Abstract
Water-soluble polyphosphazene polyacids, such as poly[di(carboxylatophenoxy)phosphazene] (PCPP), have been of significant interest due to their unique immunoadjuvant and vaccine delivery properties. We report that PCPP can spontaneously self-assemble into intermolecular complexes with common formulation excipients − polyethers in aqueous solutions at neutral pH through the establishment of hydrogen bonds. The resulting advanced PCPP delivery modalities can range from macromolecular assemblies at the nanoscale level to physically cross-linked hydrogels and the physical state can be modulated through varying polymer ratios and molecular weight of polyether. It has been demonstrated that such macromolecular complexes maintain protein-binding ability − a key characteristics of the delivery system. Importantly, the non-covalent modification of PCPP immunoadjuvant with polyethers introduces pH dependent membrane disruptive activity, which is not characteristic for PCPP itself, and is typically correlated to the ability of macromolecular carrier to facilitate endosomal escape. This can potentially affect the mechanism of immunoadjuvant action displayed by PCPP, afford means for its fine-tuning, as well as provide important insights for understanding the relationship between fundamental physico-chemical characteristics of polyphosphazene immunoadjuvants and their activity in vivo.
Keywords: Biochemistry, Immunology, Pharmaceutical science, Biotechnology, Bioengineering, Physical chemistry
1. Introduction
Polyphosphazene polyacids, a class of polyelectrolytes with an inorganic backbone, have gained significant attention primarily due to their unique immunoadjuvant activity in vivo. When administered with vaccine antigens, these ionic macromolecules display a dramatic immunopotentiating effect manifested in improved magnitude, quality, onset, and duration of immune responses, as well as in a substantial vaccine sparing capability [1, 2, 3, 4]. The lead polyphosphazene adjuvant − poly[di(carboxylatophenoxy)phosphazene], PCPP has been advanced into clinical trials and PCPP adjuvanted vaccines were reported to be safe and immunogenic in humans [5, 6]. From a physico-chemical perspective, the ability of this polymer to spontaneously bind antigenic proteins, as well as to interact with immunocompetent cells, which in turn leads to cell maturation and more effective antigen processing, plays a fundamental role in biological activity of PCPP [7, 8]. Further studies of this polymer explored its protein stabilization effect [9], hydrolytic degradation [10], microencapsulation [11], and microfabrication for transdermal delivery of vaccines [12]. However, there is only limited knowledge on the solution behavior of PCPP, its interactions with formulation components and excipients, and the effect of such interactions on biological activity of polyphosphazene.
It has been already observed that functional properties of PCPP can be significantly altered upon introduction of certain formulation excipients. For example, an addition of non-ionic surfactants or poly(ethylene oxide) (PEO) causes a dramatic enhancement of protein-stabilizing properties and modifies degradation profiles of polyphosphazenes in an aqueous environment at neutral pH [9, 10]. Although the importance of such additives and their effect on functional activity of polyphosphazene is clear, the mechanism of these phenomena is poorly understood. In this regard, the formation of interpolymer hydrogen bonds in such formulations can provide a reasonable explanation for prior findings. However, it is well established that the formation of interpolymer complexes depends on the degree of ionization of poly(carboxylic acid) and thus on environmental pH. Typically, such complexes are only formed in weakly or strongly acidic media and dissociate upon increase in pH, which limits their in vitro and in vivo utility [13, 14, 15].
In light of the above, it was important to explore if PCPP has a potential to self-assemble at neutral pH through the formation of hydrogen bonds. Moreover, it remained unclear if the formation of such complexes can result in the modulation of fundamental properties related to its immunomodulating activity. The present paper investigates interactions between immunopotentiating polyphosphazene polyelectrolyte, PCPP, and polyethers in aqueous solutions. Here, we report on the spontaneous self-assembly of polyphosphazene macromolecule and PEO at neutral pH into interpolymer complexes and we investigate some of their critical biological properties, such as protein binding affinity and pH dependent membrane activity in interactions with cells.
2. Materials and methods
2.1. Materials
Poly[di(carboxyatophenoxy)phosphazene], sodium salt (PCPP), molecular weight 800,000 g/mol, was synthesized as described previously [16]. Polyoxyethylene (PEO), poly(ethylene oxide) or poly(ethylene glycol), with molecular weights of 8,000, 300,000, 665,000 (Alfa Aesar, Ward Hill, MA), 35,000 (EMD Chemical Inc. Gibbstown, NJ), 100,000 g/mol (Sigma-Aldrich, St. Louis, MO), Albumin from Bovine Serum, FITC conjugate, FITC-BSA (Sigma-Aldrich, St. Louis, MO), sodium phosphate dibasic heptahydrate; sodium phosphate monobasic; potassium phosphate monobasic (Sigma, St Louis, MO); and Dulbecco’s Phosphate Buffered Saline, PBS (Sterile, without Calcium or Magnesium, Lonza, Walkersville, MD), 10% suspension of Porcine Red Blood Cells (RBC) in PBS (Innovative Technology Inc., Novi, MI) were used as received.
