Abstract
Genetic polymorphisms in the repeat upstream region of the serotonin transporter gene (SLC6A4) are associated with individual differences in stress reactivity, vulnerability to affective disorders, and response to pharmacotherapy. However, the molecular, neurodevelopmental and psychopharmacological mechanisms underlying the link between SLC6A4 polymorphisms and the emotionally vulnerable phenotype are not fully understood. Thus, using the marmoset monkey Callithrix jacchus we characterize here a new neurobiological model to help to address these questions. We first sequenced the marmoset SLC6A4 promoter and identified a double nucleotide polymorphism (−2053AC/CT) and two single-nucleotide polymorphisms (−2022C/T and −1592G/C) within the repeat upstream region. We showed their association with gene expression using in vivo quantitative PCR and with affective behavior using a primate test of anxiety (human intruder test). The low-expressing haplotype (AC/C/G) was linked with high anxiety while the high-expressing one (CT/T/C) was associated with an active coping strategy in response to threat. Pharmacological challenge with an acute dose of the selective serotonin reuptake inhibitor, citalopram, revealed a genotype-dependent behavioral response. While individuals homozygous for the high anxiety-related haplotype AC/C/G exhibited a dose-dependent, anxiogenic response, individuals homozygous for the low anxiety-related haplotype CT/T/C showed an opposing, dose-dependent anxiolytic effect. These findings provide a novel genetic and behavioral primate model to study the molecular, neurodevelopmental, and psychopharmacological mechanisms that underlie genetic variation-associated complex behaviors, with specific implications for the understanding of normal and abnormal serotonin actions and the development of personalized pharmacological treatments for psychiatric disorders.
Introduction
Serotonin regulates multiple physiological and behavioral processes crucial for emotional homeostasis and survival. Among these, the serotoninergic system orchestrates the response to threats and its adjustment to specific contexts. However, an exacerbated response to uncertain, but non-threatening, stimuli can lead to negative adaptive outcomes and constitutes one of the core features of anxious behavior (Grupe and Nitschke, 2013). Subjects suffering from affective disorders, including anxiety and depression, present alterations in serotonin-related functions. Of particular importance is the serotonin transporter, a key protein responsible for the reuptake of serotonin from the synaptic cleft and termination of its action (Kristensen et al, 2011).
Genetic polymorphisms in the serotonin transporter gene (SLC6A4) have long been associated with individual differences in reactivity to early-life stress and vulnerability to affective disorders in humans (Canli and Lesch, 2007; Caspi et al, 2010; Lesch et al, 1996). The most studied polymorphism is a variable number of tandem repeats (VNTR) within the upstream repeat region, with short alleles being linked to emotionally vulnerable phenotypes and reduced SLC6A4 expression, both in humans and in macaques (Lesch et al, 1996; Spinelli et al, 2007). However, even after taking into account gene × environment interactions (Caspi et al, 2003, 2010) and a single-nucleotide polymorphism in the human long allele that confers a short allele phenotype (Hu et al, 2006), discrepancies exist among these gene–behavioral association studies (Munafò and Flint, 2004). Despite these discrepancies, in vitro expression analyses have shown that the SLC6A4 repeat region regulates gene expression and that genetic variation at this locus has an impact on expression levels (Bennett et al, 2002; Heils et al, 1996).
In addition, correlational imaging studies have shown that even though there are no consistent genotype-dependent changes in serotonin transporter binding in the adult brain, there are chemical, structural, and functional alterations in neural circuits involved in emotional processing in short allele carriers (Jedema et al, 2010; Pacheco et al, 2009; Pezawas et al, 2005). Serotonin levels early in life are crucial for the development of the central nervous system and the emotional profile in adulthood (Suri et al, 2015). Thus, it has been proposed that the short-allele adult phenotype may be the result of changes in neural circuitry due to increased serotonin levels during neurodevelopment and the interaction between this emotionally vulnerable brain with stressful early life experiences (Ansorge et al, 2007).
More recently, large pharmacogenomic studies have shown an interaction between SCL6A4 genotype and the efficacy of selective serotonin reuptake inhibitors (SSRIs) in the chronic treatment of anxiety and depression, with short-allele carriers presenting a lower remission rate (Keers et al, 2011; Serretti et al, 2007). It has also been theorized (Harmer and Cowen, 2013) that individual differences in subsequent treatment efficacy may be related to an initial anxiogenic effect seen during the early stages of SSRI treatment in various patient populations (Kent et al, 1998) and healthy volunteers (Bigos et al, 2008; Browning et al, 2007; Grillon et al, 2007). However, whether the acute anxiogenic effect of SSRIs is related to the SCL6A4 VNTR remains unknown, with few studies having been performed in healthy volunteers (Hinkelmann et al, 2010).
Despite this increasing body of evidence strongly linking polymorphisms within the SCL6A4 VNTR with genotype-specific gene regulation, neurodevelopmental changes, vulnerability to psychiatric disorders, and response to pharmacotherapy, our understanding of the underlying molecular, neurodevelopmental, and neuropsychopharmacological mechanisms is poor. Hence, it is crucial to characterize an animal model that will allow us to advance our understanding of these SLC6A4 polymorphisms and vulnerable phenotypes associations, in order to develop effective psychopharmacological treatments. We characterize here such a novel genetic and behavioral model in the common marmoset Callithrix jacchus, a species that has gained increasing popularity in molecular neuroscience since the completion of the marmoset genome (Flicek et al, 2011) and the generation of the first transgenic marmoset (Sasaki et al, 2009). Moreover, given their small size, fast reproductive rate, short developmental period, and similarity of brain organization with that of humans, marmosets are becoming an ideal primate model to study the neurobiological mechanisms underlying affective disorders (Sawada et al, 2014).
In the present study, we characterize the entire marmoset SLC6A4 repeat upstream region, identify novel sequence polymorphisms, and show their association with both gene expression levels and affective responses to threat. In addition, we reveal a variant-dependent behavioral response to threat after an acute dose of an SSRI, citalopram. Altogether, these findings establish a robust primate model for future molecular and developmental studies of the neurobiological mechanisms underlying individual differences in affective behavior, vulnerability to affective disorders, and pharmacotherapeutic efficacy.
Materials and Methods
Animals and Housing
Common marmosets C. jacchus (age: 29.9±1.2 months, weight: 433.8±9.1 g; see Supplementary Table S1 for sample size summary) were bred on site at the Innes Marmoset Colony (Behavioral and Clinical Neuroscience Institute). Genotyped animals were housed in pairs or in families. Animals included in the SLC6A4 gene expression, behavioral, and pharmacological studies were only housed in pairs. Family relationship information among these animals can be found in Supplementary Figure S1. Temperature (24 °C) and humidity (55%) conditions were controlled and a dawn/dusk-like 12 h-period was maintained. They were provided with a balanced diet and water ad libitum. All procedures were performed in accordance with the project and personal licenses held by the authors under the UK Animals (Scientific Procedures) Act 1986.
