Abstract
The current study was designed to examine the protective efficacy of DNA vaccines based on gp63 and Hsp70 against murine visceral leishmaniasis. Inbred BALB/c mice were immunized subcutaneously twice at an interval of three weeks with pcDNA3.1(+) encoding T cell epitopes of gp63 and Hsp70 individually and in combination. Animals were challenged intracardially with 107 promastigotes of Leishmania donovani 10 days post immunization and sacrificed 1, 2 and 3 months post challenge. The immunized animals revealed a significant reduction (P < 0.05) in splenic and hepatic parasite burden as compared to the infected controls. Maximum reduction in parasite load (P < 0.05) was observed in animals treated with a combination of pcDNA/gp63 and pcDNA/Hsp70. These animals also showed heightened DTH response, increased IgG2a, elevated Th1 cytokines (IFN-γ and IL-2) and reduced IgG1 and IL-10 levels. Thus, mice immunized with the cocktail vaccine exhibited significantly greater protection in comparison to those immunized with individual antigens.
Keywords: visceral leishmaniasis, DNA vaccine, T-cell epitopes, gp63, Hsp70
Introduction
Leishmaniasis is a group of diseases comprised of three clinical entities: visceral leishmaniasis (VL), cutaneous leishmaniasis (CL) and muco-cutaneous leishmaniasis (MCL). The severest form of leishmaniasis is VL, or kala-azar, which is usually fatal if left untreated[1]. In India, the state of Bihar and adjoining areas of West Bengal, Jharkhand and Uttar Pradesh account for about half of the world’s burden of VL[2]. Intensive efforts have been devoted to vaccine development against the disease because of the lack of effective and low-cost treatments and the irreversibility of tissue damage during infection. Because DNA vaccines elicit a strong Th1 bias in the immune response, they appear particularly promising in the case of Leishmania[3]. Indeed, the superior efficacy, compared to their recombinant counterparts, of DNA vaccines encoding gp63, PSA-2 and LACK demonstrated the potential of this approach[4-8]. DNA vaccines induce a full spectrum of immune responses that include cytolytic T cells, helper T cells and antibodies[9]. The ability of plasmid DNA encoding specific antigen to induce both CD4+ and CD8+ T cells suggests that this approach will be of particular use for protection against diseases that require cell-mediated immunity, such as leishmaniasis. In a study by Rafati, the protective potential of an immunogenic gene, known as SPase (signal peptidase type I) from Leishmania major (L. major), was evaluated using three different vaccination strategies (DNA/DNA, protein/protein, and DNA/protein) against L. major infection. The results suggested that a DNA/DNA strategy induced greater protection than the other two approaches[10]. The membrane protease gp63 is present in promastigotes of all species and is one of the parasite receptors for host monoclonal antibodies. Parasite mutants lacking the protein are avirulent[11]. It induced Th1 type of immune responses when used as vaccine in human models[5]. Recombinant BCG expressing the Leishmania surface antigen gp63 induced a protective immune response against L. major infection in BALB/c mice[12]. In fact, gp63 or leishmaniolysin was the first recombinant antigen used to vaccinate against leishmaniasis.
Hsp70 has been shown to be a potent activator of innate immunity. An Leishmania donovani (L. donovani) antigen belonging to the Hsp70 family is recognized by sera from the majority of patients with visceral leishmaniasis[13]. Leishmania infantum (L. infantum) Hsp70 has been described as an immunodominant antigen in both humans and dogs suffering from VL. It has been found to be a major target of the humoral immune response during Leishmania infections[14]. In addition, Hsp70 has been found to activate the complement system[15] and in the development of protective immune response. In our previous study, we reported the protective efficacy of a gp63 and Hsp70 cocktail vaccine against experimental murine VL[16], but the vaccine did not provide complete protection from Leishmania infection. DNA vaccines may be able to induce a better immunity than protein vaccine; thus, in this study, the immunogenicity and efficacy of DNA vaccines encoding gp63 along with Hsp70 as an adjuvant was tested and compared with the protein counterparts to define the most promising antigen against L. donovani.
Materials and methods
Parasite
Promastigotes of L. donovani, strain MHOM/IN/80/Dd8 were grown at ambient temperature of 22°C±1°C in RPMI-1640 medium supplemented with 10% FCS. The L. donovani strain was maintained by serial subcultures in the same medium after every 48-72 hours.
Animals
Inbred BALB/c mice of either sex, weighing 20-25 g, were obtained from the Central Animal House of Panjab University, Chandigarh, India. They were fed with water and food ad libitum. The ethical clearance to conduct the experiments was obtained from Institutional Animal Ethics Committee, Panjab University, Chandigarh and the study was done according to the established guidelines of CPCSEA (110/CAH).
Preparation of vaccines
Isolation of genomic DNA
Approximately 2×108 log phase promastigotes were harvested by centrifugation at 2,000 g for 15 mintues at 4°C. The pellet was washed once with phosphate buffer saline (PBS, pH 7.2) and finally suspended in the same. Totally 1×106 promastigotes were taken in 800 μL of lysis buffer provided with the genomic DNA extraction kit (Zymo Research, USA). The isolated DNA was quantitated by NanodropTM.
PCR
The T cell epitopes of genes encoding gp63 (GenBank accession No. M60048) and Hsp70 (GenBank accession No. X85798) were identified using “MHC Pred” online software. The T cell epitopes were identified on the basis of prediction score for both mouse alleles, i.e. IAd and IEd. The primers were designed from the gene sequences available in the database using IDT “Scitools". The restriction enzyme sites for BamHI and HindIII and kozak sequences were added in the primers. The following primers were designed for amplification of 612 bp sequence of Hsp70: forward primer, 5′-GCA GGA TCC GCC ACC ATG GGC AAC GAG ATC-3′ and reverse primer - 5′-GCG CAG CTC GAG TTA GAG GTC CTG GAA GAT-3′. The following primers were designed for amplification of 576 bp sequence of gp63: forward primer, 5′-GCA GGA TCC GCC ACC ATG GAC AAG CGC-3′ and reverse primer 5′-CAG CTC GAG TTA GTG CCC TTC TCC TCC-3′. The following conditions were used for the amplification of the coding region of T cell epitopes of Hsp70 and gp63. Initial denaturation was performed at 94°C for 10 minutes and then for 1 minute. Annealing was carried out for one minute at 55.4°C for Hsp70 and 57°C for gp63. Extension was carried out at 72°C for 1 minute and final extension was done at the same temperature for 10 minutes. PCR was carried out for 40 cycles.
