Abstract
Situations that ordinarily necessitate consideration of anticoagulation, such as arterial and venous thrombotic events and prevention of stroke in atrial fibrillation, become challenging in patients with inherited bleeding disorders such as hemophilia A, hemophilia B, and von Willebrand disease. There are no evidence-based guidelines to direct therapy in these patients, and management strategies that incorporate anticoagulation must weigh a treatment that carries a risk of hemorrhage in a patient who is already at heightened risk against the potential consequences of not treating the thrombotic event. In this paper, we review atherothrombotic disease, venous thrombotic disease, and atrial fibrillation in patients with inherited bleeding disorders, and discuss strategies for using anticoagulants in this population using cases to illustrate these considerations.
Introduction
The primary, and most evident, complication in patients with inherited bleeding disorders such as hemophilia A, hemophilia B, and von Willebrand disease (VWD), is spontaneous and/or posttraumatic bleeding. Although patients with these disorders are relatively protected from thrombosis, both arterial and venous thromboses do occur on occasion, necessitating management decisions on anticoagulation. Furthermore, consideration may need to be given to prophylactic anticoagulation in situations in which it might normally be indicated, for example, in the presence of concomitant atrial fibrillation (AF).
In the absence of evidence-based management guidelines, we present our approach to anticoagulation in patients with inherited bleeding disorders for various indications, including arterial thrombotic events, venous thromboembolism (VTE), and AF, and review management strategies using illustrative clinical scenarios.
Case 1
A 68-year-old man with mild hemophilia A (baseline factor VIII [FVIII] activity of 8%) and no history of inhibitor presents with acute onset of chest pressure associated with shortness of breath. An electrocardiogram performed in the emergency department demonstrates ST segment elevation, and blood work returns with a troponin level elevated to 2.9 ng/mL. He needs to undergo urgent cardiac catheterization to reestablish coronary artery perfusion.
Case 2
A 69-year-old man with severe hemophilia A and high-titer inhibitor (historical peak of 3239 Bethesda units [BU]/mL, currently 128 BU/mL), hypertension (HTN), and diabetes mellitus (DM) presents with black tarry stools for several days. Blood work demonstrates a hemoglobin level of 7.2 g/dL, representing a 6g/dL drop over the past 6 months, and troponin is elevated to 0.39 ng/mL, consistent with a non-ST-elevation myocardial infarction (NSTEMI). Positron emission tomography stress test demonstrates partially reversible large defects in the apical, septal, and anteroseptal segments. Angiogram demonstrates significant coronary artery disease (CAD), including 90% occlusion of the ostial left main coronary artery, 99% occlusion of the proximal main coronary artery, and 70% occlusion of the ostial right coronary artery.
Atherothrombosis in bleeding disorders
Improved treatment and management of patients with hemophilia has resulted not only in an increase in life expectancy approaching that of people without hemophilia,1 but also in an increase in age-related comorbidities. One such comorbidity is cardiovascular disease (CVD). The lifetime prevalence of CVD has been estimated to be as high as 19.5% in persons with hemophilia,2 and the prevalence of ischemic heart disease (IHD) is estimated to be 15% in those older than 60 years.3 Published literature demonstrates that CVD and arterial occlusive events occur in VWD as well, albeit at a reduced prevalence compared with reference populations: the rate of arterial thrombotic events was found to be 3.3% in a cohort study of more than 600 adult VWD patients, significantly lower than 2 reference populations,4 and the prevalence of CVD was found to be 15% in a cross-sectional study of more than 7500 patients with VWD, compared with 26% in non-VWD patients.5
Although epidemiologic data clearly demonstrate that CVD occurs in inherited bleeding disorders, studies on the protective effect of hemophilia and VWD against the development of atherosclerosis have generated conflicting data. Studies using animal models have shown both protection6 and lack of protection7,8 on the development of atherosclerosis. Earlier human studies using arterial intimal medial thickness (IMT) as a surrogate for atherosclerosis are similarly conflicting, with some suggesting a protective effect and others failing to demonstrate protection.9 More recent studies using IMT in humans, however, have failed to demonstrate a protective effect: a systematic review found similar mean artery IMTs in patients with hemophilia and VWD compared with healthy controls and concluded that hemophilia or low VWF levels had little effect on the development of atherosclerosis, although the presence of atherosclerotic plaques was less prevalent in patients with hemophilia.10 Another study found a similar degree of coronary calcium deposition and severe calcification in patients with hemophilia aged 59 years or older compared with matched controls without hemophilia.11 Although hemophilia does not seem to protect against the development of atherosclerosis, several studies suggest that it may protect against mortality from IHD.9,12-14 A large systematic review comprising more than 19 000 patients with hemophilia A, hemophilia B, and VWD or who were hemophilia carriers found that >90% of the studies reported a reduction in mortality from IHD in persons with hemophilia compared to the general population, and calculated a weighted standardized mortality ratio of 0.51 (95% confidence interval, 0.24-1.09).10 The reduced mortality rate in hemophilia is postulated to be due to fewer occlusive thrombi on the surface of ruptured atherosclerotic plaques as a result of low levels of FVIII/FIX.10,15
The development of atherosclerosis in hemophilia and VWD can be attributed to the presence of traditional cardiovascular risk factors, which have been shown to occur at similar or higher rates in persons with hemophilia and the general population.3,16-19 Furthermore, these risk factors have been associated with CVD in persons with hemophilia: a recent cross-sectional study of men with moderate to severe hemophilia found that smoking significantly increased the odds of a cardiovascular event,20 and in a study of a large cohort of males with hemophilia who had been hospitalized at least once, IHD was significantly associated with DM, HTN, and hyperlipidemia compared with other cardiac diseases.3 Similarly, VWD patients with CVD have significantly increased rates of HTN, hyperlipidemia, and DM than do VWD patients without CVD; however, the overall risk of CVD remains lower in VWD than in non-VWD patients after adjustment for age, sex, and cardiovascular risk factors.5
Case 3
A 32-year-old woman with type 3 VWD is admitted for esophagogastroduodenoscopy (EGD) with biopsies for evaluation of dysphagia. She has been treated in the past with FVIII-VWF plasma-derived concentrate for episodes of menorrhagia and epistaxis. She receives FVIII-VWF concentrate before the EGD, which is continued for 5 days postprocedure. Tissue biopsy samples taken during the EGD are benign, and she does not have bleeding complications postprocedure. Six days after the procedure, she develops left-leg swelling. Duplex ultrasound reveals an acute thrombus in the left popliteal vein.