Cloning and Sequencing of the Marmoset SLC6A4 Repeat and Promoter Regions
A 2.4 kb-fragment spanning from −2.3 kb to the first exon of the SLC6A4 marmoset gene was cloned using a PCR-based strategy. Briefly, the exon 1 and the proximal promoter region were amplified using the following primers: SLC6A4-AF, SLC6A4-AR, SLC6A4-BF1, SLC6A4-BR2, SLC6A4-CF1, SLC6A4-CF2, and SLC6A4-CR (Supplementary Table S2). HotStarTaq DNA Polymerase (Qiagen, UK) was used in a MJ Research PTC-200 thermal cycler (conditions: activation 16 min at 94 °C, 50 cycles of 30 s at 94 °C, 30 s at TA1 °C (TA1=from 54 to 60 °C, according to each primer Tm) and 1 min at 72 °C; and termination 5 min at 72 °C). The 300 bp upstream region of the promoter was cloned using inverse PCR methods with NspI and the following primers: IPCR-F1 and IPCR-R1 (conditions: activation 16 min at 94 °C, 30 cycles of 30 s at 94 °C, 30 s at TA2 °C (TA2= from 74 to 58 °C step-down program) and 4 min at 72 °C; and termination 5 min at 72 °C). The PCR products were cloned using the TOPO TA cloning kit system (Invitrogen Ltd, UK). Inserts were sequenced by GeneService Ltd (Cambridge) and consensus sequence was annotated in Nucleotide database (http://www.ncbi.nlm.nih.gov/nuccore) with the accession number HG515029 (Supplementary Figure S2).
Determination of the SLC6A4 Transcription Start Site
Total RNA was extracted from marmoset Raphe nuclei by homogenization with TRI Reagent (Sigma) using the MagNA Lyser Instrument (Roche), followed by chloroform extraction and ethanol precipitation. RNA pellets were washed in 70% EtOH and resuspended in 50 μl of water and stored at −80 °C. To amplify the 5′ cDNA sequence of marmoset SLC6A4, a system for rapid amplification of cDNA ends (5′RACE) was used on total RNA extract using First ChoiceRLM-RACE kit (Ambion), following the manufacturer's protocol (see Supplementary Materials and Methods and Supplementary Table S2).
Repeat Region Sequence Alignment
Marmoset repeats were characterized by multiple sequence alignments. C. jacchus gene sequences were obtained from our own SLC6A4 clone sequencing and from the Ensembl database (www.ensembl.org). Human and other primate SLC6A4 sequences (Macaca mulatta and Pongo pygmaeus) were obtained from Ensembl database. The Gorilla gorilla (accession number AB061805.1) and Saguinus oedipus (accession number AB326308.1) were obtained from the NCBI website (www.ncbi.nlm.nih.gov/nuccore). To determine the first and last repeats of the marmoset region, a preliminary alignment was performed with the human, macaque, and marmoset sequences using Clustal Omega online (http://www.ebi.ac.uk/Tools/msa/clustalo/) (Supplementary Figure S3). The internal repeats were aligned manually for all primate species based on the Lesch et al (1997) repeat consensus sequence unit (Supplementary Figure S4).
Genotyping
Blood samples were taken from the femoral vein under sedation (0.1 ml i.m., Vetalar V 100 mg/ml; Pfizer, UK). A syringe was prefilled with ACD (acid–citrate–dextrose: 12.5 g/l Na citrate, 10 g/l D-glucose, 6.85 g/l citric acid) anticoagulant. Genomic DNA (gDNA) extraction was performed using Dneasy Blood & Tissue kit (Qiagen) (yield 2–6 μg per sample). Hair follicles were plucked from the animal's back. Samples were processed using the QIAamp DNA Micro kit for forensic casework samples (Qiagen) (yield 0.5–1.2 μg per sample). Primers were designed to flank the SLC6A4 repeat region: RPRF and RPRR (Supplementary Table S2). HotStarTaq Plus DNA Polymerase (Qiagen) was used in a BioRad C1000 thermal cycler (conditions: activation 15 min at 94 °C; 44 cycles of 30 s at 94 °C, 30 s at 55 °C and 1 min at 72 °C; and termination 5 min at 72 °C). The PCR product was visualized in an agarose gel, purified using the Mini Elute PCR Purification Kit (Qiagen) and sent for sequencing (Source BioScience, Cambridge, UK). Primers used for sequencing can be found in Supplementary Table S2.
Chimaerism
When studying the behavioral and pharmacological effects, which are dependent upon the brain, animals were genotyped using hair follicles, which showed the lowest level of chimaerism (Benirschke et al, 1962; Sasaki et al, 2009), and the same genotype as the brain (Supplementary Table S3). When measuring RNA in blood, samples were genotyped using genomic DNA extracted from the same blood tissue, taking into account the chimaerism. Captive (Bethesa, USA) and free-range (Rio Grande do Norte, Brazil) populations of marmosets were genotyped using hair follicles (Supplementary Table S4).
Expression Assay with qPCR
Total RNA was extracted using the QIAamp RNA Blood Mini kit (Qiagen). Samples were stored at −80 °C till use. cDNA synthesis and real time qPCR was performed using Brilliant II SYBR Green QRT-PCR Master Mix Kit, 1-Step (Agilent Technologies, UK) using primers spanning SLC6A4 exons 12–13: SLC6A4-F and SLC6A4-R (Supplementary Table S2). Porphobilinogen deaminase gene (PBGD) was used as the reference gene using primers spanning exons 13–14: PBGD-F and PBGD-R. All primer combinations were designed to span exon–exon boundaries. All reactions were performed in duplicate for each individual and controls (conditions: cDNA synthesis 30 min at 50 °C, activation step 10 min at 95 °C, 40 two-step cycles of denaturation 30 s at 95 °C and combined annealing/extension 1 min at 60 °C, final melting curves to check specificity of the product) in a DNA Engine Opticon 2 thermocycler (MJ Research). Results were compared with a gene-specific standard curve and normalized to the expression of PBGD (van Lelyveld et al, 2008).
Data analysis
Results were analyzed with one-way ANOVA followed by LSD post hoc contrasts (SPSS Statistics 22.0). Data are presented as mean±SEM. A p<0.05 was considered statistically significant.
