Despite low prevalence of methicillin-resistant Staphylococcus aureus (MRSA; 0.7%), nearly one-third of adults hospitalized with community-acquired pneumonia (CAP) received anti-MRSA antibiotics. Characteristics of MRSA and pneumococcal CAP overlapped substantially, highlighting the challenge of accurately targeting anti-MRSA antibiotics using clinical criteria.
Keywords: pneumonia, Staphylococcus aureus, antibiotics
Abstract
Background. Prevalence of Staphylococcus aureus community-acquired pneumonia (CAP) and its clinical features remain incompletely understood, complicating empirical selection of antibiotics.
Methods. Using a multicenter, prospective surveillance study of adults hospitalized with CAP, we calculated the prevalence of methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus (MSSA) among all CAP episodes. We compared the epidemiologic, radiographic, and clinical characteristics of S. aureus CAP (per respiratory or blood culture) with those of pneumococcal (per respiratory or blood culture or urine antigen) and all-cause non-S. aureus CAP using descriptive statistics.
Results. Among 2259 adults hospitalized for CAP, 37 (1.6%) had S. aureus identified, including 15 (0.7%) with MRSA and 22 (1.0%) with MSSA; 115 (5.1%) had Streptococcus pneumoniae. Vancomycin or linezolid was administered to 674 (29.8%) patients within the first 3 days of hospitalization. Chronic hemodialysis use was more common among patients with MRSA (20.0%) than pneumococcal (2.6%) and all-cause non-S. aureus (3.7%) CAP. Otherwise, clinical features at admission were similar, including concurrent influenza infection, hemoptysis, multilobar infiltrates, and prehospital antibiotics. Patients with MRSA CAP had more severe clinical outcomes than those with pneumococcal CAP, including intensive care unit admission (86.7% vs 34.8%) and in-patient mortality (13.3% vs 4.4%).
Conclusions. Despite very low prevalence of S. aureus and, specifically, MRSA, nearly one-third of adults hospitalized with CAP received anti-MRSA antibiotics. The clinical presentation of MRSA CAP overlapped substantially with pneumococcal CAP, highlighting the challenge of accurately targeting empirical anti-MRSA antibiotics with currently available clinical tools and the need for new diagnostic strategies.
During the past decade, several reports described the emergence of community-acquired pneumonia (CAP) caused by Staphylococcus aureus and, specifically, methicillin-resistant S. aureus (MRSA) as a cause of severe pneumonia that leads to critical illness and death [1–6]. Although the prevalence of MRSA among acute cases of CAP has not been fully elucidated, recent studies suggest MRSA is an uncommon cause of CAP in the United States [7–11]. Nonetheless, clinicians often use vancomycin or linezolid empirically to treat adults with CAP due to concerns about the potential of MRSA pneumonia [12–16].
Although current guidelines from the Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) do not recommend routine empirical anti-MRSA antibiotics for CAP, addition of vancomycin or linezolid to standard therapy is recommended if MRSA pneumonia is clinically suspected [17]. Prior studies have described clinical features associated with MRSA CAP, such as hemodialysis, influenza infection, hemoptysis, multifocal or cavitary infiltrates, and recent antibiotic use [1–3, 7, 17–19]. However, these associations have not been rigorously evaluated, and whether the clinical syndrome of MRSA CAP is distinct from other types of CAP remains unclear [14]. Therefore, we estimated the prevalence of MRSA and methicillin-susceptible S. aureus (MSSA) among adults hospitalized with CAP and compared their clinical features with those of CAP patients without S. aureus, including all-cause CAP and pneumococcal CAP.
METHODS
We used data from the Etiology of Pneumonia in the Community (EPIC) study, a multicenter prospective active surveillance study of patients hospitalized with CAP funded by the Centers for Disease Control and Prevention (CDC) [9]. Enrollment of adult patients was conducted from 1 January 2010 to 30 June 2012 at 3 hospitals in Chicago, Illinois, and 2 hospitals in Nashville, Tennessee. Ethics approval for the study protocol was obtained at the CDC and each enrolling institution. Written informed consent was obtained from all participants or their representatives.
Population
Detailed inclusion and exclusion criteria for the EPIC study cohort have been previously described [9]. In brief, adults (aged ≥18 years) were eligible if they were admitted to a study hospital, resided in the study catchment area, had clinical evidence of an acute respiratory infection, and had radiographic evidence of pneumonia as interpreted by a study-dedicated thoracic radiologist. Patients with any of the following criteria were excluded: recent hospitalization (<28 days for immunocompetent patients and <90 days for immunosuppressed patients), nursing home resident not functionally independent [20], tracheostomy, gastrostomy, cystic fibrosis, cancer with neutropenia, solid organ or hematopoietic stem-cell transplant within the previous 90 days, active graft-vs-host disease, bronchiolitis obliterans, or human immunodeficiency virus infection with a CD4 cell count <200 mm3. The study population for the current analysis included adults in the EPIC study cohort who underwent at least 1 diagnostic test for both bacteria and viruses, which are outlined below.
Diagnostic Testing
As previously described [9], specimens were systematically evaluated for pathogen detection using blood, respiratory, and urine samples collected as soon as possible after hospital presentation.
Bacterial testing included blood cultures, sputum cultures (limited to high-quality samples [21]), urinary antigen tests for Streptococcus pneumoniae and Legionella pneumophila (BinaxNow, Alere) [22, 23], and detection of Chlamydophila pneumoniae and Mycoplasma pneumoniae by real-time polymerase chain reaction (RT-PCR) of naso/oropharyngeal (NP/OP) swabs [24]. Sputum was also tested for Legionella with a RT-PCR assay regardless of sputum quality [24]. Additionally, endotracheal aspirates, pleural fluid, and bronchoalveolar lavage (BAL) specimens obtained for routine clinical care underwent bacterial culturing. Pleural fluid samples obtained as part of clinical care also underwent testing with RT-PCR assays targeting S. aureus, S. pneumoniae, other streptococcus species, Enterobacteriaceae, Haemophilus influenzae, and Pseudomonas aeruginosa [25–27].
Viral testing included RT-PCR assays on NP/OP swab specimens for adenovirus, coronaviruses, human metapneumovirus, human rhinovirus, influenza, parainfluenza, and respiratory syncytial virus [28, 29]. Paired acute and convalescent serology was conducted for adenovirus, human metapneumovirus, influenza, parainfluenza, and respiratory syncytial virus [30, 31].
