Abstract
Polygalacturonase inhibiting proteins (PGIPs) are major defensive proteins produced by plant cell walls that play a crucial role in pathogen resistance by reducing polygalacturonase (PG) activity. In the present study, a novel PGIP gene was isolated from tobacco (Nicotiana tabacum), hereafter referred as NtPGIP. A full-length NtPGIP cDNA of 1,412 bp with a 186 bp 5′-untranslated region (UTR), and 209 bp 3′-UTR was cloned from tobacco, NtPGIP is predicted to encode a protein of 338 amino acids. The NtPGIP sequence from genomic DNA showed no introns and sequence alignments of NtPGIP’s deduced amino acid sequence showed high homology with known PGIPs from other plant species. Moreover, the putative NtPGIP protein was closely clustered with several Solanaceae PGIPs. Further, the expression profile of NtPGIP was examined in tobacco leaves following stimulation with the oomycete Phytophthora nicotianae and other stressors, including salicylic acid (SA), abscisic acid (ABA), salt, and cold treatment. The results showed that all of the treatments up-regulated the expression of NtPGIP at different times. To understand the biochemical activity of NtPGIP gene, a full-length NtPGIP cDNA sequence was subcloned into a pET28a vector and transformed into E. coli BL21 (DE3). Recombinant proteins were successfully induced by 1.0 nmol/L IPTG and the purified proteins effectively inhibited Phytophthora capsici PG activity. The results of this study suggest that NtPGIP may be a new candidate gene with properties that could be exploited in plant breeding.
Keywords: Plant biology, Biological sciences
1. Introduction
In plants, the cell wall functions as a primary barrier opposing pathogenic attacks (De Lorenzo et al., 2001). During the early stages of a phytopathogenic fungal attack, the fungi secrete polygalacturonases (PGs) which cleave α-(1–4) linkages between D-galacturonic acid residues in homogalacturonan, causing the cell wall to collapse (Bezier et al., 2002; Jones and Jones, 1997; Lang and Dornenburg, 2000). This plant cell wall degradation by PGs facilitates further fungal colonization and invasion (Karr and Albersheim, 1970). Consequently, PGs are regarded as potential pathogenicity factors (D’Ovidio et al., 2004; Bezier et al., 2002).
Plant cell walls combat phytopathogenic fungal invasion by producing polygalacturonase-inhibiting proteins (PGIPs) that specifically inhibit fungal PGs and promote oligogalacturonides (OGs) accumulation (Wang et al., 2013; Di et al., 2012; Hwang et al., 2010; Jones and Jones, 1997; Federici et al., 2006). While the mechanisms by which OGs activate a defence response remain unknown, OGs are believed to be important elicitors of plant defense responses (Federici et al., 2006; D’Ovidio et al., 2004). Apart from PGIP’s role in defense mechanism against fungal pathogens, it has also been reported that PGIP genes are involved in fundamental biological processes such as flower development (Gamboa et al., 2001) and response to stress stimuli in plants (Wang et al., 2013; Ahsan et al., 2005; Cheng et al., 2008), as well as implicated in inhibition of PGs from Oomycetes such as Phytophthora capsici (Wang et al., 2013).
While PGIP genes from different plant species have a high degree of sequence homology, the encoded proteins have distinct recognition specificities against fungal PGs (Wang et al., 2013; Cheng et al., 2008; Ahsan et al., 2005; De Lorenzo et al., 2001). The structure of a typical PGIP is characterized by the presence of 9–10 repeats, each being derived from a 24-amino acid leucine-rich with repeat (LRR) (De Lorenzo and Ferrari, 2002; D’Ovidio et al., 2004; De Lorenzo et al., 2001). Each LRR motif consists of a consensus GxIPxxLGxLxxLxxLxLxxNxLT/S sequence, with the hypervariable xxLxLxx region predicted to form a beta-strand structure and considered responsible for PG recognition (De Lorenzo and Ferrari, 2002; Di Matteo et al., 2003).
Growing evidence showed that PGIP genes are potential resources for the development of new cultivars with high resistance to fungal pathogens (Manfredini et al., 2005). PGIP overexpression in tomato (Lycopersicon esculentum) (Powell et al., 2000), tobacco (Nicotiana tabacum) (Joubert et al., 2006) and Arabidopsis thaliana (Manfredini et al., 2005) has been shown to improve resistance to Botrytis cinerea infection, whereas antisense PGIP expression in A. thaliana enhanced its susceptibility to B. cinerea (Ferrari et al., 2006). Moreover, persimmon expressing pear PGIP showed an increased inhibitive ability towards B. cinerea PGs (Tamura et al., 2004). Heterologous expression of Malus domestica Mdpgip1 gene in tobacco and purification of matured MdPGIP1 proteins from transgenic tobacco resulted in inhibition of PGs from Colletotrichum lupini, Botryosphaeria obtusa and Diaporthe ambigua (Oelofse et al., 2006). In transgenic tobacco heterologous expression of Capsicum annuum CaPGIP1 showed enhanced resistance to Alternaria alternata and Colletotrichum nicotianae PGs, with a significant reduction in the number of infection sites, lesions and average lesion size on the leaves (Wang et al., 2013). Therefore, characterization and functional analysis of these genes is essential to deepen our understanding of plant defense responses and the molecular basis of pathogen-plant interactions (Liu et al., 2013).
Tobacco, Nicotiana tabacum, is a widespread plant, a major commercial crop with substantial economic value and serves as an important resource for scientific research (Ren and Timko, 2001). However, several soil-borne pathogens can result in severe annual losses because of inadequate disease management strategies. One such soil-borne pathogen, Phytophthora nicotianae is the causal agent of black shank disease of tobacco (Kosola et al., 1995). Black shank is one of the most destructive and common tobacco diseases, and it can lead to losses at all growth stages ranging from minor injury to complete destruction of a tobacco plant (Cartwright and Spurr, 1998). Therefore, it is crucial to find resistance genes involved in plant defense mechanisms against black shank. While a few studies have been performed to investigate the role of PGIP in other plants (Wang et al., 2013; Liu et al., 2013; De Lorenzo et al., 2001; Hu et al., 2012; Machinandiarena et al., 2001; Berger et al., 2000; Faize et al., 2003; Ferrari et al., 2006; Cheng et al., 2008), its exact function in tobacco biotic stress responses is still not fully understood. Therefore, efforts to investigate the molecular stress adaptation mechanisms and PGIP functions in this host are of fundamental importance.
In this study, the NtPGIP gene was cloned using the RACE-PCR technique and the gene structure was examined. NtPGIP expressional patterns against various biotic and abiotic stress factors were characterized. Additionally, NtPGIP was cloned into an expression vector and transformed into Escherichia coli. The recombinant protein was purified that showed inhibition of P. capsici PG activity. In summary, these results suggested that NtPGIP play an important role in plant biotic and abiotic resistance, and provide a good candidate for our further research on construction of transgenic plants.
2. Materials and methods
2.1. Plant materials and cultivation of plant pathogens
Tobacco seeds (N. tabacum var. NC89) were germinated after surface-sterilization by immersion in sodium hypochlorite (0.5% vol/vol) for 30 min followed by a thorough rinsing in sterile water. The seedlings were cultured in a tray containing heat-sterilized soil/sand (1:1) mixed at 28 °C (16 h light period) in a growth chamber. Single seedlings at the three leaf stage were then transplanted into plastic trays and grown for eight weeks under the same conditions. The strain JM-1 of P. nicotianae (Iso-Z0) was cultured and used for infection experiments (Li et al., 2011), while P. capsici strain, SD33 was used for PG induction (Sun et al., 2009) and was routinely cultured on 10% V8-juice agar medium at 25 °C (Kim and Hwang, 1992).