Human Intruder Test
Anxious behavior was assessed using the human intruder test (HIT) (Agustín-Pavón et al, 2012). Marmosets were separated from their cage mate and restricted to the upper right-hand quadrant of their home cage (separated phase). After 8 min, an unfamiliar person entered the room. The intruder stood 40 cm from the front of the cage and stared at the marmoset, maintaining eye contact, for 2 min (intruder phase). Marmoset performance was recorded with a HD video camera (Genie CCTV-C5351/12, Korea) and a shotgun condenser microphone (Pulse-NPM702, Taiwan) with a preamplifier (Pulse-PLS00335, China). Once the intruder had left the room, recording went on for 5 min to observe the recovery of normal behavior. Several measures were scored off line by an experimenter blind to the genotype using the program JWatcher V1.0 (http://www.jwatcher.ucla.edu/): average distance (mean of the proportion of time spent in each of 15 locations with respect to the cage front); locomotion (proportion of time spent in translational movements between locations); jumps (number of jumps made to the front of the cage, towards the human intruder); bobbing event (number of rapid and repetitive side-to-side movements of the upper body while sitting and staring at the object of interest); and number of vocalizations (tsik, egg, tsik-egg and tse-like calls). For more details see Supplementary Materials and Methods and Supplementary Figure S5.
Data analysis
Distance and locomotion were scored and analyzed with repeated measures ANOVA for both separated and intruder phases. For the intruder phase, a principal component analysis (PCA) was performed on all eight variables, to extract the behavioral dimensions underlying the response to threat (Supplementary Table S5). The PC1 and PC2 derived from the PCA corresponded to Anxiety and Coping Strategy, respectively, based on the behavioral variable loadings (Agustín-Pavón et al, 2012). A two-way ANOVA was used to compare the two component scores (PC1 and PC2) between the SLC6A4 genotypes followed by LSD pairwise comparisons. One-way ANOVA (or non-parametric test when normality was not achieved) followed by post hoc pairwise comparisons were also performed for each individual variable. All statistical analyses were performed with SPSS Statistics 22.0. Data are presented as mean±SEM. A p<0.05 was considered statistically significant.
Pharmacological Manipulation on HIT
Twelve homozygous marmosets were included in this study (Supplementary Table S1). Animals were injected i.m. with citalopram (2.5 or 10 mg/kg) or vehicle (0.01 M PBS-HCl) 25 min before the intruder phase. We selected citalopram as it is a commonly used SSRI in the clinic and has been used when studying the impact of SLC6A4 VNTR on treatment efficacy (Keers et al, 2011). HIT procedures were exactly the same as described above. To avoid habituation to the human intruder across sessions the intruder wore different realistic rubber human masks each session (Greyland Film spol. s r.o., Czech Republic). The experimental design was a latin square randomized by sex, genotype, and masks. Treatment order was the same for all individuals (lower dose, higher dose, and vehicle) with 2 weeks between each session.
Data analysis
To calculate the PCA scores for each treatment, the variable values were standardized using the mean and standard deviation of the control condition (injection with vehicle) of the experimental subpopulation used in this study (N=12). These standardized values were then used in a PCA function derived from the previously performed PCA that included the whole population (N=52). PCA scores and variables were analyzed using repeated measure ANOVA with one between subject factor (haplotype) and one within subject factor (treatment), using SPSS Statistics 22.0. Data are presented as mean±SEM. A p<0.05 was considered statistically significant.
Results
New Sequence Polymorphisms in the Marmoset SLC6A4 Upstream Repeat Region are Linked to Gene Expression Levels
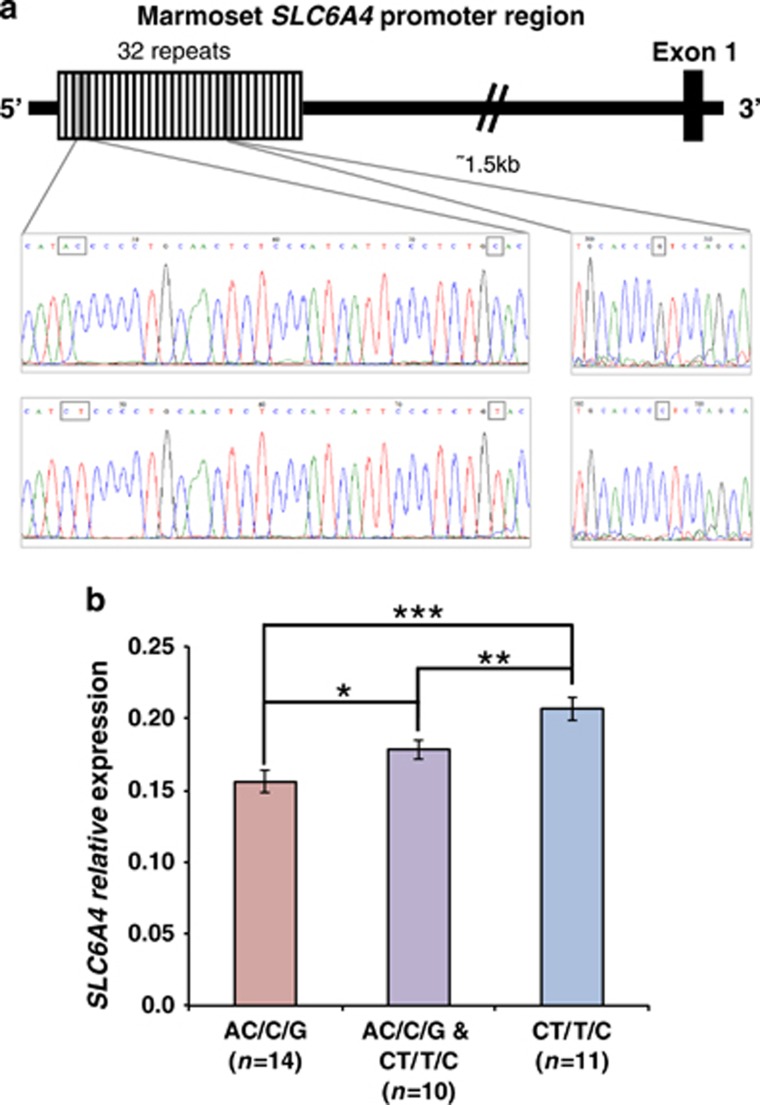
To characterize genetic variation at the marmoset SLC6A4 repeats, we cloned the promoter region from −2.3 kb to the first exon using a PCR-based method (Supplementary Table S2) and generated a consensus sequence (accession number HG515029) (Supplementary Figure S2). Using the 5′-RACE technique we experimentally identified the marmoset SLC6A4 transcription start site (highlighted in Supplementary Figure S2) and determined the repeat boundaries by performing a series of sequence alignments, revealing the presence of 32 repeats (Supplementary Figures S3 and S4). We found no variation in the number of tandem repeats in our colony (N=144 animals). Instead, we identified sequence polymorphisms: one dinucleotide polymorphism in the third repeat (−2053AC/CT) and two single-nucleotide polymorphisms in the fourth (−2022C/T) and the 23rd (−1592G/C) repeats (Figure 1a). The haplotypes AC/C/G and CT/T/C showed high frequencies in our colony (49.6 and 42.4%, respectively) while the CT/C/G haplotype was less common (8%) and no other combination was detected (Supplementary Table S1). These haplotypes were also found in 62 animals from the marmoset colony maintained at the National Institute of Neurological Disorders and Stroke (Bethesda, United States) and the AC/C/G and CT/T/C haplotypes were also present in 47 individuals from free-ranging marmoset families living at the FLONA of Nísia Floresta field station—ICMBio (Rio Grande do Norte, Brazil). Genotypic frequencies of these captive and free-ranging populations are provided in Supplementary Table S4.