Case 4
A 55-year-old man with severe hemophilia A and high-titer FVIII inhibitor (currently at a peak of 200 BU/mL) is hospitalized for a spontaneous left-knee hemarthrosis. He is treated with recombinant FVIIa, with minimal response, and, therefore, transitioned to FVIII inhibitor bypass activity treatment (ie, FEIBA) with resolution of bleeding. On the day of discharge, he complains of pain and swelling in the right upper thigh; venous duplex ultrasound demonstrates an acute deep vein thrombosis (DVT) in the femoral vein.
VTE in bleeding disorders
In addition to arterial thrombotic events, patients with inherited bleeding disorders occasionally develop VTE. Most venous events in this population occur in the presence of an additional risk factor for VTE, such as the postoperative setting or during clotting factor replacement. The largest case series in patients with hemophilia reports 27 cases (12 in hemophilia A and 15 in hemophilia B) of non-catheter-associated venous thrombosis, including 18 DVT or pulmonary emboli and 5 superficial thromboses (with the remaining DVTs occurring in unusual venous locations).21 In only 3 cases could a risk factor for VTE not be identified, and 19 of 27 events occurred while the patient was receiving factor or bypassing agents. Only a handful of cases of spontaneous VTE in (untreated) hemophilia have been reported in the literature.22,23 Spontaneous VTE is also rare in patients with VWD: in a recently published review of 486 VWD patients at a single institution, for instance, there were no cases of venous thrombosis.24 A literature review within the same publication reported 33 cases of VWD and venous thrombosis, with all but 7 cases having identifiable risk factors; the most common risk factor (N = 14) was factor replacement therapy consisting of VWF with FVIII. The association between VTE and factor replacement is thought to be a consequence of high FVIII levels achieved during infusion. Although factor replacement serves to restore levels necessary for hemostasis, replacement to levels above normal may instead create an acquired thrombophilic state. Indeed, high FVIII levels are a well-established risk factor for VTE, with a relative risk of thrombosis of 4.8 with FVIII levels of >150 IU/dL compared to FVIII levels <100 IU/dL.25-27
Patients with hemophilia may be at increased risk of VTE when undergoing hip and knee arthroplasty, in which both surgery and perioperative factor replacement contribute to the thrombotic risk. Small sample size numbers limit the estimation of the incidence of postoperative VTE, and reported estimates vary widely. One prospective single-institution study of 29 orthopedic surgeries found that asymptomatic distal DVT occurred in 10% of patients with hemophilia undergoing orthopedic surgery.28 However, several studies have found no cases of VTE in patients with hemophilia undergoing surgery in the absence of prothrombin-complex concentrates.29,30 An aggregate analysis of published cases in the literature estimates the symptomatic VTE rate in hemophilia patients undergoing hip or knee arthroplasty (usually in the absence of pharmacologic thromboprophylaxis) to be 0.5%, much lower than the estimated cumulative rate of 4.3% in the general population.31 The relative “protection” against VTE in hemophilia A may be a result of the relative concentration of FVIII in plasma: as an acute phase reactant, FVIII is typically significantly elevated postoperatively in the nonhemophilia population; however, patients with hemophilia A have FVIII levels carefully monitored and controlled, thereby avoiding excessively high levels for prolonged periods.
Case 5
A 71-year-old man with mild hemophilia A (baseline factor level, 17%) and AF is started on warfarin by his internist for stroke prevention, given his CHA2DS2-VASc (congestive heart failure, HTN, age ≥75 years [doubled], diabetes, stroke/transient cerebral ischemia/thromboembolism [doubled], vascular disease [previous myocardial infarction, peripheral artery disease, or aortic plaque], age 65-74 years, sex category [female]) score of 2. He remains on warfarin for several years without stroke complications or major bleeding; however, he has developed chronic arthropathy, thought to be hemophilia related, in both elbows since starting warfarin and recently experienced a right ankle bleed.
