Abstract
Background
Relationships of thrombin generation (TG) with cardiovascular disease risk are under-evaluated in population-based cohorts.
Objectives
Evaluate the relationships of TG influenced by the contact and tissue factor coagulation pathways ex vivo with common SNPs and incident cardiovascular disease and stroke.
Patients/Methods
We measured peak TG (pTG) in baseline plasma samples of Cardiovascular Health Study participants (n=5,411), both with and without inhibitory anti-FXIa antibody (pTG/FXIa−). We evaluated their associations with ~50K SNPs using the IBCv2 genotyping array, and with incident cardiovascular disease and stroke events over a median follow-up of 13.2-years.
Results
The minor allele for a SNP in the coagulation factor XII gene (F12), rs1801020, was associated with lower pTG in European-Americans (β=−34.2 nM ± 3.5 nM; p=3.3×10−22; minor allele frequency (MAF) =0.23) and African-Americans (β=−31.1 nM ± 7.9 nM; p=9.0×10−5; MAF=0.42). Lower FXIa-independent pTG (pTG/FXIa−) was associated with the F12 rs1801020 minor allele, and higher pTG/FXIa− was associated with the ABO SNP rs657152 minor allele (β=16.3 nM; p=4.3×10−9; MAF=0.37). The risk factor-adjusted ischemic stroke hazard ratio (95% confidence interval) was 1.09 (1.01, 1.17; p=0.03) for pTG, 1.06 (0.98, 1.15; p=0.17) for pTG/FXIa−, and 1.11 (1.02, 1.21; p=0.02) for FXIa-dependent pTG (pTG/FXIa+), per 1-SD increment (n=834 ischemic strokes). In a multi-cohort candidate gene analysis, rs1801020 was not associated with incident ischemic stroke (β= −0.02; (SE=0.08); p=0.81).
Conclusions
These results support the importance of contact activation pathway-dependent TG as a risk factor for ischemic stroke and indicate the importance of F12 SNPs on TG ex vivo and in vivo.
Keywords: Cardiovascular Diseases, Epidemiology, Factor XIIa, Single Nucleotide Polymorphisms, Thrombin
Introduction
The proteolytic conversion of prothrombin to thrombin is the central event of hemostatic activation [1]. Thrombin plays an essential role in clot formation by converting soluble fibrinogen to insoluble fibrin and regulating coagulation by activating platelets, protein C, thrombin activatable fibrinolysis inhibitor, and coagulation factors V, VIII, and XI [1]. Therefore, inter-individual differences in thrombin generation (TG) may be important for hemorrhagic or thrombotic risk.
Increasing evidence suggests TG measurements are useful in assessing hemophilia [2, 3], von Willebrand disease [4], thrombophilia [5, 6], and venous thromboembolism (VTE) [7, 8]. Some evidence suggested higher TG was associated with increased risk of ischemic stroke [9] and myocardial infarction (MI) [10]. The relationships of TG with cardiovascular disease risk, however, are currently under-evaluated in population-based studies. The genetic associations with TG are also limited.
Currently, a majority of evidence suggests the tissue factor (TF)-VIIa-mediated coagulation pathway is the most relevant for hemostatic activation in vivo. Accumulating evidence implicates the coagulation factor XIIa (FXIIa)-dependent contact pathway in fibrin clot structure and stability [11, 12], and suggests elevated coagulation factor XI (FXI) may be a risk factor for VTE and ischemic stroke [13, 14]. The importance of the contact activation pathway towards cardiovascular disease risk remains uncertain.
We used a commercial assay to measure peak thrombin generation (pTG) in baseline plasma samples of European-American (EA) and African-American (AA) older adults from the Cardiovascular Health Study (CHS). The contributions of the contact and FVIIa-TF coagulation pathways were evaluated using an inhibitory anti-FXI antibody to measure pTG generated independent of FXIa and to calculate a pTG phenotype that was dependent on FXIa. We conducted SNP association scans with these pTG phenotypes, evaluated their cross-sectional relationships with cardiovascular disease risk factors, and examined their associations with incident cardiovascular disease and stroke during up to 22-years of follow-up.
Methods
Study Population
The CHS is a prospective population-based cohort study of cardiovascular disease risk factors in older American adults [15]. CHS recruited 5,201 men and women (original cohort, 1988-1989) aged 65 years and older from 4 U.S. field centers: Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA. An additional cohort of 687 primarily AA participants was recruited in 1992-1993, for a total cohort of 5,888 participants. Baseline evaluation included demographic information, standardized clinic examination, lifestyle and medical histories, fasting blood collection, and assessment of subclinical atherosclerosis by carotid ultrasound [16].
Additional experiments were performed using plasma samples from apparently healthy local volunteers. All subjects gave written informed consent for participation and all procedures were conducted under institutionally approved protocols at each center.
Thrombin Generation Assay
Citrated blood was collected at the CHS baseline exam using standardized protocols. All technicians completed centralized training and certification [17]. Timing between venipuncture and plasma processing (≤ 15 minutes) and between plasma processing and aliquot storage at −70°C (≤ 10 minutes) were standardized [17]. Plasma was processed and aliquoted at room temperature to limit the cold-activation of FVII [18].
Three different estimates of thrombin generation were evaluated: pTG was determined by the peak height on the thrombin generation curve. We defined pTG/FXIa− as pTG measured in the presence of inhibitory FXI antibody. The anti-FXI antibody completely inhibited FXIa [19], therefore this phenotype represented pTG minus any contribution of FXIa. pTG/FXIa+ was defined as the residual pTG after subtracting pTG/FXIa− from pTG (pTG/FXIa+ = pTG - pTG/FXIa−), and represented pTG that was primarily dependent on the contribution of FXIa.
TG was measured in three analytical batches by the Thrombin Generation Assay (TGA) (Technoclone, Vienna, Austria) according to the manufacturer’s instructions. The first batch included a random subset of 998 EA baselines samples; the second batch included 600 samples from the primarily AA cohort; and, the third batch included 3,813 remaining baseline samples. Assay standardization across batches was achieved using control samples provided with the TGA and with pooled normal plasma (George King Bio-Medical, Inc.; Overland Park, KS).
Plasma aliquots were thawed and equilibrated to room temperature. Technoclone Reagent C Low was used as a source of recombinant human TF (71.6 pM) and phospholipid micelles (3.2 μM) [7, 8]. Reagent C Low (10 uL) and Technoclone fluorogenic substrate (50 uL) were added to 40 uL plasma on a microtiter plate, resulting in a final tissue factor concentration of 7.2 pM. Fluorogenic substrate cleavage was measured immediately using a Polarstar Optima microplate reader (BMG Labtech; Ortenberg, Germany) analyzed in 1-minute intervals for 60 minutes at 37°C. Data were assessed by Technothrombin TGA evaluation software (Technoclone) using a thrombin calibration curve reference. All samples were run in duplicate. The assay coefficient of variation (CV) ranged from 10.9%−29.0% (Supporting Information Table S1). We evaluated the correlation between thrombin peak height and endogenous thrombin potential (ETP) using TGA measurements from local volunteers (n=47). The correlation was r= 0.80. Given the strong correlation, we did not analyze both parameters in CHS.