2.2. Preparation of PCPP-PEO and PCPP-PEO-protein formulations
Formulations were prepared by mixing aqueous solutions of PCPP and PEO in the appropriate media, typically 50 mM phosphate buffer or PBS, and vortexing them for 0.5 min. For protein containing formulations, solutions of Cytochrome C in aqueous PBS (pH 7.4) were added. Formulation components were filtered using 0.22 μm Millex syringe filters (EMD Millipore, Billerica, MA) before mixing.
2.3. Evaluation of membrane disruptive activity
The membrane disruptive activity of multifunctional carriers, which can be correlated to the ability of the carrier to facilitate endosomal escape and cytosolic delivery of pharmaceutical agent, was tested as described previously [17, 18, 19]. 100 μL of fresh Porcine Red Blood Cells (RBC) as a 10% suspension in phosphate buffered saline (PBS) (Innovative Technology Inc., Novi, MI) was re-suspended in 900 μL of PBS. 50 μL of re-suspended RBC was added to 950 μL of the PCPP-PEG or PCPP formulation in PBS at the appropriate pH, inverted several times for mixing, and incubated in a 37 °C for 60 min. Cells were then centrifuged at 14,000 rpm for 5 min, and the absorbance of the supernatant was then measured at 541 nm using Multiskan Spectrum microplate spectrophotometer (ThermoFisher Scientific, Waltham, MA). To determine 100% hemolysis, RBCs were suspended in distilled water and lysed by ultrasound (Branson Sonifier, Model 450). All hemolysis experiments were done in triplicate.
2.4. Dynamic light scattering (DLS) analysis
Hydrodynamic radii and Zeta potentials of polymers in aqueous solutions was carried out using ZetaSizer Nano series, ZEN3500, (Malvern Instruments Ltd., Worcestershire, UK). Formulation components were filtered using 0.22 μm Millex syringe filters (EMD Millipore, Billerica, MA) before mixing.
2.5. Analysis of protein content using high performance liquid chromatography
Size exclusion − high performance liquid chromatography (SEC-HPLC) of the formulation was performed using a Hitachi HPLC system with an L-2450 diode array detector (Hitachi LaChrom Elite system, Hitachi, San Jose, CA) equipped with an Ultrahydrogel Linear size exclusion column (Waters Corporation, Milford, MA) at 25 °C using 1x PBS as a mobile phase with a flow rate of 0.75 mL/min. Peak area and retention time of the peak attributed to Cytochrome C was monitored at 280 nm. The content of bound protein was determined by subtracting the amount of Cytochrome C detected by HPLC and the total amount of protein used in the formulation. The loading of protein was calculated as a weight ratio between bound Cytochrome C and a complex and the efficiency of protein binding was defined as a weight ratio between bound and total amount of protein added to the system.
2.6. Turbidimetric titration
Turbidimetric titration was performed at an ambient temperature by measuring the absorbance/transmittance (T) of the mixture at 420 nm (UV/Vis Spectrophotometer, HITACHI U-2810, Hitachi, San Jose, CA) using 2 ml cuvettes with 1 cm path length. Polymer solutions were filtered using 0.2 μm Millex filters (EMD Millipore, Billerica, MA) prior to titration. Solutions were vortexed for 15 seconds after addition of the titrant and monitored in the spectrophotometer until a stable turbidity reading (±0.1% T) was obtained.
2.7. Potentiometric titration
Potentiometric titration was conducted using Mettler-Toledo FiveEasy® FE20 pH Meter equipped with InLab® MicroPro pH electrode (Mettler-Toledo, Columbus, OH). Aliquots of PEO solutions were added to 0.5 mg/mL solutions of PCPP in deionized water in the presence or absence of 0.9% sodium chloride. After each addition of the titrant, the pH was allowed to equilibrate until stable reading was obtained, typically for approximately 2 minutes. The initial pH of PCPP solutions of PCPP was adjusted to pH 7.40–7.45 by adding 0.01 N solution of hydrochloric acid.
2.8. Fluorescent microscopy
Fluorescent microscopy examination was conducted using ECLIPSE 80I fluorescent microscope (Nikon Instruments, Melville, NY).
3. Results and discussion
3.1. PCPP-PEO complex formation at neutral pH: phase separation, coacervation, and macromolecular self-assembly in solutions
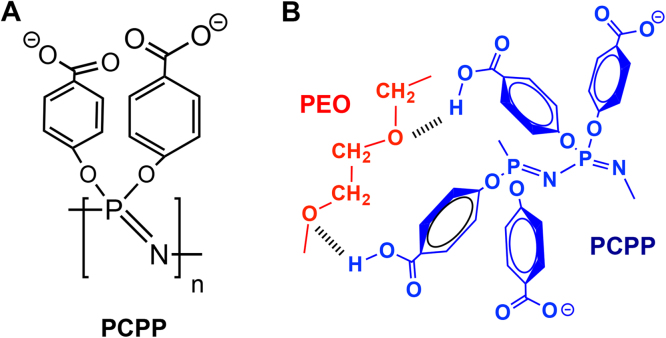
The ability of PCPP (Fig. 1A) to form interpolymer complexes with PEO at neutral pH through the establishment of hydrogen bonds (Fig. 1B) has been investigated using DLS, fluorescent microscopy, turbidimetric, and potentiometric titration methods. Method selection was dictated primarily by the solubility and physical state of the complex, which varied depending on the reaction conditions, ratio of reagents, and their molecular weights. Such diversity can potentially offer ample opportunities for fine-tuning formulations for various drug delivery applications.