Etiology Group Definitions
Staphylococcus aureus CAP was defined as detection of S. aureus from a respiratory or blood specimen as described above. Patients with codetection of S. aureus and other pathogens were included in the S. aureus CAP group. Methicillin resistance was defined according to Clinical and Laboratory Standards Institute interpretive criteria for results of S. aureus in vitro antimicrobial susceptibility testing [32, 33]. Both MRSA and MSSA were included in the S. aureus CAP group. Additionally, MRSA and MSSA CAP subgroups were also reported separately.
Initially, the characteristics of S. aureus CAP and MRSA CAP were compared with characteristics of all CAP patients without S. aureus, defined as the all-cause non-S. aureus CAP group. This group included patients with other bacteria, viruses, fungi, mycobacteria, and unknown etiology. This comparison aimed to determine if MRSA CAP had clinical features distinctive enough to identify MRSA cases among a heterogeneous group of CAP patients.
Because certain clinical features may be associated with bacterial pneumonia in general, we also used pneumococcal CAP, the most common bacterial CAP detected in adults, as a second comparison group. Pneumococcal CAP was defined as a positive culture for S. pneumoniae in blood or respiratory specimen or a positive urinary antigen test as described above. Pneumococcal CAP was a subgroup of all-cause non-S. aureus CAP.
Prevalence and Seasonality
Prevalence of MRSA and MSSA was calculated by dividing the number of cases with each of these bacteria by the total study population of hospitalized adults with CAP. Prevalence calculations were also performed after stratifying the study population by age group (18–49, 50–64, 65–79, and ≥80 years), site of hospitalization (intensive care unit [ICU] vs general floor), and chronic hemodialysis use. Binomial 95% confidence intervals (CIs) were calculated for prevalence estimates. MRSA and MSSA prevalences were compared with S. pneumoniae prevalence, which has been previously reported in this study population [9].
To examine variation in S. aureus CAP across seasons and its temporal association with influenza activity, we plotted the number of S. aureus CAP and influenza-associated CAP cases by month during the study.
Clinical Characteristics and Outcomes
Patient characteristics, antibiotics administered, and clinical outcomes were systematically ascertained via patient interviews and medical chart abstractions using standardized definitions and data collection instruments [9]. For this analysis, use of empirical anti-MRSA antibiotics was defined as the administration of ≥1 dose of vancomycin or linezolid during the first 3 days of hospitalization, a time when culture results are typically incomplete and thus antibiotic selection is empirical.
Chest radiography findings were based on interpretations of study-dedicated thoracic radiologists. Pneumonia severity scores, including the pneumonia severity index (PSI) [34], CURB-65 (confusion, blood urea nitrogen ≥20 mg/dL, respiratory rate ≥30, systolic blood pressure <90 mmHg, age ≥65 years) [35], and ATS severe CAP minor criteria [17], were calculated from data at the time of initial hospital presentation.
Clinical characteristics and outcomes were compared among the CAP etiology groups. We compared the MRSA group with the pneumococcal and all-cause non-S. aureus groups for specific clinical features previously reported as potential risk factors for MRSA, including hemodialysis use [17, 18], seizure disorder [17], diabetes mellitus [17], recurrent soft tissue infections [1, 3], hemoptysis [1, 3], daily alcohol use [17], multilobar or cavitary infiltrates [1–3, 7, 19], pleural effusions [2, 3, 19], concurrent influenza infection [1–3, 17], proton pump inhibitor use [18], and use of antibiotics prior to hospital presentation [17]. Comparisons were conducted with the Wilcoxon rank-sum, χ2, or Fisher exact test as appropriate. Analyses were performed with Stata 12.0 (StataCorp, College Station, Texas).
RESULTS
During the 2.5-year study period, 3634 potentially eligible adults were identified; 2259 (62.2%) of these were enrolled, confirmed to have radiographic pneumonia, and underwent etiologic testing for both bacteria and viruses (study population). Eighty-seven (3.2%) patients within this study population chronically used hemodialysis and 12 (0.5%) were independently functioning nursing home residents.
Prevalence
Overall, 37 (1.6%; 95% CI, 1.2, 2.3) patients were identified as having S. aureus CAP, including 15 (0.7%; 95% CI, .4, 1.1) with MRSA and 22 (1.0%; 95% CI, .6, 1.5) with MSSA (Table 1). These S. aureus cases included 21 with S. aureus cultured from blood, 13 from high-quality sputum, 6 from BAL, and 2 from pleural fluid; 4 patients had S. aureus cultured from multiple sample types.
Table 1.
Prevalence of Staphylococcus aureus Community-Acquired Pneumonia (CAP), Including Methicillin-Resistant S. aureus and Methicillin-Susceptible S. aureus Subgroups, and Pneumococcal CAP Among Adults Hospitalized With CAP
| Population | Total CAP Cases |
Staphylococcus aureus CAP, n (row %) |
Pneumococcal CAP, n (row %) | ||
|---|---|---|---|---|---|
| Methicillin-Resistant S. aureus | Methicillin-Susceptible S. aureus | All S. aureus | |||
| All adults | 2259 | 15 (0.7) | 22 (1.0) | 37 (1.6) | 115 (5.1) |
| By age group, y | |||||
| 18–49 | 681 | 2 (0.3) | 7 (1.0) | 9 (1.3) | 31 (4.6) |
| 50–64 | 773 | 7 (0.9) | 11 (1.4) | 18 (2.3) | 41 (5.3) |
| 65–79 | 506 | 4 (0.8) | 2 (0.4) | 6 (1.2) | 34 (6.7) |
| ≥80 | 299 | 2 (0.7) | 2 (0.7) | 4 (1.3) | 9 (3.0) |
| By admission type | |||||
| Intensive care unit | 482 | 13 (2.7) | 10 (2.1) | 23 (4.8) | 40 (8.3) |
| General floor | 1777 | 2 (0.1) | 12 (0.7) | 14 (0.8) | 75 (4.2) |
| By chronic hemodialysis use | |||||
| Hemodialysis user | 87 | 3 (3.5) | 2 (2.3) | 5 (5.8) | 3 (3.5) |
| Not hemodialysis user | 2172 | 12 (0.6) | 20 (0.9) | 32 (1.5) | 112 (5.2) |
Abbreviation: CAP, community-acquired pneumonia.