Preparation of DNA vaccine
DNA vaccines were prepared with pcDNA3.1 (+) vector. The fragments of genes encoding gp63 and Hsp70 were amplified using primers. The cloning of these fragments was done according to the manufacturer’s instructions (Lucigen). Briefly, the PCR products were run on agarose gel electrophoresis and the desired DNA fragments were isolated and purified from the gel using a commercial DNA purification kit (Geneaid, Taiwan, China) following the manufacturer’s instructions to avoid any contamination of unused primers and primer dimers. The purified DNA was quantified using NanodropTM.
Restriction digestions of PCR products and plasmid DNA were performed per the manufacturer’s instructions (MBI Fermentas, Germany). The reaction mixture was mixed gently and incubated at 37°C. Double digestion of PCR products and the plasmid was carried out with BamH1 and HindIII. The double digested plasmid DNA and PCR products were electrophoresed and purified from the rest of the reaction mixtures with Gel Extraction kit (Geneaid) using the manufacturer’s protocol. Ligation reactions were set up using the linearized pcDNA3.1(+) and double digested PCR products. The ligation mixture was incubated at 16°C for 16 hours in water bath.
Transformation efficiency of the competent cells was checked by transforming them with an uncut plasmid and calculating the cfu/μg of DNA. The transformation efficiency of 1×108 cfu/μg DNA and above was taken as a good competent cell preparation. Transformation of E. coli DH5α cells was carried out per the instructions given by Sambrook and Russell[50]. The presence of the gp63 and Hsp70 genes in pcDNA3.1(+) vector was determined by restriction enzyme digestion (data not shown) and then by PCR primers. Furthermore, the in vitro expression of the inserted genes in vector pcDNA3.1 was checked by transfection of plasmid constructs in J774.2 cell lines from mouse macrophages.
In vivo immunization and challenge infection
Immunization and infection of mice
Each group had 6 BALB/c mice. They were immunized by subcutaneous injections of 100 μg of pcDNA/gp63 and pcDNA/Hsp70, free or in combination. Group I animals were immunized with gene encoding gp 63 alone, Group II were immunized with genes encoding Hsp70 alone and the animals in Group III were given the combination of both genes. Two injections were given at an interval of 3 weeks. Animals receiving only PBS served as unimmunized infected controls. Ten days after the booster, the immunized and unimmunized infected control mice were intracardially challenged with 107 promastigotes.
Assessment of parasite load
Animals were sacrificed on 30, 60 and 90 days post infection/challenge. The livers and spleens of all animals were removed and weighed. Impression smears of the liver and spleen were made and the parasite load was assessed in terms of L. donovan Units (LDU) by the method of Bradley and Kirkley[17].
Delayed type hypersensitivity (DTH) responses to Leishmania
All groups of mice were challenged in the right foot pad with a subcutaneous injection of leishmanin. The left foot pads were inoculated with PBS. After 48 hours, the thickness of the right and left foot pad was measured using a pair of Vernier calipers. Results were expressed as mean ± S.D. of percentage increase in the thickness of the right foot pad as compared to the left footpad of mice[18-19].
ELISA
The specific serum immunoglobulin G (IgG) isotype antibody response was measured by conventional ELISA using commercially available kits (Bangalore Genei, India). ELISA plates were coated with crude antigen. Serum samples were added at two fold serial dilutions, followed by washes and addition of isotype specific HRP-conjugated secondary antibodies (rabbit anti-mouse IgG1 or IgG2a) after which the substrate and chromogen were added and absorbance was read on an ELISA plate reader at 450 nm.
For determination of vaccine-induced cytokine production, the lymphocytes from spleens of mice were cultured in 24 well plates in 1 mL of RPMI-1640 containing 20 mmol/L NaHCO3, 10 mmol/L HEPES, 10 U/mL of penicillin, 100 μg/mL streptomycin, 2 mmol/L L-glutamine and 10% fetal calf serum. Cells were stimulated with 50 μg/mL of 63 kDa, 70 kDa or 63+70 kDa proteins. Cells were then incubated at 37°C for 72 hours and supernatant was collected and stored at -20°C. This was then assayed for IL-2, IL-4, IL-10 and IFN-γ by using ELISA kits (BenderMed Systems, Diaclone).
Statistical analysis
All the experiments were performed three times independently. All the data were analyzed using two-way analysis of variance (ANOVA). Post hoc test was used for multiple comparisons. The results were considered statistically significant when P < 0.05.
Results
Parasite load
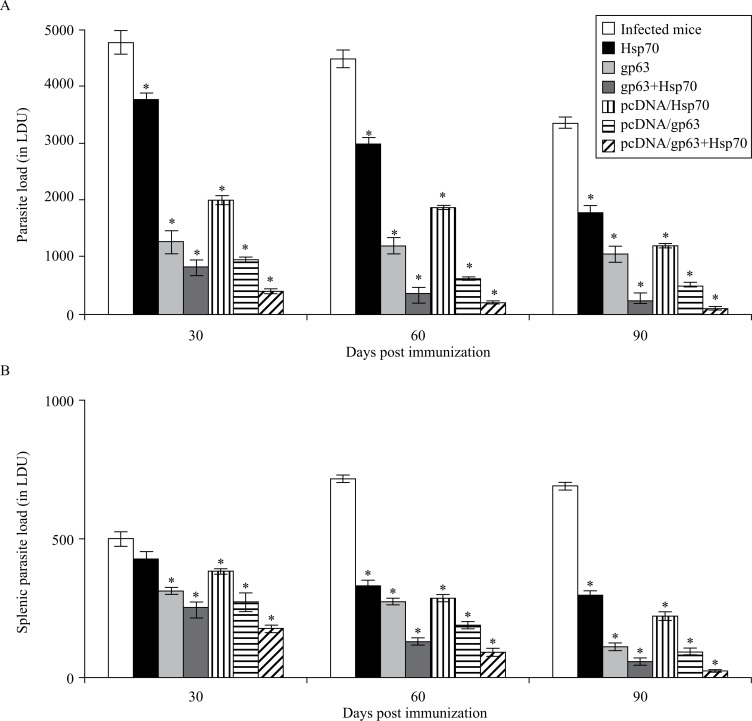
The splenic parasite load increased significantly (P < 0.05) from 30 to 60 days post infection and then decreased significantly (P < 0.05) from 60 to 90 days post infection in the unimmunized infected mice. However, in the immunized animals, the splenic parasite burden declined significantly (P < 0.05) from 30 to 90 days post challenge. A significant reduction in parasite load was observed in animals immunized with pcDNA/gp63 as compared to the infected controls. The pcDNA/Hsp70 genetic vaccine also caused a decline in the parasite burden. However, mice immunized with the cocktail DNA vaccine showed the least parasite burden as compared to those immunized with individual genetic vaccines (Fig. 1A).