Fig. 1.
Schematic presentation of (A) PCPP and (B) formation of PCPP-PEO complex through hydrogen bonds.
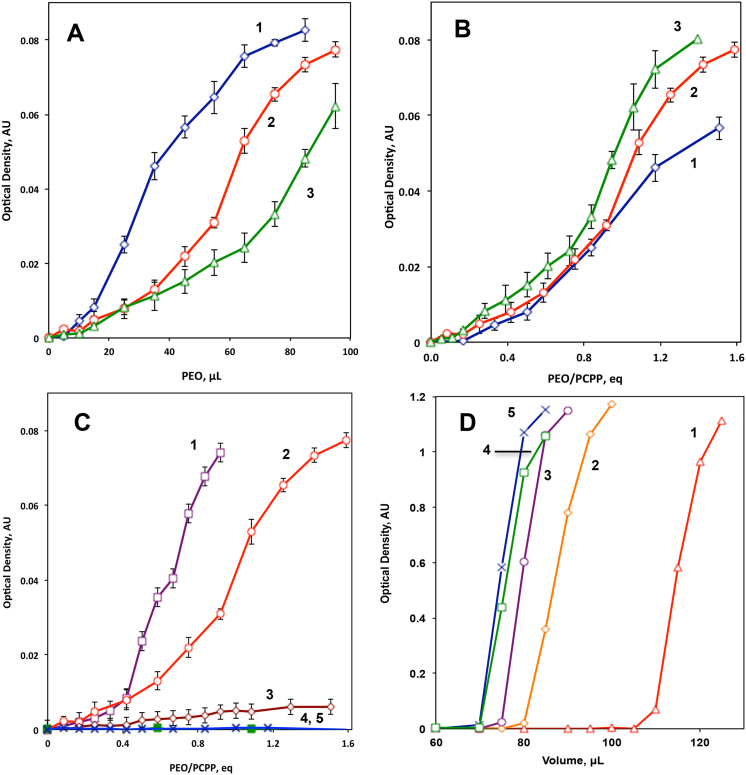
The formation of insoluble complexes in the system was studied using turbidimetric titration of PCPP solutions with PEO in phosphate buffer at pH 7.4. The addition of polyether with the molecular weight of 300,000 g/mol to PCPP solution led to distinct phase separation at various concentrations of PEO (Fig. 2A). The section of the curves corresponding to low ether to acid equivalent ratio (below unity in Fig. 2B) was characterized with a very low optical density, which suggests the presence of colloidal solution. It was found that the formation of insoluble complexes is only limited to polyethers of high molecular weight (Fig. 2C) with no phase separation detected for PEO with the molecular weight below 35,000 g/mol.
Fig. 2.
Turbidimetric titration of (A, B) PCPP with PEO at various PCPP concentrations − 0.025% (1), 0.05% (2) and 0.075% (3) (0.2% PEO of 300,000 g/mol); (C) PCPP with PEO of various molecular weights − 665,000 (1), 300,000 (2), 100,000 (3), 35,000 (4), and 8,000 (5) g/mol (0.05% PCPP, 0.25% PEO, 420 nm, 0.05 M phosphate buffer, pH 7.4), and (D) PCPP with sodium chloride in the presence of 0% (1), 0.002% (2), 0.005% (3) 0.01% (4), 0.02% (5) of PEO (0.05% PCPP, molecular weight of polyether − 35,000 g/mol, 10% sodium chloride, 0.05 M phosphate buffer, pH 7.4).
Soluble PCPP-PEO formulations were also titrated with aqueous sodium chloride, which, as was shown previously [16], can cause precipitation of PCPP. Phase separation was observed in all formulations, however distinct shifts in turbidimetric titration profiles were detected, which were proportional to the amount of PEO added to the system (Fig. 2D). Since precipitation of PCPP with sodium chloride can be explained by electrostatic screening of the repulsion interactions along the polymer chain [16], the differences in titration curves can be the result of varying charge density of PCPP as a function of PEO concentration. A reduction in the number of ionized carboxyl groups of PCPP in the presence of PEO may indicate the occurrence of interactions in soluble PCPP-PEO systems as well.