Streptococcus pneumoniae was identified in 115 (5.1%; 95% CI, 4.2, 6.1) patients. These detections included 26 by blood culture, 8 from high-quality sputum, 3 from BAL, 1 from pleural fluid, 56 by urine antigen, and 21 by multiple modalities.
Prevalence of MRSA CAP was <1% in each age group, among patients admitted to the general medical floor, and among those who did not chronically use hemodialysis (Table 1). Prevalence of MRSA was lower than pneumococcus among CAP patients admitted to an ICU (2.7% vs 8.3%; P < .01) and similar among those chronically using hemodialysis (3.5% vs 3.5%; P = 1.0). None of the 12 independently functioning nursing home residents were found to have MRSA, MSSA, or pneumococcal CAP.
Seasonality and Codetections
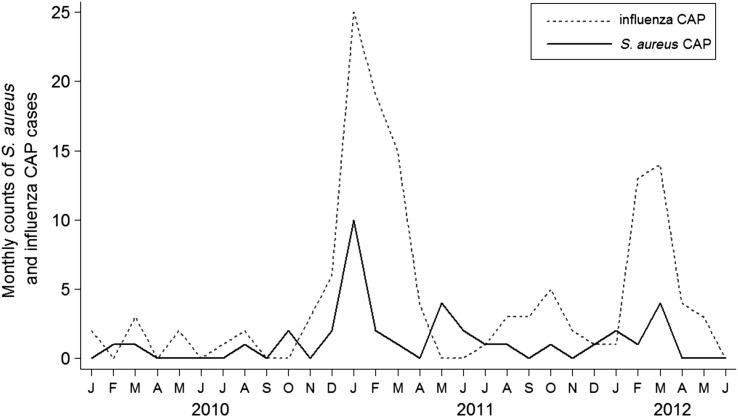
Staphylococcus aureus CAP cases were identified sporadically throughout the study, with 3 peaks of increased activity, including December 2010–February 2011, May 2011, and January–March 2012. Two of these peaks closely coincided with increased numbers of influenza CAP (Figure 1).
Figure 1.
Counts of Staphylococcus aureus and influenza community-acquired pneumonia (CAP) hospitalizations by month.
Prevalence of codetection of another pathogen was similar in S. aureus CAP (13 of 37 patients, 35.1%) and pneumococcal CAP (35 of 115 patients, 30.4%; P = .68). Influenza virus was the most common copathogen in S. aureus CAP, codetected in 3 (8.1%) cases (Table 2). None of the influenza–S. aureus or influenza–S. pneumoniae codetections occurred in patients on chronic hemodialysis. Four (10.8) patients with S. aureus CAP had codetection of a gram-negative rod (2 with Klebsiella pneumoniae, 1 with Escherichia coli, and 1 with Pseudomonas aeruginosa).
Table 2.
Pathogen Codetections Among Adults Hospitalized With Community-Acquired Pneumonia, by Etiology Group
| Pathogen |
Staphylococcus aureus CAP, n (Column %) |
Pneumococcal CAP, n (Column %); n = 115b | ||
|---|---|---|---|---|
| Methicillin-Resistant S. aureus (n = 15) | Methicillin-Susceptible S. aureus (n = 22)a | All S. aureus (n = 37)a | ||
| Any codetection | 3 (20.0) | 10 (45.5) | 13 (35.1) | 35 (30.4) |
| Viruses | ||||
| Influenza | 1 (6.7) | 2 (9.1) | 3 (8.1) | 4 (3.5) |
| Parainfluenza | 1 (6.7) | 1 (5.4) | 2 (5.4) | 7 (6.1) |
| Coronavirus | 0 | 1 (4.6) | 1 (2.7) | 2 (1.7) |
| Human metapneumovirus | 0 | 1 (4.6) | 1 (2.7) | 7 (6.1) |
| Respiratory syncytial virus | 0 | 1 (4.6) | 1 (2.7) | 2 (1.7) |
| Human rhinovirus | 0 | 0 | 0 | 14 (12.2) |
| Bacteria | ||||
| Klebsiella pneumoniae | 1 (6.7) | 1 (4.6) | 2 (5.4) | 0 |
| Escherichia coli | 0 | 1 (4.6) | 1 (2.7) | 0 |
| Mycoplasma pneumoniae | 0 | 1 (4.6) | 1 (2.7) | 0 |
| Pseudomonas aeruginosa | 0 | 1 (4.6) | 1 (2.7) | 0 |
| Viridans group Streptococcus | 0 | 1 (4.6) | 1 (2.7) | 0 |
| Haemophilus influenzae | 0 | 0 | 0 | 1 (0.87) |
| Legionella pneumophila | 0 | 0 | 0 | 1 (0.87) |
| Neisseria meningitidis | 0 | 0 | 0 | 1 (0.87) |
Abbreviation: CAP, community-acquired pneumonia.
a One patient with methicillin-susceptible S. aureus (MSSA) CAP had the following 3 pathogens detected: MSSA, Escherichia coli, and Pseudomonas aeruginosa. Therefore, among the 22 patients with MSSA CAP there were 11 codetections in 10 patients. Among 37 patients with all S. aureus CAP, there were 14 codetections in 13 patients.
b Four patients with pneumococcal CAP had 3 pathogens detected; these coinfections included 1 of each of the following combinations: Streptococcus pneumoniae/Haemophilus influenzae/Neisseria meningitidis; Streptococcus pneumoniae/influenza/human rhinovirus; Streptococcus pneumoniae/coronavirus/human rhinovirus; Streptococcus pneumoniae/human metapneumovirus/human rhinovirus. Therefore, among the 115 patients with pneumococcal CAP, there were 39 codetections in 35 patients.