2.2. DNA isolation and cDNA synthesis
Genomic DNA was isolated from young tobacco leaves using a DNA isolation kit (Solarbio, Shanghai, China). Total RNA was extracted from 30 mg of leaves using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA samples were treated with gDNA eraser from the PrimeScript RT reagent kit (Takara, Dalian, China) to eliminate genomic DNA contamination prior to reverse transcription. cDNA synthesis was carried out using oligod (T)15 according to manufacturer’s instructions, with samples stored at −80 °C until further use.
2.3. Primer design, NtPGIP fragment amplification and RACE-PCR
A pair of forward and reverse degenerate primers (FP and RP, Table 1), were designed according to conserved PGIP sequences from other species, such as Malus pumila (GenBank: JQ001783), Capsicum annuum (HM132879), Vitis vinifera (JN797496), Pyrus pyrifolia (JF727573) and Carica papaya (HQ290129). Both the FP primer and the RP primer are located about 120 bp near to the 5′-end and 3′-end of the ORF. Rapid amplification of cDNA ends (RACE) was performed to isolate full-length NtPGIP cDNA, with gene-specific primers P-GSP5 (5′-RACE primer) and P-GSP3 (3′-RACE primer), synthesized based on the obtained cDNA fragment sequence (Table 1). 5′- and 3′-RACE-PCR amplifications of the NtPGIP gene were performed using a Smart RACE cDNA amplification kit (Clontech, Mountain View, CA, USA) according to the manufacturer’s instructions. Touchdown PCR was used for RACE-PCR using the following protocol: 5 cycles at 94 °C for 3 min, 94 °C for 1 min, 70 °C for 45 s and 72 °C for 2 min; followed by 30 cycles at 94 °C for 1 min, 64 °C for 50 s and 72 °C for 1 min; and a final elongation at 72 °C for 10 min. PCR products were purified using the Zymoclean Gel DNA Recovery kit (ZYMO, Irvine, CA, USA), cloned into pEASY-T1 (Transgen, Beijing, China) vectors and transformed into E. coli DH5α competent cells. At least three clones were sequenced using the ABI3730 automated sequencer with M13F primer.
Table 1.
Primers used in this study to isolate, clone and for expression analysis of NtPGIP.
| Primer name | Primers sequences (5′-3′) | Utility in this study |
|---|---|---|
| P-F P-R P-ORF-F P-ORF-R P-GSP5 P-GSP3 P-28-F P-28-R 18s-F 18s-R rt-PGF1 rt-PGR1 |
GAMGACAAAAAGTYCTCCT CCACACAHCTGTTGTAGCTCAC ATGCTTCATAAAATGAAAACCTC TCACGATTTACAAGGTGGCAA GGCTTCAACCCAATCAGTACAACAATCGG ACAACCATGGCAGCTCTTCAACGTG GCCGAATTCGAAAGATGCAATCCAAATGA CCGAAGCTTCGATTTACAAGGTGGCAG GGATAGATCATTGCAATTGTTGG GGTTCAATGGACTTCTCGCGAC CTAGAAACATGCTTGAAGGAGA GGCAACGGAGAGTCACACAA |
Amplify cDNA fragment Amplify ORF and introns Amplify the 5′-UTR Amplify the 3′-UTR Amplify expression fragment Expression of 18s rDNA RT-qPCR expression |
Explanations: Y represents C or T; M represents A or C; H represents T, A or C. The restriction enzyme sites are underlined and bold, with EcoRI site added to the forward primer (p-28-F) and a HindIII site added to the reverse primer (p-28-R).
2.4. Genomic sequences of the tobacco NtPGIP
Based on the full-length NtPGIP cDNA sequence, P-ORF-F and P-ORF-R were used to amplify the gene from tobacco DNA to understand its structure (Table 1). The PCR conditions were as follows: initial denaturation at 94 °C for 4 min, followed by 35 cycles of 94 °C for 30 s, 57 °C for 35 s and 72 °C for 2 min 30 s, followed by one extension cycle at 72 °C for 10 min.
2.5. Sequence and phylogenetic analyses
The deduced PGIP amino acid sequences were aligned using Clustal W in MEGA version 4.0 (Tamura et al., 2007), with a phylogenetic tree constructed using the neighbor-joining method (Saitou and Nei, 1987) and bootstrap tests replicated 1000 times to achieve a desirable confidence level. The PGIP LRR domains were predicated using the SMART server (http://smart.embl-eidelberg.de) and signaling peptide sequence were predicted using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/).
2.6. Different stress treatments
The leaves of the plants at ten- to twelve-leaf stages were mutilated with the use of sterile forceps. Low temperature, salt, salicylic acid (SA) and abscisic acid (ABA) treatments were carried out as environmental stresses. P. nicotianae spores were induced as previously reported by Li et al. (2011) and the spore infection concentration was adjusted to 2 × 104 spores/ml. Small wounds were induced on the leaf surfaces using a 20 μL pipettor without punching out the tissue, with ∼1 wound/leaf tissue produced. A 5 μL droplet of spores was spotted onto each wound and the leaves were incubated at 25 °C with a 16/8 h photoperiod. As a control, leaves were mock-inoculated in the same way with sterile uninoculated medium. Defense response stresses such as 5 mM salicylic acid (SA) (Li et al., 2003), 100 μM abscisic acid (ABA) (Cheng et al., 2008) and 300 mM NaCl (Ahsan et al., 2005) were sprayed on the leaf surfaces, with leaves of the same age inoculated with double distilled water as a control. Following each treatment, the leaves were covered with polyethylene bags to provide adequate humidity. During cold exposure, the leaves were kept at 4 °C for 3 days under 16 h daylight conditions in a cold room. Leaves were harvested at different time points, snap frozen in liquid nitrogen and stored at −80 °C prior to RNA extraction.
2.7. Expression analysis of NtPGIP
Real-time quantitative reverse transcription PCR (RT-qPCR) was performed to determine NtPGIP expression profiles. Total RNA was extracted from leaves treated with various stressors and cDNAs were synthesized using the PrimeScriptRT reagent kit (Takara, Dalian, China) according to the manufacturer’s instructions. Primers, rt-PGF1 and rt-PGR1 (Table 1), were designed to amplify the NtPGIP gene fragments of 310 bp. The 18 s rRNA of tobacco genome was used as a constitutively expressed endogenous control, and the expression levels of the NtPGIP in different lines were determined relative to 18 s rRNA. Six no template control (NTC) wells were prepared using nuclease-free water to establish baseline fluorescence without either mimic or sample RNA. RT-PCR was performed on a 7500 Real-time PCR system (Applied Biosystems, USA). The 20 μL reaction system contained 1 μL cDNA template, 10 μL SYBR Premix Ex TaqTM (Takara), 0.4 μL ROX reference dye II, 0.4 μL of each primer and 7.8 μL of sterile H2O. Reactions carried out without the template were used as a blank control. PCR was performed in triplicate wells, three samples per treatment, using the following sequence: 30 s at 95 °C, followed by 40 cycles consisting of 5 s at 95 °C, 25 s at 54 °C and 1 min at 72 °C. Dissociation curve analysis was performed after each assay to determine target specificity. The threshold cycle (CT) values were determined automatically by instrument, and the fold changes of NtPGIP were calculated using the equation 2−⊿⊿CT (Livak and Schmittgen, 2001). Data are represented as mean ± SD of the values obtained from triplicate experiments.
2.8. Recombinant vector construction and SDS-PAGE analysis of the recombinant protein
The sequence encoding the mature peptide sequence without the signaling sequence was amplified with a P-28-F primer containing an EcoRI restriction site and P-28-R primer containing a HindIII restriction site (Table 1). The amplification products were cloned into a pEASY-T1 vector (Transgen, Beijing, China), sequenced and digested. The digested fragment was ligated into a bacterial expression vector pET-28a (Invitrogen, Carlsbad, CA, USA) pre-digested with EcoRI and HindIII. The recombinant plasmid contained the NtPGIP sequence fused upstream of an encoded 6 × His-tag. Correct insertion of the fragment was confirmed by DNA sequencing and the recombinant plasmid was named pET-PGIP. The E. coli BL21 (DE3) strain was transformed with recombinant plasmid and cultured until an OD600 of 0.6 was reached. Then IPTG (1 nmol/L) was added into the culture and cell growth was continued at 37 °C for 4 h to induce NtPGIP protein expression. The NtPGIP protein from E. coli was analyzed by SDS-PAGE along with E. coli BL21 (DE3) harboring pET-28a (negative control) and E. coli BL21 (DE3) harboring pET-28a without induction (positive control).
