 (0.66-1.16) | 0.366 | 0.91 (0.64-1.31) | 0.593 | 0.86 (0.54-1.37) | 0.549 |
| Model II OR (95%CI) | 0.91 (0.66-1.25) | 0.575 | 1.01 (0.67-1.52) | 0.955 | 0.83 (0.49-1.39) | 0.496 |
| Model III OR (95%CI) | 0.96 (0.68-1.34) | 0.800 | 1.09 (0.71-1.68) | 0.744 | 0.82 (0.47-1.43) | 0.494 |
| Total fruit consumption | ||||||
| Model I OR (95%CI) | 0.41 (0.30-0.56) | < 0.001 | 0.52 (0.36-0.75) | 0.001 | 0.34 (0.21-0.57) | < 0.001 |
| Model II OR (95%CI) | 0.57 (0.41-0.81) | 0.002 | 0.73 (0.49-1.10) | 0.148 | 0.52 (0.30-0.92) | 0.032 |
| Model III OR (95%CI) | 0.59 (0.41-0.85) | 0.005 | 0.73 (0.47-1.13) | 0.179 | 0.57 (0.31-1.05) | 0.089 |
| Potatoes | ||||||
| Model I OR (95%CI) | 0.82 (0.61-1.10) | 0.114 | 0.60 (0.41-0.87) | 0.003 | 1.19 (0.73-1.93) | 0.444 |
| Model II OR (95%CI) | 0.79 (0.57-1.09) | 0.105 | 0.55 (0.36-0.85) | 0.003 | 0.99 (0.57-1.70) | 0.867 |
| Model III OR (95%CI) | 0.91 (0.64-1.29) | 0.458 | 0.65 (0.41-1.02) | 0.034 | 1.10 (0.61-1.97) | 0.572 |
| Sweet potatoes | ||||||
| Model I OR (95%CI) | 0.57 (0.42-0.77) | < 0.001 | 0.54 (0.37-0.80) | < 0.001 | 0.71 (0.44-1.16) | 0.244 |
| Model II OR (95%CI) | 0.62 (0.44-0.87) | 0.002 | 0.60 (0.39-0.92) | 0.003 | 0.68 (0.39-1.18) | 0.196 |
| Model III OR (95%CI) | 0.69 (048-1.00) | 0.018 | 0.66 (0.42-1.05) | 0.016 | 0.76 (0.42-1.37) | 0.294 |
| Korean cabbage kimchi | ||||||
| Model I OR (95%CI) | 1.08 (0.81-1.43) | 0.547 | 0.90 (0.63-1.28) | 0.572 | 1.47 (0.91-2.39) | 0.087 |
| Model II OR (95%CI) | 1.08 (0.79-1.48) | 0.629 | 0.91 (0.61-1.35) | 0.693 | 1.41 (0.81-2.43) | 0.163 |
| Model III OR (95%CI) | 1.11 (0.80-1.55) | 0.511 | 0.99 (0.65-1.51) | 0.976 | 1.27 (0.71-2.28) | 0.342 |
| Green pepper | ||||||
| Model I OR (95%CI) | 0.85 (0.64-1.14) | 0.252 | 0.78 (0.54-1.12) | 0.141 | 0.99 (0.60-1.62) | 0.894 |
| Model II OR (95%CI) | 0.87 (0.64-1.20) | 0.328 | 0.74 (0.49-1.12) | 0.090 | 0.99 (0.57-1.72) | 0.973 |
| Model III OR (95%CI) | 0.81 (0.57-1.13) | 0.167 | 0.67 (0.44-0.04) | 0.037 | 0.93 (0.51-1.68) | 0.844 |
| Radish | ||||||
| Model I OR (95%CI) | 0.97 (0.72-1.31) | 0.599 | 0.89 (0.62-1.28) | 0.468 | 1.22 (0.72-2.06) | 0.870 |
| Model II OR (95%CI) | 0.92 (0.67-1.28) | 0.348 | 0.90 (0.60-1.35) | 0.489 | 1.16 (0.65-2.07) | 0.799 |
| Model III OR (95%CI) | 0.98 (0.69-1.39) | 0.495 | 0.92 (0.60-1.43) | 0.578 | 1.27 (0.68-2.35) | 0.893 |
| Spinach | ||||||
| Model I OR (95%CI) | 0.77 (0.58-1.03) | 0.173 | 0.62 (0.44-0.90) | 0.024 | 0.93 (0.58-1.51) | 0.923 |
| Model II OR (95%CI) | 0.80 (0.58-1.09) | 0.283 | 0.66 (0.45-0.99) | 0.071 | 1.02 (0.59-1.77) | 0.851 |
| Model III OR (95%CI) | 0.86 (0.61-1.20) | 0.532 | 0.78 (0.51-1.20) | 0.360 | 0.94 (0.52-1.70) | 0.821 |
| Radish kimchi | ||||||
| Model I OR (95%CI) | 0.82 (0.62-1.10) | 0.193 | 0.69 (0.48-0.99) | 0.038 | 1.33 (0.80-2.22) | 0.592 |
| Model II OR (95%CI) | 0.82 (0.59-1.12) | 0.195 | 0.70 (0.47-1.05) | 0.090 | 1.21 (0.69-2.11) | 0.937 |
| Model III OR (95%CI) | 0.80 (0.57-1.12) | 0.192 | 0.73 (0.47-1.13) | 0.142 | 1.06 (0.58-1.94) | 0.816 |