Fig. 1. Parasite load in terms of L. donovan units (LDU) in BALB/c mice immunized with different vaccine formulations.
Infected mice vs. mice immunized with Hsp70 or mice immunized with gp63 or mice immunized with gp63+Hsp70 or mice immunized with pcDNA/Hsp70 or mice immunized with pcDNA/gp63 or mice immunized with pcDNA/gp63+pcDNA/Hsp70; * P < 0.05.
The hepatic parasite load decreased significantly from 30 to 90 days post challenge/infection. However, the decrease was more significant (P < 0.05) in immunized animals as compared to the unimmunized infected mice. Similar to parasite burden of the spleen, minimum parasite load was observed in mice immunized with the combination of pcDNA/gp63 and pcDNA/Hsp70 (Fig. 1B).
DTH response to leishmanin
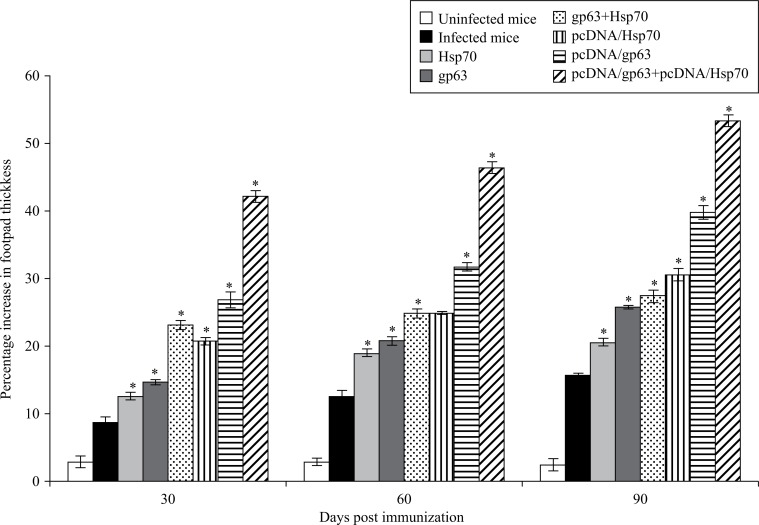
Immunization with vaccine containing pcDNA/gp63 and pcDNA/Hsp70 induced the highest level of DTH response which was significantly higher as compared to mice immunized with individual DNA vaccines (P < 0.05), suggesting the generation of cell-mediated immune responses. The DTH responses in immunized animals were significantly higher than the unimmunized infected and unimmunized uninfected animals (P < 0.05). In immunized animals, the DTH responses were significantly increased from 30 to 90 days post challenge (Fig. 2).
Fig. 2. DTH responses in BALB/c mice immunized with different vaccine formulations.
Infected mice vs mice immunized with Hsp70 or mice immunized with gp63 or mice immunized with gp63+Hsp70 or mice immunized with pcDNA/Hsp70 or mice immunized with pcDNA/gp63 or mice immunized with pcDNA/gp63+pcDNA/Hsp70; * P < 0.05.
Parasite-specific IgG1 and IgG2a isotypes
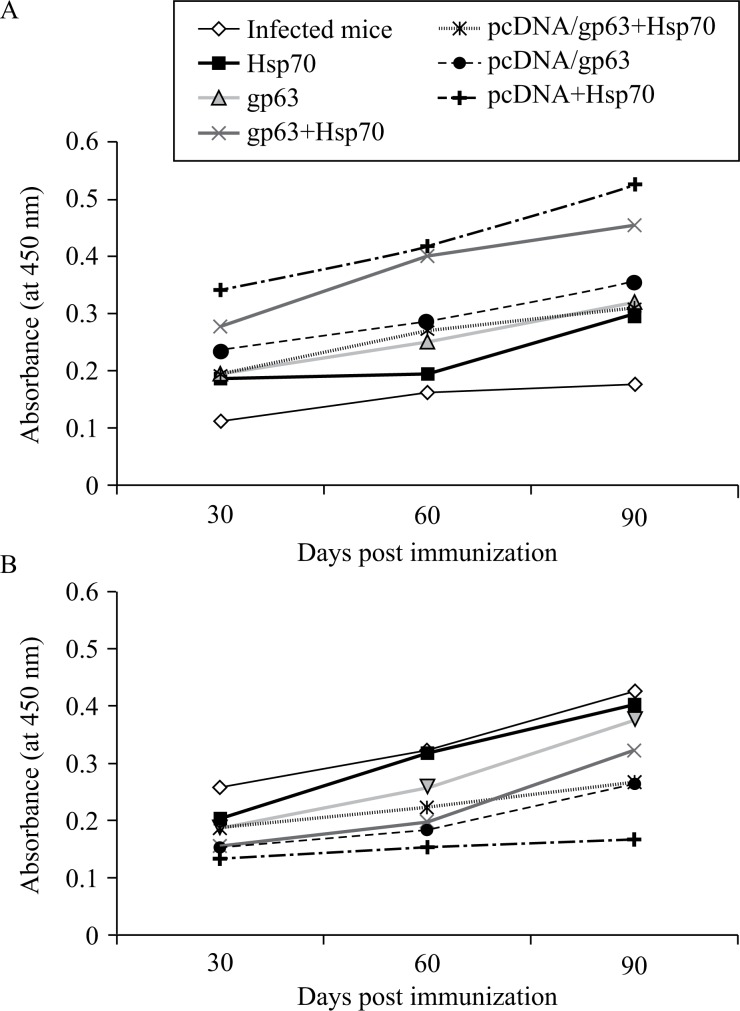
IgG1 and IgG2a antibody responses were also evaluated by ELISA using specific anti-mouse isotype antibodies. Mice immunized with combination of DNA vaccines revealed the highest level of Th1 regulated antibody, IgG2a. Th2 regulated antibody, IgG1, was the lowest in mice immunized with pcDNA/gp63 and pcDNA/Hsp70. IgG1 levels were significantly higher in the unimmunized infected animals as compared to the immunized mice (P < 0.05). Both IgG1 and IgG2a levels increased from 30 to 90 days post challenge (Fig. 3).