Clinical Characteristics and Outcomes
Overall, patients with S. aureus CAP, including those with both MRSA and MSSA, had similar demographic characteristics, comorbidities, and presenting signs and symptoms compared with patients with all-cause non-S. aureus and pneumococcal CAP (Table 3). However, patients with S. aureus CAP, especially MRSA, tended to have higher severity scores than patients with non-S. aureus and pneumococcal CAP (Table 3). For example, 73.3% of MRSA and 45.2% of pneumococcal CAP patients had a PSI risk class ≥4 (P = .04). Among clinical characteristics previously described as potential risk factors for MRSA CAP, only chronic hemodialysis use and diabetes mellitus were significantly more common in MRSA CAP compared with pneumococcal CAP (Table 4).
Table 3.
Presenting Clinical Characteristics of Adults Hospitalized With Community-Acquired Pneumonia, by Etiology Group
| Characteristic |
Staphylococcus aureus CAP |
CAP Without Staphylococcus aureus |
|||
|---|---|---|---|---|---|
| Methicillin-Resistant S. aureus (n = 15) | Methicillin-Susceptible S. aureus (n = 22) | All S. aureus (n = 37) | Pneumococcal (n = 115) | All-Cause non-S. aureus (n = 2222) | |
| Demographics | |||||
| Median age (IQR), y | 62 (54, 76) | 56 (48, 64) | 60 (50, 65) | 59 (48, 67) | 57 (46,71) |
| Female, n (%) | 9 (60.0) | 12 (54.6) | 21 (56.8) | 55 (47.8) | 1134 (51.0) |
| Independently functioning nursing home resident | 0 | 0 | 0 | 0 | 12 (0.5) |
| Race/Ethnicity, n (%) | |||||
| White non-Hispanic | 8 (53.3) | 13 (59.1) | 21 (56.8) | 54 (47.0) | 1033 (46.5) |
| Black non-Hispanic | 3 (20.0) | 8 (36.4) | 11 (29.7) | 44 (38.3) | 863 (38.8) |
| Hispanic | 3 (20.0) | 1 (4.6) | 4 (10.8) | 14 (12.2) | 234 (10.5) |
| Other | 1 (6.7) | 0 | 1 (2.7) | 3 (2.6) | 92 (4.1) |
| Medical conditions, n (%) | |||||
| Asthma | 4 (26.7) | 3 (13.6) | 7 (18.9) | 24 (20.9) | 577 (26.0) |
| Chronic obstructive pulmonary disease | 3 (20.0) | 4 (18.2) | 7 (18.9) | 34 (29.6) | 513 (23.1) |
| Chronic heart failure | 4 (26.7) | 6 (27.3) | 10 (27.0) | 21 (18.3) | 420 (18.9) |
| Diabetes mellitus | 7 (46.7) | 8 (36.4) | 15 (40.5) | 23 (20.0) | 569 (25.6) |
| Chronic kidney disease | 4 (26.7) | 6 (27.3) | 10 (27.0) | 25 (21.7) | 346 (15.6) |
| Chronic hemodialysis | 3 (20.0) | 8 (36.4) | 11 (29.7) | 3 (2.6) | 82 (3.7) |
| Chronic liver disease | 0 | 1 (4.6) | 1 (2.7) | 6 (5.2) | 125 (5.6) |
| Any immunodeficiency | 3 (20.0) | 4 (18.2) | 7 (18.9) | 16 (13.9) | 361 (16.3) |
| Human immunodeficiency virus infection | 0 | 1 (4.6) | 1 (2.7) | 2 (1.7) | 65 (2.9) |
| Cancer | 5 (33.3) | 3 (13.6) | 8 (21.6) | 22 (19.1) | 397 (17.9) |
| Seizure disorder | 1 (6.7) | 1 (4.6) | 2 (5.4) | 4 (3.5) | 85 (3.8) |
| Current smoker | 1 (6.7) | 3 (13.6) | 4 (10.8) | 46 (40.0) | 591 (26.6) |
| Body mass index ≥30 kg/m2 | 5 (33.3) | 9 (40.9) | 14 (37.8) | 26 (22.6) | 789 (35.5) |
| Symptoms | |||||
| Median duration of symptoms prior to presentation (IQR), days | 4.7 (3.7, 7.5) | 4.8 (2.5, 8.7) | 4.7 (2.9, 7.8) | 4.8 (2.8, 8.8) | 4.8 (2.8, 8.8) |
| Fever | 9 (60.0) | 14 (63.6) | 23 (62.2) | 81 (70.4) | 1523 (68.5) |
| Cough with sputum production | 8 (53.3) | 8 (36.4) | 16 (43.2) | 69 (60.0) | 1235 (55.6) |
| Hemoptysis | 2 (13.3) | 3 (13.6) | 5 (13.5) | 13 (11.3) | 192 (8.6) |
| Shortness of breath | 11 (73.3) | 16 (72.7) | 27 (73.0) | 86 (74.8) | 1733 (78.0) |
| Chest pain | 5 (33.3) | 8 (36.4) | 13 (35.1) | 60 (52.2) | 1100 (49.5) |
| Rash | 2 (13.3) | 1 (4.5) | 3 (8.1) | 4 (3.5) | 73 (3.3) |
| Clinical signs at hospital presentation | |||||
| Confusion | 1 (6.7) | 3 (13.6) | 4 (10.8) | 7 (6.1) | 149 (6.7) |
| Wheezing | 4 (26.7) | 7 (31.8) | 11 (29.7) | 33 (28.7) | 636 (28.6) |
| Median Temperature (IQR), °F | 99.9 (97.9, 101.2) | 98.2 (97.2, 99.8) | 98.7 (97.8, 100.9) | 99.4 (98.1, 100.6) | 98.9 (98.0, 100.4) |
| Median systolic blood pressure (IQR), mmHg | 142 (110, 159) | 113 (101, 157) | 119 (102, 159) | 120 (103, 139) | 131 (115, 149) |
| Median oxygen saturation (IQR), % | 93 (88, 95) | 94 (91, 96) | 93 (89, 96) | 95 (92, 98) | 95 (93, 97) |
| Laboratory results at hospital presentation | |||||
| Median white blood cell count (IQR), 103 cells/μL | 12.7 (7.7, 16.8) | 13.4 (9.6, 17.2) | 12.8 (9.6, 16.8) | 13.5 (9.0, 19.9) | 11.4 (7.9, 14.9) |
| Median hematocrit (IQR), % | 36.0 (35.0, 40.0) | 37.8 (33.0, 39.5) | 38.0 (34.6, 39.6) | 37.0 (33.1, 41.0) | 38.0 (34.1, 41.5) |
| Median platelet count (IQR), 103 cells/μL | 168 (130, 305) | 225 (194, 295) | 219 (161, 295) | 216 (165, 276) | 237 (179, 306) |
