Abstract
The integrin α6β4 (referred to as β4) is expressed in epithelial cells where it functions as a laminin receptor. Although in vitro studies have implicated β4 in the biology of mammary epithelial cells, its contribution to mammary gland development has not been settled. To address this problem, we generated and analyzed itgb4flox/flox MMTV-Cre− and itgb4flox/flox MMTV-Cre+ mice. The salient features of embryonic mammary tissue from itgb4flox/flox MMTV-Cre+ mice were significantly smaller mammary buds and increased apoptosis in the surrounding mesenchyme. Also, compared to control glands, the itgb4-deleted mammary buds lacked expression of the progenitor cell marker CK14 and they were unable to generate mammary glands upon transplantation into cleared fat pads of recipient mice. Analysis of mammary glands at puberty and during pregnancy revealed that itgb4-diminished mammary tissue was unable to elongate and undergo branching morphogenesis. Micro-dissection of epithelial cells in the mammary bud and of the surrounding mesenchyme revealed that loss of β4 resulted in a significant decrease in the expression of parathyroid hormone related protein (PTHrP) in epithelial cells and of target genes of the PTHrP receptor in mesenchymal cells. Given that the phenotype of the itgb4-deleted mammary tissue mimicked that of the PTHrP knockout, we hypothesized that β4 contributes to mammary gland development by sustaining PTHrP expression and enabling PTHrP signaling. Indeed, the inability of itgb4-deleted mammary buds to elongate was rescued by exogenous PTHrP. These data implicate a critical role for the β4 integrin in mammary gland development and provide a mechanism for this role.
Introduction
The α6β4 integrin (referred to as β4 because there is only 1 β4 integrin heterodimer) is a structural and functional anomaly among the integrin family of receptors (Hemler et al., 1989). This integrin, which is expressed primarily on the basal surface of most epithelia and in a few other cell types, is defined as an adhesion receptor for most of the known laminins (LMs) (Mercurio, 1995). A primary function of β4, revealed definitively by studies on knockout and transgenic mice, is to maintain the integrity of epithelia, especially the epidermis (Dowling et al., 1996; van der Neut et al., 1996). In the absence of β4 expression, skin morphogenesis appears normal but the epidermis detaches in response to mechanical stress, a condition that results in death shortly after birth (Dowling et al., 1996; van der Neut et al., 1996). This critical role for β4 derives from its ability to mediate the formation of stable and rigid adhesive structures termed hemidesmosomes (HDs) on the basal cell surface that link the intermediate filament cytoskeleton with laminins in the basement membrane (BM) (Borradori and Sonnenberg, 1999) (Green and Jones, 1996). Such structures are most pronounced in stratified epithelia, but rudimentary forms exist in most epithelia (Green and Jones, 1996).
Although the contribution of β4 to epithelial biology has been investigated for over two decades, its role in mammary gland biology is unclear and confusing. Normal mammary glands are composed of a branched system of excretory ducts and secretory alveoli. The epithelium of ducts and secretory alveoli consists of two layers of cells: a layer of luminal cells, which is responsible for the synthesis and secretion of milk components, and a layer of myoepithelial cells (Gjorevski and Nelson, 2011). β4 is expressed primarily in myoepithelial cells that anchor to the basement membrane and generate contractility for milk ejection. Seminal studies by Bissell and others using three-dimensional cultures revealed the importance of laminins in mammary morphogenesis and the contribution of the α6 integrins (α6β4 and α6β1) to this process (Nistico et al., 2014). Based on these findings and the essential role of β4 in other epithelia, the inference can be made that α6β4 is essential for mammary gland development and function. Surprisingly, however, it was reported that mammary gland development and differentiation are not dependent on the α6 integrins based on the analysis of knockout mice and transplantation studies (Klinowska et al., 2001). These findings are provocative because they indicate that mammary gland development and differentiation occur without the participation of the major α6 integrin laminin receptors, which are critical for the development of other epithelia. Given our long-standing interest in α6β4, we sought to re-investigate this issue making use of conditional β4 knockout mice.