The pTG/FXIa− phenotype was evaluated in 4,340 re-frozen and thawed samples from Batches 2 (n=575) and 3 (n=3,765) by the addition of a monoclonal inhibitory anti-human FXI antibody to the plasma during the TG assay (Haematologic Technologies, Inc.; Essex Junction, VT). No samples were remaining from Batch 1 (n=998) to allow experiments with the anti-FXI antibody. Anti-FXI antibody was diluted in TGA buffer (Hepes-NaCl buffer containing 1.0% BSA; Technoclone) and added to 100 µL of plasma at a final concentration of 0.1 mg/mL. The CV of the assay with the addition of anti-FXI antibody ranged from 32.5%−66.0% (Supporting Information Table S1).
In additional experiments performed in plasma samples from local volunteers (n=47), FXIIa was inhibited by the addition of corn trypsin inhibitor (CTI) (50 µg/mL) (Haematologic Technologies, Inc.) [20] to the TG assay as described above for anti-FXI antibody. CTI was added alone or together with anti-FXI antibody. An equivalent volume of TGA buffer without inhibitors was used as a control.
Blood was also collected into citrate venipuncture tubes containing 50 µg/mL CTI (Haematologic Technologies, Inc.) and into citrate tubes with a manual addition of CTI (50 µg/mL) performed 30-seconds post-draw (n=8). CHS venipuncture and sample processing protocols were followed for all experiments with local volunteers.
Cardiovascular Disease-Related Biomarkers and Genotyping
Biomarkers of inflammation and coagulation were measured at baseline [17, 21-24]. Fibrinogen was measured in a BBL fibrometer (Becton Dickinson, Cockeysville, MD), CV 3.4%; FVIIc and FVIIIc were measured on the Coag-A-Mate X2 (Organon-Teknika, Durham, NC), CVs 5.9% and 10.4%. Interleukin-6 (IL-6) (CV 6.3%), C-reactive protein (CRP) (CV 8.9%), and D-dimer (CV 7.0%) were measured by ELISA using commercial (Quantikine IL-6, R&D Systems, Minneapolis, MN, USA) and in-house assays [21, 22, 24], respectively. IL-6 and D-dimer were measured only in subjects enrolled in a case-cohort sub-study (n=2,454). FVIIa was measured in n=3,486 citrated plasma samples collected at the 1992-1993 (CHS year 5) clinical exam using the Staclot VIIa-rTF assay (Diagnostica Stago, Inc.) (inter-assay CV 8.7%). Hematocrit, hemoglobin, and platelet count, were measured on automated instruments at local laboratories.
Genotyping was performed on 3,673 EA and 676 AA CHS participants who provided informed consent for participation in DNA studies using the ITMAT-Broad-CARe genotyping array version 2 (IBCv2) (Illumina; San Diego, CA) containing ~50,000 SNPs (~2,100 loci) selected for their relevance to cardiovascular, metabolic, and inflammatory syndromes [25]. Individuals were excluded due to inadequate DNA samples or missing TGA data. SNPs were excluded when monomorphic, the call rate was <95%, or when significant departures from expected Hardy-Weinberg equilibrium (HWE) genotype proportions were observed (p<10–5 in EAs). Given the genetic admixture in AAs, there was no HWE filter used for these samples. After these exclusions were applied, data remained on 42,011 SNPs.
Event Ascertainment and Definitions
Incident cardiovascular disease and stroke events were reported during semiannual telephone contacts by participant or proxies or at clinical visits. The primary clinical endpoints for this study were: incident non-medical-procedure-related fatal or nonfatal MI; incident fatal or nonfatal ischemic stroke; incident fatal or nonfatal coronary heart disease (CHD; defined as MI, coronary artery angioplasty or bypass grafting, or angina); and cardiovascular disease- or stroke-related mortality. Medical records were obtained to confirm the diagnosis, and events were adjudicated by a physician review panel [26, 27]. For suspected stroke events, information was collected from the participant or proxy, medical records, the participant's physician, and CT and/or MRI scans when available. Final adjudication was performed by vascular neurologists at a consensus conference using all available information [28]. For this analysis, hemorrhagic strokes (n=114) and strokes of unknown subtype (n=73) were excluded. Censoring date was defined as death, loss to follow-up, study drop out, or event date, occurring through December 31, 2011.
Statistical Analysis
Cross-sectional relationships were evaluated using linear regression with pTG phenotypes modeled as the outcome variable, adjusted for age, sex, and race. Independent variables were analyzed per standard deviation (SD) increment higher. Triglycerides, CRP, IL-6, and D-dimer had a non-normal distribution and were natural log-transformed. To correct for multiple testing, results were considered statistically significant if p<0.005. T-tests were used to compare unadjusted pTG means by sex.
Associations of pTG phenotypes and IBCv2 SNPs were performed using linear regression, implemented in PLINK [29], stratified by race with covariate adjustment for age, sex, clinic, and TGA analytical batch (EAs) or the first 10 eigenvectors derived from principal components analysis (AAs). SNPs were analyzed under an additive model. Results were considered statistically significant if p≤ 2.2×10−6 [30]. To evaluate whether FVIIa mediated any of the association of rs1801020 with pTG, separate linear regression models were analyzed with FVIIa included as a covariate (modeled per 1-SD increment higher).
Relationships of pTG phenotypes with incident cardiovascular disease, stroke, and cardiovascular- or cerebrovascular-related mortality were evaluated using Cox proportional hazards ratios, modeled per SD increment higher or by quartiles of the distribution. Participants with adjudicated disease at baseline relevant for each outcome were excluded (n=516 participants with MI; n=223 with stroke, and n=1045 with CHD at baseline). Demographic-adjusted models included age, sex, and race. Risk factor-adjusted models included age, sex, race, smoking status, diabetes status (defined as the use of insulin, oral hypoglycemic medications, or a fasting glucose level 126 ≥ mg/dL), hypertension status (defined as systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or current use of antihypertensive medication), systolic blood pressure, and LDL-cholesterol. Mortality models included adjustment for prevalent MI, stroke, and CHD at baseline.
Genetic Analyses in GAIT-2 and CHARGE
SNPs identified as statistically significant in CHS were genotyped in the Genetic Analysis of Idiopathic Thrombophilia (GAIT)-2 study cohort [31] and evaluated with pTG, ETP, TG lag time, activated partial thromboplastin time (aPTT), FXIIc, FXIc, and FIXc using linear regression. Thrombin generation in GAIT-2 was measured in plasma as described by Hemker et al. [32] using the Fluoroskan Ascent assay (ThermoLab systems, Helsinki, Finland) with 5 pM TF and 4 µM phospholipids.