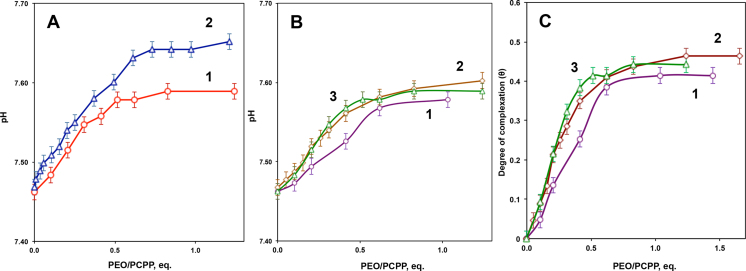
Further studies of soluble and colloidal systems were carried out using potentiometric titration in deionized water. Prior to titration, the pH of PCPP solution was adjusted to pH 7.4. The analysis was performed on the basis of well-established fact that hydrogen bond formation shifts polyacid dissociation equilibrium towards the non-dissociated form resulting in an increase of the solution pH [20, 21].
The potentiometric profiles demonstrate a pronounced increase in pH, which was dependent on the concentration of PCPP (Fig. 3A). This effect was observed for polyethers with molecular weights ranging between 30,000 and 300,000 (Fig. 3B), which demonstrates formation of water-soluble complexes even with low molecular weight polyethers. The change in pH for the investigated systems was leveled off upon achieving the ratio of ether to acid groups at approximately 1:2, which can be considered as an endpoint of titration [20, 21]. The degree of complex conversion (the fraction of bonded carboxylic groups) for PCPP titration with polyethers of various molecular weights levels off at approximately 0.4 (Fig. 3C) indicating that almost half of functional groups of PCPP can be involved in the complex formation.
Fig. 3.
Potentiometric titration of PCPP with PEO at (A) various concentrations of PCPP − 0.05% (1) and 0.2% (2) (molecular weight of PEO − 300,000 g/mol), and (B) various molecular weights of PEO − 8,000 (1), 100,000 (2), 300,000 (3) (1% PEO, deionized water, 25 °C). (C) Degree of complexation (θ) as a function of the ratio of functional groups in PEO and PCPP for various molecular weights − 8,000 (1), 100,000 (2), 300,000 g/mol (3) of PEO (0.05% PCPP, deionized water, 25 °C).
Formulations in the intermediate PEO:PCPP range, which displayed some turbidity in the absence of precipitation, were also studied using fluorescent microscopy. To visualize the particulates, a fluorescently labeled protein, FITC-BSA, was added to PCPP-PEO formulations. The particulates in micrometer size range were observed (Fig. 4), although with a somewhat broad size distribution. The primarily spherical shape of stained microparticulates, which can be seen in the Figure, suggests coacervation of hydrogen bond complexes under these conditions and some internalization of fluorescently labeled protein in a polymer rich phase. These findings may indicate a potential of PCPP-PEO system for protein encapsulation and drug delivery applications.
Fig. 4.
Physical states of PCPP-PEO system at various ratios between carboxyl and ether groups (pH 7.4, 0.05% of PCPP, molecular weight of PEO − 300,000 g/mol, 0.05 M phosphate buffer).
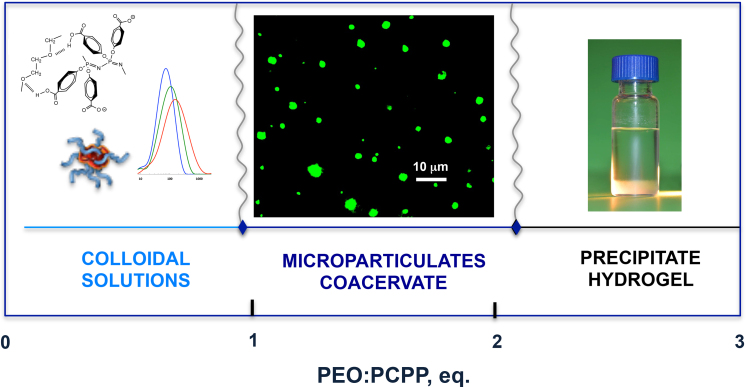
The above characterization results suggest the occurrence of three distinct physical states for PCPP-PEO systems in aqueous media at neutral pH depending on the concentrations and molecular weights of the polymers (Fig. 4). Clear or colloidal macromolecular solutions with self-assemblies in the 100–1000 nm size range are typical for low molecular weight polyethers or at a low PEO:PCPP ratio. Microparticles or coacervate systems can be obtained at intermediate ether-acid ratios for high molecular weight polyethers. Finally, the precipitate can be also formed in the systems with a large excess of high molecular weight PEO. Interestingly, a slightly transparent appearance of the precipitate suggests its high water content. The apparent hydrogel characteristics of the insoluble complex suggest physical cross-linking in the system, which can be further explored for biomaterials applications. These three physical states and their boundaries are schematically exemplified for the PEO with the molecular weight of 300,000 g/mol (Fig. 4) and designated on the basis of commonly accepted classification of particulate systems [22]. Although coacervates and hydrogels also present significant interest as delivery systems and biomaterials, our present studies were further focused on soluble and colloidal formulations due to well-established immunoadjuvant properties of water-soluble PCPP.