Results and Discussion
Integrin β4 contributes to the development of the mammary bud
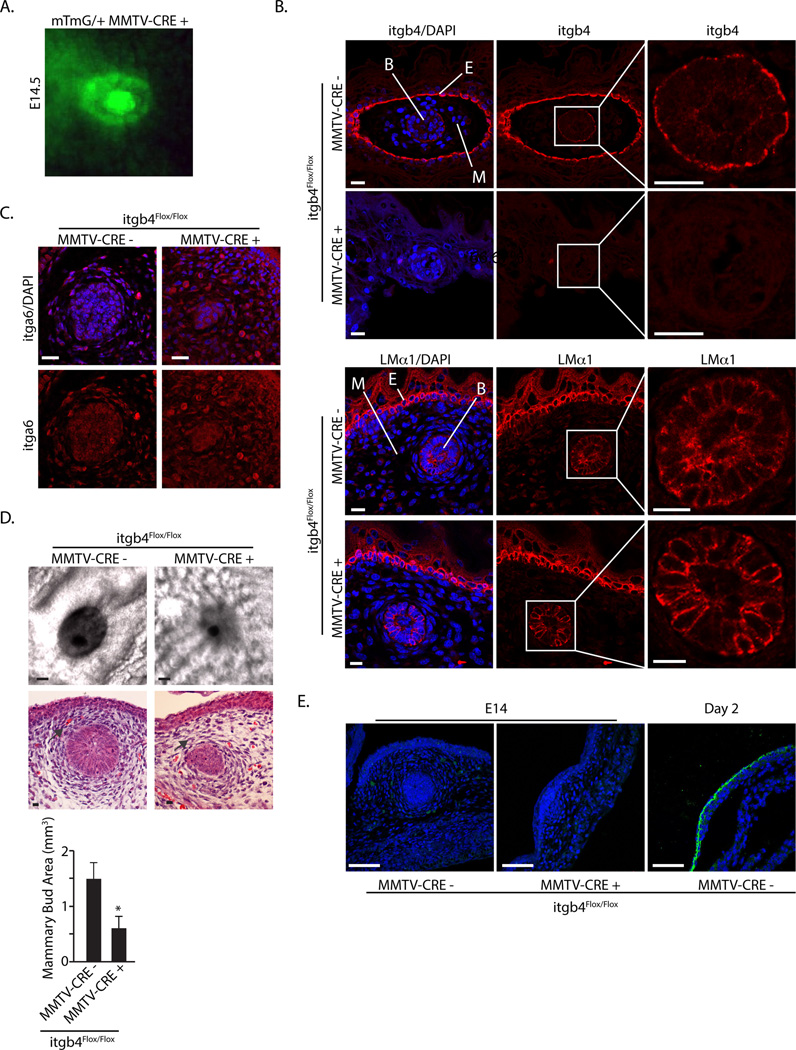
The nascent mammary bud begins to form at embryonic day 12.5 (E12.5) and it is clearly evident at E14.5 before it begins to elongate to form the rudimentary mammary gland (Watson and Khaled, 2008). Given that β4 is expressed in the mammary bud at E14.5 and its expression is sustained in the adult gland (Huang and Ip, 2001; Wansbury et al., 2011), we sought initially to evaluate the contribution of β4 to the development and elongation of the mammary bud. For this purpose, we crossed C57BL/6 itgb4flox/flox mice with C57BL/6 MMTV-Cre mice to generate itgb4flox/flox MMTV-Cre− and itgb4flox/flox MMTV-Cre+ mice. To verify that MMTV-driven Cre is expressed in the embryonic mammary bud, we crossed MMTV-Cre mice with Rosa26 mTmG mice and observed GFP expression in the E14.5 mammary buds (Fig. 1A).
Figure 1. Conditional deletion of itgb4 reduces size of embryonic mammary buds.