Figure 1.
Genetic variation in the repeat upstream region of the marmoset SLC6A4 gene and its association with gene expression levels. (a) Schematic representation of the marmoset SLC6A4 promoter region showing 32 repeats. Third, fourth, and 23rd repeats containing the double and the two single-nucleotide polymorphisms, respectively, are shaded in grey. Representative examples of electropherograms of the most frequent haplotypes AC/C/G and CT/T/C are shown. (b) Relative expression values are shown (mean±SEM). One-way ANOVA F(2,32)=11.40, p<0.001 followed by post hoc LSD: AC/C/G vs CT/T/C p<0.001 (***), CT/T/C vs AC/C/G&CT/T/C p=0.020 (**), AC/C/G vs AC/C/G&CT/T/C p=0.047 (*).
Owing to the low frequency of the CT/C/G haplotype and subsequent low number of CT/C/G carriers in our colony, we focused our subsequent analysis on the most frequent haplotypes, AC/C/G and CT/T/C, the genotypic frequencies of which followed Hardy–Weinberg equilibrium (X(1)2=3.06) (Supplementary Table S1). Quantitative PCR studies have shown that SLC6A4 mRNA levels in human brain and lymphoblast cultures are similar (Gyawali et al, 2010) and more recent studies have proposed lymphocytes as an alternative peripheral model of central serotonin function (Marazziti et al, 2013). Thus, we assessed differential gene expression between SLC6A4 genotypes using marmoset lymphocytes (N=35). Marmosets homozygous for the AC/C/G haplotype showed a significant reduction of 25% in SLC6A4 gene expression compared with individuals homozygous for the CT/T/C variant, with samples from heterozygous marmosets expressing intermediate levels (Figure 1b). This effect was independent of sex or age (Supplementary Table S6).
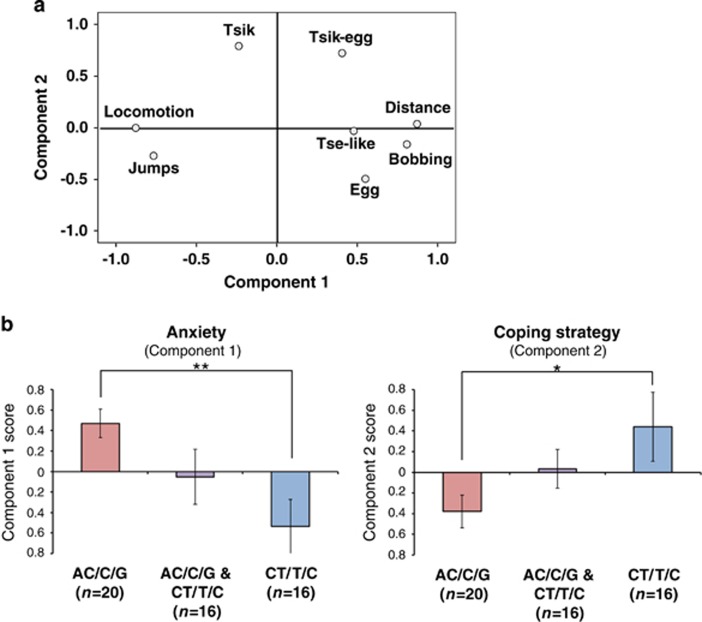
The Marmoset SLC6A4 Polymorphisms are Associated with Anxiety and Coping Strategy in Response to Threat
To investigate the contribution of the marmoset SLC6A4 polymorphisms to affective behavior, we used the HIT (N=52) (Agustín-Pavón et al, 2012). During the intruder phase, the marmosets spent more time at the back of the cage, maintaining some distance between them and the unfamiliar person (increased average distance) and moved around much less (reduced locomotion) compared with the separated phase (Table 1). The two principal components (PC1=anxiety and PC2=coping strategy) derived from the PCA explained together over 63% of the total variance (Figure 2a and Supplementary Table S5). When comparing individual PC scores between genotypes, marmosets homozygous for the low-expressing AC/C/G haplotype displayed significantly higher anxiety (higher PC1 scores, Figure 2b, left) and a more passive coping strategy (lower PC2 scores, Figure 2b, right) than the CT/T/C homozygotes, which showed the opposite behavioral pattern. This effect was independent of sex or age (Supplementary Table S6).
Table 1. Human Intruder Test Performance Summary.
|
SLC6A4
genotypes |
|||
|---|---|---|---|
| AC/C/G homozygous | AC/C/G and CT/T/C heterozygous | CT/T/C homozygous | |
| Separated phase | |||
| Locomotion (s)a | 10.04±1.75 | 13.44±2.45 | 17.66±2.53 |
| Distance (cm)b | 71.00±4.06 | 68.00±4.45 | 64.34±4.62 |
| Intruder phase | |||
| Locomotion (s)a | 5.88±0.91c | 8.17±2.05 | 13.27±2.43 |
| Distance (cm)b | 87.75±2.56 | 81.57±4.98 | 75.13±4.46 |
| Bobbing | 54.00±5.43d | 31.00±5.80 | 25.37±5.20 |
| Jumps | 0.90±0.25 | 1.44±0.62 | 2.31±0.72 |
| Egg calls | 21.55±3.31e | 11.87±2.95 | 8.94±2.46 |
| Tse-like calls | 8.35±2.62 | 12.37±±4.70 | 4.31±1.04 |
| Tsik calls | 0.65±0.36f | 3.67±1.54 | 6.87±2.59 |
| Tsik-Egg calls | 13.35±3.69 | 10.94±3.78 | 17.50±6.00 |
Mean±SEM for each variable during separated and human intruder phases.
Repeated measures ANOVA, square root transformed, separated vs test phases, significant main effect F(1,49)=10.10, p=0.003.
Repeated measures ANOVA, square transformed, separated vs test phases, significant main effect F(1,49)=36.41, p=0.000, p<0.001.
ANOVA, square root transformed, F(2,49)=4.20, p=0.021 followed by post hoc LSD: AC/C/G vs CT/T/C p=0.008, heterozygous vs CT/T/C p=0.036.