A candidate gene look-up of rs1801020 with ischemic stroke risk was assessed within the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium [33, 34]. Genomewide association data generated from four prospective cohorts, including CHS, was used to impute to the 2.5 million nonmonomorphic, autosomal SNPs described in the HapMap’s Centre d’Etude du Polymorphisme Humain collection panel among a discovery sample of 19,602 EA participants. Analyses were performed using linear regression adjusted for age and sex.
Results
Associations of peak thrombin generation (pTG) with cardiovascular disease risk factors and common single nucleotide polymorphisms (SNPs)
Baseline characteristics of CHS participants are summarized in Supporting Information Table S2. pTG values (n=5,411) were approximately normally distributed with a mean (SD) of 495 nM (142 nM) in the overall study population. pTG was higher in women than men, and among participants with younger age. The unadjusted mean (SD) pTG values in women was 500 nM (142 nM) and 488 nM (142 nM) in men (p=0.003). In demographic-adjusted models, pTG was positively associated with BMI, total- and LDL-cholesterol, triglycerides, CRP, IL-6, fibrinogen, D-dimer, FVIIc, FVIIa, FVIIIc, and platelet count, and inversely associated with HDL-cholesterol, hematocrit and hemoglobin (Table 1).
Table 1.
Associations of peak thrombin generation (pTG) phenotypes with cardiovascular disease risk factors
| Variable (standard deviation) |
pTG (nM) (n=5411) β (SE) |
pTG/FXIa− (nM) (n=4340) β (SE) |
pTG/FXIa+ (nM) (n=4339) β (SE) |
|||
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age (5.6 years) | −13.2 (1.9)† | −1.8 (1.8) | −11.1 (1.3)† | |||
| Female gender | 9.2 (3.9) | 13.2 (3.6)* | −8.0 (2.6)* | |||
| Current Smoking | 4.6 (5.9) | −12.8 (5.5) | 18.7 (3.9)† | |||
| BMI (4.7 kg/m2) | 12.2 (2.0)† | −1.3 (1.8) | 12.5 (1.3)† | |||
| Hypertension | 10.2 (3.9) | −0.49 (3.6) | 11.8 (2.6)† | |||
| Systolic BP (21.4 mmHg) | 3.3 (2.0) | 3.6 (1.9) | 0.72 (1.3) | |||
| Lipids | ||||||
| Cholesterol-total (39.2 mg/dL) | 12.2 (2.0)† | −2.6 (1.8) | 14.9 (1.3)† | |||
| HDL-cholesterol (15.8 mg/dL) | −15.5 (2.0)† | −0.70 (1.9) | −16.4 (1.4)† | |||
| LDL-cholesterol (24.8 mg/dL) | 7.0 (1.4)† | −3.1 (1.3) | 9.9 (0.88)† | |||
| lnTriglycerides (0.44) | 25.4 (1.9)† | 4.5 (1.8) | 21.8 (1.2)† | |||
| Inflammation and Coagulation | ||||||
| lnCRP (0.99) | 31.6 (1.8)† | 15.6 (1.7)† | 17.6 (1.2)† | |||
| lnIL-6 (0.58) | 19.4 (1.9)† | 10.1 (1.7)† | 11.4 (1.2)† | |||
| Platelet count (76.0×103/mm3) | 21.6 (1.9)† | 8.2 (1.9)† | 12.5 (1.3)† | |||
| Fibrinogen (67.4 mg/dL) | 35.8 (1.9)† | 10.4 (1.8)† | 26.1 (1.2)† | |||
| lnD-dimer (0.82) | 19.3 (2.9)† | 6.3 (2.8) | 10.9 (2.1)† | |||
| FVIIc (29.6%) | 36.1 (2.0)† | 17.2 (1.9)† | 16.0 (1.4)† | |||
| FVIIa (25.3 mU/mL) | 18.5 (2.4)† | 13.1 (2.2)† | 1.8 (1.7) | |||
| FVIIIc (37.1%) | 31.6 (2.0)† | 22.7 (1.8)† | 10.6 (1.4)† | |||
| Hematocrit (3.9%) | −10.7 (2.1)† | −24.3 (2.0)† | 12.9 (1.4)† | |||
| Hemoglobin (1.4 g/dL) | −14.9 (2.2)† | −28.0 (2.0)† | 12.1 (1.4)† | |||
| Subclinical Atherosclerosis and Diabetes | ||||||
| Common carotid intima medial thickness (IMT) (0.22 mm) |
(2.0) | 5.0 | 0.16 (1.9) | - | (1.4)* | 5.1 |
| Diabetes | 6.6 (5.3) | −0.39 (4.8) | 15.2 (3.4)† |
Independent variables were analyzed per standard deviation increment higher (shown in parentheses). Analyses were adjusted for age, sex, and race (models of age and sex were only adjusted for the two remaining variables). Triglycerides, C-reactive protein (CRP), interleukin-6 (IL-6), and D-dimer were natural log-transformed (ln).
: P<0.005;
: P<0.0001.
In genotyping analyses of 3,673 EA and 676 AA participants, two SNPs were significantly associated with pTG in EAs: rs1801020 (p=3.3×10−22) and rs2545801 (p=3.1×10−21) (Table 2). The two SNPs are located in the F12 gene encoding FXII and are in strong linkage disequilibrium (LD) (r2 =0.96). Rs1080210 is a functional SNP located four bases upstream of the F12 transcriptional start site [35]; rs2545801 is a non-coding SNP located in the 5’ region upstream of the transcriptional start site.
Table 2.
Single nucleotide polymorphisms (SNPs) significantly associated with peak thrombin generation (pTG) in European-American participants of the Cardiovascular Health Study
| SNP | Chr | Position | Gene | A1 | A2 | MAF | Beta (SE) [nM Thrombin] |
P-Value |
|---|---|---|---|---|---|---|---|---|
| rs1801020 | 5 | 176769138 | F12 | A | G | 0.23 | −34.2 (3.5) | 3.3×10−22 |
| rs2545801 | 5 | 176773945 | F12 | T | C | 0.24 | −33.0 (3.5) | 3.1×10−21 |
Chr, chromosome; A1, allele 1 (major allele); A2, allele 2 (minor allele); MAF, minor allele frequency.
The mean (SD) pTG value was 501 nM (133 nM) for rs1801020 major allele homozygotes, 477 nM (134 nM) for heterozygotes, and 415 nM (138 nM) for minor allele homozygotes (p<0.0001). The same alleles of rs1801020 and rs2545801 were associated with lower pTG in AAs. However, the allele frequencies and LD patterns were different than those observed for EAs (r2 =0.48), and the associations were of nominal significance. An additional F12 SNP, rs17876032, was also associated with higher pTG in AAs (β=45.0 nM, p=2.0×10−5) (Supporting Information Table S3). The associations of rs2545801 (p=0.22) and rs17876032 (p=0.10) were not conditionally independent of rs1801020 in AAs.