(A) E14.5 mammary tissue was microdissected from Rosa26mTmG−/+/MMTV-Cre mice (n=3) and photographed. Note the strong expression of GFP in the representative mammary bud shown. (B) E14.5 mammary buds from itgb4flox/flox MMTV-Cre− (n=7) and itgb4flox/flox MMTV-Cre+ mice (n=10) were stained with antibodies specific for the β4 integrin and LM α1 subunits, and counter-stained with DAPI. The mammary buds (boxes) were enlarged to highlight the staining of these structures. (C) E14.5 mammary buds from itgb4flox/flox MMTV-Cre− (n=5) and itgb4flox/flox MMTV-Cre+ (n=7) mice were stained with antibodies specific for the α6 integrin subunit, and counter-stained with DAPI. (D) Phase-contrast (upper panels) and H&E stained (lower panels) of E14.5 mammary buds from itgb4flox/flox MMTV-Cre− (n=24) and itgb4flox/flox MMTV-Cre+ mice (n=24). The bar graph represents the quantification of bud area based on the analysis of 24 mice for each genotype. Scale bars: 100 um.
Cre-mediated excision of itgb4 was verified by immunofluorescence. Integrin β4 staining was not detectable in itgb4-deleted mammary buds compared to control buds (Fig. 1B). MMTV-Cre is also expressed in the embryonic epidermis (Jiao et al., 2012) and, as expected, itgb4-deleted tissue exhibited a loss of β4 staining in the skin (Fig. 1B). Interestingly, however, integrin α6 expression was not diminished by deletion of β4 (Fig. 1C) indicating that the α6β1 integrin is expressed in the itgb4-deleted mammary buds. Laminin111 is expressed in the E14.5 mammary bud (Fig. 1B) and deletion of β4 had no effect on the expression or localization of this laminin suggesting that it may engage α6β1 in the β4-deleted buds. Laminin 332 expression, however, was not evident in E14.5 mammary buds (Fig. 1E).
We observed a significant decrease in the size of the itgb4flox/flox MMTV-Cre+ mammary buds compared to control buds (Fig. 1D). Also, there is less condensation in the surrounding mesenchyme in the itgb4-deleted compared to control tissue (Fig. 1D). Condensation is indicated by dense mammary mesenchyme arranged radially around the bud (Hens and Wysolmerski, 2005). The fact that β4 is not expressed in this mesenchyme (Fig. 1B) suggests that the observed effect on condensation is a consequence of the loss of β4 in the mammary bud (see below). The possibility that the observed decrease in the size of the mammary buds resulted from increased apoptosis upon loss of β4 was assessed by staining for cleaved caspase 3. Surprisingly, loss of β4 did not increase apoptosis in the mammary buds but it did in the surrounding mesenchyme (Fig. 2A). BrdU staining, however, revealed a significant decrease in proliferation in the itgb4-deleted buds (Fig. 2A). Decreased BrdU staining was also evident in the mesenchyme (Fig. 2A).
Figure 2. Effect of itgb4 deletion on the properties of mammary buds.
(A) E14.5 mammary buds from itgb4flox/flox MMTV-Cre− (n=7) and itgb4flox/flox MMTV-Cre+ (n=7) mice were stained for cleaved caspase 3 and counter-stained with DAPI (upper panels) or stained for BrdU (lower panel). Representative images are shown. (B) E14.5 mammary buds from itgb4flox/flox MMTV-Cre− (n=7) and itgb4flox/flox MMTV-Cre+ (n=7) mice were stained with an antibody specific for K14 and counter-stained with DAPI. Representative images are shown. (C) Control (n=25) and itgb4-deleted mammary buds (n=25) were isolated from E14.5 itgb4flox/flox MMTV-Cre− (n=4) and itgb4flox/flox MMTV-Cre+ (n=7) mice, respectively. Mammary buds were transplanted into cleared fat pads of virgin nu/nu mice (5 mice/genotype) and each mouse was implanted with 5 mammary buds. After 8 weeks, the recipient tissue was harvested and stained with H&E as shown. itgb4flox/flox MMTV-Cre− mammary buds expanded a simple ductal structure and increased proliferation. In contrast, itgb4flox/flox MMTV-Cre+ mammary buds were unable to generate new glands. Representative images are shown and the results were consistent for all transplants. Scale bars: 100 um.
