Abstract
The central melanocortin (MC) system mediates its effects on food intake via MC3 (MC3R) and MC4 receptors (MC4R). Although the role of MC4R in meal size determination, satiation, food preference, and motivation is well established, the involvement of MC3R in the modulation of food intake has been less explored. Here, we investigated the role of MC3R on the incentive motivation for food, which is a crucial component of feeding behavior. Dopaminergic neurons within the ventral tegmental area (VTA) have a crucial role in the motivation for food. We here report that MC3Rs are expressed on VTA dopaminergic neurons and that pro-opiomelanocortinergic (POMC) neurons in the arcuate nucleus of the hypothalamus (Arc) innervate these VTA dopaminergic neurons. Our findings show that intracerebroventricular or intra-VTA infusion of the selective MC3R agonist γMSH increases responding for sucrose under a progressive ratio schedule of reinforcement, but not free sucrose consumption in rats. Furthermore, ex vivo electrophysiological recordings show increased VTA dopaminergic neuronal activity upon γMSH application. Consistent with a dopamine-mediated effect of γMSH, the increased motivation for sucrose after intra-VTA infusion of γMSH was blocked by pretreatment with the dopamine receptor antagonist α-flupenthixol. Taken together, we demonstrate an Arc POMC projection onto VTA dopaminergic neurons that modulates motivation for palatable food via activation of MC3R signaling.
INTRODUCTION
The central melanocortin (MC) system has an integral role in the regulation of food intake (Cone, 2005). MCs are products of the pro-opiomelanocortin (POMC) gene, which is expressed in neurons within the arcuate nucleus of the hypothalamus (Arc) and the nucleus of the solitary tract (NTS) (Cone, 2005). Through projections from the Arc and NTS to various brain nuclei, MCs regulate various aspects of food intake such as satiation (Zheng et al, 2005; Fan et al, 2004; Sutton et al, 2005), food motivation (Vaughan et al, 2006; Davis et al, 2011; Pandit et al, 2013), and food preference (Mul et al, 2012; Hagan et al, 2001). MCs mediate their effects within the brain via the MC3 and MC4 receptors (MC3R and MC4R) that are differentially expressed in the hypothalamus and the limbic system (Mountjoy et al, 1994; Roselli-Rehfuss et al, 1993).
Interestingly, in addition to regulating homeostatic aspects of feeding, the MC system has also been implicated in the hedonic and incentive motivational properties of food (Davis et al, 2011; Figlewicz et al, 2013). In the past decade, pharmacological and genetic manipulations have been used to investigate the role of MC4Rs in regulation of hedonic food intake (Butler and Cone 2002; Zheng et al, 2010; Figlewicz et al, 2013). Furthermore, we recently showed that central infusion of αMSH, an MC4R agonist, decreases the motivation for sucrose and that this effect is mediated by MC4R in the nucleus accumbens (NAc) (Pandit et al, 2015). In contrast to the well-established role of MC4R in the motivation for food, the involvement of MC3Rs in the modulation of food intake remains largely unexplored. Peripheral administration of the selective MC3R agonist γMSH has been shown to increase food intake in freely moving animals (Marks et al, 2006), but the role of MC3Rs in the incentive motivation for food is not known.
Within the Arc, MC3Rs are expressed in POMC neurons where they are suggested to function as auto-receptors (Cowley et al, 2001). MC3Rs are also expressed in dopaminergic neurons within the ventral tegmental area (VTA; Roselli-Rehfuss et al, 1993; Lippert et al, 2014), a neuronal population that has a key role in the incentive motivational processes (Berridge, 2007; Kelley, 2004; Salamone and Correa, 2012; Barbano and Cador, 2007). In a recent publication, Lippert et al (2014) showed that up to 37% of neurons expressing tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of dopamine, contained MC3Rs. Furthermore, several studies have suggested a cross-talk between MC3Rs and the dopaminergic system. For instance, overexpression of POMC within the VTA increases TH expression (Andino et al, 2011), suggesting modulation of dopaminergic signaling by MCs. In line with this, genetic deletion of MC3Rs decreases dopamine release within the NAc (Lippert et al, 2014). In contrast, pharmacological activation of intra-VTA MC3Rs by infusion of γMSH or αMSH increases NAc dopamine levels (Jansone et al, 2004; Torre and Celis, 1988). Although these data suggest that MC3R signaling within the VTA potentiates dopaminergic signaling, direct evidence to support a role for MC3R signaling in modulating VTA dopaminergic activity is lacking. It is also unknown whether VTA dopaminergic neurons are innervated by POMC neurons and whether this physiological interaction regulates the motivation for food rewards via the MC3Rs. Therefore, in the present study, we investigated the role of MC3Rs within the VTA on the motivation to respond for sucrose under a progressive ratio (PR) schedule of reinforcement. We hypothesized that stimulation of MC3Rs within the VTA increases motivation for palatable food rewards through modulation of dopaminergic neurotransmission.
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles River, Germany) weighing 200–225 g on arrival in our laboratory were used for behavioral studies. TH::Cre heterozygous transgenic rats on a Long-Evans background (1–2 months old) were used for slice electrophysiology and viral retrograde tracing experiments. For Cholera toxin B (CTB)-based retrograde tracing experiments, adult male Wistar rats (300–350 g) were used. The animals were individually housed (Macrolon cages; 40 × 26 × 20 cm) in a temperature (21±2 °C) and humidity (60–70%) controlled room under a 12-h reversed light/dark cycle (lights on: 1900 h). Rats had ad libitum access to rat chow (3.31 kcal/g, Standard Diet Service, UK) and tap water. All behavioral experiments were performed in the dark phase of the day–night cycle (0800 h to 1700 h). Experimental procedures were approved by the Animal Ethics Committee of Utrecht University and were in agreement with Dutch laws and European regulations (Guideline 86/609/EEC).
Surgery
Surgery was performed when the animals weighed between 275 and 300 g. Rats were anesthetized with 0.1 ml/100 g intramuscular injection of fentanyl/fluanisone (Hypnorm, Janssen Pharmaceutica, Belgium). For implantation of intracerebroventricular (i.c.v.) cannulas, the skull was exposed and a 10 mm stainless steel guide cannula (Plastics One, USA) was inserted into the lateral ventricle (1 mm lateral and 1 mm posterior from bregma) via a craniotomy. Cannulas were fixed to the skull with stainless steel screws and dental cement. For VTA cannulations, animals were positioned in a stereotaxic apparatus (David Kopf, USA) and stainless steel guide cannulas (26 GA, 8 mm; Plastics One, USA) were implanted bilaterally, 1 mm above the VTA (AP: −5.40, ML: +2.20, DV: −7.90, angle 10°, Paxinos and Watson, 2008). Perioperatively, rats received a single injection of carprofen (5 mg/kg, s.c.) for two consecutive days. Behavioral experiments commenced following a 10–14 day recovery period.
