Abstract
Objective
To identify subgroups of U.S. children with special health care needs (CSHCN) and characterize key outcomes.
Data Source
Secondary analysis of 2009–2010 National Survey of CSHCN.
Study Design
Latent class analysis grouped individuals into substantively meaningful classes empirically derived from measures of pediatric medical complexity. Outcomes were compared among latent classes with weighted logistic or negative binomial regression.
Principal Findings
LCA identified four unique CSHCN subgroups: broad functional impairment (physical, cognitive, and mental health) with extensive health care (Class 1), broad functional impairment alone (Class 2), predominant physical impairment requiring family‐delivered care (Class 3), and physical impairment alone (Class 4). CSHCN from Class 1 had the highest ED visit rates (IRR 3.3, p < .001) and hospitalization odds (AOR: 12.0, p < .001) and lowest odds of a medical home (AOR: 0.17, p < .001). CSHCN in Class 3, despite experiencing more shared decision making and medical home attributes, had more ED visits and missed school than CSHCN in Class 2 (p < .001); the latter, however, experienced more cost‐related difficulties, care delays, and parents having to stop work (p < .001).
Conclusions
Recognizing distinct impacts of cognitive and mental health impairments and health care delivery needs on CSHCN outcomes may better direct future intervention efforts.
Keywords: Children's health, special health care needs, latent class analysis
Children with medical complexity (CMC) are a population of increasing clinical, research, and policy interest (Burke and Alverson 2010; Cohen et al. 2011; Simon, Mahant, and Cohen 2012; Berry et al. 2013) due to their disproportionately large and long‐term health service needs (Newacheck and Kim 2005; Simon et al. 2010; Cohen et al. 2012), which account for up to one‐third of total child health care spending (Neff et al. 2004; Cohen et al. 2012). Research suggests that CMC experience highly fragmented care with significant negative impacts on family well‐being (Bramlett et al. 2009; Kuo et al. 2011; Berry et al. 2013). Furthermore, ongoing advances in neonatology, pediatric care, and medical technology are steadily increasing the prevalence of such children (Burke and Alverson 2010; Cohen et al. 2011).
Despite this interest, a consensus definition for “medical complexity” has been elusive. Two broad strategies have been pursued: diagnosis‐based (“categorical”) and consequences‐based (“noncategorical”) systems, each having unique strengths and weaknesses. Diagnosis‐based schemas allow researchers, policy makers, and analysts to stratify populations and conduct analyses of large administrative datasets (Neff et al. 2002). Common examples of such systems include complex chronic conditions, clinical risk groups, and the chronic illness and disability payment system (Feudtner, Christakis, and Connell 2000; Kronick et al. 2000; Feudtner et al. 2002; Neff et al. 2002) among others.
The other common approach is to define medical complexity using a “consequences‐based strategy” in which the consequences of the condition establish complexity rather than the diagnoses themselves. Functional limitations or combinations of high subspecialty use and organ system involvement are some examples of noncategorical complexity definitions (Palfrey et al. 2004; Gordon et al. 2007; Bramlett et al. 2009). Though potentially more inclusive, this approach is difficult to apply to large administrative datasets (Neff et al. 2002; Bramlett et al. 2009; Cohen et al. 2011). While both strategies are highly valuable, each has important limitations in sensitivity and specificity for identifying CMC (Berry et al. 2013).
How one defines CMC has many important implications for performing risk stratification, comparing outcomes across studies, and facilitating program planning. The CMC research field may benefit from efforts to identify this population using a conceptually based and empirically rigorous approach. Over the last several years, a framework for conceptualizing CMC has been developed and proposed (van der Lee et al. 2007; Cohen et al. 2011) though not well tested in a large cohort of children. In this framework, medical complexity is described through characteristic manifestations within each of four domains—chronic conditions, health‐related needs, functional limitations, and health care use.
The purpose of this study was to identify and characterize CMC and their outcomes from a nationally representative sample of children with special health care needs (CSHCN) by operationalizing the CMC conceptual framework using a rigorous, empirical, consequences‐based analytical method. This study provides a novel means to identify CMC across the United States, and it also identifies other meaningful groups of children having unique aspects of special health care needs.
Methods
Study Population
We conducted a secondary analysis of the 2009–2010 National Survey of Children with Special Health Care Needs (NS‐CSHCN). This nationally representative survey is funded and administered by the Maternal and Child Health Bureau (MCHB) and conducted by the National Center for Health Statistics using random‐digit‐dialing methods, with separate random‐digit‐dial samples for landline and cell‐phone numbers (van Dyck et al. 2002; Blumberg et al. 2003, 2008; U.S. Department of Health and Human Services 2013).
In the NS‐CSHCN, children are defined as having special health care needs based on currently experiencing one or more of five health consequences attributable to a medical, behavioral, or other health condition that has lasted or is expected to last for at least 12 months. Consequences include ongoing limitations in ability to do things most children of the same age can do; prescription medications; specialized therapies; more medical, mental health, or educational services than are usual for most children of the same age; or behavioral, emotional, or developmental conditions requiring treatment or counseling (Bethell et al. 2002; Blumberg et al. 2003). From 196,159 households with children, 372,698 children were screened and 40,242 parents completed in‐depth NS‐CSHCN interviews between July 2009 and March 2011. One CSHCN was randomly selected in cases where a household had multiple CSHCN. The completion rate after a child was selected was 80.8 percent (83.6 percent for landline and 76.6 percent for cell‐phone samples) (U.S. Department of Health and Human Services 2013).
Latent Class Analysis
Latent class analysis (LCA) is a nonparametric statistical technique that identifies otherwise unobservable (i.e., latent) groups based on individuals' patterns of responses to multiple observable variables. Thus, substantively meaningful subgroups within a population can be empirically identified using LCA (Lanza et al. 2007; Nylund, Asparoutiov, and Muthen 2007; Collins and Lanza 2010). While related to factor analysis, LCA is based on categorical (as opposed to continuous) latent variables (Collins and Lanza 2010). The approach results in mutually exclusive latent classes of individuals based on their responses to observed variables (Lanza et al. 2007). Because LCA allows one to study the patterns of a set of variables on an outcome, it is useful for studying multifaceted constructs such as medical complexity.
From the 2009–2010 NS‐CSHCN, we identified items for inclusion in LCA modeling that represented the different domains of the conceptual framework for medical complexity (van der Lee et al. 2007; Cohen et al. 2011). In this framework, children are described as having characteristic patterns of (1) chronic conditions, (2) health‐related needs, (3) functional limitations, and (4) health care use. The NS‐CSHCN does not identify a comprehensive set of chronic condition diagnoses; however, as the CSHCN screener used to select participants explicitly identifies children with chronic conditions (Bethell et al. 2002), we assumed all respondents of the NS‐CSHCN fell within this domain. To avoid endogeneity, we then excluded from the remaining list of items those that were also considered primary study outcomes. For example, emergency department visits were not included as an item within the health care use domain because we planned to use it as a primary study outcome. The final list for inclusion in LCA modeling contained 17 items across the three domains (Table 1), a number that falls within typical maximum limits for LCA.
Table 1.