Fig. 3. Levels of Leishmania-specific antibodies (A: IgG2a, B: IgG1) in serum samples on different days post challenge in BALB/c mice immunized with different vaccine formulations.
Vaccine induced cytokine responses
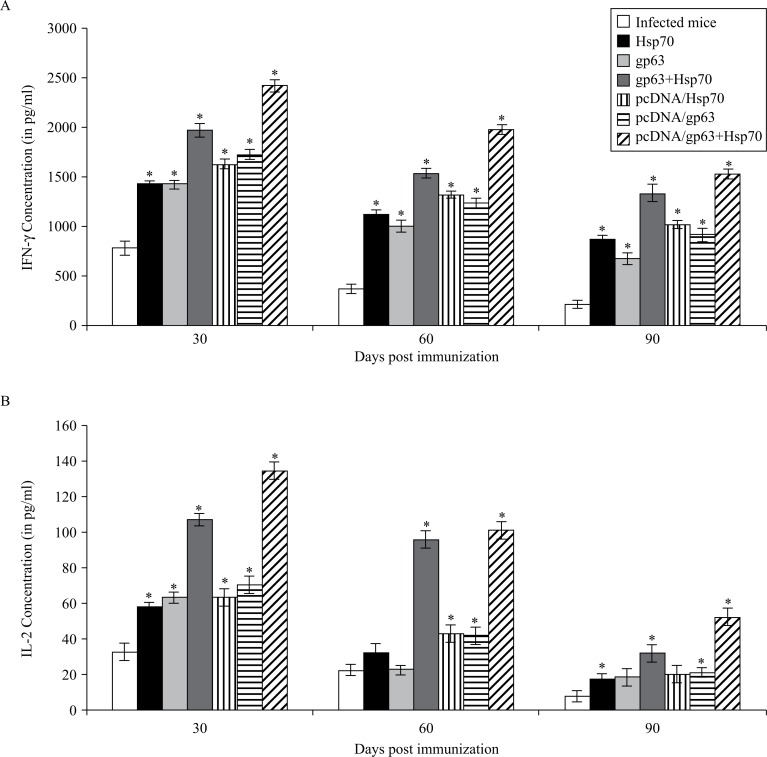
Th1 specific cytokines, that is, IFN-γ and IL-2, were significantly higher in immunized mice as compared to the unimmunized infected and unimmunized uninfected animals (P <0.05). Maximum concentrations of these cytokines were found in animals immunized with the combination of DNA vaccines in comparison to those immunized by pcDNA/gp63 or pcDNA/Hsp70 individually (Fig. 4). The difference within the groups was significant (P < 0.05).
Fig. 4. Concentration of Th1 cytokines (A: IFN-γ, B: IL-2) in the supernatants of cultures of splenocytes of all groups of animals.
The splenocytes were prepared from spleen collected on different days post challenge in BALB/c mice immunized with different vaccine formulations. Infected mice vs mice immunized with Hsp70 or mice immunized with gp63 or mice immunized with gp63+Hsp70 or mice immunized with pcDNA/Hsp70 or mice immunized with pcDNA/gp63 or mice immunized with pcDNA/gp63+pcDNA/Hsp70; * P < 0.05.
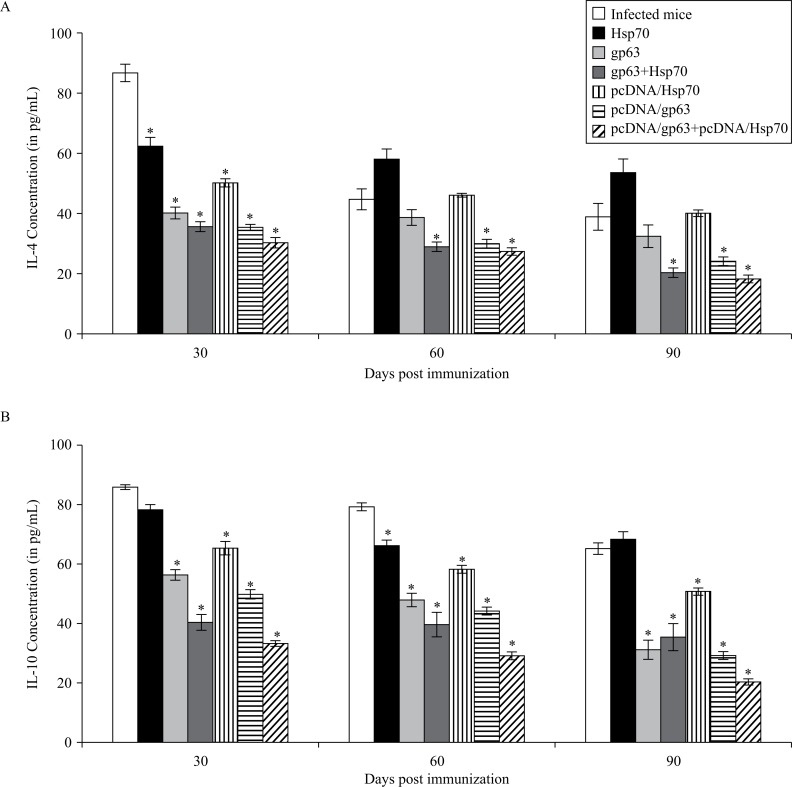
Th2 regulated cytokines IL-4 and IL-10 levels were the lowest in mice immunized with combination of pcDNA/gp63 and pcDNA/Hsp70 (Fig. 5). Mice immunized with individual genetic vaccines showed significantly greater levels of these cytokines as compared to those immunized with the combination of antigens (P < 0.05).