TH::Cre rats (n=9) weighing 40–70 g were deeply anesthetized as described before and placed in a stereotaxic frame and craniotomies were performed using VTA coordinates (AP: −4.8, ML: 1.0, DV: −7.1 from bregma, angle 5°). A Cre-dependent adeno-associated virus (AAV) expressing mCherry (AAV-DIO-mCherry; UNC Vector Core, USA) was bilaterally injected in the VTA as described previously (Boender et al, 2014). Briefly, virus (109 genomic copies/μl) was injected using a 2 μl Hamilton syringe at a rate of 0.2 μl/min for a total volume of 1 μl. Injection needles were left in place for 5 min to prevent backflow. After the injection, the skin was sutured and the rats allowed to recover for at least 2 weeks before the electrophysiological recordings were performed.
Drugs and Microinfusions
αMSH (Tocris, UK), HS014 (Tocris, UK), D-trp8-γMSH (γMSH; Tocris, UK,), AGRP (83–132)-amide (Phoenix Pharmaceuticals, USA), and α-flupenthixol dihydrochloride (Sigma Aldrich, USA) were dissolved in sterile saline. 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, Tocris, UK), DL-2-Aminophosphonopentanoic acid (APV, Tocris, UK), and bicuculline (Tocris, UK) were prepared as a stock solution.
For i.c.v. infusions, rats were briefly restrained and 2 μl of drug solution was injected over 10 s into the ventricular cavity. Intra-VTA infusions were performed through an injector (9 mm, 33GA, Plastics One, USA) inserted into the guide cannula. Bilateral infusions (300 nl over 30 s) were made using a syringe pump with the injectors left in place for another 30 s to allow for diffusion. α-Flupenthixol dihydrochloride was injected intraperitoneally (i.p.) 30 min before intra-VTA infusions. All animals received all drug doses/combinations, according to a Latin-square design. Behavioral testing commenced 5 min after drug infusions and a minimum 1 day drug-free period was maintained between infusions during which the animals were trained but not tested.
Electrophysiology
Horizontal slices of the midbrain (300 μm) were prepared from TH::Cre rats (5–7 weeks old) using a vibratome (HM650V; Microm) in ice-cold modified artificial cerebrospinal fluid (ACSF) containing (in mM): 92N-methyl-D-glucamine (NMDG), 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Napyruvate, 0.5 CaCl2.4H2O, and 10 MgSO4.7H2O, bubbled with 95% O2 and 5% CO2 (pH 7.3–7.4). Slices were initially recovered in carbogenated modified ACSF for 15 min at 34 °C and then transferred into a holding chamber containing standard ACSF (in mM): 126 NaCl, 3 KCl, 2 MgSO4, 2 CaCl2, 10 glucose, 1.25 NaH2PO4, and 26 NaHCO3 bubbled with 95% O2 and 5% CO2 (pH 7.3) at room temperature for at least 1 h. The slices were transferred one at a time to the recording chamber perfused with standard ACSF continuously bubbled with 95% O2 and 5% CO2 at 30–32 °C. Whole-cell patch-clamp recordings were made from VTA dopamine neurons visualized with an Olympus BX61W1 microscope using infrared video microscopy and differential interference contrast (DIC) optics. VTA dopaminergic neurons were identified by mCherry fluorescence. Patch electrodes were pulled from borosilicate glass capillaries; they had a resistance of 3–5 MΩ when filled with intracellular solutions. Internal solution contained (in mM): 140 K-gluconate, 1 KCl, 10 HEPES, 0.5 EGTA, 4 MgATP, 0.4 Na2GTP, 4 phosphocreatine (pH 7.3 with KOH). Signals were amplified filtered at 3 kHz and digitized at 10 kHz using an EPC-10 patch-clamp amplifier and PatchMaster v2x73 software. Series resistance was constantly monitored, and the cells were rejected from analysis if the resistance changed by >20%. No series resistance compensation was used. Resting membrane potential was measured in bridge mode (I=0) immediately after obtaining whole-cell access. The basic electrophysiological properties of the cells were determined from the voltage responses to a series of hyperpolarizing and depolarizing square current pulses. Input resistance was determined by the slope of the linear regression line through the voltage–current curve. The voltage sag was measured as the difference between peak and steady-state voltage response to a hyperpolarizing current step of −150 pA. The action potential amplitude was defined as the difference between the threshold and the action potential peak voltage. The action potential duration was measured at half of the peak amplitude of the action potential. The after hyperpolarizing potential (AHP) was measured as the peak hyperpolarizing deflection from action potential threshold.
Experimental Designs
Central effects of MC3R stimulation on responding for sucrose
To investigate the effects of MC3R stimulation on the motivation for sucrose, rats were tested under a progressive ratio (PR) schedule of reinforcement (Hodos, 1961), according to previously published procedures (la Fleur et al, 2007). Briefly, rats were trained under a fixed ratio 1 schedule of reinforcement in two-lever operant conditioning chambers (30.5 × 24.1 × 21.0 cm., Med-Associates, USA), whereby each active lever press (ALP) led to retraction of the levers and the delivery of a 45 mg sucrose pellet (Noyes precision pellets Formula F, Research Diet, USA). Twenty seconds after pellet delivery, levers were reinserted into the chamber. Inactive lever presses (ILP) were counted but had no programmed consequences. Allocation of the left and right lever as active and inactive lever was counterbalanced between animals. After acquisition of responding under the fixed ratio 1 schedule, a PR schedule of reinforcement was introduced, in which the number of lever presses required to obtain successive rewards increased according to the following equation: response ratio (5e(0.2 × reward number))−5 through the following series: 1, 2, 4, 9, 12, 15, 20, 25 (Richardson and Roberts, 1996; la Fleur et al, 2007). Sessions ended whenever the animal failed to earn a reward within 60 min. After 10 training sessions, the rats were tested during a period of 3–5 weeks with five to seven training sessions/week. All infusions were performed once responding under the PR schedule had stabilized. Responding was considered stable when the number of ALP did not differ by more than 15% between three consecutive sessions and there was no consistent up- or downward trend.
To investigate the role of MC3R stimulation on responding for food, rats (n=18) were treated with the MC3R/MC4R agonist αMSH (2 nmol, 2 μl), with or without the MC4R antagonist HS014 (1.2 nmol, 2 μl; Schioth et al, 1999), 5 min before operant testing. One rat was excluded from the analysis because of unstable baseline responding. A separate group of rats (n=12) also received either saline (2 μl) or γMSH (0.5 or 1 nmol) according to a Latin square design. Three rats were excluded from the analysis because of blocked cannulas and one was excluded because of unstable baseline responding. After completion of operant testing, animals were placed back in their home cages with a pre-weighed amount of chow. Chow consumption was determined 20 h later.