3.2. DLS studies of PCPP-PEO complex formation at near physiological conditions
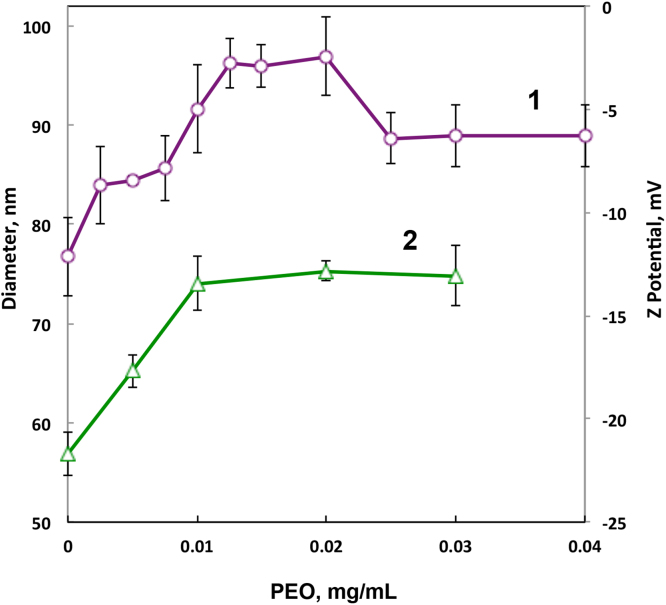
Formation of soluble PCPP-PEO complexes in aqueous solutions at neutral pH can potentially present new opportunities for formulating this important immunoadjuvant. Therefore, it was important to investigate if such macromolecular interactions take place under physiological conditions. To that extent, phosphate buffered saline, PBS is an important medium as its relatively high salt content can have a significant effect on the degree of dissociation and thereby on the formation of the complex. Fig. 5 shows DLS results for PCPP-PEO formulations at various molar ratios in PBS. The well-pronounced initial increase of the hydrodynamic diameter upon addition of PCPP correlates with the results for the complex formation obtained in phosphate buffer and indicates the apparent non-covalent ‘grafting’ of PEO chains on PCPP. Some decrease in the molecular size at higher PEO:PCPP ratios may be associated with moderate compaction of the assembly due to a larger number of non-covalent cross-links similar to those described previously for PCPP-protein complexes [7]. The formation of PCPP-PEO complex under physiological conditions is further supported by a decline in the Z-potential absolute value indicating the decrease in the surface concentration of negative charges of PCPP upon complexation.
Fig. 5.
Hydrodynamic diameter (1) and zeta potential (2) of PCPP-PEO complexes as a function of PEO concentration (0.0025% PCPP, PBS, molecular weight of PEO − 100,000 g/mol, pH 7.1).
3.3. Protein binding capability of PCPP-PEO complexes
The ability of PCPP to form complexes with proteins in aqueous solutions is one of key features, which is primarily responsible for a peculiar biological behavior of this macromolecular carrier. Non-covalent interactions with proteins have been correlated with immunoadjuvant activity, as well as ability to stabilize proteins in solution and during drying processes [7, 9]. Therefore, it was important to examine whatever PCPP-PEO complexes are capable of maintaining this vital function of the polyphosphazene carrier.
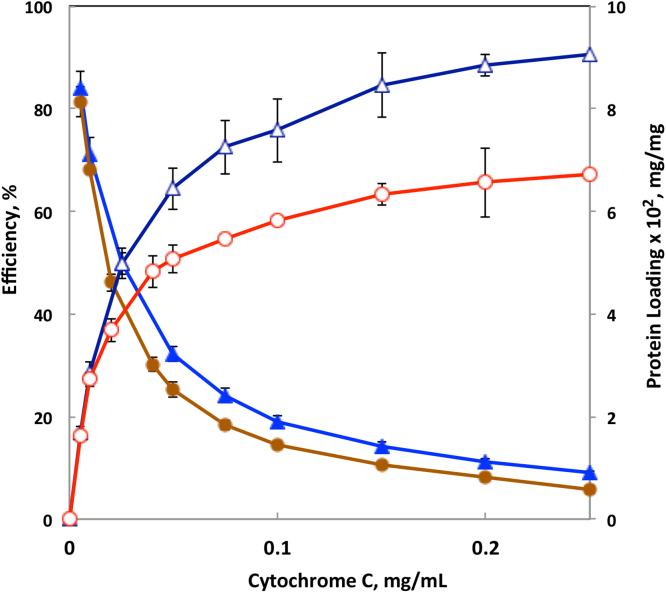
Cytochrome C, a protein capable of inducing apoptosis in cancer cells, was used as a model to investigate binding ability of soluble PCPP-PEO complexes. Fig. 6 shows the results of protein affinity studies in PBS (pH 7.4). The content of protein in the complex (loading) was calculated on the basis of unbound Cytochrome C in the system, which was determined using size exclusion HPLC with diode array detection. As seen from the Figure, PCPP-PEO complexes maintain the ability to bind protein and its loading grows with the increase in Cytochrome C concentration. The results are similar to those for PCPP, which are also presented in Fig. 6; however, the binding affinity of the complex is somewhat lower. This can be explained by the fact that some of the potentially reactive sites on PCPP are already consumed in the formation of PCPP-PEO complex. The efficiency of protein binding, calculated as a percent of bound protein in the formulation, also follows PCPP profile, though also at a somewhat lower level. Overall, although some decrease in the protein binding capacity of PCPP-PEO complexes compared to PCPP was observed, the system is definitely capable of serving as a protein delivery vehicle.
