Statins were underutilized among veterans infected with human immunodeficiency virus and hepatitis C virus according to the 2004 Adult Treatment Panel guidelines. Newer guidelines substantially expand statin recommendations and potentially widen the gap of underutilization in these chronically infected veterans.
Keywords: human immunodeficiency virus, hepatitis C virus, cardiovascular disease, hydroxymethylglutaryl-CoA reductase inhibitors, statins
Abstract
Background. Human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infections are associated with increased risk of cardiovascular disease (CVD). The potential impact of recently updated cholesterol guidelines on treatment of HIV- and HCV-infected veterans is unknown.
Methods. We performed a retrospective cohort study to assess statin use and recommendations among 13 579 HIV-infected, 169 767 HCV-infected, and 6628 HIV/HCV-coinfected male veterans aged 40–75 years. Prior 2004 Adult Treatment Panel (ATP-III) guidelines were compared with current 2013 American College of Cardiology/American Heart Association (ACC/AHA) cholesterol guidelines and 2014 US Department of Veterans Affairs (VA)/US Department of Defense (DoD) joint clinical practice guidelines using laboratory, medication, and comorbidity data from the VA Clinical Case Registry from 2008 through 2010.
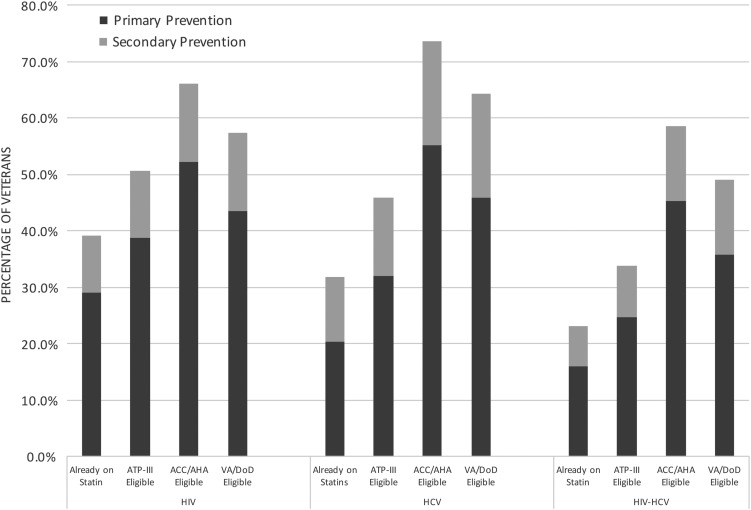
Results. Using risk criteria delineated by the ATP-III guidelines, 50.6% of HIV-infected, 45.9% of HCV-infected, and 33.8% of HIV/HCV-coinfected veterans had an indication for statin therapy. However, among those eligible, 22.7%, 30.5%, and 31.5%, respectively, were not receiving ATP-III recommended statin therapy. When current cholesterol guidelines were applied by VA/DoD and ACC/AHA criteria, increases in recommendations for statins were found in all groups (57.3% and 66.1% of HIV-infected, 64.4% and 73.7% of HCV-infected, 49.1% and 58.5% of HIV/HCV-coinfected veterans recommended).
Conclusions. Statins were underutilized among veterans infected with HIV, HCV, and HIV/HCV according to previous ATP-III guidelines. Current VA/DoD and ACC/AHA guidelines substantially expand statin recommendations and widen the gap of statin underutilization in all groups. These gaps in care present an opportunity to improve CVD prevention efforts in these at-risk populations.
Persons living with human immunodeficiency virus (HIV) infection are enjoying greater longevity but are also experiencing a disproportionate burden of other HIV-associated non-AIDS–related conditions such as cardiovascular disease (CVD). Chronic hepatitis C virus (HCV) infection has also been associated with an increased incidence of major CVD events [1, 2]. Although controversial, recent clinical studies suggest that persons coinfected with HIV and HCV may be at greater risk for major CVD events compared with persons infected only with HIV [3, 4]. Therefore, effective preventative measures are critical in these high-risk patients.
