Abstract
Melatonin plays a critical role in regulating photoperiodic signals and has recently been shown to decrease immunosenescence with age. In this study, we examined whether melatonin activates T lymphocytes as major adaptive immune cells in in vitro and in vivo models. Splenocytes, CD4+, and naïve CD4 T lymphocytes were isolated from the spleen of BALB/c mice and the cell population patterns and mRNA profiles associated with T cell activation (CD28 and p21) and the melatonin receptor (MT1A and MT1B) were assessed. The T cell activation-related proteins Ki67 and Bcl2 were also evaluated to confirm the relationship between gene and protein levels. Our data clearly revealed that CD28, p21, MT1A, and MT1B mRNA were highly expressed in the presence of melatonin. Co-culture of CD4+ T lymphocyte and peritoneal macrophage 7 days after melatonin administration to young and aged mice significantly increased APRIL mRNA, suggesting induction or maintenance of T lymphocyte responses. We also found that the intracellular amount of Ki67 and Bcl2 proteins were significantly upregulated in aged CD4+ T lymphocytes, suggesting enhancing T cell proliferation and ling-term maintenance of memory T cells. Taken together, we conclude that melatonin supplementation may enhance immunity in aged individuals by upregulating immunosenescence indices in association with T lymphocytes and may be an attractive pharmacological candidate for aged and immunocompromised individuals.
Keywords: melatonin, aging, CD4+, naïve CD4, melatonin receptor
Introduction
The functional versatility of melatonin, a derivative of serotonin, as an environmental clock and calendar is reflected in its wide distribution within phylogenetically distant organisms, ranging from bacteria to plants and humans[1–2]. Accumulating studies indicate that melatonin induces circadian changes in organisms via receptor-mediated mechanisms, regulates seasonal reproduction, modulates sleep cycles, and influences bone growth[3–4]. Moreover, locally produced melatonin in organs throughout the body protects against nitro-oxidative stress and free radical scavengers[5–6]. This may be particularly valid in the gastrointestinal tract and skin because these organs are constantly exposed to external insults, such as food pollutants, microorganisms, and ultraviolet energy[7–9]. Hormones play a role in the aging process by altering metabolisms due to lowered hormone levels[10]. Among other metabolic hormones, melatonin is a key hormone to provide the concept of an age clock[11]. Melatonin also has an immunomodulatory role via immunocompetent cells, such as T helper cells, by binding the melatonin receptor[12]. Caroleo et al. reported that melatonin restores T cell-mediated immune function to the young adult level when exogenously administered to old mice[13]. Immunosenescence has been linked to downregulation of immune function with age, and thereby decreased protection against infectious diseases and cancer[14–15]. Decreased production of interleukin-2 (IL-2), low affinity IL-2 receptors and the T lymphocyte (T cell) proliferative response to novel antigens with aging are associated with a decrease in the number of naïve T cells[16]. Although these studies highlight age-related immunosuppression and the potential of melatonin in immune stimulation and aging-associated immunosuppression, a correlation between immunity and aging and the effect of melatonin on T cell differentiation and proliferation has not been reported. Therefore, this study determined whether melatonin plays a role in T cell proliferation and thereby enhances or restores immunity in an aged model.
Materials and methods
Melatonin treatment
Splenocytes and T cells prepared from BALB/c mice were cultured in Roswell Park Memorial Institute (RPMI) media with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA). Melatonin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in dimethyl sulfoxide to prepare a stock solution, and the cells were treated with 100~500 μmol/L melatonin for 24~48 hours. To determine the T cell activation, mouse monoclonal anti-CD3 and anti-CD28 (eBioscience, San Diego, CA, USA) were used as a positive control.
Animals
Five-week old male BALB/c strains (Central Lab. Animal Inc., Seoul, Republic of Korea) were housed in polysulfone cages, fed a standard mice diet with filtered tap water, and grown to 10 weeks (young adult) and > 100 weeks (aged). The animal room was maintained at a constant temperature (22°C±2°C), relative humidity (50%±10%), and a 12-hour light/dark cycle of⩾300 lux. The animal experiments were approved by the Gangneung-Wonju National University Animal Care and Use Committee (approval No. GWNU-2015-4).
