Key Points
Idelalisib as upfront therapy for CLL caused an early hepatotoxicity in a subset of primarily younger patients with IGHV-mutated disease.
Multiple lines of evidence suggest that this adverse effect is immune mediated, perhaps through inhibition of regulatory T cells.
Abstract
Idelalisib is a small-molecule inhibitor of PI3Kδ with demonstrated efficacy for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL). To evaluate idelalisib as front-line therapy, we enrolled 24 subjects in a phase 2 study consisting of 2 months of idelalisib monotherapy followed by 6 months of combination therapy with idelalisib and the anti-CD20 antibody ofatumumab. After a median follow-up period of 14.7 months, hepatotoxicity was found to be a frequent and often severe adverse event. A total of 19 subjects (79%) experienced either grade ≥1 ALT or AST elevation during the study, and 13 subjects (54%) experienced grade ≥3 transaminitis. The median time to development of transaminitis was 28 days, occurring before ofatumumab introduction. Younger age and mutated immunoglobulin heavy chain status were significant risk factors for the development of hepatotoxicity. Multiple lines of evidence suggest that this hepatotoxicity was immune mediated. A lymphocytic infiltrate was seen on liver biopsy specimens taken from 2 subjects with transaminitis, and levels of the proinflammatory cytokines CCL-3 and CCL-4 were higher in subjects experiencing hepatotoxicity. All cases of transaminitis resolved either by holding the drug, initiating immunosuppressants, or both, and rates of recurrent toxicity were lower in patients taking steroids when idelalisib was reinitiated. A decrease in peripheral blood regulatory T cells was seen in patients experiencing toxicity on therapy, which is consistent with an immune-mediated mechanism. These results suggest that caution should be taken as drugs within this class are developed for CLL, particularly in younger patients who have not received prior disease-specific therapy. This study was registered at www.clinicaltrials.gov as #NCT02135133.
Introduction
A major recent advancement in therapy for chronic lymphocytic leukemia (CLL) has been the development of small-molecule inhibitors of the p110 δ isoform (p110δ) of phosphatidyl-inositol-3-kinase (PI3K). These inhibitors block signaling from a variety of prosurvival B-cell surface receptors that converge on the PI3K pathway. Idelalisib is a highly selective oral inhibitor of p110δ that, in combination with anti-CD20 monoclonal antibodies, has shown efficacy for the treatment of relapsed/refractory CLL. Even in heavily pretreated patient populations, responses to these drug combinations are both frequent and durable, with overall response rates of 70% to 80% and median progression free survival in the range of 18 to 19 months.1-3 This success in relapsed, refractory disease has prompted evaluation of idelalisib in the upfront setting, with particular interest for older patients who may have multiple other medical comorbidities. Given this, tolerability is crucial. Major toxicities of p110δ inhibition with idelalisib are enterocolitis, transaminitis, and pneumonitis. In the relapsed/refractory setting, these toxicities have been manageable, with grade ≥3 diarrhea/colitis seen in ∼14% of patients, grade ≥3 transaminitis in 14%, and any-grade pneumonitis in 3%.4 However, the rates of such toxicities when idelalisib is used as upfront therapy for previously untreated disease have not been well established. A phase 2 study has examined front-line idelalisib in patients age 65 or older, with rates of grade ≥3 diarrhea/colitis up to 42% and grade ≥3 transaminitis up to 23%.5,6 These data suggest that toxicity rates may be higher in the front-line setting.
A better understanding of the pathogenesis of these adverse events may facilitate treatment or avoidance of them. An autoimmune mechanism for idelalisib-associated enterocolitis has been hypothesized. Mice with genetic inactivation of p110δ develop an autoimmune colitis.7 Histopathologic data from patients with idelalisib-associated colitis show an intraepithelial lymphocytosis.8,9 Some patients with late-onset idelalisib-associated diarrhea that does not respond to antidiarrheal or empiric antimicrobial therapy are anecdotally responsive to steroids.10 It is unknown if idelalisib-related transaminitis occurs via a similar mechanism.
Given the known efficacy of idelalisib in relapsed/refractory CLL, but also given concern about its toxicities, we report here an initial safety analysis of a phase 2 clinical trial of front-line idelalisib used in combination with the anti-CD20 monoclonal antibody ofatumumab, with the finding that an immune-mediated transaminitis in this setting is frequent and often severe.
Patients and methods
The study was initiated on June 16, 2014 (study 13-309; www.clinicaltrials.gov identifier NCT02135133). Of the 24 patients enrolled in the trial, 23 had CLL, and 1 patient was later shown to have lymphoplasmacytic lymphoma and removed from study. The target enrollment was 50 subjects between the Dana-Farber Cancer Institute and Massachusetts General Hospital Cancer Center, but all upfront combination studies of idelalisib were discontinued by the sponsor in March 2016 due to safety concerns. This analysis is based on data up to November 2015. Institutional review boards at each study site approved the protocol. All authors had full access to study data and were involved in data interpretation, manuscript preparation, revision, and final approval.
Eligibility criteria
Eligible patients were older than 18 years and had previously untreated CLL with an indication for treatment according to the 2008 International Workshop on CLL guidelines.11 Subjects were required to have measurable disease (lymphocytosis >5000 cells/µL or palpable/computed tomography–measurable lymphadenopathy > 1.5 cm) and an Eastern Cooperative Oncology Group performance status of 0 to 2. Exclusion criteria included the following: prior systemic therapy for CLL, serum creatinine ≥2.0 times the upper limit of normal (ULN), total bilirubin ≥1.5 ULN, alanine aminotransferase ≥2.5 ULN, alkaline phosphatase ≥2.5 ULN, positive test result for active hepatitis B or C, active chronic infections requiring treatment, and clinically significant cardiovascular disease. All patients provided written informed consent.
Study treatments
A schematic of the trial design is provided in supplemental Figure 1 (available on the Blood Web site). For the first 56 days, subjects received 150 mg idelalisib twice daily, followed by 6 months of idelalisib plus ofatumumab combination therapy, then followed by idelalisib monotherapy continued indefinitely until progression or toxicity. The initial dose of ofatumumab was 300 mg; all subsequent doses were 1000 mg. Infection prophylaxis was initially optional, but the protocol was subsequently amended to require prophylaxis against Pneumocystis jirovecii pneumonia and herpes simplex virus/varicella-zoster virus after 2 cases of P jirovecii pneumonia occurred.
After identification of hepatotoxicity as a frequent adverse event, the trial protocol was modified in September 2014 to enhance early recognition of transaminitis and standardize treatment. Liver function tests were monitored twice weekly from week 3 to 16. The development of grade 1 transaminitis was treated with 40 mg prednisone daily. Idelalisib was held for the development of grade 2 transaminitis or worsening of grade 1 transaminitis while on steroids. Grade ≥3 transaminitis was treated with 1 mg/kg prednisone in addition to discontinuation of idelalisib. For patients with grade ≥3 transaminitis without immediate response to steroids, mycophenolate mofetil was considered.