2.9. Recombinant protein purification and Western blotting of NtPGIP
Cultures were started from single colonies, grown in LB broth containing 100 μg/mL kanamycin at 37 °C, and then diluted to 1:100 at OD600 = 0.6. The cultures were then grown to a density of OD600 = 0.6, induced with 1 mmol/L IPTG and grown for an additional 12 h at 28 °C. The cultures (1 L) were harvested by centrifugation for 15 min at 5000 g. The expression and purification of PGIPs were performed under denaturing and refolding conditions as previously reported (Wang et al., 2013; Yang et al., 2009) with slight modification. The inclusion bodies were run on a 12% SDS–PAGE gel. Harvested cells were washed twice in 25 mM Tris buffer, then resuspensed in binding buffer (25 mM Tris-HCl pH 8.0 containing 10 mM NaCl, 6 M urea, 20 mM imidazole and 5 mM β-mercaptoethanol, 0.5% Tween 20), and lysed by sonication. The lysate was centrifuged at 12,000 g for 15 min at 4 °C and the supernatants were loaded onto a 5.0 mL Ni–NTA fastflow column (GE Healthcare) pre-equilibrated with binding buffer. Bound fusion protein was eluted several times with wash buffer (25 mM Tris-HCl pH 8.0 containing 10 mM NaCl, 6 M urea, 20 mM imidazole). Recombinant proteins were eluted with 15 mL elution buffer (25 mM Tris-HCl pH 8.0 containing 10 mM NaCl, 6 M urea, 100 or 200 or 400 mM imidazole). The purity of the refolded protein was analyzed via 12% SDS-PAGE. Protein concentrations were determined via Bradford assay, with bovine serum albumin used as a standard, and protein aliquots were stored at −70 °C. Western blotting was conducted as described by Fan et al. (2007) using a mouse anti-His primary antibody (TIANGEN, Beijing, China).
2.10. PG preparation from P. capsici
The P. capsici strain SD33 was cultured due to its high PG activity as previously reported (Wang et al., 2013; Sun et al., 2009) and PGs were collected as described by Sella et al. (2004). P. capsici PGs were extracted from culture filtrates. Cultures were incubated on a rotary shaker at 25 °C for 5 days and centrifuged at 12,000 g for 15 min at 4 °C. The supernatants were then filtered using a 0.44 μm Whatman GF/Aglass filter paper and dialyzed against 0.1 M NaAc at 4 °C, with crude PG filtrates assayed for activity.
2.11. Agarose diffusion assay and PG inhibition activity of NtPGIP
An agarose diffusion assay was performed as reported by Wang et al. (2013) with little modification. An agarose sheet was prepared by pouring 40 mL melted 1% agarose and 0.5% polygalacturonic acid (PGA; Sigma company, America) in 0.05 M sodium acetate buffer (pH 5.0) into a 10 cm × 8 cm Plexiglas frame. When the agar was solidified, 5 mm diameter wells were cut in the agar sheet using a cup plate and 60 μL of extracted P. capsici PGs were loaded into the wells. Next, purified recombinant NtPGIP and Tris-HCl (pH 8.0) were added to each well to achieve a total volume of 100 μL, with final recombinant NtPGIP concentrations adjusted to 0.1 μg/μL, 0.05 μg/μL, 0.025 μg/μL and 0.0125 μg/μL. As a control, heated 40 μL of killed NtPGIP (boiled for 10 min) was used. Each mixture was incubated at 30 °C for 12 h and the gel was stained with ruthenium red (0.05% w/v in water) and rinsed thoroughly with water (Taylor and Secor, 1988). Each experiment was performed in triplicate and a smaller ring diameter corresponded to a higher amount of NtPGIP.
The inhibition activity assay for the NtPGIP protein was performed by quantifying end groups with a modified DNS reagent (Taylor and Secor, 1988; Sathiyaraj et al., 2010; Wang et al., 2013). The reaction mixture consisted of 400 μL of PGA (Sigma), 300 μL of PGs and 100 μL of 0.05 M NaAC buffer (pH 5.0). For the assay, 200 μL of NtPGIP was added to the reaction mixture to achieve a final concentration of recombinant NtPGIP adjusted to 0.1 μg/μL, 0.05 μg/μL, 0.025 μg/μL and 0.0125 μg/μL. As controls, 200 μL of heat killed PGIP (boiled for 10 min) was used as a negative control and 300 μL of heat killed PGs was used as a positive control. One unit of PGIP was defined as the amount of inhibitor required to reduce one unit of PG activity by 50%. The enzyme assay mixture was maintained without inhibitors. Released reducing sugars were quantified using a standard calibration curve obtained with galacturonic acid as a standard. Controls were maintained with 0 h reaction mixtures whose reactions were terminated with DNS after adding enzyme and the OD read at 575 nm. PG activity was expressed as reducing units (RU), with one RU defined as the amount of enzyme required to release reducing groups at 1 mol/min using D-galacturonic acid as a standard.
3. Results
3.1. Isolation and cloning of NtPGIP
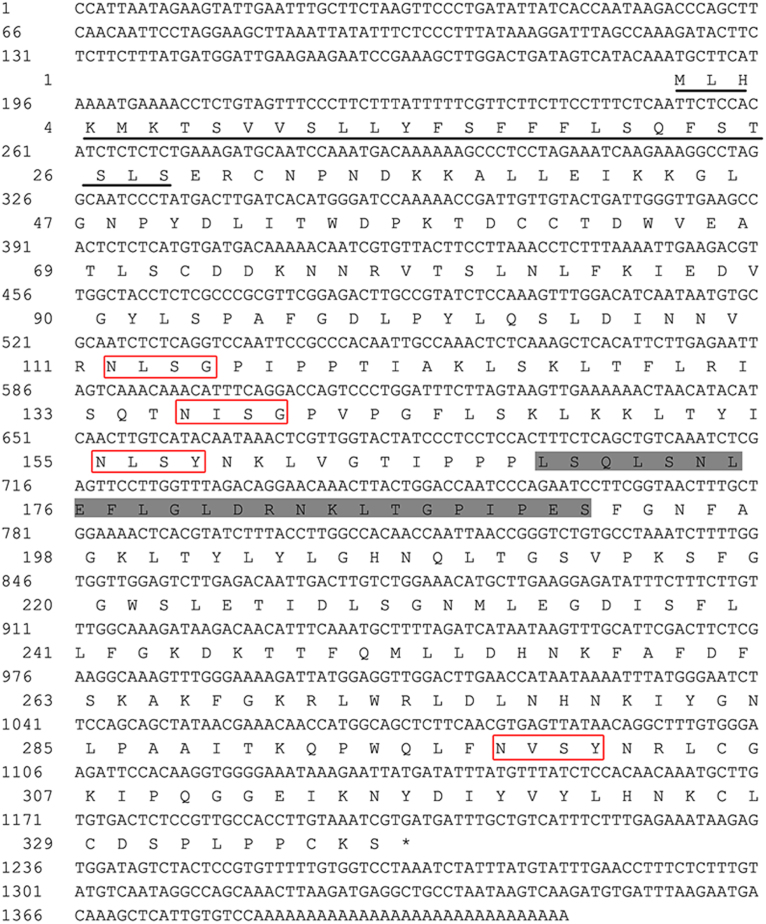
A fragment of 800 bp PGIP was obtained using the degenerate primers FP and RP (Table 1). Based on the cDNA fragment, 5′ RACE-PCR and 3′ RACE-PCR were performed. The complete ORF was verified by amplification using primer pair P-ORF-F/P-ORF-R and sequencing. Sequence analysis showed that the isolated cDNA, designated NtPGIP (GeneBank accession# KF500525), is 1,412 bp in length and contains a 186-bp 5′-untranslated region (5′-UTR) and a 209-bp 3′-UTR. Its ORF is 1,017 bp that encodes 338 amino acids with a predicted molecular weight of 38.09 kDa and an isoelectric point of 9.25. Furthermore, the genomic NtPGIP sequence was confirmed using gene-specific primers and the genomic sequence shared complete identity with the isolated cDNA fragments. This showed that no introns were present in the genomic NtPGIP sequence (Fig. 1).