Central effects of γMSH on sucrose free-feeding
Free-feeding experiments were conducted to determine whether the effects of γMSH were specific to the animal's motivation for palatable food or associated with general effects on feeding behavior. In these experiments, animals had free access to sucrose pellets for 60 min every day, ie, the animals did not need to perform an operant task to obtain the sucrose pellets. Ad libitum fed rats were introduced into an empty cage with a suspended steel receptacle containing 45 mg sucrose pellets. After 60 min, sucrose intake was measured. To accustom rats to the procedure, 4–5 training sessions were performed before testing. Following stabilization of sucrose intake, rats (n=8) were injected with saline (2 μl) and two doses of γMSH (0.5, 1 nmol) according to a Latin square design.
The role of VTA MC3Rs in responding for sucrose
To investigate the effects of activation of VTA MC3Rs on motivation for sucrose, rats (n=11) were bilaterally infused with saline or γMSH (0.1 or 0.2 nmol) into the VTA and tested under the PR schedule of reinforcement. Two rats were excluded from the analysis because of unstable baseline responding. The effects of VTA MC3R stimulation on responding for sucrose was also investigated using αMSH and the MC3R/MC4R inverse agonist AGRP in a separate group of rats (n=11). In another group of animals (n=7), effect of MC3R stimulation by γMSH on sucrose free-feeding was determined. Furthermore, the interactions between MC3R and dopamine signaling were investigated by pre-treating rats (n=23) with saline or α-flupenthixol (0.125 mg/kg, i.p.) 30 min before intra-VTA saline or γMSH (0.2 nmol) infusions. The dose of α-flupenthixol was based on previous studies and did not affect responding for sucrose by itself (Veeneman et al, 2012; Pandit et al, 2015; Pandit et al, 2014). Five minutes after intra-VTA infusions, responding for sucrose under the PR schedule was tested as outlined above. One rat was removed from the analysis because of unstable baseline responding.
Cannula placements: At the end of the experiment, animals equipped with i.c.v. cannulas were killed with carbon dioxide and received an i.c.v. injection with 2 μl of ink. Brains were then removed and the lateral ventricles opened to check for ink staining. Rats equipped with VTA cannulas were killed with carbon dioxide, decapitated, the brains removed and frozen on dry ice. Next, cryostat sections (16 μm) were stained with cresyl violet to determine cannula locations (See Supplementary Figures S1 and 2).
MC3R Expression in the VTA
In this experiment, a separate group of rats (n=7) was used that was not subjected to behavioral testing. Following decapitation, the brains were frozen on dry ice. Coronal sections (16 μm) were fixed with 4% paraformaldehyde (10 min), subsequently washed with PBS (3 ×, 10 min) and further acetylated (10 min). Following acetylation, slices were preincubated with a prehybridization mix (50% deionized formamide, 5 × SSC, 5 × Denhardt's solution, 250 μg/ml tRNA baker's yeast, 500 μg/ml sonicated salmon sperm DNA final concentrations in MilliQ). Next, the sections were incubated overnight at 40 °C in 120 μl hybridization mix (Panomics) containing the probe sets (concentration dopamine D2 receptor (D2R)-1 : 33, MC3R/MC4R-1 : 50) and washed. Probe sets used to detect the desired rat mRNAs were designed by Panomics (Santa Clara, USA), using published sequences (Oude Ophuis et al, 2014; see Supplementary Data). Sections were then incubated for 1.5 h at 40 °C in 120 μl PreAmplifier Mix (Panomics) containing preamplification oligos (PreAmp TYPE4 1 : 20, PreAmp TYPE 6 1 : 50, PreAmp TYPE 8 1 : 33) and washed. Then, sections were incubated for 1.5 h at 40 °C in 120 μl Amplifier Mix (Panomics) containing amplification oligos (Amp TYPE4 1 : 20, Amp TYPE 6 1 : 50, Amp TYPE 8 1 : 33) and washed. Subsequently, sections were incubated for 1.5 h at 40 °C in 120 μl in Label Probe Mix (Panomics) containing label oligos (LP TYPE4 1 : 20, LP TYPE 8 1 : 33, LP TYPE 6 1 : 50). After washing, sections were incubated in 750 μl PBS supplemented with 4′,6-diamidino-2-phenylindole (DAPI) (6.7 μg/ml, Sigma Aldrich, St Louis, USA) for 5 min. Finally, slices were washed and embedded in Mowiol. Sections were visualized and images were obtained with a Zeiss AxioScope A1 microscope (Carl Zeiss, Germany). Images were processed using ImageJ (version1.43r) software.
For double-labeling immunohistochemistry of POMC and tyrosine hydroxylase (TH) proteins, rats (n=2) were perfused with ice-cold 4% paraformaldehyde (PFA) in PBS. Brains were removed and overnight post-fixated in 4% PFA and then embedded in a 30% sucrose solution in PBS. Brains were subsequently sliced using a cryostat to obtain free-floating 40 μm thin slices. Briefly, slices were washed with 0.5 M Tris-buffered solution (TBS; 3 × 10 min), and then blocked with super-mix (0.5 M TBS, 0.25% gelatin and 0.1% Triton X) for 30 min. Following blocking, slices were incubated with primary antibody against POMC (1 : 1000, Rabbit, H-029-30, Phoenix Pharmapseuticals, USA) and TH (1 : 1000, Mouse, MAB318, Millipore, USA; 4 °C, overnight) on a shaker. Subsequently, the slices were washed (TBS, 3 × 10 min) and incubated with fluorescent secondary antibody mix (chicken anti rabbit, 488, A-21441 and goat anti mouse 568, A-11004, Alexa Fluor, Life Technologies, USA) for 1 h at room temperature. Slices were further washed (3 × 10 min) with TBS, put on glass slides, and embedded with Fluorsave (Merck Milipore, USA). Images were made using Zeiss AxioScope A1 microscope (Carl Zeiss, Jena, Germany) and processed with ImageJ software.
A cholera toxin B (CTB)-based retrograde tracing strategy was used to study whether Arc POMC neurons projected to the VTA. To label perikarya of neurons in the Arc nucleus projecting to the VTA, the rats (n=6) were treated with CTB (0.5% solution; #103, List Biological Laboratories, Campbell, CA) via unilateral iontophoresis (5 μA, 7 s on–off) into the VTA for 20 min. For targeting both mid-rostral and mid-caudal VTA regions, the following stereotaxic coordinates were used, respectively, with reference to the interaural (IA) planes; AP: +3.8 mm or +2.96 mm, ML: +0.6 mm, DV: +2 mm or +1.8 mm. Ten days after tracer injections, the animals were perfused transcardially with 200 ml of 4% paraformaldehyde solution in 0.1 M sodium phosphate buffer (PBS, pH 7.4). The brains were collected and soaked into 20% sucrose overnight, snap-frozen and cut in the coronal plane by a Leica SM 2000R freezing microtome (Leica Microsystems Nussloch GmbH, Nussloch, Germany). Thirty-micrometer-thick sections were cut and transferred into a six-well plate in a consecutive manner to produce six representative collections of sections from each brain. One out of the six groups of sections from each brain was processed to reveal CTB and POMC immunoreactive neurons in the Arc. The immunofluorescent double labeling and the subsequent confocal microscopic analyses were carried out as described before (Kallo et al, 2012). Supplementary Table S3 contains a list of antibodies used for this study.