Correlative studies
Blood samples were obtained from enrolled subjects and processed by the CLL Research Consortium Tissue Core at the UC San Diego Moores Cancer Center. Ficoll-Hypaque density-gradient centrifugation was used to obtain mononuclear cells. ZAP-70 status and immunoglobulin heavy chain variable (IGHV) mutation status were assessed by the Consortium Tissue Core as per established criteria (see Rassenti et al12,13 for ZAP-70 and Ghia et al,14 Widhopf et al,15 Giudicelli et al,16 and Lefranc et al17 for IGHV). Sequences with <98% homology to the corresponding germline IGHV gene were considered mutated.
Peripheral blood mononuclear cells were isolated from 16 subjects at baseline, 15 subjects at day 28 (±14 days, depending on when toxicity developed), and 5 subjects at day 130 (±21 days, depending on when toxicity developed). At these time points, the patients were experiencing toxicity, but the drug had not yet been held or steroids initiated. Mass cytometric (CyTOF) analysis was performed with a panel of monoclonal antibodies targeting 26 surface-membrane and 9 intracellular markers. The Wilcoxon matched-pairs signed rank test was used to compare percentages of T-cell subsets obtained from CyTOF analysis as well as cytokine concentrations; any samples without a matched baseline time point were not included in significance calculations, and samples with values from day 28 and day 130 were used twice.
Cytokine analysis was performed on serum collected from subjects at the indicated time using the Magnetic Luminex Performance Assay (catalog number FCSTM03-13, R&D Biosystems). Each sample was analyzed in duplicate. Concentrations reported are the average of all values. Mann-Whitney U test was used for statistical comparison.
Statistical analysis
All patients who received any amount of study treatment were included in the analysis. The median time on therapy was 7.7 months (range, 0.7-16.1 months), and median follow-up time was 14.7 months (range, 1.2-16.8 months). All reported P values are 2 sided, and no adjustments have been made for multiple comparisons.
Results
Patient characteristics
At the time of data cutoff, 24 patients had enrolled. Baseline subject characteristics are provided in Table 1. The 24 subjects enrolled had a median age of 67 years (range, 58 to 85 years) and included 6 women and 18 men. Seventeen subjects (71%) had high-risk Rai stage 3-4 disease, and 29% had bulky lymphadenopathy defined by the presence of at least 1 lymph node ≥5 cm. From the 21 patients with bone marrow biopsy specimens at enrollment, lymphocytes comprised a median of 80% (range, 35% to 95%) of the intertrabecular space. Two patients (8%) had del 11q, and an additional four patients (17%) had either del 17p, a TP53 mutation, or both.
Table 1.
Baseline demographic and clinical characteristics of enrolled patients
| Characteristics | N = 24 |
|---|---|
| Age (y) | |
| Median | 67 |
| Range | 58-85 |
| Sex, n (%) | |
| Male | 18 (75%) |
| Female | 6 (25%) |
| Rai stage, n (%) | |
| 0 | 0 (0%) |
| I-II | 7 (29%) |
| III-IV | 17 (71%) |
| Lymphocytes in bone marrow at enrollment (n = 21) | |
| Median | 80% |
| Range | 35%-95% |
| β2 microglobulin (mg/L) | |
| Median | 4.9 |
| Range | 2.4-13.7 |
| IGHV, n (%) | |
| Mutated >2% | 12 (50%) |
| Unmutated | 12 (50%) |
| NOTCH1 c.7541-7542delCT, n (%) | |
| Mutated | 3 (13%) |
| Unmutated | 17 (71%) |
| Unknown | 4 (17%) |
| TP53 mutation or 17p deletion, n (%) | |
| No | 20 (83%) |
| Yes | 4 (17%) |
| 11q deletion, n (%) | |
| No | 22 (92%) |
| Yes | 2 (8%) |
| 13q deletion, n (%) | |
| No | 8 (33%) |
| Yes | 16 (67%) |
| Trisomy 12, n (%) | |
| No | 18 (75%) |
| Yes | 6 (25%) |
| Extent of CLL, n (%) | |
| Bulky lymphadenopathy (≥1 node ≥5 cm diameter) | 7 (29%) |
| Thrombocytopenia (platelets <100 × 109/L) | 14 (58%) |
| Anemia (hemoglobin <11 g/dL) | 7 (29%) |
| Neutropenia (ANC <1.5 × 109/L) | 0 (0%) |
| Absolute lymphocyte count (×109 cells/L) | |
| Median | 44.2 |
| Range | 1.8-236.9 |
| Baseline absolute CD4+ count (×106 cells/L) | |
| Median | 1199 |
| Range | 45-6714 |
| Baseline immunoglobulin G level (mg/dL) | |
| Median | 567 |
| Range | 316-1111 |
ANC, absolute neutrophil count.
Frequency, severity, and timing of hepatotoxicity
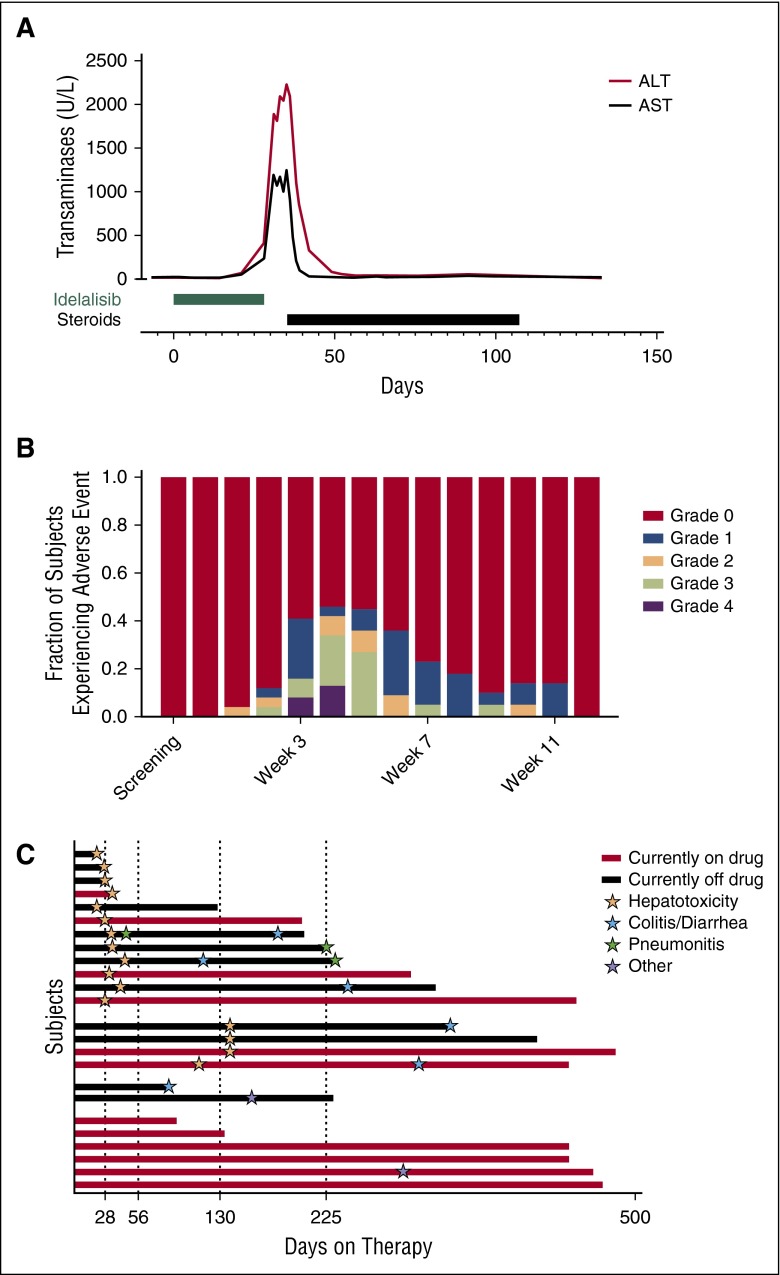
Multiple subjects developed severe hepatotoxicity. In a representative index case, the patient suddenly developed a grade 3 alanine aminotransferase (ALT) and asparate aminotransferase (AST) elevation on day 28 of idelalisib monotherapy (Figure 1A). The drug was stopped. Despite the drug being held, the transaminitis worsened, reaching a maximum AST of 1251 U/L and ALT of 2237 U/L on day 35. On day 34, the subject underwent a liver biopsy, and on day 35, steroids were initiated. The liver function tests normalized after 3 weeks of steroid treatment.