AF in bleeding disorders
A cross-sectional survey from 14 European hemophilia centers found an overall prevalence of AF in patients with hemophilia of 0.84%,32 which is comparable to that found in the general population.33 Just as in the general population, the prevalence of AF in persons with hemophilia rises with increasing age, with a prevalence of 3.4% in subjects >60 years old compared to 0.2% in patients ≤60 years old.32
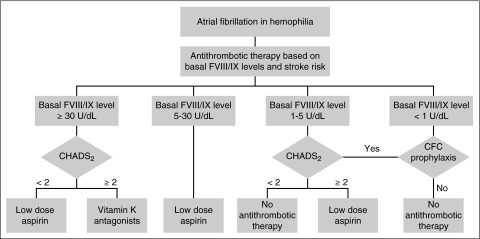
In usual medical practice, treatment decisions regarding anticoagulation in AF are determined by weighing an individual’s stroke risk as calculated by the CHA2DS2-VASc score against an estimated bleeding risk occurring as a consequence of anticoagulation therapy. However, validated bleeding scores for hemophilia patients with AF do not exist; although the HAS-BLED score estimates risk of bleeding with anticoagulation for AF in the general population, concerns exist that the score underestimates the bleeding risk in hemophilia. In the absence of validated bleeding scores in persons with hemophilia, algorithms for medical treatment of stroke prevention have been proposed, with initial management decisions based on baseline clotting factor levels to approximate risk of bleeding (Figure 1).34,35
Figure 1.
Recommended management algorithm for AF in hemophilia. CFC, clotting factor concentrate; CHADS2, congestive heart failure, hypertension, age ≥75, diabetes, stroke/transient cerebral ischemia (doubled) score. Reprinted with permission from Mannucci et al.34
Considerations when using antithrombotic therapy in patients with inherited bleeding disorders
When considering anticoagulant or anti-platelet therapy in patients with inherited bleeding disorders, we first evaluate the following 4 principles: bleeding phenotype of the patient, characteristics of the anticoagulant, intensity of anticoagulant therapy, and duration of planned therapy. Here, we use cases of VWD and hemophilia as illustrative examples of primary and secondary bleeding disorders, respectively; these are also the most common inherited bleeding disorders that carry a definitive diagnosis in clinical practice. As such, experience in using anticoagulants is most extensive in these 2 disorders, and we believe that the principles (if not the specifics) behind our approach to decision making is generalizable to the broader scope of inherited bleeding disorders.
1. Bleeding phenotype.
The primary consideration in our decision whether to initiate anticoagulation is the bleeding phenotype. Consideration of how frequently and how severely the patient bleeds and whether bleeding is spontaneous or occurs only when provoked (eg, by trauma) is important. In addition, for less severely affected patients in particular, the ease of achieving hemostasis in response to past hemostatic challenges (such as dental procedures and surgeries) is also critical. Hemophilia patients without an inhibitor typically have a predictable response to clotting factor replacement, and bleeding can be reliably prevented or treated. Patients with inhibitors, however, have a much less predictable hemostatic response to bypassing agents; studies have found that ∼10% to 20% of bleeding with inhibitors is either unresponsive or only partially responsive to bypassing agents.36-38 The risk of uncontrolled bleeding, therefore, almost always outweighs the benefit to anticoagulation in patients with inhibitors.
2. Characteristics of anticoagulant/antiplatelet agent.
Consideration should be given to the bleeding risk associated with the anticoagulant, as well as its reversibility and half-life, when choosing a product for a patient with a bleeding disorder. We prefer anticoagulants that are more easily reversed and have shorter half-lives in patients with bleeding disorders; thus, we prefer parenteral unfractionated heparin (UFH) or low-molecular-weight heparin over parenteral drugs with longer half-lives, such as fondaparinux. If oral therapy is chosen, we prefer to use vitamin K antagonists or dabigatran, because these agents currently have available antidotes. We do not typically use direct FXa inhibitors, because specific reversal agents for these agents are not yet approved for use, although this situation is likely to change in the near future.