Fig. 6.
Cytochrome C loading and efficiency of protein binding for PCPP-PEO complexes (1, 2) and PCPP (3, 4) as a function of protein concentration (0.025% PCPP, 0.01% PEO, molecular weight of PEO − 100,000 g/mol, PBS, pH 7.4).
3.4. PCPP-PEO complexation introduces pH dependent membrane disruptive activity
One of prerequisites for the development of effective protein drug delivery vehicles is the ability of macromolecular carrier to facilitate endosomal escape and deliver payload into the cytosol [23, 24, 25]. This can be achieved through the pH dependent swelling or aggregation of macromolecule so that the transition occurs at the pH range corresponding to the pH of early endosomal environment, usually in the pH range of 5.8–6.6. The feature can be also of importance for vaccine delivery. Cross-presentation of antigens by dendritic cells plays central role in the induction of efficient immune responses, especially CD8+ T-cell responses, which are critical for the immunological control of tumors and infectious diseases. It has been shown that polymer carriers can enhance the efficacy of presentation of soluble protein antigen directing it into the major histocompatibility complex (MHC) class I antigen presentation pathway [26]. This has been attributed to the ability of polymeric nanoparticulates or liposomes to increase the amount of antigen that escapes from endosomes into the cytoplasm [26, 27, 28]. Some polyacids have a potential to facilitate endosomal escape of proteins through pH sensitive membrane active behavior, typically through the pH triggered conformational changes and formation of hydrophobic aggregates during the acidification in an early endosomal environment [17]. However, the membrane disruptive properties are typically displayed by hydrophobic macromolecules and their activity does not always correlate with the desirable physiological range.
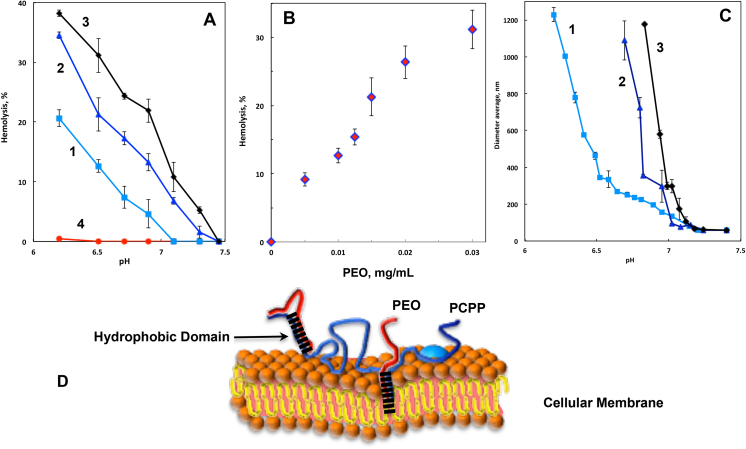
We investigated the ability of PCPP and its complexes with PEO to disrupt eukaryotic cell membranes. These studies were conducted using red blood cells (RBC) as endosomal membrane models [17, 29]. It was found that PCPP does not possess pH dependent membrane disruptive activity in the pH range 6.3–6.6 and is essentially insoluble at more acidic solutions (Fig. 7A). However, introduction of polyether and subsequent formation of complexes dramatically alters this fundamental property of PCPP delivery system. PCPP-PEO complexes display significant pH dependent membrane destabilizing activity with a pH threshold dependent on the content of polyether (Fig. 7A). At pH 6.5, the percent of hemolysis caused by PCPP-PEO complexes correlates well with the amount of PEO and increases as the concentration of polyether rises (Fig. 7B). It appears that membrane disruptive activity also follows the pH triggered growth in the size of aggregates, as detected by DLS (Fig. 7C). It can be assumed that interpolymer ether − acid bonds in the system result in the establishments of hydrophobic domains and such hydrophobic modification plays critical role in interactions with cellular membranes (Fig. 7D).
Fig. 7.
Hemolysis of red blood cells by (A) PCPP-PEO complexes as a function of pH at 0.01 (1), 0.02 (2), 0.03 (3) and 0 (4) mg/mL PEO (0.0025% PCPP, PBS); (B) PEO concentration (0.0025% PCPP, PBS, pH 6.5, molecular weight of PEO − 100,000 g/mol) and (C) hydrodynamic diameter of complexes as a function of pH at 0.01 (1), 0.02 (2) and 0.03 mg/mL PEO (3). (D) Schematic presentation of PCPP-PEO complex interaction with cellular membrane.
The results demonstrate that preparation of PCPP-PEO complexes introduces pH dependent membrane disruptive activity into this important immunoadjuvant and macromolecular delivery system providing new information for further advancement of mechanistic studies. Moreover, this important biological property can be easily modulated through variations in the polyether content of the system suggesting a potential dial-in pH switch approach, which can be applied more broadly to delivery of macromolecular drugs.