The mammary bud is comprised of cytokeratin 14 (K14)-positive progenitor cells that give rise to both myoepithelial cells and luminal cells (Inman et al., 2015). To assess whether β4 contributes to sustaining this population, control and itgb4-deleted mammary buds were stained for K14. Indeed, loss of β4 resulted in a loss of K14 expression (Fig. 2B). This finding suggested that β4 is necessary for mammary gland formation. This possibility was evaluated by isolating control and itgb4-deleted E14.5 mammary buds and transplanting them into cleared mammary fat pads of virgin recipient mice. The recipients were maintained for 8 weeks prior to removal and analysis by H&E staining. Transplanted control mammary buds developed glandular structures. In contrast, no such structures were evident in the transplanted itgb4-deleted buds (Fig. 2C). This result also indicates that the observed effect of β4 loss on the mammary bud is intrinsic to the bud and not the result of Cre-mediated deletion of β4 in another tissue.
Subsequently, we analyzed the mammary glands from itgb4flox/flox MMTV-Cre− and itgb4flox/flox MMTV-Cre+ mice post partum to determine the consequent effect of β4 loss on mammary gland elongation and development. Given that newborn itgb4flox/flox MMTV-Cre+ mice die by 3 days after birth because of skin blistering, we analyzed the mammary structures collected from 2 day-old control and itgb4flox/flox MMTV-Cre+ mice by whole mounting and H&E staining. Control mice displayed normal mammary gland trees (Fig. 3A). In contrast, mammary buds were still evident in the itgb4flox/flox MMTV-Cre+ mice indicating that these buds were unable to elongate and develop in the absence of the β4 integrin (Fig. 3A). Given that the mammary gland is a skin appendage (Macias and Hinck, 2012), the possibility existed that the loss of β4 expression in the epidermis at E14.5 (Fig. 1B) and the post-natal skin blistering we observed in itgb4flox/flox MMTV-Cre+ mice affected mammary gland development. Interestingly, however, the epidermis appeared normal in both control (itgb4flox/wt MMTV-Cre−) and heterozygous (itgb4flox/Wt MMTV-Cre+) tissue (Fig. 3B). As expected, detachment of the dermal-epidermal layer was observed in itgb4flox/flox MMTV-Cre+ tissue from 2 day-old mice but not E14.5 embryos (Fig. 3B). The fact that the skin is normal in the heterozygous tissue and these mice do not die after birth enabled us to use them to compare mammary gland development at different stages. We observed that mammary gland development is impaired significantly in heterozygous glands compared to control glands despite the fact that their skin is normal. This effect was evident at puberty and in virgin glands, and it continued during pregnancy where a marked reduction in alveologenesis was observed (Fig. 3C). Lastly, expression of MMTV-Cre alone had no effect on mammary gland development (Fig. 3C) discounting the possibility that Cre expression itself contributed to the results observed.
Figure 3. Conditional deletion of itgb4 impedes post-partum mammary gland development.
(A) Whole-mount (left panels) and H&E stained (right panels) images of mammary glands isolated from 2 day old itgb4flox/flox MMTV-Cre− (n=10) and itgb4flox/flox MMTV-Cre+ (n=17) mice. The boxes on the whole-mount images depict the area of the mammary gland, which is absent in the itgb4flox/flox MMTV-Cre+ mice. (B) H&E stained images of the skin from 2 day old itgb4flox/wt MMTV-Cre− (n=10), itgb4flox/wt MMTV-Cre+ (n=10) and itgb4flox/flox MMTV-Cre+ mice (n=17). As shown, itgb4flox/flox MMTV-Cre+ mice exhibited detachment of the dermal-epidermal layer (arrow) that was not evident in itgb4flox/wt MMTV-Cre+ mice. (C) Whole-mount images of mammary glands at the stages indicated obtained from itgb4flox/Wt MMTV-Cre− (n=10), itgb4flox/Wt MMTV-Cre+ (n=10) and itgb4wt/wt MMTV-Cre+ mice (n=10). Scale bars: 200 um. Representative images are shown for all experiments.
