Abstract
In this study, to obtain biomedical polyurethane elastomers with good mechanical properties and biocompatibility, a series of polycarbonate urethanes were synthesized via a two-step solution of polymerization method using the poly(1,6-hexanediol)carbonate diols (PCDL) as the soft segment, 4,4′-methylenebis(cyclohexyl isocyanate) (H12MDI), 1,6-hexamethylene diisocyanate (HDI) and 1,4-butanediol (BDO) as the hard segment with dibutyltin dilaurate as the catalyst. In this article, we illustrated the physical behaviors were obviously influenced by synthetic routes. And their chemical and physical structures were investigated by gel permeation chromatograph (GPC), differential scanning calorimeter (DSC), fourier transform infrared spectrography (FT-IR) and mechanical properties tests. The surface wettability were studied by contact angle measurement (CA). As a kind of short-term implant biomaterial, the results of the hemolysis and platelet adhesive tests were recorded by spectrophotometer and scanning electron microscopy (SEM), indicating the materials have a great potential for developments and applications in biomedical field.
Keywords: Bioengineering, Developmental biology
1. Introduction
Biomedical polyurethanes, due to a combination of excellent physical properties and good biocompatibility, have been deeply investigated and used in medical fields for its non-toxic and biodegradable properties (Christenson et al., 2005; Hasirci and Aksoy, 2007; Fragiadakis and Runt, 2013; Mathur et al., 1997). Scientists have dedicated themselves to this field for at least 50 years (Boretos and Pierce, 1968), which has led to a large number of understandings of their excellent properties, and all of these phenomena were related to the synthetic process and structure of the polyurethanes (Gisselfält et al., 2002; Lee et al., 2011; Li et al., 2012). The unique physical and chemical properties are directly related to their microphase-separated morphology between the soft and hard segment, and also the nature of chain extender indeed influence the properties (Lamba et al., 1997). In order to meet specific property requirements, a variety of polyurethanes were synthesized and countless researches were focused on changing the structures of polyurethanes by introducing different types of diisocyanates or adjusting the proportions of hard segment and soft segment for meeting applications including vascular scaffolds (Sang et al., 2008; Crapo and Wang, 2010), artificial heart valves, adhesives and coating and pacemaker leads (Vlad et al., 2009; Chen et al., 2012; Leila et al., 2010; O'Brien and Carroll, 2009).
Poly(1,6-hexanediol) carbonate diols (PCDL) are biostable elastic materials which have been widely used to synthesize polycarbonate urethanes for biomedical applications, such as the evaluation in vivo of biodegradative stress cracking in polyurethanes elastomer and exposure to hydrolytic, oxidative, peroxidative and biological solutions. (Henryk et al., 2002; Kultys et al., 2009; Seifalian et al., 2003). However, few attentions have been paid to the investigation of the relationship between chemical structures and polyurethane performance, particularly the thermal property, surface behavior and blood compatibility. The objective of the present study in this work was to synthesize and investigate biomedical polyurethanes with the same content of hard segment in weight. The materials were synthesized by a two-step solution polymerization method by using the PCDL (Mn = 2000 Da) as the soft segment, H12MDI, HDI and BDO as the hard segment. The physicochemical properties were investigated by GPC, FTIR, DSC and tensile tests in this study and the maximum tensile elongation was about 850%. A comparative analysis was made between the synthesized materials and available commercial medical polyurethane materials, Pellethane 2363-80A, indicating the poly(carbonate-urethane) elastomers can achieve the same level or even better in mechanical properties. And at the same time, the results of hemolysis and platelet adhesive tests indicated the materials were in accordance with the standard for biomaterials (Kuo et al., 2012; Solanki et al., 2014). The results of the investigation may lay a preliminary basis for future researches and applications in biomedical field.
2. Materials and methods
2.1. Preparation of reagents
The poly(1,6-hexanediol)carbonate diols (Mn = 2000 Da) was supplied by Hersbit Chemical Co., Ltd (Shanghai, China) and stored at room temperature in vacuum. And it was dried at 115 °C under nearly vacuum atmosphere for 24 h before use. The dibutyltin dilaurate (DBTDL), N,N-dimethyl formamide (DMF), N,N-dimethyl acetamide (DMAc), tetrahydrofuran(THF), diethyl ether and 1,4-butanediol (BDO) with chemically pure were all attained from Sinopharm Chemical Reagent Co., Ltd (Beijing, China) without further purification. The diisocyanates, 1,6-hexamethylene diisocyanate (90 wt% purity) and 4,4′-methylenebis(cyclohexyl isocyanate) (99 wt% purity) was purchased from Tokyo Chemical Industry (Tokyo, Japan) and stored at almost 0 °C in the refrigerator to keep it away from water molecule. And H12MDI was properly purified by reducing pressure distillation at 210–220 °C before use. The adult rabbit blood were obtained from Biomedical Materials and Engineering Research Center of Hubei Province, China.