Furthermore, to determine whether POMC neurons projecting to the VTA made synaptic contacts with VTA dopaminergic neurons, we used a rabies virus-based trans-synaptic retrograde tracing approach as described earlier (Wickersham et al, 2007). Briefly, TH::Cre rats (n=3) were injected with cre-dependent helper viruses AAV-DIO-TVA-mCherry and AAV-DIO-RG (UNC Vector Core, USA) unilaterally into the VTA (AP: −5.40, ML: +2.20, DV: −8.90, angle 10°, Paxinos and Watson, 1998), which later restricts the rabies virus infection to Cre-positive TH cells. Two weeks later, the rats were injected in the VTA with a modified rabies virus (EnvA-ΔG-mCherry, 105 genomic copies/μl) that infects Cre-positive TH cells (DA neurons) and can be retrogradely transported across one synapse. Thus, neurons providing synaptic input to TH positive neurons can be visualized using this technique. Seven days following the second injection, rats were perfused with 4% PFA as described earlier. Immunohistochemistry was performed and staining was visualized for POMC and mCherry (1 : 500, monoclonal mouse, Abcam, UK) with an Olympus Fluoview FV1000 confocal microscope (Olympus, Japan).
Statistical Analysis
For the analysis, data were excluded from animals that had either unstable responding under the PR schedule of reinforcement (ie, >15% variation between three consecutive sessions and/or a consistent upward or downward trend in the number of rewards earned) or blocked cannulas. All the data were analyzed using one-way repeated-measures ANOVA of two-way ANOVA followed by a Dunnett's post hoc test. Effects of AGRP infusion on responding for sucrose were analyzed using paired t-tests. Differences were considered significant at p<0.05. All statistical analyses and graphical representations were performed using Graphpad software (v 6.03, USA). Data are expressed as mean±SEM.
RESULTS
Central Stimulation of MC3Rs Increases Responding for Sucrose but not Sucrose Free-Feeding
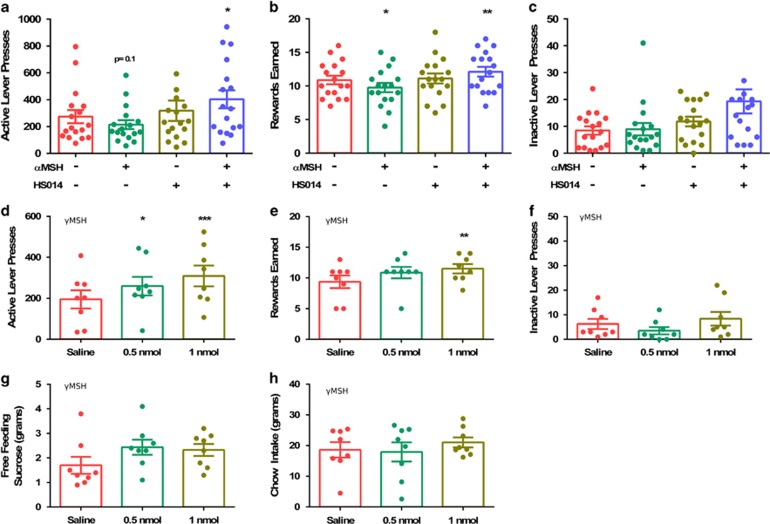
As reported earlier (Pandit et al, 2015), i.c.v infusion of αMSH decreased the number of ALP (F(3,16)=4.03, p=0.036, Figure 1a) and rewards earned (F(3,16)=7.62, p=0.002, Figure 1b). Interestingly, co-injection of the specific MC4R antagonist HS014 together with αMSH, increased both ALP and rewards earned. The number of ILP following combined HS014 and αMSH injections was increased (F(3,16)=4.06, p=0.029, Figure 1c); however, post hoc tests revealed no significant differences between the experimental groups.
Figure 1.
Central stimulation of MC3Rs increases motivation and free intake of sucrose. Effects of i.c.v. infusion of αMSH in combination with a MC4R blocker (HS014) on ALPs (a), rewards earned (b), and ILPs (c) (n=17). Effects of i.c.v. infusion of saline or γMSH on ALPs (d), rewards earned (e), ILPs (f), sucrose free feeding (g), and 24 h chow intake (h) (n=8). One-way ANOVA, *p<0.05, **p<0.01, ***p<0.001 compared with saline condition.
I.c.v. infusion of the selective MC3R agonist γMSH increased both ALPs (F(2,7)=10.36, p=0.002) and rewards earned (F(2,7)=6.19, p=0.012) in a dose-dependent manner (Figure 1d and e). The number of ILPs remained unchanged (F(3,7)=1.44, p=0.270, Figure 1f). In the free-feeding paradigm, γMSH did not affect sucrose (F(2,7)=2.56, p=0.112, Figure 1g) or overnight chow F(2,7)=0.75, p=0.489, Figure 1h) consumption.
POMC Neurons from the Arc Nucleus make Synaptic Contact with Dopaminergic Neurons
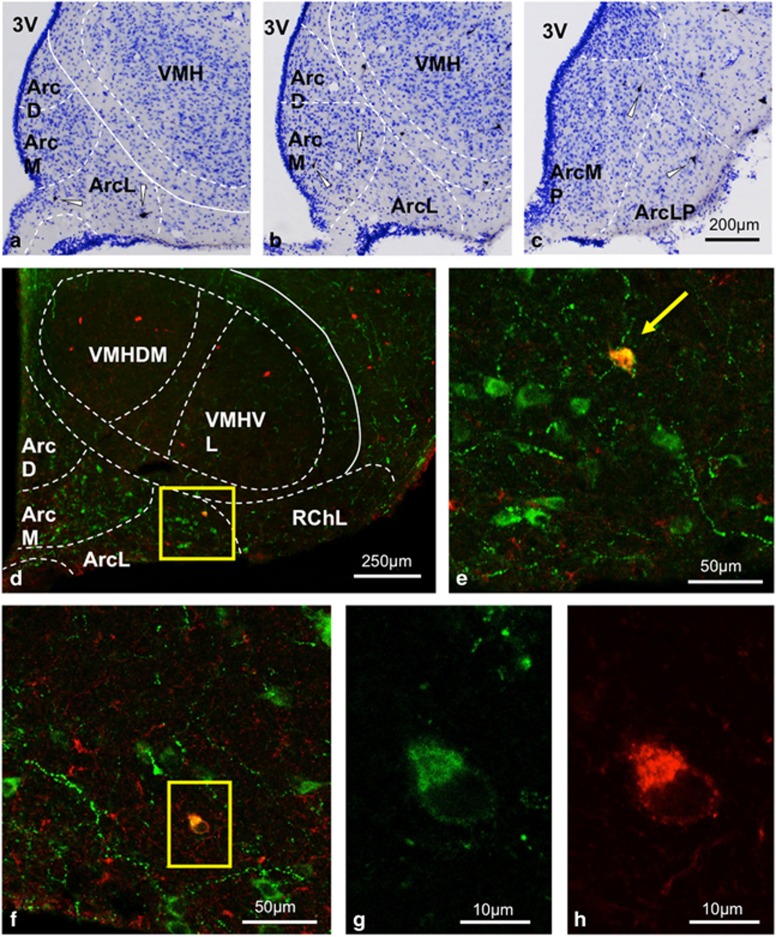
The injection of CTB into the VTA labeled several neurons within the Arc. Analyses of sections immunolabeled for both POMC and CTB in this region revealed that 30.07±3.25% of the CTB-immunoreactive neurons were positive for POMC (Figure 2). We also found 1.09±0.07% of POMC cells to be reterogradely labeled with CTB. Immunohistochemical studies further confirmed the presence of POMC fibers interspaced between the dopaminergic neurons within the VTA (Supplementary Figure S4). Furthermore, synaptic connectivity between the VTA dopaminergic neurons and the Arc POMC neurons was established using trans-synaptic retrograde tracing experiments. Thus, rabies virus injection into VTA-TH-positive neurons retrogradely transported mCherry in a subset of POMC neurons within the Arc (Supplementary Figure S5). Although a few mCherry-positive neurons were sporadically observed in the NTS region, none of these neurons were POMC positive (Supplementary Figure 6).