PTHrP mediates the contribution of integrin β4 to mammary gland development
The effect of β4 loss on mammary bud development is reminiscent of the contribution of parathyroid hormone related protein (PTHrP) to mammary gland development (Dunbar and Wysolmerski, 1999). PTHrP and its receptor (PTH1R) are critical for the formation of the mammary mesenchyme and outgrowth of the nascent mammary ducts (Dunbar and Wysolmerski, 1999). PTHrP normally does not circulate, but rather is secreted locally to exert autocrine, paracrine and intracrine functions (Hens and Wysolmerski, 2005). PTHrP is produced and secreted by epithelial cells in the mammary bud and it interacts with PTH1R in surrounding mesenchymal cells to promote condensation and differentiation (Hens and Wysolmerski, 2005). As a result, mesenchymal cells secrete factors that maintain the mammary bud and enable it to elongate. To address the hypothesis that the effects of β4 on mammary bud development are mediated by PTHrP, we micro-dissected epithelial cells in the mammary bud and surrounding mesenchymal cells from E14.5 control and itgb4-deleted mammary tissue and analyzed the expression of key genes by qPCR. The results obtained revealed a significant decrease in the expression of PTHrP upon loss of β4 expression (Fig. 4A). A decrease in the expression of two key PTH1R target genes (estrogen receptor α and androgen receptor) (Dunbar et al., 1999) was seen in mesenchymal cells isolated from itgb4-deleted tissue (Fig. 4A). The observed decrease in PTHrP mRNA expression was substantiated by immunofluorescence staining. A dramatic reduction in PTHrP staining was seen in itgb4-deleted buds compared to control buds (Fig. 4B). PTHrP staining was diminished in the heterozygous (itgb4flox/Wt MMTV-Cre+) buds (Fig. 4B). The critical experiment that arises from these findings is whether exogenous PTHrP can rescue itgb4-deleted mammary buds and enable them to elongate. For this purpose, micro-dissected itgb4-deleted mammary buds and surrounding mesenchyme from E14.5 embryos were cultured in the presence or absence of PTHrP. After 9 days, control buds cultured in the absence of PTHrP produced elongated sprouts with several primary branches (Figure. 4C). The itgb4-deleted buds, in contrast, failed to elongate. However, these buds elongated in response to exogenous PTHrP (Fig. 4C).
Figure 4. The effect of itgb4 on mammary bud elongation is mediated by PTHrP.
(A) Epithelial and mesenchymal cells were microdissected from E14.5 itgb4flox/flox MMTV-Cre− (n=5) and itgb4flox/flox MMTV-Cre+ embryos (n=9) and RNA isolated from these cells was used to quantify the expression of the mRNAs indicated by qPCR. (B) E14.5 mammary buds from itgb4flox/flox MMTV-Cre− (n=5), itgb4flox/wt MMTV-Cre+ (n=5) and itgb4flox/flox MMTV-Cre+ mice were stained with an antibody specific for PTHrP and counter-stained with DAPI. Scale bar: 100um. (C) Mammary tissue (bud and surrounding mesenchyme) was isolated from E14.5 itgb4flox/flox MMTV-Cre− (n =11) and itgb4flox/flox MMTV-Cre+ (n=19) embryos, cultured in DMEM containing 10% FBS for 9 days with or without 10−7 M PTHrP and phase-contrast images were taken as shown. Representative images are shown and similar results were obtained with the other cultures. Scale bar: 200 um.
The data we obtained reveal the importance of the β4 integrin to mammary gland development, especially to the properties and function of the embryonic mammary bud. This conclusion differs from a previous study, which concluded that mammary gland development and differentiation occur in the absence of the α6 integrins, as well as the α3β1 integrin (Klinowska et al., 2001). Although we cannot explain the discrepancy between our results, it would be surprising if mammary gland development were independent of the major integrin laminin receptors given the wealth of data on the importance of these integrins and laminins to the biology of mammary epithelial cells (Lambert et al., 2012; Nistico et al., 2014). Indeed, a more recent study has implicated the α3β1 integrin in the contractile function of myoepithelial cells during lactation (Raymond et al., 2011). The potential contribution of the α6 integrins, however, had not been re-examined. In this direction, an important aspect of our data is that expression of the α6β1 integrin is sustained in itgb4-deleted mammary glands but it cannot suffice for the loss of α6β4. Clearly, the presence of the β4 subunit confers distinct properties that are essential for mammary gland development.