2.2. Characterization methods
2.2.1. Fourier transform infrared spectroscopy (FT-IR)
The FT-IR spectra were obtained using a VERTEX80v Fourier transform infrared spectroscopy (Bruker, Germany), spectrum range 400–4000 cm−1 with a resolution of 4 cm−1. The data were processed by Origin-Pro 8.5.
2.2.2. Differential scanning calorimeter measurements (DSC)
Thermal behaviors were analyzed with a DSC (Perkin-Elmer, America). Liquid N2 was used to cool the DSC cell to reach sub-ambient temperature. All the samples were placed in aluminum plate with weighing range from 9 to 13 mg heated from −150 °C to 200 °C. The experiments were carried out under nitrogen atmosphere flow with a heating rate of 10 °C/min. In order to eliminate the thermal history, only the second heating run was used for further investigation to obtain the glass transition temperature after 2 min station behind the first heating.
2.2.3. Molecular weight determination
Gel permeation chromatography (GPC) measurements were carried out on Alliance high performance liquid chromatography analyzer (Waters, America) taking tetrahydrofuran solution as eluent at a flow rate of about 1.00 ml/min at 30 °C after calibration with monodisperse polystyrene distribution curve as a general calibration parameter to get inquired data based on ASTM D6579-6.
2.2.4. Mechanical testing
Mechanical properties of the films were performed at room temperature by M-30A computer control hydraulic universal testing machine (Shenzhen, China) with a cross-head speed of 50 mm min−1. At least 3 specimens for each type of films were tested to obtain the average.
2.2.5. Contact angles measurement (CA)
The OCA-35 goniometer (Dataphysics, Germany) was used to measure water contact angles of the samples. For each sample, 4 measurements were taken on the air-exposed side to obtain the average value.
2.2.6. Scanning electron microscopy (SEM)
SEM were used to investigate the platelet adhesion on different films recorded on a Hitachi S-4800 SEM (Hitachi, Japan).
2.3. Synthesis and preparation of polyurethane films
The following parameters were guaranteed to be the same during the preparation of the polymer, which were the hard segment of 30 wt% as well as the isocyanate index of 1.05. The polyurethane elastomers were prepared using a two-step solution polymerization method. The reactants were mixed according to the molar ratio of (HDI or H12MDI)/PCDL (1.3, 1.4, 1.5):1 in the first step and then the rest of the chemicals were added based on the isocyanate index of 1.05. Here, we called the molar ratio of (HDI or H12MDI)/PCDL in the first step as R-value. The weight ratio of the hard segment was chosen according to our former work, which showed the better mechanical properties. The procedures of the reaction was divided into two parts: firstly, HDI or H12MDI with PCDL based on different R-value were added in a round-flask in proportion at 40 °C or 70 °C, and then, the BDO and remaining HDI or H12MDI according to the requirement were added to the prepared solution under stirring for 12 h at 40–45 °C or 80–85 °C. In the process of the whole reaction, reaction vessels were protected by nitrogen to prevent water molecule from entering. The simplified reaction procedure was illustrated in Fig. 1.
Fig. 1.
The synthetic procedure for polyurethanes.
The polyurethane films can be easily synthesized via a two-step reaction using PCDL and diisocyanate coupled with a chain extender BDO. In order to keep the experiment work properly, we should make sure all the materials for experiment were fully dry and carried out under nitrogen protection. And all the reagents added to the reacting round-bottomed flask were dissolved in DMAc (for HDI) or DMF (for H12MDI) with the mass percentage points 30%. After that, the mixture was cast into a beaker for precipitation, with ether-water solution in it, And then the polymer was washed 3 times by ultrapure water and placed in Soxhlet extrator using deionized water as extracing agent for removing small molecules for at least 24 h. And then, after being dissolved in THF (7.5 wt%) with stirring at 800 r/min for 2 h, the polyurethane solution was placed in a vacuum oven at 45 °C. Finally, in order to keep the surface of the polyurethane flat, the prepared polymer solution was put in homemade molds carefully for drying at room temperature. The tests on mechanical properties could be carried out after drying in the vacuum oven at 40 °C for at least 5 days.