Hydroxymethylglutaryl-CoA reductase inhibitors (statins) have been a cornerstone of primary CVD prevention for the last 2 decades [5]. Unfortunately, HIV-infected veterans, particularly those with HCV coinfection, are less likely to receive statins as recommended by guidelines [6]. In 2013, the American College of Cardiology together with the American Heart Association (ACC/AHA) released new guidelines for statin use, replacing the Adult Treatment Panel III (ATP-III) guidelines last updated in 2004 [7, 8]. These new guidelines call for broad expansions in the number of adults eligible to receive statins, with treatment now recommended for 45 million Americans for primary prevention of atherosclerotic cardiovascular disease (ASCVD). This increase in eligibility was primarily due to the number of adults newly recommended for statin therapy based on their predicted 10-year risk of CVD as calculated by the pooled cohort equations [9]. In 2014, the US Department of Veterans Affairs (VA) and the US Department of Defense (DoD) approved a joint clinical practice guidelines for primary prevention of CVD for veterans with increased CVD risk (Supplementary Table 1 for comparison of ATP-III, ACC/AHA, and VA/DoD criteria) [10]. Despite significant changes in the indications for statin therapy in the general population, the current models do not account for the effects of HIV or HCV infection and may, in fact, underestimate CVD risk in HIV-infected patients [11]. At this time, the impact of these guideline changes on persons chronically infected with HIV and/or HCV remains unclear. Here, we investigate the impact of recent statin therapy guideline changes on recommendations for treatment in these high-risk populations.
The VA is the largest single provider of HIV and HCV care in the United States [12]. The Clinical Case Registry (CCR) is a national database of more than 60 000 HIV-infected and 320 000 HCV-infected veterans that includes electronic medical record data on diagnosis codes, laboratory results, medications, and procedures [13]. The CCR provides an excellent study cohort for investigating the application of statin guidelines given its size and stringent data collection methods.
METHODS
Study Population
All HIV-infected, HCV-infected, and HIV/HCV-coinfected males aged 40–75 years with data available in the CCR for the period 2008–2010 were eligible for inclusion. HIV-infected patients met criteria with confirmed positive HIV testing (HIV-1 enzyme-linked immunosorbent assay, Western blot, or HIV viral load) or documentation from International Classification of Diseases, ninth revision (ICD-9), for HIV (042 or V08). HCV infection was confirmed by positive HCV RNA or genotype test. Exclusion criteria included female sex or incomplete data (including comorbidities, laboratory data, and prescribed medications) that would limit the ability to assess cardiovascular risk. Due to the small proportion of women in the cohort, they were excluded from the analysis. The age range was selected due to the emphasis on adults aged 40–75 years in the recent ACC/AHA guidelines. The institutional review board at the Durham VA Medical Center approved the study.
Determination of Recommendations for Statins
Statin recommendations were determined using criteria from the updated 2004 ATP-III guidelines, the 2013 ACC/AHA guidelines, and the 2014 VA/DoD guidelines. Diagnoses of coronary heart disease or comorbidities were identified by ICD-9 code based on problem list or discharge codes (Supplementary Table 2).
For evaluation of the ATP-III guidelines, we assessed for presence of coronary heart disease (CHD), CHD risk equivalents, or CHD risk factors, together with the calculated Framingham risk score and low-density lipoprotein cholesterol (LDL-C) level (Supplementary Table 1). If veterans were already on statin therapy as of 2008–2010, we assumed they were being treated based on ATP-III criteria, as those were the guidelines relevant during that time period. To apply the ACC/AHA guidelines, we assessed 10-year ASCVD risk scores using the pooled cohort equations. We similarly applied the new VA/DoD guidelines using the same criteria but with a higher ASCVD risk score threshold of 12% derived using the pooled cohort equations.
Assessment of Laboratory and Pharmacologic Data
Clinical characteristics were evaluated, including HIV-specific measurements of viral load (copies/mL) and CD4 lymphocyte count (cells/mm3). For veterans infected with HCV, we calculated noninvasive markers of fibrosis, the aspartate aminotransferase-to-platelet ratio index (APRI) as well as the fibrosis-4 (FIB-4) score (Supplementary Table 3) [14, 15]. For all laboratory data, we used the most recently available values during the study period. We assessed the rates of statin prescription based on documentation of a statin on the medication list at any point during the time period from 2008 to 2010. Pharmacy data were used to determine the proportion on antihypertensive therapy and, where applicable, antiretroviral therapy.
Statistical Analyses
Demographic and clinical characteristics are presented as medians with 25% and 75% percentiles for continuous variables and frequencies with proportions for categorical variables. We also performed descriptive analyses with medians and percentiles for veterans recommended for statins based on ATP-III criteria, grouped by those receiving statins and those not receiving statins. To further explore predictors for statin underutilization, multivariable logistic regression was used to determine patient-level covariates associated with receiving recommended statin therapy according to ATP-III guidelines. The response variable was not receiving vs receiving statin therapy, and independent variables are those listed in Table 2. Age and body mass index were modeled with linear and quadratic terms. “Engagement in care” was defined as consecutive annual visits during all years of the study period. Separate models were fit for HIV-monoinfected, HCV-monoinfected, and HIV/HCV-coinfected veterans. Final model selection was performed using backward elimination of variables and included only those variables that were significant at P < .05. All analyses were performed using SAS statistical software version 9.4.