The impact of β4 loss on the mammary bud is striking as evidenced by a significant reduction in the size of the itgb4-deleted buds and the inability of these buds to develop into glandular structures post-partum. Although β4 is deleted in the epidermis by MMTV-Cre (Fig. 1), the data we obtained from heterozygous (itgβ4flox/Wt MMTV-Cre+) mice (Fig. 3) discount an indirect effect of β4 loss in the skin on the observed effect of itgb4-deletion on the mammary bud. Moreover, the fact that itgb4-deleted buds were unable to form mammary glands upon transplantation strengthens the hypothesis that the observed defects are intrinsic to the mammary bud and surrounding mesenchyme.
A major conclusion of this study is that integrin β4 regulation of PTHrP contributes to the ability of this integrin to regulate mammary bud development. Indeed, the ability of PTHrP to regulate mammary bud development through paracrine interactions with the mesenchyme is established (Hens and Wysolmerski, 2005), but the ability of a specific integrin to control this paracrine mechanism is novel. Interestingly, it was reported previously that PTHrP can regulate β4 expression based on in vitro data (Mula et al., 2010; Shen et al., 2007), a result contrary to our finding. It may be possible, however, that a feedback loop exists between β4 and PTHrP. Nonetheless, our data reveal an unexpected mechanism for the regulation of mammary bud development by the β4 integrin. It is also probable that this integrin contributes to embryonic mammary gland development by additional mechanisms.
Materials and Methods
Mouse breeding
All mouse experiments were conducted following a protocol approved by the Institution Animal Care and Use Committee (IACUC) at the University of Massachusetts Medical School. C57BL/6 MMTV-Cre (Line F) mice and B6.129 Rosamtmg/mtmg mice were obtained from Ingolf Bach and Jaime Rivera, respectively at the University of Massachusetts Medical School. The generation of itgb4flox/flox C57/B6 mice has been described previously (Nodari et al., 2008). Briefly, loxP sites were inserted between the 1.4 kb fragment that contains the itgb4 exon2 and the ATG start codon. Itgb4flox/flox mice were crossed with MMTV-Cre mice to generate itgb4flox/WtMMTV-Cre mice. itgb4flox/WtMMTV-Cre male and female mice were crossed and their offspring were analyzed. Rosamtmg/mtmg mice were crossed with MMTV-Cre mice to verify Cre expression in the embryonic mammary glands. We determined the sex of the mouse embryos by assaying for the presence of a unique sequence on the Y chromosome by PCR as described (Lambert et al., 2000).
Antibodies
Rat anti-mouse integrin β4 (346-11A-3C3) was a gift from Rita Falcioni (Regina Elena Cancer Institute, Rome, Italy). The following antibodies were purchased from the companies indicated: rat anti-mouse integrin α6 (GoH3, EMD Millipore, MA), cleaved caspase-3 (Cell Signaling Technology; cat# 9661S); rabbit anti-keratin 14 (Gene Tex; cat# GTX12975); rabbit anti-cre recombinase (Abcam; cat# ab 137240); mouse anti-PTHrP [a gift from Dr. Kremer (McGill University)]; donkey anti-rabbit conjugated TRITC, donkey anti-rabbit conjugated FITC and donkey anti-rat conjugated-TRITC (Jackson Immune Research Laboratories); goat anti-mouse conjugated 488, goat anti-mouse conjugated cy3 and goat anti-rabbit conjugated 488 (Invitrogen Life Sciences).
Tissue analysis
Histology, immunohistochemistry (IHC) and immunofluorescence (IF) staining were performed as described (Li et al., 2011). Mammary buds, mammary glands and skin samples were fixed overnight in 4% paraformaldehyde, embedded in paraffin (5 um sections) or whole-mounted, and stained with carmine alum red (Sigma).