Contributions of coagulation factor XIIa and factor XIa to pTG
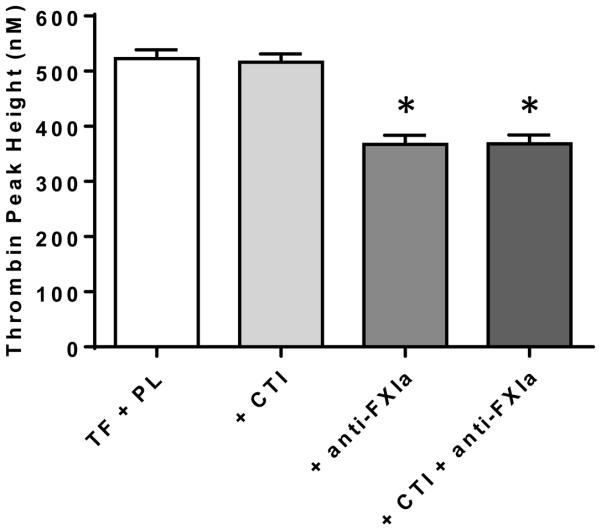
Associations of F12 SNPs with pTG suggested activated FXII (FXIIa) was important for TG ex vivo. To test this hypothesis, we performed the TG assay in plasma samples (n=47) with the addition of CTI to the assay (50 µg/mL). The addition of CTI had no effect on pTG (Figure 1), suggesting a potential role of FXIa, a coagulation factor further downstream in the contact coagulation pathway. To evaluate this, we performed the TG assay with the addition of inhibitory anti-human FXIa antibody (± CTI) to the assay. Inhibition of FXIa resulted in a mean ~30% reduction of pTG; co-treatment with CTI had no additional effect (Figure 1). These results indicated the importance of FXIa on TG ex vivo.
Figure 1.
Inhibition of coagulation factor XIa (FXIa), but not coagulation factor XIIa (FXIIa), during the thrombin generation assay effects peak thrombin generation (pTG) ex vivo. FXIIa was inhibited by the addition of corn trypsin inhibitor (CTI; 50 µg/mL) to the plasma samples during the thrombin generation assay. FXIa was inhibited by the addition of inhibitory monoclonal anti-human FXI antibody (anti-FXIa; 100 µg/mL) to samples during the assay (± CTI). Data are means (SEM) (n=47). PL, phospholipids; TF, tissue factor. * <0.0001 compared to TF + PL.
To determine the influence of FXII activation during sample collection and processing prior to inhibition during the TG assay, we collected blood directly into CTI-containing phlebotomy tubes (n=8). We also collected blood into citrate tubes and manually added CTI (50 µg/mL) 30-seconds post-draw, prior to processing and freezing (n=8). Collection into CTI-containing tubes resulted in an ~80% reduction of pTG; the post-draw addition of CTI resulted in an ~27% reduction (Supporting Information Figure S1). These data indicated the immediate activation of FXII in vitro during sample collection. These results also suggested the FXIIa-dependent activation of FXIa during sample collection and processing.
Associations of FXIa-independent pTG (pTG/FXIa−) and FXIa-dependent pTG (pTG/FXIa+) with cardiovascular disease risk factors and common SNPs
We next performed the TG assay in 4,340 available CHS baseline samples with the addition of inhibitory anti-FXIa antibody to the assay. Since the addition of anti-FXIa antibody to the assay completely inhibited FXIa [19], this phenotype represented pTG minus any contribution of FXIa. The mean (SD) value of pTG/FXIa− in the overall study population was 165 nM (119 nM); mean values were higher in women [171 nM (SD 124 nM)] than men [156 nM (SD 110nM)] (p<0.0001) (Table 1).
A third phenotype, pTG/FXIa+, was calculated by subtracting pTG/FXIa− from pTG. This phenotype represented the fraction of the total pTG that was dependent on contributions of FXIa. In the overall study population, the mean (SD) pTG/FXIa+ value was 333 nM (85 nM). pTG/FXIa+ was higher with older age, with minor sex differences [women: 331 nM (SD 86 nM); men: 338 (SD 83 nM)] (p=0.006).
The cross-sectional correlates of pTG/FXIa− and pTG/FXIa+ phenotypes with cardiovascular disease risk factors are shown in Table 1. Notably, current smoking and higher BMI, total- and LDL-cholesterol, hemoglobin, and hematocrit were associated with lower pTG/FXIa−, but higher pTG/FXIa+. Higher common carotid intima media thickness, hypertension, and diabetes were associated with pTG/FXIa+, but not pTG/FXIa− (Table 1).
Three SNPs were significantly associated with pTG/FXIa− in EAs (Table 3), including rs1801020 (p=3.9×10−14) and rs2545801 (p=5.2×10−14), the F12 SNPs associated with pTG; and, rs657152 (p=4.3×10−9) a tag SNP for blood group O [36], located in an intronic region in the ABO gene encoding a glycosyltransferase (Table 3). Among AAs, the strongest association of pTG/FXIa− was with rs1801020 (p=2.9×10−8) (Table 3). No SNPs were significantly associated with pTG/FXIa+ in either EAs or AAs (Supporting Information Table S4).
Table 3.
Top single nucleotide polymorphisms (SNPs) associated with FXIa-independent peak thrombin generation (pTG/FXIa-) in participants of the Cardiovascular Health Study
| Population | SNP | Chr | Position | Gene | A1 | A2 | MAF | Beta (SE) [nM Thrombin] |
P-Value |
|---|---|---|---|---|---|---|---|---|---|
| European- Americans |
|||||||||
| rs1801020 | 5 | 176769138 | F12 | A | G | 0.23 | −24.7 (3.2) | 3.9×10−14 | |
| rs2545801 | 5 | 176773945 | F12 | T | C | 0.24 | −24.2 (3.2) | 5.2×10−14 | |
| rs657152 | 9 | 135129086 | ABO | A | C | 0.37 | 16.3 (2.8) | 4.3×10−9 | |
| African- Americans |
|||||||||
| rs1801020 | 5 | 176769138 | F12 | A | G | 0.42 | −38.5 (6.8) | 2.9×10−8 |
Chr, chromosome; A1, allele 1 (major allele); A2, allele 2 (minor allele); MAF, minor allele frequency.
In analyses of EAs, associations of rs1801020 with pTG phenotypes were not attenuated by adjustment for FVIIa. With FVIIa included in the models (adjusted for age, sex, and clinic), the associations of rs1801020 with pTG (β=−33.1 nM; SE=4.0 nM; p<0.0001) and pTG/FXI− (β=−24.2. nM; SE=3.6 nM; p<0.0001) were not substantially altered. These results suggested the relationships of F12 SNPs with pTG were not mediated by FVIIa.