Figure 1.
Transaminitis is frequent and severe in subjects receiving idelalisib monotherapy. (A) An index case of idelalisib-related transaminitis. (B) The fraction of subjects experiencing an ALT elevation of the indicated grade at the indicated time. (C) Swim plot of the indicated toxicities of grade ≥2 at the time that they were first experienced by subjects enrolled in the trial. Other toxicities include one subject with grade 3 rash and 1 subject with grade 3 oral ulcers.
Cumulatively, 19 subjects (79%) experienced at least grade 1 ALT or AST elevation during the study, with 13 subjects (54%) experiencing grade ≥3 transaminitis. The median time to initial development of any grade ≥2 transaminitis was 27 days (range, 14-133 days). This was before the initial administration of ofatumumab on day 56, exonerating ofatumumab as the cause. At week 5, the time of maximum incidence, 11 subjects (46%) had an elevated ALT, with 5 subjects (21%) experiencing grade 3 ALT elevations and 3 subjects (13%) experiencing grade 4 ALT elevations (Figure 1B). The fraction of subjects with ALT abnormalities decreased over time, but this reflected active intervention rather than self-resolution of the process. The pattern of liver injury was hepatocellular, with more severe elevations in the transaminases compared with alkaline phosphatase and bilirubin (supplemental Figure 2). Patients could be divided into 4 groups based on the adverse effects they experienced (Figure 1C). In addition to the 12 subjects who developed early grade ≥2 transaminitis around day 28 of idelalisib therapy, a second group of 4 subjects developed a delayed grade ≥1 hepatotoxicity around day 130. A third group of subjects did not experience hepatotoxicity but discontinued the study due to other adverse events (colitis and rash). A fourth group of subjects tolerated the therapy well, with 4 subjects remaining on therapy for >1 year without experiencing transaminitis, pneumonitis, or colitis.
Frequency of other toxicities
The incidence of any grade adverse event, regardless of whether the event was considered related to the study treatment, was 100%. The incidence of any adverse event of grade 3 was 50% and any adverse event of grade 4 was 38% (Table 2). Transaminase elevations were the most frequent toxicity. Diarrhea and colitis were also frequent, with 46% of patients experiencing diarrhea/colitis (17% grade ≥3). The median time to onset of grade ≥2 diarrhea/colitis was 228 days (range, 100-392 days). Pneumonitis was seen in 13% of patients (8% grade ≥3), with a median time to onset of 117 days (range, 40-223 days). Hematologic toxicities included anemia (8%, with 4% grade ≥3), neutropenia (46%, with 29% grade ≥3), and thrombocytopenia (8%, with 0% grade ≥3). Opportunistic infections included 2 cases of P jirovecii pneumonia, 1 case of Aspergillus pneumonia, 1 brain abscess due to an unknown organism (thought likely to be P jirovecii) that resolved on trimethoprim/sulfamethoxazole, 1 case of cytomegalovirus colitis, and 1 case of herpes simplex virus esophagitis. There were no deaths on study or during the follow-up period.
Table 2.