Table 2.
Characteristics of Veterans Recommended for Statin Therapy Based on by 2004 Adult Treatment Panel Guidelines: Prescribed Statins vs Not Prescribed Statins
| Variablea | HIV Infection |
HCV Infection |
HIV/HCV Coinfection |
|||
|---|---|---|---|---|---|---|
| Statin (n = 5310) | No Statin (n = 1557) | Statin (n = 54 144) | No Statin (n = 23 813) | Statin (n = 1534) | No Statin (n = 706) | |
| Age (y) | 57.7 (50.9–63.3) | 58.4 (51.8–63.4) | 59.0 (55.1–62.2) | 58.8 (55.1–61.9) | 57.7 (53.6–61.6) | 58.0 (54.3–61.3) |
| Black race | 39.5% | 45.7% | 33.5% | 30.1% | 60.3% | 61.6% |
| Cardiovascular disease | 25.5% | 15.4% | 36.2% | 15.8% | 31.0% | 17.1% |
| Cholesterol (mg/dL) | ||||||
| Median total cholesterol | 183 (157–213) | 200 (181–224) | 163 (138–194) | 189 (173–209) | 172 (146–200) | 193 (175–214) |
| Median high-density lipoprotein cholesterol | 40 (33–49) | 38 (31–47) | 39 (32–48) | 39 (32–47) | 40 (32–50) | 39 (31–48) |
| Median low-density lipoprotein cholesterol | 104 (83–129) | 124 (112–142) | 93 (72–119) | 121 (109–137) | 96 (76–117) | 119 (108–135) |
| Hypertension | ||||||
| Systolic blood pressure (mmHg) | 127 (117–137) | 133 (122–144) | 130 (119–140) | 135 (124–146) | 129 (118–138) | 134 (122–145) |
| On anti-hypertension therapy | 74.5% | 68.7% | 88.5% | 77.8% | 83.8% | 79.0% |
| Diabetes | 26.3% | 18.2% | 44.0% | 22.4% | 37.0% | 25.1% |
| Body mass index (kg/m2) | 26.7 (23.6–30.2) | 25.9 (23.1–29.4) | 28.8 (25.5–32.7) | 28.0 (24.9–31.5) | 26.0 (23.2–29.4) | 25.5 (22.7–28.3) |
| Smoking | 14.0% | 26.7% | 20.2% | 34.9% | 17.0% | 30.0% |
| Engaged in care | 92.2% | 84.5% | 91.2% | 83.8% | 93.3% | 90.1% |
| HIV | ||||||
| CD4 (cells/mm3) | 537 (364–742) | 461 (295–653) | n/a | n/a | 523 (349–723) | 440 (276–639) |
| CD4 nadir (cells/mm3) | 222 (96–372) | 201 (69–349) | n/a | n/a | 185 (70–310) | 163 (55–300) |
| Viral suppression | 80.40% | 69.90% | n/a | n/a | 76.20% | 66.60% |
| Antiretroviral therapy | 87.20% | 76.00% | n/a | n/a | 79.80% | 73.20% |
| Protease inhibitor | 51.80% | 51.30% | n/a | n/a | 54.90% | 56.10% |
| HCV | ||||||
| Fibrosis-4 | n/a | n/a | 1.57 (1.14–2.27) | 1.77 (1.25–2.77) | 1.77 (1.24–2.55) | 1.90 (1.36–3.08) |
| Aminotransferase-to- platelet ratio index | n/a | n/a | 0.38 (0.26–0.62) | 0.49 (0.30–0.96) | 0.44 (0.30–0.74) | 0.49 (0.32–0.98) |
| Cirrhosis | n/a | n/a | 11.1% | 17.8% | 14.7% | 21.5% |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; n/a, not applicable.
a Values in the tables are shown as counts and percentages for the categorical variables and median [25%, 75%] for continuous measures.