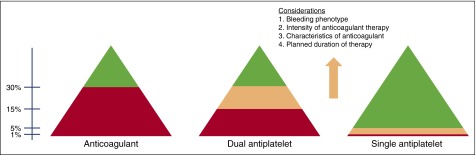
Broadly, we follow an approach to anticoagulation as illustrated in Figure 2.39 In hemophilia and VWD, we feel comfortable using anticoagulants under factor protection when trough FVIII/FIX and VWF activity levels are maintained at >30%, although decisions for individual patients are made on a case-by-case basis. We avoid single-agent antiplatelet therapy in severe hemophilia (factor levels <1%), when the risk of spontaneous bleeding is potentially quite high in the absence of ongoing prophylactic clotting factor therapy. Low-dose aspirin is usually well tolerated in patients with factor levels >5%, whereas its use for patients with factor levels between 1% and 5%, or for those on continuous prophylaxis, must be made on an individual basis with consideration given to the aforementioned principles, including bleeding phenotype and planned duration of therapy. Because dual antiplatelet therapy has a bleeding risk comparable to warfarin,40 we do not typically use dual antiplatelet therapy without prophylactic factor replacement, especially in patients with more-severe variants of VWD. Although published approaches have recommended maintenance of a trough factor level at >30% in hemophilia (Table 1),1,34,41-43 we have found that patients tolerate dual antiplatelet therapy with somewhat lower troughs, and by doing so, we avoid the frequent higher peak levels of FVIII that are necessary to achieve troughs of this magnitude, thereby improving patient compliance and reducing the thrombotic risk. Further, data from The Netherlands have shown that spontaneous (although not traumatic or anticoagulant-induced) bleeding is eradicated in mild to moderate hemophilia when the factor level is >15%44; therefore, we extrapolate that the risk of spontaneous bleeding is minimal at 10% to 15% trough levels.
Figure 2.
Approach to FVIII or FIX goals for the use of anticoagulant and antiplatelet therapy in hemophilia. Red, avoid use; green, likely okay to use; peach, decisions on use made after considerations as outlined in the figure.
Table 1.
Common features in published approaches to treatment of CVD in hemophilia
| Intervention/medication | Factor replacement goal |
|---|---|
| Percutaneous coronary intervention with therapeutic antithrombotic (UFH, bivalirudin, glycoprotein IIb/IIIa inhibitors) | Peak level of 80-100%, continue as long as therapeutic doses of antithrombotic are used (usually at least 48 h) |
| Dual antiplatelet therapy (aspirin + P2Y12 inhibitors) | Trough level of ≥30% |
| Single-agent antiplatelet therapy | Trough level of ≥5% |
| Coronary bypass surgery | Peak level of 80-100% by continuous infusion before, during, and after until sufficient wound healing has taken place |
3. Intensity of anticoagulant therapy.
A third consideration is the required intensity of anticoagulant therapy, which can be broadly categorized as high intensity (eg, UFH regimens used for acute coronary syndromes and loading bolus doses used during initiation of VTE treatment), standard intensity (eg, doses used in the treatment of VTE), or low intensity (eg, prophylactic-dose anticoagulation for VTE and single-agent antiplatelet therapy). The bleeding risk associated with each level of intensity roughly parallels the intensity of antithrombotic therapy, with the risk increased in high-intensity regimens compared with low-intensity regimens. Our approach is to use high-intensity anticoagulation for the minimal duration and under replacement clotting factor protection. Further, we consider lowering the intensity of anticoagulation in patients with a history of significant or frequent bleeding, for example, by eliminating the UFH bolus in initiation of anticoagulation for VTE.
4. Duration of antithrombotic therapy.
The planned duration of therapy must be considered when using anticoagulants. Duration can range from very short (eg, during a procedure such as percutaneous coronary intervention) to a prolonged or even indefinite duration for a chronic condition (eg, chronic antiplatelet therapy for CAD or anticoagulation for a prosthetic cardiac valve). It is easier to replace missing clotting factor to levels that minimize bleeding risk with anticoagulation over a period of days than it is to replace it long-term, when the logistics and expense of factor replacement might lead to reduced adherence in the setting of ongoing anticoagulation, thereby outweighing any benefit of anticoagulation. Therefore, for patients with inherited bleeding disorders who require factor protection in order to receive anticoagulation (as discussed in the previous paragraph), we typically treat for the shortest reasonable duration; for example, we use standard doses of anticoagulation but may only treat for 6 to 8 weeks for a provoked VTE rather than the standard recommendation of 12 weeks.