| Cabbage | ||||||
| Model I OR (95%CI) | 0.34 (0.25-0.46) | < 0.001 | 0.37 (0.26-0.53) | < 0.001 | 0.33 (0.19-0.55) | < 0.001 |
| Model II OR (95%CI) | 0.45 (0.32-0.63) | < 0.001 | 0.50 (0.34-0.75) | 0.004 | 0.45 (0.25-0.81) | 0.016 |
| Model III OR (95%CI) | 0.50 (0.35-0.72) | 0.001 | 0.53 (0.35-0.82) | 0.015 | 0.54 (0.29-1.00) | 0.094 |
| Chonggak kimchi | ||||||
| Model I OR (95%CI) | 0.83 (0.62-1.10) | 0.215 | 0.69 (0.48-0.99) | 0.038 | 1.33 (0.80-2.20) | 0.589 |
| Model II OR (95%CI) | 0.83 (0.60-1.04) | 0.253 | 0.69 (0.46-1.04) | 0.077 | 1.21 (0.69-2.11) | 0.933 |
| Model III OR (95%CI) | 0.81 (0.58-1.13) | 0.244 | 0.72 (0.47-1.11) | 0.113 | 1.06 (0.58-1.94) | 0.818 |
| Zucchini | ||||||
| Model I OR (95%CI) | 1.01 (0.76-1.35) | 0.898 | 0.96 (0.67-1.37) | 0.772 | 1.38 (0.84-2.25) | 0.195 |
| Model II OR (95%CI) | 1.09 (0.79-1.51) | 0.783 | 0.99 (0.66-1.48) | 0.846 | 1.87 (1.06-3.28) | 0.026 |
| Model III OR (95%CI) | 1.11 (0.78-1.56) | 0.749 | 1.97 (0.63-1.50) | 0.784 | 1.82 (1.00-3.30) | 0.045 |
| Chinese cabbage | ||||||
| Model I OR (95%CI) | 0.64 (0.47-0.86) | < 0.001 | 0.53(0.36-0.78) | < 0.001 | 0.80 (0.49-1.31) | 0.342 |
| Model II OR (95%CI) | 0.58 (0.41-0.81) | < 0.001 | 0.49(0.32-0.76) | < 0.001 | 0.72 (0.41-1.25) | 0.115 |
| Model III OR (95%CI) | 0.62 (0.44-0.89) | < 0.001 | 0.57(0.36-0.90) | 0.005 | 0.67 (0.37-1.22) | 0.092 |
| Lettuce | ||||||
| Model I OR (95%CI) | 0.64 (0.48-0.86) | 0.008 | 0.58 (0.41-0.82) | 0.013 | 0.77 (0.47-1.26) | 0.301 |
| Model II OR (95%CI) | 0.67 (0.49-0.93) | 0.026 | 0.58 (0.39-0.86) | 0.023 | 0.79 (0.45-1.36) | 0.365 |
| Model III OR (95%CI) | 0.68 (0.48-0.95) | 0.031 | 0.58 (0.38-0.88) | 0.023 | 0.78 (0.43-1.40) | 0.337 |
| Onion | ||||||
| Model I OR (95%CI) | 1.06 (0.79-1.42) | 0.817 | 0.90 (0.62-1.30) | 0.436 | 1.21 (0.73-1.99) | 0.539 |
| Model II OR (95%CI) | 1.09 (0.79-1.51) | 0.693 | 0.84 (0.56-1.27) | 0.320 | 1.33 (0.76-2.33) | 0.344 |
| Model III OR (95%CI) | 1.13 (0.80-1.59) | 0.572 | 0.90 (0.58-1.40) | 0.524 | 1.27 (0.71-2.30) | 0.457 |
| Mustard leaf Kimchi | ||||||
| Model I OR (95%CI) | 0.84 (0.62-1.12) | 0.099 | 0.87 (0.60-1.25) | 0.220 | 0.66 (0.40-1.08) | 0.207 |
| Model II OR (95%CI) | 0.76 (0.55-1.06) | 0.018 | 0.84 (0.56-1.27) | 0.089 | 0.60 (0.34-1.04) | 0.076 |
| Model III OR (95%CI) | 0.76 (0.54-1.08) | 0.038 | 0.90 (0.58-1.40) | 0.180 | 0.57 (0.32-1.04) | 0.093 |
| Green onion | ||||||
| Model I OR (95%CI) | 1.03 (0.76-1.38) | 0.909 | 0.92 (0.64-1.33) | 0.527 | 1.21 (0.73-2.01) | 0.612 |
| Model II OR (95%CI) | 1.02 (0.73-1.41) | 0.807 | 0.94 (0.62-1.42) | 0.588 | 1.11 (0.64-1.95) | 0.731 |
| Model III OR (95%CI) | 1.01 (0.71-1.44) | 0.744 | 0.94 (0.60-1.46) | 0.582 | 1.18 (0.65-2.13) | 0.650 |
| Mandarins | ||||||
| Model I OR (95%CI) | 0.60 (0.44-0.80) | 0.001 | 0.79 (0.55-1.12) | 0.356 | 0.42 (0.25-0.71) | 0.002 |
| Model II OR (95%CI) | 0.74 (0.53-1.04) | 0.061 | 0.97 (0.66-1.44) | 0.941 | 0.60 (0.34-1.07) | 0.101 |