ANOVA, F(2,49)=8.03, p=0.001 followed by post hoc LSD: AC/C/G vs heterozygous p=0.004, AC/C/G vs CT/T/C p=0.001.
ANOVA, square root transformed, F(2,49)=5.95, p=0.005 followed by post hoc LSD: AC/C/G vs heterozygous p=0.013, AC/C/G vs CT/T/C p=0.002.
Kruskal–Wallis X2(2)=10.46, p=0.005 followed by Mann–Whitney AC/C/G vs CT/T/C (sig. two-tailed) p=0.001.
Figure 2.
The marmoset SLC6A4 polymorphisms are associated with individual differences in anxiety and coping strategy, assessed using the human intruder test (HIT). (a) Component plot in rotated space (variable loadings plot) illustrating the relationship of the individual behavioral measures with the two components derived from the principal component analysis (PCA). (b) Comparison of component behavioral scores (mean±SEM) derived from the PCA of the HIT performance. Left panel: Component 1 ‘Anxiety'. Right panel: Component 2 ‘Coping Strategy'. Two-way ANOVA, genotype × component interaction F(2,49)=9.36, p<0.001 (Power 97%), followed by post hoc LSD: AC/C/G vs CT/T/C, Component 1 p=0.002 (**), Component 2, p=0.014 (*).
The increased PC1 scores in AC/C/G reflected reduced locomotion, increased distance from the intruder, and high numbers of head and body bobbing, and alarm calls (Figure 2a and Table 1), which are behaviors corresponding to high anxiety (Agustín-Pavón et al, 2012; Carey et al, 1992). In contrast, the increased PC2 scores in CT/T/C homozygous were related to high numbers of mobbing vocalizations implicated in active coping in response to stress (Bezerra and Souto, 2008; Cross and Rogers, 2006) Supplementary Videos S1 (for homozygous AC/C/G) and S2 (for homozygous CT/T/C) show representative examples of these two distinct behavioral phenotypes.
The Marmoset SLC6A4 Polymorphisms Modulate the Effect of an Acute Dose of a Serotonin Reuptake Inhibitor on the Response to Threat
To determine whether the marmoset SLC6A4 polymorphisms influence the effect of SSRIs on affective responses, we compared HIT performance across homozygous genotypes after an acute dose of citalopram (N=12). Neither of the two acute doses of citalopram tested had any impact on the behaviors measured during the separated phase (Supplementary Table S7) nor on the PCA factor scores during the intruder phase (Supplementary Table S8), although PC1 scores were higher overall in the AC/C/G compared with the CT/T/C marmosets, replicating our previous finding in this subgroup. However, consistent with previous studies showing that distance from the human intruder is highly sensitive to anxiolytics (Carey et al, 1992), citalopram had a genotype-dependent effect specifically on average distance (Figure 3a and b). Specifically, there was a dose-dependent increase in average distance in high trait anxious AC/C/G marmosets, such that they spent more time away from the human intruder at the cage front, positioning themselves in the middle of the cage following the low dose (Figure 3c, middle panel) and at the back of the cage following the high dose (Figure 3c, right panel), indicative of heightened anxiety. In contrast, the CT/T/C homozygous marmosets exhibited the opposite behavioral pattern, moving closer to the intruder and thus reducing the average distance with increasing doses of citalopram, indicative of a reduction in anxiety (Figure 3d, right panel). This effect was independent of sex and age (Supplementary Table S6). Both genotypes showed reduced locomotion and numbers of jumps to the front in response to the acute citalopram (Supplementary Figure S6).
Figure 3.
SLC6A4 variant-specific anxiety response to an acute dose of a selective serotonin reuptake inhibitor, citalopram. Human intruder test (HIT) was used to assess anxiety levels in response to vehicle (V) and to a single dose of 2.5 mg/kg (D1) or 10 mg/kg (D2) citalopram, 25 min prior to the intruder phase. (a) Schematics of the home cage test quadrant in the HIT. High anxiety-related locations are shaded in red (high and rear) and low anxiety-related locations are shaded in blue (low and front). (b) Average distance. Repeated measures ANOVA, with within factor ‘treatment' and between factor ‘genotype'. Treatment × genotype interaction F(2)=4.214, p=0.030; followed by LSD pairwise comparisons. D1: AC/C/G vs CT/T/C p=0.017; D2: AC/C/G vs CT/T/C p=0.012; AC/C/G: V vs D2 p=0.012, and CT/T/C: V vs D2 p=0.031. (c, d) Proportion of time spent at different locations (mean±SEM): Front, middle and back, for each group of homozygous AC/C/G (c) and CT/T/C (d). Two-way repeated measures ANOVA with ‘location' (front, middle, back) and ‘treatment' (V, D1, D2) as within factors and ‘genotype' as between factor (AC/C/G, CT/T/C). Genotype × treatment × location interaction F(4)=6.530, p<0.001, followed by LSD post hoc comparisons. (c, middle panel) AC/C/G D1: front vs middle p=0.004; middle vs back p=0.027. (c, right panel) AC/C/G D2: front vs back p=0.001; middle vs back p=0.026. (d, right panel) CT/T/C D2: front vs back p=0.040. *p<0.05; **p<0.005.
Discussion
We report here, a new genetic and behavioral primate model highly relevant for the study of the underlying mechanisms of gene-affective behavior associations. We identify novel functional sequence polymorphisms within the marmoset SCL6A4 upstream repeat region and show their association with individual differences in gene expression and negative affective behavior. In addition, we reveal a genotype-specific behavioral effect of an acute dose of the SSRI citalopram, with individuals carrying the low-expressing haplotype showing a dose-dependent anxiogenic response, in contrast to the anxiolytic response in those carrying the high-expressing haplotype.
Genetic variation in the upstream repeat region of the SCL6A4 gene has been extensively investigated in both human and non-human primates (Inoue-Murayama et al, 2000; Lesch et al, 1997). In particular, a recent study in the marmoset showed no variation in the number of repeats; however, their sequence was incomplete (Pascale et al, 2012). Here, we have determined the full length of the marmoset repeat region with a total number of 32 repeats. Instead of a VNTR, we have identified three sequence polymorphisms within the repeat region that parallel the behavioral and gene expression effects associated with the human and macaque VNTRs. The importance of sequence variation in this region is highlighted by the presence of a single-nucleotide polymorphism within the human repeat polymorphic region that is also linked to SCL6A4 expression and psychiatric disorders (Hu et al, 2006). Moreover, the marmoset sequence polymorphisms reported here showed haplotype-specific gene expression levels that correspond with the expression changes associated with repeat length and sequence polymorphisms using human lymphocyte cell lines (Bennett et al, 2002; Hu et al, 2006; Lesch et al, 1996).