2.4. In vitro blood compatibility evaluation
2.4.1. Hemolytic test
The fresh blood sample was prepared by mixing 1 ml rabbit blood with 0.1 mg heparin sodium and was diluted with 1.25 ml 0.9% NaCl solution. In the first step, the film pieces were placed in 10 ml 0.9% NaCl solution for 24 h after rinsing for three times by it. And before 0.2 ml blood sample was added, the mixture was placed aside in a thermostatic water bath for 30 min at 37 °C. After incubation for 60 min, diluted fresh blood was subjected to centrifugation at 2500 r/min for 5 min. The upper clear solution of the blood mixture was characterized at an absorption frequency of 542 nm by a spectrophotometer. The sample for positive control was prepared by mixing 10 ml distilled water with 0.2 ml whole blood sample and the sample for negative control was just a mixture of 0.2 ml whole blood sample with 10 ml 0.9% NaCl solution. Six parallel experiments were performed for each film sample.
2.4.2. Platelet adhesion
The degree of blood platelet adhesion and spreading investigations on the various membranes were conducted according to the observations by SEM. The polyurethane films with a real thickness of about 5 μm were prepared in exactly the same way as described previously. The samples were washed at least three times by ultrapure water with the size of membranes were about 4 mm × 4 mm and then equilibrated with phosphate buffer saline (PBS, PH = 7.4) for 2 h at 37 °C. After that, the films were soaked in freshly prepared platelet-rich plasma (PRP) for 1 h at 37 °C and then non-adherent platelets were removed by washing three times with fresh PBS through mild shaking and fixed with 3.8% (w/v) sodium citrate solution. The surface of the membranes were dehydrated in an ethanol-graded series for 30 min each and placed in a dry vessel at ordinary temperature. Adhesion and deformation of platelets on surfaces were examined with a Japan Hitachi S-4800 SEM after gold-sputtering treatment.
3. Results and discussion
3.1. FT-IR analysis
Fig. 2 shows the FT-IR spectra of PCDL and prepared polyurethane films with different R-value. After testing several samples, it could be clearly seen that the characteristic vibrational absorption bands of polyurethane films were almost in the same position. The absorption peaks of the spectra were at 3320–3360 cm−1 (N-H stretching, combined with hydrogen bond) for urethane groups, at 2860–2870 cm−1 and 2930–2939 cm−1 (CH2- stretching and CH2- asymmetrical stretching, respectively) from polycarbonate diol component, at 1710–1738 cm−1 (NHCCO stretching, urethane groups combined with carbonyl groups of HDI or H12MDI), 1523–1537 cm−1 (C-N stretching, combined with N-H in-plane bending). For the location of 1731 cm−1, both of the absorption peak were much broader than PCDL’s and split into 1738 cm−1 and 1680 cm−1. The characteristic absorption peaks of 906 cm−1 and 1039 cm−1 were standard double loop structure supported by H12MDI. And also, 791 cm−1 and 790 cm−1 (O═C-O asymmetric bending), 1242 cm−1 (C-O-C asymmetrical stretching) were supported by polycarbonate diol component proving the successfully synthesis of the structure of carbonate urethane.
Fig. 2.
FT-IR spectra of PCDL and the polyurethanes with different R-value.
Normally, hydrogen bonding also exists in the internal of the polyurethanes, involving the carbonyl groups as the acceptor, and the amide group as the donor. Based on the comparison of the structure of HDI and H12MDI, the HDI has more different molecular conformations resulting in a more flexible of the molecular chain in the hard segment (lower position 1680 cm−1 in the FT-IR). The reduced degree of polarity of carbonyl group can form much more tight structure leading to stronger hydrogen bonding interaction, which also make harder access of the soft segment. The peak of NHCCO stretching combined with carbonyl groups has been lowered by the reinforcement of hydrogen bonding interactions resulting in the better arrangement of hard segment in order. Therefore, the larger degree of microphase separation was resulted from the inhibition of combination of the hard segment and soft segment by hydrogen bonds environment. From what we have discussed above, we can easily come to the conclusion that the polycarbonate polyurethane films have been successfully synthesized according to the procedures depicted in Fig. 1.