Associations of pTG phenotypes with incident cardiovascular disease and ischemic stroke
Of the 5,411 CHS participants with pTG measurements, there were 944 incident MI, 834 incident ischemic strokes, 1,665 incident CHD, and 1,705 cardiovascular- or cerebrovascular-related deaths over a maximum 22.5-years of follow-up (median 13.2 years). In demographic-adjusted Cox models, pTG was positively associated with incident ischemic stroke risk, but not MI, CHD, or event-related mortality. The stroke hazard ratio (HR) (95% confidence interval (CI)) per SD higher pTG was 1.10 (1.03, 1.19; p=0.006). Results were not materially altered after adjustment for risk factors (Table 4). Comparing the fourth to the first quartile in risk factor-adjusted models, the pTG HR for ischemic stroke was 1.25 (1.02, 1.54; p=0.04) (Supporting Information Table S5).
Table 4.
Cox proportional hazards ratios for associations between 1-SD unit higher peak thrombin generation (pTG) and incident cardiovascular disease, ischemic stroke, and event-related mortality
| Phenotype (standard deviation) |
MI (n=944) HR (95% CI) |
Stroke (n=834) HR (95% CI) |
CHD (n=1,665) HR (95% CI) |
CVD-Related
Mortality (n=1,705) HR (95% CI) |
|---|---|---|---|---|
| Model 1 | ||||
| pTG (142 nM) | 1.03 (0.96, 1.10) | 1.10 (1.03, 1.19) | 1.03 (0.98, 1.09) | 1.01 (0.96, 1.06) |
| pTG/FXIa- (118 nM) | 0.98 (0.91, 1.05) | 1.04 (0.96, 1.12) | 1.00 (0.95, 1.05) | 1.01 (0.96, 1.07) |
| pTG/FXIa+ (84 nM) | 1.05 (0.98, 1.13) | 1.14 (1.05, 1.23) | 1.08 (1.02, 1.14) | 1.10 (1.03, 1.16) |
| Model 2 | ||||
| pTG (142 nM) | 1.03 (0.96, 1.10) | 1.09 (1.01, 1.17) | 1.02 (0.97, 1.07) | 1.00 (0.95, 1.06) |
| pTG/FXIa− (118 nM) | 1.01 (0.93, 1.08) | 1.06 (0.98, 1.15) | 1.01 (0.95, 1.07) | 1.01 (0.95, 1.07) |
| pTG/FXIa+ (84 nM) | 1.02 (0.94, 1.10) | 1.11 (1.02, 1.21) | 1.04 (0.98, 1.10) | 1.06 (1.00, 1.13) |
Analyses using Cox proportional hazards ratios (HR) and 95% confidence intervals (CI). Peak thrombin generation (pTG) phenotypes were evaluated per standard deviation (SD) increment higher value (shown in parentheses). Participants with adjudicated relevant disease prevalent at baseline were excluded from the analyses. The number of measurements varies across TG categories: pTG: n=5,411; pTG/FXIa−: n=4,340; pTG/FXIa+: n=4,339.
Model 1: Age, sex, race.
Model 2: model 1 + smoking status, diabetes status, hypertension, systolic blood pressure, and LDL-cholesterol; mortality models also included prevalent MI, stroke, and CHD at baseline. CHD indicates coronary heart disease; CVD-related mortality indicates cardiovascular or cerebrovascular disease-related mortality; LDL, low-density lipoprotein; MI, myocardial infarction.
In demographic-adjusted models, pTG/FXIa+ was positively associated with incident ischemic stroke, CHD, and event-related mortality. After adjustment for risk factors, pTG/FXIa+ remained significantly associated with ischemic stroke. The ischemic stroke HR (95% CI) per SD higher pTG/FXIa+ was 1.11 (1.03, 1.21; p=0.02). Comparing the fourth to the first quartile, the stroke HR (95% CI) was 1.17 (0.93, 1.47; p=0.19). pTG/FXIa− was not significantly associated with cardiovascular or stroke events (Table 4; Supporting Information Table S5).
In a candidate gene look-up performed in CHARGE (n=19,602) [34], the rs1801020 SNP was not associated with incident ischemic stroke (n=1164) (β= −0.02; (SE=0.08); p=0.81) (adjusted for age and sex).
Assessment of associations in GAIT-2
In analyses performed in GAIT-2 [31], rs1801020 was associated with lower FXIIc (β=−30.9%; p=1.3×10−45), lower FIXc (β=−9.0%; p=9.0×10−6), and longer aPTT (β=0.06 s; p=1.4×10−15), but not pTG, ETP, or TG lag time (p >0.40). Rs657152 was associated with higher pTG (β=16.3 nM; p=0.01), shorter aPTT (β=−0.03 s; p=9.8×10−8), and higher FXIc (β=0.03%; p=0.02), but not ETP or TG lag time (p >0.40) (Supporting Information Table S6).
Discussion
We have demonstrated that pTG was associated with incident ischemic stroke risk. pTG/FXIa+, but not pTG/FXIa−, was associated with ischemic stroke suggesting an important contribution of the intrinsic coagulation pathway. Further, we identified two SNPs in the gene for coagulation FXII associated with lower pTG.
Our findings are consistent with the Three-City Cohort study which demonstrated higher TG was associated with increased risk of acute ischemic stroke, but not CHD [9]. These consistent associations may suggest the potential utility of TG assays in stroke risk assessment. To the best of our knowledge, by measuring both standard and FXIa-inhibited TG, our study is the first to provide evidence that TG influenced by the intrinsic coagulation system was prospectively associated with ischemic stroke risk in a population of older adults. Our observational study, however, cannot establish causality.
There is accumulating evidence implicating the intrinsic coagulation system in atherothrombosis. Mice with genetic ablation of FXII or FXI were protected from carotid arterial occlusion [37, 38] and cerebral artery ischemia-reperfusion injury [39]. Epidemiologically, higher levels of FXI were associated with increased stroke risk [40, 41] and FXI deficiency was associated with reduced risk [13]. FXI was associated with risk of MI in some studies [42], but not all [43, 44]. The relationships of FXII with arterial thrombosis are uncertain [45], although recent evidence supports a role of FXII in the regulation of fibrin clot structure and stabilization [11, 12, 46]. Relationships of F12 SNPs with thrombotic outcomes are conflicting [47].
We identified associations of two SNPs located in the F12 gene, rs1801020 and rs2545801, with pTG. Rs1801020 is a well-studied common variant causing a C to T transition 4 nucleotides upstream of the transcription start codon. Carriers of the T variant lack a Kozak consensus sequence, resulting in decreased translation and lower circulating FXII [35]; rs2545801 is in strong LD with rs1801020. Our findings are consistent with previous studies identifying relationships of F12 SNPs with pTG [48, 49] and aPTT [49-52], and support the importance of F12 SNPs on TG ex vivo. Associations of rs1801020 with TG measurements were not replicated in GAIT-2. This discrepancy may reflect the different TG assays utilized by CHS and GAIT-2. The associations of rs1801020 with FXIIc, FIXc, and aPTT in GAIT-2, however, are consistent with a role of F12 in coagulation ex vivo.