Evidence that the haplotypes AC/C/G, CT/T/C and CT/C/G are not restricted to our colony is provided by their detection within the marmoset-breeding colony at the National Institute of Neurological Disorders and Stroke. The former two haplotypes are also present in five free-ranging marmoset families living at the FLONA of Nísia Floresta field station—ICMBio. The failure to detect the low frequent CT/C/G haplotype suggests that it may not be present in wild populations.
Consistent with reports in humans and macaques that have linked the short alleles with emotionally vulnerable phenotypes and anxiety traits, the low-expressing haplotype in the marmoset (AC/C/G) was also associated with high anxiety. However, this contrasts with a study in macaques which detected no association between the SLC6A4 VNTR and ‘anxious temperament' using a non-eye contact HIT (Oler et al, 2010), although differential neural circuitry have been reported (Kalin et al, 2008). One possible explanation may be that the macaque and marmoset behavioral models are characterizing distinct trait anxiety phenotypes (Shackman et al, 2013; Shiba et al, 2014) that are differentially sensitive to SLC6A4 VNTR. This is particularly likely since these primate models measure different behaviors in response to the human intruder in two quite distinct contexts, in the home cage in case of the marmoset and in a separate isolated room in macaques.
In this study we have also revealed an effect of the SCL6A4 polymorphism on a second dimension of the affective response, namely coping strategy. The high-expressing haplotype CT/T/C was associated with an active coping strategy in response to threat. Although a role for SLC6A4 variation in coping strategy has not been previously reported, differences in passive and active coping styles have been related to altered serotonin release in the dorsal raphe nucleus in response to stress (Amat et al, 2005). Moreover, an active coping style has been associated with increased aggressive behavior and reduced brain serotonin levels (Koolhaas et al, 2010).
An important question arising from these findings is why a gene–behavioral association was revealed in the marmoset, apparently, relatively easily compared with the mixed findings in humans? The answer may lie in the more complex phenotype of human, compared with marmoset, emotional behavior that is influenced not only by environmental and genetic factors but also a rich life experience. The contribution of genetic variation to individual differences in human behavior is thus attenuated by these other influences as well as by compensatory and homeostatic mechanisms. This can be offset, however, by studying such behavior in a non-human primate species where the emotional behavior is a less complex phenotype and the enormous variation in environment and life experiences between individuals can be dramatically reduced by studying a purpose-bred primate colony in a controlled environmental setting, as described here. Thus, we would argue for these reasons the effects of genotype–behavior relationships have been more easily revealed. In addition, when studying emotional behavior it is also important to recognize the variable array of responses that are displayed by individual animals to threat. Accordingly, when characterizing the repertoire of behaviors that marmosets display in response to a human intruder we took into account the ethological behavior of a marmoset when facing predators in the natural environment (Ferrari and Ferrari, 1990; Stevenson and Poole, 1976) and under experimental conditions (Barros, 2002; Cilia and Piper, 1997). Moreover, by using PCA, we were able to reveal the genotype-specific, haplotype dose-dependent effect of SLC6A4 genetic variation on two dimensions of the threat response in the marmoset, emotionality and coping strategy.
Nonetheless, the putative contribution of each marmoset polymorphism to gene expression and behavior needs to be further investigated using, for example, in vitro controlled studies in marmoset cell culture (Shimada et al, 2012). In addition, the promoter region proximal to the transcription start site should also be explored for genetic variation possibly contributing to the phenotypes described here. Finally, we cannot rule out the possibility that a different genetic locus, co-segregating with the marmoset SLC6A4 polymorphisms, may be also contributing to the genotype–phenotype associations. The development of transgenic and genome editing technologies will enable us to confirm these genetic–behavior associations in this primate model.
In addition to the association with distinct behavioral traits, we showed that the marmoset SLC6A4 polymorphisms were also associated with individual differences in response to an SSRI. The AC/C/G high-trait anxious marmosets showed a dose-dependent anxiogenic response, as measured by average distance from the anxiety-provoking human. This result is of particular interest given a recent report showing increased fear reactivity after short-term citalopram administration in individuals high in neuroticism (Di Simplicio et al, 2014), an anxiety trait dimension associated with the SLC6A4 VNTR in humans (Lesch et al, 1996). Contrary to this anxiogenic effect, the low-trait anxious CT/T/C marmosets exhibited a reduction in anxiety, that is, reduced distance from the anxiety-provoking human, in response to the acute high dose of citalopram. This latter effect is consistent with the early changes in cognitive and neurobiological processing of emotional stimuli detected after short-term SSRI administration (Harmer et al, 2006; Murphy et al, 2009) that may account for the later improvement of the clinical symptoms observed in long allele carriers.
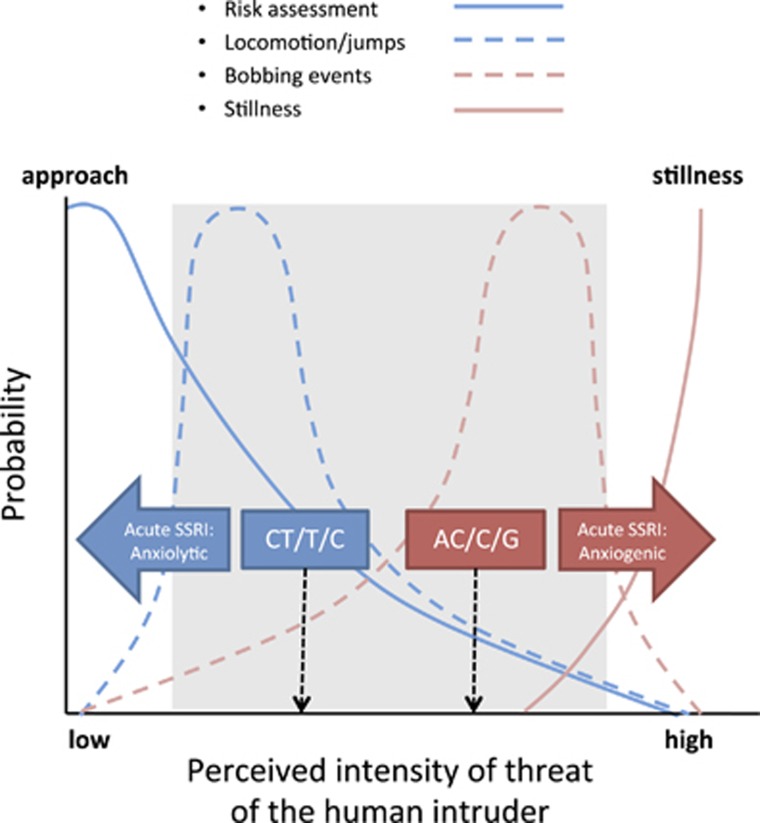
Although acute SSRI treatment produced genotype-dependent opposing effects on approach-avoidance behavior, its effects on other threat-related measurements were in the same direction, that is, reduction in both locomotion and jumps to the front. However, a reduction in the latter behaviors can be brought about by both increases and decreases in anxiety. The type of defense response adopted by an animal depends upon the result of the risk assessment performed, which takes into account the likelihood and proximity of the threat to determine whether to avoid immediately or to approach and gather more information (Blanchard et al, 2011; McNaughton and Corr, 2004). A high-threat risk assessment of the stimulus results in immediate avoidance behavior. In the current study, high anxious AC/C/G animals in response to an acute SSRI spent more time at the back of the cage (reduced risk assessment) and stayed very still, as reflected by reduced locomotion and jumps to the front (Figure 4). On the contrary, a low-threat risk assessment results in approach behavior and increased attention. This was the pattern of behavior observed in the low trait anxious CT/T/C group in response to acute SSRI, resulting incidentally in reduced locomotion and jumps to the front as they remained at the front for a large percentage of the time attending to the intruder.