3.2. Molecular weight determination
Table 1 shows the molecular mass of polyurethane samples with R-value, showing the weight average molecular and number average molecular increased with R-value rose. The number-average molecular weight (Mn) are in the range of 0.56 × 104-0.92 × 104 and the molecular weight distributions are between 1.23 and 1.39 for HDI-PUs. The number-average molecular weight (Mn) are in the range of 0.75 × 104-1.93 × 104 and the molecular weight distributions are between 1.51 and 1.44 for H12MDI-PUs. Maybe, the designation of the reaction routes can increase the degree of the reaction making almost all of −OH and −NCO groups fully reacted, therefore, reducing the three-dimensional steric effect on the macromolecular main chain of polyurethanes. In general, a relatively large molecule weight will be more beneficial to physical performance. The molecular weight has certain relations with mechanical properties making a great difference for applications in the field of biomedical materials (Taeyi et al., 2011; Doulabi et al., 2013).
Table 1.
Composition of different step of the reaction and molecular weight distribution of the polyurethanes.
| Molar ratio (in the first step) |
Molar ratio (in the second step) |
||||
|---|---|---|---|---|---|
| Samplea | diisocyanate: polydiolb | diisocyanate: chain extender | Mn × 104(Da) | Mw × 104(Da) | Mw/Mn |
| HDI 1.3-PU | 1.300: 1 | 2.434: 2.556 | 0.56 | 0.78 | 1.39 |
| HDI 1.4-PU | 1.400: 1 | 2.334: 2.556 | 0.66 | 0.89 | 1.34 |
| HDI 1.5-PU | 1.500: 1 | 2.234: 2.556 | 0.92 | 1.14 | 1.23 |
| H12MDI 1.3-PU | 1.303: 1 | 1.420: 1.593 | 0.75 | 1.13 | 1.51 |
| H12MDI 1.4-PU | 1.402: 1 | 1.321: 1.593 | 1.24 | 1.81 | 1.46 |
| H12MDI 1.5-PU | 1.501: 1 | 1.221: 1.593 | 1.93 | 2.78 | 1.44 |
HDI/H12MDI-PCDL polyurethanes are denoted as HDI/H12MDI x-PU, where x is the molar ratio of NCO/OH in the first step of the reaction.
Polydiol refers to PCDL.
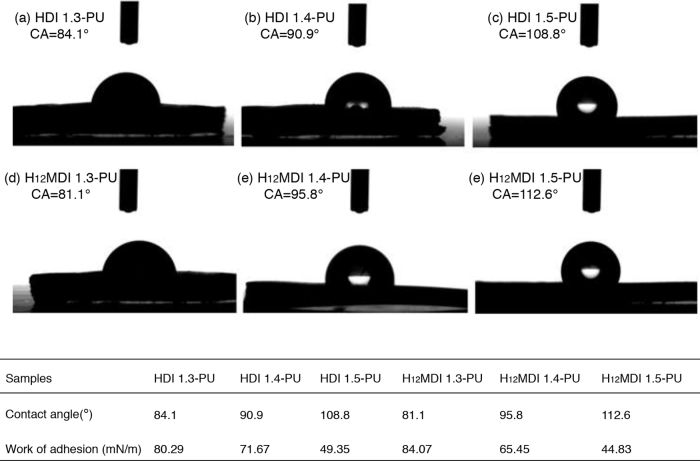
3.3. Contact angles of polyurethanes
As we have known, the surface properties, especially roughness of the membranes will eventually affect the blood-membrane interaction. Generally speaking, it is claimed that the water contact weight can be attributed to many impacts, such as the forming of urea by the water molecule in the raw material with HDI or H12MDI and macrodiol. As directly impacted by chemical bonds, the molecular angles are smaller on smooth surfaces than on rough surfaces resulting from the wetting hysteresis reduced by the surface smoothness (Extrand and Kumagai, 1997). The larger the surface roughness is, the smaller possibility the blood clots to form. In order to get better blood-compatible materials, it is necessary to observe and investigate the surface wettability of the films. The contact angles of water were measured at normal temperatures and pressures on the surface of the films, and work of adhesion values were calculated on the basis of the following equation:
where , and θ represented the work of adhesion, surface tension of water (72.81 mN/m) and contact angle of water, respectively (Sheikhy et al., 2013).
