Key Points
Patients homozygous for HLA-C2 group alleles have worse outcomes after CBT.
CB selection based on the combination of NK licensing and activating KIRs may improve outcomes after CBT.
Abstract
The ability of cord blood transplantation (CBT) to prevent relapse depends partly on donor natural killer (NK) cell alloreactivity. NK effector function depends on specific killer-cell immunoglobulin-like receptors (KIR) and HLA interactions. Thus, it is important to identify optimal combinations of KIR-HLA genotypes in donors and recipients that could improve CBT outcome. We studied clinical data, KIR and HLA genotypes, and NK-cell reconstitution in CBT patients (n = 110). Results were validated in an independent cohort (n = 94). HLA-KIR genotyping of recipient germline and transplanted cord blood (CB) grafts predicted for large differences in outcome. Patients homozygous for HLA-C2 group alleles had higher 1-year relapse rate and worse survival after CBT than did HLA-C1/C1 or HLA-C1/C2 (HLA-C1/x) patients: 67.8% vs 26.0% and 15.0% vs 52.9%, respectively. This inferior outcome was associated with delayed posttransplant recovery of NK cells expressing the HLA-C2-specific KIR2DL1/S1 receptors. HLA-C1/x patients receiving a CB graft with the combined HLA-C1-KIR2DL2/L3/S2 genotype had lower 1-year relapse rate (6.7% vs 40.1%) and superior survival (74.2% vs 41.3%) compared with recipients of grafts lacking KIR2DS2 or HLA-C1. HLA-C2/C2 patients had lower relapse rate (44.7% vs 93.4%) and better survival (30.1% vs 0%) if they received a graft with the combined HLA-C2-KIR2DL1/S1 genotype. Relapsed/refractory disease at CBT, recipient HLA-C2/C2 genotype, and donor HLA-KIR genotype were independent predictors of outcome. Thus, we propose the inclusion of KIR genotyping in graft selection criteria for CBT. HLA-C1/x patients should receive an HLA-C1-KIR2DL2/L3/S2 CB graft, while HLA-C2/C2 patients may benefit from an HLA-C2-KIR2DL1/S1 graft.
Introduction
Natural killer (NK) cells are an important component of the graft-versus-leukemia response, which is critical to preventing relapse after allogeneic hematopoietic stem cell transplantation (HSCT) for high-risk hematologic malignancies.1,2 Each mature NK cell expresses a wide array of activating and inhibitory killer immunoglobulin-like receptors (KIRs), which are specific for different HLA class I molecules.3-5 The ability of NK cells to recognize and kill malignant cells is governed by complex and poorly understood interactions between inhibitory signals resulting from the binding of inhibitory KIRs with their cognate HLA class I ligands, and activating signals from both the inhibitory KIRs that fail to interact with their appropriate HLA-ligand (“missing self”) and those from activating receptors.1,5,6 The inhibitory KIR2DL group of receptors interacts with HLA-C molecules, which can be divided into 2 mutually exclusive groups, based on a polymorphism in the α-1 chain.7,8 KIR2DL2 and KIR2DL3 recognize HLA molecules in the HLA-C1 group, whereas KIR2DL1 and the activating KIR2DS1 receptors recognize the HLA-C2 group.9,10 To become functional, NK cells must undergo a continuous process of “education” or “licensing.”11-13 This phenomenon is achieved when inhibitory KIRs interact with their cognate ligand (eg, KIR2DL2 with an HLA-C molecule in the C1 group) during development. This process directs NK cell functional maturation and enables their discrimination of self from missing-self.14 Despite evidence that specific combinations of HLA and KIR molecules in the donor are related to transplantation outcomes,1,15-17 the incorporation of KIR genotype in donor selection criteria has been slow to gain traction.
Cord blood (CB) transplantation (CBT) has extended access to HSCT for many patients with hematologic malignancies who lack an HLA-matched donor, especially those from racial and ethnic minorities.18-20 Because of its less stringent HLA matching requirements, this procedure affords a choice of multiple suitable CB units, potentially allowing the selection of units that could maximize the graft-versus-leukemia response based on favorable combinations of activating and inhibitory KIRs and their HLA ligands. We therefore studied specific combinations of KIR receptors and HLA ligands in patients undergoing CBT to identify those most closely linked to clinical outcome.
Methods
Patients
All patients who received a CBT at MD Anderson Cancer Center (MDACC) between July 2005 and December 2012 under standardized protocols for the treatment of different hematologic malignancies were eligible for this analysis. HLA genotypes were provided by the HLA Typing Laboratory at MDACC. KIR genotyping was performed with the sequence-specific priming KIR genotyping kit (Invitrogen, Carlsbad, CA) as described previously,21 based on the availability of specimens and without preference for patients with particular clinical characteristics. For the discovery and validation studies, patients were selected sequentially, and no patient was included in both studies.
Our discovery cohort included 110 patients who had undergone CBT between 2009 and 2012 and had available genomic DNA from both the recipient and the CB graft (Table 1). The median follow-up for surviving patients at the time of analysis was 14 months (range, 2-64 months). An independent cohort of 94 consecutive patients who received CBT between 2005 and 2009 and had features similar to those of the study group (Table 2) was used to validate our results. This study was performed in accord with the Declaration of Helsinki following informed consent and was approved by the local institutional review board.
Table 1.
Patient characteristics and outcomes in the discovery (n = 110) and validation (n = 94) cohorts: discovery cohort
| 1-y overall survival | 1-y relapse rate | ||||
|---|---|---|---|---|---|
| n | Probability (%) | HR (95%CI) (risk of death) | Cumulative incidence (%) | HR (95%CI) (risk of relapse) | |
| Age* | P = .08 | P = .74 | |||
| ≤40 y | 59 | 52.4 | 1 | 32.7 | 1 |
| >40 y | 51 | 35.6 | 1.57 (0.94-2.63) | 36.0 | 1.12 (0.56-2.22) |
| Sex | P = .23 | P = .24 | |||
| Male | 48 | 40.6 | 1 | 40.5 | 1 |
| Female | 62 | 47.9 | 0.73 (0.44-1.22) | 35.1 | 0.66 (0.23-1.61) |
| Diagnosis | P = .75 | P = .40 | |||
| AML | 44 | 41.5 | 1 | 41.0 | 1 |
| Acute lymphoblastic leukemia | 24 | 41.7 | 0.95 (0.49-1.84) | 23.1 | 0.51 (0.18-1.38) |