4. Conclusions
The importance of polyphosphazene polyacids, such as PCPP, as clinically proven potent immunoadjuvants, dictates the need for a better understanding of their mechanism of action and formulation behavior. One of the important aspects of this research is the investigation of macromolecular interactions between polyphosphazenes and formulation excipients. Although, it has been previously observed that the addition of hydrogen bonding compounds leads to significant changes in protein stabilizing properties and degradation profiles of these macromolecules, little was known on the physico-chemical basis of these phenomena.
The results of present studies provide unambiguous proof of PCPP-PEO complexation at near physiological conditions. Formation of intermolecular complexes between polyacids and polyethers, although well studied under acidic conditions, is highly unusual at neutral pH. Such unique behavior of PCPP can be probably explained by high ionic density of this macromolecule resulting in low dissociation constant and, as a consequence, the ability of un-dissociated acidic groups to form hydrogen bonds in aqueous solutions at neutral pH. Most importantly, various physical states of the complexes, which span from nanosized assemblies in solutions to coacervate systems and hydrogel matrices, provide opportunities for designing new drug delivery systems and biomaterials. Moreover, it was found that the formation of complexes does not prohibit the important biologically relevant properties of the carrier, such a protein binding, which was demonstrated for both soluble and coacervate formulations.
One of the most important findings of the present study is that a simple addition of polyethers to polyphosphazene carriers can introduce new biological properties in the system. The pH dependent membrane disruptive activity, which is not characteristic for PCPP itself, is clearly demonstrated for its interpolymer complexes. This can be especially important for the design of new intracellular macromolecular delivery systems, development of new approaches for modulating biological properties of the existing systems, and provide important insights for understanding the relationship between fundamental physico-chemical characteristics of polyphosphazene immunoadjuvants and their activity in vivo.
Declarations
Author contribution statement
Alexander K. Andrianov: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Alexander Marin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Thomas R. Fuerst: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Powell B.S., Andrianov A.K., Fusco P.C. Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes. Clin. Exp. Vaccine Res. 2015;4:23–45. doi: 10.7774/cevr.2015.4.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrianov A.K. Polyphosphazene vaccine delivery vehicles: state of development and perspectives. In: Andrianov A.K., editor. Polyphosphazenes for biomedical applications. Wiley; Hoboken: 2009. pp. 47–63. [Google Scholar]
- 3.Payne L.G., Jenkins S.A., Woods A.L., Grund E.M., Geribo W.E., Loebelenz J.R. Poly[di(carboxylatophenoxy)phosphazene] (PCPP) is a potent immunoadjuvant for an influenza vaccine. Vaccine. 1998;16:92–98. doi: 10.1016/s0264-410x(97)00149-7. [DOI] [PubMed] [Google Scholar]
- 4.Eng N.F., Garlapati S., Gerdts V., Potter A., Babiuk L.A., Mutwiri G.K. The potential of polyphosphazenes for delivery of vaccine antigens and immunotherapeutic agents. Curr. Drug Deliv. 2010;7:13–20. doi: 10.2174/156720110790396481. [DOI] [PubMed] [Google Scholar]
- 5.Bouveret Le Cam N.N., Ronco J., Francon A., Blondeau C., Fanget B. Adjuvants for influenza vaccine. Res. Immunol. 1998;149:19–23. [Google Scholar]
- 6.Thongcharoen P., Suriyanon V., Paris R.M., Khamboonruang C., de Souza M.S., Ratto-Kim S. A Phase 1/2Comparative Vaccine Trial of the Safety and Immunogenicity of a CRF01_AE (Subtype E) Candidate Vaccine: ALVAC-HIV (vCP1521) Prime With Oligomeric gp160 (92TH023/LAI-DID) or Bivalent gp120 (CM235/SF2) Boost. J. Acquir. Immune Defic. Syndr. 2007;46:48–55. doi: 10.1097/QAI.0b013e3181354bd7. [DOI] [PubMed] [Google Scholar]
- 7.Andrianov A.K., Marin A., Roberts B.E. Polyphosphazene polyelectrolytes: A link between the formation of noncovalent complexes with antigenic proteins and immunostimulating activity. Biomacromolecules. 2005;6:1375–1379. doi: 10.1021/bm049329t. [DOI] [PubMed] [Google Scholar]