The obtained results were shown in Fig. 3, revealing the wettability depends on the diisocyanate nature and R-value. And Fig. 3 showed the contact angles of polyurethane films with different R-value, HDI 1.3-PU<HDI 1.4-PU<HDI 1.5-PU and H12MDI 1.3-PU<H12MDI 1.4-PU<H12MDI 1.5-PU. We could get the results H12MDI-PUs were better in hydrophobic property. In general, the value of angles increased as the molecular weight increased, indicating the surface hydrophobic property’s rising. The increase of contact angle and the decrease of work of adhesion could be responsible for the weaker capability of the PU surface to generate more hydrogen bonds with water molecules. Furthermore, for the smaller molecular weight of the polyurethanes, the existence of more smaller side chains might be another significant factor which would given rise to more hydrophilic in controlling contact angles.
Fig. 3.
Visual performance of the water droplet contact angles and work of adhesion on the different surfaces of membranes: (a) HDI 1.3-PU, (b) HDI 1.4-PU, (c) HDI1.5-PU, (d) H12MDI 1.3-PU, (e) H12MDI 1.4-PU and (f) H12MDI 1.5-PU.
3.4. Differential scanning calorimetry
To obtain further insight into the microphase separation of polyurethanes based on H12MDI or HDI/PCDL, DSC analysis was employed to study their glass transition temperatures (Eceiza et al., 2008). The relationship between R-value and degree of microphase separation could be deduced from a comparison with the Tg of PCDL at −70–66.4 °C. Fig. 4 displays the DSC thermograms of them and only the second heating run was used for further investigation so that the thermal history of the representative samples could be eliminated.
Fig. 4.
The DSC thermograms of polyurethanes based on H12MDI or HDI/PCDL.
A much wider glass transition region can be seen for these polyurethanes based on H12MDI and HDI/PCDL, displaying approximately 16 °C and −42 °C, respectively. And the H12MDI/PCDL moved progressively to higher temperatures, indicating the better compatibility between the soft segment and the hard segment. With the increase of R-value, the temperature of Tg slightly increased resulting from the improvement of the microphase separation by increasing R-value. Hence, the results demonstrated the microphase separation structures existed, and this was in accordance with the mechanical properties since the membrane with higher R-value possessed better physical behavior. In addition, some detectable corresponding peak for melting endotherms can be observed in the range of 90 °C–110 °C for HDI/PCDL and 80 °C–100 °C for H12MDI/PCDL. These phenomena should be probably attributed to the higher crosslinking and lower mobility of polymer chains at higher temperature, indicating the R-value has a certain effect on the contribution to the glass transition properties of polyurethanes under investigation (Kalogeras and Hagg Lobland, 2012; Choi et al., 2010). Some small melting endotherms are observed in the range of 40–50 °C and the glass transition temperature are to be approximately 25 °C. The results demonstrated the being of HDI-BDO sequence into the soft phase was existed. In my personal perspective, the increase of R-value could be favourable to the transformation of dispersed phase to continuous phase which enhanced the aggregation effects the level of order of the hard segment.
3.5. Mechanical testing
Whether a kind of biomedical material can be put into application depends largely on its mechanical properties (Martin et al., 2000). The material will crack when the local strength is more than the limited strength resulting from the rupture of local molecular chains. As we know, that is a fact that elongation is inversely proportional to brittleness. Many factors could play a crucial part in deciding the mechanical properties of the prepared polyurethanes, such as the crosslink density and the proportion of the hard segment (Abraham et al., 1997; Jeong et al., 2000).
Fig. 5 depicted the stress-strain curves for films and the corresponding tensile characteristic parameters for elastomers were collected in Table 2. Clearly presented in Fig. 5, the series of polyurethane films could reach the same level or even better in mechanical properties than Pellethane 2363–80A did, especially for H12MDI 1.5-PU. It displayed tensile strength of less than 10 MPa and showed the best properties in elongation at break. With the increase of the R-value, the elongation at break of synthetic products had regular changes in Table 2 and it should be gradually reduced in theory (Groot et al., 1997; Narkis et al., 2006). With the degree of microphase separation increased, it may lead to more crystallization appearing at the same time. Even after the microphase separation, the possibility of soft segment crystalline has improved and there is no three-dimensional steric effect on the macromolecular main chain so as to make the material much more harder and brittle, and also resulting in the decrease of elongation at break.
Fig. 5.
Stress-strain curves of polyurethane films and Comparison of mechanics properties tests between polyurethane membranes and Pellethane 2363-80A.
Table 2.