| Myelodysplastic syndromes | 19 | 40.6 | 1.00 (0.49-2.0) | 47.6 | 1.06 (0.45-2.48) |
| Lymphoproliferative disorder†I | 22 | 43.3 | 0.75 (0.28-1.51) | 30.3 | 0.44 (0.13-1.50) |
| Chronic myeloid leukemia | 1 | 100.0 | — | 0.0 | — |
| Disease status at transplant | P = .06 | P = .006 | |||
| Complete remission | 67 | 52.9 | 1 | 23.2 | 1 |
| Refractory/relapsed disease | 43 | 31.9 | 1.63 (0.97-2.73) | 51.3 | 2.55 (1.27-5.10) |
| Conditioning regimen | P = .20 | P = .55 | |||
| Myeloablative | 79 | 38.1 | 1 | 46.3 | 1 |
| Nonmyeloablative | 31 | 22.1 | 1.19 (0.67-2.09) | 40.9 | 0.56 (0.23-1.36) |
| Graft | P = .22 | P = .20 | |||
| Double cord | 105 | 45.9 | 1 | 32.6 | 1 |
| Single cord | 5 | 20.0 | 1.36 (0.82-2.27) | 0.60 | 1.51 (0.84-2.74) |
| CMV status‡ | P = .84 | P = .43 | |||
| Seronegative | 11 | 53.0 | 1 | 56.2 | 1 |
| Seropositive | 97 | 43.6 | 1.10 (0.44-2.75) | 32.0 | 0.66 (0.23-1.89) |
| HLA match between recipient and dominant CB unit§ | P = .50 | P = .34 | |||
| 7-8/8 | 16 | 61.4 | 1 | 25.1 | 1 |
| 5-6/8 | 46 | 47.1 | 1.41 (0.48-4.13) | 30.1 | 1.33 (0.42-6.01) |
| ≤4/8 | 38 | 40.8 | 1.80 (0.61-5.27) | 39.6 | 1.60 (0.61-4.81) |
| Total mononuclear cells infused|| | P = .90 | P = .99 | |||
| ≤4.1 × 108/kg | 56 | 46.1 | 1 | 34.2 | 1 |
| >4.1 × 108/kg | 54 | 42.6 | 1.035 (0.62-1.73) | 33.5 | 0.99 (0.50-1.96) |
| Patient HLA C group | P < .001 | P < .001 | |||
| C1/C1 | 31 | 59.9 | 1 | 27.3 | 1 |
| C1/C2 | 55 | 48.7 | 1.35 (0.68-2.67) | 24.9 | 0.92 (0.38-2.26) |
| C2/C2 | 24 | 15.0 | 4.33 (2.10-8.94) | 67.8 | 4.05 (1.66-9.87) |
| Patient HLA C group | P < .001 | P < .001 | |||
| C1/x | 86 | 52.9 | 1 | 26.0 | 1 |
| C2/C2 | 24 | 15.0 | 3.56 (2.05-6.18) | 67.8 | 4.25 (2.09-8.63) |
| Patients receiving CB grafts with the combined HLA-C1-KIR2DL2/L3/S2 genotype¶ | P = .002 | P = .009 | |||
| Yes | 67 | 64.6 | 1 | 46.9 | 1 |
| No | 37 | 34.3 | 2.65 (1.39-5.03) | 16.0 | 3.07 (1.26-7.47) |
| HLA-C1/x patients receiving CB grafts with the combined HLA-C1-KIR2DL2/L3/S2 genotype¶ | P = .003 | P = .002 | |||
| Yes | 31 | 74.2 | 1 | 6.7 | 1 |
| No | 49 | 41.3 | 3.31 (1.45-7.50) | 40.1 | 6.98 (1.61-30.25) |
| Patients receiving CB grafts with the combined HLA-C2-KIR2DL1/S1 genotype¶ | P = .17 | P = .72 | |||
| Yes | 47 | 51.9 | 1 | 35.2 | 1 |
| No | 57 | 39.1 | 1.45 (0.85-2.49) | 34.0 | 1.13 (0.56-2.27) |
| Number of CB units with haplotype B¶ | P = .45 | P = .30 | |||
| 2 CB units | 88 | 44.9 | 1 | 31.2 | 1 |
| 1 CB units | 14 | 42.9 | 0.961 (0.45-2.03) | 43.6 | 1.37 (0.18-10.15) |
| 0 CB units | 2 | 50.0 | 1.12 (0.09-16.27) | 50.0 | 2.12 (0.91-4.93) |
The median age was 38 y (range, 2-73).
Six patients had Hodgkin disease; 4 had chronic lymphocytic leukemia, and 12 had non-Hodgkin lymphoma.
Two patients had missing data.
The identity of the dominant CB unit could not be ascertained in 14 cases.
The median value for the total nucleated cells infused was 4.1 × 108/kg (range, 2.0 × 108/kg to 19.5 × 108/kg).
Six patients had missing data.
Table 2.
Patient characteristics and outcomes in the discovery (n = 110) and validation (n = 94) cohorts: validation cohort
| 1-y overall survival | 1-y relapse | ||||
|---|---|---|---|---|---|
| n | Probability (%) | HR (95%CI) (risk of death) | Cumulative incidence (%) | HR (95%CI) (risk of relapse) | |
| Age* | P = .09 | P = .35 | |||
| ≤40 y | 44 | 55.0 | 1 | 21.1 | 1 |
| >40 y | 50 | 31.1 | 1.27 (0.72-1.90) | 25.5 | 1.02 (0.45-2.30) |
| Sex | P = .61 | P = .19 | |||
| Male | 54 | 40.0 | 1 | 19.5 | 1 |
| Female | 40 | 43.7 | 0.78 (.45-1.37) | 27.3 | 1.67 (0.77-3.61) |
| Diagnosis | P = .51 | P = .23 | |||
| AML | 39 | 43.6 | 1 | 33.3 | 1 |
| Acute lymphoblastic leukemia | 25 | 40.0 | 0.96 (0.50-18.5) | 40.1 | 1.27 (0.53-2.78) |
| Myelodysplastic syndromes | 5 | 80.0 | 0.26 (0.21-1.81) | 20.0 | 0.55 (0.07-4.20) |
| Lymphoproliferative disorder† | 16 | 37.5 | 1.19 (0.57-2.53) | 16.2 | 0.14 (0.2-1.18) |
| Chronic myeloid leukemia | 9 | 56.6 | 0.62 (.21-1.81) | 12.5 | 0.30 (0.04-2.32) |
| Disease status at transplant | P = .04 | P = .02 | |||
| Complete remission | 52 | 48.9 | 1 | 17.8 | 1 |
| Refractory/relapsed disease | 42 | 34.2 | 1.47 (1.09-2.56) | 33.2 | 1.84 (1.13-4.22) |
| Conditioning regimen | P = .87 | P = .65 | |||
| Myeloablative | 73 | 40.9 | 1 | 25.1 | 1 |
| Nonmyeloablative | 21 | 47.4 | 1.09 (0.63-1.71) | 17.6 | 0.85 (0.42-1.88) |
| Graft | P = .73 | P = .73 | |||
| Double cord | 88 | 40.5 | 1 | 31.9 | 1 |
| Single cord | 6 | 66.7 | 0.91 (0.50-1.68) | 16.7 | 0.84 (0.23-1.86) |
| CMV status‡ | P = .78 | P = .44 | |||
| Seronegative | 16 | 42.9 | 1 | 43.2 | 1 |
| Seropositive | 76 | 42.3 | 0.99 (0.77-1.36) | 29.1 | 0.65 (0.20-1.91) |
| HLA match between recipient and dominant CB unit§ | P = .11 | P = .26 | |||
| 7-8/8 | 10 | 75.1 | 1 | 20.1 | 1 |
| 5-6/8 | 31 | 36.8 | 1.61 (0.96-4.71) | 36.8 | 1.4 (0.84-2.33) |
| ≤4/8 | 43 | 38.2 | 1.79 (0.91-3.54) | 18.6 | 0.99 (0.70-1.24) |
| Total mononuclear cells infused|| | P = .91 | P = .73 | |||
| ≤3.8 × 108/kg | 42.9 | 1 | 26.8 | 1 | |
| >3.8 × 108/kg | 39.0 | 0.97 (0.55-1.70) | 24.4 | 0.862 (0.37-2.03) | |
| Patient HLA C group¶,# | P = .002 | P < .001 | |||
| C1/C1 | 38 | 50.0 | 1 | 22.2 | 1 |
| C1/C2 | 40 | 52.0 | 0.91 (0.47-1.76) | 18.1 | 0.83 (0.30-2.34) |
| C2/C2 | 16 | 12.5 | 2.73 (1.30-5.75) | 68.7 | 5.34 (1.96-14.50) |
| Patient HLA C group | P < .001 | P < .001 | |||
| C1/x | 78 | 51.3 | 1 | 19.5 | 1 |
| C2/C2 | 16 | 12.5 | 2.90 (1.56-5.38) | 68.7 | 5.98 (2.73-13.10) |
| Patients receiving CB grafts with the combined HLA-C1-KIR2DL2/L3/S2 genotype | P = .07 | P = .05 | |||
| Yes | 37 | 56.8 | 1 | 16.2 | 1 |
| No | 57 | 36.8 | 1.70 (0.94-3.07) | 35.7 | 2.38 (0.96-5.94) |
| HLA-C1/x patients receiving CB grafts with the combined HLA-C1-KIR2DL2/L3/S2 genotype | P = .02 | P = .01 | |||
| Yes | 31 | 67.7 | 1 | 6.5 | 1 |
| No | 47 | 40.4 | 2.33 (1.13-4.81) | 28.3 | 5.02 (1.13-22.26) |
| HLA match between recipient and dominant CB unit§ | P = .11 | P = .26 | |||
| 7-8/8 | 10 | 75.1 | 1 | 20.1 | 1 |
| 5-6/8 | 31 | 36.8 | 1.61 (0.96-4.71) | 36.8 | 1.4 (0.84-2.33) |
| ≤4/8 | 43 | 38.2 | 1.79 (0.91-3.54) | 18.6 | 0.99 (0.70-1.24) |
| Patients received CB grafts with the combined HLA-C2-KIR2DL1/S1 genotype | P = .54 | P = .20 | |||
| Yes | 35 | 46.6 | 1 | 23.1 | 1 |
| No | 59 | 41.7 | 1.19 (0.68-2.07) | 34.1 | 1.31 (0.76-2.01) |
Median age was 41.0 y (range, 1-73).