Discussion
Cases 1 and 2: management of CAD
In case 1, a patient with mild hemophilia A and no history of inhibitor presented with an ST-elevation myocardial infarction (STEMI) and underwent angiography for revascularization. Although there are no evidence-based guidelines for the treatment of CVD in patients with bleeding disorders, several authors have published suggested treatment algorithms for patients with hemophilia that include recommendations for factor coverage during anticoagulant and antiplatelet therapy (Table 1). Although the literature is even less robust for treatment in VWD, there have been published reports of patients with VWD who have been successfully treated with antiplatelet and anticoagulant therapy.4 Our approach for patients with hemophilia undergoing coronary angiography is to infuse factor with a goal peak FVIII level of >80% before initiation of anticoagulant/antiplatelet agents and before starting the procedure. Anticoagulation with UFH or bivalirudin can then be initiated and used for the duration of the revascularization procedure. We feel comfortable with usual doses of anticoagulation, unless inhibitors are present (as in case 2, below). We prefer the radial approach to angiography, because direct pressure is more easily applied and signs/symptoms of bleeding are more easily monitored compared with the femoral approach. When stenting is necessary, we prefer bare metal stents (BMSs) over drug-eluting stents in most patients with bleeding disorders, because the minimum time for required dual antiplatelet therapy is shorter for BMSs (1 month) compared with drug-eluting stents (1 year). After catheterization is completed, care is taken to maintain pressure on the arterial puncture site until hemostasis has been secured. Continuous factor is maintained postprocedure to target levels of >80% for 48 hours and until signs of hemostasis (ie, lack of bleeding at the insertion site, hemoglobin stability, and blood transfusion requirements similar to the nonhemophilic patient)45 are maintained; we check factor activity levels at 8 hours after starting the continuous infusion and then every 24 hours to ensure that target levels are met. If continuous factor infusion is not accessible, bolus infusion should continue postprocedure with a goal peak level of >80% and a trough level of >30% for 48 hours. Our approach to the factor coverage of VWD patients undergoing angiogram is similar to that of hemophilia patients, because FVIII levels have been shown to be predictive of bleeding associated with surgical procedures in patients with VWD.46 We aim to keep peak FVIII levels >80% and trough FVIII level >30%, as well as VWF activity levels >50% before the procedure, for 48 hours postprocedure, and until hemostasis is achieved (consistent with existing recommendations for high-risk procedures).47,48
Dual antiplatelet therapy is continued for 4 to 6 weeks in the setting of BMS replacement, and we replace FVIII to a goal trough of 10% to 15% for the duration of dual antiplatelet therapy. We continue secondary prophylaxis with aspirin (81 mg daily) indefinitely, as long as it is tolerated without bleeding. Given this patient’s baseline factor activity of >5%, we would not replace factor for single-agent antiplatelet therapy; however, in patients with factor levels between 1% and 5%, we would consider the benefit of adding continuous factor prophylaxis on an individual basis. This patient was also prescribed proton-pump inhibitor therapy while on antiplatelet therapy.
Case 2 presents a much more complicated situation, given the presence of an inhibitor in the setting of concurrent gastrointestinal bleeding and an NSTEMI with multivessel disease. In this situation, we would not give factor replacement before the procedure, because the NSTEMI occurred with the use of bypassing agents. Cardiac catheterization was performed via a radial approach, using a moderated dose of heparin (about 75% of usual bolus doses of 60 to 70 U/kg, followed by a maintenance dose of 12 U/kg per hour), given his recent bleeding and the inhibitor. Although a patient with this severity of CAD would ordinarily be considered for coronary artery bypass grafting, he elected to undergo percutaneous intervention after discussion of the risks/benefits of both approaches. Two BMSs were placed, and heparin was reversed immediately after completion of the procedure. He started daily aspirin (81 mg), along with a beta-blocker and a statin. We advised avoidance of a second antiplatelet agent that would otherwise be considered standard therapy, because we believe the high risk of bleeding and the unpredictability of achieving hemostasis in the setting of inhibitor outweigh the 2% improvement in outcome with dual antiplatelet therapy compared with single antiplatelet therapy (0.5% vs 3.5%).49 The patient continued on daily aspirin for 6 weeks, after which the dose was reduced to every other day indefinitely. He was not administered prophylactic factor replacement and had no evidence of thrombosis or bleeding complications after 7 months.
Cases 3 and 4: management of VTE
As with the management of arterial thrombosis, there are no guidelines for the treatment of VTE in hemophilia and VWD. Published approaches to VTE management in hemophilia and VWD vary widely,28,50-52 and the data are insufficient to compare the outcomes (risks and benefits) of these strategies. In case 3, the patient has a moderate bleeding phenotype, requiring occasional clotting factor replacement for bleeding. She now has a proximal DVT, which carries a significant risk of pulmonary embolism, the risk of which is thought to outweigh the risk of bleeding in the short-term.
Our approach is to initiate anticoagulation with a therapeutic dose of UFH without a loading bolus, and if no bleeding occurs within the first 24 to 48 hours, we initiate warfarin with a goal international normalized ratio range of 2.0 to 3.0. We aim for at least 1 month of therapy, after which the risk of recurrent thrombosis drops dramatically.53 We use short-term factor prophylaxis while anticoagulating with VWF-containing concentrate 3 times per week, aiming for a trough activity level of 30% for both VWF and FVIII. Although the benefit of anticoagulation outweighs the bleeding risk in the acute setting, the relative benefit of anticoagulation in a patient with an increased risk of bleeding will decrease over time such that the risk of bleeding with standard duration of therapy (3 months for provoked VTE) is greater than the benefit of anticoagulation therapy. If this patient’s bleeding phenotype had been less pronounced, however, she may have been able to better tolerate anticoagulation for the recommended standard duration.
Case 4 illustrates alternative strategies for the management of VTE in patients who are at high risk of spontaneous bleeding. This patient has severe hemophilia, recent bleeding, and the presence of an inhibitor. Furthermore, the proximal DVT developed in the setting of use of bypassing agents, so he would not be a candidate for further bypassing agents in the acute setting. Therefore, we would not use anticoagulation and, instead, would recommend a temporary inferior vena cava filter, with plans to remove it after 3 months.