Figure 2.
POMC neurons from the Arc project to the VTA: Cholera toxin B (CTB)-immunoreactive (IR) neurons in the arcuate nucleus after injecting the retrograde tracer CTB into the ventral tegmental area (VTA). A few scattered CTB-IR neurons (arrowheads) appear in the arcuate nucleus as demonstrated at three rostro-caudal levels (a: Bregma −2.04 mm, b: Bregma −2.40 mm, and c: Bregma −3.84 mm). (d) Immunofluorescent detection of POMC (green) and CTB (red) in hypothalamic sections shows co-localization (yellow in color) in the arcuate nucleus. (e) The enframed area in d is further magnified to demonstrate the double-labeled cell (yellow arrow). (f) Another arcuate section showing POMC-IR neurons (green); one of these neurons also immunoreactive for CTB (in the enframed area; (g and h)).
MCRs are Expressed in Mesolimbic Dopaminergic Neurons
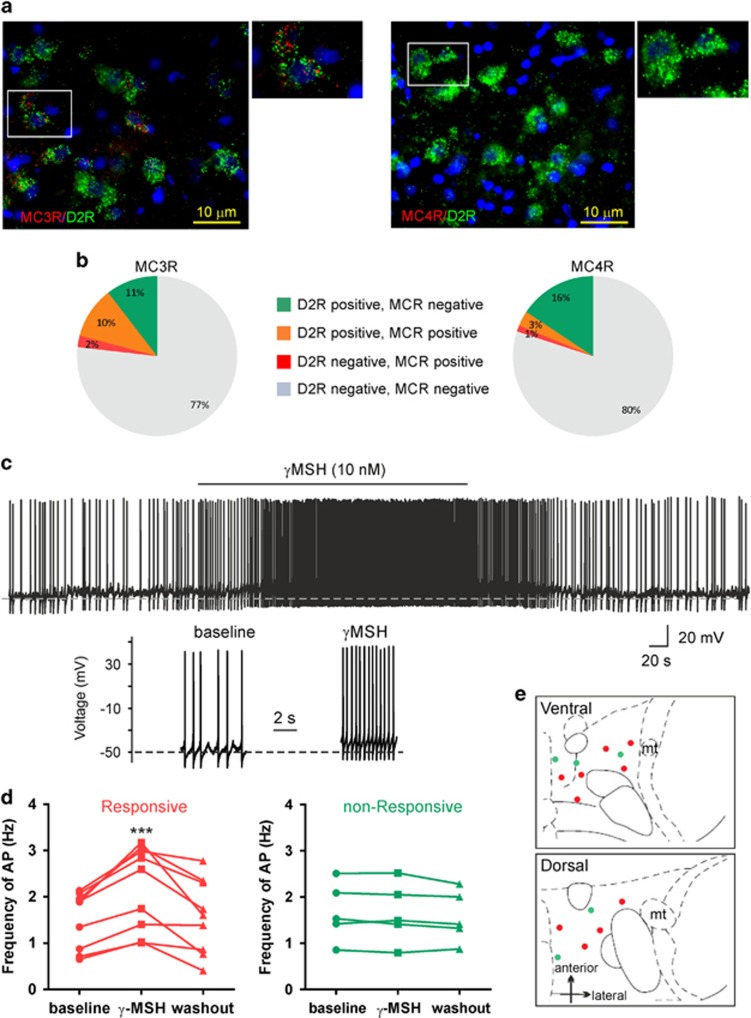
Using in situ hybridization and double-labeling fluorescence immunohistochemistry for D2Rs and MCRs, we found an abundant expression of MC3Rs in the VTA (Figure 3a and b). The majority of MC3Rs (more than 90%) co-localized with D2R-expressing cells (48±6% of D2R-positive cells also express MC3Rs). In line with previous reports (Mountjoy, 2010), we found that relatively low numbers of MC4Rs are expressed within the VTA. Furthermore, the MC4Rs were more abundantly expressed in D2R positive than in D2R negative cells (Figure 3a and b).
Figure 3.
MCRs are expressed in mesolimbic dopaminergic neurons. (a) Images showing co-localization of MC3R (left) and MC4R (right) with D2R in the VTA. Boxed areas shows digitally zoomed images of the co-localizations. (b) Percentage of D2R-positive, MC3R-positive, MC4R-positive cells from total number of DAPI-positive cells. (c) Sample current-clamp trace of spontaneous action potentials from a VTA dopamine neuron before and after γMSH application. Expanded sections are shown underneath the trace. (d) Effect of γMSH on frequency of spontaneous action potentials of VTA dopamine neurons before, during, and after bath application of γMSH in both responsive (red) and non-responsive cells (green). (e) The distribution of all recorded dopamine neurons plotted on horizontal midbrain slices with colors indicating the response to γMSH. One-way ANOVA, ***p<0.001 compared with baseline condition.
γMSH Modulates Dopamine Neuronal Firing in the VTA
To selectively record from VTA dopaminergic (DA) neurons, we injected a Cre-inducible AAV expressing mCherry into the VTA of TH::Cre rats. After 2–3 weeks, whole-cell current clamp recordings were made from mCherry-positive neurons in horizontal midbrain slices. The mCherry-positive/DA neurons were located in both lateral and medial regions of the anterior and posterior VTA. Given the heterogeneity of dopaminergic neurons (Lammel et al, 2008), basic electrophysiological properties of recorded neurons were determined before application of γMSH (see ‘Materials and methods' section for details). Under baseline conditions, dopaminergic neurons had a resting membrane potential of −46.1±2.7 mV, and mostly showed spontaneously firing action potentials at a rate of 1.57±0.16 Hz (n=14). To determine the direct effect of γMSH on dopaminergic neurons, synaptic inputs onto these neurons were blocked using inhibitors of synaptic transmission (CNQX 10 μM, APV 50 μM, and bicuculline 20 μM). γMSH (10 nM) depolarized dopaminergic neurons by 4.3±0.55 mV and increased the frequency of spontaneous action potentials to 147%±4.5% of baseline in 9 out of 14 neurons, which was reversed upon washout of γMSH (F(2,16)=14.62, p=0.0006 and F(2,8)=2.79, p=0.14 for responsive and nonresponsive cells, respectively, Figure 3c and d). Responsive and nonresponsive neurons were located in both lateral and medial VTA regions (Figure 3e, Supplementary Figure 7) and were not significantly different in their electrophysiological properties (Supplementary Figure 7).