Five patients had chronic lymphocytic leukemia; 5 patients had Hodgkin disease, and 6 patients had non-Hodgkin lymphoma.
Two patients had missing data.
The identity of the dominant CB unit could not be ascertained in 10 cases.
The median value for the total of nucleated cells infused was 3.8 × 108/kg (range, 1.5 × 108/kg to 34.2 × 108/kg).
The P values for the comparisons of outcomes in C1/C1 vs C2/C2 patients were P = .007 for OS and P < .001 for cumulative incidence of progression.
The P values for the comparisons of outcomes in C1/C2 vs C2/C2 patients were P = .001 for OS and P < .001 for cumulative incidence of progression.
Cord unit dominance, achieved by most patients, was defined as the unit with >90% chimerism in the total DNA fraction at the time the assay was performed.
In vitro NK differentiation from CD34+ CB-derived progenitor cells
CB units for research were provided by the MDACC Blood Bank under a protocol approved by the institutional review board. CB mononuclear cells were isolated by density-gradient technique (Ficoll-Histopaque; Sigma-Aldrich, St. Louis, MO), and CD34+ progenitor cells were selected with the CD34 Progenitor Cell Isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). CD34+ purity was confirmed by flow cytometry and was consistently >98%. Isolated CD34+ cells (5 × 104) were cultured in 0.1-mL serum-free media (CellGro; Mediatech, Manassas, VA) supplemented with 100 U/mL penicillin/streptomycin and 10% fetal bovine serum, recombinant human stem cell factor (30 ng/mL), Flt3 ligand (50 ng/mL), interleukin-15 (50 ng/mL), and insulin-like growth factor-1 (100 ng/mL) in 96-well culture plates at 37°C in a humidified atmosphere with 5% CO2 for 4 weeks. The culture medium was renewed with cytokines every other day, and KIR protein expression was monitored by flow cytometry for 4 weeks with use of anti-CD56-BV605 (clone HCD56), anti-CD3-PECy5 (clone UCHT1), anti-KIR3DL1-AlexaFluor700 (clone DX9; Biolegend, San Diego, CA), anti-KIR2DL1-APC (clone REA284), anti-KIR2DL3-Biotin (clone REA147 with streptavidin-APC-Cy7; Miltenyi Biotec), anti-NKG2A-PECy7 (clone Z199), and anti-KIR2DL2/L3/S2-PECy5.5 (clone GL183; Beckman Coulter, Pasadena, CA).
NK cell phenotyping and functional assays
NK cell cytotoxicity and cytokine production were assessed by preincubating peripheral blood mononuclear cells either alone (negative control) or with target K562 cells (effector to target ratio of 10:1) for 5 hours at 37°C in the presence of anti-CD107a-PECF594 (clone H4A3), GolgiStop/monensin (both from BD Biosciences, San Jose, CA), and Brefeldin A (Sigma-Aldrich). Phorbol myristate acetate/ionomycin stimulation was used as a positive control. After coculture, cells were stained with a live/dead aqua viability marker (Life Technologies, Frederick, MD), anti-CD56-BV605 (clone HCD56), anti-CD3-PECy5 (clone UCHT1), anti-CD16-BV650 (clone 3G8), anti-NKG2A-PECy7 (clone Z199; Beckman Coulter), and with mAbs against activating and inhibitory KIR receptors, including anti-KIR2DL1-APC (clone REA284), anti-KIR2DL3-Biotin (clone REA147, combined with streptavidin-APC-Cy7; Miltenyi Biotec), anti-KIR2DL1/2DS1-PE (clone EB6), anti-KIR2DL2/S2/L3-PECy5.5 (clone GL183), anti-KIR3DL1/DS1 (clone Z27.3.7), and anti-KIR3DL1-AlexaFluor700 (clone DX9; Biolegend). Cells were then fixed and permeabilized with fluorescence-activated cell sorter lysing and permeabilizing solution (both from BD Biosciences). Cytokine production was measured by intracellular staining with anti-IFNγ-V450 (clone B27; BD Biosciences) and anti-TNFα-PerCPCy5.5 (clone MAB11; Biolegend). All flow cytometry data were acquired on an LSRFortessa (BD Biosciences) and analyzed on Flowjo software (Treestar).
Phenotyping of AML blasts
The expression of HLA class I on the surface of acute myeloid leukemia (AML) blasts and normal myeloid cells was assessed with use of anti-CD33-PE-Cy7 (clone P67.6; BD Biosciences), anti-CD34-PerCP (clone 8G12; BD Biosciences), anti-CD13-PE (clone WM15; BioLegend), and anti-HLA-ABC-APC (clone G46-2.6; Pharmingen).
Target cells and culture conditions
K562 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 1% penicillin, streptomycin, and l-glutamine and incubated at 37°C with 5% CO2.
Statistical methods
The probability of overall survival (OS) was calculated by the Kaplan-Meier method. The probabilities of disease relapse and transplant-related mortality (TRM) were calculated by the cumulative incidence procedure. For disease relapse, relapse was considered the event of interest, and death prior to relapse was considered the competitor. For TRM, death not caused by disease relapse was considered the event of interest, and death caused by the malignancy was considered the competitor. Univariate analysis was performed with standard statistical methodology. Variables found to be significant at the P < .10 level were included in the multivariate analysis, where OS was examined with a Cox regression model and relapse by Fine-Gray regression analysis. Categorical data were compared with Fisher’s exact test and quantitative data were compared with the Mann-Whitney or the Kruskal-Wallis test. Hazard ratios (HR) are reported with 95% confidence intervals (CI). All P values are 2-sided.