Weighted Latent Class Item Probabilities and Corresponding Complexity Domains from the Conceptual Framework
| CMC Conceptual Framework Domain | Broad Functional Impairment Requiring Extensive Health Care “CMC” (N = 1,969,057) | Broad Functional Impairment Alone (N = 3,766,077) | Predominant Physical Impairment Requiring Family‐Delivered Care (N = 2,277,867) | Physical Impairment Alone (N = 3,088,750) | |
|---|---|---|---|---|---|
| Class 1 | Class 2 | Class 3 | Class 4 | ||
| Difficulty with specific physical functiona | Functional limits | 0.869 | 0.528 | 0.965 | 0.515 |
| Difficulty with specific cognitive functionb | Functional limits | 0.963 | 0.957 | 0.243 | 0.206 |
| Difficulty with specific mental health functionc | Functional limits | 0.903 | 0.856 | 0.328 | 0.223 |
| Health conditions consistently affect daily activities | Functional limits | 0.870 | 0.579 | 0.423 | 0.073 |
| Use prescription medications | Health care use | 0.940 | 0.745 | 0.973 | 0.907 |
| See specialty doctor | Health care use | 0.795 | 0.379 | 0.576 | 0.379 |
| Receive PT/OT/speech therapy | Health care use | 0.636 | 0.370 | 0.080 | 0.055 |
| Receive home health | Health care use | 0.169 | 0.017 | 0.025 | 0.002 |
| Receive durable medical equipment | Health care use | 0.269 | 0.009 | 0.261 | 0.016 |
| Family provides health care at home | Needs | 0.753 | 0.328 | 0.768 | 0.306 |
| Providers need to communicate with school, early intervention, rehabilitation, child care providers | Needs | 0.704 | 0.378 | 0.199 | 0.056 |
| Health condition caused financial problems | Needs | 0.614 | 0.162 | 0.207 | 0.033 |
| Need extra help arranging or coordinating care | Needs | 0.535 | 0.151 | 0.083 | 0.012 |
| Need family mental health due to child's condition | Needs | 0.375 | 0.133 | 0.023 | 0.007 |
| Health care needs change all the time | Needs | 0.312 | 0.113 | 0.163 | 0.022 |
| ≥8 hours per week spent coordinating care by family | Needs | 0.215 | 0.049 | 0.033 | 0.008 |
| Need family respite care | Needs | 0.289 | 0.044 | 0.020 | 0.004 |
Bold text identifies item response probabilities over 50%.
Breathing or respiration, swallowing or digestion, blood circulation, chronic physical pain, seeing even when wearing glasses or contacts, hearing even when using a hearing aid.
Self‐care, coordination/moving around, using hands, learning, understanding or paying attention, speaking, communicating, or being understood.
Feeling anxious or depressed, acting‐out, fighting, bullying or arguing, making and keeping friends, age 18 months–17 years.
CMC, children with medical complexity.
LCA created a variety of candidate models, each of which divided study participants into a discrete number of mutually exclusive classes based on their responses to the 17 items. Candidate latent class models with 1–7 different classes were evaluated. Model fit criteria, including the Akaike information criteria (AIC) and Bayesian information criteria (BIC), as well as the Lo–Mendell–Rubin and Vuong–Lo–Mendell–Rubin likelihood ratio tests, were used to narrow down the best models (Schwarz 1978; Sclove 1987; Lo, Mendell, and Rubin 2001; Nylund, Asparoutiov, and Muthen 2007). For these likelihood ratio tests, models with k classes were compared to those with k − 1 classes. A p‐value <.05 indicated that the model with k classes provided a better fit than the model with k − 1 classes. When the difference in fit between two models was no longer significant, the more parsimonious model (k − 1) was favored. We assessed item‐response patterns to identify a final model with the best combination of homogeneity (i.e., dominant pattern within classes) and separation (i.e., distinction across classes) (Collins and Lanza 2010). Descriptive labels characterized the high‐probability items within each class.
Outcomes
Outcomes were grouped into three categories: utilization, child and family, and medical home. Utilization outcomes included number of emergency department visits and presence of a hospitalization in the past 12 months (hospitalization data provided from California only). Child and family outcomes included number of missed school days, parents stopping work due to the child's condition, difficulties or delays in care related to cost, and shared decision making. Medical home outcomes included each of the subcomponents for assessing the medical home concept as defined by the MCHB, including personal MD or RN, family‐centered care, no problem with referrals, usual source of care and coordinated care and a composite measure. Additional information on the medical home component definitions can be found elsewhere (2009).
Statistical Analysis
After selecting the best‐fit LCA model, descriptive statistics were used to characterize relevant demographic covariates across each latent class. Covariates included age, gender, race/ethnicity, primary language, payer category, poverty level, household education level, and household structure, consistent with other studies (Bethell et al. 2002; Inkelas et al. 2005; Kuo et al. 2011). Outcomes were compared among different latent classes with weighted logistic or negative binomial regression after adjusting for statistically significant covariates (bivariate relationships with p < .05). We used Mplus (version 7, Muthen & Muteh, Los Angeles, CA, USA) to perform LCA, and STATA (SE version 12.1, Stata Corp, College Station, TX, USA) to perform remaining analyses. Survey procedures based on sampling weights provided by the NS‐CSHCN were used to account for complex survey design to generate population estimates (van Dyck et al. 2002; Blumberg et al. 2008). The results were summarized in terms of adjusted incidence rate ratios (IRR) or adjusted odds ratios (AOR) along with the corresponding 95 percent confidence intervals (CI). This secondary data analysis of publicly available data was exempt from IRB review, in accordance with University of Wisconsin policy.
Results
Model Selection
The AIC and BIC best‐fit statistics leveled off at 4–5 classes (Appendix SA3), a result that was also supported by likelihood ratio tests. A four‐class LCA model was chosen after reviewing item‐response probabilities showing best homogeneity and separation with the four‐class solution. For each class, over 93 percent of children assigned to it had posterior probabilities >50 percent of being assigned to that class. Table 1 illustrates this model's conditional item‐response probabilities for each indicator of medical complexity.
Medical Complexity and Latent Classes
Within classes, items clustered around functional impairments and health care use and needs (Table 1).
Functional Impairments consisted of physical, cognitive, or mental health impairments, as well as the consistency with which the child's daily activities were impacted by their condition. Children in Classes 1 and 2 had high response probabilities across every one of these functional domains and were therefore labeled as having broad functional impairment. Children in Class 3 were noted to have predominant physical impairments (item‐response probability 0.965).
Health Care Use and Needs in our model classes included family delivery of health care at home, need for extra help coordinating care, provider need to communicate with community resources including early intervention, school, or rehabilitation child need to see subspecialists, and health conditions causing financial problems. Children in Class 1 had high probabilities in many areas and were therefore labeled as having extensive health care, while children in Class 3 had particular need for family‐delivered care at home (item‐response probability 0.768). Children in Class 2 did not have any unique health care needs.
Children in Class 4 were not characterized by any distinguishing items; however, consistent with all classes, they identified some physical functional impairment and need for prescription medications (item‐response probabilities 0.515 and 0.907, respectively).
Classes were labeled as follows:
Class 1 (17.7 percent): Broad Functional Impairment Requiring Extensive Health Care
Class 2 (27.8 percent): Broad Functional Impairment Alone
Class 3 (33.9 percent): Predominant Physical Impairment Requiring Family‐Delivered Care
Class 4 (20.5 percent): Physical Impairment Alone
Children in Class 1, having high‐response probabilities across items from all domains of the CMC conceptual framework, were identified as the CMC population.