- 8.Palmer C.D., Ninković J., Prokopowicz Z.M., Mancuso C.J., Marin A., Andrianov A.K. The effect of stable macromolecular complexes of ionic polyphosphazene on HIV Gag antigen and on activation of human dendritic cells and presentation to T-cells. Biomaterials. 2014;35:8876–8886. doi: 10.1016/j.biomaterials.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 9.Marin A., DeCollibus D.P., Andrianov A.K. Protein Stabilization in Aqueous Solutions of Polyphosphazene Polyelectrolyte and Non-Ionic Surfactants. Biomacromolecules. 2010;11:2268–2273. doi: 10.1021/bm100603p. [DOI] [PubMed] [Google Scholar]
- 10.DeCollibus D.P., Marin A., Andrianov A.K. Effect of Environmental Factors on Hydrolytic Degradation of Water-Soluble Polyphosphazene Polyelectrolyte in Aqueous Solutions. Biomacromolecules. 2010;48:487–492. doi: 10.1021/bm100395u. [DOI] [PubMed] [Google Scholar]
- 11.Andrianov A.K., Chen J. Polyphosphazene microspheres: Preparation by ionic complexation of phosphazene polyacids with spermine. J. Appl. Polym. Sci. 2006;101:414–419. [Google Scholar]
- 12.Andrianov A.K., DeCollibus D.P., Gillis H.A., Kha H.H., Marin A., Prausnitz M.R. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc. Natl. Acad. Sci. USA. 2009;106:18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khutoryanskiy V.V. Hydrogen-bonded interpolymer complexes as materials for pharmaceutical applications. Int. J. Pharm. 2007;334:15–26. doi: 10.1016/j.ijpharm.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 14.Such G.K., Johnston A.P.R., Caruso F. Engineered hydrogen-bonded polymer multilayers: from assembly to biomedical applications. Chem. Soc. Rev. 2011;40:19–29. doi: 10.1039/c0cs00001a. [DOI] [PubMed] [Google Scholar]
- 15.Kharlampieva E., Kozlovskaya V., Sukhishvili S.A. Layer-by-Layer Hydrogen-Bonded Polymer Films: From Fundamentals to Applications. Adv. Mater. 2009;21:3053–3065. [Google Scholar]
- 16.Andrianov A.K., Svirkin Y.Y., LeGolvan M.P. Synthesis and biologically relevant properties of polyphosphazene polyacids. Biomacromolecules. 2004;5:1999–2006. doi: 10.1021/bm049745d. [DOI] [PubMed] [Google Scholar]
- 17.Yessine M.-A., Leroux J.-C. Membrane-destabilizing polyanions: interaction with lipid bilayers and endosomal escape of biomacromolecules. Adv. Drug Deliv. Rev. 2004;56:999–1021. doi: 10.1016/j.addr.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Lackey C.A., Murthy N., Press O.W., Tirrell D.A., Hoffman A.S., Stayton P.S. Hemolytic activity of pH-responsive polymer-streptavidin bioconjugates. Bioconjug. Chem. 1999;10:401–405. doi: 10.1021/bc980109k. [DOI] [PubMed] [Google Scholar]
- 19.Rozema D.B., Ekena K., Lewis D.L., Loomis A.G., Wolff J.A. Endosomolysis by masking of a membrane-active agent (EMMA) for cytoplasmic release of macromolecules. Bioconjug. Chem. 2003;14:51–57. doi: 10.1021/bc0255945. [DOI] [PubMed] [Google Scholar]
- 20.Bokias G., Staikos G., Iliopoulos I., Audebert R. Interpolymer association between acrylic acid copolymers and polyethylene glycol: effects of the copolymer nature. Macromolecules. 1994;27:427–431. [Google Scholar]
- 21.Krupers M.J., Van Der Gaag F.J., Feijen J. Complexation of poly (ethylene oxide) with poly (acrylic acid-co-hydroxyethyl methacrylate)s. Eur. Polym. J. 1996;32:785–790. [Google Scholar]
- 22.Messner M., Kurkov S.V., Jansook P., Loftsson T. Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 2010;387:199–208. doi: 10.1016/j.ijpharm.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Breunig M., Bauer S., Göpferich A. Polymers and nanoparticles: intelligent tools for intracellular targeting? Eur. J. Pharm. Biopharm. 2008;68:112–128. doi: 10.1016/j.ejpb.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Varkouhi A.K., Scholte M., Storm G., Haisma H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 25.El-Sayed M.E., Hoffman A.S., Stayton P.S. Smart polymeric carriers for enhanced intracellular delivery of therapeutic macromolecules. Expert Opin. Biol. Ther. 2005;5:23–32. doi: 10.1517/14712598.5.1.23. [DOI] [PubMed] [Google Scholar]
- 26.Shen H., Ackerman A.L., Cody V., Giodini A., Hinson E.R., Cresswell P. Enhanced and prolonged cross‐presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stier E.M., Mandal M., Lee K.-D. Differential cytosolic delivery and presentation of antigen by listeriolysin O-liposomes to macrophages and dendritic cells. Mol. Pharm. 2005;2:74–82. doi: 10.1021/mp049896v. [DOI] [PubMed] [Google Scholar]
- 28.Lin M.-L., Zhan Y., Villadangos J.A., Lew A.M. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol. Cell Biol. 2008;86:353–362. doi: 10.1038/icb.2008.3. [DOI] [PubMed] [Google Scholar]
- 29.Yessine M.-A., Lafleur M., Meier C., Petereit H.-U., Leroux J.-C. Characterization of the membrane-destabilizing properties of different pH-sensitive methacrylic acid copolymers. BBA)-Biomembranes. 2003;1613:28–38. doi: 10.1016/s0005-2736(03)00137-8. [DOI] [PubMed] [Google Scholar]