Results
Recipient HLA-C genotypes are associated with distinct transplantation outcomes
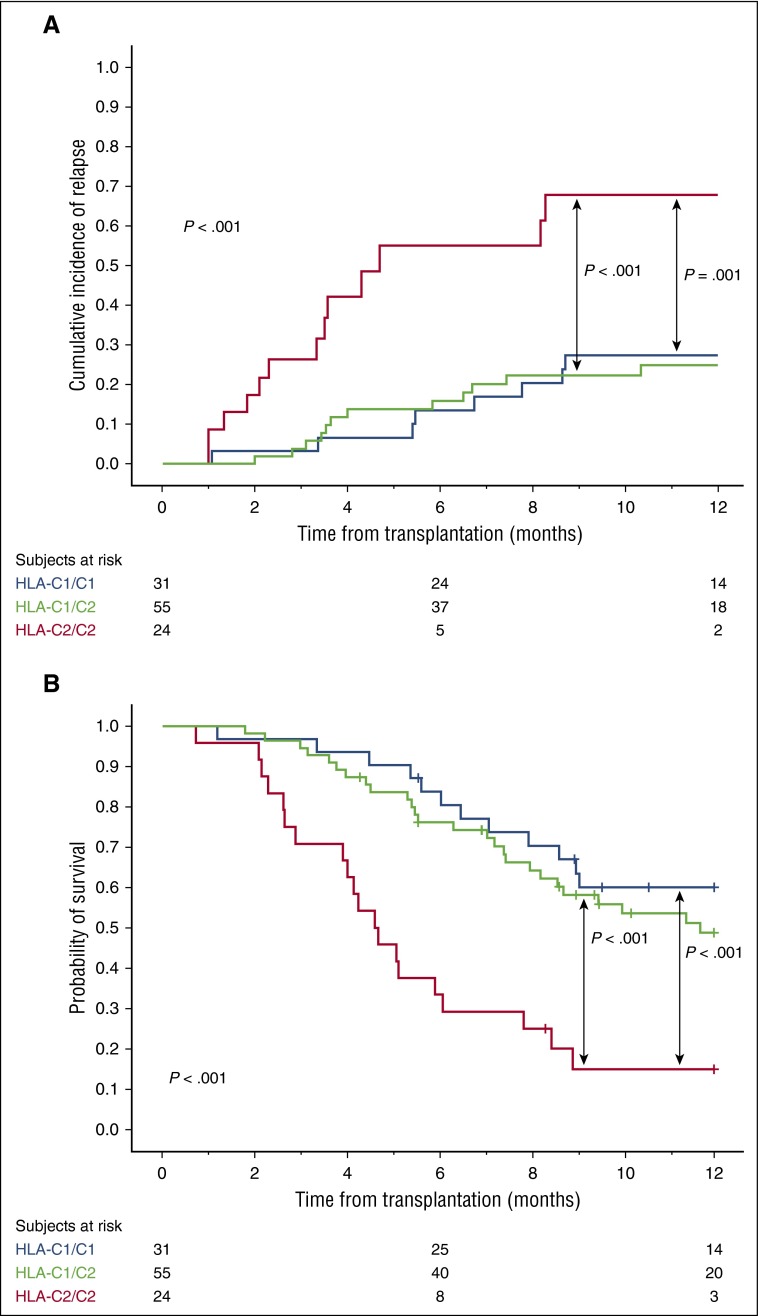
We first classified patients according to the presence of genes encoding recipient HLA-C ligands for donor inhibitory KIRs. Supplemental Table 1, available on the Blood Web site, summarizes the HLA-C group 1 (C1) and group 2 (C2)-related alleles. The 24 patients who were HLA-C2 homozygous had a significantly higher risk of relapse and a worse OS than the 31 patients with HLA-C1/C1 or the 55 patients with HLA-C1/C2 genotypes (Table 1; Figure 1), regardless of whether the underlying malignancy was of myeloid or lymphoid origin (supplemental Table 2). We validated these findings in an independent cohort of 94 CBT patients (Table 2).
Figure 1.
Effect of recipient HLA-C genotype on clinical outcome after CBT in the 110 patients in the discovery cohort. (A) One-year cumulative incidence of relapse. (B) One-year probability of OS.
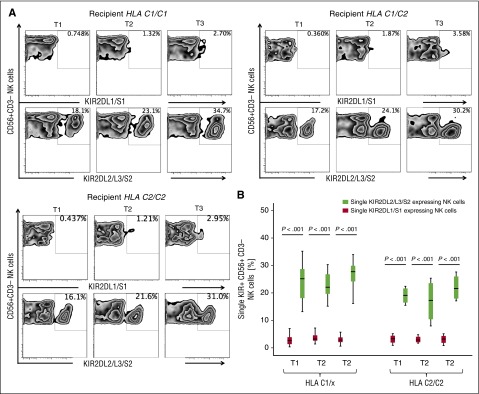
KIR2DL2/L3/S2-expressing NK cells emerge as the dominant NK cell subset after CBT regardless of recipient HLA-C genotype
To determine whether the effect of the HLA-C genotype on outcome is related to biased expression of HLA-C-specific KIRs during NK cell development, we differentiated NK cells from CB-derived CD34+ hematopoietic progenitors (n = 8) in vitro and determined the order of KIR acquisition. HLA-C1-specific KIR2DL2/L3/S2-expressing NK cells appeared significantly earlier and in greater numbers than C2-specific KIR2DL1/S1-expressing cells, irrespective of the HLA-C or the KIR genotype of the CB units used for NK cell differentiation (supplemental Figure 2). Using 14-color multiparameter flow cytometry, we then studied KIR expression on single cells in peripheral blood samples from 20 patients at 3 post-CBT intervals. As shown in Figure 2A-B, KIR2DL2/L3/S2-expressing NK cells dominated the NK cell repertoire, regardless of the recipient’s HLA-C group. These findings support our in vitro model favoring the generation of C1-specific NK cells early after CBT.
Figure 2.
KIR2DL2/L3/S2-expressing NK cells dominate the reconstituting NK repertoire after CBT. (A) Representative fluorescence-activated cell sorter plots of reconstituting KIR2DL1/S1 and KIR2DL2/L3/S2 expressing NK cells in PBMCs from 7 HLA-C1/C1, 9 HLA-C1/C2, and 4 HLA-C2/C2 patients collected at different post-CBT intervals (median times 50.0 [T1], 97.5 [T2], and 189.5 [T3] days). (B) Frequency of KIR2DL2/L3/S2 vs KIR2DL1/S1 expressing NK cells at different time points post-CBT. Box plots represent the first and third quartiles, and lines inside the boxes represent the median values; whiskers extend to 1.5 times the interquartile range.
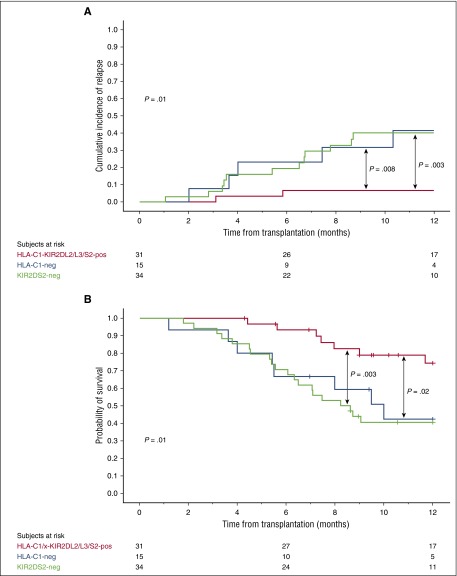
Combined HLA-C1-KIR2DL2/L3 and KIR2DS2 genotype in the CB graft is associated with a lower risk of relapse and superior OS in HLA-C1/x CBT recipients
Licensed NK cells are functionally more responsive to their targets.11-13 We therefore hypothesized that protection from relapse in HLA-C1/x (HLA-C1/C1 or HLA-C1/C2) recipients would be improved if the donor NK cells are predicted to be licensed for KIR2DL2 or KIR2DL3 (ie, HLA-C1 positive) and the activating KIR2DS2 is present (combined genotype KIR2DL2 or KIR2DL3 positive [or both] and KIR2DS2 positive, referred to as KIR2DL2/L3/S2 for the remainder of the paper). To test this hypothesis, we analyzed the outcome of CBT in the 80 HLA-C1/x patients in the discovery cohort. The 34 patients who received units with the genotype HLA-C1-KIR2DL2/L3 but lacking KIR2DS2 and the 15 patients who received HLA-C1-negative (unlicensed) grafts had a significantly higher relapse rate and a worse OS than the 31 patients receiving at least one CB unit with the combined donor genotype of HLA-C1-KIR2DL2/L3/S2. The HR for relapse were HR = 7.04 (CI, 1.57-31.47; P = .002) and HR = 6.87 (CI, 1.33-35.37; P = .01), respectively. The HR for OS were HR = 3.46 (CI, 1.46-8.20; P = .005) and HR = 3.00 (CI, 1.109-8.431; P = .03), respectively. Figure 3 shows the plots for the 1-year cumulative incidence of relapse and probability of OS. TRM rates among the 3 groups were similar (data not shown). Because patients who received KIR2DS2-negative or unlicensed grafts had a similar outcome, we combined them into a single cohort for the remainder of the analysis. These 49 patients had a higher risk of relapse (HR = 6.98; CI, 1.61-30.25; P = .002) and a worse OS (HR = 3.31; CI, 1.45-7.50; P = .003) than the 31 who received HLA-C1-KIR2DL2/L3/S2 grafts (Table 1). There was no impact of either donor HLA-C or KIR2DL2/L3/S2 alone, or indeed other hapolotype B–defining genes on outcomes after CBT (supplemental Table 3).
Figure 3.