The population estimates of each specific condition evaluated by the NS‐CSHCN and their respective proportions in each class are shown in Appendix SA2. The conditions having the highest proportion in the complex class included cerebral palsy (67.4 percent), brain injury (62.7 percent), and muscular dystrophy (62.5 percent); while the conditions with the lowest proportions in the complex class included asthma (16.8 percent), allergies (19.4 percent), and diabetes (25.2 percent). Similarly, the conditions with lowest proportions in the noncomplex Class 4 included muscular dystrophy (1.0 percent), intellectual disability (1.1 percent), and brain injury (1.2 percent); while the highest proportions in this class were allergies (28.3 percent), asthma (26.3 percent), and diabetes (22.5 percent). The conditions with the highest proportions in Class 2 (broad functional impairment alone) and Class 3 (predominant physical impairment requiring family‐delivered care) were attention‐deficit/hyperactivity disorder and cystic fibrosis, respectively.
Figure 1 illustrates relationships among the latent classes, as well as manifestation of several conditions within the latent classes (cystic fibrosis, asthma and Down syndrome). For example, children with cystic fibrosis varied across the breadth of functional impairment (48.4 percent in Class 1 and 45.6 percent in Class 3, both of which are classes having higher need for and use of health care).
Figure 1.
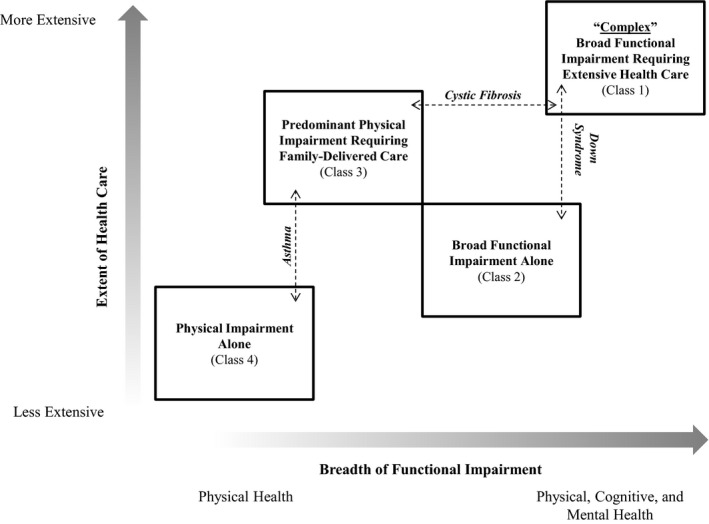
- Notes. aHealth Care Use and Needs: family delivers health care at home, family needs extra help coordinating care, providers need to communicate with community resources, including early intervention, school, or rehabilitation, child needs to see subspecialists, or health condition causes financial problems. Having multiple factors corresponds to more extensive health care. bFunctional Impairment: Physical, Cognitive and Mental Health. Having multiple impairments corresponds to broader overall functional impairment.
Population
Each complexity class had unique demographic profiles (Table 2). Compared to the noncomplex Class 4, children in the complex class were younger and more often male, publicly insured or uninsured, and impoverished. They were also less often white, non‐Hispanic, from a two‐parent household, or household with a parent educated beyond high school.
Table 2.
Characteristics of the U.S. CSHCN Population by Complexity Class (all data are weighted)
| Class 1 | Class 2 | Class 3 | Class 4 | |
|---|---|---|---|---|
| Broad Functional Impairment Requiring Extensive Health Care (“Complex”) | Broad Functional Impairment Alone | Predominant Physical Impairment Requiring Family‐Delivered Care | Physical Impairment Alone | |
| Population estimate (N) | 1,969,057 | 3,766,077 | 2,277,867 | 3,088,750 |
| Age | ||||
| 0–5 years | 21.5 | 16.2 | 28.9 | 19.9 |
| 6–11 years | 39.1 | 42.3 | 36.6 | 35.5 |
| >11 years | 39.4 | 41.4 | 34.5 | 44.7 |
| Gender | ||||
| Male | 61.7 | 64.7 | 54.1 | 54.6 |
| Female | 38.2 | 35.1 | 45.7 | 45.2 |
| Race/ethnicity | ||||
| White, non‐Hispanic | 54.4 | 57.7 | 55.0 | 67.4 |
| Black, non‐Hispanic | 16.0 | 17.0 | 18.7 | 13.3 |
| Hispanic | 20.4 | 18.0 | 17.9 | 12.1 |
| Multiple or other | 9.2 | 7.3 | 8.4 | 7.2 |
| Primary language | ||||
| English | 91.2 | 91.9 | 93.0 | 96.0 |
| Other | 8.8 | 8.1 | 7.0 | 4.0 |
| Payer category | ||||
| Private | 46.6 | 53.5 | 64.1 | 75.5 |
| Public | 49.1 | 42.0 | 32.3 | 22.5 |
| Uninsured | 4.3 | 4.5 | 3.6 | 2.0 |
| Poverty level, % | ||||
| 0–99% FPL | 31.8 | 26.5 | 21.2 | 11.7 |
| 100–199% FPL | 25.6 | 24.4 | 20.7 | 17.2 |
| 200–399% FPL | 25.0 | 26.7 | 30.3 | 31.7 |
| ≥400% FPL | 17.6 | 22.4 | 27.8 | 39.4 |
| Household educational level | ||||
| <High school | 14.5 | 14.4 | 9.9 | 5.9 |
| High school graduate | 21.2 | 23.6 | 18.8 | 15.4 |
| >High school | 64.2 | 62.1 | 71.3 | 78.7 |
| Household structure | ||||
| Parent household (biological or adopted) | 47.1 | 49.7 | 62.1 | 68.0 |
| Two‐parent stepfamily household | 11.1 | 11.0 | 7.6 | 8.4 |
| Mother only household | 32.7 | 29.0 | 24.9 | 17.9 |
| Other | 9.1 | 10.3 | 5.3 | 5.7 |
| No. of specialists in last 12 months, mean | 2.7 | 1.8 | 1.7 | 1.4 |
Outcomes
For every outcome measured, children from the complex class fared the worst (Tables 3, 4).
Table 3.