Fig. 5. Concentration of Th2 cytokines (A: IL-4, B: IL-10) in the supernatants of cultures of splenocytes of all groups of animals.
The splenocytes were prepared from spleen collected on different days post challenge in BALB/c mice immunized with different vaccine formulations. Infected mice vs mice immunized with Hsp70 or mice immunized with gp63 or mice immunized with gp63+Hsp70 or mice immunized with pcDNA/Hsp70 or mice immunized with pcDNA/gp63 or mice immunized with pcDNA/gp63+pcDNA/Hsp70; * P < 0.05.
Discussion
DNA vaccination was introduced in 1990 by a study that demonstrated the induction of protein expression upon direct intramuscular injection of plasmid DNA in myocytes[20]. Several features of DNA vaccines have made them an attractive alternative to conventional methods of vaccination. These include the ability of DNA vaccines to express native protein antigens in situ for recognition by B cells and presentation by MHC class I and II molecules to prime helper T cells and CTLs (cytotoxic T lymphocytes), elicit robust immune responses in animal species, and be efficiently manufactured and well characterized[21]. DNA vaccines have been shown to induce a preferentially Th1 immune response, which is necessary for elimination of intracellular parasites[22-24]. DNA plasmids are good priming agents since they are internalized by antigen presenting cells and can induce antigen presentation via MHC class I or II. DNA plasmid backbones are immunogenic due to the presence of stimulatory unmethylated CpG motifs that readily induce Th-1 cytokine expression, leading to cell mediated immunity. Because cytokines or co-stimulatory cell surface molecules play a crucial role in generation of effector T-cell subsets and in determining the magnitude of response, plasmid DNA encoding various cytokines, or co-stimulatory molecules like IL-12, IFN-γ, GM- CSF, and TGF-β, etc. have been used to enhance or bias the immune response generated by DNA vaccination[25]. Cationic liposomes containing soluble Leishmania antigens and CpG ODNs have been reported to induce Th1 immune response and protection against leishmaniasis[26]. Recombinant gp63 has been found to be a potential vaccine candidate for CL[12]. It has been shown to be immunogenic and shows extensive cross reactions among different Leishmania species[27]. gp63 has been found to stimulate expression of Th1 related cytokines which is a known indicator of protective immunity against leishmaniasis[28]. Also, genetic adjuvants have been found to increase the protective efficacy of DNA vaccines. A L. donovani antigen, belonging to Hsp70 family, has been described which is recognized by sera from patients with VL[13]. Hsp70 acts as chaperones and the adjuvant effect of Hsp70 has been demonstrated after immunization with Plasmodium peptides[29] and L. infantum[30]. It has been used as an adjuvant along with DNA immunization with P4[31]. Therefore, the present study was conducted to test the protective efficacy of a DNA vaccine encoding gp63 along with gene encoding Hsp70 as an adjuvant against visceral leishmaniasis. The existence of drug-resistant strains of L. donovani and the increased prevalence of VL among HIV-positive patients have provided a new impetus to the search for a vaccine[32]. In a previous study, we worked on the immunoprophylactic potential of a gp63 and Hsp70 cocktail vaccine in L. donovani infected BALB/c mice[16]. The vaccine imparted significant protection against the disease, but it could not eliminate the parasite completely. Therefore, to impart complete protection against L. donovani, we vaccinated the mice subcutaneously with DNA vaccines comprising of a T-cell epitopic region of gp63 and Hsp70 genes individually and in combination. Subcutaneous route was chosen because, compared to the intramuscular route, the subcutaneous route is easier and safer to administer. Earlier workers have also reported protection against VL and CL with the help of DNA vaccines inoculated subcutaneously in mice[23,33]. The protective efficacy of the three vaccines in our study was assessed by the percentage reduction in the hepatic and splenic parasite load and immunogenicity was assessed through the detection of delayed type hypersensitivity responses, production of anti-leishmanial antibodies (IgG1 and IgG2a) and cytokines such as IFN-γ, IL-2, IL-4 and IL-10.
In the present study, immunization of mice with gp63 and Hsp70 based DNA vaccines conferred significant protection against a progressive infection with L. donovani. The genetic vaccine comprising both pcDNA/gp63 and pcDNA/Hsp70 significantly protected the BALB/c mice against L. donovani infection and reduced the parasite load in the liver by 99.13% and in the spleen by 97.35%. The level of protection was enhanced in comparison to the protein vaccines used in our previous study where, in the animals immunized with a cocktail vaccine of gp63 and Hsp70, the parasite load declined by 82.21% to 92.61% as compared to the infected controls[16]. Similar to our study, Mazumder et al.[34] also reported durable protection against L. donovani challenge till 12 weeks post vaccination with CpG based gp63 vaccine. Similarly, cocktail DNA vaccine encoding cysteine proteinase I, II and III with solid nanoparticles imparted protection in mice against L. major infection[35]. In another study, C57BL/6 mice were vaccinated subcutaneously with a mixture of plasmid DNAs encoding the Leishmania Ags LACK, LmSTI1, and TSA (AgDNA), which led to a complete protection against the development of dermal lesions, and a 100-fold reduction in peak dermal parasite loads compared with controls[51]. Moreover, Guha et al.[52] observed that immunization with HbR-DNA (hemoglobin receptor-DNA) induces complete protection against virulent L. donovani infection in both BALB/c mice and hamsters.