One-year cumulative incidence of relapse and probability of OS in the 80 HLA-C1/x patients grouped according to the KIR-HLA genotype of the donor CB graft. The 31 HLA-C1/x patients receiving at least one CB unit with the HLA-C1-KIR2DL2/L3/S2 (red line) have a significantly lower incidence of relapse (A) and better OS (B) compared with the 15 patients receiving CB grafts that were predicted to be unlicensed (HLA-C1-negative, blue line) and to the 34 patients receiving KIR2DS2-negative grafts (green line). Tick marks on the lines indicate censored patients.
These findings were confirmed in the 78 HLA-C1/x CBT recipients of the validation cohort. The 47 patients who received KIR2DS2-negative or unlicensed grafts had a higher risk of relapse (HR = 5.02; CI, 1.13-22.26; P = .01) and a worse OS (HR = 2.33; CI, 1.13-4.81; P = .02) than the 31 who received an HLA-C1-KIR2DL2/L3/S2 graft (Table 2).
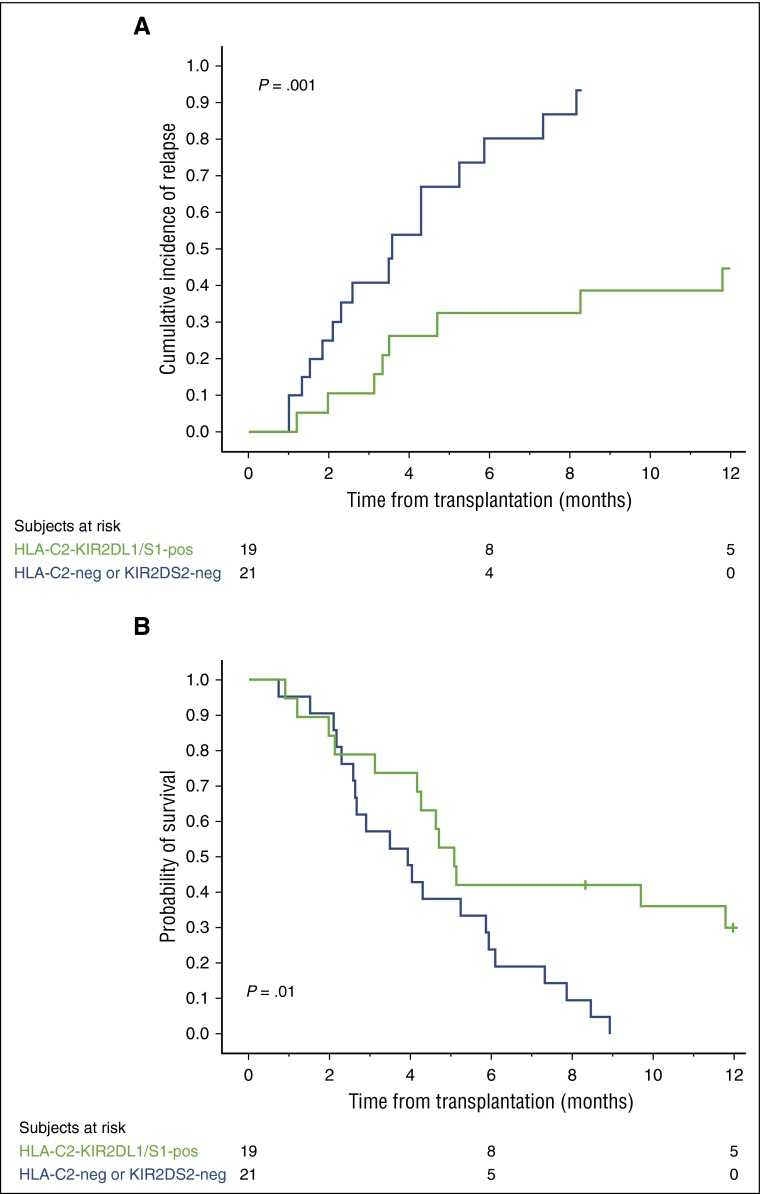
Combined donor HLA-C2-KIR2DL1 and KIR2DS1 genotype may improve outcome in HLA-C2 homozygous CBT recipients
Based on the above results, we reasoned that HLA-C2 homozygous patients would have a better outcome if they receive a CB unit predicted to be licensed for KIR2DL1 (HLA-C2/x) and positive for KIR2DS1. Because of the relatively small number of HLA-C2 homozygous patients in our series, we combined the discovery and validation cohorts to address this question.
The 21 of the 40 HLA-C2 homozygous patients who received grafts that were either HLA-C2-negative or KIR2DS1-negative had a significantly higher relapse rate (HR = 4.21; CI, 1.67-10.61; P = .002) and worse OS (HR = 2.48; CI, 1.18-5.25; P = .01) than the 19 patients who received at least one CB unit possessing the combined genotype of HLA-C2-KIR2DL1 and KIR2DS1. Figure 4A-B shows the curves for the 1-year cumulative incidence of relapse and probability of OS. We could not identify any protective effect of the donor HLA-C1-KIR2DL2/L3/S2 genotype on outcome in HLA-C2 homozygous recipients (supplemental Figure 1).
Figure 4.
HLA-C2 homozygous patients receiving a CB graft with the combined HLA-C2-KIR2DL1/S1 genotype have a lower 1-year cumulative incidence of relapse and better probability of OS. We combined HLA-C2 homozygous patients in the discovery and validation cohorts (n = 40) for this analysis. The 19 HLA-C1/x patients receiving at least one CB unit with the combined HLA-C2-KIR2DL1/S1 genotype (green line) have a significantly lower incidence of relapse (A) and a better OS (B) compared with the 21 patients receiving CB grafts that were either KIR2DS1-negative or predicted to be unlicensed (HLA-C2-negative) (blue line). Tick marks on the lines indicate censored patients.
The combined HLA-KIR genotype of the dominant CB graft determines outcome after double CBT
In double cord HSCT, one CB unit becomes dominant as the main source of hematopoiesis.22 We therefore performed a 6-month landmark analysis to examine the influence of the dominant CB unit’s KIR-HLA genotype on outcome. The 25 HLA-C1/x patients, in whom the dominant unit was negative for HLA-C1/x or KIR2DS2, had a significantly greater risk of relapse (HR = 5.05; CI, 1.15-30.99; P = .03) and a trend toward worse OS (HR = 2.50; CI, 0.89-36.51; P = .08) than the 23 HLA-C1/x patients in whom the dominant unit was positive for the combined genotype of HLA-C1-KIR2DL2/L3/S2. By 6 months, the majority of HLA-C2 homozygous patients had relapsed, preventing any further analysis of this subset.
Combined HLA-C1-KIR2DL2/L3/S2 genotype in the CB graft, recipient HLA-C2 homozygosity, and disease status at transplant are major factors associated with outcome
To identify key contributors to outcome, we performed a multivariate analysis that included the variables emerging from the univariate analysis (Table 1), as described in “Methods.” Active disease at transplantation, recipient HLA-C2 homozygosity, and not receiving a graft with an HLA-C1-KIR2DL2/L3/S2 genotype were the only independent predictors of relapse and OS. The influence of HLA-C1-KIR2DL2/L3/S2 was only observed in HLA-C1/x patients. Briefly, receiving a graft lacking the HLA-C1-KIR2DL2/L3/S2 genotype had a significant impact on relapse (HR = 6.27; CI, 1.44-17.29; P = .01) and OS (HR = 3.06; 95% CI 1.32-7.09; P = .009) in HLA-C1/x patients but did not influence relapse (HR = 0.87; CI, 0.41-1.84; P = .72) or OS (HR = 0.66; CI, 0.17-3.11; P = .84) in HLA-C2 homozygous patients (supplemental Figure 1).