Utilization, Child and Family Outcomes (Multivariate Adjusted and Weighted Regression)a
| ED Visits, IRR (95% CI) | Hospitalizationb OR (95% CI) | Missed School Days, IRR (95% CI) | Parent Stopped Working due to Condition, OR (95% CI) | Difficulties or Delays Related to Cost, OR (95% CI) | Shared Decision Making, OR (95% CI) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| 0–5 years | Ref | Ref | –c | Ref | Ref | Ref |
| 6–11 years | 0.62 (0.57–0.68) | 0.36 (0.15–0.85) | Ref | 0.69 (0.60–0.81) | 1.52 (1.28–1.81) | 0.98 (0.87–1.11) |
| >11 years | 0.63 (0.58–0.68) | 0.27 (0.11–0.67) | 1.07 (1.03–1.10) | 0.58 (0.49–0.68) | 1.52 (1.29–1.80) | 1.00 (0.89–1.14) |
| Race/ethnicity | ||||||
| White, non‐Hispanic | Ref | Ref | Ref | Ref | Ref | Ref |
| Hispanic | 0.99 (0.88–1.12) | 1.29 (0.44–3.73) | 0.90 (0.85–0.96) | 1.39 (1.14–1.69) | 1.12 (0.92–1.38) | 0.81 (0.70–0.94) |
| Black, non‐Hispanic | 1.35 (1.22–1.48) | 3.82 (0.85–17.1) | 0.77 (0.73–0.82) | 1.06 (0.88–1.28) | 0.90 (0.73–1.11) | 0.76 (0.66–0.87) |
| Multiple or other | 1.06 (0.93–1.22) | 1.46 (0.42–5.14) | 0.91 (0.85–0.97) | 1.29 (1.03–1.61) | 1.08 (0.88–1.32) | 0.76 (0.64–0.90) |
| Primary language | ||||||
| English | Ref | Ref | Ref | Ref | Ref | Ref |
| Other | 0.81 (0.68–0.97) | 0.81 (0.31–2.14) | 0.81 (0.73–0.91) | 2.00 (1.52–2.64) | 0.80 (0.58–1.09) | 0.80 (0.63–1.02) |
| Payer category | ||||||
| Private | Ref | Ref | Ref | Ref | Ref | Ref |
| Public | 1.28 (1.17–1.39) | 1.05 (0.44–2.49) | 1.10 (1.04–1.16) | 1.47 (1.24–1.74) | 0.85 (0.72–1.01) | 0.89 (0.78–1.01) |
| Uninsured | 1.10 (0.89–1.37) | 1.29 (0.25–6.54) | 1.10 (0.98–1.23) | 1.61 (1.09–2.36) | 7.3 (5.55–9.51) | 0.63 (0.49–0.81) |
| Poverty level, % | ||||||
| 0–99% FPL | 1.65 (1.46–1.88) | 0.64 (0.15–2.73) | 1.14 (1.05–1.23) | 1.65 (1.31–2.09) | 1.69 (1.32–2.17) | 0.86 (0.72–1.02) |
| 100–199% FPL | 1.37 (1.23–1.52) | 1.07 (0.27–4.15) | 1.02 (0.96–1.09) | 1.41 (1.15–1.73) | 2.18 (1.78–2.66) | 0.89 (0.77–1.03) |
| 200–399% FPL | 1.19 (1.09–1.31) | 0.40 (0.10–1.60) | 1.02 (0.98–1.07) | 1.14 (0.95–1.36) | 1.87 (1.59–2.19) | 0.9 (0.80–1.01) |
| ≥400% FPL | Ref | Ref | Ref | Ref | Ref | Ref |
| Household educational level | ||||||
| <High school | 1.23 (1.08–1.41) | 2.69 (0.73–9.95) | 1.01 (0.93–1.10) | 0.97 (0.76–1.24) | 0.74 (0.57–0.97) | 0.84 (0.70–1.01) |
| High school graduate | 1.21 (1.11–1.33) | 4.26 (1.44–12.65) | 1.02 (0.97–1.07) | 0.84 (0.72–0.99) | 0.74 (0.62–0.88) | 0.91 (0.81–1.03) |
| >High school | Ref | Ref | Ref | Ref | Ref | Ref |
| Household structure | ||||||
| Parent household | Ref | Ref | Ref | Ref | Ref | Ref |
| Two‐parent stepfamily | 1.29 (1.16–1.45) | 0.92 (0.26–3.21) | 0.96 (0.91–1.02) | 0.84 (0.69–1.02) | 0.92 (0.75–1.12) | 0.86 (0.74–1.00) |
| Mother only household | 1.20 (1.10–1.30) | 0.71 (0.24–2.10) | 1.08 (1.03–1.13) | 0.74 (0.63–0.86) | 1.19 (1.02–1.38) | 0.88 (0.78–0.98) |
| Other | 0.98 (0.85–1.12) | 0.24 (0.04–1.48) | 0.84 (0.77–0.91) | 0.48 (0.37–0.64) | 0.91 (0.70–1.18) | 0.89 (0.75–1.06) |
| Complexity | ||||||
| Physical impairment alone | Ref | Ref | Ref | Ref | Ref | Ref |
| Broad functional impairment alone | 1.43 (1.30–1.58) | 0.88 (0.27–2.81) | 1.34 (1.28–1.41) | 6.14 (4.79–7.89) | 3.50 (2.85–4.28) | 0.49 (0.44–0.55) |
| Predominant physical impairment requiring family‐delivered care | 2.12 (1.94–2.34) | 1.96 (0.66–5.87) | 1.79 (1.71–1.89) | 4.28 (3.28–5.59) | 2.88 (2.33–3.57) | 0.73 (0.64–0.83) |
| Broad functional impairment requiring extensive health care | 3.34 (3.03–3.70) | 11.97 (4.27–33.53) | 2.20 (2.09–2.32) | 29.48 (23.02–37.75) | 10.88 (8.89–13.33) | 0.31 (0.27–0.36) |
All data are weighted. Logistic regression used for dichotomous outcomes, and negative binomial regressions used for count data that was not normally distributed (ED visits). Analyses were adjusted by age, gender, race/ethnicity, payer category, poverty level, household educational level, household structure, and complexity. Variables with p > .05 in the univariate analysis were not included in multivariate analysis unless conceptually important.
Respondents from California only.
Ages 0–5 excluded from analysis of missed school days.
Table 4.
Medical Home Outcomes (Multivariate Adjusted and Weighted Regression)a
| Medical Home OR (95%CI) | Personal MD or RN OR (95% CI) | Family‐Centered Care OR (95% CI) | No Problem with Referrals OR (95% CI) | Usual Source of Care OR (95% CI) | Coordinated Care OR (95% CI) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| 0–5 years | Ref | Ref | Ref | Ref | Ref | Ref |
| 6–11 years | 0.95 (0.85–1.07) | 0.99 (0.81–1.23) | 0.92 (0.82–1.03) | 0.78 (0.63–0.97) | 1.24 (1.05–1.47) | 0.87 (0.76–0.99) |
| >11 years | 0.91 (0.81–1.02) | 0.85 (0.69–1.05) | 0.87 (0.77–0.98) | 0.73 (0.59–0.90) | 1.06 (0.89–1.26) | 0.82 (0.72–0.94) |
| Race/ethnicity | ||||||
| White, non‐Hispanic | Ref | Ref | Ref | Ref | Ref | Ref |
| Hispanic | 0.73 (0.63–0.84) | 0.87 (0.65–1.16) | 0.71 (0.62–0.82) | 0.81 (0.62–1.06) | 0.85 (0.68–1.07) | 0.93 (0.79–1.09) |
| Black, non‐Hispanic | 0.63 (0.55–0.71) | 0.76 (0.61–0.93) | 0.55 (0.48–0.62) | 1.06 (0.82–1.37) | 0.57 (0.48–0.69) | 0.82 (0.71–0.96) |
| Multiple or other | 0.72 (0.62–0.84) | 0.65 (0.49–0.85) | 0.68 (0.58–0.79) | 0.86 (0.66–1.11) | 0.73 (0.58–0.90) | 0.82 (0.68–0.98) |
| Primary language | ||||||
| English | Ref | Ref | Ref | Ref | Ref | Ref |
| Other | 0.64 (0.50–0.84) | 2.35 (1.52–3.63) | 0.82 (0.65–1.04) | 0.48 (0.34–0.68) | 0.68 (0.51–0.92) | 0.75 (0.56–1.01) |
| Payer category | ||||||
| Private | Ref | Ref | Ref | Ref | Ref | Ref |
| Public | 0.89 (0.79–1.00) | 0.85 (0.67–1.07) | 0.91 (0.81–1.03) | 0.92 (0.75–1.14) | 0.98 (0.82–1.17) | 0.90 (0.78–1.03) |
| Uninsured | 0.49 (0.38–0.62) | 0.28 (0.20–0.39) | 0.55 (0.43–0.70) | 0.39 (0.24–0.62) | 0.38 (0.29–0.50) | 0.50 (0.37–0.68) |
| Poverty level, % | ||||||
| 0–99% FPL | 0.86 (0.74–1.01) | 0.49 (0.36–0.68) | 0.74 (0.63–0.87) | 0.88 (0.65–1.21) | 0.64 (0.51–0.82) | 1.18 (0.98–1.42) |
| 100–199% FPL | 0.88 (0.78–1.00) | 0.69 (0.52–0.91) | 0.74 (0.64–0.85) | 0.81 (0.63–1.05) | 0.85 (0.69–1.06) | 1.01 (0.87–1.18) |
| 200–399% FPL | 0.99 (0.91–1.10) | 0.73 (0.58–0.93) | 0.85 (0.76–0.95) | 0.98 (0.78–1.23) | 0.87 (0.72–1.03) | 1.04 (0.92–1.17) |
| ≥400% FPL | Ref | Ref | Ref | Ref | Ref | Ref |
| Household educational level | ||||||
| <High school | 0.86 (0.71–1.03) | 0.65 (0.50–0.86) | 0.71 (0.60–0.85) | 0.93 (0.67–1.31) | 0.50 (0.40–0.63) | 1.32 (1.07–1.63) |
| High school graduate | 0.94 (0.83–1.05) | 0.73 (0.60–0.88) | 0.85 (0.76–0.95) | 1.17 (0.94–1.46) | 0.75 (0.63–0.88) | 1.14 (1.00–1.31) |