Adverse events experienced by at least 2 subjects during the trial
| Event | Any grade | Grade 3 or 4 |
|---|---|---|
| ALT increase | 19 (79%) | 13 (54%) |
| AST increase | 19 (79%) | 10 (42%) |
| Colitis/diarrhea/enteritis | 11 (46%) | 4 (17%) |
| Blood bilirubin level increase | 10 (42%) | 1 (4%) |
| Rash | 8 (33%) | 3 (13%) |
| Alkaline phosphatase level increase | 8 (33%) | 0 (0%) |
| Nausea/vomiting | 8 (33%) | 0 (0%) |
| Abdominal pain | 7 (29%) | 0 (0%) |
| Constipation | 7 (29%) | 0 (0%) |
| Myalgia | 5 (21%) | 1 (4%) |
| Arthralgia | 5 (21%) | 0 (0%) |
| Cough | 5 (21%) | 0 (0%) |
| Edema | 5 (21%) | 0 (0%) |
| Chills | 4 (17%) | 0 (0%) |
| Dysgeusia | 4 (17%) | 0 (0%) |
| Night sweats | 4 (17%) | 0 (0%) |
| Insomnia | 4 (17%) | 0 (0%) |
| Weight loss | 4 (17%) | 0 (0%) |
| Lung infection | 3 (13%) | 3 (13%) |
| Hyperglycemia | 3 (13%) | 2 (8%) |
| Pneumonitis | 3 (13%) | 2 (8%) |
| Hypotension | 3 (13%) | 1 (4%) |
| Infusion-related reaction | 3 (13%) | 1 (4%) |
| Anorexia | 3 (13%) | 0 (0%) |
| Dizziness | 3 (13%) | 0 (0%) |
| Dry mouth | 3 (13%) | 0 (0%) |
| Dry skin | 3 (13%) | 0 (0%) |
| Dyspnea | 3 (13%) | 0 (0%) |
| GERD | 3 (13%) | 0 (0%) |
| Hyponatremia | 2 (8%) | 2 (8%) |
| Anemia | 2 (8%) | 1 (4%) |
| Hypertension | 2 (8%) | 1 (4%) |
| Oral mucositis | 2 (8%) | 1 (4%) |
| Alopecia | 2 (8%) | 0 (0%) |
| Back pain | 2 (8%) | 0 (0%) |
| Bronchial infection | 2 (8%) | 0 (0%) |
| Depression | 2 (8%) | 0 (0%) |
| Nasal congestion | 2 (8%) | 0 (0%) |
| Platelet count decreased | 2 (8%) | 0 (0%) |
| Polyuria | 2 (8%) | 0 (0%) |
| White blood cell count decreased | 2 (8%) | 0 (0%) |
Clinical risk factors for early hepatotoxicity
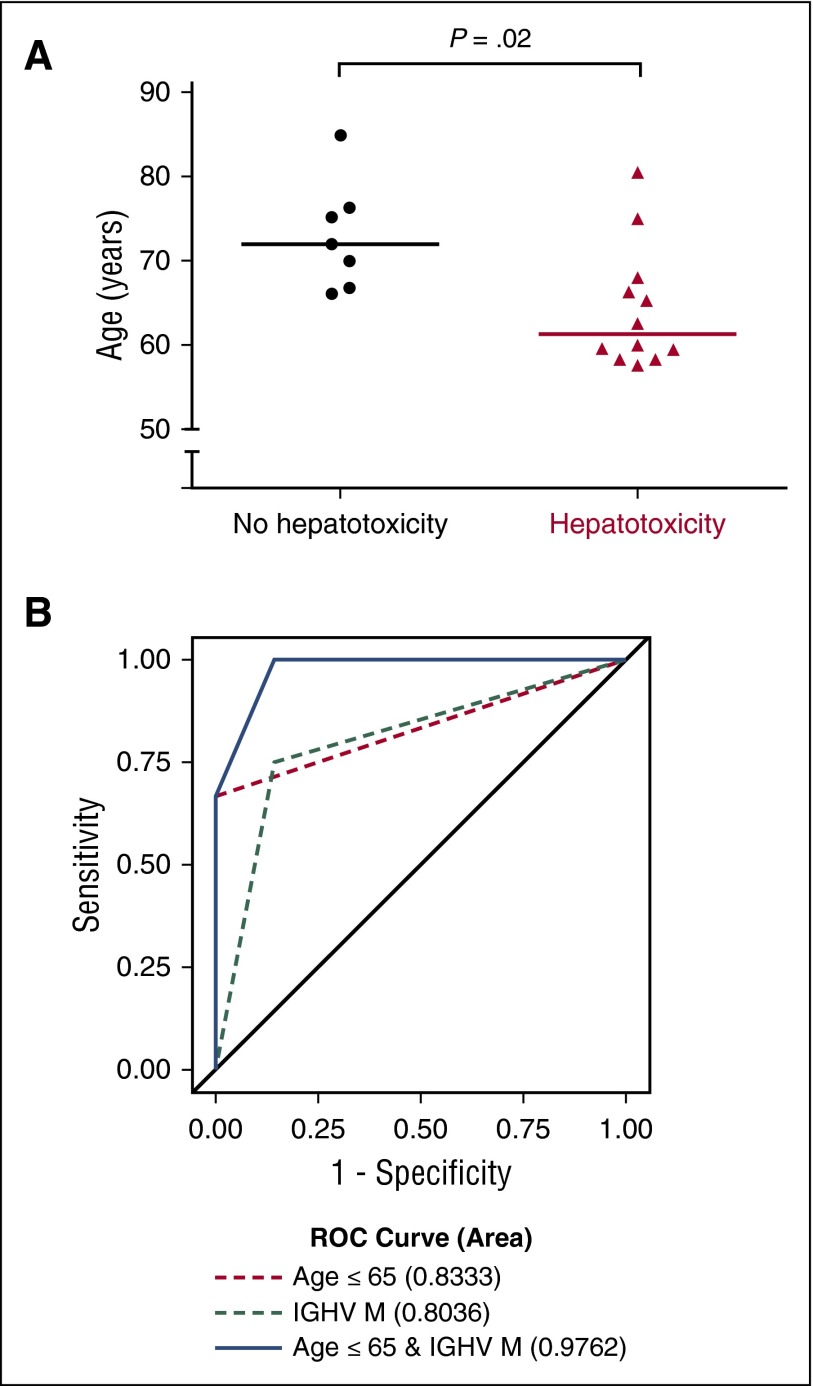
A younger age was a significant risk factor for the development of early hepatotoxicity (Figure 2A). The median age of subjects who did not have any hepatotoxicity was 72 years, whereas the median age of subjects who did have early hepatotoxicity was 61 years (P = .02). All subjects under age 65 required systemic steroids for immune-mediated toxicities at some point during the trial. A total of 75% of patients with mutated IGHV experienced early hepatotoxicity, whereas only 25% of patients with unmutated IGHV experienced early hepatotoxicity (P = .039). The combination of age and IGHV status was highly predictive of early hepatotoxicity, with an area under the receiver-operating characteristic curve of 0.9762 (Figure 2B). Measurements of disease burden, including β2-microglobulin, LDH, absolute lymphocyte count, Rai stage at baseline, intertrabecular bone marrow involvement at screening, and computed tomography–assessed lymph node size burden were not predictive of early hepatotoxicity (supplemental Figure 3).
Figure 2.
Clinical factors are associated with the development of early hepatotoxicity. (A) Significant difference in age at enrollment between subjects who experienced no toxicity on trial and those who experienced early hepatotoxicity (P = .02, Mann-Whitney U test). (B) Receiver-operating characteristic (ROC) curves for mutated (M) IGHV status, age ≤65 years, or a combination of the two for predicting the occurrence of early hepatotoxicity.
Histologic findings on liver biopsy of affected subjects
No viral etiology for the transaminitis could be identified, and no other drug culprits were suspected. All subjects tested negative for hepatitis B surface antigen and hepatitis C antibody at enrollment. Hepatitis B surface antibody alone was positive in 5 subjects, consistent with immunization. Hepatitis B surface antibody and core antibody were positive at enrollment in 2 subjects who developed transaminitis, but one of these subjects was on monthly intravenous immunoglobulin infusions (a possible source of false-positive serologies), and in both cases, the viral load remained undetectable. Upon developing transaminitis, 4 subjects underwent repeat testing for hepatitis A, B, and C, as well as EBV and cytomegalovirus, which in all cases was negative.