RESULTS
Demographic and Clinical Characteristics
From a cohort of 253 866 veterans, 63 892 were excluded due to incomplete data. The final study cohort included 189 974 male veterans: 13 579 HIV-infected, 169 767 HCV-infected, and 6628 HIV/HCV-coinfected male veterans aged 40–75 years. Baseline characteristics of each study population are provided in Table 1. Forty-seven percent of HIV-infected persons, 34% of HCV-infected persons, and 63% of coinfected persons were black. Relative to veterans with HIV monoinfection, veterans infected with HCV had lower baseline total cholesterol and LDL-C values and higher rates of diabetes. The majority of veterans with HIV and HIV/HCV had undetectable HIV viral loads (71.8% and 67.2%, respectively). Among HCV-infected veterans, the rate of cirrhosis (APRI > 2 or FIB4 > 3.25) was 19.5% for HCV-monoinfected and 24.5% for HIV/HCV-coinfected veterans. Statins were prescribed for 39.1% of HIV-infected, 31.9% of HCV-infected, and 23.1% of HIV/HCV-coinfected veterans. Numbers of new statin prescriptions among the HIV, HCV, and coinfected cohorts varied by year during the study period (Supplementary Figure 1).
Table 1.
Demographics and Characteristics of Veterans’ Affairs Clinical Case Registry Cohort
| Variablea | HIV | HCV | HIV/HCV |
|---|---|---|---|
| Infection (n = 13 579) | Infection (n = 169 767) | Coinfection (n = 6628) | |
| Age (y) | 53.9 (48.1–61.3) | 57.9 (54.1–61.3) | 56.3 (52.5–60.3) |
| Black race | 47.0% | 34.0% | 63.4% |
| Cardiovascular disease | 13.8% | 18.4% | 13.2% |
| Cholesterol (mg/dL) | |||
| Median total cholesterol | 179 (154–206) | 162 (139–188) | 160 (136–187) |
| Median high-density lipoprotein cholesterol | 40 (32–50) | 41 (33–51) | 41 (32–51) |
| Median low-density lipoprotein cholesterol | 103 (83–125) | 92 (72–115) | 87 (66–109) |
| Hypertension | |||
| Systolic blood pressure (mmHg) | 126 (117–136) | 130 (119–140) | 127 (116–138) |
| Antihypertensive treatment | 57.4% | 74.1% | 68.0% |
| Diabetes | 15.6% | 26.9% | 20.5% |
| Body mass index (kg/m2) | 25.9 (23.0–29.3) | 27.6 (24.3–31.4) | 25.0 (22.2–28.2) |
| Smoking | 15.8% | 22.3% | 20.5% |
| Engaged in care | 87.8% | 86.1% | 89.7% |
| HIV | |||
| CD4 (cells/mm3) | 486 (307–687) | n/a | 434 (264–636) |
| CD4 nadir (cells/mm3) | 207 (76–357) | n/a | 169 (61–295) |
| Viral suppression | 71.8% | n/a | 67.2% |
| Antiretroviral therapy | 80.6% | n/a | 74.1% |
| Protease inhibitor | 50.9% | n/a | 55.3% |
| HCV | |||
| Fibrosis-4 | n/a | 1.78 (1.24–2.89) | 2.02 (1.40–3.28) |
| Aminotransferase-to-platelet ratio index | n/a | 0.48 (0.30–0.96) | 0.56 (0.34–1.05) |
| Cirrhosisb | n/a | 19.5% | 24.2% |
| Prescribed statins | 39.1% | 31.9% | 23.1% |
Abbreviations: APRI, aspartate aminotransferase-to-platelet ratio index; FIB-4, fibrosis-4; HCV, hepatitis C virus; HIV, human immunodeficiency virus; n/a, not applicable.
a Values in the tables are shown as counts and percentages for the categorical variables and median [25%, 75%] for continuous measures.
b Cirrhosis defined as FIB-4 > 3.25 or APRI > 2.
ATP-III Guidelines and Underutilization of Statins
The proportions of each group already receiving statin therapy and recommended for statin therapy under each set of guidelines, according to primary or secondary prevention, are shown in Figure 1. Based on ATP-III guidelines, 50.6% of HIV-infected, 45.9% of HCV-infected, and 33.8% of HIV/HCV-coinfected veterans were eligible for statin therapy at the time of observation. We assessed those for whom statins were underutilized, that is, “ATP-III statin eligible but not on therapy”; 22.7% of HIV-infected, 30.6% of HCV-infected, and 31.5% of HIV/HCV-coinfected veterans recommended for statins were not receiving therapy. Compared with ATP-III statin-eligible veterans on therapy, statin-eligible veterans not on therapy had lower rates of CVD and diabetes but higher rates of smoking (Table 2). HIV-infected veterans who were statin eligible but not on therapy were less likely to have an undetectable viral load compared with those receiving statin therapy as recommended by ATP-III guidelines. HCV-infected veterans who were ATP-III statin eligible but not on therapy had higher noninvasive markers of fibrosis than HCV-infected veterans receiving recommended statin therapy (Table 2). In a multivariable logistic regression model, smoking and age were independently associated with increased risk of statin underutilization based on ATP-III guidelines, while engagement in care was associated with decreased risk of underutilization. Among HIV-monoinfected and HIV/HCV-coinfected veterans, detectable HIV viral load was a predictor of statin underutilization, as was cirrhosis for HCV-monoinfected and HIV/HCV-coinfected veterans (Table 3).