Intra-VTA MC3R Stimulation Increases Responding for Sucrose but not Sucrose Free-Feeding
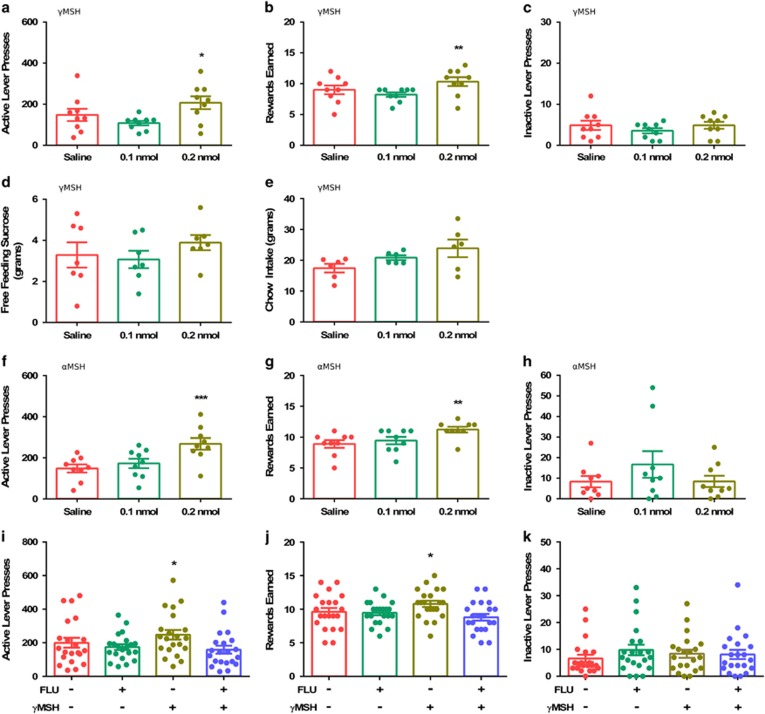
Intra-VTA infusion of γMSH increased ALPs for sucrose (F(2,8)=10.57, p=0.001, Figure 4a) and rewards earned (F(2,8)=11.60, p=0.001, Figure 4b) without increasing ILPs (F(2,8)=5.33, p=0.465, Figure 4c). In contrast to its effects on responding for sucrose, intra-VTA injections of γMSH failed to increase free intake of sucrose (F(2,6)=1.18, p=0.340, Figure 4d) or chow (F(2,5)=2,72, p=0.114, Figure 4e).
Figure 4.
Intra-VTA MC3R stimulation increases motivation but not free feeding of sucrose. Effects of intra-VTA injections of γMSH on ALPs (a), rewards earned (b), ILPs (c), sucrose free-intake (d), and 24 h chow intake (e) (n=7–9). Effects of intra-VTA injections of αMSH on ALPs (f), rewards earned (g), and ILPs (h) (n=9). Effects of α-flupenthiixol (FLU) pretreatment on the number of ALPs (i), rewards earned (j), and ILPs (k) following intra-VTA γMSH infusion (n=21). One-way ANOVA, *p<0.05 **p<0.01, ***p<0.001 compared with saline condition.
Interestingly, the effects of intra-VTA γMSH infusion on responding for sucrose could be replicated using αMSH (ALP: F(2,8)=12.10, p=0.001, Figure 4f; rewards earned: (F(2,8)=8.25, p=0.003, Figure 4g). ILPs after αMSH treatment (F(2,8)=1.44, p=0.267) remained unaltered (Figure 4h). Furthermore, infusion of AGRP, an inverse agonist for the MC3R reduced responding for sucrose (ALP: t(10)=2.83, p=0.018, Supplementary Figure 8a); rewards earned: t(10)=2.51, p=0.031, Figure Supplementary Figure 8b, ILP: t(10)=0.91, p=0.380, Supplementary Figure 8c).
MC3R Stimulation of Responding for Sucrose is Dopamine-Dependent
Pretreatment of animals with α-flupenthixol before infusions of γMSH prevented the increase in responding for sucrose by γMSH as reflected by the amounts of ALPs (F(3,20)=8.09, p=0.000, Figure 4i) and rewards earned (F(3,20)=11.11, p=0.001, Figure 4j) while the ILP remained unchanged (F(3,20)=0.99, p=0.397, Figure 4k). A two-way ANOVA showed an interaction between γMSH and α-flupenthixol for both ALPs (F(1, 20)=5.92, p=0.024) and rewards earned (F(1, 20)=14.12, p=0.001), but not ILPs (F(1, 20)=2.86, p=0.107).
DISCUSSION
In the present study, we provide evidence that stimulation of MC3Rs in the VTA increases the motivation for palatable food, through a dopaminergic mechanism of action. Rats centrally treated with the MC3R agonist γMSH displayed increased responding for sucrose but not free consumption of sucrose or chow, indicating that MC3R stimulation enhances motivation for sucrose. In contrast, we observed that infusion of the MC4R/MC3R agonist αMSH reduced responding for sucrose. However, when αMSH was co-administered with a MC4R antagonist, motivation for sucrose was enhanced, further supporting a role of MC3Rs in increasing the motivation for food reward.
It is known that the VTA dopaminergic neurons projecting to the NAc have a crucial role in incentive motivation for rewards (Barbano and Cador, 2007; Kelley, 2004; Salamone and Correa, 2012). Therefore, we determined whether MC3R signaling within the VTA influences motivation for sucrose. To assess the physiological relevance of MC3R signaling within the VTA, we first confirmed the presence of POMC immunoreactivity within the VTA. Second, consistent with earlier reports (Roselli-Rehfuss et al, 1993), we confirmed the expression of MC3Rs in this area. Last, using CTB injections and trans-synaptic retrograde tracing technology, we showed that Arc POMC neurons make synaptic contact with dopamine neurons in the VTA. Furthermore, we did not observe any co-localization of mCherry and POMC neurons in the NTS, making the VTA-NTS projection an unlikely candidate for mediating the motivational effects of melanocortins.
To expand these neuroanatomical findings, we investigated the physiological effects of MC3R stimulation in dopaminergic neurons. Similar to the effects observed in POMC neurons (Smith et al, 2007), γMSH directly depolarized and increased the firing frequency of dopaminergic neurons in the VTA. Altogether, these neuroanatomical and electrophysiological data suggest that Arc POMC neurons can directly regulate VTA dopaminergic activity via MC3R signaling and as such mediate dopamine-dependent behaviors.