Fig. 1.
cDNA sequence of the tobacco NtPGIP gene. The stop codon is indicated by an asterisk, the signal peptide sequence is underlined, boxes designate N-linked glycosylation sites. The LRR motifs are highlighted in the dark gray box.
3.2. Protein sequence analysis of NtPGIP
The analysis of secondary structure of the deduced NtPGIP protein sequence revealed a high degree of similarity with previously reported PGIPs, and it also possessed structural characteristics common to all other PGIPs. NtPGIP has four potential N-glycosylation sites (NXS/T) (Fig. 1) and a number of potential phosphorylation sites, and eight highly conserved cysteine residues, with four in the N-terminal region and four in the C-terminal region of the mature peptide (Fig. 2). Based on the SignalP-HMM (Hidden Markov Models) prediction, the NtPGIP N-terminus contains a 28 amino acid signaling peptide with a cleavage site between amino acids Ser28 and Glu29 (Fig. 1). The NtPGIP peptide has a high leucine content, with 49 amino acids out of 338 (14.5%), and 28 (57.1%) of the 49 leucine residues were conserved among other plant PGIPs. Moreover, the NtPGIP sequence showed 10 LRR domains that might be involved in protein–protein interaction (De Lorenzo et al., 2001). The xxLxLxx repeats determine the repeating structure formation, β-sheet/β-loop, where leucine residues form the hydrophobic center. A classic extracellular LRR consensus sequence LxxLxxLxxLxLxxNxLxGxIPxx was found in the ORF domain (Fig. 2).
Fig. 2.
Alignment of NtPGIP amino acid sequence with other known PGIPs. Species abbreviations are Nt (Nicotiana tabacum), Ca (Capsicum annuum), Md (Malus domestica), Pm (Prunus mahaleb), Eg (Eucalyptus grandis), St (Solanum_torvum) and Gb (Gossypium barbadense). Conserved cysteine and leucine sites are indicated with a hashtag and asterisk, respectively and the LRR motifs are shown in red boxes.
3.3. Homologous alignment and phylogenetic analysis of the NtPGIP gene
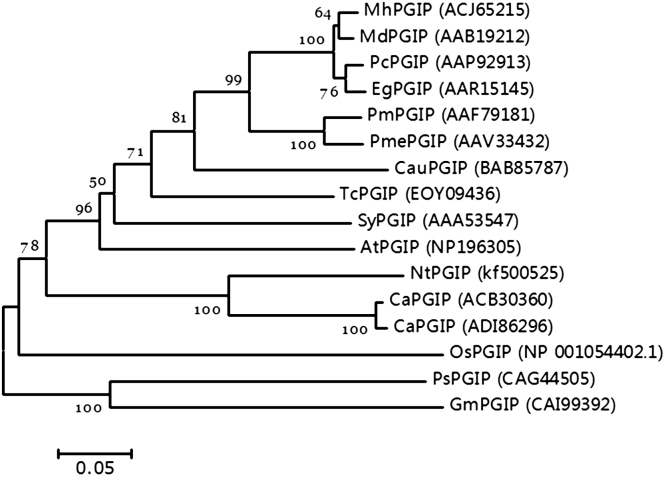
Phylogenetic analysis demonstrated that the deduced tobacco NtPGIP amino acid sequence shared identity with pepper (79.6%), soybean (46.7%), pea (45.8%), crabapple (52.9%), apple (52.0%), pear (51.6%), alpine ash (51.8%), cherry (51.5%), Japanese apricot (51.3%), lime (53.6%), tomato (57.9%), cacao (55.6%), Arabidopsis (54.3%) and rice 42.7%. Moreover, the phylogenetic trees exhibited a close relationship with previously reported PGIP genes from Solanaceae (Fig. 3).
Fig. 3.
Phylogenetic tree construction of the deduced PGIP amino acid sequence via the neighbor-joining method. Genetic distance was calculated based on the nucleotide differences (p-distance) with the complete deletion of gaps. The number at each node indicates the bootstrapping percentage of 1000 replicates. Species abbreviations are Nt (Nicotiana tabacum), At (Arabidopsis thaliana), Ca (Capsicum annuum), Cau (Citrus aurantiifolia), Sy (Solanum lycopersicum), Tc (Theobroma cacao), Mh (Malus hupehensis), Md (Malus domestica), Pm (Prunus mahaleb), Pme (Prunus mume), Eg (Eucalyptus grandis), Pc (Pyrus communis), Ps (Pisum sativum), Gm (Glycine max) and Os (Oryza sativa).
3.4. Expressional analysis of NtPGIP in response to stress factors
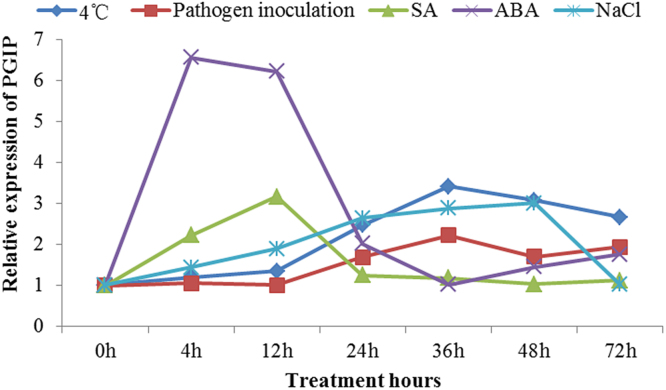
RT-qPCR was performed to examine the NtPGIP expression in leaves under biotic stresses and various treatments. Salicylic acid (SA), abscisic acid (ABA), salt, low temperature and wounding treatments were carried out as chemical and environmental stresses. The results showed that NtPGIP transcriptional levels were up-regulated when tobacco leaves encountered any of the examined stress factors (Fig. 4). After cold treatment, the NtPGIP expression was gradually up-regulated between 4 and 36 h, followed by a moderate decrease from 36 to 72 h, with the highest expressional level reached at 36 h post-treatment (3.4-fold > control; Fig. 4). Tobacco challenged with P. nicotianae also showed a slight up-regulation of NtPGIP after 24 h. During the early stage of infection, the expression was almost unchanged up to 12 h post-infection, with a peak in expression noted after 36 h of treatment (2.2-fold > control) followed by a slowly decline (Fig. 4). SA treatment resulted in a marked NtPGIP expressional pattern change, with expression levels initially up-regulated from 0 h to 12 h, then acutely decreased between 12 and 24 h and recovered to a normal level after 48 h. Expression peaked at 12 h post-treatment and was 3.2-fold higher than the control (Fig. 4). The ABA stress response was very similar to the SA response, with NtPGIP expression initially up-regulated from 0 h to 12 h, then acutely decreased between 12 and 36 h and moderately increase after 36 h. Expression peaked at 4 h post-treatment and was 6.6-fold higher than the control (Fig. 4). Salt stimulated NtPGIP expression, with levels peaking at 48 h and then sharply declined to near pre-infection levels at 72 h (Fig. 4). These results suggest that NtPGIP might be involved in plant responses to different abiotic stresses and play a role in the plant defense system
Fig. 4.
RT-qPCR expression analysis of NtPGIP in response to different stressors. a) Low temperature exposure 4 °C; b) Phytophthora nicotianae infection; c) SA treatment; d) ABA treatment; and e) salt treatment. All sample expression levels were compared with that of the control samples. 18s rRNA gene was used as a reference control.