| Median blood urea nitrate concentration (IQR), mg/dL | 34 (17, 62) | 17 (11, 43) | 29 (15, 43) | 20 (13, 32) | 15 (10, 23) |
| Median glucose concentration (IQR), mg/dL | 130 (111, 170) | 141 (115, 174) | 137 (115, 170) | 115 (100, 151) | 115 (99, 144) |
| Chest X-ray findings | |||||
| Cavitation/Necrosis, n (%) | 0 (0) | 0 | 0 (0) | 0 (0) | 34 (1.5) |
| Multilobar infiltrates, n (%) | 5 (33.3) | 7 (31.8) | 12 (32.4) | 39 (33.9) | 647 (29.1) |
| Pleural effusion, n (%) | 4 (26.7) | 5 (22.7) | 9 (24.3) | 41 (35.7) | 687 (30.9) |
| Pneumonia severity scores | |||||
| Median pneumonia severity index score (IQR) | 107 (98, 132) | 88 (74, 115) | 99 (76,118) | 83 (61.11) | 75 (52, 102) |
| Pneumonia severity index risk class 4 or 5, n (%) | 11 (73.3) | 11 (50.0) | 22 (59.5) | 52 (45.2) | 762 (34.3) |
| ≥3 CURB-65 criteria, n (%) | 6 (40.0) | 3 (13.6) | 9 (24.3) | 20 (17.4) | 243 (10.9) |
| ≥3 American Thoracic Society CAP minor criteria, n (%) | 3 (20.0) | 4 (18.2) | 7 (18.9) | 13 (11.3) | 153 (6.9) |
Abbreviations: CAP, community-acquired pneumonia; CURB-65IQR, confusion, blood urea nitrogen ≥20 mg/dL, respiratory rate ≥30, systolic blood pressure <90 mmHg, age ≥65 years; IQR, interquartile range.
Table 4.
Prevalence of Previously Reported Potential Risk Factors for Methicillin-Resistant Staphylococcus aureus Community-Acquired Pneumonia, by Etiology Group
| Characteristic | MRSA CAP, n (%) (n = 15) | Methicillin-Susceptible Staphylococcus aureus CAP, n (%) (n = 22) | Pneumococcal CAP, n (%) (n = 115) | P Valuea (MRSA vs Pneumococcal) | All-Cause non- Staphylococcus aureus CAP, n (%) (n = 2222) | P Valuea (MRSA vs All-Cause non- Staphylococcus aureus) |
|---|---|---|---|---|---|---|
| Hemodialysis use | 3 (20.0) | 2 (9.1) | 3 (2.6) | 0.02 | 82 (3.7) | 0.02 |
| Seizure disorder | 1 (6.7) | 1 (4.6) | 4 (3.5) | 0.46 | 85 (3.8) | 0.45 |
| Diabetes mellitus | 7 (46.7) | 8 (36.4) | 23 (20.0) | 0.04 | 569 (25.6) | 0.08 |
| Recurrent soft tissue infections | 1 (6.7) | 4 (18.2) | 9 (7.8) | 1.00 | 145 (6.5) | 1.00 |
| Hemoptysis | 2 (13.3) | 3 (13.6) | 13 (11.3) | 0.68 | 192 (8.6) | 0.38 |
| Daily alcohol use | 1 (6.7) | 3 (13.6) | 11 (9.6) | 1.00 | 156 (7.0) | 1.00 |
| Multilobar or cavitary infiltrates | 5 (33.3) | 7 (31.8) | 39 (33.9) | 1.00 | 667 (30.0) | 0.78 |
| Pleural effusion | 4 (26.7) | 5 (22.7) | 41 (35.7) | 0.58 | 687 (30.9) | 1.00 |
| Concurrent influenza infection | 1 (6.7) | 2 (9.1) | 4 (3.5) | 0.46 | 129 (5.8) | 0.59 |
| Current proton pump inhibitor use prior to admission | 5 (33.3) | 5 (22.7) | 18 (15.6) | 0.14 | 505 (22.7) | 0.35 |
| Outpatient antibiotic use prior to admission | 2 (13.3) | 0 | 15 (13.0) | 1.00 | 440 (19.8) | 0.75 |
Abbreviations: CAP, community-acquired pneumonia; MRSA, methicillin-resistant Staphylococcus aureus.
a P values calculated using Fisher exact test.
Empirical use of anti-MRSA antibiotics was common, with 29.8% of all enrolled patients receiving vancomycin or linezolid within the first 3 days of hospital admission despite only 0.7% ultimately found to have MRSA (Table 5). Anti-MRSA antibiotics were administered within the first 3 days of admission in 93.3% of patients with MRSA CAP compared with 47.0% with pneumococcal CAP (P = .001).
Table 5.
Use of Anti-Methicillin-Resistant Staphylococcus aureus Antibiotics (Vancomycin or Linezolid) Within 3 Days of Hospital Presentation, by Etiology Group
| Etiology Group | Patients, n | Anti-MRSA Antibiotics, n (row %) |
|---|---|---|
| All community-acquired pneumonia | 2259 | 674 (29.8) |
| Staphylococcus aureus | 37 | 34 (91.9) |
| MRSA | 15 | 14 (93.3) |
| MSSA | 22 | 20 (90.9) |
| All-cause non-Staphylococcus aureus | 2222 | 640 (28.8) |
| Pneumococcal | 115 | 54 (47.0) |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus.
Analyses to evaluate the prevalence of traditional MRSA risk factors and use of anti-MRSA antibiotics were repeated after excluding the 87 patients who chronically used hemodialysis and 12 independently functioning nursing home residents. These results (Supplementary Tables 1 and 2) were similar to the results with the full study population.
Among the 37 patients with S. aureus CAP, 4 (10.8%) died during the index hospitalization (Table 6). These 4 deaths included 1 patient with codetection of MRSA and K. pneumoniae, 1 with codetection of MSSA and viridans group Streptococcus, 1 with detection of MRSA alone, and 1 with detection of MSSA alone.
Table 6.