| >High school | Ref | Ref | Ref | Ref | Ref | Ref |
| Household structure | ||||||
| Parent household | Ref | Ref | Ref | Ref | Ref | Ref |
| Two‐parent stepfamily | 0.88 (0.77–1.02) | 0.57 (0.45–0.72) | 0.84 (0.73–0.97) | 0.88 (0.67–1.17) | 1.01 (0.81–1.26) | 1.10 (0.94–1.29) |
| Mother only household | 0.87 (0.78–0.97) | 0.87 (0.70–1.07) | 0.82 (0.73–0.91) | 1.05 (0.85–1.30) | 1.05 (0.89–1.25) | 0.87 (0.77–0.99) |
| Other | 0.80 (0.68–0.94) | 0.70 (0.52–0.94) | 0.87 (0.73–1.03) | 0.81 (0.60–1.11) | 1.04 (0.81–1.33) | 0.99 (0.82–1.20) |
| Complexity | ||||||
| Physical impairment alone | Ref | Ref | Ref | Ref | Ref | Ref |
| Broad functional impairment alone | 0.40 (0.37–0.44) | 0.83 (0.69–1.00) | 0.50 (0.44–0.55) | 0.57 (0.43–0.75) | 0.94 (0.80–1.10) | 0.32 (0.28–0.37) |
| Predominant physical impairment requiring family‐delivered care | 0.69 (0.62–0.77) | 1.24 (0.98–1.57) | 0.76 (0.67–0.86) | 0.73 (0.55–0.97) | 1.30 (1.08–1.56) | 0.49 (0.42–0.58) |
| Broad functional impairment requiring extensive health care | 0.17 (0.15–0.19) | 1.53 (1.19–1.96) | 0.35 (0.31–0.40) | 0.29 (0.22–0.38) | 1.12 (0.92–1.36) | 0.10 (0.09–0.12) |
All data are weighted. Analyses were adjusted by age, gender, race/ethnicity, payer category, poverty level, household educational level, household structure, and complexity. Variables with p > .05 in the univariate analysis were not included in multivariate analysis unless conceptually important. Respondents who were legitimately skipped from an outcome were excluded from analysis on that outcome (e.g., those without need for referrals were excluded from analysis of the “no problem with referrals” outcome).
Utilization
The complex class had the highest ED visit rates (IRR: 3.34 vs. the noncomplex Class 4, 95 percent CI: 3.03–3.70) and odds of hospitalizations (AOR: 11.97, 95 percent CI: 4.27–33.53). Class 3 (Predominant physical impairment requiring family‐delivered care) had the second highest ED rates (IRR: 2.12, 95 percent CI: 1.94–2.34).
Child and Family
All classes were significantly worse across each outcome than the noncomplex class. Those from the complex class missed the most school days (IRR 2.20, 95 percent CI: 2.09–2.32), had the lowest odds of shared decision making (AOR: 0.31, 95 percent CI: 0.27–0.36), and had the highest odds of a parent stopping work due to the child's condition (AOR: 29.48, 95 percent CI: 23.02–37.75) and facing difficulties or delays in care related to cost (AOR: 10.88, 95 percent CI: 8.89–13.33). In fact, the predicted probability for at least one parent to stop working due to the child's condition was 41 percent in the complex class.
Medical Home
The odds of having an MCHB‐defined medical home were significantly lower for every class than for noncomplex CSHCN. The noncomplex group had a 63 percent predicted probability of having a medical home. Despite having the highest odds of reporting a personal MD or RN (AOR: 1.53, 95 percent CI: 1.19–1.96), those in the complex class only had a 20 percent predicted probability of having a medical home. Of note, Class 3 (predominant physical impairment requiring family‐delivered care) was the only class to have significantly higher odds of having a usual source of care than the noncomplex class (AOR: 1.30, 95 percent CI: 1.08–1.56).
Class 2 versus Class 3 Subanalysis
When comparing Class 2 (broad functional impairment—physical, cognitive, and mental health—without substantial health care use or needs) and Class 3 (predominant physical impairment requiring family‐delivered care) to one another, Class 2 reported fewer ED visits (IRR: 0.67, p < .001) and missed school days (IRR: 0.75, p < .001). Class 2, however, also reported lower odds of shared decision making (AOR: 0.68, p < .001), and higher odds of difficulties/delays in care due to cost (AOR: 1.21, p = .024) and parents stopping work due to the condition (AOR: 1.44, p < .001). Class 2 was also less likely to report having a medical home (AOR: 0.58, p < .001) and had 0.65–0.78 times the odds of experiencing any of the medical home subcomponents. Class 2's predicted probability of having a medical home was 38 percent, while Class 3's was 52 percent.
Discussion and Conclusions
Using LCA, we were able to describe otherwise unobservable complexity subgroups from within the broader CSHCN population. This study is the first to our knowledge that operationalizes the CMC conceptual framework (van der Lee et al. 2007; Cohen et al. 2011) to characterize outcomes related to medical complexity using a robust analytical approach. Though others have identified latent constructs from the NS‐CSHCN, we are not aware of previous efforts to identify latent classes of children with complexity from this population. Blumberg and Carle modeled the well‐being of the health care environment for CSHCN and their families (Blumberg and Carle 2009), finding that family‐centered care and adequate health insurance were associated with better levels of the well‐being latent construct while unmet family support, no usual place for care and significant time spent by the family coordinating care were associated with lower levels of the well‐being construct.
Previous researchers have modeled complexity within the NS‐CSHCN along a unidimensional continuum (Carle, Blumberg, and Poblenz 2011). While it is appealing to consider CSHCN along a single spectrum of complexity, the unique perspective offered by this work is that complexity manifests along multiple dimensions for CSHCN and their families. Our data suggested that characterizing CSHCN according to both the breadth of the condition's functional impact and the extent of the child's health care use and needs identified unique populations with distinct outcomes. Likewise, although there does appear to be one class with the highest complexity and another with the lowest complexity, two other classes appear to describe separate populations that do not track along a single unidirectional continuum (Figure 1). This finding is supported further by the lack of consistent dose–response relationships in the odds ratios between Classes 2 and 3 across outcomes. The demographic characteristics of each group also differed significantly. Finally, if each class represented a subsequently more complex version of another class (i.e., along a single continuum), one might expect item‐response patterns to simply build off one another, or at least to relate closely to one another. Instead, we observed classes to have different response patterns altogether, most notably when comparing Classes 1, 2, and 3.