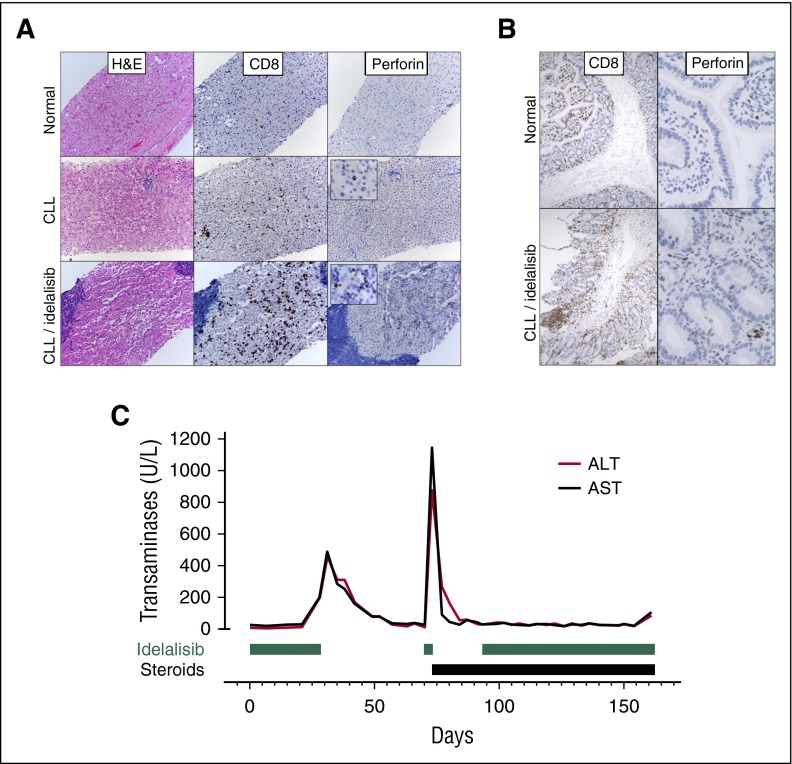
In order to determine the cause of hepatotoxicity, 2 patients who developed severe transaminitis underwent liver biopsy. These specimens were compared with specimens taken from a normal liver and from the liver of an otherwise healthy patient with CLL. No significant mononuclear infiltrate was seen in healthy liver, whereas clusters of CD20+ periportal lymphocytes and scattered intraparenchymal CD8+, perforin-negative cytotoxic T cells were seen in the patient with CLL, consistent with previous reports.18 However, biopsy specimens from the CLL patients experiencing idelalisib-related transaminitis showed an increased infiltrate of CD8+ cytotoxic T cells that also stained positive for perforin, indicating an activated state (Figure 3A). Similar biopsy findings were noted in sections of duodenum taken from a subject on trial experiencing idelalisib-related enteritis, with CD8+ cytotoxic lymphocytes infiltrating the lamina propria (Figure 3B).
Figure 3.
Tissue biopsies and response to steroids suggest an immune-mediated cause for hepatotoxicity. (A) Liver biopsy specimens taken from a normal patient, an otherwise healthy patient with CLL, and a subject with persistent transaminitis after discontinuation of idelalisib. Liver biopsy specimens from patients with CLL demonstrate lymphoid aggregates on hematoxylin and eosin staining (composed of CD20+ B cells, data not shown) as well as increased CD3+ T cells scattered throughout the parenchyma that are increased in number and activation (as assessed by perforin staining) in a subject on idelalisib. (B) A duodenal biopsy specimen from a subject with diarrhea on idelalisib reveals an activated (perforin-positive) CD8+ lymphocytic infiltrate, which is not present in a control sample taken from a subject without CLL. (C) Transaminase levels over time for a subject who, after an unsuccessful attempt at idelalisib reintroduction, was successfully reintroduced to the drug while on steroids.
Use of steroids to treat hepatotoxicity
All cases of transaminitis resolved by holding the drug, initiating immunosuppression (1 subject required mycophenolate mofetil in addition to steroids), or both. Many subjects were initiated on steroids around day 28, and a second group of subjects required steroids around day 130 (supplemental Figure 4). The median time from initiation of steroids to reduction of transaminitis to grade ≤1 was 8 days (range, 3-21 days). Patients who developed hepatotoxicity but were able to remain on study drug at the time of data cutoff had a short time from onset of transaminitis to initiation of steroids (median, 0 days; range, 0-4 days) and had long steroid tapers (median, 129 days; range, 31-235 days). In comparison, subjects who developed hepatotoxicity and eventually had to discontinue drug had a longer time to steroid initiation (median, 7 days; range, 0-17 days) and shorter steroid tapers (median, 70 days; range, 7-198 days). By the end of the trial, 16 subjects (67%) had received steroids for transaminitis and 19 subjects (79%) had received steroids for presumed autoimmune toxicities.
In 12 instances, subjects who developed grade ≥2 transaminitis were rechallenged with idelalisib after it was held for a median of 21 days (range, 10-43 days). Transaminases had normalized for at least 1 week prior to the reintroduction of idelalisib. Six subjects developed recurrent transaminitis. In all cases of recurrent transaminitis, the elevated liver enzymes developed within 1 to 4 days of re-exposure to idelalisib (for example, Figure 3C). A total of 5 subjects were rechallenged while off steroids, and 4 developed recurrent transaminitis (grade 2, 1; grade 3, 2; and grade 4, 1). Seven subjects were rechallenged while on steroids, and 2 developed recurrent transaminitis (grade 3, 1; grade 2, 1). This protective effect of steroids was also noted when all episodes of recurrent transaminitis, pneumonitis, or colitis were examined in aggregate. In total, after an initial episode of transaminitis, pneumonitis, or colitis, resumption of idelalisib led to 22 subsequent, separate additional grade ≥2 toxicities (including only transaminitis, pneumonitis, or colitis). Of these, 17 occurred while subjects were off steroids (grade 2, 9; grade 3, 7; grade 4, 1), and 5 occurred while subjects were on steroids (grade 2, 3; grade 3, 2; and grade 4, 0).
Cytokine analysis
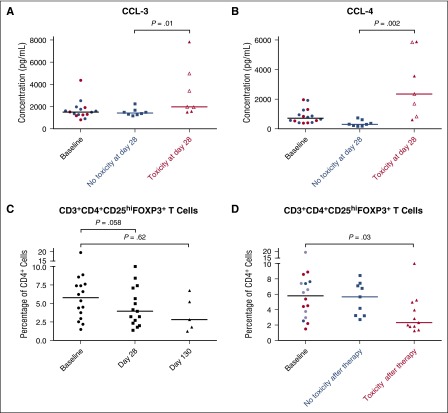
We measured concentrations of a panel of cytokines in serum obtained from patients at baseline and at day 28, the time of maximum incidence of hepatotoxicity. At day 28, patients experiencing hepatotoxicity, compared with those without hepatotoxicity, had significantly higher levels of the proinflammatory cytokines CCL-3 (median, 1983 pg/mL vs 1429 pg/mL, P = .01; Figure 4A) and CCL-4 (median, 2351 pg/mL vs 303 pg/mL, P = .002; Figure 4B), consistent with an underlying inflammatory process as the cause of the transaminitis. Subjects with and without hepatotoxicity showed no significant difference in the other tested cytokines (CCL-2, CXCL-5, and vascular endothelial growth factor) at baseline or at day 28 (supplemental Figure 5).