Clinical Outcomes of Adults Hospitalized With Community-Acquired Pneumonia, by Etiology Group
| Outcome |
Staphylococcus aureus CAP |
CAP without Staphylococcus aureus |
|||
|---|---|---|---|---|---|
| Methicillin-Resistant S. aureus (n = 15) | Methicillin-Susceptible S. aureus (n = 22) | All S. aureus (n = 37) | Pneumococcal (n = 115) | All-cause non-S. aureus (n = 2222) | |
| Median hospital length of stay (IQR), days | 9 (8, 13) | 6 (4, 10) | 8 (4, 11) | 4 (2, 7) | 3 (2, 6) |
| Intensive care unit admission, n (%) | 13 (86.7) | 10 (45.5) | 23 (62.2) | 40 (34.8) | 459 (20.7) |
| Empyema, n (%) | 4 (26.7) | 4 (18.2) | 8 (22.2) | 17 (14.8) | 187 (8.4) |
| Invasive mechanical ventilation, n (%) | 5 (33.3) | 4 (18.2) | 9 (24.3) | 14 (12.2) | 108 (4.8) |
| Acute respiratory distress syndrome, n (%) | 1 (6.7) | 1 (4.5) | 2 (5.4) | 5 (4.3) | 71 (3.2) |
| Septic shock with vasopressor use, n (%) | 3 (20.0) | 3 (13.6) | 6 (16.2) | 13 (11.3) | 81 (3.6) |
| In-hospital mortality, n (%) | 2 (13.3) | 2 (9.1) | 4 (10.8) | 5 (4.4) | 45 (2.0) |
Abbreviations: CAP, community-acquired pneumonia; IQR, interquartile range.
In-patient mortality for MRSA CAP (13.3%) was similar to that for MSSA CAP (9.1%; P = 1.00) but was higher than that for pneumococcal (4.4%; P = .19) and all-cause non-S. aureus (2.0%; P = .04) CAP. Patients with both MRSA and MSSA CAP also had longer hospital length of stay and were more likely to be admitted to the ICU than those with all-cause non-S. aureus CAP (Table 6).
DISCUSSION
In this multicenter, prospective active surveillance study at 5 US hospitals with systematic testing for pneumonia pathogens, S. aureus infection was uncommon among adults hospitalized with CAP, accounting for <2% of hospitalized CAP cases. MRSA was identified in <1% of adults hospitalized with CAP. Although clinical outcomes of MRSA CAP tended to be more severe than of other types of CAP, the initial presentation of MRSA and MSSA CAP overlapped substantially with one another and also with all-cause non-S. aureus CAP, especially that caused by S. pneumoniae.
Although the identification of risk factors and development of clinical prediction models for MRSA CAP have generated great interest recently [16, 18, 36–39], our results suggest it is unlikely that a clinical prediction model could be developed that accurately identifies MRSA CAP at hospital admission due to the nonspecificity of common MRSA features (influenza coinfection, multilobar infiltrates) and uncommon occurrence of more specific features (massive hemoptysis, cavitary pneumonia).
Despite the low prevalence of MRSA CAP, empirical anti-MRSA antibiotics were frequently used, with 29.8% of all patients in this study receiving vancomycin or linezolid during the first 3 days of hospitalization. MRSA CAP was uncommon among patients not admitted to an ICU, with a prevalence of 2 (0.1%) MRSA cases out of 1777 patients in this study, providing support for the current IDSA/ATS CAP guidelines [17] that do not recommend routine anti-MRSA antibiotics for patients admitted to a general medical floor. The prevalence of MRSA CAP among patients admitted to an ICU was 2.7%, and their clinical outcomes were particularly severe. Therefore, empirical anti-MRSA therapy for critically ill CAP patients appears to be an important consideration until results of diagnostic testing are available, especially since failure of initial empirical antibiotics to cover for the infecting pathogen appears to be associated with increased mortality in sepsis [40].
Overuse of antibiotics has been linked to several negative outcomes, including antibiotic resistance, medication-related toxicities, Clostridium difficile infections, and increased cost [41]. The difficulty of identifying MRSA CAP based on epidemiologic and clinical features and the overuse of anti-MRSA antibiotics highlight the need for the rapid diagnostic tests to help guide antibiotic selection [42] and stewardship programs to minimize the use of unnecessary antibiotics [12].
Prevalence of S. aureus and MRSA CAP in our study was comparable to that found by Moran et al [7], who reported a 3.9% prevalence of S. aureus and a 2.4% prevalence of MRSA among 595 adults hospitalized with CAP at 12 US hospitals during the 2005–2006 and 2006–2007 influenza seasons. Our results complement and expand on those published by Moran et al [7] in several important respects; namely, we studied a larger population 4–6 years later in 2 cities not evaluated in the prior study, enrolled a population-based cohort year-round for 2.5 consecutive years, and performed systematic influenza testing to understand the frequency of S. aureus and influenza coinfections. Similar to our results, Moran et al [7] also found that patients with MRSA CAP had high pneumonia severity scores at presentation, but that epidemiologic and clinical features were not particularly helpful for identifying MRSA cases for the purposes of guiding empirical antibiotic selection. Interestingly, in-patient mortality for MRSA CAP in our study (13.3%) and that of Moran et al [7] (14%) was similar and substantially lower than in older MRSA CAP case series [2, 3, 43, 44].
Prior studies emphasized the potential role of influenza infection as a predisposing factor for S. aureus CAP and frequent coinfection with influenza and S. aureus [1–3, 44]. We systematically tested for influenza, allowing direct evaluation of influenza–S. aureus coinfection in individual patients and seasonal variation in the detection of these pathogens. As illustrated in Figure 1, the highest levels of detection for influenza and S. aureus tended to cluster together in the same months, but cases of S. aureus CAP occurred year-round. Influenza was codetected in only 3 (8.1%) patients with S. aureus CAP. This pattern suggests that while S. aureus CAP may be more common during influenza peaks, the disease is not limited to patients with active influenza infection.