The humoral responses to the vaccines were characterized by analyzing the distribution of IgG1 and IgG2a specific antibodies in the sera of immunized and control animals. ELISA results show that immunized groups developed higher level of antibody responses than control animals. IgG1 response is an indicator of Th2 type of immune response and IgG2a antibody response corresponds to Th1 response which is protective. Among the immunized groups, the highest IgG1 response was induced in mice immunized with pcDNA/Hsp70 followed by gp63 pcDNA/gp63 and the least antibody response was observed in the animals immunized with the combination of both the vaccines. In contrast, the IgG2a antibody production was found to be the maximum in mice immunized with pcDNA/gp63+ pcDNA/Hsp70 genetic vaccine indicating towards the generation of Th1 response. This corresponds to a previous study where mice injected i.m. with 100 Lg L. mexicana cDNA demonstrated high levels of IgG2a isotype antibody and low levels of IgG1 antibody[36]. Abdian et al.[37] also reported higher antibody levels in BALB/c mice immunized with LPG3 based DNA/DNA vaccine as compared to the DNA/protein vaccine approach.
A typical DTH reaction is characterized by activation and recruitment, predominantly of T cells and macrophages, at the intradermal injection site in a previously sensitized host[38]. Peak DTH responses were observed in animals immunized with the combination of pcDNA/gp63 and pcDNA/Hsp70 and these were followed by animals immunized with pcDNA/gp63 DNA vaccine. The immunized mice developed a strong cell mediated immune response and thereby resisted the challenge by parasites. The results demonstrated a positive correlation between enhanced DTH response and reduced parasite load for all groups. These results correspond with an earlier study where mongrel dogs infected with L. chagasi amastigotes and vaccinated with NH36-DNA vaccine developed a significant increase in DTH reactions[39].
The immune response (Th1 or Th2) generated by various vaccine formulations was assessed by quantifying the cytokines (IFN-γ, IL-2 and IL-10) produced by splenocytes of vaccinated animals. Mice immunized with pcDNA/gp63 and pcDNA/Hsp70 in combination showed a greater concentration of IFN-γ, the Th1 specific cytokine, in comparison to mice immunized with the individual genes. High levels of IFN-γ indicate the development of a protective Th1 immune response. Similar to our study, Gurunathan et al.[23] observed that the control of disease progression and parasitic burden in mice vaccinated with LACK DNA was associated with enhancement of antigen-specific IFN-γ production. This IFN-γ is found to come from natural killer cells and is thought to play an important role as a component of innate immune mechanism for the activation of macrophages[40]. Immunization of animals significantly increased the concentration of IL-2 in comparison to the infected controls. Similarly, the IL-2 levels were also found to be maximum in animals immunized with the combination of pcDNA/gp63 and pcDNA/Hsp70 plasmids. IL-2 plays a critical role in priming naive CD4+ T cells to become IL-4 or IFN-γ producers[41] and it is a principal T cell growth factor for Th1 type of immune response. In contrast, IL-4 and IL-10 response was found to be maximum in infected control animals but declined significantly in immunized mice. The lowest concentrations of these two cytokines were observed in the animals immunized with the combination of pcDNA/gp63 and pcDNA/Hsp70. IL-10 represents the main macrophage-deactivating cytokine and plays an important regulatory role in the progression of VL[42]. Our results are consistent with those obtained from the murine leishmaniasis model of both cutaneous and visceral diseases where expression of IFN-γ is associated with control of infection[43,44]. Studies on cytokine profiles in canine VL have established the predominance of the Leishmania-specific Th1 response in asymptomatic dogs and the role of IFN-γ as the key cytokine involved in the activation of macrophages and the killing of the intracellular amastigotes[45]. High levels of IL-10 in infected control animals supported the view that upregulation of this cytokine is accompanied by disease progression and depressed Th1 type of cell mediated immunity with decreased production of IFN-γ and IL-12[46-47]. Previous studies have also confirmed that IL-10 suppresses Th1 response, which plays a protective role against active VL[48]. Cytotoxic T lymphocyte activity of splenocytes was observed in mice immunized either with L. mexicana gp63 cDNA or SLA and long-lived CTL activity was observed in immunized and⁄or re-challenged mice but not naive mice infected with the parasite[35]. In BALB⁄ c mice, the intramuscular injection of a L. major gp63 plasmid DNA construct resulted in partial protection against Leishmania infection, where IFN- γ but not IL-4 was produced by immune CD4+ cells upon restimulation with parasite antigens, suggesting a bias towards a Th1 response[49-50].
Vaccination is the most cost-effective way of controlling infectious diseases. A successful vaccine would be one which is able to generate a long lasting immunity. DNA vaccines, in this regard, are more stable and have lower production costs compared to recombinant vaccines. These vaccines induce stronger and more durable immune responses through triggering innate immune responses. In the present study, the genes encoding T cell epitopes of gp63 and Hsp70 have been used for the first time. Moreover, the immune response generated suggests them as promising candidates for vaccine trials but further studies are needed in higher animal models before coming to a logical conclusion.
Acknowledgments
The authors acknowledge the support provided by the Indian Council of Medical Research, Department of Health Research, India for providing financial support for this study under project ref. 5/8-7(77)/2006-ECD-||.