Splenocyte isolation from BALB/c mouse spleen
Mouse spleens were aseptically isolated from BALB/c mice after sacrifice under deep ether anesthesia, and splenocytes were prepared by mechanical dissociation by pushing with syringe plunger through a 300 mesh in cold phosphate buffered saline (PBS) at pH 7.2. Erythrocytes were depleted using a red blood cell lysis buffer containing ammonium chloride (eBioscience), which lyses red blood cells with minimal effects on T cells. Then, splenocytes were cultured in RPMI1640 complete medium with 10% FBS and maintained at 37°C in a humidified atmosphere containing 5% CO2. The splenocytes were cultured at 5 × 105 cells/mL in a 96-well plate or 5 × 106 cells/mL in a 60 mm culture dish for gene expression or Western blot assays.
Splenocyte-derived CD4+ and naïve CD4 T cells
CD4+ T cells were isolated from BALB/c splenocytes using negative selection and a CD4+ T cell isolation kit (MiltenyiBiotec, Redwood, CA, USA). CD4+ naïve T cells were isolated from pure CD4+ T cells using CD44 micro beads (MiltenyiBiotec). All procedures were performed with a MACS® separation column according to the manufacturer's instructions. CD4+ T cells and naïve CD4+ T cells were cultured in RPMI1640 complete medium with 10% FBS and maintained at 37°C in a humidified atmosphere containing 5% CO2. CD4+ T cells and naïve CD4+ T cells were cultured at 2 × 106 cells/mL in 12-well plates for mRNA expression.
Total splenocytes, splenocyte-derived CD4+, and complete blood count
Melatonin (10 mg/kg) or saline (0.2 mL, normal control) was administered orally to aged BALB/c mice for 7 days to evaluate T cell proliferation in splenocytes[17]. One day after the final treatment, all splenocytes and the isolated CD4+ T cells were counted after 1% trypan blue staining using the Cellometer® mini (Nexcelcom Biosciences, Lawrence, MA, USA). Blood was drawn from the abdominal inferior vena cava (500 μL) 1 day after final treatment and the blood samples were analyzed to evaluate the number of ex vivo white blood cells.
Collection of peritoneal macrophages
Peritoneal macrophages were isolated from melatonin-treated mice to detect the immunomodulating activity of melatonin by co-culturing CD4+ T cells and melatonin-activated macrophages. In brief, melatonin (10 mg/kg) was administered orally to young adult and aged BALB/c mice for 7 days. Ten mL of FBS-free RPMI 1640 media was injected intraperitoneally, the abdomen was massaged for 10 minutes, and the media was recovered and centrifuged at 1,800 × g for 10 minutes at 4°C. The cell pellets were resuspended in fresh FBS-free RPMI 1640 media. Peritoneal macrophages (1.5 × 106 cells/well, 1.5~2 × 106 cells/mouse) were seeded on 12-well plates for 2 hours. The non-adherent cells were removed and refreshed with RPMI 1640 media containing 10% FBS and maintained at 37°C in a humidified atmosphere containing 5% CO2. After replacement with fresh RPMI 1640 media, peritoneal macrophages and CD4+ T cells (1:1 ratio) from the spleens of the same mice were co-cultured for 24 hours.
Cell viability and cytotoxicity assay
To assess cell viability and cytotoxicity, splenocytes isolated from BALB/c mice were incubated for 24 hours at 37°C in a humidified 5% CO2 incubator. In brief, cell viability was investigated in response to melatonin stimulation (31.1~1000 μmol/L) in 96-well microtiter plates (1 × 106 cells/mL) following a 24-hour culture using the Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). This system uses WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt]. The plates were measured at 450 nm (Spectra Max 340, Molecular Devices, Sunnyvale, CA, USA). Data from triplicate cultures are expressed as percent viability vs. the control. Cytotoxicity induced by melatonin was quantitated by measuring lactate dehydrogenase (LDH) release. LDH content was determined using a commercial non-radioactive LDH assay kit (CytoTox 96®; Promega, Madison, WI, USA), which is based on a coupled enzymatic reaction that results in conversion of a tetrazolium salt into a red formazan product. Formazan was quantified spectrophotometrically by measuring absorbance at 490 nm (Spectra Max 340). Cytotoxicity in the experimental samples was determined as %LDH release compared with cells treated with 5 mmol/L H2O2.