Figure 1.
Veterans receiving statins and eligible for statins under 2004 Adult Treatment Panel (ATP-III), American College of Cardiology/American Heart Association (ACC/AHA), and US Department of Veterans Affairs (VA)/Department of Defense guidelines. Abbreviations: DoD, US Department of Defense; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Table 3.
Multivariable Logistic Regression Model Demonstrating Significant Predictors of Underutilization of 2004 Adult Treatment Panel–Recommended Statin Therapy
| HIV |
HCV |
HIV/HCV Coinfection |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | Variable | OR | 95% CI | Variable | OR | 95% CI |
| Age | 1.156 | 1.040–1.284 | Age | 1.278 | 1.223–1.336 | Age | 1.381 | 1.077–1.771 |
| Age squared | 0.999 | .998–1.000 | Age squared | 0.998 | .998–.999 | Age squared | 0.998 | .995–1.000 |
| Black race | 1.180 | 1.024–1.360 | Black race | 0.960 | .922–.999 | CVD | 0.562 | .428–.738 |
| CVD | 0.570 | .476–.681 | CVD | 0.391 | .374–.409 | TC | 0.992 | .987–.998 |
| TC | 0.997 | .994–1.000 | TC | 0.993 | .992–.994 | HDL-C | 0.991 | .983–.999 |
| HDL-C | 0.982 | .978–.987 | HDL-C | 0.989 | .988–.991 | LDL-C | 1.038 | 1.031–1.045 |
| LDL-C | 1.020 | 1.016–1.024 | LDL-C | 1.031 | 1.030–1.032 | SBP | 1.017 | 1.011–1.023 |
| SBP | 1.020 | 1.016–1.024 | SBP | 1.017 | 1.016–1.018 | BMI | 0.972 | .950–.994 |
| Diabetes | 0.737 | .618–.879 | Anti-HTN therapy | 0.779 | .741–.819 | Smoking | 2.253 | 1.742–2.914 |
| BMI | 0.977 | .963–.991 | Diabetes | 0.526 | .504–.548 | CD4 | 0.999 | .999–1.000 |
| Smoking | 2.306 | 1.958–2.715 | BMI | 0.979 | .976–.983 | Detectable HIV viral load | 1.298 | 1.009–1.670 |
| CD4 | 0.999 | .999–1.000 | Smoking | 1.972 | 1.893–2.054 | Cirrhosis | 1.732 | 1.296–2.313 |
| Detectable HIV viral load | 1.282 | 1.092–1.505 | Cirrhosis | 2.301 | 2.184–2.424 | Engaged in care | 0.687 | .457–1.035 |
| ART | 0.610 | .501–.744 | Engaged in Care | 0.591 | .559–.625 | |||
| Engaged in care | 0.673 | .538–.841 | ||||||
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; HCV, hepatitis C virus; HDL-C, high-density lipoprotein cholesterol; HIV, human immunodeficiency virus; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; OR, odds ratio; SBP, systolic blood pressure; TC, total cholesterol.