| Model III OR (95%CI) | 0.71 (0.50-1.01) | 0.038 | 0.95 (0.62-1.44) | 0.961 | 0.54 (0.29-0.99) | 0.061 |
| Strawberries | ||||||
| Model I OR (95%CI) | 0.44 (0.33-0.60) | < 0.001 | 0.40 (0.27-0.58) | < 0.001 | 0.39 (0.23-0.67) | 0.001 |
| Model II OR (95%CI) | 0.56 (0.40-0.78) | 0.001 | 0.49 (0.32-0.74) | 0.001 | 0.52 (0.29-0.93) | 0.026 |
| Model III OR (95%CI) | 0.61 (0.43-0.86) | 0.009 | 0.50 (0.32-0.79) | 0.004 | 0.57 (0.30-1.07) | 0.065 |
| Orange juice | ||||||
| Model I OR (95%CI) | 0.43 (0.32-0.59) | < 0.001 | 0.36 (0.25-0.53) | < 0.001 | 0.50 (0.30-0.85) | 0.006 |
| Model II OR (95%CI) | 0.61 (0.44-0.85) | 0.003 | 0.47 (0.30-0.71) | 0.001 | 0.83 (0.46-1.51) | 0.294 |
| Model III OR (95%CI) | 0.65 (0.46-0.94) | 0.014 | 0.49 (0.31-0.77) | 0.002 | 0.98 (0.52-1.86) | 0.677 |
| Watermelon | ||||||
| Model I OR (95%CI) | 0.59 (0.44-0.78) | 0.003 | 0.61 (0.43-0.87) | 0.032 | 0.63 (0.39-1.02) | 0.117 |
| Model II OR (95%CI) | 0.69 (0.50-0.95) | 0.065 | 0.71 (0.48-1.06) | 0.211 | 0.72 (0.42-1.24) | 0.309 |
| Model III OR (95%CI) | 0.65 (0.46-0.92) | 0.043 | 0.67 (0.44-1.03) | 0.132 | 0.71 (0.40-1.27) | 0.292 |
| Apples | ||||||
| Model I OR (95%CI) | 0.40 (0.29-0.54) | < 0.001 | 0.38 (0.26-0.55) | < 0.001 | 0.43 (0.26-0.71) | 0.005 |
| Model II OR (95%CI) | 0.60 (0.43-0.85) | 0.006 | 0.57 (0.37-0.87) | 0.026 | 0.64 (0.37-1.11) | 0.204 |
| Model III OR (95%CI) | 0.64 (0.45-0.92) | 0.028 | 0.53 (0.34-0.83) | 0.013 | 0.82 (0.46-1.47) | 0.705 |
| Persimmons | ||||||
| Model I OR (95%CI) | 0.49 (0.36-0.66) | < 0.001 | 0.62 (0.43-0.89) | 0.026 | 0.40 (0.24-0.66) | 0.002 |
| Model II OR (95%CI) | 0.56 (0.40-0.78) | 0.001 | 0.72 (0.48-1.08) | 0.151 | 0.46 (0.26-0.80) | 0.018 |
| Model III OR (95%CI) | 0.55 (0.38-0.78) | 0.001 | 0.67 (0.44-1.03) | 0.086 | 0.45 (0.25-0.82) | 0.028 |
| Bananas | ||||||
| Model I OR (95%CI) | 0.32 (0.24-0.44) | < 0.001 | 0.33 (0.22-0.47) | < 0.001 | 0.26 (0.15-0.46) | < 0.001 |
| Model II OR (95%CI) | 0.40 (0.29-0.57) | < 0.001 | 0.41 (0.27-0.62) | < 0.001 | 0.34 (0.19-0.63) | 0.001 |
| Model III OR (95%CI) | 0.44 (0.31-0.63) | < 0.001 | 0.41 (0.27-0.64) | 0.001 | 0.44 (0.23-0.83) | 0.014 |
| Citrus tea | ||||||
| Model I OR (95%CI) | 0.64 (0.48-0.87) | 0.002 | 0.56 (0.38-0.81) | 0.001 | 0.81 (0.49-1.34) | 0.281 |
| Model II OR (95%CI) | 0.78 (0.56-1.09) | 0.048 | 0.68 (0.44-1.04) | 0.017 | 1.00 (0.57-1.76) | 0.669 |
| Model III OR (95%CI) | 0.83 (0.59-1.18) | 0.161 | 0.71 (0.45-1.11) | 0.040 | 1.14 (0.63-2.09) | 0.992 |
OR for the association with the lowest tertile group compared with the highest tertile group;
Trends were calculated using the median intake for each category of vitamin C-contributing food consumption as a continuous variable: Model I: Unadjusted; Model II: Adjusted by first-degree family history of gastric cancer, education level, job, household income, smoking status, regular exercise; Model III: Additionally adjusted for H. pylori infection.