Figure 4.
Increased inflammatory cytokine levels and decreased regulatory T-cell levels are associated with the development of toxicities while on idelalisib. (A) Serum CCL-3 and (B) CCL-4 levels in subjects at various time points on idelalisib therapy. Baseline values indicated in red are subjects who experienced toxicity at day ∼28, and blue represents subjects who did not experience early hepatotoxicity. Open symbols represent levels drawn when subjects have held idelalisib for 3 to 9 days due to toxicity; closed symbols represent samples drawn while subjects remain on idelalisib. (C) Percentage of CD4+ T cells that are FoxP3+CD25hi regulatory T cells in subjects on idelalisib. (D) Percentage of CD4+ T cells that are CD3+CD4+ FoxP3+CD25hi regulatory T cells in subjects on idelalisib, stratified by toxicity. At baseline, values indicated in red are subjects who experienced toxicity at day ∼28, blue symbols are subjects who did not experience toxicity, and purple symbols are subjects who experienced delayed toxicity and thus contributed data points to the no toxicity group at day ∼28 and the toxicity group at day ∼130.
Changes in regulatory T cells on idelalisib
Inactivation of p110δ in mice decreases the number and function of regulatory T cells (Tregs).19 Through the use of mass cytometry, we investigated the effects of idelalisib on T cells in patients exposed to idelalisib. Samples of peripheral blood mononuclear cells had been collected from patients at baseline, day 28, and day 130 (at the time of late hepatotoxicity). At day 28, the fraction of CD3+ T cells that were CD4+ helper cells trended higher, whereas CD8+ cytotoxic cells trended lower (supplemental Figure 6A-B). Decreases in the Treg population were frequent. At baseline, the median percentage of total CD4+ T cells that were CD3+CD4+ FoxP3+CD25hi Treg cells was 5.8%. This decreased to a median of 4.0% after 28 days on therapy and 2.8% after 130 days on therapy, although these decreases were not statistically significant (Figure 4C). Out of 19 matched pairs, 13 patients (68%) experienced a decrease in Treg percentage while on therapy, with a median relative loss of 42% of the Treg fraction. When patients were stratified by toxicity at the time of sample collection (including those with transaminitis or colitis), those without toxicity had a median Treg percentage of 5.7%, whereas those experiencing toxicity had a significantly lower median Treg percentage of 2.3% (p=.03, Figure 4D). In patients experiencing toxicity on idelalisib, there was a trend toward a lower Treg:CD4+ ratio (supplemental Figure 6C).
Discussion
Idelalisib is a p110δ inhibitor that has shown impressive efficacy for the treatment of relapsed, refractory CLL. Here, we found that idelalisib used as upfront therapy for CLL caused an early, severe (grade ≥3) hepatotoxicity in 54% of patients. Subjects who are younger and subjects with IGHV mutated disease were more likely to experience this early hepatotoxicity. Steroids were effective at treating the transaminitis once it developed, and the incidence of recurrent hepatotoxicity was lower if subjects were receiving steroids at the time of reintroduction of the study drug. In keeping with preclinical data, idelalisib caused a reduction in the number of Tregs in the peripheral blood, particularly in patients experiencing toxicities.
Multiple lines of evidence point to an autoimmune mechanism as the cause of the hepatotoxicity seen. Perhaps surprisingly, younger age was identified as a risk factor for early hepatitis, consistent with the view that younger subjects may have a more robust immune system than older subjects. An activated T-cell infiltrate is seen on liver biopsy specimens of patients who experienced idelalisib-related hepatitis, but not in otherwise healthy patients with CLL. Immunosuppression with steroids (and, in a single case, mycophenolate mofetil) was effective at treating the transaminitis and decreasing the incidence of subsequent transaminitis upon re-exposure to the drug. The observation that the transaminitis takes weeks to develop but then rapidly recurs upon re-exposure to idelalisib is suggestive of liver injury secondary to an adaptive immune response.20 Cytokine analysis demonstrated increased levels of CCL-3 and CCL-4, which are known to play proinflammatory roles in murine21 and human22 immune-mediated hepatitis. CyTOF analysis of the peripheral blood did show a decrease in Tregs, which would correlate with an inflammatory state.
Preclinical evidence also supports the conclusion that on-target inhibition of p110δ by idelalisib can cause autoimmune toxicities and furthermore that the mechanism underlying this could be due to effects on Tregs. Mice with genetic inactivation of p110δ develop an autoimmune colitis characterized by numerous intraepithelial lymphocytes.7,23 Systemic p110δ inactivation has broad effects on the immune system, decreasing B-cell function and number as well as decreasing the ability of naive T cells to differentiate into both Th1 and Th2 subtypes.7,24,25 However, on an organismal level, this impaired effector cell function is often counterbalanced by inhibition of Tregs, as p110δ is also critical for the survival and function of Tregs.19 For example, despite the fact that both CD8+ and CD4+ T cells from p110δ-deficient mice demonstrate reduced production of cytotoxic mediators and interferon-γ, respectively, mice with genetic inactivation of p110δ are resistant to tumorigenesis. Adoptive transfer experiments showed that p110δ inactivation in Tregs is both necessary and sufficient to confer this resistance to tumor growth.26 In agreement with this, germline genetic mutations that disrupt Treg function in mice and humans, including FOXP3, lead to autoimmune syndromes with hepatitis, enteritis, and pneumonitis.27,28
The rates of grade ≥3 transaminitis reported here are higher than rates previously reported for this drug, as summarized in Table 3. In the phase 1 trial, subjects were the most heavily pretreated, with a median of 5 prior therapies, and the rate of grade ≥3 hepatotoxicity was the lowest, at 2%.29 When toxicity data were aggregated from 8 clinical trials examining idelalisib used as later-line treatment of indolent B-cell malignancies, the rate of grade ≥3 transaminitis was 14%.4 In addition to the trial presented here, one other published trial examined idelalisib in the upfront setting, given to patients age 65 or older. In the cohort treated with combination rituximab therapy, 67% of subjects experienced a transaminase elevation of any grade (23% grade ≥3)6; in the cohort treated with idelalisib alone, 24% experienced a transaminase elevation (22% grade ≥3).5 Thus, as the median age and number of prior therapies increased, the frequency of immune-mediated adverse events decreased. This may reflect the long-lasting effects that some CLL therapies, and the disease itself, can have on the immune system, as well as an accruing immune senescence with age. For example, for at least 2 years after fludarabine therapy, absolute CD4+ and CD8+ T-cell counts remain less than half pretreatment levels.30 In keeping with these observations, when idelalisib was given to healthy young volunteers, toxicity was frequent, with 5 subjects out of 24 (21%) experiencing grade 3 transaminitis after only 7 to 9 days on 150 mg idelalisib twice daily.31 The association between IGHV mutation status and early hepatotoxicity may reflect a unique interaction between mutated IGHV neoplastic cells and T-cell subsets.32 For example, 2 studies have previously reported that patients with mutated IGHV CLL have reduced numbers of Tregs.33,34 We did find a lower baseline median percentage of Tregs in our patients with IGHV mutated disease (data not shown), although the difference was not statistically significant.