Case 5: management of AF in hemophilia and VWD
The patient in case 5 developed arthropathy from presumed intra-articular bleeding only after the initiation of anticoagulation. This pattern of bleeding would be considered unusual in a mild hemophilia patient with a baseline FVIII level of 17%. We therefore assume that his bleeding phenotype has been exacerbated by warfarin therapy. In our practice, we would typically treat this patient (with a factor activity level between 5% and 30%) with aspirin rather than warfarin. Although one could cautiously consider warfarin on an individual basis, taking into consideration bleeding phenotype, he now has bleeding complications while on warfarin, shifting the balance of the risk/benefit ratio away from full-intensity anticoagulation, particularly given the low CHA2DS2-VASc score of 2. Therefore, we would favor switching to aspirin.
In some instances, rhythm-control strategies such as cardioversion or ablation may be chosen for management of symptomatic AF. We approach management of these cases as in other procedures, with factor replaced by bolus to a goal peak activity of >80% at least 30 minutes before procedure, followed by continuous infusion to maintain a factor activity level of >80% for 48 hours and until hemostasis is secured. Rhythm control does not always obviate the need for therapeutic anticoagulation, however, particularly in patients at high risk for thromboembolism, and we concurrently replace factor to a goal activity level of 30% for the duration of therapeutic anticoagulation. Left atrial appendage occlusion may be an option for patients at high risk of bleeding and thromboembolism, because periprocedural anticoagulation is reduced to ∼45 days, but this should be done at an experienced facility to reduce procedural complications.41
Acknowledgment
This study was supported by National Institutes of Health National Heart, Lung, and Blood Institute grant T32 HL007149 (K.M.).
Authorship
Contribution: K.M. and N.S.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel S. Key, Division of Hematology/Oncology, Department of Medicine, University of North Carolina, 1079 Genetic Medicine Building, CB #7035, Chapel Hill, NC 27599; e-mail: nigel_key@med.unc.edu.
References
- 1.Mannucci PM, Mauser-Bunschoten EP. Cardiovascular disease in haemophilia patients: a contemporary issue. Haemophilia. 2010;16(Suppl 3):58-66. [DOI] [PubMed]
- 2.Sharathkumar AA, Soucie JM, Trawinski B, Greist A, Shapiro AD. Prevalence and risk factors of cardiovascular disease (CVD) events among patients with haemophilia: experience of a single haemophilia treatment centre in the United States (US). Haemophilia. 2011;17(4):597-604. [DOI] [PubMed]
- 3.Kulkarni R, Soucie JM, Evatt BL Hemophilia Surveillance System Project Investigators. Prevalence and risk factors for heart disease among males with hemophilia. Am J Hematol. 2005;79(1):36–42. doi: 10.1002/ajh.20339. [DOI] [PubMed] [Google Scholar]
- 4.Sanders YV, Eikenboom J, de Wee EM, et al. WiN Study Group. Reduced prevalence of arterial thrombosis in von Willebrand disease. J Thromb Haemost. 2013;11(5):845–854. doi: 10.1111/jth.12194. [DOI] [PubMed] [Google Scholar]
- 5.Seaman CD, Yabes J, Comer DM, Ragni MV. Does deficiency of von Willebrand factor protect against cardiovascular disease? Analysis of a national discharge register. J Thromb Haemost. 2015. [DOI] [PubMed]
- 6.Khallou-Laschet J, Caligiuri G, Tupin E, et al. Role of the intrinsic coagulation pathway in atherogenesis assessed in hemophilic apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25(8):e123–e126. doi: 10.1161/01.ATV.0000171995.22284.9a. [DOI] [PubMed] [Google Scholar]
- 7.Badimon L, Steele P, Badimon JJ, Bowie EJ, Fuster V. Aortic atherosclerosis in pigs with heterozygous von Willebrand disease. Comparison with homozygous von Willebrand and normal pigs. Arteriosclerosis. 1985;5(4):366–370. doi: 10.1161/01.atv.5.4.366. [DOI] [PubMed] [Google Scholar]
- 8.Fabri DR, de Paula EV, Costa DS, Annichino-Bizzacchi JM, Arruda VR. Novel insights into the development of atherosclerosis in hemophilia A mouse models. J Thromb Haemost. 2011;9(8):1556–1561. doi: 10.1111/j.1538-7836.2011.04410.x. [DOI] [PubMed] [Google Scholar]
- 9.Tuinenburg A, Mauser-Bunschoten EP, Verhaar MC, Biesma DH, Schutgens RE. Cardiovascular disease in patients with hemophilia. J Thromb Haemost. 2009;7(2):247–254. doi: 10.1111/j.1538-7836.2008.03201.x. [DOI] [PubMed] [Google Scholar]
- 10.Biere-Rafi S, Zwiers M, Peters M, et al. The effect of haemophilia and von Willebrand disease on arterial thrombosis: a systematic review. Neth J Med. 2010;68(5):207–214. [PubMed] [Google Scholar]