DISCUSSION
In our study, we found a negative association between vitamin C intake and gastric cancer in the crude model and the adjusted model. The association became less apparent after an additional adjustment for H. pylori status. After adjustment for confounders, vitamin C intake showed no protective effect for participants infected with H. pylori. The consumption of cabbage, strawberries, and bananas had inverse associations with gastric cancer risk based on the results of the overall adjusted model and for both genders.
The association between vitamin C intake and the risk of gastric cancer is supported by many observational and meta-analysis studies. In a meta-analysis of 11 observational studies, a dose-response analysis was conducted for vitamin C intake (100 mg/d), which showed a significant reduction in the risk of gastric cancer [RR (95%CI): 0.74 (0.69-0.79)][6]. An inverse association between the intake of vitamin C and the risk of gastric cancer was consistent among case-control studies[7-13] and cohort studies[14,15]. For example, in a Spanish study, the strongest protective effects were observed for vitamin C from fruits and vegetables[12]. Another case-control study in Italy reported that increased vitamin C consumption exhibited an inverse relationship to the risk of gastric cancer[13]. Our result is consistent with a cohort study from Netherlands that reported that an inverse association between vitamin C and the risk of gastric carcinoma was found in age- and gender-adjusted analyses. However, this association became weaker and was of borderline significance in the multivariate analysis (which included age, gender, smoking history, education, stomach disorders, and family history of gastric cancer) [RR (95%CI): 0.70 (0.50-1.00)][14]. Therefore, it appears that vitamin C is among the most consistent protective factors against gastric carcinogenesis. This protective effect may be related to the antioxidant effects of vitamin C, free radical scavenger effects, and the inhibition of nitrosamine formation[30,31]. Another biological explanation for the inverse association is the direct action of vitamin C on the growth of H. pylori[32]. However, no clear protective effect of vitamin C intake was observed in participants infected with H. pylori in our study.
In contrast, some observational studies did not successfully demonstrate a significant association between vitamin C intake and gastric cancer. Two case-control studies conducted in Mexico and Italy that included a small number of participants, showed no protective effect of vitamin C[16,17]. The Shanghai Women’s and Men’s Health study showed that none of the dietary nutrients examined, including vitamin A, vitamin C, vitamin E, carotene, retinol, selenium, or folic acid, were associated with the distal gastric cancer risk among men or women[18].
In the present study, we failed to find a protective effect of vitamin C against gastric cancer in participants infected with H. pylori. At least three explanations for this finding should be considered. First, the consumption of fruits and vegetables, which are the main sources of vitamin C, is highly prevalent among the Korean population[33]. In our study, a difference between case and control groups was observed only for total fruit consumption. Therefore, if an association between vitamin C intake and gastric cancer truly exists, the small difference between the case and control groups in our study may have limited the statistical power to detect this association. Second, this finding may be related to the Korean habit of eating pickled or processed vegetables, which includes many types of kimchi. Kimchi is a fermented vegetable with a high concentration of salt and pepper, which are important risk factors for gastric cancer[3]. Moreover, a high dietary salt intake can exacerbate H. pylori infection in gastric cancer patients[34]. Therefore, it is not surprising that no difference in vegetable consumption was observed between the case and control groups, which may weaken the protective effect of vitamin C. Additionally, the exacerbating role of H. pylori infection may modify the true association between vitamin C intake and gastric cancer risk in the adjusted model. Therefore, the protective effect of vitamin C should be considered in the model without adjusting for H. pylori status. Finally, the amount of vitamin C consumed by the participants with H. pylori infection could explain this finding. A Korean case-control study reported that consuming over 170 mg/d of vitamin C could protect people with H. pylori infection against the risk of gastric cancer [0.10 (0.02-0.63)][10]. Hence, in our analysis, vitamin C doses of 120 mg/d may not be high enough to show protective effect of vitamin C in participants infected with H. pylori.