Table 3.
Idelalisib-related toxicities compared across multiple clinical trials
| Phase 1 | Overall relapsed | Upfront patients ≥65 y with rituximab | Upfront patients ≥65 y idelalisib monotherapy | Current trial | |
|---|---|---|---|---|---|
| Number of subjects | 54 | 760 | 64 | 41 | 24 |
| Median prior therapies | 5 (2-14) | ≥1 | 0 | 0 | 0 |
| Median age | 63 (37-82) | 66 (21-91) | 71 (65-90) | 71 (65-84) | 67 (58-85) |
| Median time on therapy (mo) | 15 (0.2-48.7) | — | 22.4 (0.8-45.8) | 9.3 (1.4-17.4) | 7.7 (0.7-16.1) |
| Any grade transaminitis | 28% | 48% | 67% | 24% | 79% |
| Grade ≥3 transaminitis | 1.9% | 14% | 23% | 22% | 54% |
| Grade ≥3 colitis/diarrhea | 5.6% | 14% | 42% | 27% | 13% |
| Any grade pneumonitis | 5.6% | 3% | 3% | 5% | 13% |
| Reference | Brown et al29 | Coutre et al4 | O’Brien et al6 | Zelenetz et al5 |
Transaminitis is seen when other drugs within this class are tested in the upfront setting, as would be expected if hepatotoxicity is due to on-target p110δ inhibition. TGR-1202 is a p110δ inhibitor with hepatotoxicity seen in 2% of patients treated for relapsed, refractory disease.35 However, a recent trial combining TGR-1202 with obinutuzumab and chlorambucil for CLL treatment had rates of grade ≥3 transaminitis of 28%.36 Eighty-three percent of the subjects in this 18-patient trial were treatment naive. Our group recently reported preliminary results of the phase 1 portion of a trial of the p110δ/γ inhibitor duvelisib in combination with fludarabine, cyclophosphamide, and rituximab for the upfront treatment of CLL. We observed a 29% rate of grade ≥3 transaminitis, occurring at time points similar to those reported here.37 In this duvelisib trial, the combination of p110δ inhibition with chemoimmunotherapy or concomitant p110γ inhibition may have decreased the overall rates of autoimmune toxicity.
Limitations of the study include the small number of patients enrolled and the correspondingly small number of samples available for correlative studies. Our analysis here focuses on the characteristics of the early hepatotoxicity seen at day 28 and may not be applicable to other toxicities seen with the drug, including enterocolitis, pneumonitis, and the delayed hepatotoxicity occurring around day 130. Correlative studies regarding the mechanism of drug-mediated hepatotoxicity were analyzed retrospectively, and while these findings are important for future hypothesis generation, they cannot show causation.
While this manuscript was in preparation, Gilead closed seven randomized trials of idelalisib in B-cell malignancies (5 in treatment-naive patients) due to an excess of infectious deaths. In our study, opportunistic infections were noted prior to mandating prophylaxis. Further studies will be required to determine any relationship between these infectious toxicities and the autoimmune toxicities seen here, but both may relate to the immunologic effects of inhibiting p110δ. As additional drugs are developed within this class, subjects should be closely monitored for infection as well as transaminitis (and other autoimmune phenomena), with a low threshold for infectious prophylaxis for the former and immunosuppressants for the latter, based on the likely immune-mediated origin of this toxicity.
Acknowledgments
The authors thank all the patients and their families who participated in this trial and donated extra blood and bone marrow for analysis.
This study is an investigator-initiated trial run by J.R.B. and funded by Gilead and Novartis; both companies provided their study drug at no cost. Correlative studies were funded by the Dana-Farber Cancer Institute Medical Oncology Grant program 2015, as well as the Melton Family Fund for CLL Research and the Susan and Gary Rosenbach Fund for Lymphoma Research. This work was supported in part by a grant from the National Institutes of Health, National Cancer Institute (PO1-CA81534) of the CLL Research Consortium (L.R. and T.J.K.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.R.B. designed the trial; B.L.L., S.N.K., T.R.M., E.A.M., J.R., and J.R.B. performed the research and analyzed data; J.F. and J.H. collected and analyzed data; M.S.D., D.C.F., A.S.F., C.A.J., P.A., J.S.A., J.E.A., and J.R.B. enrolled patients; L.R., S.F., and T.J.K. managed sample collection for correlative analyses; H.T.K., S.K., and T.M. performed statistical analyses; B.L.L. wrote the manuscript with input from J.R.B.; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: J.R.B. has served as a consultant for Gilead, Infinity, Janssen, Pharmacyclics, and Roche/Genentech. M.S.D. has served as a consultant for Gilead, Infinity, Janssen, Pharmacyclics, TG Therapeutics, Abbvie, and Roche/Genentech. J.S.A has served as a consultant for Gilead, Infinity, and Pharmacyclics. The remaining authors declare no competing financial interests.
The current affiliation for T.R.M. is Instituto de Medicina Molecular, Faculdade de Medicina da Universidade de Lisboa, Lisbon, Portugal.
The current affiliation for J.F. is Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Correspondence: Jennifer R. Brown, Dana-Farber Cancer Institute, Mayer Building Room 226, 450 Brookline Ave, Boston, MA 02215; e-mail: jennifer_brown@dfci.harvard.edu.