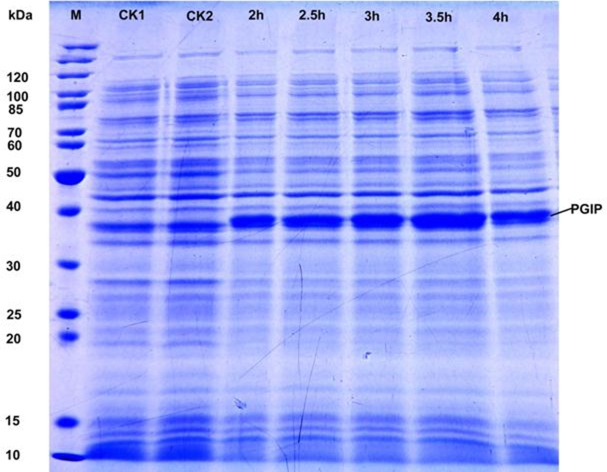
3.5. NtPGIP expression in E.coli, recombinant protein purification and Western blot analysis
Compared with the negative and positive controls, the recombinant NtPGIP protein was highly expressed in IPTG induced E. coli BL21 (DE3) at different sampling times. The predicted weight of the NtPGIP protein was 34.84 kDa, with the 6xHis-tag adding ∼2.5 kDa for a total of ∼37.3 kDa (Fig. 5). Following SDS-PAGE, the detected fusion protein band was consistent with the predicted molecular weight (Fig. 5). pET-PGIP transformed E. coli were grown and gene expression was induced under optimal conditions. Harvested cells were lysed by sonication and the supernatants were loaded onto a Ni–NTA fastflow column. Bound recombinant protein was eluted and the final preparation, which contained 400 mmol/L imidazole, gave a single band on the SDS–PAGE gel (Fig. 6).
Fig. 5.
SDS-PAGE analysis of induced NtPGIP. The samples were collected at 2, 2.5, 3, 3.5 and 4 h post-induction with 1 mol/mL IPTG, negative control (CK1) and positive control (CK2).
Fig. 6.

SDS–PAGE of purified NtPGIP recombinant protein via Ni-Sepharose affinity chromatography. Lane 1: product eluted with 20 mmol/L imidazole; Lane 2: product eluted with 100 mmol/L imidazole; Lane 3: product eluted with 200 mmol/L imidazole; and Lane 4: product eluted with 400 mmol/L imidazole. S: supernatants; P: precipitation; N: total protein from strains harboring pET-PGIP after induction for 4 h under 37 °C; M: Protein molecular weight marker. A full, unmodified version of this figure is available as Supplementary File 1.
Western blot analysis showed that both of the post-induction purified protein and the total protein from pET-PGIP harboring strains post-induction reacted with the mouse anti-His antibody displaying an expected ∼37 kDa band, while the same band was not detected in either the positive (i.e E. coli BL21 (DE3) harboring pET-28a) or negative (i.e E. coli BL21 (DE3) harboring pET-28a without induction) controls (Fig. 7). These results suggest that the pET-PGIP expression vector was successfully constructed and that the recombinant protein could be used for further analysis.
Fig. 7.
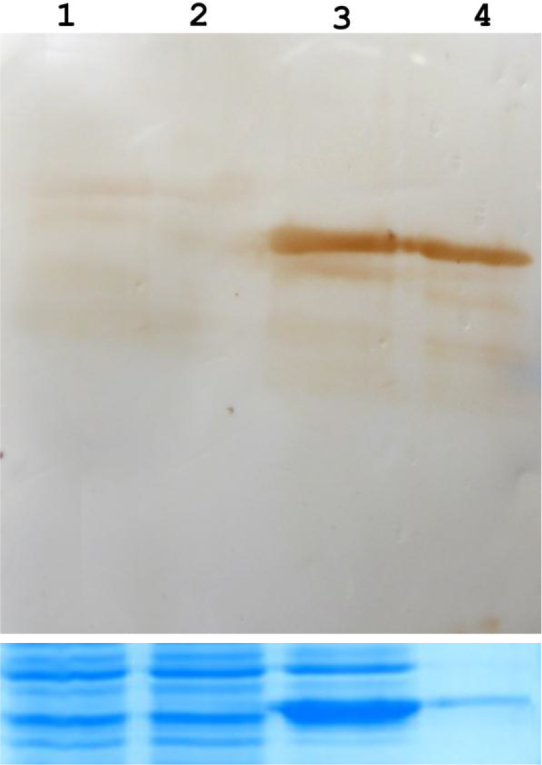
Western-blot analysis of purified and total recombinant proteins. Lane 1: negative control (E. coli BL21 (DE3) harboring pET-28a); Lane 2: positive control (E. coli BL21 (DE3) harboring pET-28a without induction); Lane 3: total protein from strains harboring pET-PGIP after induction; and Lane 4: purified protein. Full, unmodified versions of this figure are available as Supplementary File 2 (top) and Supplementary File 3 (bottom).
3.6. Inhibition of P. capsici PGs by recombinant NtPGIP
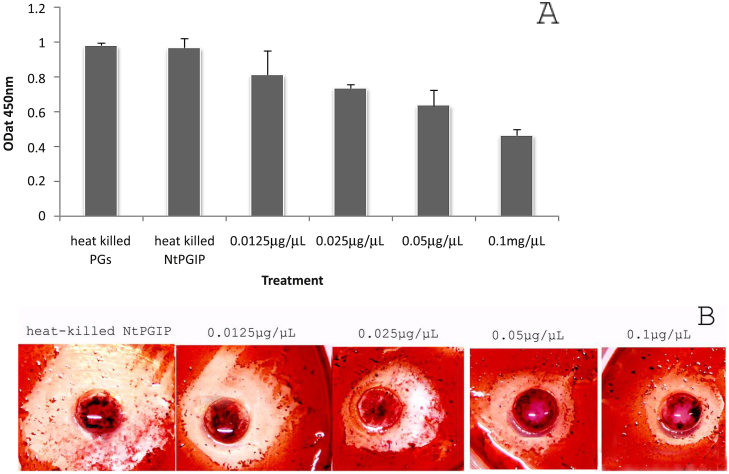
An agarose diffusion plate assay showed that tobacco NtPGIP recombinant proteins can inhibit P. capsici PG activity; the higher the recombinant protein concentration, the smaller the ring diameter (Fig. 8B). Additionally, NtPGIP inhibitory activity against P. capsici PGs (PCPGs) was examined, with different concentrations displaying a clear inhibition. Moreover, the inhibitory effects were in a concentration dependent manner, with 10 μg of purified protein being able to inhibit PCPG activity by 52.6% (Fig. 8A).
Fig. 8.
Inhibition activity analysis of recombinant NtPGIP. A. Inhibition of PGs from P.capsici. B. Agarose diffusion plate assay. The assay was conducted in triplicate and three samples were analyzed with values represented as a mean ± SD. The OD value for each bar represents the mean of three independent experiments and the error bars represent the standard deviations (SDs). Heat killed PGs were used as a positive control and heat killed NtPGIPs were used as a negative control. A full, unmodified version of this figure is available as Supplementary File 4.
4. Discussion
In the present study, the tobacco NtPGIP gene of cultivar NC89 was cloned, its expression pattern was examined under different stressors and its in vitro function was explored. The NtPGIP gene is 1,412 bp long and contains a 186-bp 5′-UTR, a 209-bp 3′-UTR and 1,017 bp ORF that encodes 338 amino acids with a predicted molecular weight of 38.09 kDa and an isoelectric point of 9.25. The NtPGIP gene showed a high degree of homology with other PGIPs from the Solanaceae family that had been previously cloned. Furthermore, the consensus NtPGIP LRR domain was homologous to other characterized PGIPs and PGIP-like plant protein LRR regions (Yang and Chen, 1997). This result suggested that NtPGIP should also be a prominent defense reactive molecule like as it has been reported previously (Di Matteo et al., 2006).