- 11.Tuinenburg A, Rutten A, Kavousi M, et al. Coronary artery calcification in hemophilia A: no evidence for a protective effect of factor VIII deficiency on atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(3):799–804. doi: 10.1161/ATVBAHA.111.238162. [DOI] [PubMed] [Google Scholar]
- 12.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 13.Plug I, Van Der Bom JG, Peters M, et al. Mortality and causes of death in patients with hemophilia, 1992-2001: a prospective cohort study. J Thromb Haemost. 2006;4(3):510–516. doi: 10.1111/j.1538-7836.2006.01808.x. [DOI] [PubMed] [Google Scholar]
- 14.Triemstra M, Rosendaal FR, Smit C, Van der Ploeg HM, Briët E. Mortality in patients with hemophilia. Changes in a Dutch population from 1986 to 1992 and 1973 to 1986. Ann Intern Med. 1995;123(11):823–827. doi: 10.7326/0003-4819-123-11-199512010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Ragni MV. Aging in haemophilia: getting to the heart of the matter. Thromb Haemost. 2011;105(2):207–208. doi: 10.1160/TH10-12-0818. [DOI] [PubMed] [Google Scholar]
- 16.Foley CJ, Nichols L, Jeong K, Moore CG, Ragni MV. Coronary atherosclerosis and cardiovascular mortality in hemophilia. J Thromb Haemost. 2010;8(1):208–211. doi: 10.1111/j.1538-7836.2009.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ragni MV, Moore CG. Atherosclerotic heart disease: prevalence and risk factors in hospitalized men with haemophilia A. Haemophilia: the official journal of the World Federation of Hemophilia. 2011;17(6):867-871. [DOI] [PMC free article] [PubMed]
- 18.Minuk L, Jackson S, Iorio A, et al. doi: 10.1111/hae.12768. Cardiovascular disease (CVD) in Canadians with haemophilia: Age-Related CVD in Haemophilia Epidemiological Research (ARCHER study). Haemophilia. 2015;21(6):736-741. [DOI] [PubMed] [Google Scholar]
- 19.Biere-Rafi S, Baarslag MA, Peters M, et al. Cardiovascular risk assessment in haemophilia patients. Thromb Haemost. 2011;105(2):274–278. doi: 10.1160/TH10-07-0460. [DOI] [PubMed] [Google Scholar]
- 20.Sood SL, Cheng D, Ragni MV, et al. doi: 10.1182/bloodadvances.2018018226. A cross-sectional analysis of cardiovascular disease in the hemophilia population [abstract]. Blood. 2015;124(21):2836. Abstract 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girolami A, Scandellari R, Zanon E, Sartori R, Girolami B. Non-catheter associated venous thrombosis in hemophilia A and B. A critical review of all reported cases. J Thromb Thrombolysis. 2006;21(3):279–284. doi: 10.1007/s11239-006-6556-7. [DOI] [PubMed] [Google Scholar]
- 22.Dargaud Y, Cruchaudet BB, Lienhart A, Coppere B, Ninet J, Negrier C. Spontaneous proximal deep vein thrombosis in a patient with severe haemophilia A. Blood Coagul Fibrinolysis. 2003;14(4):407-409. [DOI] [PubMed]
- 23.Kashyap R, Sharma LM, Gupta S, Saxena R, Srivastava DN. Deep vein thrombosis in a patient with severe haemophilia A. Haemophilia. 2006;12(1):87-89. [DOI] [PubMed]
- 24.Girolami A, Tasinato V, Sambado L, Peroni E, Casonato A. Venous thrombosis in von Willebrand disease as observed in one centre and as reported in the literature. Blood Coagul Fibrinolysis. 2015;26(1):54-58. [DOI] [PubMed]
- 25.van der Meer FJ, Koster T, Vandenbroucke JP, Briët E, Rosendaal FR. The Leiden Thrombophilia Study (LETS). Thromb Haemost. 1997;78(1):631–635. [PubMed] [Google Scholar]
- 26.Kraaijenhagen RA, in’t Anker PS, Koopman MM, et al. High plasma concentration of factor VIIIc is a major risk factor for venous thromboembolism. Thromb Haemost. 2000;83(1):5–9. [PubMed] [Google Scholar]
- 27.Koster T, Blann AD, Briët E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet. 1995;345(8943):152–155. doi: 10.1016/s0140-6736(95)90166-3. [DOI] [PubMed] [Google Scholar]
- 28.Hermans C, Hammer F, Lobet S, Lambert C. Subclinical deep venous thrombosis observed in 10% of hemophilic patients undergoing major orthopedic surgery. J Thromb Haemost. 2010;8(5):1138–1140. doi: 10.1111/j.1538-7836.2010.03829.x. [DOI] [PubMed] [Google Scholar]
- 29.Takedani H, Ohnuma K, Hirose J. Deep venous thrombosis was not detected after total knee arthroplasty in Japanese patients with haemophilia. Haemophilia. 2015;21(5):585-588. [DOI] [PubMed]
- 30.Kasper CK. Postoperative thromboses in hemophilia B. N Engl J Med. 1973;289(3):160. doi: 10.1056/NEJM197307192890321. [DOI] [PubMed] [Google Scholar]