In the vitamin C-contributing food consumption analyses, our findings are consistent with a meta-analysis of prospective cohort studies that reported an inverse association between fruit intake and gastric cancer incidence [RR (95%CI): 0.82 (0.73-0.93)] that was stronger for follow-up periods of ≥ 10 years [0.66 (0.52-0.83)]; however, no such association was observed for vegetable consumption [0.88 (0.69-1.13)][35]. Another meta-analysis of 8 observational studies of Korean and Japanese populations also showed that an increased intake of fresh vegetables was significantly associated with a decreased risk of gastric cancer [OR (95%CI): 0.62 (0.46-0.85)][36]. Other meta-analyses of observational studies have supported the protective effect of fruits and vegetables against gastric cancer[37-40]. Additionally, the protective effect of fruits and vegetables has been consistently reported in many other case-control studies[8,11,41-46] and prospective cohort studies[47-50]. However, some cohort studies did not find this association[51-54]. For example, our findings are inconsistent with a cohort study from Japan that reported non-significant associations for the consumption between fruit and vegetable consumption and gastric cancer incidence[54]. This finding is comparable with the results of the Netherlands Cohort Study, which showed inverse associations between gastric cancer and the consumption of total vegetables, pulses, raw leaf vegetables, total fruits, citrus fruits, and apples and pears in the crude analysis that became weaker or disappeared in the multivariate analysis[51].
The methods used to cook fruits and vegetables may play an important role in the relationship between fruit and vegetable consumption and gastric cancer risk. Some studies have reported that an increased consumption of pickled or processed vegetables increases the risk of gastric cancer[36,46,55-57]. A meta-analysis of 14 observational studies demonstrated that an increased intake of pickled vegetables was significantly associated with an increased risk of gastric cancer [OR (95%CI): 1.28 (1.06-1.53)][36]. Moreover, a Korean study reported that increased intake of salt-fermented fish and kimchi was associated with an elevated risk of early gastric cancer[46]. These findings explain the non-significant associations in our study because Koreans frequently consume processed vegetables, such as cooked, salted, or pickled vegetables, instead of fresh vegetables, and these often include a high concentration of salt. This increased salt consumption could weaken the protective effect of vegetables against gastric cancer.
Some strengths of the present study include the use of a comprehensive, validated FFQ to assess of the exposure to factors of interest. Additionally, we collected information from the participants about the prevalence of H. pylori infection, which an IARC monograph names as a cause of stomach cancer[19,20].
However, some potential limitations are also present in our hospital-based case-control study, such as selection and recall bias. Selection bias occurs in a case-control study when subjects in the “control” group are not truly representative of the population that is included in the case group. The hospital-based control group may not represent the Korean population. Moreover, the small number of participants in our study may not be sufficient to detect the protective effects of vitamin C and vitamin C-contributing foods on the gastric cancer risk. Finally, subgroup analyses by anatomical site (cardia vs non-cardia) or histological type (intestinal vs diffuse) would be helpful because these factors may modify the epidemiological characteristics of gastric cancer.
In conclusion, an inverse association was found between vitamin C and the risk of gastric cancer. Sufficient evidence is lacking to support the protective effect of vitamin C intake in participants infected with H. pylori. The total fruit consumption and some vitamin C-contributing foods showed a negative association with gastric cancer. Further studies that replicate our results in larger sample are required.
COMMENTS
Background
Vitamin C is one of the most common antioxidants in fruits and vegetables and it may exert a chemopreventive effect. However, the association between vitamin C intake and gastric cancer risk has been inconsistent among epidemiological studies.
Research frontiers
The authors conducted a case-control study to investigate the association between vitamin C, foods containing vitamin C consumption and gastric cancer risk.
Innovations and breakthroughs
Protective effect of vitamin C and some vitamin C-contributing foods against gastric cancer risk was observed in this study. Additionally, the authors collected information from the participants about the prevalence of H. pylori infection, which an IARC monograph names as a cause of stomach cancer. However, they failed to find a protective effect of vitamin C against gastric cancer in participants infected with H. pylori.
Applications
Results of this study support for using vitamin C and some vitamin C-contributing foods to protect people against gastric cancer risk.
Terminology
Dietary vitamin C intake has a chemopreventive effect, which may reduce gastric cancer risk. The normal metabolism in human body or exposure to well-known carcinogenesis can produce reactive oxygen species. At a cellular level, these species cause various mutations and other consequences in the DNA. Vitamin C plays a role in blocking carcinogenesis to protect cells from this damage and development of gastric cancer.
Peer-review
The manuscript by Hoang and colleagues describes the risk of gastric cancer as a function of vitamin C intake, an epidemiological study involving more than 1200 cases who participated in the study through questionaires and other tests. The study is well performed, and the manuscript is written in good and logical order easy for the reader to digest.
Footnotes
Institutional review board statement: The study was reviewed and approved by the National Cancer Center Institutional Review Board.