Previous studies examining PGIPs from a variety of plant species have indicated that PGIP genes are expressed in various organs and tissues and inferred that the observed organ-specific distribution plays an important role in biotic resistance during plant growth and development (De Lorenzo and Ferrari, 2002; Gomathi et al., 2006). Some reports have indicated that PGIPs are mostly expressed in tomato (Powell et al., 2000), apple (Yao et al., 1999), raspberry (Johnston et al., 1993) and strawberry (Di et al., 2006) fruit tissues. However, other reports have shown that PGIPs expression is most abundant in young leaves and less in mature leaves (Abu-Goukh et al., 1983; Johnston et al., 1993; Hu et al., 2012), while ginseng PGIP are highly expressed in the roots (Sathiyaraj et al., 2010). In the present study, NtPGIP expression patterns in leaves that were treated with various abiotic and biotic stresses were investigated.
PGIPs may be essential for general resistance to biotic stresses, with PGIP levels generally correlated with the degree of resistance. PGIP transcript accumulation was also noted in potato leaves induced by wounding, salicylic acid or infection with P. infestans (Machinandiarena et al., 2001). The three pepper CaPGIPs were up-regulated at different times following stimulation of the pepper leaves by P. capcisi and abiotic stresses, including salicylic acid, methyl jasmonate, abscisic acid, wounding and cold treatment (Wang et al., 2013). Moreover, Arabidopsis AtPGIP1 and AtPGIP2 transcription levels were up-regulated in response to infection with S. solani (Di et al., 2012). In the present study, tobacco NtPGIP expression was unchanged during the early stage of P. capsici infection and subsequently rose to peak (2.2-fold > control) after 36 h of treatment. This result suggests that this expression pattern is observed probably due to a weaker pathogenicity of the JM-1(Iso-Z0) strain (Li et al., 2011). SA plays a critical role in the defense signaling pathway (Johnson et al., 2003; Grüner et al., 2003; D’Ovidio et al., 2004) and induces PGIP, that reinforces the hypothesis that PGIP functions in plant resistance to fungal attacks (Machinandiarena et al., 2001). In this study, SA treatment induced NtPGIP up-regulation with a peak reached at 12 h, suggesting that NtPGIP may be involved in a SA-induced response (Wang et al., 2013). In mulberry, PGIP expression significantly increased under SA and NaCl stresses relative to the controls (Liang et al., 2005), whereas ABA stress resulted in a significant decrease (Hu et al., 2012). Our study showed that NaCl stress up-regulated NtPGIP expression, which was consistent to what had been found in Brassica campestris (Ahsan et al., 2005). Our results also showed that ABA and SA stresses resulted in an up-regulation of NtPGIP expression, with a peak reached at 12 h post-treatment, which was consistent with previous reports (Wang et al., 2013). However, these findings differed from findings in Phaseolus vulgaris (Bergmann et al., 1994) and in Prunus persica (Liang et al., 2005) where PGIPs were down-regulated following ABA stress. Following cold treatment, NtPGIP expression was up-regulated during the early stage of treatment, followed by a gradual decline to pre-infection levels, which was consistent with the expression profiles of the three CaPGIPs (Wang et al., 2013) and what had been reported in Brassica campestris (Ahsan et al., 2005). All of these results suggest that NtPGIP may be involved in plant abiotic stress responses.
PGIPs from European pear (Stotz et al., 1993), apple (Yao et al., 1999), Japanese’s pear (Faize et al., 2003), Chinese cabbage-pak-choi (Huang et al., 2011; Liu et al., 2007) and poplar (Cheng et al., 2008) are all part of a gene cluster. So far, more than seven PGIPs have been isolated from pepper (Wang et al., 2013), which suggests that PGIP polymorphisms are present in tobacco. While PGIP genes from different plant species share sequence homology, they display versatile recognition specificities against fungal PGs (Wang et al., 2013; Cheng et al., 2008; Ahsan et al., 2005; De Lorenzo et al., 2001; Machinandiarena et al., 2001; Cook et al., 1999; Sathiyaraj et al., 2010). Different PGIPs display distinct inhibitive abilities against PGs, with a diverse range of inhibition. The three pepper CaPGIP proteins showed incompatible interactions with Alternaria alternata, Colletotrichum nicotianae and Phytophthora capsici PGs (Wang et al., 2013). Furthermore, potato PGIP has a broad inhibitive spectrum and almost completely inhibits F. moniliforme, F. solani and A. niger PGs, but shows a more limited ability to inhibit PGs from F. solani f. sp eumatii (Machinandiarena et al., 2001). Similarly, PGIPs from the bean cultivars Blue Lake and Pinto have a broad range of inhibition and are able to inhibit PGs from several fungi (Cook et al., 1999; Machinandiarena et al., 2001). However, there are many specific PGIPs that are highly specific (Desiderio et al., 1997; Cook et al., 1999; Leckie et al., 1999), such as four P. vulgaris PGIPs that display different inhibitory activities and specific recognition abilities against endoPGs from various sources (Leckie et al., 1999; D’Ovidio et al., 2004; Mariotti et al., 2008). Additionally, PGs with variably shaped surfaces have different electrostatic potentials and may be differentially recognized by the broad interacting surfaces of PGIPs (Protsenko et al., 2008). Moreover, one or more point mutations may confer new PGIP recognition specificities and allow interactions with different parts of the PG molecule (Federici et al., 2006). Hence, the various abilities of PGIPs to inhibit a wide spectrum of fungal PGs may be the sum of the abilities of individual PGIPs with different specificities, with each contributing to confer a broad range of inhibitory activities (Desiderio et al., 1997). In the present study, NtPGIP effectively inhibited P. capsici PG activity (PCPG), with 10 μg of purified protein able to inhibit PCPG activity by 52.6%. These results suggest that the NtPGIP gene might be a good candidate to introduce into pepper through molecular breeding. However, further studies should be carried out to investigate the NtPGIP inhibitory spectrum and to further examine tobacco PGIP molecular polymorphism and their specific functions.
In conclusion, tobacco NtPGIP full-length cDNA and gDNA was cloned and showed that NtPGIP expression was up-regulated in response to biotic and abiotic stresses at different times post-treatment. Furthermore, NtPGIPs had an effective inhibitory effect on P. capsici PGs. Overall, these results suggest that tobacco NtPGIP could serve as a new candidate for plant molecular breeding and that characterization of the multivariate functions of NtPGIP may provide valuable insight into the physiological significance of PGIPs in plant disease resistance and stress tolerance.
Declarations
Author contribution statement
Chengsheng Zhang: Analyzed and interpreted the data; Wrote the paper.
Chao Feng, Jing Wang, Fanyu Kong: Contributed reagents, materials, analysis tools or data.
Wenxiu Sun: Performed the experiments.
Fenglong Wang: Conceived and designed the experiments.
Funding statement
This work was supported by a grant from the National Natural Science Foundation of China (31000878).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Chengsheng Zhang, Email: zhangchengsheng@caas.cn.
Fenglong Wang, Email: wangfenglong@caas.cn.