- 31.Perez Botero J, Spoon DB, Patnaik MS, Ashrani AA, Trousdale RT, Pruthi RK. Incidence of symptomatic venous thromboembolism in patients with hemophilia undergoing joint replacement surgery: a retrospective study. Thromb Res. 2015;135(1):109–113. doi: 10.1016/j.thromres.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Schutgens RE, Klamroth R, Pabinger I, Malerba M, Dolan G ADVANCE Working Group. Atrial fibrillation in patients with haemophilia: a cross-sectional evaluation in Europe. Haemophilia. 2014;20(5):682-686. [DOI] [PubMed]
- 33.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 34.Mannucci PM, Schutgens RE, Santagostino E, Mauser-Bunschoten EP. How I treat age-related morbidities in elderly persons with hemophilia. Blood. 2009;114(26):5256–5263. doi: 10.1182/blood-2009-07-215665. [DOI] [PubMed] [Google Scholar]
- 35.Aguilar C. Antithrombotic therapy in a patient with mild haemophilia A and atrial fibrillation: case report and brief review of the literature. Blood Coagul Fibrinolysis. 2015;26(3):346-349. [DOI] [PubMed]
- 36.Dimichele D, Negrier C. A retrospective postlicensure survey of FEIBA efficacy and safety. Haemophilia. 2006;12(4):352-362. [DOI] [PubMed]
- 37.Key NS, Aledort LM, Beardsley D, et al. Home treatment of mild to moderate bleeding episodes using recombinant factor VIIa (Novoseven) in haemophiliacs with inhibitors. Thromb Haemost. 1998;80(6):912–918. [PubMed] [Google Scholar]
- 38.Astermark J, Donfield SM, DiMichele DM, et al. FENOC Study Group. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood. 2007;109(2):546–551. doi: 10.1182/blood-2006-04-017988. [DOI] [PubMed] [Google Scholar]
- 39.Schutgens RE, Tuinenburg A, Fischer K, Mauser-Bunschoten EP. Anticoagulation therapy in haemophilia. Managing the unknown. Hamostaseologie. 2013;33(4):299–304. doi: 10.5482/HAMO-12-08-0015. [DOI] [PubMed] [Google Scholar]
- 40.Connolly S, Pogue J, Hart R, et al. ACTIVE Writing Group of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367(9526):1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 41.Schutgens RE, Tuinenburg A, Roosendaal G, Guyomi SH, Mauser-Bunschoten EP. Treatment of ischaemic heart disease in haemophilia patients: an institutional guideline. Haemophilia. 2009;15(4):952-958. [DOI] [PubMed]
- 42.Staritz P, de Moerloose P, Schutgens R, Dolan G. Applicability of the European Society of Cardiology guidelines on management of acute coronary syndromes to people with haemophilia – an assessment by the ADVANCE Working Group. Haemophilia. 2013;19(6):833-840. [DOI] [PubMed]
- 43.Ferraris VA, Boral LI, Cohen AJ, Smyth SS, White GC., II Consensus review of the treatment of cardiovascular disease in people with hemophilia A and B. Cardiol Rev. 2015;23(2):53–68. doi: 10.1097/CRD.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.den Uijl IE, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41-44. [DOI] [PubMed]
- 45.Blanchette VS, Key NS, Ljung LR, Manco-Johnson MJ, van den Berg HM, Srivastava A Subcommittee on Factor VIII, Factor IX and Rare Coagulation Disorders of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939. doi: 10.1111/jth.12672. [DOI] [PubMed] [Google Scholar]
- 46.Mannucci PM. Treatment of von Willebrand’s Disease. N Engl J Med. 2004;351(7):683–694. doi: 10.1056/NEJMra040403. [DOI] [PubMed] [Google Scholar]
- 47.Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171-232. [DOI] [PubMed]
- 48.Pasi KJ, Collins PW, Keeling DM, et al. Management of von Willebrand disease: a guideline from the UK Haemophilia Centre Doctors’ Organization. Haemophilia. 2004;10(3):218-231. [DOI] [PubMed]
- 49.Leon MB, Baim DS, Popma JJ, et al. Stent Anticoagulation Restenosis Study Investigators. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. N Engl J Med. 1998;339(23):1665–1671. doi: 10.1056/NEJM199812033392303. [DOI] [PubMed] [Google Scholar]
- 50.Goddard NJ, Mann HA, Lee CA. Total knee replacement in patients with end-stage haemophilic arthropathy: 25-year results. J Bone Joint Surg Br. 2010;92-B(8):1085–1089. doi: 10.1302/0301-620X.92B8.23922. [DOI] [PubMed] [Google Scholar]
- 51.Franchini M, Krampera M, Veneri D. Deep vein thrombosis after orthopedic surgery in a patient with type 1 von Willebrand disease and mutations in the MTHFR and beta-fibrinogen genes. Thromb Haemost. 2003;90(5):963–964. [PubMed] [Google Scholar]
- 52.Bowen D, Dasani H, Yung B, Bloom A. Deep venous thrombosis and pulmonary embolism in a patient with type III von Willebrand’s disease, protein C and antithrombin III deficiency. Br J Haematol. 1992;81(3):446–447. doi: 10.1111/j.1365-2141.1992.tb08255.x. [DOI] [PubMed] [Google Scholar]
- 53.Heit JA, Mohr DN, Silverstein MD, Petterson TM, O’Fallon WM, Melton LJ., III Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160(6):761–768. doi: 10.1001/archinte.160.6.761. [DOI] [PubMed] [Google Scholar]