The distinction between CAP and pneumonia that developed as a result of exposure to the healthcare system, termed healthcare-associated pneumonia (HCAP) [45], has not been fully delineated [46, 47]. In this study, we standardized enrollment criteria, aiming to capture patients who developed pneumonia outside the healthcare environment [9]. Patients with strong risk factors for multidrug-resistant pathogens, including recent hospitalization, tracheostomy, gastrostomy, and nursing home residents unable to perform activities of daily living, were excluded from this study [18, 36–38]. However, some patients with traditional HCAP criteria outlined by the IDSA/ATS pneumonia guidelines [45] were eligible for inclusion, including functionally independent nursing home residents and patients with chronic hemodialysis use. These patients were included to allow for more comprehensive evaluation of characteristics associated with S. aureus CAP. Hemodialysis use was significantly associated with S. aureus infection, supporting the recommendation to consider empiric anti-MRSA antibiotics in hemodialysis patients who are hospitalized for pneumonia [45]. Importantly, when analyses were repeated after patients with these traditional HCAP risk factors (hemodialysis use and nursing home residency) were excluded, the prevalence of MRSA was very low (0.6%) and the use of anti-MRSA antibiotics remained high (28.0%).
This study had limitations. First, 68% of patients identified as eligible were enrolled; among these, 91% of patients met the final radiographic criteria and also had appropriate testing for both bacteria and viruses and thus were included in this analysis. Nonenrollment was largely due to patients or surrogates declining participation in the study; nonenrolled patients were older, more likely to require invasive mechanical ventilation, and more likely to die in the hospital [17]. Whether patients with S. aureus CAP were differentially enrolled in the study is unknown. Second, detection of S. aureus was based on cultures of blood and respiratory secretions, which may have missed the presence of S. aureus at the site of disease pathology in the lung. Results from invasive sampling, such as BAL and pleural fluid, were only included when these specimens were obtained for routine clinical care. This may have contributed to more S. aureus detection in patients with ICU admission and higher severity scores. Third, S. aureus can colonize the respiratory tract without causing illness. We attempted to minimize misclassification of colonizing S. aureus as S. aureus CAP by only including sputum and endotracheal samples that met high-quality criteria and BAL cultures with moderate or heavy growth [9]. Fourth, the number of S. aureus cases was small, limiting the precision of statistical comparisons and precluding more robust multivariable analyses. Fifth, we evaluated for concurrent influenza and S. aureus infections but could not assess for influenza infection as a precipitating event before S. aureus CAP because we did not obtain samples prior to hospital presentation. Sixth, our study population may not be fully representative of other geographic regions or time periods.
In conclusion, S. aureus (1.6%), including MRSA (0.7%), was uncommon in this large population-based study of adults hospitalized with CAP. Despite this low prevalence, anti-MRSA antibiotics were frequently used (29.8%). While MRSA CAP patients generally had high severity of illness, clinical and epidemiologic characteristics overlapped substantially with non-S. aureus CAP, particularly pneumococcal CAP, making it difficult to distinguish between etiologic types of pneumonia clinically. Low prevalence of MRSA combined with a lack of highly distinctive clinical features make accurate targeting of empirical anti-MRSA antibiotics very difficult. Development of diagnostic tests capable of rapidly and accurately identifying S. aureus could greatly improve the current approach to CAP management and reduce overuse of anti-MRSA antibiotics.
Supplementary Data
Supplementary materials are available at http://cid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Author Contributions. Study concept and design: W. H. S., R. G. W., D. J. W., E. J. A., J. D. C., D. M. C., S. M., S. J., K. M. E., and C. G. G. Acquisition of data: W. H. S., R. G. W., D. J. W., R. A. B., S. S. F., J. D. C., D. M. C., C. T., G. W. W., A. B., S. J., and K. E. M. Statistical analysis: W. H. S., G. C., Y. Z., A. B., and C. G. G. Interpretation of data: W. H. S., R. G. W., D. J. W., G. C., Y. Z., E. J. A., R. A. B., S. S. F., J. D. C., D. M. C., C. T., G. W. W., S. M., S. J., K. M. E., and C. G. G. Drafting of the initial manuscript: W. H. S. Critical revision of manuscript: All authors. Obtained funding: R. G. W., S. J., and K. M. E. Study supervision: R. G. W., S. J., K. M. E., C. G. G. W. H. S. takes responsibility for the manuscript as a whole, including the data and analyses.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC).
Role of the sponsor. Investigators from the CDC were involved in all phases of the study, including protocol development, study execution, data analysis, and decision to pursue publication.
Financial support. This work was supported by a cooperative agreement with the CDC (U18 IP000299). W. H. S. was supported in part by the National Institute of General Medical Sciences (K23GM110469). C. G. G. was supported in part by the National Institute on Aging (R01AG043471).