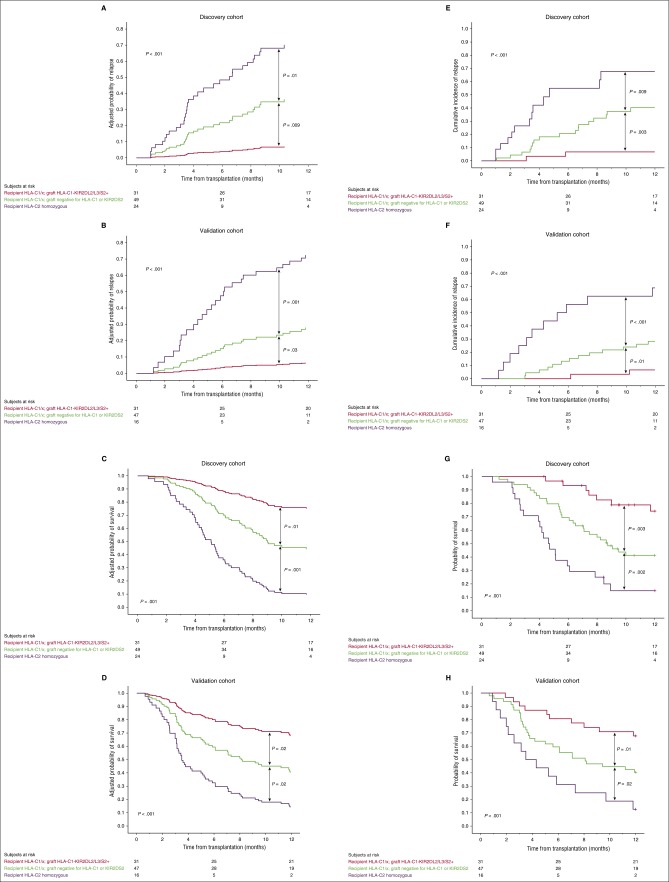
As the influence of HLA-C1-KIR2DL2/L3/S2 on both outcomes was only observed in HLA-C1/x individuals, patients were classified into 3 categories that were associated with distinct outcomes: HLA-C1/x patients who received an HLA-C1-KIR2DL2/L3/S2 graft, HLA-C1/x patients who did not receive an HLA-C1-KIR2DL2/L3/S2 graft, and HLA-C2 homozygous patients. The adjusted HR for relapse for the 49 HLA-C1/x patients who did not receive an HLA-C1-KIR2DL2/L3/S2 graft and the 24 HLA-C2 homozygous patients compared with the 31 HLA-C1/x patients who received an HLA-C1-KIR2DL2/L3/S2 graft were 6.18 (CI, 1.42-21.85; P = .01) and 16.55 (CI, 3.69-74.21; P < .001), respectively. Similarly, the adjusted HR for OS were 2.85 (CI, 1.22-6.63; P = .01) and 8.21 (CI, 3.44-19.60; P < .001), respectively. The adjusted HRs for relapse and OS of the 24 HLA-C2 homozygous patients compared with the 49 HLA-C1/x patients who did not receive an HLA-C1-KIR2DL2/L3/S2 graft were 2.68 (CI, 1.28-5.59; P = .009) and 2.88 (CI, 1.57-5.30; P = .001), respectively. Patients with relapsed/refractory disease at CBT had a higher risk of relapse and a worse OS: adjusted HR 2.34 (CI, 1.14-4.75; P = .02) and adjusted HR 1.60 (1.09-2.79; P = .04), respectively. These relationships are shown graphically in Figure 5A,C,E,G together with side-by-side confirmation from analysis of the validation cohort (Figure 5B,D,F,H).
Figure 5.
Prognostic impact of key HLA and KIR genotypes emerging from multivariate analysis. Adjusted (A-B and C-D) and unadjusted (E-F and G-H) 1-year probabilities of relapse and OS in the discovery vs validation cohorts according to HLA and KIR genotype. Patients in each cohort were classified into 3 categories: HLA-C1/x receiving an HLA-C1-KIR2DL2/L3/S2 graft (red line), HLA-C1/x receiving an HLA-C1-KIR2DL2/L3/S2 graft (green line), and HLA-C2 homozygous patients (purple line).
The clinical characteristics of patients were similar whether stratified by their HLA-C group (supplemental Table 4) or their CB graft HLA-KIR genotype (supplemental Table 5).
KIR2DL2/L3/S2-expressing NK cells reconstituting from CB units with the combined HLA-C1-KIR2L2/L3/S2 genotype possess enhanced effector function in HLA-C1/x recipients
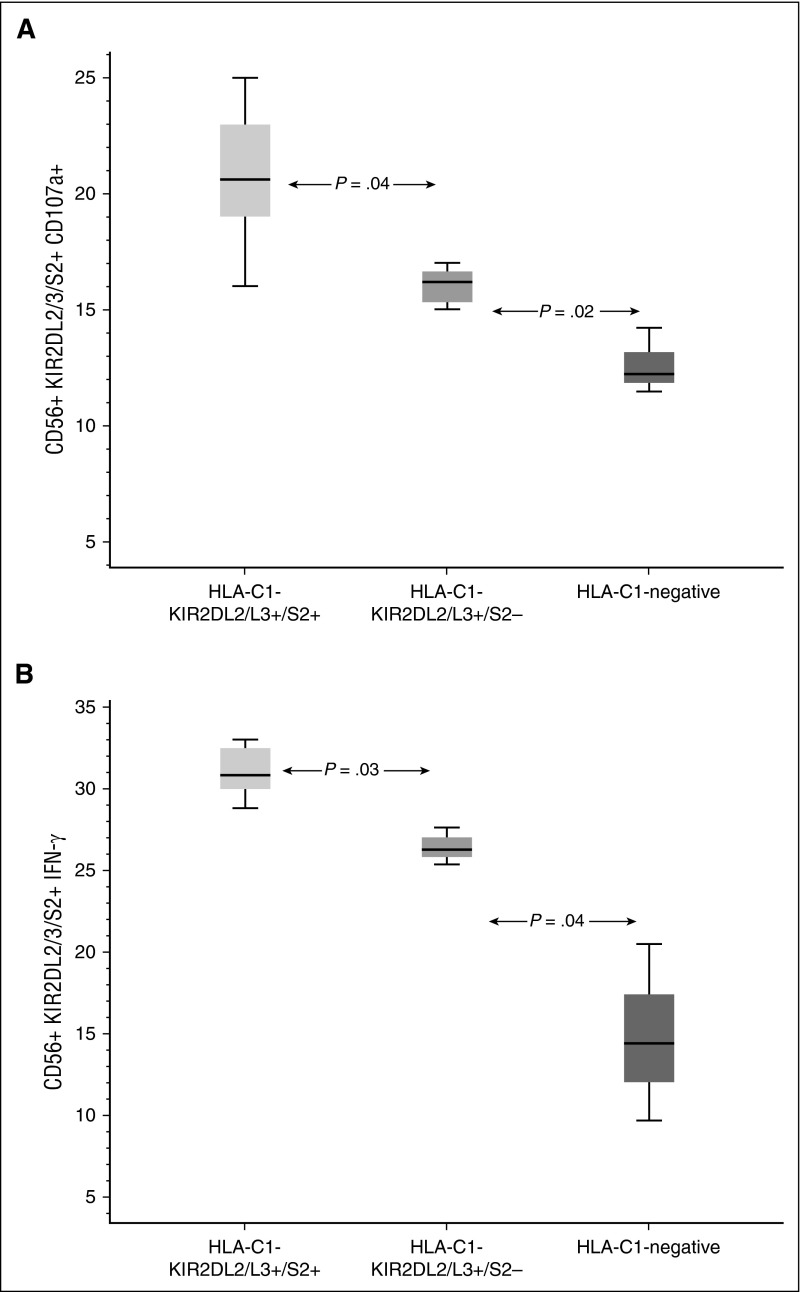
We hypothesized that NK cells from an HLA-C1-KIR2DL2/L3/S2 CB unit are more effective in preventing relapse in HLA-C1/x recipients because they have enhanced effector function. This idea was tested by analyzing NK effector function in 27 samples from HLA-C1/x patients in the first 100 days post-CBT. The median frequency of CD107+ KIR2DL2/L3/S2+ NK cells in the 9 samples from HLA-C1-KIR2DL2/L3/S2 CB recipients was 20.6% (range 16% to 25%) compared with 16.2% (range 13% to 17%; P = .041) in the 15 samples from recipients of HLA-C1-KIR2DL2/L3-positive but KIR2DS2-negative grafts, and to 12.2% (11.5% to 14.2%; P = .018) for the 3 samples from recipients of unlicensed (HLA-C1-negative) units. Similar results were obtained when we examined the interferon-γ response in the 3 groups: 30.8% (range 28.8% to 33.0%) compared with 26.3% (range, 25.4% to 27.6%; P = .029) and 14.4% (range, 9.7% to 20.5%; P = .036) respectively (Figure 6; supplemental Figure 3).