Figure 4.
Schematic depicting the response to threat during the human intruder anxiety test. The graph indicates the probability of engaging in different behaviors in relation to the perceived intensity of threat. The normal range population described by our principal component analysis is shaded in gray. The AC/C/G group perceive the human intruder as a relatively high threat, showing high numbers of bobbing events, reduced locomotion and jumps, and primarily avoidance of the threat (reduced risk assessment). The CT/T/C group perceive the human intruder as a lower threat, showing fewer numbers of bobbing events, increased locomotion and jumps, and approach behavior to the threat (increased risk assessment/approach). Acute selective serotonin reuptake inhibitor (SSRI) in the AC/C/G group produces an anxiogenic effect with avoidance of the threat and further reduction in locomotion, jumps, and numbers of bobbing events, leading to an anxious state of stillness. In contrast, acute SSRI in the CT/T/C group induces an anxiolytic effect leading to increased approach behavior (increased inspection/risk assessment of the human intruder), with concomitant reduction in locomotion and jumps.
Together, these findings bridge the gap between the finding of reduced responsivity to chronic SSRI treatment in short-allele carriers with anxiety disorders (Perna et al, 2005) and depression (Keers et al, 2011; Porcelli et al, 2012) and the individual differences in sensitivity to the anxiogenic effect of acute SSRIs (Harmer and Cowen, 2013). Currently, there are no studies that have specifically considered the relationship between the acute behavioral effects of SSRIs and SLC6A4 genotypes with respect to treatment efficacy in patients with mood and anxiety disorders. This is an important issue though, since it is clinical practice to initiate SSRI treatment below therapeutic doses if anxiety symptoms are elicited in the early stages of treatment. By demonstrating genome-dependent differential effects of the SLC6A4 polymorphism on the behavioral effects of acute SSRIs in marmosets, the present study provides support for the proposal that individual differences in the acute SSRI-induced anxiogenic effect may predict subsequent treatment efficacy (Harmer and Cowen, 2013).
In conclusion, our findings link genetic functional polymorphisms to differential SLC6A4 expression and individual differences in complex affective primate behaviors, including sensitivity to pharmacotherapies. This new primate genetic model provides a unique tool for future investigations into the neurodevelopmental changes and physiological endophenotypes associated with serotonin genetic variation that leads to emotional vulnerability. Importantly, it also provides a model to study the neurochemical and neurobiological mechanisms of SSRI actions and their interactions with serotonin genetic variation. Finally, with the recent development of transgenic (Sasaki et al, 2009) and stem cell (Shimada et al, 2012) biotechnologies in marmosets, it will be possible in the future to use this model to further characterize the molecular mechanisms regulating the serotoninergic system and develop more efficient and specific molecular therapies for the treatments of mood and affective disorders.
Funding and disclosure
This work was supported by an MRC Programme (ACR;G0901884) and performed within the Behavioural and Clinical Neuroscience Institute, University of Cambridge, funded jointly by the Wellcome Trust and MRC. AMS was supported by a McDonnell Foundation grant (PIs: EPhelps, TW Robbins; co-investigators: ACR and JLeDoux; 22002015501) and currently supported by MRC; YS supported by the Long Term Student Support Program provided by Osaka University and the Ministry of Education, Culture, Sports, Science and Technology of Japan; HFC supported by MRC Career Development Award and ACFS/MI supported by grants from the MRC and Wellcome Trust. GC supported by the Behavioural and Clinical Neuroscience Institute, Cambridge, UK. EHSS was self-funded. The authors declare no conflict of interest.
Acknowledgments
We thank Prof Maria De Fatima Arruda (Laboratory of Behavioral Biology, Universidade Federal do Rio Grande do Norte, Brazil) for providing samples from free ranging marmosets and Dr Afonso C Silva (NINDS, NIH, Bethesda, USA) and Dr Annabelle (Mimi) M Belcher (Department of Psychiatry, University of Maryland School of Medicine) for providing samples from captive marmosets. We also thank Prof M Haggard and R Cardinal for advice on statistical analysis, and L O'Dea, T Jacques, Sufia Rahman, C Windle, and D Theobald for technical assistance.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Agustín-Pavón C, Braesicke K, Shiba Y, Santangelo AM, Mikheenko Y, Cockroft G et al (2012). Lesions of ventrolateral prefrontal or anterior orbitofrontal cortex in primates heighten negative emotion. Biol Psychiatry 72: 266–272. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF (2005). Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8: 365–371. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Hen R, Gingrich JA (2007). Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol 7: 8–17. [DOI] [PubMed] [Google Scholar]
- Barros M (2002). Reactions to potential predators in captive-born marmosets (Callithrix penicillata). Int J Primatol 23: 443–454. [Google Scholar]
- Benirschke K, Anderson JM, Brownhill LE (1962). Marrow chimerism in marmosets. Science 138: 513–515. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE et al (2002). Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry 7: 118–122. [DOI] [PubMed] [Google Scholar]
- Bezerra BM, Souto A (2008). Structure and usage of the vocal repertoire of Callithrix jacchus. Int J Primatol 29: 671–701. [Google Scholar]
- Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR (2008). Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology 33: 3221–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Pobbe R, Blanchard RJ (2011). Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev 35: 991–998. [DOI] [PubMed] [Google Scholar]
- Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ (2007). A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol 21: 684–690. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch K-P (2007). Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 10: 1103–1109. [DOI] [PubMed] [Google Scholar]
- Carey GJ, Costall B, Domeney AM, Jones DN, Naylor RJ (1992). Behavioural effects of anxiogenic agents in the common marmoset. Pharmacol Biochem Behav 42: 143–153. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE (2010). Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry 167: 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H et al (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301: 386–389. [DOI] [PubMed] [Google Scholar]
- Cilia J, Piper DC (1997). Marmoset conspecific confrontation: an ethologically-based model of anxiety. Pharmacol Biochem Behav 58: 85–91. [DOI] [PubMed] [Google Scholar]
- Cross N, Rogers LJ (2006). Mobbing vocalizations as a coping response in the common marmoset. Horm Behav 49: 237–245. [DOI] [PubMed] [Google Scholar]
- Ferrari SF, Ferrari MAL (1990). Predator avoidance behaviour in the buffy-headed marmoset, Callithrix flaviceps. Primates 31: 323–338. [Google Scholar]