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA from splenocyte-derived CD4+, naïve CD4, and splenocytes in the presence or absence of melatonin (100 and 250 µmol/L) was prepared using Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNAs were synthesized from RNA by reverse transcription of 1 µg total RNA using the Improm-II reverse transcription system (Promega) and oligo-dT primers in a total volume of 20 µL. PCR was performed. Quantitative real-time PCR reactions were run on a Rotor-Gene 6000 (Qiagen, Valencia, CA, USA) using a SensiMixTM SYBR HiROX kit (Bioline, London, UK) in 20 μL reaction mixtures. Each real-time PCR master mix contained 10 μL of 2 × enzyme mastermix, 7 μL RNase free water, 1 μL of each primer (10 pmol/L each), and 1 μL diluted template. The PCR was performed with an initial pr-incubation step for 15 minutes at 95°C, followed by 45 cycles of 95°C for 15 seconds, annealing at 52°C for 15 seconds, and extension at 72°C for 10 seconds. Melting curve analysis was used to confirm formation of the expected PCR products, and products from all assays were tested with 1.2% agarose gel electrophoresis to confirm the correct lengths. An inter-run calibrator was used, and a standard curve was created for each gene to obtain PCR efficiencies. Relative sample expression levels were calculated using Rotor-Gene 6000 Series Software 1.7, expressed relative to β-actin, and corrected for between-run variability. Data for the experimental samples are expressed as fold units of the internal control gene. The primers for the target genes (Bioneer, Daejeon, Republic of Korea) are listed in Table 1.
Table 1. Primer sequences of real-time polymerase chain reaction.
| Gene | Sequence | Product size (bp) | Accession No. |
|---|---|---|---|
| CD28 | 5′-GACGTGGAAGTCTGTGTCGG-3′ 5′-TTCCATTGCTCCTCTCGTTG-3′ |
217 | NM_007642 |
| CTLA4 | 5′-CAACCTTCAGTGGTGTTGGC-3′ 5′-TCCTTGGATGGTGAGGTTCA-3′ |
243 | NM_009843 |
| MKI67 | 5′-GATGGAAGCAAGCCAACAGA-3′ 5′-CATGCCCTGATGAGTCTTGG-3′ |
228 | NM_001081117 |
| p21 | 5′-AGTGTGCCGTTGTCTCTTCG-3′ 5′-TCAAAGTTCCACCGTTCTCG-3′ |
107 | AB017817 |
| APRIL | 5′-TTTCACAATGGGTCAGGTGG-3′ 5′-CCCGAGGAATTTTGACAGTG-3′ |
169 | NM_001159505 |
| Bcl2 | 5′-GGCTGGGGATGACTTCTCTC-3′ 5′-CACCCCATCCCTGAAGAGTT-3′ |
129 | NM_009741 |
| β-actin | 5′-TACAGCTTCACCACCACAGC-3′ 5′-AAGGAAGGCTGGAAAAGAGC-3′ |
205 | NM_007393 |
Cell cycle assay
A cell cycle analysis kit and a premixed reagent (Millipore, Billerica, MA, USA) were used for nuclear DNA intercalating staining with propidium iodide (PI), which discriminates cells at different stages of the cell cycle based on differential DNA (G0/G1, S and G2/M). CD4+ T cells were cultured for 20 hr. Isolated CD4+ cells were fixed in 70% ice-cold ethanol overnight and washed twice in PBS. The cells were analyzed on a Muse flow cytometer (Millipore) fitted with a 532 nm green diode using the cell cycle assay software. The cell cycle analysis was performed according to manufacturer's instructions.
Western blot analysis
Splenocytes were lyzed in 1% RIPA buffer containing protease and phosphatase inhibitors (Roche, Mannheim, Germany), and whole-cell lysates were separated on 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. After electrophoresis, the proteins were electrotransferred to polyvinylidene difluoride membranes and detected with anti-Bcl2 and anti-Ki67 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by reaction with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Danvers, MA, USA). Blots were developed using an enhanced chemiluminescence solution (SuperSignal® West Dura Extended Duration Substrate, Thermo Scientific, Rockford, IL, USA). The intensities of bands was quantified by densitometry (GelQuant Pro software (DNR Bio Imaging Ltd. Jerusalem, Israel)).
Statistical analysis
The Kruskal–Wallis one-way analysis of variance and Dunnett's post-hoc test were used to detect differences between groups using Graphpad v. 5.1 software (GraphPad Software, La Jolla, CA, USA). A P < 0.05 was considered significant.