Dissection and analysis of embryonic mammary buds
The dissection of embryo mammary buds was performed as described (Veltmaat, 2013). Briefly, females were evaluated for the presence of a vaginal plug after mating. The uterus was removed from pregnant mice at gestational day 14 under sterile conditions and transferred to sterile DPBS in a 100 mm petri dish and dissected using a Leica MZ10 F Stereo Microscope. In some experiments, epithelial and mesenchymal compartments were microdissected and used for qPCR analysis as described below. In other experiments, the intact mammary bud was removed and transplanted into the cleared mammary fat pads of 3–4 week old female nu/nu mice (Jackson Laboratories). Specifically, E14.5 mammary buds obtained from itgb4flox/floxMMTV-Cre+ and itgb4flox/floxMMTV-Cre− were transplanted into cleared fat pads of recipient mice (5 mice/genotype, 5 buds/mouse). The recipients were kept 8 weeks before sacrifice. The recipient glands were dissected and analyzed by H&E staining. In the last set of experiments, the entire bud was removed and placed on track-etched membrane cell culture inserts (0.4 mm pores; Becton Dickinson) in 12-well plates. These buds were cultured in DMEM media containing 10% FBS and antibiotics for 7–9 days with or without 10−7 M PTHrP (Sigma). The medium was changed every other day for the length of the experiment. The cultures were fixed in 4% paraformaldehyde and stained with carmine alum red (Sigma).
RNA isolation and qPCR
Total RNA was isolated using nucleoSpin (Clontech Laboratories Inc, Germany) from epithelial and mesenchymal cells that had been micro-dissected from E14.5 mammary buds. RT-PCR was performed by standard methods using the transcriptor first strand cDNA sysnthesis kit (Roche) according to the manufacturer's instructions. qPCR was performed using a SYBR green master mix (Applied Biosystems). We generated the following primer sets for SYBR-Green-based qRT-PCR: ITGB4-E2 forward primer 5-GAGGAGGAGGAGGATGG-3 and reverse primer 5–GGTCTCCAGGGAGGCTGGC-3, PTHLH forward primer 5-CATCAGCTACTGCATGACAAGG-3 and reverse primer 5-CTGTGTGGATCTCCGCCGCGAT-3; ITGA6 forward primer 5-CGGGATATGCCTCAAGGTTA-3 and reverse primer 5-TGCCTTTTTGAATTGGAAGG-3; AR forward primer 5-CAGCATTATTCCAGTGGATGG-3 and reverse primer 5-GGGCACTTGCACAGAGATG-3; ERα forward primer 5-GACCAGATGGTCAGTGCCTT-3 and reverse primer 5-ACTCGAGAAGGTGGACCTGA-3; GAPGH forward primer 5-AGGTCGGTGTGAACGGATTTG-3 and reverse primer 5-GGGGTCGTTGATGGCAACA-3. Samples were analyzed by using software DataAssist V3.01 (Applied Biosystems).
Acknowledgments
NIH Grants CA168464 (AMM) and R01 NS045630 (MLF) supported this work. We thank Hira Lal Goel and Ingolf Bach for providing valuable insight and advice.
Footnotes
Author Contributions: Jiarong Li performed all of the experiments and analyzed the data. Huayan Sun contributed to many of the experiments and data analysis. M. Laura Feltri provided the itgb4flox/flox mice. Arthur M. Mercurio supervised the project, data analysis and preparation of the manuscript.