- Flicek P, Amode MR, Barrell D, Beal K, Brent S, Chen Y et al (2011). Ensembl 2011. Nucleic Acids Res 39: D800–D806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS (2007). A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology 32: 225–231. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat Rev Neurosci 14: 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyawali S, Subaran R, Weissman MM, Hershkowitz D, McKenna MC, Talati A et al (2010). Association of a polyadenylation polymorphism in the serotonin transporter and panic disorder. Biol Psychiatry 67: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Cowen PJ (2013). “It”s the way that you look at it'—a cognitive neuropsychological account of SSRI action in depression. Philos Trans R Soc Lond B Biol Sci 368: 20120407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM (2006). Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59: 816–820. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D et al (1996). Allelic variation of human serotonin transporter gene expression. J Neurochem 66: 2621–2624. [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Dragoi L, Gompf J, Muhtz C, Demiralay C, Yassouridis A et al (2010). Decreased recognition of negative affect after selective serotonin reuptake inhibition is dependent on genotype. Psychiatry Res 177: 354–357. [DOI] [PubMed] [Google Scholar]
- Hu X-Z, Lipsky RH, Zhu G, Akhtar L a, Taubman J, Greenberg BD et al (2006). Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet 78: 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Murayama M, Niimi Y, Takenaka O, Okada K, Matsuzaki I, Ito S et al (2000). Allelic variation of the serotonin transporter gene polymorphic region in apes. Primates 41: 267–273. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Gianaros PJ, Greer PJ, Kerr DD, Liu S, Higley JD et al (2010). Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry 15: 512–522 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ (2008). The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry 13: 1021–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keers R, Uher R, Huezo-Diaz P, Smith R, Jaffee S, Rietschel M et al (2011). Interaction between serotonin transporter gene variants and life events predicts response to antidepressants in the GENDEP project. Pharmacogenomics J 11: 138–145. [DOI] [PubMed] [Google Scholar]
- Kent JM, Coplan JD, Gorman JM (1998). Clinical utility of the selective serotonin reuptake inhibitors in the spectrum of anxiety. Biol Psychiatry 44: 812–824. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B (2010). Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31: 307–321. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jørgensen TN, Sørensen L, Eriksen J, Loland CJ et al (2011). SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev 63: 585–640. [DOI] [PubMed] [Google Scholar]
- Lelyveld N, van, Linde J, Ter, Schipper M, Samsom M (2008). Serotonergic signalling in the stomach and duodenum of patients with gastroparesis. Neurogastroenterol Motil 20: 448–455. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S et al (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527–1531. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flügge G, Hinney A, Hebebrand J et al (1997). The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm 104: 1259–1266. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Landi P, Baroni S, Vanelli F, Bartolommei N, Picchetti M et al (2013). The role of platelet/lymphocyte serotonin transporter in depression and beyond. Curr Drug Targets 14: 522–530. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ (2004). A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev 28: 285–305. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Flint J (2004). Meta-analysis of genetic association studies. Trends Genet 20: 439–444. [DOI] [PubMed] [Google Scholar]
- Murphy SE, Yiend J, Lester KJ, Cowen PJ, Harmer CJ (2009). Short-term serotonergic but not noradrenergic antidepressant administration reduces attentional vigilance to threat in healthy volunteers. Int J Neuropsychopharmacol 12: 169–179. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ et al (2010). Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature 466: 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM (2009). Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J Neurosci 29: 6229–6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale E, Lucarelli M, Passarelli F, Butler RH, Tamellini A, Addessi E et al (2012). Monomorphic region of the serotonin transporter promoter gene in new world monkeys. Am J Primatol 74: 1028–1034. [DOI] [PubMed] [Google Scholar]
- Perna G, Favaron E, Bella D, Di, Bussi R, Bellodi L (2005). Antipanic efficacy of paroxetine and polymorphism within the promoter of the serotonin transporter gene. Neuropsychopharmacology 30: 2230–2235. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS et al (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8: 828–834. [DOI] [PubMed] [Google Scholar]
- Porcelli S, Fabbri C, Serretti A (2012). Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur Neuropsychopharmacol 22: 239–258. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M et al (2009). Generation of transgenic non-human primates with germline transmission. Nature 459: 523–527. [DOI] [PubMed] [Google Scholar]
- Sawada K, Hikishima K, Murayama AY, Okano HJ, Sasaki E, Okano H (2014). Fetal sulcation and gyrification in common marmosets (Callithrix jacchus) obtained by ex vivo magnetic resonance imaging. Neuroscience 257: 158–174. [DOI] [PubMed] [Google Scholar]
- Serretti A, Kato M, De Ronchi D, Kinoshita T (2007). Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with selective serotonin reuptake inhibitor efficacy in depressed patients. Mol Psychiatry 12: 247–257. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH (2013). Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc Natl Acad Sci USA 110: 6145–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Santangelo AM, Braesicke K, Agustín-Pavón C, Cockcroft G, Haggard M et al (2014). Individual differences in behavioral and cardiovascular reactivity to emotive stimuli and their relationship to cognitive flexibility in a primate model of trait anxiety. Front Behav Neurosci 8: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H, Okada Y, Ibata K, Ebise H, Ota S, Tomioka I et al (2012). Efficient derivation of multipotent neural stem/progenitor cells from non-human primate embryonic stem cells. PLoS One 7: e49469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simplicio M, Di, Norbury R, Reinecke A, Harmer CJ (2014). Paradoxical effects of short-term antidepressant treatment in fMRI emotional processing models in volunteers with high neuroticism. Psychol Med 44: 241–252. [DOI] [PubMed] [Google Scholar]
- Spinelli S, Schwandt ML, Lindell SG, Newman TK, Heilig M, Suomi SJ et al (2007). Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev Psychopathol 19: 977–987. [DOI] [PubMed] [Google Scholar]
- Stevenson MF, Poole TB (1976). An ethogram of the common marmoset (Calithrix jacchus jacchus): general behavioural repertoire. Anim Behav 24: 428–451. [DOI] [PubMed] [Google Scholar]
- Suri D, Teixeira CM, Cagliostro MKC, Mahadevia D, Ansorge MS (2015). Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology 40: 88–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.