Hematolysis ratio of membranes.
| Samples | Average optical density | Negative | Positive | Hemolysis ratio (100%) |
|---|---|---|---|---|
| HDI 1.3-PU | 0.4319 | 0.0401 | 0.3722 | 1.3004 |
| HDI 1.4-PU | 0.4079 | 0.0401 | 0.3722 | 1.1076 |
| HDI 1.5-PU | 0.3140 | 0.0401 | 0.3722 | 0.8250 |
| H12MDI 1.3-PU | 0.4064 | 0.0401 | 0.3722 | 1.1031 |
| H12MDI 1.4-PU | 0.3341 | 0.0401 | 0.3722 | 0.8854 |
| H12MDI 1.5-PU | 0.3049 | 0.0401 | 0.3722 | 0.7973 |
3.6. Hemolytic tests
Hemolysis of the blood is an extremely significant problem associated with biocompatibility of materials faced by biomedical investigators. Red blood cells may rupture and then release the blood platelet and hemoglobin when contacting with blood incompatible implant materials, causing blood clots (Wen et al., 2010; Wanqing et al., 2012). It is of vital importance to study the hematolysis ratio of the membranes. The hematolysis ratio of material indicates the extent of red blood cell broken when contacting with blood. That is to say, the HR value of the material represents the blood compatibility of the material on some level. Generally speaking, the greater HR value is, the worse the blood compatibility of the material has. The value of HR must be ensured at less than 5 so that the material can be used for wide-ranging applications in biomedical field (Fromstein and Woodhouse, 2002). The haemolysis ratio (HR) could be calculated from the following equation:
HR(%) = [(Asample-Anegative)/(Apositive-Anegative)] x 100%
where Asample is the absorbance of the PUs, Apositive and Anegative are the absorbance of positive and negative controls, respectively.
It was found that the HR value of all materials were below 5 from the analysis of Table 2, indicating only little hemolysis were taken on the materials and the hemolysis rate less obviously decreased when the R-value increased. Maybe, this phenomenon can be attributed to the number of side chains of the polymer. Therefore, causing less damage of red blood cells, the HDI 1.5-PU and H12MDI 1.5-PU exhibited desirable blood compatibility.
3.7. Platelet adhesion
The microphotographs came from the observation of SEM of polyurethane membranes with different R-values after 60 min of blood contact. The observation clearly showed lots of platelets adhered to the polyurethane film with the R-value of 1.3 and some of them were gathered together while the number of platelet adhesion on surface obviously declined with the R-value increased. And the pictures of Fig. 6(c, f) illustrated clearly that there was very little adhesion on the surface. This result was also in accordance with the results of the hemolytic tests inducing the higher R-value could slightly reduce the number of adhered platelets on the surface of films which was benefit for potential use as biomaterial devices with favorable blood compatibility. It’s obviously that the wettability of materials has great relationship with the adhesion of platelet in the blood. Generally speaking, when the contact angle gets smaller, it benefits from the adhesion procedure that will result in higher probability to blockages in blood vessels to make medical operation more difficult to carry out. What’s more, some enlightenment should be gained to improve blood compatibility of organisms in the future by modifications. Based on these considerations, we’ll concentrate on doing some researches to enhance the hydrophilicity and improve the biocompatibility expecting little release of the mechanical properties.
Fig. 6.
SEM microphotographs depicting the morphologies of platelet rich plasma contacted surfaces: (a) HDI 1.3-PU, (b) HDI 1.4-PU, (c) HDI 1.5-PU, (d) H12MDI 1.3-PU, (e) H12MDI 1.4-PU and (f) H12MDI 1.5-PU.
4. Conclusions
In this article, a series of different polyurethane membranes were successfully synthesized using HDI or H12MDI, BDO and PCDL via a two-step solution polymerization. The structure of the polymers was proved by the analysis of FT-IR spectra. The wettability and mechanical properties of them were also investigated, displaying a certain capacity for the short-term biomedical application. The physical properties of our prepared polymers were even better in flexibility and elongation at break after the comparison with a commercial biomedical polyurethane. The results of the hemolytic tests and platelet adhesion experiments indicated the materials were of great potential for use as biomaterials with good blood compatibility. Our ongoing work have demonstrated that H12MDI 1.5-PU has great potential in biomedical field. In order to get better applications as a candidate for elastic biomedical material, some modifications are still should be made to improve their biocompatibility.
Declarations
Author contribution statement
Rong Zhu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yiyu Wang: Contributed reagents, materials, analysis tools or data.
Zongrui Zhang: Analyzed and interpreted the data.
Daiwei Ma: Performed the experiments.
Xinyu Wang: Conceived and designed the experiments.