References
- [1].Ravindran R, Ali N. Progress in vaccine research and possible effector mechanisms in visceral leishmaniasis[J]. Curr Mol Med, 2004, 4(6): 697-709. [DOI] [PubMed] [Google Scholar]
- [2].Sundar S, Chatterjee M. Visceral leishmaniasis-current therapeutic modalities[J]. Indian J Med Res, 2006, 123(3): 345-352. [PubMed] [Google Scholar]
- [3].Dumonteil E, RSM Jesus, EO Javier, et al. DNA vaccines induce partial protection against[J]. Leishmania Mexicana Vaccine, 2003, 21(17-18): 2161-2168. [DOI] [PubMed] [Google Scholar]
- [4].Gurunathan S, Prussin C, Sacks DL, et al. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection[J]. Nat Med, 1998, 4(12): 1409-1415. [DOI] [PubMed] [Google Scholar]
- [5].Olobo JO, Anjili CO, Gicheru MM, et al. Vaccination of vervet monkeys against cutaneous leishmaniasis using recombinant Leishmania major “surface glycoprotein” (gp63)[J]. Vet Parasitol, 1995, 60(3-4): 199-212. [DOI] [PubMed] [Google Scholar]
- [6].Rafati S, Kariminia A, Seyde-Eslami S, et al. Recombinant cysteine proteinases-based vaccines against Leishmania major in Balb/c mice: the partial protection relies on interferon gamma producing CD8+ T Lymphocyte activation[J]. Vaccine, 2002, 20(19-20): 2439-2447. [DOI] [PubMed] [Google Scholar]
- [7].Sjolander A, Baldwin TM, Curtis JM, et al. Vaccination with recombinant parasite surface antigen 2 from Leishmania major induces a Th1 type of immune response but does not protect against infection[J]. Vaccine, 1998, 16(20): 2077-2084. [DOI] [PubMed] [Google Scholar]
- [8].Sjolander A, Baldwin TM, Curtis JM, et al. Induction of a Th1 immune response and simultaneous lack of activation of a Th2 response are required for generation of immunity to leishmaniasis[J]. J Immunol, 1998, 160(8): 3949-3957. [PubMed] [Google Scholar]
- [9].Kowalczyk DW, Ertl HCJ. Immune responses to DNA vaccines. Cell Mol Life Sci, 1998, 55(5): 751-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rafati S, Ghaemimanesh F, Zahedifard F. Comparison of potential protection induced by three vaccination strategies (DNA/DNA, Protein/Protein and DNA/ Protein) against Leishmania major infection using signal peptidase type I in BALB/c mice[J]. Vaccine, 2006, 24(16): 3290-3297. [DOI] [PubMed] [Google Scholar]
- [11].Russell DG, Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes[J]. J Immunol, 1988, 140(4): 1274-1279. [PubMed] [Google Scholar]
- [12].Abdelhak S, Louzir H, Timm J, et al. Recombinant BCG expressing the Leishmania surface antigen gp63 induces protective immunity against Leishmania major infection in BALB/c mice[J]. Microbiol, 1995, 141 (Pt 7): 1585-1592. [DOI] [PubMed] [Google Scholar]
- [13].Macfarlane J, Blaxter ML, Bishop RP, et al. Identification and characterization of a Leishmania donovani antigen belonging to the 70 kDa heat shock protein family[J]. Eur J Biochem, 1990, 190(2): 377-384. [DOI] [PubMed] [Google Scholar]
- [14].Quijada L, Requena JM, Soto M, et al. Analysis of the antigenic properties of the L. infantum Hsp70: design of synthetic peptides for specific serodiagnosis of human leishmaniasis[J]. Immunol Lett, 1998, 63(3): 169-174. [DOI] [PubMed] [Google Scholar]
- [15].Prohaszka Z, Singh M, Nagy K, Kiss E, et al. Heat shock protein 70 is a potent activator of the human complement system[J]. Cell Stress Chaperone, 2002, 7(1): 17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kaur T, Sobti RC, Kaur S. Cocktail of gp63 and Hsp70 induces protection against Leishmania donovani in BALB/c mice[J]. Parasite Immunol, 2011, 33(2): 95-103. [DOI] [PubMed] [Google Scholar]
- [17].Bradley DJ, Kirkley J. Regulation of Leishmania populations within host I. the variable course of Leishmania donovani infections in mice[J]. Clin Exp Immunol, 1977, 30(1): 119-129. [PMC free article] [PubMed] [Google Scholar]
- [18].Nagill R, Kaur S. Enhanced efficacy and immunogenicity of 78 kDa antigen formulated in various adjuvants against murine visceral leishmaniasis[J]. Vaccine, 2010, 28(23): 4002-4012. [DOI] [PubMed] [Google Scholar]
- [19].Kaur S, Kaur T, Garg N, et al. Effect of dose and route of inoculation on the generation of CD4+ Th1/Th2 type of immune response in murine visceral leishmaniasis[J]. Parasitol Res, 2008, 103(6): 1413-1419. [DOI] [PubMed] [Google Scholar]
- [20].Wolff JA, Malone RW, Williams P, et al. Direct gene transfer into mouse muscle in vivo[J]. Science, 1990, 247(4949 Pt 1): 1465-1468. [DOI] [PubMed] [Google Scholar]
- [21].Donnelly JJ, Liu MA, Ulmer JB. Antigen Presentation and DNA vaccines[J]. Am J Respir Crit Care Med, 2000, 162(4 Pt 2): S190-S193. [DOI] [PubMed] [Google Scholar]
- [22].Campos-Neto A, Webb JR, Greeson K, et al. Vacination with plasmid DNA encoding TSA/LmSTII leishmanial fusion proteins confer protection against Leishmania major infection in Balb/c mice[J]. Infect Immun, 2002, 70(6): 2828-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gurunathan S, Sacks DL, Brown DR, et al. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major[J]. J Exp Med, 1997, 186(7): 1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seder RA, Hill AVS. Vaccine against intracellular infections requiring cellular immunity. Nature, 2000, 406: 793-8. [DOI] [PubMed] [Google Scholar]
- [25].Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application and optimization[J]. Annu Rev Immunol, 2000, 18: 927-974. [DOI] [PubMed] [Google Scholar]
- [26].Heravi Shargh V, Jaafari MR, Khamesipour A, et al. Cationic liposomes contatining soluble Leishmania antigens (SLA) plus CpG ODNs induce protection against murine model of leishmanaisis[J]. Parasitol Res, 2012, 111(1): 105-114. [DOI] [PubMed] [Google Scholar]
- [27].Jaffe CL, Bennett E., Grimaldi G Jr, et al. Production and characterization of species specific monoclonal antibodies against Leishmania donovani for immunodiagnosis[J]. J Immunol, 1984, 133(1): 440-447. [PubMed] [Google Scholar]