Results
The 7-day oral administration of melatonin (10 mg/kg) to aged mice (n = 3 in each group) increased the numbers of splenocytes and CD4+ T cells. Total splenocytes and splenocyte-derived CD4+ T cells increased by 57% and 147% after the melatonin treatment compared to those in the control group (saline) (Table 2). The complete blood count remained unchanged, except neutrophils (↑27.8%) compared to those in the control group (Table 3).
Table 2. Cell counts of splenocytes and splenocyte-derived CD4+ cells in aged mice (n = 3).
| Treatment | Dose (mg/kg) | Cell types | Cell counts | % vs. control |
|---|---|---|---|---|
| Saline | – | Splenocytes | 1.48 × 107±7.14 × 106 | 100 |
| Melatonin | 10 | Splenocytes | 2.33 × 107±6.13 × 106 | 157a) |
| Saline | – | CD4+ | 3.35 × 106±1.03 × 106 | 100 |
| Melatonin | 10 | CD4+ | 8.27 × 106±3.55 × 106 | 247b) |
Note: a) P<0.01 vs. splenocyte control (saline), b) P<0.001 vs. CD4+ control (saline)
Table 3. Complete blood counts in aged mice (n = 3).
| Treatment | Dose (mg/kg) | Neutrophil (%) | Lymphocytes (%) | Monocytes (%) | Eosinophil (%) | Basophil % |
|---|---|---|---|---|---|---|
| Saline | – | 34.2 | 48.6 | 3.1 | 12.4 | 0.3 |
| Melatonin | 10 | 43.7 | 49.6 | 3.3 | 12.7 | 0.4 |
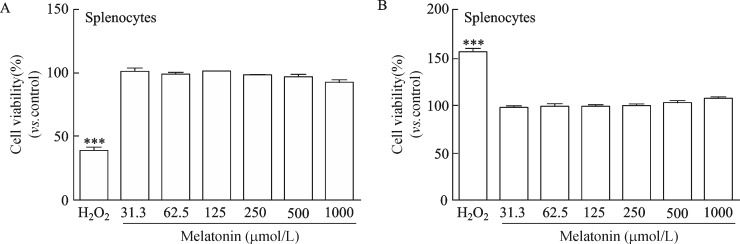
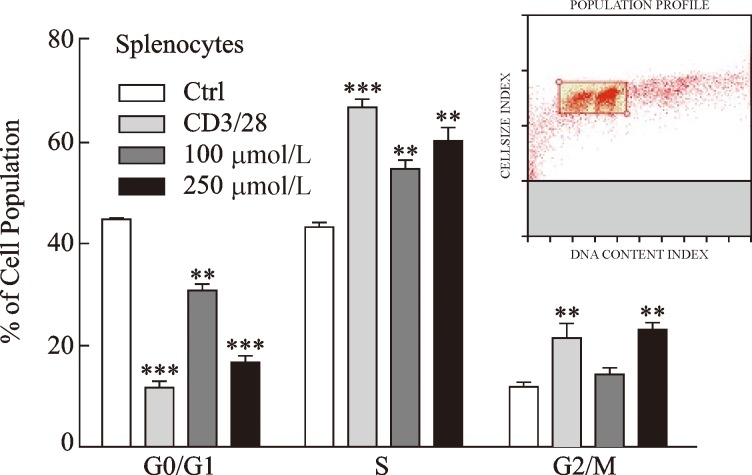
To investigate cell viability and toxicity of melatonin, we incubated splenocytes with various concentrations of melatonin (31.3~1,000 μmol/L). As shown in Fig. 1A and 1B, melatonin maintained cell viability at the level of the normal control and was not toxic for up to 24-hour exposure at concentrations<1000 μmol/L compared to that in the hydrogen peroxide positive control. The distribution of cell cycle phases was monitored during the checkpoints at the transition from the G0/G1 to the S phases. Cell proliferation regulated by sequential progression was analyzed by flow cytometry through the G0/G1-S-G2/M phase by treating cells with melatonin (100 or 250 μmol/L) (Fig. 2).
Fig. 1. Cell viability and cytotoxicity of various doses of melatonin after 24 hour incubation.
The percentage of cell viability (A) and cytotoxicity (B) was measured by the CCK and lactate dehydrogenase assays. Data are presented as means±standard deviations from three separate experiments. ***P < 0.001 vs. control (H2O2).