Figure 6.
NK response post CBT. Recovering NK cells from CB units with a combined HLA-C1-KIR2DL2/L3/2DS2 genotype express more CD107a (A) and interferon-γ (B) in response to stimulation with K562 targets than those from CB units that were either predicted to be unlicensed (HLA-C1-negative) or were KIR2DS2-negative. Boxes represent first and third quartiles; lines inside boxes represent median values, with whiskers extending to 1.5 times the interquartile range.
Discussion
At the start of this study, the impact of KIR-HLA genetics on the outcome of CBT was unclear. Whereas, in a large retrospective registry study, CBT from KIR ligand-incompatible donors for acute leukemia patients in complete remission was associated with decreased relapse and improved survival rates,23 in another study, KIR-ligand mismatch was associated with the development of severe acute graft-versus-host disease and the risk of death after double CBT with a reduced-intensity conditioning regimen.24 A limitation of these studies is that they only take into account KIR-ligand mismatch, without consideration for the role of activating KIRs or NK licensing. Moreover, they are exclusively based on genetic studies and lack functional correlates and a plausible mechanistic basis for the observed effects of KIR genotype on outcome. Our study is the first to assess the impact of NK licensing and activating KIRs in combination with functional measures of NK reconstitution after CBT, using an independent cohort to validate results. We report that patients with an HLA-C1/C1 or HLA-C1/C2 genotype have a significantly lower relapse rate and higher OS when they receive CB grafts with an HLA-C1/KIR2DL2/L3/S2 genotype. This protective effect was lost if the CB grafts were HLA-C1-KIR2DL2/L3-positive but lacked the activating KIR2DS2, or if they were unlicensed (HLA-C1-negative). All these results were validated in an independent cohort of patients. Conversely, patients homozygous for HLA-C2 had a superior outcome when transplanted with an HLA-C2/KIR2DL1/S1-positive CB graft. Of note, there was no impact of KIR-HLA genotype on TRM or the risk of acute or chronic graft-versus-host disease (data not shown).
The notion that the presence of single or multiple activating KIR genes, such as KIR2DS1 or donor haplotype B, is associated with protection against leukemic relapse after HSCT is not new.15,16,25,26 In our study, we did not observe an impact of haplotype B on outcome in either the discovery or the validation cohorts (Tables 1 and 2; supplemental Table 3). On the other hand, KIR2DS2 and KIR2DS1 positivity in the CB graft was associated with a lower relapse rate in specific cohorts of patients. The protective effect of KIR2DS2 was limited to patients with the HLA-C1/x genotype, whereas that of KIR2DS1 was limited to HLA-C2 homozygous patients. Notably, this protection was seen only in the presence of NK licensing, consistent with studies in both humans and murine models showing that unlicensed NK cells are hyporesponsive to activating stimuli, and that this hyporesponsiveness can only be partially reversed by the expression of an activating receptor.11,27 Indeed, we show that in HLA-C1/x recipients, the effector function of donor NK cells from HLA-C1-KIR2DL2/L3/S2-positive CB grafts is superior to that of NK cells that were either unlicensed (HLA-C1/x negative) or lacked KIR2DS2 (Figure 6; supplemental Figure 3). Finally, we did not observe any beneficial effect of KIR-ligand mismatch (data not shown), as reported in early studies post-HSCT.1,28
These observations emphasize the importance of the combination of NK licensing and activating KIRs, as opposed to only KIR-ligand mismatch or the presence of an activating KIR per se, to alloreactivity in the post-CBT setting. Moreover, HLA class I molecules may be downregulated on leukemic cells, creating a situation of de facto KIR-ligand mismatch, irrespective of whether such a mismatch exists at the genotype level. In the absence of a KIR-ligand mismatch, NK cells are capable of killing leukemic blasts with low expression of HLA class I molecules more efficiently than cells with high HLA class I expression.29,30 Indeed, our analysis of HLA class I expression on primary blasts from patients with acute lymphoblastic leukemia31 or AML (supplemental Figure 4) showed significantly lower expression on leukemic blasts compared with cells from healthy donors. Thus, we propose that in a setting where cancer cells downregulate HLA class I molecules, the licensing status of NK cells and coexpression of activating KIRs will be the major determinants of alloreactivity. This novel observation confirms experimental work in a murine model27 that the recognition of self-MHC class I and licensing leads to enhanced function of NK cell activation receptors.
Our data also support the hypothesis that NK licensing is determined by the HLA group of the donor rather than the recipient. We found that in HLA-C1/x patients, NK cells reconstituted from HLA-C1-negative (unlicensed) CB units had significantly inferior effector function in vitro compared with those regenerated from licensed (HLA-C1/x) grafts. Moreover, HLA-C1/x patients receiving HLA-C1-negative CB units fared worse after CBT. The dominant role of donor MHC class I molecules in the licensing of NK cells is supported by studies in murine models32 and functional studies in HSC recipients.33
In this study, HLA-C2 homozygous patients had a significantly higher relapse rate after CBT than did HLA-C1/x-positive patients, as reported for patients receiving stem cell transplants other than CB.17,34 However, the effect of HLA-C2 homozygosity on outcome after CBT appeared to be much more dramatic than previously reported, showing independent prognostic significance in a multivariate analysis, and was observed to the same extent in patients with lymphoid or myeloid malignancies. Our data further support a role for NK cells in protection against relapse following HSCT in lymphoid malignancies, as also reported by others.24,35 CB differs from peripheral blood in ways that could influence the quality of NK cell reconstitution following transplantation (eg, less mature NK functional phenotypes, infusion of fewer mature NK cells, and a smaller hematopoietic progenitor cell dose). Thus, to explore the underlying mechanism for the poor prognostic impact of HLA-C2 homozygosity, we investigated the sequential acquisition of KIRs on NK cells, both in vitro and in vivo, and found earlier recovery and higher frequencies of HLA-C1-specific KIR2DL2/L3/S2-expressing NK cells compared with HLA-C2-specific KIR2DL1/S1-positive cells. Thus, we attribute the worse outcome of HLA-C2 homozygous patients to the delayed recovery of KIR2DL1-expressing NK cells.
CBT, unlike other approaches to HSCT, allows a choice of multiple donors for transplantation. Historically, these units have been selected on the basis of 4/6 HLA match and cell dose.18,19,36,37 Inexpensive methods for KIR genotyping are available at many centers and can be easily applied concomitantly to HLA genotyping, without a delay in CB selection. We therefore propose that KIR genotyping should be combined with HLA-matching in the selection of the most appropriate CB units for use in transplantation. HLA-C1/x patients should benefit from units positive for the HLA-C1-KIR2DL2/L3/S2 genotype (while those homozygous for HLA-C2 may benefit from an HLA-C2-KIR2DL1/S1-positive CB graft). Important biological differences exist between CBT and other types of HSCT. CB contains unique populations of NK cell progenitors that are either absent or present in minute numbers in peripheral blood.38-40 Furthermore, in CBT, significantly fewer progenitor cells and T cells (most of which are naïve) are infused. Thus, it is not clear whether our findings can be applied to other transplant modalities.
Our data also support a role for NK cell immunotherapy after transplantation. We recently reported the feasibility of expanding clinically relevant doses of NK cells from CB units using a GMP-compliant procedure.41 We now propose that the infusion of mature NK cells expanded from HLA-C2-KIR2DL1/S1-positive CB units immediately after CBT may overcome the delayed recovery of HLA-C2-specific KIR2DL1/S1-expressing NK cells, thus preventing disease relapse in HLA-C2 homozygous patients. A clinical trial investigating the 2 strategies, CB selection based on HLA-KIR typing and adoptive therapy with CB-derived NK cells, is underway at this center.
Acknowledgments
The authors thank the patients who agreed to participate in this study, and the teams of nurses, pharmacists, midlevel practitioners, and physicians for their patient care.