Within specific conditions, CSHCN frequently fit into different classes. For example, cystic fibrosis, often a multisystem and severe illness, is known to clinically manifest in children in distinct ways with varying levels of severity. Our model reflected this reality with about half of children with cystic fibrosis falling into the complex class, a similar proportion in Class 3, and almost none in Classes 2 or 4. Figure 1 highlights similar examples with asthma and Down syndrome. We suspect that if individual children were followed longitudinally, some would oscillate among different classes depending on how their health and needs changed with time. These findings further illustrate how diagnosis‐based systems to identify CMC can oversimplify categorization, in some instances overestimating and in others underestimating complexity.
Though innovative in approach, ours is not the first study attempting to create subgroups from the NS‐CSHCN. Other researchers have applied various criteria, most commonly using the CSHCN screener questions, to identify more complex populations of CSHCN. When a child meets the NS‐CSHCN criteria on at least two (and especially when >2) screener items, a population with more health care needs and negative family impact, worse health outcomes, less adequate insurance, and higher annual expenditures is identified (Bramlett et al. 2009; Bethell et al. 2014). Others have augmented screener responses with additional factors such as device needs and/or subspecialist use to more closely resemble types of criteria used to enroll CSHCN into complex care clinical programs (Kuo et al. 2011, 2014). Functional limitations specifically, appears to be an indicator of more complex CSHCN (Mulvihill et al. 2005; Nageswaran, Silver, and Stein 2008; Bramlett et al. 2009), identifying children having similar negative outcomes to those described above. Less complex CSHCN have been identified when only having the need for prescription medications (Bramlett et al. 2009; Carle, Blumberg, and Poblenz 2011), which we also observed (Class 4).
Our work offers a number of unique perspectives from the approaches described above using the CSHCN screener. First, a key distinction was the process for defining medical complexity. This approach established more than a complex / noncomplex dichotomy and was grounded in and provides empirical support for the proposed CMC conceptual framework (van der Lee et al. 2007; Cohen et al. 2011; Berry et al. 2013). Second, this analysis likely provides a finer level of detail with respect to teasing out differential effects of functional limitations on CSHCN. While our most complex population demonstrated significant functional impairment, we identified another group with broad functional impairment but substantially less health care use and needs (Class 2). In fact, each group in our study had some degree of functional impairment. A more nuanced appreciation for the manifestations of complexity and expected outcomes would likely be achieved by considering the breadth of functional impairment and the extent of health care delivery resulting from the child's condition. Finally, when comparing our results to the previously described approaches for identifying complexity subgroups, we found that, similar to previous work, the majority of CSHCN in our complex class (74 percent) met >2 CSHCN screener criteria, compared to only 3 percent of CSHCN in Class 4. However, we observed that 34 percent of Class 2 and only 10 percent of Class 3 met >2 screener criteria, which on a simple complexity continuum might suggest that CSHCN in Class 2 were more complex than those in Class 3.
Comparisons between Classes 2 and 3, however, suggest that such a conclusion might be misleading. While Class 2 (broad physical, cognitive, and mental health impairment alone) reported worse experiences with the health care system than Class 3 (predominant physical impairment requiring family‐delivered care), especially with respect to medical home and shared decision making, Class 2 nevertheless reported fewer ED visits and fewer missed school days. If one were to misinterpret CSHCN in Class 2, for instance, as simply having more functional limitations than those in Class 3, our findings might then also be misinterpreted as contradicting studies in which CSHCN with more functional limitations have required more frequent health services (Bramlett et al. 2009).
The fact that children in Class 3 reported more ED visits while simultaneously reporting the highest overall rates of having a usual source of care as well as better social outcomes (e.g., family income, insurance status) also made these findings surprising. This observation runs somewhat counter to analyses of children with Medicaid and commercial insurance in which increased continuity of care has been associated with lower ED and hospital use, including among subgroups having diabetes and asthma (Christakis et al. 1999, 2001a,b). Whether there is a protective effect from having a medical home on subsequent health services use among CSHCN is not firmly established (Homer et al. 2008), and there may be interaction effects based on the breadth of a child's functional impairment and extent of their health care needs.
Despite fewer ED visits and missed school days, CSHCN in Class 2 did report significantly worse family impacts than Class 3, with parents experiencing more difficulties/delays related to cost, and more parents having to stop working due to the child's condition. This is consistent with other work suggesting that functional impairment resulting from a condition may have the most influence on family impact (Bumbalo et al. 2005).
These comparisons between Classes 2 and 3 suggest that, even with fewer family impacts and more positive experiences with the health care system, children with predominant physical impairments and significant needs for health care delivered by their families at home may simply be more vulnerable to severe natural exacerbations of their underlying illness or to interruptions in the care they depend on at home, ultimately resulting in ED visits and missed school. Those with significant cognitive and mental health impairment in addition to functional impairment (but without significant need for family‐delivered care at home) may be more vulnerable to having family disruption and difficulty finding care that meets their needs, even though such challenges may not translate directly to ED use or missed school. We were surprised to not identify a group having cognitive or mental health impairment alone (i.e., without physical impairment). Research designed to unravel the causal pathways and directions of associations between these variables is critically needed.
Limitations
Our findings must be interpreted with caution. Any survey has a number of biases, including those due to sampling methods and respondent recall. We were not able to verify parent‐reported health services use, including hospital or ED visits, or specific diagnoses. While this telephonic survey accounted for landline and cell‐phone recruitment, it is unclear how the increase in cell‐phone use might impact selection bias in surveys such as this one. We would expect that any child with medical complexity would screen positive on the CSHCN screener, and therefore we would not expect there to be a subset of CMC who could have been systematically excluded from this survey. The cross‐sectional design does not capture the dynamic nature of medical complexity, which may wax and wane over time, even for children with special health care needs. A longitudinal study assessing temporal changes in complexity, and how duration of complexity relates to important outcomes would provide particularly useful information.
In addition, because of the nature of LCA modeling and item selection, there is some subjectivity in interpreting latent classes. There is a possibility of misclassification, and the identified classes may not fully represent all of the subgroups that could exist in the population (Collins and Lanza 2010). Furthermore, the NS‐CSHCN was not designed with the intent to include a comprehensive set of items targeting the CMC conceptual framework upon which to perform LCA. As such, further work with a refined set of items might improve the validity of this approach.
Nevertheless, this study's person‐centered framework (LCA), grounded in a conceptual model of medical complexity, provides a unique and in‐depth understanding of medical complexity among CSHCN. Rather than simply identifying complex and noncomplex groups, we have identified distinct populations that may not be appropriately described on a one‐dimensional scale of complexity. This approach provides a rich and potentially more informative categorization of medical complexity than disease‐specific strategies, capturing that a given condition can lead to medical complexity in one patient but not another.