Fig. 2. Melatonin affects splenocyte proliferation by inducing the transition from the G0/G1 to S cell cycle phases.
Splenocytes were treated with melatonin for 24 hours at the indicated concentrations, followed by propidium iodide staining and flow cytometry. Monoclonal antibodies (anti-CD3/CD28, 1 μg/mL each) were as a positive control. Bar graph represents the quantified values of the flow cytometry data. Data are representative of one experiment. The graphs show means±standard deviations of three independent cell cycle experiments (n = 3). **P < 0.01, **P < 0.01, ***P < 0.001 vs. control; Ctrl, control.
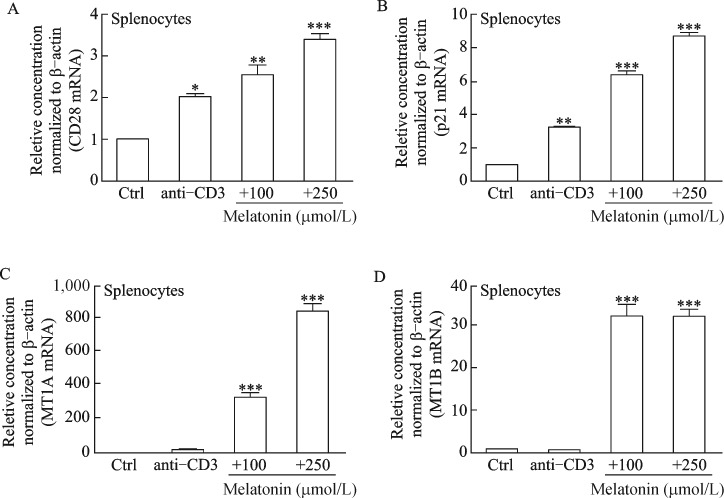
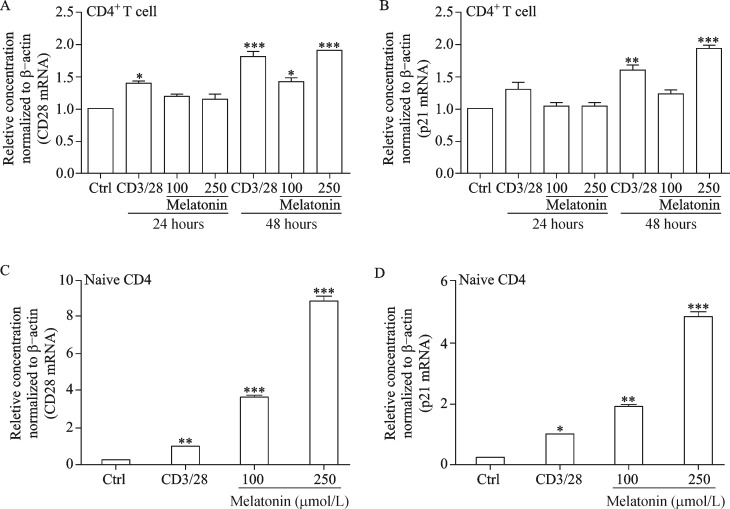
Our results show that melatonin decreased the G0/G1 cell population with a concomitant increase in the S and G2/M phase, suggesting that cells were leaving the G0/G1 phase and starting to proliferate. CD28 and p21 gene expression levels were investigated to confirm if melatonin provides the co-stimulatory signal required for T cell activation and plays a regulatory role in S phase DNA replication. Our results clearly demonstrate that melatonin increased CD28 and p21 expression significantly compared to that in the normal and positive controls (Fig. 3A and 3B). Interestingly, melatonin markedly increased melatonin membrane receptor (e.g. MT1A and MT1B) gene expression which decreases with increasing age[18] (Fig. 3C and 3D). Coincidently, melatonin significantly increased CD28 and p21 mRNA levels in splenocyte-derived CD4+ and naïve CD4 T cells (Fig. 4A-4D), suggesting that melatonin actively contributes to T cell activation.
Fig. 3. mRNA profiles of T cell activation/proliferation markers and melatonin receptors in splenocytes.
Splenocytes were treated with melatonin (100 and 250 μmol/L) for 24 hours and anti-CD3 was used as a positive control. (A) T cell activation (CD28), (B) cell proliferation marker (p21), and (C, D) the high affinity melatonin receptors (MT1A and MT1B) were analyzed by quantitative polymerase chain reaction analysis. Results are means±standard deviations (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; Ctrl, control.