This work was funded in part by Leukemia & Lymphoma Society grant LLS 6470-15, American Cancer Society grant ACS RSG-15-218-01-LIB, and National Institutes of Health, National Cancer Institute grants P01 CA148600-02 and RO1 CA061508-18.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.S. performed experiments, designed, interpreted, analyzed, and wrote the manuscript; D.M. interpreted, analyzed, and wrote the manuscript; L.L., P.M., H.S., and C.S., provided advice and performed experiments; R.J., B.O., C.H., G.R., A.A., S.P., K.C., B.A., U.P., P.K., M.M., R.B., K.K., Y.N., N.S., A.O., A.A., E.L., A.S., S.P., D.A.-J., N.I., J.M., R.C., and E.J.S. provided advice on experiments and commented on the manuscript; and K.R. designed and directed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests
Correspondence: Katayoun Rezvani, MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77004; e-mail: krezvani@mdanderson.org.
Reference List
- 1.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 2.Savani BN, Rezvani K, Mielke S, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107(4):1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yawata M, Yawata N, Draghi M, Partheniou F, Little AM, Parham P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008;112(6):2369–2380. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ljunggren HG, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 7.Winter CC, Long EO. A single amino acid in the p58 killer cell inhibitory receptor controls the ability of natural killer cells to discriminate between the two groups of HLA-C allotypes. J Immunol. 1997;158(9):4026–4028. [PubMed] [Google Scholar]
- 8.Colonna M, Borsellino G, Falco M, Ferrara GB, Strominger JL. HLA-C is the inhibitory ligand that determines dominant resistance to lysis by NK1- and NK2-specific natural killer cells. Proc Natl Acad Sci USA. 1993;90(24):12000–12004. doi: 10.1073/pnas.90.24.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moesta AK, Norman PJ, Yawata M, Yawata N, Gleimer M, Parham P. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J Immunol. 2008;180(6):3969–3979. doi: 10.4049/jimmunol.180.6.3969. [DOI] [PubMed] [Google Scholar]
- 10.Marcenaro E, Pesce S, Sivori S, Carlomagno S, Moretta L, Moretta A. KIR2DS1-dependent acquisition of CCR7 and migratory properties by human NK cells interacting with allogeneic HLA-C2+ DCs or T-cell blasts. Blood. 2013;121(17):3396–3401. doi: 10.1182/blood-2012-09-458752. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 12.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253(5016):199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 13.Anfossi N, André P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Brodin P, Kärre K, Höglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30(4):143–149. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Cooley S, Weisdorf DJ, Guethlein LA, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116(14):2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367(9):805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102(3):814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 18.Rocha V, Labopin M, Sanz G, et al. Acute Leukemia Working Party of European Blood and Marrow Transplant Group; Eurocord-Netcord Registry. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22):2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 19.Barker JN. Umbilical Cord Blood (UCB) transplantation: an alternative to the use of unrelated volunteer donors? Hematology Am. Soc. Hematol. Educ. Program. 2007:55-61. [DOI] [PubMed]
- 20.Rocha V, Gluckman E. Outcomes of transplantation in children with acute leukaemia. Lancet. 2007;369(9577):1906–1908. doi: 10.1016/S0140-6736(07)60892-7. [DOI] [PubMed] [Google Scholar]
- 21.Marin D, Gabriel IH, Ahmad S, et al. KIR2DS1 genotype predicts for complete cytogenetic response and survival in newly diagnosed chronic myeloid leukemia patients treated with imatinib. Leukemia. 2012;26(2):296–302. doi: 10.1038/leu.2011.180. [DOI] [PubMed] [Google Scholar]
- 22.Saliba RM, Rezvani K, Leen A, et al. General and Virus-Specific Immune Cell Reconstitution after Double Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(7):1284–1290. doi: 10.1016/j.bbmt.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farag SS, Bacigalupo A, Eapen M, et al. KIR Study Group, Center for International Blood and Marrow Transplantation Research. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol Blood Marrow Transplant. 2006;12(8):876–884. doi: 10.1016/j.bbmt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Hsu KC, Gooley T, Malkki M, et al. International Histocompatibility Working Group. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Marrow Transplant. 2006;12(8):828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Stringaris K, Adams S, Uribe M, et al. Donor KIR Genes 2DL5A, 2DS1 and 3DS1 are associated with a reduced rate of leukemia relapse after HLA-identical sibling stem cell transplantation for acute myeloid leukemia but not other hematologic malignancies. Biol Blood Marrow Transplant. 2010;16(9):1257–1264. doi: 10.1016/j.bbmt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooley S, Trachtenberg E, Bergemann TL, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113(3):726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebihara T, Jonsson AH, Yokoyama WM. Natural killer cell licensing in mice with inducible expression of MHC class I. Proc Natl Acad Sci USA. 2013;110(45):E4232–E4237. doi: 10.1073/pnas.1318255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willemze R, Rodrigues CA, Labopin M, et al. Eurocord-Netcord and Acute Leukaemia Working Party of the EBMT. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23(3):492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlegel P, Ditthard K, Lang P, et al. NKG2D Signaling Leads to NK Cell Mediated Lysis of Childhood AML. J.Immunol.Res. 2015;2015:473175. [DOI] [PMC free article] [PubMed]
- 30.Feuchtinger T, Pfeiffer M, Pfaffle A, et al. Cytolytic activity of NK cell clones against acute childhood precursor-B-cell leukaemia is influenced by HLA class I expression on blasts and the differential KIR phenotype of NK clones. Bone Marrow Transplant. 2009;43(11):875–881. doi: 10.1038/bmt.2008.398. [DOI] [PubMed] [Google Scholar]
- 31.Rouce RH, Shaim H, Sekine T, et al. The TGF-β/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia. 2016;30(4):800–811. doi: 10.1038/leu.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sungur CM, Tang-Feldman YJ, Zamora AE, Alvarez M, Pomeroy C, Murphy WJ. Murine NK-cell licensing is reflective of donor MHC-I following allogeneic hematopoietic stem cell transplantation in murine cytomegalovirus responses. Blood. 2013;122(8):1518–1521. doi: 10.1182/blood-2013-02-483503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haas P, Loiseau P, Tamouza R, et al. NK-cell education is shaped by donor HLA genotype after unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2011;117(3):1021–1029. doi: 10.1182/blood-2010-02-269381. [DOI] [PubMed] [Google Scholar]
- 34.Fischer JC, Kobbe G, Enczmann J, Haas R, Uhrberg M. The impact of HLA-C matching depends on the C1/C2 KIR ligand status in unrelated hematopoietic stem cell transplantation. Immunogenetics. 2012;64(12):879–885. doi: 10.1007/s00251-012-0648-7. [DOI] [PubMed] [Google Scholar]
- 35.Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172(1):644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 36.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117(8):2332–2339. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gluckman E, Rocha V, Arcese W, et al. Eurocord Group. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol. 2004;32(4):397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Bradstock KF, Luxford C, Grimsley PG. Functional and phenotypic assessment of neonatal human leucocytes expressing natural killer cell-associated antigens. Immunol Cell Biol. 1993;71(Pt 6):535–542. doi: 10.1038/icb.1993.59. [DOI] [PubMed] [Google Scholar]
- 39.Gaddy J, Broxmeyer HE. Cord blood CD16+56- cells with low lytic activity are possible precursors of mature natural killer cells. Cell Immunol. 1997;180(2):132–142. doi: 10.1006/cimm.1997.1175. [DOI] [PubMed] [Google Scholar]
- 40.Miller JS, McCullar V. Human natural killer cells with polyclonal lectin and immunoglobulinlike receptors develop from single hematopoietic stem cells with preferential expression of NKG2A and KIR2DL2/L3/S2. Blood. 2001;98(3):705–713. doi: 10.1182/blood.v98.3.705. [DOI] [PubMed] [Google Scholar]
- 41.Shah N, Martin-Antonio B, Yang H, et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One. 2013;8(10):e76781. doi: 10.1371/journal.pone.0076781. [DOI] [PMC free article] [PubMed] [Google Scholar]