Implications
This work has a number of important research, policy, and clinical implications. Based on our findings, CMC may be as high as 2–3 percent of the U.S. population, a higher figure than with other CMC definitions in which prevalence has been 0.5–1 percent (Neff et al. 2006; Cohen et al. 2012; Berry et al. 2013). These children have dramatically higher rates of ED and hospital use, significant financial family impacts, and lower rates of experiencing coordinated care within a medical home, despite having similar or better rates of having a personal provider and usual source of care compared to noncomplex CSHCN. Reasons for health services use such as ED visits likely differ in the different classes and might be predicted by breadth of functional impairment and extent of their health care delivery needs. Strategies to improve such outcomes must be appropriately tailored to the populations most likely to benefit from the intervention.
While we confirm that broad health systems improvements are essential to ensure all children receive care within a medical home, further research is needed to understand the disparate experiences of children in different classes and whether there is any causal relationship between the two. Does lack of a medical home lead to complexity, or does complexity make it extremely difficult to achieve the medical home ideal?
In addition, parent‐reported survey measures have been shown to add important information to financial risk‐adjustment models for CSHCN, and they may improve capitation payment accuracy (Yu and Dick 2010), though the survey items included in such models to date still only account for a small proportion of the variance in expenditures. Augmenting current diagnosis‐based risk‐adjustment methods with classifications such as these could eventually improve the precision of risk adjustment. Continued survey measure refinement, and inclusion of items similar to those studied here, may lead to improved model performance. A next step to improving risk‐adjustment modeling could be to refine these 17 items to a manageable and more limited subset of essential items.
Beyond health plan or administrator risk adjustment, the indicators identified in this study could be used by health care providers or complex care programs to classify patients not only by diagnosis but also by different complexity groups. Though it is likely impractical for providers to administer a questionnaire to score these 17 items and determine their patients' latent class designations, they can likely make an estimation of which class best approximates a given patient, and therefore forecast the types of outcomes that patient is at higher risk to experience. Again, refinement of these 17 items to a more limited reliable and valid subset may eventually lead to a more practical tool for use directly in the clinical setting. Testing the validity of these categorizations in a cohort of patients with chart review and patient interview as a gold standard would be a useful step, with an ultimate goal of developing improvement strategies based on class rather than diagnosis.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2. Weighted U.S. Population Estimates of Specific Conditions among CSHCN and Proportions within Each Latent Class.
Appendix SA3. Comparison of Latent Class Models of Medical Complexity.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: The authors have no funding sources to be acknowledged. This research was supported by the Departments of Pediatrics at the University of Wisconsin, Madison School of Medicine and University of California, Los Angeles, through salary support and administrative support for the authors.
Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
Disclosures: The authors have no conflicts of interest to disclose.
References
- Berry, J. G. , Agrawal R. K., Cohen E., and Kuo D. Z.. 2013. The Landscape of Medical Care for Children with Medical Complexity. Alexandria, VA; Overland Park, KS: Children's Hospital Association. [Google Scholar]
- Bethell, C. D. , Read D., Stein R. E., Blumberg S. J., Wells N., and Newacheck P. W.. 2002. “Identifying Children with Special Health Care Needs: Development and Evaluation of a Short Screening Instrument.” Ambulatory Pediatrics 2 (1): 38–48. [DOI] [PubMed] [Google Scholar]
- Bethell, C. D. , Blumberg S. J., Stein R. E., Strickland B., Robertson J., and Newacheck P. W.. 2014. “Taking Stock of the CSHCN Screener: A Review of Common Questions and Current Reflections.” Academic Pediatric 15 (2): 165–76. doi: 10.1016/j.acap.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg, S. J. , and Carle A. C.. 2009. “The Well‐Being of the Health Care Environment for CSHCN and Their Families: A Latent Variable Approach.” Pediatrics 124 (Suppl 4): S361–7. [DOI] [PubMed] [Google Scholar]
- Blumberg, S. J. , Olson L., Frankel M., Osborn L., Becker C. J., Srinath K. P., and Giambo P.. 2003. “Design and Operation of the National Survey of Children with Special Health Care Needs, 2001.” Vital Health Statistics 1 (41): 1–136. [PubMed] [Google Scholar]
- Blumberg, S. J. , Welch E. M., Chowdhury S. R., Upchurch H. L., Parker E. K., and Skalland B. J.. 2008. “Design and Operation of the National Survey of Children with Special Health Care Needs, 2005‐2006.” Vital Health Statistics 1 (45): 1–188. [PubMed] [Google Scholar]
- Bramlett, M. D. , Read D., Bethell C., and Blumberg S. J.. 2009. “Differentiating Subgroups of Children with Special Health Care Needs by Health Status and Complexity of Health Care Needs.” Maternal and Child Health Journal 13 (2): 151–63. [DOI] [PubMed] [Google Scholar]
- Bumbalo, J. , Ustinich L., Ramcharran D., and Schwalberg R.. 2005. “Economic Impact on Families Caring for Children with Special Health Care Needs in New Hampshire: The Effect of Socioeconomic and Health‐Related Factors.” Maternal and Child Health Journal 9 (2 Suppl): S3–11. [DOI] [PubMed] [Google Scholar]
- Burke, R. T. , and Alverson B.. 2010. “Impact of Children with Medically Complex Conditions.” Pediatrics 126 (4): 789–90. [DOI] [PubMed] [Google Scholar]
- Carle, A. C. , Blumberg S. J., and Poblenz C.. 2011. “Internal Psychometric Properties of the Children with Special Health Care Needs Screener.” Academic Pediatric 11 (2): 128–35. [DOI] [PubMed] [Google Scholar]
- The Child and Adolescent Health Measurement Initiative Oregon Health and Science University. 2009. Measuring Medical Home for Children and Youth: Methods and Findings from the National Survey of Children with Special Health Care Needs and the National Survey of Children's Health. [accessed November 11, 2015]. Available at http://www.childhealthdata.org/docs/medical-home/mhmanual_withappendices-updated-12-7-10-pdf
- Christakis, D. A. , Wright J. A., Koepsell T. D., Emerson S., and Connell F. A.. 1999. “Is Greater Continuity of Care Associated with Less Emergency Department Utilization?” Pediatrics 103 (4 Pt 1): 738–42. [DOI] [PubMed] [Google Scholar]
- Christakis, D. A. , Feudtner C., Pihoker C., and Connell F. A.. 2001a. “Continuity and Quality of Care for Children with Diabetes Who Are Covered By Medicaid.” Ambulatory Pediatrics 1 (2): 99–103. [DOI] [PubMed] [Google Scholar]
- Christakis, D. A. , Mell L., Koepsell T. D., Zimmerman F. J., and Connell F. A.. 2001b. “Association of Lower Continuity of Care with Greater Risk of Emergency Department Use and Hospitalization in Children.” Pediatrics 107 (3): 524–9. [DOI] [PubMed] [Google Scholar]