References
- 1.Sharman JP, Coutre SE, Furman RR, et al. Second interim analysis of a phase 3 study of idelalisib (ZYDELIG®) plus rituximab (R) for relapsed chronic lymphocytic leukemia (CLL): Efficacy analysis in patient subpopulations with del(17p) and other adverse prognostic factors [abstract]. Blood. 2014;124(21) Abstract 330. [Google Scholar]
- 2.Coutre SE, Leonard JP, Furman RR, et al. Update on a phase 1 study of the selective PI3K-delta inhibitor, idelalisib (GS-1101) in combination with ofatumumab in patients with relapsed or refractory chronic lymphocytic leukemia. Haematologica. 2013;98(suppl 1):1. [Google Scholar]
- 3.De Vos S, Leonard JP, Barrientos JC, et al. A phase 1 study of the selective PI3Kδ inhibitor idelalisib (GS-1101) in combination with therapeutic anti-CD20 antibodies (rituximab or ofatumumab) in patients with relapsed or refractory chronic lymphocytic leukemia [abstract]. Blood. 2013;122(21) Abstract 4180. [Google Scholar]
- 4.Coutre S, Barrientos JC, Brown JR, et al. Safety of idelalisib in B-cell malignancies: Integrated analysis of eight clinical trials. ASCO Meeting Abstracts. 2015;33(15_suppl):e18030. [Google Scholar]
- 5.Zelenetz AD, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib monotherapy in previously untreated patients ≥65 years with chronic lymphocytic leukemia or small lymphocytic lymphoma. Paper presented at the Annual Meeting of the American Society of Hematology. December 6, 2014. San Francisco, CA. [Google Scholar]
- 6.O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–2694. doi: 10.1182/blood-2015-03-630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297(5583):1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 8.Louie CY, DiMaio MA, Matsukuma KE, Coutre SE, Berry GJ, Longacre TA. Idelalisib-associated enterocolitis: Clinicopathologic features and distinction from other enterocolitides. Am J Surg Pathol. 2015;39(12):1653–1660. doi: 10.1097/PAS.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 9.Weidner AS, Panarelli NC, Geyer JT, et al. Idelalisib-associated colitis: histologic findings in 14 patients. Am J Surg Pathol. 2015;39(12):1661–1667. doi: 10.1097/PAS.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 10.Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56(10):2779–2786. doi: 10.3109/10428194.2015.1022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallek M, Cheson BD, Catovsky D, et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351(9):893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 13.Rassenti LZ, Jain S, Keating MJ, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112(5):1923–1930. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghia EM, Widhopf GF, II, Rassenti LZ, Kipps TJ. Analyses of recombinant stereotypic IGHV3-21-encoded antibodies expressed in chronic lymphocytic leukemia. J Immunol. 2011;186(11):6338–6344. doi: 10.4049/jimmunol.0902875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widhopf GF, II, Rassenti LZ, Toy TL, Gribben JG, Wierda WG, Kipps TJ. Chronic lymphocytic leukemia B cells of more than 1% of patients express virtually identical immunoglobulins. Blood. 2004;104(8):2499–2504. doi: 10.1182/blood-2004-03-0818. [DOI] [PubMed] [Google Scholar]
- 16.Giudicelli V, Chaume D, Jabado-Michaloud J, Lefranc MP. Immunogenetics sequence annotation: The strategy of IMGT based on IMGT-ONTOLOGY. Stud Health Technol Inform. 2005;116:3–8. [PubMed] [Google Scholar]
- 17.Lefranc MP, Giudicelli V, Kaas Q, et al. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 2005;33(Database issue):D593–D597. doi: 10.1093/nar/gki065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumhoer D, Tzankov A, Dirnhofer S, Tornillo L, Terracciano LM. Patterns of liver infiltration in lymphoproliferative disease. Histopathology. 2008;53(1):81–90. doi: 10.1111/j.1365-2559.2008.03069.x. [DOI] [PubMed] [Google Scholar]
- 19.Patton DT, Garden OA, Pearce WP, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177(10):6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 20.Holt MP, Ju C. Mechanisms of drug-induced liver injury. AAPS J. 2006;8(1):E48–E54. doi: 10.1208/aapsj080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajuebor MN, Hogaboam CM, Le T, Proudfoot AE, Swain MG. CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4(+) T cells to the liver. Eur J Immunol. 2004;34(10):2907–2918. doi: 10.1002/eji.200425071. [DOI] [PubMed] [Google Scholar]
- 22.Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104(1):49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitamura K, Farber JM, Kelsall BL. CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J Immunol. 2010;185(6):3295–3304. doi: 10.4049/jimmunol.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soond DR, Bjørgo E, Moltu K, et al. PI3K p110delta regulates T-cell cytokine production during primary and secondary immune responses in mice and humans. Blood. 2010;115(11):2203–2213. doi: 10.1182/blood-2009-07-232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clayton E, Bardi G, Bell SE, et al. A crucial role for the p110delta subunit of phosphatidylinositol 3-kinase in B cell development and activation. J Exp Med. 2002;196(6):753–763. doi: 10.1084/jem.20020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali K, Soond DR, Piñeiro R, et al. Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer. Nature. 2014;510(7505):407–411. doi: 10.1038/nature13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nat Rev Immunol. 2014;14(5):343–349. doi: 10.1038/nri3650. [DOI] [PubMed] [Google Scholar]
- 28.Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120(4):744–750, quiz 751-752. doi: 10.1016/j.jaci.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 29.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keating MJ, O’Brien S, Lerner S, et al. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92(4):1165–1171. [PubMed] [Google Scholar]
- 31.Jin F, Robeson M, Zhou H, et al. Clinical drug interaction profile of idelalisib in healthy subjects. J Clin Pharmacol. 2015;55(8):909–919. doi: 10.1002/jcph.495. [DOI] [PubMed] [Google Scholar]
- 32.Zaborsky N, Holler C, Geisberger R, et al. B-cell receptor usage correlates with the sensitivity to CD40 stimulation and the occurrence of CD4+ T-cell clonality in chronic lymphocytic leukemia. Haematologica. 2015;100(8):e307–e310. doi: 10.3324/haematol.2015.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motta M, Rassenti L, Shelvin BJ, et al. Increased expression of CD152 (CTLA-4) by normal T lymphocytes in untreated patients with B-cell chronic lymphocytic leukemia. Leukemia. 2005;19(10):1788–1793. doi: 10.1038/sj.leu.2403907. [DOI] [PubMed] [Google Scholar]
- 34.Weiss L, Melchardt T, Egle A, Grabmer C, Greil R, Tinhofer I. Regulatory T cells predict the time to initial treatment in early stage chronic lymphocytic leukemia. Cancer. 2011;117(10):2163–2169. doi: 10.1002/cncr.25752. [DOI] [PubMed] [Google Scholar]
- 35.Burris HA, Patel MR, Fenske TS, et al. TGR-1202, A novel once daily PI3K-delta inhibitor, demonstrates clinical activity with A favorable safety profile, lacking hepatotoxicity, in patients with CLL and B-cell lymphoma. Haematologica. 2015;100(suppl 1):1–3. [Google Scholar]
- 36.Mahadevan D, Pauli EK, Cutter K, et al. A phase I trial of TGR-1202, a next generation once daily PI3K-delta inhibitor in combination with obinutuzumab plus chlorambucil, in patients with chronic lymphocytic leukemia [abstract]. Blood. 2015;126(23) Abstract 2942. [Google Scholar]
- 37.Davids MS, Kim HT, Gilbert E, et al. Preliminary results of a phase ib study of duvelisib in combination with FCR (dFCR) in previously untreated, younger patients with CLL [abstract]. Blood. 2015;126(23) Abstract 4158. [Google Scholar]