As phasic firing of dopaminergic neurons underlies reward-seeking behavior (Tsai et al, 2009; Adamantidis et al, 2011; Nishino et al, 1987), we hypothesized that stimulation of MC3Rs in the VTA would increase the motivation for sucrose. Indeed, intra-VTA infusion of γMSH or αMSH increased responding for sucrose under a PR schedule of reinforcement, whereas infusion of the MC3R inverse agonist AGRP reduced responding. Finally, the increase in incentive motivation after intra-VTA γMSH treatment was blocked by systemic pretreatment with the dopamine receptor antagonist α-flupenthixol, demonstrating that the motivation-enhancing effects of intra-VTA γMSH infusion are dopamine-dependent. The finding that free-feeding remained unchanged upon γMSH infusion into the VTA, is consistent with the notion that VTA dopaminergic neurons modulate the incentive motivation for food but not its actual consumption (Adamantidis et al, 2011; Salamone et al, 2001; Kelley 2004).
As VTA dopaminergic neurons projecting to the NAc express D2Rs and respond to rewarding stimuli (Lammel et al, 2008; Lammel et al, 2011), we sought to determine whether these neurons specifically express MC3Rs. We here show MC3R co-localization predominantly in D2R-containing neurons in the VTA. Furthermore, our electrophysiological data confirmed the presence of functional MC3Rs on dopaminergic neurons, indicated by a change in the membrane potential and frequency of action potentials on γMSH application. Importantly, based on anatomical locations and electrophysiological properties, some of the responsive neurons correspond to the VTA-NAc projection neurons described previously (Lammel et al, 2008). Moreover, as stimulation of VTA-NAc projections increases the motivation for sucrose (Sokolowski et al, 1998; Salamone et al, 2001; Randall et al, 2012; Boender et al, 2014), and as local infusion of D2R antagonist into the NAc is known to reduce this motivation (Salamone et al, 1991), our data strongly suggest that the motivational effects of intra-VTA MC3Rs are mediated via dopamine signaling in the NAc. In support of this notion, it has been recently reported that knocking out MC3Rs decreases dopamine levels in the NAc (Lippert et al, 2014). It is worth noting that we also saw a sparse expression of MC3Rs in D2R-negative neurons that possibly are VTA neurons projecting to the medial prefrontal cortex (mPFC) (Lammel et al, 2008). In conclusion, although the VTA-NAc dopaminergic pathway is the most likely candidate to mediate the effects of γMSH on responding for sucrose, the involvement of the dopaminergic projection from the VTA to the mPFC in the motivational effects of γMSH cannot be excluded (Baldwin et al, 2002; Lex and Hauber 2010).
In the current study, the effects of central MC3R stimulation were limited to responding for food reward, as we did not observe effects on free consumption of sugar or chow, suggesting a rather selective role for MC3Rs in food motivation. These findings fit well into the general idea that different aspects of feeding (eg, homeostatic, motivational, and hedonic processes) are mediated by distinct neural systems (Schwartz et al, 2000; Kelley et al, 2005; Barbano and Cador 2007). Clearly, however, these different neuronal systems do not work in isolation, as the coordination of the different subprocesses is essential to obtain an adaptive pattern of feeding behavior. It is also worth noting that other factors such as, liking, choice, cost/benefit analysis, extent of hunger, satiety, etc could affect neuronal circuits involved in feeding behavior.
In this regard, it is of particular interest that Marks et al (2006) have reported that peripheral injections of γMSH increase food intake in wild type but not MC3R knockout mice, indicating that γMSH can work via MC3Rs to increase food intake. This apparent discrepancy might result from the fact that peripheral (Marks et al, 2006) vs central infusions (present study) were used so that the effects on food intake could be a result from peripheral or brain-stem-mediated effects of γMSH. In addition, species differences between rats and mice cannot entirely be excluded. Taken together, this suggests that depending on the site of action (central vs peripheral or VTA vs Arc), even the same molecule can function to influence different aspects of feeding.
Considering the physiological role of a MC3R–dopamine system interaction, one might find the effects of γMSH on food motivation confusing, not least since MCs in general are considered to be anorexigenic peptides (Cone, 2005). Our results, viewed in light of a recent finding, where Arc POMC neurons increase their firing frequency following food presentation (Chen et al, 2015), would suggest that the motivation to eat following food presentation could be mediated by the VTA MC3Rs. As POMC neurons are known to be activated by leptin and inhibited by ghrelin (Cowley et al, 2003), during positive energy balance (when leptin levels are high), activation of POMC neurons would support motivation for food via MC3R signaling within the VTA at times of energy abundance. Thus, seen from the perspective of evolutionary biology where an organism, although satiated, should maintain its intrinsic motivation to seek out food sources and consume them in order to increase the chances of survival, the MC3Rs in the VTA might have an important role in maintaining this motivational drive in times of energy abundance. Furthermore, as opposed to the motivation-enhancing effects of MC3 signaling in the VTA, MC4R signaling in the NAc decreases motivation for palatable rewards, implying that MCs may fine-tune motivation for palatable rewards based on the type of MC receptor expression in different brain nuclei.
In short, MCs regulate diverse physiological processes ranging from behaviors (food intake, anxiety, memory) to thermoregulation and cardiovascular functions (Mountjoy, 2010). These processes are modulated by POMC neurons through their projections to the different brain structures in which MC3Rs and MC4Rs are differentially expressed.
Altogether, the current study identifies a mechanism through which central MC3Rs influence food intake, ie, by affecting motivation for palatable food. Furthermore, these data add to our existing understanding about MC3R–dopamine crosstalk, in which absence of MC3R signaling is correlated with attenuated dopamine-dependent behaviors such as decreased locomotion and sucrose reference (Butler and Cone, 2002). Moreover, our study shows that alongside other feeding-related peptides such as ghrelin and neuropeptide Y (Skibicka et al, 2012; Pandit et al, 2014), MCs within the VTA regulates motivational aspects of food intake.