Our results demonstrated that while inhibition of FXIIa by CTI during the TG assay did not effect pTG, inhibition by CTI during blood collection had a significant effect. Inhibition of FXIa during the TG assay also significantly reduced pTG. These data suggested the rapid activation of FXIIa and subsequent FXIIa-dependent activation of FXIa in vitro during our standardized sample collection and processing steps. Associations of F12 SNPs with lower pTG indicated that genetic variation in FXII influenced levels of FXIa ex vivo [53], which in turn effected thrombin generation ex vivo. Unexpectedly, the pTG/FXIa+ phenotype was not associated with F12 SNPs. These null results are likely the consequence of variable inhibition of FXIa by plasma serpins during phlebotomy and sample collection prior to inhibition during the assay [19], and may also reflect variability resulting from pTG/FXIa+ being a calculated phenotype.
F12 SNPs were also associated with the pTG/FXIa− phenotype. These results implicated FXIIa and/or FXIa in influencing activation of TF-FVIIa-mediated coagulation pathway components ex vivo. Our results indicated relationships of F12 SNPs with pTG were not mediated by FVIIa. Possible mechanisms explaining these relationships may include FXIa-dependent activation of FIX or inactivation of tissue factor pathway inhibitor [54] during sample collection and processing prior to FXIa inhibition during the assay.
The rs1801020 SNP was not associated with stroke in CHARGE. These null results do not implicate casual relationships between F12 and ischemic stroke. Relationships among F12, pTG, and stroke, may be mediated by factors further down the coagulation pathway or reflect coinheritance with other functional variants [55], and interpretation of the complex relationships of the F12 rs1801020 SNP with thrombosis remains difficult [47].
FXIIa and FXIa were not inhibited during blood collection in CHS and the potential for residual activity prior to their inhibition during the assay is a technical limitation of our study. Despite this limitation, blood collection and processing in CHS were standardized, comparable to standardized protocols for many clinical and research assays. We used a commercially available TG assay which contained a TF concentration that allowed for a low sensitivity to the intrinsic coagulation pathway [56]. As such, our associations with the intrinsic pathway are likely underestimates.
pTG/FXIa± measurements were performed in 1,000 fewer samples than pTG, preventing direct comparisons of stroke hazard ratios. We may have been underpowered to detect associations of pTG/FXI− with stroke and cannot rule out its potential importance. The TG phenotypes were also highly correlated and caution is warranted in the interpretation of the results. Information regarding ischemic stroke subtypes, and levels of FXII, FXI, prekallikrein, and high-molecular-weight-kininogen are not available in CHS. Finally, we could not evaluate racial differences due to the TG data being generated in separate analytical batches.
Strengths of our study include the field center technician training, standardization and quality-assurance methods for blood collection, processing, shipping, and storage in CHS [17]. The prospective study design and use of specific coagulation factor inhibitors are additional strengths.
In conclusion, we have demonstrated that pTG was associated with increased risk of ischemic stroke. Our data suggested contributions of the contact coagulation pathway were primarily responsible for these relationships. Further, the minor alleles for SNPs in the gene for coagulation FXII were associated with lower pTG. These data support the importance of contact pathway-dependent TG as a risk factor for ischemic stroke and indicate the importance of F12 SNPs on thrombin generation both ex vivo and in vivo.
Supplementary Material
Acknowledgements
The authors acknowledge Cathy Tilley for technical assistance with the Technoclone TGA. A full list of principal Cardiovascular Health Study (CHS) investigators and institutions can be found at http://www.chs-nhlbi.org/pi. The authors thank the staff and participants of each of the studies participating in the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium for their important contributions and all members of neurology working group of the CHARGE consortium.
This study was supported by an R01 (HL71862) from the National Heart, Lung, and Blood Institute (NHLBI) to A. P. Reiner N. C. Olson was supported by the NHLBI post-doctoral training award 5T32HL007894. G. Chauhan and S. Debette were supported by a grant from the Fondation Leducq and the Agence Nationale de la Recherche (Chaire d'Excellence). Cardiovascular Health Study: The CHS research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the NHLBI, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA).
The Genetic Analysis of Idiopathic Thrombophilia (GAIT) Project: GAIT was funded in part by FIS PI11/0184, FIS PI12/00612 and Red Investigación Cardiovascular RD12/0042/0032. Atherosclerosis Risk In Communities Study (ARIC): The Atherosclerosis Risk in Communities study was performed as a collaborative study supported by NHLBI contracts HHSN268201100005C, HSN268201100006C, HSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C, R01HL70825, R01HL087641, R01HL59367, and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health (NIH) contract HHSN268200625226C. Infrastructure was partly supported by grant No. UL1RR025005, a component of the NIH and NIH Roadmap for Medical Research. This project was also supported by NIH R01 grants HL084099 and NS087541.
Framingham Heart Study (FHS): This work was supported by the NHLBI’s Framingham Heart Study (Contract No. N01-HC-25195) and its contract with Affymetrix, Inc. for genotyping services (Contract No. N02-HL-6-4278). A portion of this research utilized the Linux Cluster for Genetic Analysis (LinGA-II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. This study was also supported by grants from the NINDS (R01 NS17950) and the NIA (R01s AG033193, AG008122, and U0149505).
Rotterdam Study: The generation and management of GWAS genotype data for the Rotterdam Study is supported by the Netherlands Organisation of Scientific Research NWO Investments (nr. 175.010.2005.011, 911-03-012). This study is funded by the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), the Netherlands Genomics Initiative (NGI)/Netherlands Organisation for Scientific Research (NWO) project nr. 050-060-810. Further funding was received from the Netherlands Heart Foundation (2009B102) and a Veni-grant (916.13.054). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam.
Footnotes
Addendum
N. C. Olson conceived the experimental design, performed statistical analyses, interpreted data, and wrote the manuscript. S. Butenas conceived the experimental design and revised the manuscript. L. A. Lange and E. A. Lange performed statistical analyses, interpreted data, and revised the manuscript. N. S. Jenny and M. Cushman revised the manuscript. J. Walston designed the study and revised the manuscript. J. C. Souto, and J. M. Soria carried out experiments, conceived the experimental design, and revised the manuscript. G. Chauhan, S. Debette and S. Seshardri conceived the experimental design and performed statistical analyses. W. T. Longstreth, conceived the study design, generated data, and adjudicated stroke events. A. P. Reiner and R. P. Tracy designed the study, conceived the research and analytical plan, performed statistical analyses, interpreted data, and wrote the manuscript.