Appendix A. Supplementary data
References
- Abu-Goukh A.A., Strand L.L., Labavitch J.M. Development related changes in decay susceptibility and PG inhibitor content of ‘Bartlett’ pear fruit. Physiol. Plant Pathol. 1983;23:101–109. [Google Scholar]
- Ahsan N., Yoon H.S., Jo J. Molecular cloning of a BcPGIP cDNA from Brassica campestris and its expression to several stresses. Plant Sci. 2005;169:1081–1089. [Google Scholar]
- Berger D.K., Oelofse D., Arendse M.S., Du Plessis E., Dubery I.A. Bean polygalacturonase inhibitor protein-1 (PGIP-1) inhibits polygalacturonases from Stenocarpella maydis. Physiol. Mol. Plant P. 2000;57:5–14. [Google Scholar]
- Bergmann C.W., Ito Y., Singer D., Albersheim P., Darvill A.G. Polygalacturonase-inhibiting protein accumulates in Phaseolus vulgaris L in response to wounding, elicitors and fungal infection. Plant J. 1994;5:625–634. doi: 10.1111/j.1365-313x.1994.00625.x. [DOI] [PubMed] [Google Scholar]
- Bezier A., Lambert B., Baillieul F. Cloning of a grapevine Botrytis-responsive gene that has homology to the tobacco hypersensitivity-related hsr203. J. Exp. Bot. 2002;53:2279–2280. doi: 10.1093/jxb/erf101. [DOI] [PubMed] [Google Scholar]
- Cartwright D.K., Spurr H.W., Jr Biological control of Phytophthora parasitica var. Nicotianae on tobacco seedlings with non-pathogenic binucleate Rhizoctonia fungi. Soil Biol. Biochem. 1998;30:1879–1884. [Google Scholar]
- Cheng Q., Cao Y.Z.H., Pan H.X., Wang M.X., Huang M.R. Isolation and characterization of two genes encoding polygalacturonase inhibiting protein from Populus deltoides. J. Genet. Genomics. 2008;35(10):631–638. doi: 10.1016/S1673-8527(08)60084-3. [DOI] [PubMed] [Google Scholar]
- Cook B.J., Clay R.P., Bergmann C.W., Albersheim P., Darvil A.G. Fungal polygalacturonase exhibit different substrate degradation patterns and differ in their susceptibilities to polygalacturonase inhibiting proteins. Mol. Plant Microb. Interact. 1999;12(8):703–711. doi: 10.1094/MPMI.1999.12.8.703. [DOI] [PubMed] [Google Scholar]
- D’Ovidio R., Raiola A., Capodicasa C., Devoto A., Pontiggia D. Characterization of the complex locus of bean encoding polygalacturonase-inhibiting proteins reveals subfunctionalization for defense against fungi and insects. Plant Physiol. 2004;135(4):2424–2435. doi: 10.1104/pp.104.044644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo G., D’Ovidio R., Cervone F. The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu. Rev. Phytopathol. 2001;39:313–335. doi: 10.1146/annurev.phyto.39.1.313. [DOI] [PubMed] [Google Scholar]
- De Lorenzo G., Ferrari S. Polygalacturonase-inhibiting proteins in defense against phytopathogenic fungi. Curr. Opin. Plant Biol. 2002;5(4):295–299. doi: 10.1016/s1369-5266(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Desiderio A., Aracri B., Leckie F., Mattei B., Salvi G. Polygalacturonase-inhibiting proteins (PGIPs) with different specificities are expressed in Phaseolus vulgaris. Mol. Plant Microbe. Interact. 1997;10(7):852–860. doi: 10.1094/MPMI.1997.10.7.852. [DOI] [PubMed] [Google Scholar]
- Di C., Zhang M., Xu S., Cheng T., An L. Role of polygalacturonase inhibiting protein in plant defense. Crit. Rev. Microbiol. 2006;32(2):91–100. doi: 10.1080/10408410600709834. [DOI] [PubMed] [Google Scholar]
- Di C.X., Zhang H., Sun Z.L., Jia H.L., Yang L.N. Spatial distribution of polygalacturonase-inhibiting proteins in Arabidopsis and their expression induced by Stemphylium solani infection. Gene. 2012;506:150–155. doi: 10.1016/j.gene.2012.06.085. [DOI] [PubMed] [Google Scholar]
- Di Matteo A., Bonivento D., Tsernoglou D., Federici L., Cervone F. Polygalacturonase-inhibiting protein (PGIP) in plant defence: a structural view. Phytochemistry. 2006;67(6):528–533. doi: 10.1016/j.phytochem.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Di Matteo A., Federici L., Mattei B., Salvi G., Johnson K.A. The crystal structure of polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein involved in plant defense. Proc. Natl. Acad. Sci. USA. 2003;100(17):10124–10128. doi: 10.1073/pnas.1733690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faize M., Sugiyama T., Faize L., Ishii H. Polygalacturonaseinhibiting protein (PGIP) from Japanese pear: possible involvement in resistance against scab. Mol. Plant Microbe. In. 2003;63:319–327. [Google Scholar]
- Fan C., Zhang S., Liu Z., Li L., Luan J. Identification and expression of a novel class of glutathione-S-transferase from amphioxus (Branchiostoma belcheri) with implications to the origin of vertebrate liver. Int. J. Biochem. Cell Biol. 2007;39:450–461. doi: 10.1016/j.biocel.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Federici L., Di Matteo A., Fernandez-Recio J., Tsernoglou D., Cervone F. Polygalacturonase inhibiting proteins: players in plant innate immunity. Trends Plant Sci. 2006;11(2):65–70. doi: 10.1016/j.tplants.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Galletti R., Vairo D., de Cervone F., Lorenzo G. Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Mol. Plant Microb. Interact. 2006;19(8):931–936. doi: 10.1094/MPMI-19-0931. [DOI] [PubMed] [Google Scholar]
- Gamboa A., Paez-Valencia J., Acevedo G.F., Vazquez-Moreno L., Alvarez-Buylla R.E. Floral transcription factor AGAMOUS interacts in vitro with a leucine-rich repeat and an acid phosphatase protein complex. Biochem. Biophys. Res. Commun. 2001;288:1018–1026. doi: 10.1006/bbrc.2001.5875. [DOI] [PubMed] [Google Scholar]
- Gomathi V., Gayathri S., Anupama B., Silva J.A.T., Gnanamanickam S.S. Molecular aspects of polygalacturonase-inhibiting proteins (PGIPs) in plant defense. In: Silva J.A.T., editor. Floriculture, ornamental and plant biotechnology. Global Science Books; London: 2006. pp. 373–379. [Google Scholar]
- Grüner R., Strompen G., Pfitzner A.J.P., Pfitzner U.M. Salicylic acid and the hypersensitive response initiate distinct signal transduction pathways in tobacco that converge on the as-1-like element of the PR-1a promoter. Euro. J. Biochem. 2003;270(24):4876–4886. doi: 10.1046/j.1432-1033.2003.03888.x. [DOI] [PubMed] [Google Scholar]
- Hu D.Q., Dai R.Q., Wang Y.H., Zhang Y.H., Liu Z.Y. Molecular Cloning, Sequence Analysis, and Expression of the Polygalacturonase-inhibiting Protein (PGIP) Gene in Mulberry. Plant Mol. Biol. Rep. 2012;30:176–186. [Google Scholar]
- Huang L., Liu Y., Yu X.L., Xiang X. A polygalacturonase inhibitory protein gene (BcMF19) expressed during pollen development in Chinese cabbage-pak-choi. Mol. Biol. Rep. 2011;38(1):545–552. doi: 10.1007/s11033-010-0139-6. [DOI] [PubMed] [Google Scholar]
- Hwang B.H., Bae H., Lim H.S., Kim K.B., Kim S.J. Overexpression of polygalacturonase-inhibiting protein 2 (PGIP2) of Chinese cabbage (Brassica rapa ssp pekinensis) increased resistance to the bacterial pathogen Pectobacterium carotovorum ssp Carotovorum. Plant Cell Tiss Organ Cult. 2010;103(3):293–305. [Google Scholar]
- Johnson C., Boden E., Arias J. Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell. 2003;15:1846–1858. doi: 10.1105/tpc.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D.J., Ramanathan V., Williamson B. A protein from immature raspberry fruits which inhibits endopolygalacturonases from Botrytis cinerea and other micro-organisms. J. Exp. Bot. 1993;44(5):971–976. [Google Scholar]
- Jones D.A., Jones J.D.G. The roles of leucine-rich repeat proteins in plant defenses. Adv. Bot. Res. 1997;24:89–167. [Google Scholar]