References
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. The Journal of investigative dermatology. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Dowling J, Yu QC, Fuchs E. Beta4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. The Journal of cell biology. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar ME, Dann PR, Robinson GW, Hennighausen L, Zhang JP, Wysolmerski JJ. Parathyroid hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development. 1999;126:3485–3493. doi: 10.1242/dev.126.16.3485. [DOI] [PubMed] [Google Scholar]
- Dunbar ME, Wysolmerski JJ. Parathyroid hormone-related protein: a developmental regulatory molecule necessary for mammary gland development. Journal of mammary gland biology and neoplasia. 1999;4:21–34. doi: 10.1023/a:1018700502518. [DOI] [PubMed] [Google Scholar]
- Gjorevski N, Nelson CM. Integrated morphodynamic signalling of the mammary gland. Nature reviews. Molecular cell biology. 2011;12:581–593. doi: 10.1038/nrm3168. [DOI] [PubMed] [Google Scholar]
- Green KJ, Jones JC. Desmosomes and hemidesmosomes: structure and function of molecular components. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Crouse C, Sonnenberg A. Association of the VLA alpha 6 subunit with a novel protein. A possible alternative to the common VLA beta 1 subunit on certain cell lines. The Journal of biological chemistry. 1989;264:6529–6535. [PubMed] [Google Scholar]
- Hens JR, Wysolmerski JJ. Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast cancer research : BCR. 2005;7:220–224. doi: 10.1186/bcr1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RY, Ip MM. Differential expression of integrin mRNAs and proteins during normal rat mammary gland development and in carcinogenesis. Cell Tissue Res. 2001;303:69–80. doi: 10.1007/s004410000293. [DOI] [PubMed] [Google Scholar]
- Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development. 2015;142:1028–1042. doi: 10.1242/dev.087643. [DOI] [PubMed] [Google Scholar]
- Jiao B, Ma H, Shokhirev MN, Drung A, Yang Q, Shin J, Lu S, Byron M, Kalantry S, Mercurio AM, Lawrence JB, Hoffmann A, Bach I. Paternal RLIM/Rnf12 is a survival factor for milk-producing alveolar cells. Cell. 2012;149:630–641. doi: 10.1016/j.cell.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinowska TC, Alexander CM, Georges-Labouesse E, Van der Neut R, Kreidberg JA, Jones CJ, Sonnenberg A, Streuli CH. Epithelial development and differentiation in the mammary gland is not dependent on alpha 3 or alpha 6 integrin subunits. Developmental biology. 2001;233:449–467. doi: 10.1006/dbio.2001.0204. [DOI] [PubMed] [Google Scholar]
- Lambert AW, Ozturk S, Thiagalingam S. Integrin signaling in mammary epithelial cells and breast cancer. ISRN oncology. 20122012:493283. doi: 10.5402/2012/493283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JF, Benoit BO, Colvin GA, Carlson J, Delville Y, Quesenberry PJ. Quick sex determination of mouse fetuses. Journal of neuroscience methods. 2000;95:127–132. doi: 10.1016/s0165-0270(99)00157-0. [DOI] [PubMed] [Google Scholar]
- Li J, Karaplis AC, Huang DC, Siegel PM, Camirand A, Yang XF, Muller WJ, Kremer R. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. The Journal of clinical investigation. 2011;121:4655–4669. doi: 10.1172/JCI46134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1:533–557. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends in cell biology. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- Mula RV, Bhatia V, Falzon M. PTHrP promotes colon cancer cell migration and invasion in an integrin alpha6beta4-dependent manner through activation of Rac1. Cancer letters. 2010;298:119–127. doi: 10.1016/j.canlet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistico P, Di Modugno F, Spada S, Bissell MJ. beta1 and beta4 integrins: from breast development to clinical practice. Breast cancer research : BCR. 2014;16:459. doi: 10.1186/s13058-014-0459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Previtali SC, Dati G, Occhi S, Court FA, Colombelli C, Zambroni D, Dina G, Del Carro U, Campbell KP, Quattrini A, Wrabetz L, Feltri ML. Alpha6beta4 integrin and dystroglycan cooperate to stabilize the myelin sheath. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6714–6719. doi: 10.1523/JNEUROSCI.0326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K, Cagnet S, Kreft M, Janssen H, Sonnenberg A, Glukhova MA. Control of mammary myoepithelial cell contractile function by alpha3beta1 integrin signalling. The EMBO journal. 2011;30:1896–1906. doi: 10.1038/emboj.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Rychahou PG, Evers BM, Falzon M. PTHrP increases xenograft growth and promotes integrin alpha6beta4 expression and Akt activation in colon cancer. Cancer letters. 2007;258:241–252. doi: 10.1016/j.canlet.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nature genetics. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Veltmaat JM. Investigating molecular mechanisms of embryonic mammary gland development by bead-implantation in embryonic flank explant cultures - a protocol. Journal of mammary gland biology and neoplasia. 2013;18:247–252. doi: 10.1007/s10911-013-9297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansbury O, Mackay A, Kogata N, Mitsopoulos C, Kendrick H, Davidson K, Ruhrberg C, Reis-Filho JS, Smalley MJ, Zvelebil M, Howard BA. Transcriptome analysis of embryonic mammary cells reveals insights into mammary lineage establishment. Breast cancer research : BCR. 2011;13:R79. doi: 10.1186/bcr2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135:995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]