Disclosures of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Crawley JT, Zanardelli S, Chion CK, Lane DA. The central role of thrombin in hemostasis. J Thromb Haemost. 2007;5(Suppl 1):95–101. doi: 10.1111/j.1538-7836.2007.02500.x. [DOI] [PubMed] [Google Scholar]
- 2.Siegemund T, Petros S, Siegemund A, Scholz U, Engelmann L. Thrombin generation in severe haemophilia A and B: the endogenous thrombin potential in platelet-rich plasma. Thromb Haemost. 2003;90:781–6. doi: 10.1160/TH03-01-0027. [DOI] [PubMed] [Google Scholar]
- 3.Dargaud Y, Beguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, Hemker HC, Negrier C. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93:475–80. doi: 10.1160/TH04-10-0706. [DOI] [PubMed] [Google Scholar]
- 4.Rugeri L, Beguin S, Hemker C, Bordet JC, Fleury R, Chatard B, Negrier C, Dargaud Y. Thrombin-generating capacity in patients with von Willebrand's disease. Haematologica. 2007;92:1639–46. doi: 10.3324/haematol.11460. [DOI] [PubMed] [Google Scholar]
- 5.Wichers IM, Tanck MW, Meijers JC, Lisman T, Reitsma PH, Rosendaal FR, Buller HR, Middeldorp S. Assessment of coagulation and fibrinolysis in families with unexplained thrombophilia. Thromb Haemost. 2009;101:465–70. [PubMed] [Google Scholar]
- 6.Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C. Use of calibrated automated thrombinography +/− thrombomodulin to recognise the prothrombotic phenotype. Thromb Haemost. 2006;96:562–7. [PubMed] [Google Scholar]
- 7.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 8.Lutsey PL, Folsom AR, Heckbert SR, Cushman M. Peak thrombin generation and subsequent venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) study. J Thromb Haemost. 2009;7:1639–48. doi: 10.1111/j.1538-7836.2009.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carcaillon L, Alhenc-Gelas M, Bejot Y, Spaft C, Ducimetiere P, Ritchie K, Dartigues JF, Scarabin PY. Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly: the Three-City cohort study. Arterioscler Thromb Vasc Biol. 2011;31:1445–51. doi: 10.1161/ATVBAHA.111.223453. [DOI] [PubMed] [Google Scholar]
- 10.Smid M, Dielis AW, Spronk HM, Rumley A, van Oerle R, Woodward M, ten Cate H, Lowe G. Thrombin generation in the Glasgow Myocardial Infarction Study. PLoS One. 2013;8:e66977. doi: 10.1371/journal.pone.0066977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuijpers MJ, van der Meijden PE, Feijge MA, Mattheij NJ, May F, Govers-Riemslag J, Meijers JC, Heemskerk JW, Renne T, Cosemans JM. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol. 2014;34:1674–80. doi: 10.1161/ATVBAHA.114.303315. [DOI] [PubMed] [Google Scholar]
- 12.Konings J, Govers-Riemslag JW, Philippou H, Mutch NJ, Borissoff JI, Allan P, Mohan S, Tans G, Ten Cate H, Ariens RA. Factor XIIa regulates the structure of the fibrin clot independently of thrombin generation through direct interaction with fibrin. Blood. 2011;118:3942–51. doi: 10.1182/blood-2011-03-339572. [DOI] [PubMed] [Google Scholar]
- 13.Salomon O, Steinberg DM, Koren-Morag N, Tanne D, Seligsohn U. Reduced incidence of ischemic stroke in patients with severe factor XI deficiency. Blood. 2008;111:4113–7. doi: 10.1182/blood-2007-10-120139. [DOI] [PubMed] [Google Scholar]
- 14.Cushman M, O'Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood. 2009;114:2878–83. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.O'Leary DH, Polak JF, Wolfson SK, Jr., Bond MG, Bommer W, Sheth S, Psaty BM, Sharrett AR, Manolio TA. Use of sonography to evaluate carotid atherosclerosis in the elderly. The Cardiovascular Health Study. CHS Collaborative Research Group. Stroke. 1991;22:1155–63. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 17.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–70. [PubMed] [Google Scholar]
- 18.Seligsohn U, Osterud B, Griffin JH, Rapaport SI. Evidence for the participation of both activated factor XII and activated factor IX in cold-promoted activation of factor VII. Thromb Res. 1978;13:1049–56. doi: 10.1016/0049-3848(78)90233-5. [DOI] [PubMed] [Google Scholar]
- 19.Butenas S, Undas A, Gissel MT, Szuldrzynski K, Zmudka K, Mann KG. Factor XIa and tissue factor activity in patients with coronary artery disease. Thromb Haemost. 2008;99:142–9. doi: 10.1160/TH07-08-0499. [DOI] [PubMed] [Google Scholar]
- 20.Rand MD, Lock JB, van't Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88:3432–45. [PubMed] [Google Scholar]
- 21.Tracy RP, Lemaitre RN, Psaty BM, Ives DG, Evans RW, Cushman M, Meilahn EN, Kuller LH. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–7. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 22.Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, Tracy RP. Fibrinolytic activation markers predict myocardial infarction in the elderly. The Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19:493–8. doi: 10.1161/01.atv.19.3.493. [DOI] [PubMed] [Google Scholar]
- 23.Tracy RP, Arnold AM, Ettinger W, Fried L, Meilahn E, Savage P. The relationship of fibrinogen and factors VII and VIII to incident cardiovascular disease and death in the elderly: results from the cardiovascular health study. Arterioscler Thromb Vasc Biol. 1999;19:1776–83. doi: 10.1161/01.atv.19.7.1776. [DOI] [PubMed] [Google Scholar]
- 24.Jenny NS, Tracy RP, Ogg MS, Luong le A, Kuller LH, Arnold AM, Sharrett AR, Humphries SE. In the elderly, interleukin-6 plasma levels and the −174G>C polymorphism are associated with the development of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2002;22:2066–71. doi: 10.1161/01.atv.0000040224.49362.60. [DOI] [PubMed] [Google Scholar]
- 25.Keating BJ, Tischfield S, Murray SS, Bhangale T, Price TS, Glessner JT, Galver L, Barrett JC, Grant SF, Farlow DN, Chandrupatla HR, Hansen M, Ajmal S, Papanicolaou GJ, Guo Y, Li M, Derohannessian S, de Bakker PI, Bailey SD, Montpetit A, Edmondson AC, et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS One. 2008;3:e3583. doi: 10.1371/journal.pone.0003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 27.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 28.Price TR, Psaty B, O'Leary D, Burke G, Gardin J. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:504–7. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 29.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo KS, Wilson JG, Lange LA, Folsom AR, Galarneau G, Ganesh SK, Grant SF, Keating BJ, McCarroll SA, Mohler ER, 3rd, O'Donnell CJ, Palmas W, Tang W, Tracy RP, Reiner AP, Lettre G. Genetic association analysis highlights new loci that modulate hematological trait variation in Caucasians and African Americans. Hum Genet. 