Expansion of Recommendations Under New Guidelines
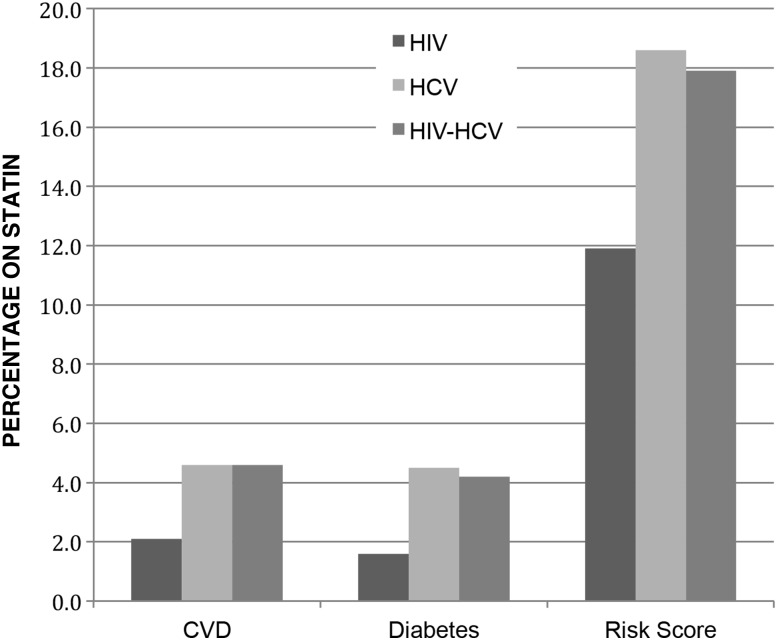
Application of the current 2013 ACC/AHA guidelines to the cohort resulted in expansion of statin eligibility relative to ATP-III guidelines in all subgroups (Figure 1). For HIV-infected, HCV-infected, and HIV/HCV-coinfected veterans, overall eligibility for statin therapy increased to 66.1%, 73.6%, and 58.5%, respectively. This represented an absolute increase in the proportion of veterans recommended for statin therapy of 15.5%, 27.7%, and 24.7%, respectively. Those newly recommended for therapy based on ACC/AHA guidelines had lower LDL-C and lower rates of diabetes than veterans meeting statin eligibility criteria by ATP-III guidelines (Supplementary Table 4). The majority of the absolute increases in statin eligibility from ATP-III guidelines resulted from expanded recommendations for primary prevention related to use of the 10-year ASCVD risk score as calculated using the pooled cohort equations (Figure 2).
Figure 2.
American College of Cardiology/American Heart Association expansions in statin recommendations: percent of veterans newly recommended for statin by specific indication. Abbreviations: CVD, cardiovascular disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Similarly, application of the new VA/DoD guidelines to the cohort resulted in expansion of statin recommendations relative to ATP-III guidelines in all subgroups (Figure 1). For HIV-infected, HCV-infected, and HIV/HCV-coinfected veterans, overall eligibility for statin therapy increased to 57.3%, 64.4%, and 49.1%, respectively. This represented an absolute increase in recommended statin therapy of 6.8%, 18.5%, and 15.3%, respectively, from ATP-III recommendations.
Impact of the New Guidelines
To estimate the likely effect of the ACC/AHA guidelines on future rates of CVD events, we applied the 10-year ASCVD risk score to all veterans without documented CVD who were newly eligible for statin therapy. We assumed statin therapy reduces the relative cardiovascular risk by 25%, as suggested in meta-analyses of statin use in primary prevention [16]. We found that among HIV-infected veterans, 14.9% would be predicted to have 10-year CVD events, resulting in a prevention rate of 3.73%. In HCV-infected veterans, 13.0% would be expected to have 10-year CVD events, resulting in a prevention rate of 3.25%. In HIV/HCV-coinfected veterans, 13.5% would be expected to have 10-year CVD events, resulting in a prevention rate of 3.38%.
DISCUSSION
In this analysis of the national Veterans Affairs CCR, approximately half of HIV- and HCV-infected veterans were eligible for statin therapy under ATP-III guidelines, whereas Pencina et al, who evaluated eligibility during a similar time period (2005–2010), found a rate of 37.4% in the general US population [9]. With application of new ACC/AHA guidelines, we found similarly high proportions to be eligible for statin therapy: 60%–74% in these chronically infected veterans vs less than half in the general population [9]. Expansion in statin recommendations in the general population is predominantly due to the implementation of the new 10-year risk calculator for ASCVD and increased emphasis on primary prevention, which has created cause for much controversy. Similarly, these large expansions in statin recommendations for HIV- and HCV-infected veterans are primarily the result of application of the new risk calculator, resulting in increased statin usage for primary prevention.
The implications of such high numbers of veterans recommended for statins (eg, almost three-quarters of those infected with HCV) are yet to be determined and may give pause to some providers. However, these chronically infected populations are at high risk for major CVD events, and given the proposed contribution of inflammation to this increased CVD risk, the anti-inflammatory properties of statins may be of additional benefit [17]. Furthermore, while there is evidence that the new pooled cohort equations may overestimate risk and thus statin recommendations in the general population [18], concern has been raised that the new ACC/AHA guidelines may still underestimate risk and statin recommendations in HIV-infected persons [11]. HIV- and HCV-infected adults stand to benefit from further research in this area, and the ongoing REPRIEVE trial (Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults; ClinicalTrials.gov identifier: NCT02344290) [19], which is testing the impact of statins on cardiovascular risk reduction in HIV-infected patients, is a clinical trial in which researchers hope to address a critical knowledge gap in understanding CVD in this population.