Funding statement
This work was supported by the HongKong, Macao and Taiwan Science & Technology Cooperation Program of China (No.2015DFH30180), and the Key Technology Research Plan of Wuhan Municipality (No.2014060202010120).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abraham G.A., Frontini P.M., Cuadrado T.R. Physical and mechanical behavior of sterilized biomedical segmented polyurethanes. J. Appl. Polym. Sci. 1997;65:1193–1203. [Google Scholar]
- Boretos J.W., Pierce W.S. Segmented polyurethane: a new elastomer for biomedical applications. Science. 1968;158:1481–1482. doi: 10.1126/science.158.3807.1481. [DOI] [PubMed] [Google Scholar]
- Chen S., Wang Q., Wang T. Preparation, tensile, damping and thermal properties of polyurethanes based on various structural polymer polyols: effects of composition and isocyanate index. J. Polym. Res. 2012;19:1–7. [Google Scholar]
- Choi T., Weksler J., Padsalgikar A., Runt J. Microstructural organization of polydimethylsiloxane soft segment polyurethanes derived from a single macrodiol. Polymer. 2010;51:4375–4382. [Google Scholar]
- Christenson E.M., Wiggins M.J., Anderson J.M., Anne H. Surface modification of poly(ether urethane urea) with modified dehydroepiandrosterone for improved in vivo biostability. J. Biomed. Mater. Res. A. 2005;73:108–115. doi: 10.1002/jbm.a.30271. [DOI] [PubMed] [Google Scholar]
- Crapo P.M., Wang Y. Physiologic compliance in engineered small-diameter arterial constructs based on an elastomeric substrate. Biomaterials. 2010;31:1626–1635. doi: 10.1016/j.biomaterials.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulabi A.H., Mirzadeh H., Imani M., Samadi N. Chitosan/polyethylene glycol fumarate blend film: physical and antibacterial properties. Carbohydr. Polym. 2013;92:48–56. doi: 10.1016/j.carbpol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Eceiza A., Martin M.D., Caba K., Kortaberria G., Gabilondo N., Corcuera M.A., Mondragon I. Thermoplastic polyurethane elastomers based on polycarbonate diols with different soft segment molecular weight and chemical structure: mechanical and thermal properties. Polym. Eng. Sci. 2008;48:297–306. [Google Scholar]
- Extrand C.W., Kumagai Y. An experimental study of contact angle hysteresis. J. Colloid Interf. Sci. 1997;191:378–383. doi: 10.1006/jcis.1997.4935. [DOI] [PubMed] [Google Scholar]
- Fragiadakis D., Runt J. Molecular dynamics of segmented polyurethane copolymers: influence of soft segment composition. Macromolecules. 2013;46:4184–4190. [Google Scholar]
- Fromstein J.D., Woodhouse K.A. Elastomeric biodegradable polyurethane blends for soft tissue applications. J. Biomater. Sci. Polym. Ed. 2002;13:391–406. doi: 10.1163/156856202320253929. [DOI] [PubMed] [Google Scholar]
- Gisselfält K., Edberg B., Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002:3951–3958. doi: 10.1021/bm025535u. [DOI] [PubMed] [Google Scholar]
- Groot J.H.D., Vrijer R.D., Wildeboer B.S., Spaans C.S., Pennings A.J. New biomedical polyurethane ureas with high tear strengths. Polym. Bull. 1997;38:211–218. [Google Scholar]
- Hasirci N., Aksoy E.A. Synthesis and modifications of polyurethanes for biomedical purposes. High Perform. Polym. 2007;19:621–637. [Google Scholar]
- Henryk J., Marianne O., Hamilton G., Seifalian A.M. Thermo-mechanical analysis of a compliant poly(carbonate-urea)urethane after exposure to hydrolytic, oxidative, peroxidative and biological solutions. Biomaterials. 2002;23:2231–2240. doi: 10.1016/s0142-9612(01)00356-8. [DOI] [PubMed] [Google Scholar]
- Jeong H.M., Kim B.K., Choi Y.J. Synthesis and properties of thermotropic liquid crystalline polyurethane elastomers. Polymer. 2000;41:1849–1855. [Google Scholar]
- Kalogeras I.M., Hagg Lobland H.E. The nature of the glassy state: structure and glass transitions. J. Mater. Educ. 2012;34:69–94. [Google Scholar]