Fig. 4. mRNA profiles of T cell activation and proliferation markers in splenocyte-derived CD4+ and naïve T cells.
Naïve T cells and CD4+ were treated with melatonin for 24 and 48 hours and anti-CD3/CD28 (1 μg/mL each) were used as a positive control. (A) T cell activation (CD28) and (B) cell proliferation marker (p21) were analyzed by quantitative polymerase chain reaction analysis of CD4+ T cells and (C, D) naïve CD4 T cells. Results are means±standard deviations (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; Ctrl, control.
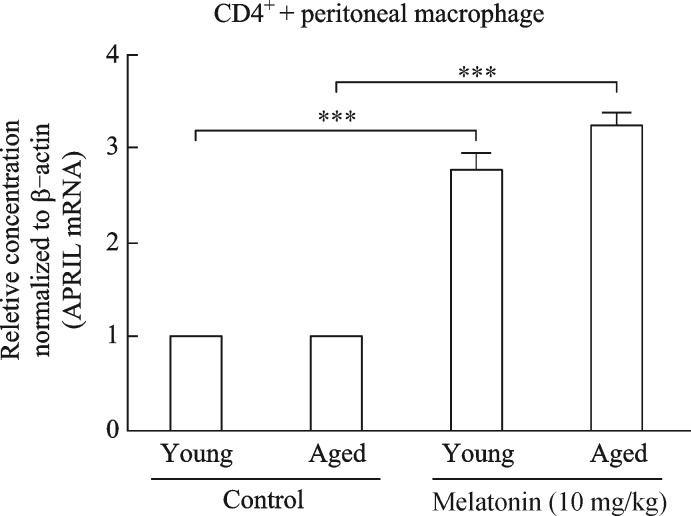
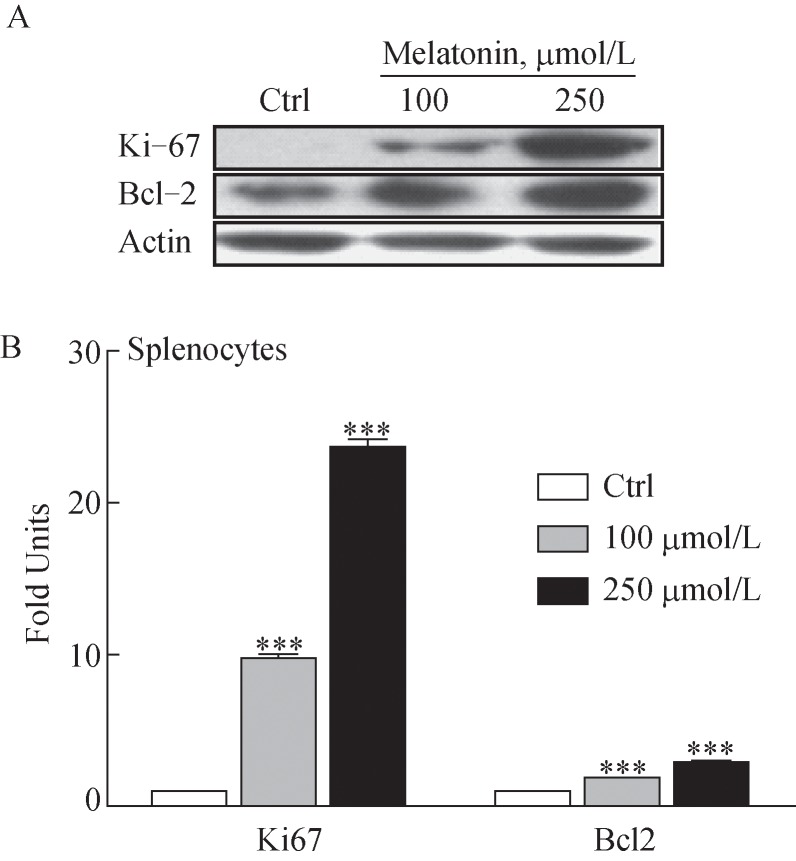
Notably, our results showed that the co-incubation of splenocytes with peritoneal macrophages 7 days after melatonin administration (10 mg/kg) significantly escalated the proliferation-inducing ligand (APRIL) mRNA, which is involved in the induction and/or maintenance of T and B cell responses by activation of macrophages (Fig. 5)[19]. Moreover, Ki67 and Bcl2 protein levels, which are involved in antigen-specific T cell proliferation and play a role in long-term maintenance of memory T cells, respectively[20–21], increased dose-dependently (Fig. 6).