- [28].Habibi GR, Khamesipour A, McMaster WR, et al. Cytokine gene expression in healing and non-healing cases of cutaneous leishmaniasis in response to in vitro stimulation with recombinant gp63 using semi-quantitative RT-PCR[J]. Scand J Immunol, 2001, 54(4): 414-420. [DOI] [PubMed] [Google Scholar]
- [29].Barrios C, Lussow AR, Van Embden J, et al. Mycobacterial heat-shock proteins as carrier molecules. II. The use of the 70 kDa mycobacterial heat shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Gue´rin priming[J]. Eur J Immunol, 1992, 22(6): 1365-1372. [DOI] [PubMed] [Google Scholar]
- [30].Rico AI, Del Real G, Soto M, et al. Characterization of the immunostimulatory properties of Leishmania infantum Hsp70 by fusion to the Escherichia coli maltose-binding protein in normal and nu/nu BALB/c mice[J]. Infect Immun, 1998, 66(1): 347-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Campbell K, Diao H, Ji J, et al. DNA immunization with the gene encoding p4 nuclease of Leishmania amazonensis protects mice against cutaneous leishmaniasis[J]. Infect Immun, 2003, 71(11): 6270-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Afrin F, Anam K, Ali N. Induction of partial protection against Leishmania donovani by promastigote antigens in negatively charged liposomes[J]. J Parasitol, 2000, 86(4): 730-735. [DOI] [PubMed] [Google Scholar]
- [33].Aguilar-Be I, Zardo RD, Paraguai de Souza E, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis[J]. Infect Immun, 2005, 73(2): 812-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mazumder S, Maji M, Das A, et al. Potency, efficacy and durability of DNA/DNA, DNA/Protein and Protein/Protein based vaccination using gp63 against Leishmania donovani in BALB/c Mice[J]. PLoS ONE, 2011, 6: e14644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Doroud D, Zahedifard F, Vatanara A, et al. Delivery of a cocktail vaccine encoding cysteine proteinases I, II and III with solid lipid nano particles potentiates protective immunity against Leishmania major infection[J]. J Con Rel, 2011, 153(2): 154-162. [DOI] [PubMed] [Google Scholar]
- [36].Ali SA, Rezvan H, Mcardle SE, et al. CTL responses to Leishmania mexicana gp63-cDNA vaccine in a murine model[J]. Parasite Immunol, 2009, 31(7): 373-383. [DOI] [PubMed] [Google Scholar]
- [37].Abdian N, Gholami E, Zahedifard F. Evaluation of DNA/DNA and prime-boost vaccination using LPG3 against Leishmania major infection in susceptible BALB/c mice and its antigenic properties in human leishmaniasis[J]. Exp Parasitol. 2011, 127(3): 627-636. [DOI] [PubMed] [Google Scholar]
- [38].Black CA. Delayed type hypersensitivity: current theories with an historic perspective[J]. Dermatol Online J, 1999, 5(1): 7. [PubMed] [Google Scholar]
- [39].Borja-Cabrera GP, Santos FB, Nico D, et al. The Leishmune Nucleoside hydrolase DNA vaccine as an aid in immunotherapy of canine visceral leishmaniasis[J]. Pro vaccinol, 2012, 6: 64-73. [Google Scholar]
- [40].Nylen S, Maasho K, Soderstrom K. Live Leishmania promastigotes can directly activate primary human natural killer cells to produce interferon -gamma[J]. Clin Exp Immunol, 2003, 131(3): 457-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Seder RA, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells[J]. Annu Rev Immunol, 1994, 12: 635-673. [DOI] [PubMed] [Google Scholar]
- [42].Tripathi P, Singh V, Naik S. Immune response to Leishmania: paradox rather than paradigm[J]. FEMS Immunol Med Microbiol, 2007, 51(2): 229-242. [DOI] [PubMed] [Google Scholar]
- [43].Heinzel FP, Sadick MD, Holaday BJ, et al. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets[J]. J Exp Med, 1989, 169(1): 59-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Squires KE, Schreiber RD, McElrath MJ, et al. Experimental visceral leishmaniasis: role of endogenous IFN-gamma in host defence and tissue granulomatous response[J]. J Immunol, 1989, 143(12): 4244-4249. [PubMed] [Google Scholar]
- [45].Carrillo E, Moreno J. Cytokine profiles in canine visceral leishmaniasis[J]. Vet Immunol Immunopathol, 2009, 128(1-3): 67-70. [DOI] [PubMed] [Google Scholar]
- [46].Carvalho EM, Bacellar O, Barral A, et al. Antigen- specific immunosuppression in visceral leishmaniasis is cell mediated[J]. J Clin Invest, 1989, 83(3): 860-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ghalib HW, Piuvezam MR, Skeiky YA, et al. Interleukin 10 production correlates with pathology in human Leishmania donovani infections[J]. J Clin Invest, 1993, 92(1): 324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Murphy ML, Wille U, Villegas EN, et al. IL-10 mediates susceptibility to Leishmania donovani infection[J]. Eur J Immunol, 2001, 31(10): 2848-2856. [DOI] [PubMed] [Google Scholar]
- [49].Walker PS, Scharton-Kersten T, Rowton ED, et al. Genetic immunization with glycoprotein 63 cDNA results in a helper T cell type 1 immune response and protection in a murine model of leishmaniasis[J]. Hum Gene Ther, 1998, 9(13): 1899-1907. [DOI] [PubMed] [Google Scholar]
- [50].Xu D, Liew FY. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major[J]. Immunology, 1995, 84(2): 173-176. [PMC free article] [PubMed] [Google Scholar]
- [51].Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual, 3rd Edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- [52].Méndez S, Gurunathan S, Kamhawi S, et al. The potency and durability of DNA and proteinbased vaccines against Leishmania major evaluated using lowdose, intradermal challenge[J]. J Immunol, 2001, 166(8): 5122-5128. [DOI] [PubMed] [Google Scholar]
- [53].Guha R, Gupta D, Rastogi R, et al. Vaccination with Leishmania Hemoglobin Receptor–Encoding DNA Protects Against Visceral Leishmaniasis[J]. Sci Translational Med, 2013, 5(202): 202ra121. [DOI] [PubMed] [Google Scholar]