Informed consent statement: All study participants or their legal guardian provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare no conflicts of interest.
Data sharing statement: The technical appendix, statistical code, and dataset are available from the corresponding author at jskim@ncc.re.kr. The participants gave informed consent for data sharing. No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 22, 2016
First decision: May 12, 2016
Article in press: June 29, 2016
P- Reviewer: Overby A S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Oh CM, Kong HJ, Cho H, Lee DH, Lee KH. Prediction of cancer incidence and mortality in Korea, 2015. Cancer Res Treat. 2015;47:142–148. doi: 10.4143/crt.2015.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC, USA: World Cancer Research Fund, American Institute for Cancer Research; 2007. [Google Scholar]
- 4.Mahdavi R, Faramarzi E, Seyedrezazadeh E, Mohammad-Zadeh M, Pourmoghaddam M. Evaluation of oxidative stress, antioxidant status and serum vitamin C levels in cancer patients. Biol Trace Elem Res. 2009;130:1–6. doi: 10.1007/s12011-008-8309-2. [DOI] [PubMed] [Google Scholar]
- 5.Pathak SK, Sharma RA, Steward WP, Mellon JK, Griffiths TR, Gescher AJ. Oxidative stress and cyclooxygenase activity in prostate carcinogenesis: targets for chemopreventive strategies. Eur J Cancer. 2005;41:61–70. doi: 10.1016/j.ejca.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 6.Kong P, Cai Q, Geng Q, Wang J, Lan Y, Zhan Y, Xu D. Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PLoS One. 2014;9:e116060. doi: 10.1371/journal.pone.0116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekström AM, Serafini M, Nyrén O, Hansson LE, Ye W, Wolk A. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: a population-based case-control study in Sweden. Int J Cancer. 2000;87:133–140. [PubMed] [Google Scholar]
- 8.Boeing H, Frentzel-Beyme R, Berger M, Berndt V, Göres W, Körner M, Lohmeier R, Menarcher A, Männl HF, Meinhardt M. Case-control study on stomach cancer in Germany. Int J Cancer. 1991;47:858–864. doi: 10.1002/ijc.2910470612. [DOI] [PubMed] [Google Scholar]
- 9.González CA, Riboli E, Badosa J, Batiste E, Cardona T, Pita S, Sanz JM, Torrent M, Agudo A. Nutritional factors and gastric cancer in Spain. Am J Epidemiol. 1994;139:466–473. doi: 10.1093/oxfordjournals.aje.a117029. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Kim MK, Chang WK, Choi HS, Choi BY, Lee SS. Effect of nutrient intake and Helicobacter pylori infection on gastric cancer in Korea: a case-control study. Nutr Cancer. 2005;52:138–146. doi: 10.1207/s15327914nc5202_4. [DOI] [PubMed] [Google Scholar]
- 11.Nomura AM, Hankin JH, Kolonel LN, Wilkens LR, Goodman MT, Stemmermann GN. Case-control study of diet and other risk factors for gastric cancer in Hawaii (United States) Cancer Causes Control. 2003;14:547–558. doi: 10.1023/a:1024887411846. [DOI] [PubMed] [Google Scholar]
- 12.Ramón JM, Serra-Majem L, Cerdó C, Oromí J. Nutrient intake and gastric cancer risk: a case-control study in Spain. Int J Epidemiol. 1993;22:983–988. doi: 10.1093/ije/22.6.983. [DOI] [PubMed] [Google Scholar]
- 13.La Vecchia C, Ferraroni M, D’Avanzo B, Decarli A, Franceschi S. Selected micronutrient intake and the risk of gastric cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:393–398. [PubMed] [Google Scholar]
- 14.Botterweck AA, van den Brandt PA, Goldbohm RA. Vitamins, carotenoids, dietary fiber, and the risk of gastric carcinoma: results from a prospective study after 6.3 years of follow-up. Cancer. 2000;88:737–748. [PubMed] [Google Scholar]
- 15.Zheng W, Sellers TA, Doyle TJ, Kushi LH, Potter JD, Folsom AR. Retinol, antioxidant vitamins, and cancers of the upper digestive tract in a prospective cohort study of postmenopausal women. Am J Epidemiol. 1995;142:955–960. doi: 10.1093/oxfordjournals.aje.a117743. [DOI] [PubMed] [Google Scholar]
- 16.López-Carrillo L, López-Cervantes M, Ward MH, Bravo-Alvarado J, Ramírez-Espitia A. Nutrient intake and gastric cancer in Mexico. Int J Cancer. 1999;83:601–605. doi: 10.1002/(sici)1097-0215(19991126)83:5<601::aid-ijc5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Pelucchi C, Tramacere I, Bertuccio P, Tavani A, Negri E, La Vecchia C. Dietary intake of selected micronutrients and gastric cancer risk: an Italian case-control study. Ann Oncol. 2009;20:160–165. doi: 10.1093/annonc/mdn536. [DOI] [PubMed] [Google Scholar]