- Joubert D.A., Slaughter A.R., Kemp G., Becker V.W.J., Krooshof G.H. The grapevine polygalacturonase-inhibiting protein (VvPGIP1) reduces Botrytis cinerea susceptibility in transgenic tobacco and differentially inhibits fungal polygalacturonases. Transgenic Res. 2006;15(6):687–702. doi: 10.1007/s11248-006-9019-1. [DOI] [PubMed] [Google Scholar]
- Karr A.L., Albersheim P. Polysaccharide-degrading enzymes are unable to attack plant cell walls without prior action by a “allmodifying enzyme”. Plant Physiol. 1970;46:69–80. doi: 10.1104/pp.46.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.S., Hwang B.K. Virulence to Korean pepper cultivars of isolates of Phytophthora capsici from different geographic areas. Plant Dis. 1992;76:486–489. [Google Scholar]
- Kosola K.R., Eissenstat M., Graham J.H. Root demography of mature citrus trees: the influence of Phytophthora nicotianae. Plant and Soil. 1995;171(2):283–288. [Google Scholar]
- Lang C., Dornenburg H. Perspectives in the biological function and the technological of polygalacturonases. Appl. Microbiol. Biotechnol. 2000;53:366–375. doi: 10.1007/s002530051628. [DOI] [PubMed] [Google Scholar]
- Leckie F., Mattei B., Capodicasa C., Hemmings A., Nuss L. The specificity of polygalacturonase-inhibiting protein (PGIP): a single amino acid substitution in the solvent-exposed b-strand/b-turn region of the leucine-rich repeats (LRRs) confers a new recognition capability. EMBO J. 1999;18(9):2352–2363. doi: 10.1093/emboj/18.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Rimmer R., Yu M., Sharpe A.G., Seguin-Swartz G. Two Brassica napus polygalacturonase inhibitory protein genes are expressed at different levels in response to biotic and abiotic stresses. Planta. 2003;217(2):299–308. doi: 10.1007/s00425-003-0988-5. [DOI] [PubMed] [Google Scholar]
- Li X.K., Kong F.Y., Li X.H., Wang J., Zhang C.S. Preliminary Report on Physiological Race of Phytophthora parasitica in Hubei. Chinese Tobacco Science. 2011;32(3):84–88. [Google Scholar]
- Liang F.S., Zhang K.C.H., Zhou C.J., Kong F.N., Li J. Cloning, characterization and expression of the gene encoding polygalacturonase-inhibiting proteins (PGIPs) of peach (Prunus persica L.) Plant Sci. 2005;168:481–486. [Google Scholar]
- Liu D.Q., Li W.X., He X., Ding Y.M., Chen C.H.Y. Characterization and functional analysis of a novel PGIP gene from Pyrus pyrifolia Nakai cv Huobali. Acta Physiol. Plant. 2013;35:1247–1256. [Google Scholar]
- Liu L., Wang Y., Zhang T., Huang L., Xiang X. Isolation and characterisation of the microspore-related gene BcMF4 in Chinese cabbage-pak-choi and its functional identification in Arabidopsis. J. Hort. Sci. Biotech. 2007;82(1):133–139. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–△△CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Machinandiarena M.F., Olivieri F.P., Daleo G.R., Oliva C.R. Isolation and characterization of a polygalacturonase-inhibiting protein from potato leaves Accumulation in response to salicylic acid, wounding and infection. Plant Physiol. Biochem. 2001;39:129–136. [Google Scholar]
- Manfredini C., Sicilia F., Ferrari S., Pontiggia D., Salvi G. Polygalacturonase-inhibiting protein 2 of Phaseolus vulgaris inhibits BcPG1, a polygalacturonase of Botrytis cinerea important for pathogenicity, and protects transgenic plants from infection. Physiol. Mol. Plant Pathol. 2005;67:108–115. [Google Scholar]
- Mariotti L., Casasoli M., Migheli Q., Balmas V., Caprari C. Reclassification of Fusarium verticillioides (syn F. moniliforme) strain FC-10 as F. phyllophilum. Mycol. Res. 2008;112(9):1010–1011. [PubMed] [Google Scholar]
- Oelofse D., Dubery I.A., Meyer R., Arendse M.S., Gazendam I. Apple polygalacturonase inhibiting protein1 expressed in transgenic tobacco inhibits polygalacturonases from fungal pathogens of apple and the anthracnose pathogen of lupins. Phytochemistry. 2006;67:255–263. doi: 10.1016/j.phytochem.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Powell A.L., Van Kan J., Ten Have A., Visser J., Greve L.C. Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol. Plant Microbe. Interact. 2000;13:942–950. doi: 10.1094/MPMI.2000.13.9.942. [DOI] [PubMed] [Google Scholar]
- Protsenko M.A., Buza N.L., Krinitsyna A.A., Bulantseva E.A., Korableva N.P. Polygalacturonase-inhibiting protein is a structural component of plant cell wall. Biochemistry. 2008;73(10):1053–1062. doi: 10.1134/s0006297908100015. [DOI] [PubMed] [Google Scholar]
- Ren N., Timko M.P. AFLP analysis of genetic polymorphism and evolutionary relationships among cultivated and wild Nicotiana species. Genome. 2001;44(4):559–571. [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method, a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sathiyaraj G., Srinivasan S., Subramanium S., Kim Y.J., Kim Y.J. Polygalacturonase inhibiting protein: isolation, developmental regulation and pathogen related expression in Panax ginseng. Mol. Biol. Rep. 2010;37(7):3445–3454. doi: 10.1007/s11033-009-9936-1. [DOI] [PubMed] [Google Scholar]
- Sella L., Castiglioni C., Roberti S., D’Ovidio R., Favaron F. An endo-polygalacturonase (PG) of Fusarium moniliforme escaping inhibition by plant polygalacturonase-inhibiting proteins (PGIPs) provides new insights into the PG–PGIP interaction. FEMS Microbiol. Lett. 2004;240:117–124. doi: 10.1016/j.femsle.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Stotz H.U., Powell A.L.T., Damon S.E., Greve L.C., Bennett A.B. Molecular characterization of a polygalacturonase inhibitor from Pyrus communis L. cv. Bartlett. Plant Physiol. 1993;102(1):133–138. doi: 10.1104/pp.102.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W.X., Jia Y.J., Feng B.Z., O’Neill N.R., Zhu X.P. Functional analysis of Pcipg2 from the straminopilous plant pathogen Phytophthora capsici. Genesis. 2009;47(8):535–544. doi: 10.1002/dvg.20530. [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;224:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tamura M., Gao M., Tao R., Labavitch J.M., Dandekar A.M. Transformation of persimmon with a pear fruit polygalacturonase inhibiting protein (PGIP) gene. Sci. Hortic. 2004;103:19–30. [Google Scholar]
- Taylor R.J., Secor G.A. An improved diffusion assay for quantifying the polygalacturonase content of Erwinia culture filtrates. Phytopathology. 1988;78(8):1101–1103. [Google Scholar]
- Wang X.J., Zhu X.P., Tooley P., Zhang X.G. Cloning and functional analysis of three genes encoding polygalacturonase-inhibiting proteins from Capsicum annuum and transgenic CaPGIP1 in tobacco in relation to increased resistance to two fungal pathogens. Plant Mol. Biol. 2013;81:379–400. doi: 10.1007/s11103-013-0007-6. [DOI] [PubMed] [Google Scholar]
- Yang B., Yajima W., Das D., Suresh M.R., Kav N.N.V. Isolation, expression and characterization of two single-chain variable fragment antibodies against an endo-polygalacturonase secreted by Sclerotinia sclerotiorum. Protein Expres Purif. 2009;64:237–243. doi: 10.1016/j.pep.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Yang C.L., Chen Z.L. LRR proteins of higher plants: structure and function. Biotechnol. Prog. 1997;17:43–47. [Google Scholar]
- Yao C., Conway W.S., Ren R., Smith D., Ross G.S. Gene encoding polygalacturonase inhibitor in apple fruit is developmentally regulated and activated by wounding and fungal infection. Plant Mol. Biol. 1999;39:1231–1241. doi: 10.1023/a:1006155723059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.