We found that almost 25% of HIV-infected veterans recommended for statin use under the 2004-updated ATP-III guidelines were “statin eligible but not on therapy” during the study period of 2008–2010, a time when the guidelines had been in place for a number of years and could have been incorporated into clinical practice. The proportion of veterans in whom statins were underutilized was even larger, roughly 33% for HCV-monoinfected and HIV/HCV-coinfected veterans. Both the new VA/DoD and ACC/AHA guidelines substantially increased (by 6%–28%) the number of veterans recommended for statin therapy, potentially widening the gap of those eligible for but not receiving therapy.
We investigated which veterans were less likely to receive statin therapy to determine subgroups that may benefit from targeted interventions. Statin-eligible veterans not on therapy were more likely to have a statin indication based on CVD risk score rather than documented CVD or diabetes (a “CHD risk equivalent”), suggesting that gaps in treatment are higher for primary, rather than secondary, prevention. Lower rates of consecutive annual visits during the study period (“engagement in care”) were seen in those not on guideline-recommended statin therapy (and similarly, engagement in care was an independent predictor of statin underutilization in our model), presumably because with fewer visits, providers have less time to address cardiovascular complications of HIV and discuss statin usage with their patients. Alternatively, engagement in care may be a surrogate marker for issues related to adherence. Notably, smoking was more common among those veterans not on recommended statin therapy. This discrepancy may reflect differences in health-seeking behaviors among smokers, an underappreciation of smoking as a CVD risk factor, or prioritization of smoking cessation over other aspects of patient care. Persons with HIV infection have high rates of smoking [20], and the attributable risk of myocardial infarction due to tobacco use may be greater in HIV-infected persons than in the general population [21], emphasizing an urgent need to better address behavioral risk factors and better understand drivers of risk in this population.
Compared with HIV-infected veterans, fewer veterans with HCV were receiving statins in 2008–2010 and, accordingly, more were recommended for statin therapy but not treated. Physicians may be more hesitant to prescribe statins to patients with chronic hepatitis and/or liver disease, especially when considering that monitoring liver function tests in patients on statins was routine even in the general population until 2012 [22]. This hesitation is further confirmed by our finding that among HCV-infected veterans, those with APRI or FIB-4 scores suggestive of cirrhosis were less likely to receive statins when recommended. However, statins have other noncardiovascular benefits in adults infected with HCV, as their use has been associated with reduced incidence of hepatocellular carcinoma [23] and decreased rate of fibrosis progression [24]. Our findings serve to emphasize an important need to educate providers about the safety of statins in patients with HCV infection and compensated cirrhosis [25].
Our study had several limitations. We excluded a large number of veterans due to incomplete data. This lack of data (eg, lipid panels) further highlights the need for more comprehensive primary care for veterans and suggests that inclusion of these veterans would be more likely to widen the gap in care described. We also could not assess whether medications had been prescribed by outside providers unless those prescriptions had been entered into the system by VA providers. We were unable to ensure that all lipid panels were ordered and collected in the fasting state, although all labs were ordered specifically for the purpose of lipid testing. We presumed that any veteran already on a statin was appropriately prescribed one based on ATP-III guidelines, which could potentially overestimate the numbers recommended per guideline criteria. The smoking prevalence in the VA electronic medical record system is known to be underreported. In order to account for this underestimation, we ran a sensitivity analysis and coded every veteran as a smoker, and our results changed negligibly. We did not have the ability to assess why veterans were not receiving guideline-recommended statin therapy; it is possible that many veterans were offered, but declined, statin therapy or had prior adverse reactions to statins. It could certainly be the case that providers had many competing priorities (eg, discussion of smoking cessation, management of uncontrolled viremia, polypharmacy) that would limit their ability to effectively prescribe statins. We did not have a control group of uninfected veterans for comparison as the CCR includes only veterans with HCV and/or HIV infections. However, it has been shown that HIV-infected veterans are less likely than uninfected veterans to receive guideline-recommended statin therapy [6]. Lastly, as we included only male veterans, our results may not be generalizable to female or civilian populations.