- Cohen, E. , Kuo D. Z., Agrawal R., Berry J. G., Bhagat S. K., Simon T. D., and Srivastava R.. 2011. “Children with Medical Complexity: An Emerging Population for Clinical and Research Initiatives.” Pediatrics 127 (3): 529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, E. , Berry J. G., Camacho X., Anderson G., Wodchis W., and Guttmann A.. 2012. “Patterns and Costs of Health Care Use of Children with Medical Complexity.” Pediatrics 130 (6): e1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, L. M. , and Lanza S. T.. 2010. Latent Class and Latent Transitional Analysis: With Applications in the Social, Behavioral, and Health Sciences. Hoboken, NJ: John Wiley & Sons Inc. [Google Scholar]
- van Dyck, P. C. , McPherson M., Strickland B. B., Nesseler K., Blumberg S. J., Cynamon M. L., and Newacheck P. W.. 2002. “The National Survey of Children with Special Health Care Needs.” Ambulatory Pediatrics 2 (1): 29–37. [DOI] [PubMed] [Google Scholar]
- Feudtner, C. , Christakis D. A., and Connell F. A.. 2000. “Pediatric Deaths Attributable to Complex Chronic Conditions: A Population‐Based Study of Washington State, 1980‐1997.” Pediatrics 106 (1 Pt 2): 205–9. [PubMed] [Google Scholar]
- Feudtner, C. , Christakis D. A., Zimmerman F. J., Muldoon J. H., Neff J. M., and Koepsell T. D.. 2002. “Characteristics of Deaths Occurring in Children's Hospitals: Implications for Supportive Care Services.” Pediatrics 109 (5): 887–93. [DOI] [PubMed] [Google Scholar]
- Gordon, J. B. , Colby H. H., Bartelt T., Jablonski D., Krauthoefer M. L., and Havens P.. 2007. “A Tertiary Care‐Primary Care Partnership Model for Medically Complex and Fragile Children and Youth with Special Health Care Needs.” Archives of Pediatrics and Adolescent Medicine 161 (10): 937–44. [DOI] [PubMed] [Google Scholar]
- Homer, C. J. , Klatka K., Romm D., Kuhlthau K., Bloom S., Newacheck P., Van Cleave J., and Perrin J. M.. 2008. “A Review of the Evidence for the Medical Home for Children with Special Health Care Needs.” Pediatrics 122 (4): e922–37. [DOI] [PubMed] [Google Scholar]
- Inkelas, M. , Smith K. A., Kuo A. A., Rudolph L., and Igdaloff S.. 2005. “Health Care Access for Children with Special Health Care Needs in California.” Maternal and Child Health Journal 9 (2 Suppl): S109–16. [DOI] [PubMed] [Google Scholar]
- Kronick, R. , Gilmer T., Dreyfus T., and Lee L.. 2000. “Improving Health‐Based Payment for Medicaid Beneficiaries: CDPS.” Health Care Financing Review 21 (3): 29–64. [PMC free article] [PubMed] [Google Scholar]
- Kuo, D. Z. , Cohen E., Agrawal R., Berry J. G., and Casey P. H.. 2011. “A National Profile of Caregiver Challenges among More Medically Complex Children with Special Health Care Needs.” Archives of Pediatrics and Adolescent Medicine 165 (11): 1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, D. Z. , Goudie A., Cohen E., Houtrow A., Agrawal R., Carle A. C., and Wells N.. 2014. “Inequities in Health Care Needs for Children with Medical Complexity.” Health Affairs (Millwood) 33 (12): 2190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza, S. T. , Collins L. M., Lemmon D. R., and Schafer J. L.. 2007. “PROC LCA: A SAS Procedure for Latent Class Analysis.” Structural Equation Modeling—A Multidisciplinary Journal 14 (4): 671–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee, J. H. , Mokkink L. B., Grootenhuis M. A., Heymans H. S., and Offringa M.. 2007. “Definitions and Measurement of Chronic Health Conditions in Childhood: A Systematic Review.” Journal of the American Medical Association 297 (24): 2741–51. [DOI] [PubMed] [Google Scholar]
- Lo, Y. T. , Mendell N. R., and Rubin D. B.. 2001. “Testing the Number of Components in a Normal Mixture.” Biometrika 88 (3): 767–78. [Google Scholar]
- Mulvihill, B. A. , Wingate M. S., Altarac M., Mulvihill F. X., Redden D. T., Telfair J., Pass M. A., and Ellis D. E.. 2005. “The Association of Child Condition Severity with Family Functioning and Relationship with Health Care Providers among Children and Youth with Special Health Care Needs in Alabama.” Maternal and Child Health Journal 9 (2 Suppl): S87–97. [DOI] [PubMed] [Google Scholar]
- Nageswaran, S. , Silver E. J., and Stein R. E.. 2008. “Association of Functional Limitation with Health Care Needs and Experiences of Children with Special Health Care Needs.” Pediatrics 121 (5): 994–1001. [DOI] [PubMed] [Google Scholar]
- Neff, J. M. , Sharp V. L., Muldoon J., Graham J., Popalisky J., and Gay J. C.. 2002. “Identifying and Classifying Children with Chronic Conditions Using Administrative Data with the Clinical Risk Group Classification System.” Ambulatory Pediatrics 2 (1): 71–9. [DOI] [PubMed] [Google Scholar]
- Neff, J. M. , Sharp V. L., Muldoon J., Graham J., and Myers K.. 2004. “Profile of Medical Charges for Children by Health Status Group and Severity Level in a Washington State Health Plan.” Health Services Research 39 (1): 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, J. M. , Sharp V. L., Popalisky J., and Fitzgibbon T.. 2006. “Using Medical Billing Data to Evaluate Chronically Ill Children over Time.” Journal of Ambulatory Care Management 29 (4): 283–90. [DOI] [PubMed] [Google Scholar]
- Newacheck, P. W. , and Kim S. E.. 2005. “A National Profile of Health Care Utilization and Expenditures for Children with Special Health Care Needs.” Archives of Pediatrics and Adolescent Medicine 159 (1): 10–7. [DOI] [PubMed] [Google Scholar]
- Nylund, K. L. , Asparoutiov T., and Muthen B. O.. 2007. “Deciding on the Number of Classes in Latent Class Analysis and Growth Mixture Modeling: A Monte Carlo Simulation Study.” Structural Equation Modeling—A Multidisciplinary Journal 14 (4): 535–69. [Google Scholar]
- Palfrey, J. S. , Sofis L. A., Davidson E. J., Liu J., Freeman L., Ganz M. L., and C. Pediatric Alliance for Coordinated . 2004. “The Pediatric Alliance for Coordinated Care: Evaluation of a Medical Home Model.” Pediatrics 113 (5 Suppl): 1507–16. [PubMed] [Google Scholar]
- Schwarz, G. 1978. “Estimating Dimension of a Model.” Annals of Statistics 6 (2): 461–4. [Google Scholar]
- Sclove, S. L. 1987. “Application of Model‐Selection Criteria to Some Problems in Multivariate‐Analysis.” Psychometrika 52 (3): 333–43. [Google Scholar]
- Simon, T. D. , Mahant S., and Cohen E.. 2012. “Pediatric Hospital Medicine and Children with Medical Complexity: Past, Present, and Future.” Current Problems in Pediatric and Adolescent Health Care 42 (5): 113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, T. D. , Berry J., Feudtner C., Stone B. L., Sheng X., Bratton S. L., Dean J. M., and Srivastava R.. 2010. “Children with Complex Chronic Conditions in Inpatient Hospital Settings in the United States.” Pediatrics 126 (4): 647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services , Health Resources and Services Administration, and Maternal and Child Health Bureau. 2013. “Technical Appendix: The National Survey of Children with Special health Care Needs Chartbook 2009‐2010” [accessed on March 18, 2013]. Available at http://mchb.hrsa.gov/cshcn0910/more/technical.html
- Yu, H. , and Dick A. W.. 2010. “Risk‐Adjusted Capitation Rates for Children: How Useful Are the Survey‐Based Measures?” Health Services Research 45 (6 Pt 2): 1948–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2. Weighted U.S. Population Estimates of Specific Conditions among CSHCN and Proportions within Each Latent Class.
Appendix SA3. Comparison of Latent Class Models of Medical Complexity.