- 18.Epplein M, Shu XO, Xiang YB, Chow WH, Yang G, Li HL, Ji BT, Cai H, Gao YT, Zheng W. Fruit and vegetable consumption and risk of distal gastric cancer in the Shanghai Women’s and Men’s Health studies. Am J Epidemiol. 2010;172:397–406. doi: 10.1093/aje/kwq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 20.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang ZW, Patchett SE, Perrett D, Katelaris PH, Domizio P, Farthing MJ. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut. 1998;43:322–326. doi: 10.1136/gut.43.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correa P, Malcom G, Schmidt B, Fontham E, Ruiz B, Bravo JC, Bravo LE, Zarama G, Realpe JL. Review article: Antioxidant micronutrients and gastric cancer. Aliment Pharmacol Ther. 1998;12 Suppl 1:73–82. doi: 10.1111/j.1365-2036.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- 23.Dabrowska-Ufniarz E, Dzieniszewski J, Jarosz M, Wartanowicz M. Vitamin C concentration in gastric juice in patients with precancerous lesions of the stomach and gastric cancer. Med Sci Monit. 2002;8:CR96–C103. [PubMed] [Google Scholar]
- 24.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjønneland A, Olsen A, Overvad K, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Carcinogenesis. 2006;27:2250–2257. doi: 10.1093/carcin/bgl096. [DOI] [PubMed] [Google Scholar]
- 25.Woo HD, Lee J, Choi IJ, Kim CG, Lee JY, Kwon O, Kim J. Dietary flavonoids and gastric cancer risk in a Korean population. Nutrients. 2014;6:4961–4973. doi: 10.3390/nu6114961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim W, Woo HD, Lee J, Choi IJ, Kim YW, Sung J, Kim J. Dietary folate, one-carbon metabolism-related genes, and gastric cancer risk in Korea. Mol Nutr Food Res. 2016;60:337–345. doi: 10.1002/mnfr.201500384. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton R, Aatonen L. Tumors of digestive system. Lyon: IARC; 2000. pp. 39–52. [Google Scholar]
- 28.Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 29.Willett W. Nutritional Epidemiology. In: Willett W, editor. Implications of total energy intake for epidemiologic analyses. New York, NY, USA: Oxford University Press; 2013. pp. 260–286. [Google Scholar]
- 30.Mirvish SS. Effects of vitamins C and E on N-nitroso compound formation, carcinogenesis, and cancer. Cancer. 1986;58:1842–1850. doi: 10.1002/1097-0142(19861015)58:8+<1842::aid-cncr2820581410>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control. 1991;2:427–442. doi: 10.1007/BF00054304. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HM, Wakisaka N, Maeda O, Yamamoto T. Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter pylori. Cancer. 1997;80:1897–1903. [PubMed] [Google Scholar]
- 33.Lee JS, Kim J. Vegetable intake in Korea: data from the Korean National Health and Nutrition Examination Survey 1998, 2001 and 2005. Br J Nutr. 2010;103:1499–1506. doi: 10.1017/S0007114509993527. [DOI] [PubMed] [Google Scholar]
- 34.Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Algood HM, Cover TL. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun. 2013;81:2258–2267. doi: 10.1128/IAI.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunet N, Lacerda-Vieira A, Barros H. Fruit and vegetables consumption and gastric cancer: a systematic review and meta-analysis of cohort studies. Nutr Cancer. 2005;53:1–10. doi: 10.1207/s15327914nc5301_1. [DOI] [PubMed] [Google Scholar]
- 36.Kim HJ, Lim SY, Lee JS, Park S, Shin A, Choi BY, Shimazu T, Inoue M, Tsugane S, Kim J. Fresh and pickled vegetable consumption and gastric cancer in Japanese and Korean populations: a meta-analysis of observational studies. Cancer Sci. 2010;101:508–516. doi: 10.1111/j.1349-7006.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertuccio P, Rosato V, Andreano A, Ferraroni M, Decarli A, Edefonti V, La Vecchia C. Dietary patterns and gastric cancer risk: a systematic review and meta-analysis. Ann Oncol. 2013;24:1450–1458. doi: 10.1093/annonc/mdt108. [DOI] [PubMed] [Google Scholar]
- 38.Bonequi P, Meneses-González F, Correa P, Rabkin CS, Camargo MC. Risk factors for gastric cancer in Latin America: a meta-analysis. Cancer Causes Control. 2013;24:217–231. doi: 10.1007/s10552-012-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, Carneiro F, Barros H. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev. 2007;16:312–327. doi: 10.1097/01.cej.0000236255.95769.22. [DOI] [PubMed] [Google Scholar]