- Kultys A., Rogulska M., Pikus S., Skrzypiec K. The synthesis and characterization of new thermoplastic poly(carbonate-urethane) elastomers derived from HDI and aliphatic?aromatic chain extenders. Eur. Polym. J. 2009;45:2629–2643. [Google Scholar]
- Kuo W.H., Wang W.H., Chang C.W., Wei T.C., Lai J.Y., Tsai W.B. Improvement of hemocompatibility on materials by photoimmobilization of poly(ethylene glycol) J. Mater. Chem. 2012;22:9991–9999. [Google Scholar]
- Lamba N.M.K., Woodhouse K.A., Cooper S.L. In: Polyurethanes in Biomedical Applications. Schapiro F., editor. CRC Press; Boca Raton, FL, USA: 1997. [Google Scholar]
- Lee C.H., Kato M., Usuki A. Preparation and properties of bio-based polycarbonate/clay nanocomposites. J. Mater. Chem. 2011;19:6844–6847. [Google Scholar]
- Leila H., Kong X., Narine S.S. Novel long chain unsaturated diisocyanate from fatty acid: synthesis, characterization, and application in bio-based polyurethane. J. Polym. Sci. Pol. Chem. 2010;48:3302–3310. [Google Scholar]
- Li Y., Thouas A., Chen Q. Biodegradable soft elastomers: synthesis/properties of materials and fabrication of scaffolds. Rsc Adv. 2012;2:8229–8242. [Google Scholar]
- Martin D.J., Warren L.A.P., Gunatillake P.A., Mccarthy S.J., Meijs G.F., Schindhelm K. Polydimethylsiloxane/polyether-mixed macrodiol-based polyurethane elastomers: biostability. Biomaterials. 2000;21:1021–1029. doi: 10.1016/s0142-9612(99)00271-9. [DOI] [PubMed] [Google Scholar]
- Mathur A.B., Collier T.O., John K.J., Wiggins M., Schubert M.A., Hiltner A., Anderson J.M. In vivo biocompatibility and biostability of modified polyurethanes. J. Biomed. Mater. Res. 1997;36:246–257. doi: 10.1002/(sici)1097-4636(199708)36:2<246::aid-jbm14>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Narkis M., Brostow W., Hagg Lobland H.E. Sliding wear, viscoelasticity, and brittleness of polymers. J. Mater. Res. 2006;21:2422–2428. [Google Scholar]
- O'Brien B., Carroll W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: a review. Acta Biomater. 2009;5:945–958. doi: 10.1016/j.actbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Sang J.L., Jie L., Oh S.H., Soker S., Atala A., Yoo J.J. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials. 2008;29:2891–2898. doi: 10.1016/j.biomaterials.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Seifalian A.M., Salacinski H.J., Tiwari A., Edwards A., Bowald S., Hamilton G. In vivo biostability of a poly(carbonate-urea)urethane graft. Biomaterials. 2003;24:2549–2557. doi: 10.1016/s0142-9612(02)00608-7. [DOI] [PubMed] [Google Scholar]
- Sheikhy H., Shahidzadeh M., Ramezanzadeh B., Noroozi F. Studying the effects of chain extenders chemical structures on the adhesion and mechanical properties of a polyurethane adhesive. J. Ind. Eng. Chem. 2013;19:1949–1955. [Google Scholar]
- Solanki A., Methta J., Thakore S. Structure property relationships and biocompatibility of carbohydrate crosslinked polyurethanes. Carbohydr. Polym. 2014;110:338–344. doi: 10.1016/j.carbpol.2014.04.021. [DOI] [PubMed] [Google Scholar]
- Taeyi C., Jadwiga W., Ajay P., James R. Novel hard-block polyurethanes with high strength and transparency for biomedical applications. J. Biomat. Sci. 2011;22:973–980. doi: 10.1163/092050610X540684. [DOI] [PubMed] [Google Scholar]
- Vlad S., Spiridon I., Grigoras C.V., Drobota M., Nistor A. Thermal, mechanical and wettability properties of some branched polyetherurethane elastomers. e-Polymers. 2009;9:37–47. [Google Scholar]
- Wanqing H., Mei T., Rong Z., Jianhao Z., Changren Z. Preparation, characterization and cytocompatibility of polyurethane/cellulose based liquid crystal composite membranes. Carbohyd. Polym. 2012;90:1353–1361. doi: 10.1016/j.carbpol.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Wen X.W., Pei S.P., Hong L., Fei A., Chen H., Li K.Y., Wang Q., Zhang Y.M. Study on an antifouling and blood compatible poly(ethylene–vinyl acetate) material with fluorinated surface structure. J. Mater. Sci. 2010;45:2788–2797. [Google Scholar]