2011;129:307–17. doi: 10.1007/s00439-010-0925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camacho M, Martinez-Perez A, Buil A, Siguero L, Alcolea S, Lopez S, Fontcuberta J, Souto JC, Vila L, Soria JM. Genetic determinants of 5-lipoxygenase pathway in a Spanish population and their relationship with cardiovascular risk. Atherosclerosis. 2012;224:129–35. doi: 10.1016/j.atherosclerosis.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Beguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 33.Psaty BM, O'Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, Uitterlinden AG, Harris TB, Witteman JC, Boerwinkle E. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, Debette S, Lumley T, Folsom AR, van den Herik EG, Bos MJ, Beiser A, Cushman M, Launer LJ, Shahar E, Struchalin M, Du Y, Glazer NL, Rosamond WD, Rivadeneira F, et al. Genomewide association studies of stroke. New Engl J Med. 2009;360:1718–28. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanaji T, Okamura T, Osaki K, Kuroiwa M, Shimoda K, Hamasaki N, Niho Y. A common genetic polymorphism (46 C to T substitution) in the 5'-untranslated region of the coagulation factor XII gene is associated with low translation efficiency and decrease in plasma factor XII level. Blood. 1998;91:2010–4. [PubMed] [Google Scholar]
- 36.Zabaneh D, Gaunt TR, Kumari M, Drenos F, Shah S, Berry D, Power C, Hypponen E, Shah T, Palmen J, Pallas J, Talmud PJ, Casas JP, Sofat R, Lowe G, Rumley A, Morris RW, Whincup PH, Rodriguez S, Ebrahim S, et al. Genetic variants associated with Von Willebrand factor levels in healthy men and women identified using the HumanCVD BeadChip. Ann Hum Genet. 2011;75:456–67. doi: 10.1111/j.1469-1809.2011.00654.x. [DOI] [PubMed] [Google Scholar]
- 37.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–81. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–6. [PubMed] [Google Scholar]
- 39.Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renne C, Gailani D, Nieswandt B, Renne T. Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med. 2006;203:513–8. doi: 10.1084/jem.20052458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suri MF, Yamagishi K, Aleksic N, Hannan PJ, Folsom AR. Novel hemostatic factor levels and risk of ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2010;29:497–502. doi: 10.1159/000297966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siegerink B, Rosendaal FR, Algra A. Antigen levels of coagulation factor XII, coagulation factor XI and prekallikrein, and the risk of myocardial infarction and ischemic stroke in young women. J Thromb Haemost. 2014;12:606–13. doi: 10.1111/jth.12531. [DOI] [PubMed] [Google Scholar]
- 42.Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood. 2006;108:4045–51. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 43.Salomon O, Steinberg DM, Dardik R, Rosenberg N, Zivelin A, Tamarin I, Ravid B, Berliner S, Seligsohn U. Inherited factor XI deficiency confers no protection against acute myocardial infarction. J Thromb Haemost. 2003;1:658–61. doi: 10.1046/j.1538-7836.2003.00195.x. [DOI] [PubMed] [Google Scholar]
- 44.Tanis B, Algra A, van der Graaf Y, Helmerhorst F, Rosendaal F. Procoagulant factors and the risk of myocardial infarction in young women. Eur J Haematol. 2006;77:67–73. doi: 10.1111/j.1600-0609.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 45.Key NS. Epidemiologic and clinical data linking factors XI and XII to thrombosis. Hematology Am Soc Hematol Educ Program. 2014;2014:66–70. doi: 10.1182/asheducation-2014.1.66. [DOI] [PubMed] [Google Scholar]
- 46.Matafonov A, Leung PY, Gailani AE, Grach SL, Puy C, Cheng Q, Sun MF, McCarty OJ, Tucker EI, Kataoka H, Renne T, Morrissey JH, Gruber A, Gailani D. Factor XII inhibition reduces thrombus formation in a primate thrombosis model. Blood. 2014;123:1739–46. doi: 10.1182/blood-2013-04-499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson CY, Tuite A, Morange PE, Tregouet DA, Gagnon F. The factor XII −4C>T variant and risk of common thrombotic disorders: A HuGE review and meta-analysis of evidence from observational studies. Am J Epidemiol. 2011;173:136–44. doi: 10.1093/aje/kwq349. [DOI] [PubMed] [Google Scholar]
- 48.Segers O, van Oerle R, ten Cate H, Rosing J, Castoldi E. Thrombin generation as an intermediate phenotype for venous thrombosis. Thromb Haemost. 2010;103:114–22. doi: 10.1160/TH09-06-0356. [DOI] [PubMed] [Google Scholar]
- 49.Corral J, Anton AI, Quiroga T, Gonzalez-Conejero R, Pereira J, Roldan V, Vicente V, Mezzano D. Influence of the F12 −4 C>T polymorphism on hemostatic tests. Blood Coagul Fibrinolysis. 2010;21:632–9. doi: 10.1097/MBC.0b013e32833a9048. [DOI] [PubMed] [Google Scholar]
- 50.Houlihan LM, Davies G, Tenesa A, Harris SE, Luciano M, Gow AJ, McGhee KA, Liewald DC, Porteous DJ, Starr JM, Lowe GD, Visscher PM, Deary IJ. Common variants of large effect in F12, KNG1, and HRG are associated with activated partial thromboplastin time. Am J Hum Genet. 2010;86:626–31. doi: 10.1016/j.ajhg.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang W, Schwienbacher C, Lopez LM, Ben-Shlomo Y, Oudot-Mellakh T, Johnson AD, Samani NJ, Basu S, Gogele M, Davies G, Lowe GD, Tregouet DA, Tan A, Pankow JS, Tenesa A, Levy D, Volpato CB, Rumley A, Gow AJ, Minelli C, et al. Genetic associations for activated partial thromboplastin time and prothrombin time, their gene expression profiles, and risk of coronary artery disease. Am J Hum Genet. 2012;91:152–62. doi: 10.1016/j.ajhg.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaunt TR, Lowe GD, Lawlor DA, Casas JP, Day IN. A gene-centric analysis of activated partial thromboplastin time and activated protein C resistance using the HumanCVD focused genotyping array. Eur J Hum Genet. 2013;21:779–83. doi: 10.1038/ejhg.2012.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renne T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–9. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puy C, Tucker EI, Matafonov A, Cheng Q, Zientek KD, Gailani D, Gruber A, McCarty OJ. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood. 2015 doi: 10.1182/blood-2014-10-604587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soria JM, Almasy L, Souto JC, Bacq D, Buil A, Faure A, Martinez-Marchan E, Mateo J, Borrell M, Stone W, Lathrop M, Fontcuberta J, Blangero J. A quantitative-trait locus in the human factor XII gene influences both plasma factor XII levels and susceptibility to thrombotic disease. Am J Hum Genet. 2002;70:567–74. doi: 10.1086/339259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008;142:889–903. doi: 10.1111/j.1365-2141.2008.07267.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.