- 40.Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: results from a meta-analysis of cohort studies. Eur J Cancer. 2014;50:1498–1509. doi: 10.1016/j.ejca.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Hoshiyama Y, Sasaba T. A case-control study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Cancer Causes Control. 1992;3:441–448. doi: 10.1007/BF00051357. [DOI] [PubMed] [Google Scholar]
- 42.Ito LS, Inoue M, Tajima K, Yamamura Y, Kodera Y, Hirose K, Takezaki T, Hamajima N, Kuroishi T, Tominaga S. Dietary factors and the risk of gastric cancer among Japanese women: a comparison between the differentiated and non-differentiated subtypes. Ann Epidemiol. 2003;13:24–31. doi: 10.1016/s1047-2797(02)00269-7. [DOI] [PubMed] [Google Scholar]
- 43.Ji BT, Chow WH, Yang G, McLaughlin JK, Zheng W, Shu XO, Jin F, Gao RN, Gao YT, Fraumeni JF. Dietary habits and stomach cancer in Shanghai, China. Int J Cancer. 1998;76:659–664. doi: 10.1002/(sici)1097-0215(19980529)76:5<659::aid-ijc8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 44.Kono S, Ikeda M, Tokudome S, Kuratsune M. A case-control study of gastric cancer and diet in northern Kyushu, Japan. Jpn J Cancer Res. 1988;79:1067–1074. doi: 10.1111/j.1349-7006.1988.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SA, Kang D, Hong WS, Shim KN, Choe JW, Choi H. Dietary Habit and Helicobacter pylori Infection in Early Gastric Cancer Patient. Cancer Res Treat. 2002;34:104–110. doi: 10.4143/crt.2002.34.2.104. [DOI] [PubMed] [Google Scholar]
- 46.Lee SA, Kang D, Shim KN, Choe JW, Hong WS, Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J Epidemiol. 2003;13:162–168. doi: 10.2188/jea.13.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chyou PH, Nomura AM, Hankin JH, Stemmermann GN. A case-cohort study of diet and stomach cancer. Cancer Res. 1990;50:7501–7504. [PubMed] [Google Scholar]
- 48.Galanis DJ, Kolonel LN, Lee J, Nomura A. Intakes of selected foods and beverages and the incidence of gastric cancer among the Japanese residents of Hawaii: a prospective study. Int J Epidemiol. 1998;27:173–180. doi: 10.1093/ije/27.2.173. [DOI] [PubMed] [Google Scholar]
- 49.Ngoan LT, Mizoue T, Fujino Y, Tokui N, Yoshimura T. Dietary factors and stomach cancer mortality. Br J Cancer. 2002;87:37–42. doi: 10.1038/sj.bjc.6600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tajima K, Tominaga S. Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya, Japan. Jpn J Cancer Res. 1985;76:705–716. [PubMed] [Google Scholar]
- 51.Botterweck AA, van den Brandt PA, Goldbohm RA. A prospective cohort study on vegetable and fruit consumption and stomach cancer risk in The Netherlands. Am J Epidemiol. 1998;148:842–853. doi: 10.1093/oxfordjournals.aje.a009709. [DOI] [PubMed] [Google Scholar]
- 52.Kneller RW, McLaughlin JK, Bjelke E, Schuman LM, Blot WJ, Wacholder S, Gridley G, CoChien HT, Fraumeni JF. A cohort study of stomach cancer in a high-risk American population. Cancer. 1991;68:672–678. doi: 10.1002/1097-0142(19910801)68:3<672::aid-cncr2820680339>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi M, Tsubono Y, Sasazuki S, Sasaki S, Tsugane S. Vegetables, fruit and risk of gastric cancer in Japan: a 10-year follow-up of the JPHC Study Cohort I. Int J Cancer. 2002;102:39–44. doi: 10.1002/ijc.10659. [DOI] [PubMed] [Google Scholar]
- 54.Sauvaget C, Lagarde F, Nagano J, Soda M, Koyama K, Kodama K. Lifestyle factors, radiation and gastric cancer in atomic-bomb survivors (Japan) Cancer Causes Control. 2005;16:773–780. doi: 10.1007/s10552-005-5385-x. [DOI] [PubMed] [Google Scholar]
- 55.Kim HJ, Chang WK, Kim MK, Lee SS, Choi BY. Dietary factors and gastric cancer in Korea: a case-control study. Int J Cancer. 2002;97:531–535. doi: 10.1002/ijc.10111. [DOI] [PubMed] [Google Scholar]
- 56.Lee JK, Park BJ, Yoo KY, Ahn YO. Dietary factors and stomach cancer: a case-control study in Korea. Int J Epidemiol. 1995;24:33–41. doi: 10.1093/ije/24.1.33. [DOI] [PubMed] [Google Scholar]
- 57.Nan HM, Park JW, Song YJ, Yun HY, Park JS, Hyun T, Youn SJ, Kim YD, Kang JW, Kim H. Kimchi and soybean pastes are risk factors of gastric cancer. World J Gastroenterol. 2005;11:3175–3181. doi: 10.3748/wjg.v11.i21.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]