Funding and disclosure
Susanne E la Fleur was supported by a ZonMw-VIDI grant (917.96.331). Imre Kallo and Zlost Liposits were supported by a grant from the National Science Foundation of Hungary OTKA K101326. The remaining authors declare no competing financial interest. The work was supported by funding from the European Community's Seventh Framework Programme (FP7/2007-2013 under grant agreement no. 245009 and 266408).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA et al (2011). Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci 31: 10829–10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andino LM, Ryder DJ, Shapiro A, Matheny MK, Zhang Y, Judge MK et al (2011). POMC overexpression in the ventral tegmental area ameliorates dietary obesity. J Endocrinol 210: 199–207. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Kelley AE (2002). Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci 22: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Cador M (2007). Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 191: 497–506. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191: 391–431. [DOI] [PubMed] [Google Scholar]
- Boender AJ, de Jong JW, Boekhoudt L, Luijendijk MC, van der Plasse G, Adan RA (2014). Combined use of the canine adenovirus-2 and DREADD-technology to activate specific neural pathways in vivo. PLoS One 9: e95392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Cone RD (2002). The melanocortin receptors: lessons from knockout models. Neuropeptides 36: 77–84. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW, Knight ZA (2015). Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160: 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD (2005). Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE (2003). Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann NY Acad Sci 994: 175–186. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL et al (2001). Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484. [DOI] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Shurdak JD, Krause EG, Fitzgerald MF, Lipton JW et al (2011). Central melanocortins modulate mesocorticolimbic activity and food seeking behavior in the rat. Physiol Behav 102: 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD (2004). Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7: 335–336. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Jay JL, Acheson MA, Magrisso IJ, West CH, Zavosh A et al (2013). Moderate high fat diet increases sucrose self-administration in young rats. Appetite 61: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ (2001). Opioid receptor involvement in the effect of AgRP- (83-132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol 280: R814–R821. [DOI] [PubMed] [Google Scholar]
- Hodos W (1961). Progressive ratio as a measure of reward strength. Science 134: 943–944. [DOI] [PubMed] [Google Scholar]
- Jansone B, Bergstrom L, Svirskis S, Lindblom J, Klusa V, Wikberg JE (2004). Opposite effects of gamma(1)- and gamma(2)-melanocyte stimulating hormone on regulation of the dopaminergic mesolimbic system in rats. Neurosci Lett 361: 68–71. [DOI] [PubMed] [Google Scholar]
- Kallo I, Vida B, Deli L, Molnar CS, Hrabovszky E, Caraty A et al (2012). Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol 24: 464–476. [DOI] [PubMed] [Google Scholar]
- Kelley AE (2004). Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27: 765–776. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ (2005). Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86: 773–795. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA (2007). A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes (Lond) 31: 1286–1294. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J (2008). Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57: 760–773. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC (2011). Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70: 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex B, Hauber W (2010). The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning. Cereb Cortex 20: 873–883. [DOI] [PubMed] [Google Scholar]
- Lippert RN, Ellacott KL, Cone RD (2014). Gender-specific roles for the melanocortin-3 receptor in the regulation of the mesolimbic dopamine system in mice. Endocrinology 155: 1718–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DL, Hruby V, Brookhart G, Cone RD (2006). The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R). Peptides 27: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG (2010). Distribution and function of melanocortin receptors within the brain. Adv Exp Med Biol 681: 29–48. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD (1994). Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 8: 1298–1308. [DOI] [PubMed] [Google Scholar]
- Mul JD, van Boxtel R, Bergen DJ, Brans MA, Brakkee JH, Toonen PW et al (2012). Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity (Silver Spring) 20: 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino H, Ono T, Muramoto K, Fukuda M, Sasaki K (1987). Neuronal activity in the ventral tegmental area (VTA) during motivated bar press feeding in the monkey. Brain Res 413: 302–313. [DOI] [PubMed] [Google Scholar]
- Oude Ophuis RJ, Boender AJ, van Rozen AJ, Adan RA (2014). Cannabinoid, melanocortin and opioid receptor expression on DRD1 and DRD2 subpopulations in rat striatum. Front Neuroanat 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, la Fleur SE, Adan RA (2013). The role of melanocortins and Neuropeptide Y in food reward. Eur J Pharmacol 719: 208–214. [DOI] [PubMed] [Google Scholar]
- Pandit R, Luijendijk MC, Vanderschuren LJ, la Fleur SE, Adan RA (2014). Limbic substrates of the effects of neuropeptide Y on intake of and motivation for palatable food. Obesity (Silver Spring) 22: 1216–1219. [DOI] [PubMed] [Google Scholar]
- Pandit R, van der Zwaal EM, Luijendijk MC, Brans MA, van Rozen AJ, Oude Ophuis RJ et al (2015). Central melanocortins regulate the motivation for sucrose reward. PLoS One 10: e0121768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2008). The rat brain in stereotaxic coordinates, 4th ed. Elsevier: San Diego, CA.
- Randall PA, Pardo M, Nunes EJ, Lopez CL, Vemuri VK, Makriyannis A et al (2012). Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One 7: e47934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB et al (1993). Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 90: 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76: 470–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, Mccullough LD, Smith P, Grebel D, Mahan K (1991). Haloperidol and nucleus-accumbens dopamine depletion suppress lever pressing for food but increase free food-consumption in a novel food choice procedure. Psychopharmacology 104: 515–521. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Wisniecki A, Carlson BB, Correa M (2001). Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ratio requirements but do not impair primary food reinforcement. Neuroscience 105: 863–870. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Muceniece R, Mutulis F, Bouifrouri AA, Mutule I, Wikberg JE (1999). Further pharmacological characterization of the selective melanocortin 4 receptor antagonist HS014: comparison with SHU9119. Neuropeptides 33: 191–196. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D Jr., Seeley RJ, Baskin DG (2000). Central nervous system control of food intake. Nature 404: 661–671. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Shirazi RH, Hansson C, Dickson SL (2012). Ghrelin interacts with neuropeptide Y Y1 and opioid receptors to increase food reward. Endocrinology 153: 1194–1205. [DOI] [PubMed] [Google Scholar]
- Smith MA, Hisadome K, Al-Qassab H, Heffron H, Withers DJ, Ashford ML (2007). Melanocortins and agouti-related protein modulate the excitability of two arcuate nucleus neuron populations by alteration of resting potassium conductances. J Physiol 578: 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Conlan AN, Salamone JD (1998). A microdialysis study of nucleus accumbens core and shell dopamine during operant responding in the rat. Neuroscience 86: 1001–1009. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Duos B, Patterson LM, Berthoud HR (2005). Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 146: 3739–3747. [DOI] [PubMed] [Google Scholar]
- Torre E, Celis ME (1988). Cholinergic mediation in the ventral tegmental area of alpha-melanotropin induced excessive grooming: changes of the dopamine activity in the nucleus accumbens and caudate putamen. Life Sci 42: 1651–1657. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de LL et al (2009). Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science 324: 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan C, Moore M, Haskell-Luevano C, Rowland NE (2006). Food motivated behavior of melanocortin-4 receptor knockout mice under a progressive ratio schedule. Peptides 27: 2829–2835. [DOI] [PubMed] [Google Scholar]
- Veeneman MM, van AM, Broekhoven MH, Limpens JH, Vanderschuren LJ (2012). Seeking-taking chain schedules of cocaine and sucrose self-administration: effects of reward size, reward omission, and alpha-flupenthixol. Psychopharmacology (Berl) 220: 771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK, Callaway EM (2007). Retrograde neuronal tracing with a deletion-mutant rabies virus. Nat Methods 4: 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Phifer CB, Berthoud HR (2005). Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289: R247–R258. [DOI] [PubMed] [Google Scholar]
- Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR (2010). High-fat intake induced by mu-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R- stimulation. Brain Res 1350: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.