CONCLUSIONS
HIV is a treatable chronic disease and HCV is curable, thus prevention of other chronic medical conditions such as CVD is critical for the long-term survival of veterans with these infections. Underscoring the increased CVD risk in HIV-infected, HCV-infected, and HIV/HCV-coinfected veterans, we found a high rate of statin eligibility in these groups, which expands substantially under the new VA/DoD and ACC/AHA guidelines. A large proportion of veterans were not treated with the recommended statin therapy under the previous ATP-III guidelines, which was most evident in HCV-infected veterans. These findings suggest that programs are needed to improve primary CVD risk assessments in the clinical care of HIV- and HCV-infected veterans and to ensure statin therapy is discussed with the patient at the time of assessment. Under all three sets of guidelines, there is substantial opportunity to better address primary CVD prevention in veterans with chronic viral infections.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Dr Lisa Backus, Palo Alto Veterans Affairs Hospital, for her support of this project and input on the Clinical Case Registry database and data management.
Financial support. This work was supported by the Interdisciplinary Research Training Program in AIDS training (grant 5T32AI007392-25 to M. E. C.) as well as through the National Institutes of Health National Heart, Lung, and Blood Institute (U01 HL123336-01 to P. S. D.).
Potential conflicts of interest. P. S. D. receives research support from Kowa Pharmaceuticals; M. J. P. receives consulting fees from McGill University Health Center/Doggone Foundation; and A. M. N. and M. J. P. receive research support from Sanofi and Regeneron Pharmaceuticals. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Vassalle C, Masini S, Bianchi F, Zucchelli GC. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart 2004; 90:565–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis 2009; 49:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiberg MS, Chang C-CH, Skanderson M et al. . The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes 2011; 4:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med 2010; 11:462–8. [DOI] [PubMed] [Google Scholar]
- 5.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–9. [PubMed] [Google Scholar]
- 6.Freiberg MS, Leaf DA, Goulet JL et al. . The association between the receipt of lipid lowering therapy and HIV status among veterans who met NCEP/ATP III criteria for the receipt of lipid lowering medication. J Gen Intern Med 2009; 24:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone NJ, Robinson J, Lichtenstein AH et al. . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 63:2889–934. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Cleeman JI, Merz CNB et al. . Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol 2004; 24:e149–61. [DOI] [PubMed] [Google Scholar]
- 9.Pencina MJ, Navar-Boggan AM, D'Agostino RB Sr et al. . Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370:1422–31. [DOI] [PubMed] [Google Scholar]
- 10.Downs JR, O'Malley PG. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2014 U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med 2015; 163:291–7. [DOI] [PubMed] [Google Scholar]
- 11.Zanni MV, Fitch KV, Feldpausch M et al. . 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS Lond Engl 2014; 28:2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HHPHP Annual Stakeholders Report, May 2015 - stakeholders-report-2015.pdf. Available at: http://www.hiv.va.gov/pdf/stakeholders-report-2015.pdf Accessed 5 August 2015.
- 13.Backus LI, Gavrilov S, Loomis TP et al. . Clinical case registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc 2009; 16:775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Ann Intern Med 2013; 159:372. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z-H, Xin Y-N, Dong Q-J et al. . Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 2011; 53:726–36. [DOI] [PubMed] [Google Scholar]
- 16.Taylor F, Huffman MD, Macedo AF et al. . Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2013; 1:CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longenecker CT, Eckard AR, McComsey GA. Statins to improve cardiovascular outcomes in treated HIV infection. Curr Opin Infect Dis 2016; 29:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFilippis AP, Young R, Carrubba CJ et al. . An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015; 162:266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitka M. Exploring statins to decrease HIV-related heart disease risk. JAMA 2015; 314:657–9. [DOI] [PubMed] [Google Scholar]
- 20.Gritz ER, Vidrine DJ, Lazev AB, Amick BC, Arduino RC. Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine Tob Res 2004; 6:71–7. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen LD, Helleberg M, May MT et al. . Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis 2015; 60:1415–23. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration. FDA drug safety communication: Important safety label changes to cholesterol-lowering statin drugs. Rockville, MD, 2012. Available at: http://www.fda.gov/drugs/drugsafety/ucm293101.htm Accessed 10 May 2015. [Google Scholar]
- 23.Tsan Y-T, Lee C-H, Ho W-C, Lin M-H, Wang J-D, Chen P-C. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol 2013; 31:1514–21. [DOI] [PubMed] [Google Scholar]
- 24.Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol 2015; 62:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobson TA. NLA task force on statin safety—2014 update. J Clin Lipidol 2014; 8:S1–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.