Potential conflicts of interest. W. H. S. reports receiving payment for serving on scientific advisory boards for BioFire Diagnostics and Venaxis, Inc. E. J. A. reports receiving grants and nonfinancial support from MedImmune and nonfinancial support from Roche and has served as a consultant for Abbvie. K. M. E. has served on a data and safety monitoring board for Novartis and her institution has received research support from Novartis. C. G. G. has served as a consultant for Pfizer Inc. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Francis JS, Doherty MC, Lopatin U et al. . Severe community onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentin leukocidin genes. Clin Infect Dis 2005; 40:100–7. [DOI] [PubMed] [Google Scholar]
- 2.Hageman JC, Uyeki TM, Francis JS et al. . Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis 2006; 12:894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kallen AJ, Brunkard J, Moore Z et al. . Staphylococcus aureus community-acquired pneumonia during the 2006 to 2007 influenza season. Ann Emerg Med 2009; 53:358–65. [DOI] [PubMed] [Google Scholar]
- 4.Kollef MH, Shorr A, Tabak YP, Gupta V, Lui LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 2005; 128:3854–62. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH, Micek ST. Staphylococcus aureus: a superbug infection in community and hospital settings. Chest 2005; 128:1093–7. [DOI] [PubMed] [Google Scholar]
- 6.Tadros M, Williams V, Coleman BL et al. . Epidemiology and outcome of pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) in Canadian hospitals. Plos One 2013; 8:e75171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moran GJ, Krishnadasan A, Gorwitz RJ et al. . Prevalence of methicillin-resistant Staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis 2012; 54:1126–33. [DOI] [PubMed] [Google Scholar]
- 8.Lobo JL, Reed KD, Wunderink RG. Expanded clinical presentation of community-acquired methicillin-resistant Staphylococcus aureus pneumonia. Chest 2010; 138:130–6. [DOI] [PubMed] [Google Scholar]
- 9.Jain S, Self WH, Wunderink RG et al. . Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postma DF, van Werkhoven CH, van Elden LJ et al. . Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med 2015; 372:1312–23. [DOI] [PubMed] [Google Scholar]
- 11.Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect 2013; 67:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fridkin S, Baggs J, Fagan R et al. . Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014; 63:194–200. [PMC free article] [PubMed] [Google Scholar]
- 13.Rosini JM, Grovola MR, Levine BJ, Jasani NB. Prescribing habits of vancomycin in the emergency department: are we dosing appropriately? J Emerg Med 2013; 44:979–84. [DOI] [PubMed] [Google Scholar]
- 14.Mandell LA, Wunderink R. Methicillin-resistant Staphylococcus aureus and community-acquired pneumonia: an evolving relationship. Clin Infect Dis 2012; 54:1134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin AT, Peyrani P, Wiemken TL, Ramirez JA, Arnold FW. Empiric therapy directed against MRSA in patients admitted to the intensive care unit does not improve outcomes in community-acquired pneumonia. Infection 2013; 41:517–23. [DOI] [PubMed] [Google Scholar]
- 16.Self WH, Wunderink RG, Williams DJ, Barrett TW, Baughman AH, Grijalva CG. Comparison of clinical prediction models for resistant bacteria in community-onset pneumonia. Acad Emerg Med 2015; 22:730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandell LA, Wunderink RG, Anzueto A et al. . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shindo Y, Ryota I, Kobayashi D et al. . Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Resp Crit Care Med 2013; 188:985–95. [DOI] [PubMed] [Google Scholar]
- 19.Jung WJ, Kang YA, Park MS et al. . Prediction of methicillin-resistant Staphylococcus aureus in patients with non-nosocomial pneumonia. BMC Infect Dis 2013; 13:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies in the aged: the index of ADL: a standardized measure of biological and psychological function. JAMA 1963; 185:914–9. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett RC. Medical microbiology: quality, cost and clinical relevance. New York: John Wiley, 1974:24–31. [Google Scholar]
- 22.Murdoch DR, Laing RTR, Mills GD et al. . Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol 2001; 39:3495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murdoch DR. Diagnosis of Legionella infection. Clin Infect Dis 2003; 36:64–9. [DOI] [PubMed] [Google Scholar]
- 24.Thurman KA, Warner AK, Cowart KC, Benitez AJ, Winchell JM. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn Microbiol Infect Dis 2011; 70:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carvalho MGS, Tondella ML, Mc-Caustland K et al. . Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol 2007; 45:2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaschke AJ, Heyrend C, Byington CL et al. . Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J 2011; 30:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaschke AJ, Heyrend C, Byington CL et al. . Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn Microbiol Infect Dis 2012; 74:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg GA, Schnabel KC, Erdman DD et al. . Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J Clin Virol 2013; 57:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dare RK, Fry AM, Chittaganpitch M, Sawanpanyalert P, Olsen SJ, Erdman DD. Human coronavirus infections in rural Thailand: a comprehensive study using real-time reverse-transcription polymerase chain reaction assays. J Infect Dis 2007; 196:1321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawatwong P, Chittaganpitch M, Hall H et al. . Serology as an adjunct to polymerase chain reaction assays for surveillance of acute respiratory virus infections. Clin Infect Dis 2012; 54:445–6. [DOI] [PubMed] [Google Scholar]
- 31.Feikin DR, Njenga MK, Bigogo G et al. . Additional diagnostic yield of adding serology to PCR in diagnosing viral acute respiratory infections in Kenyan patients 5 years of age and older. Clin Vaccine Immunol 2013; 20:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jorgensen J, Pfaller M, Carroll K et al. . Manual of clinical microbiology. 11th edition Washington, DC: ASM Press, 2015. [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 22nd informational supplement. CLSI document M100-S22. Wayne, PA: Clinical and Laboratory Standards Committee, 2012. [Google Scholar]
- 34.Fine MJ, Auble TE, Yealy DM et al. . A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336:243–50. [DOI] [PubMed] [Google Scholar]
- 35.Lim WS, van der Eerden MM, Laing R et al. . Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maruyama T, Fujisawa T, Okuno M et al. . A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug resistant pathogens to select initial empiric therapy. Clin Infect Dis 2013; 57:1373–83. [DOI] [PubMed] [Google Scholar]
- 37.Shorr AF, Zilberberg MD, Reichley R et al. . Validation of a clinical score for assessing the risk of resistant pathogens in patients with pneumonia presenting to the emergency department. Clin Infect Dis 2012; 54:193–8. [DOI] [PubMed] [Google Scholar]
- 38.Aliberti S, Di Pasquale M, Zanaboni AM et al. . Stratifying risk factors for multidrug-resistant pathogens in hospitalized patients coming from the community with pneumonia. Clin Infect Dis 2012; 54:470–8. [DOI] [PubMed] [Google Scholar]
- 39.Shorr AF, Myers DE, Huang DB, Nathanson BH, Emons MF, Kollef MH. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect Dis 2013; 13:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother 2010; 54:4851–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flanders SA, Saint S. Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med 2014; 174:661–2. [DOI] [PubMed] [Google Scholar]
- 42.Caliendo AM, Gilbert DN, Ginocchio CC et al. . Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis 2013; 57(suppl 3):S139–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gillet Y, Vanhems P, Lina G et al. . Factors predicting mortality in necrotizing community-acquired pneumonia caused by Staphylococcus aureus containing Panton-Valentine leukocidin. Clin Infect Dis 2007; 45:315–21. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza—Louisiana and Georgia, December 2006–January 2007. MMWR Morb Mortal Wkly Rep 2007; 56:355–9. [PubMed] [Google Scholar]
- 45.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388–416. [DOI] [PubMed] [Google Scholar]
- 46.Fujitani S, Yu VL. A new category–healthcare-associated pneumonia: a good idea, but problems with its execution. Eur J Clin Microbiol Infect Dis 2006; 25:627–31. [DOI] [PubMed] [Google Scholar]
- 47.Wunderink RG. Healthcare-associated bacteremia: stirring the mud. Crit Care Med 2006; 34:2685–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.