Fig. 5. Comparative analysis of APRIL mRNA expression in co-cultured cells (CD4+ and peritoneal macrophages).
CD4+ and peritoneal macrophages were prepared from spleens 7 days after administering melatonin (10 mg/kg) to young adult and aged BALB/c mice and co-cultured for 24 hours. Untreated young adult and aged mice were used as comparison for each group. Results are means±standard deviations (n = 3, three mice per group). ***P < 0.001 vs. corresponding control.
Fig. 6. Western blot analysis of Ki67 and Bcl2. Splenocytes were isolated from aged BALB/c mice and cultured for 24 hours.
(A) Immunoblotting analysis of Ki67 and Bcl-2 protein levels was conducted in the presence of melatonin (100 and 250 μmol/L). (B) Bar graph represents quantified values of the blotting data. The blot is one of three independent blots, and results are means±standard deviations (n = 3). ***P < 0.001 vs. control; Ctrl, control.
Discussion
Many studies have reported that progressive alterations in T cell function are evident in aged individuals and trigger immunosenescence that influences the innate and adaptive immune systems leading to an increased incidence of cancers and infectious and degenerative diseases[22]. Although melatonin is known to correlate with the photoperiodic signal, it also plays a profound immunomodulatory role by increasing the number of T-helper cells (particularly CD4+ cells), indicating that melatonin is an anti-aging candidate. Our results show that melatonin may play a critical role in increasing immune cell function, specifically that of CD4+ T cells, and, thus, improve the immune system in aged individuals and in immunocompromised patients.
A decrease in the CD4/CD8 ratio in elderly individuals is among the major immune alterations associated with immunosenescence[23–24]. Thus, our study was primarily designed to investigate the role of melatonin in T cell proliferation. The data revealed that melatonin did not affect cell viability or toxicity at a concentration up to 1,000 μmol/L, indicating that melatonin can be used safely at < 1,000 μmol/L.
Increasing evidence indicates that melatonin acts directly on immune tissues to modulate immune function mainly via T helper cells containing high affinity melatonin receptors (MT1A and MT1B) localized on T cells and splenocytes that presumably mediate the release cytokines when melatonin is administered[25,26].
Our data show that melatonin stimulated cells to leave G0/G1 phase and proliferate (↑ S and G2/M phase). CD28 mRNA which is an important co-stimulatory signal for T cell activation and survival, increased dose-dependently along with increased p21 mRNA after melatonin treatment, suggesting that melatonin controls T cell activation and proliferation of only activated/memory T cells[24,27]. Surprisingly, MT1A and MT1B mRNA expression in splenocytes increased dramatically in response to melatonin compared to that in the normal control, suggesting an immunoregulatory action of melatonin mediated through its specific receptors[18]. Other evidence indicates that melatonin influences activation and proliferation of CD4+ and naïve T cells in a time dependent manner via promoting differentiation into helper T cells[28].
It has also been suggested that APRIL is involved in T cell proliferation and, thus, induces T and B cell responses[19]. Our results clearly reveal that co-culture of splenocyte-derived CD4+ cells and peritoneal macrophages 7 days after melatonin administration significantly increased APRIL mRNA expression levels, indicating activation of immune cells.
A Western blot analysis revealed that Ki67, which is a proliferation marker present in all active cell cycle phases except resting cells[29], and Bcl2, of which expression decreases with age[30], was increased markedly in splenocytes. Our findings indicate that melatonin supplementation in aged mice increases T cell (particularly CD4+ and naïve CD4 T cells) activation and proliferation probably via MT1A and MT1B, which affect immune cells by inducing the expression of Th-dependent cytokines, such as IL-2[19].
In summary, our results demonstrate that melatonin has immune-enhancement properties by activating proliferation markers, CD28, p21, KI-67, Bcl2, and melatonin receptors on splenocytes, CD4+, and naïve CD4 T cells. Therefore, we conclude that melatonin is an attractive candidate to enhance immunity in aged individual by upregulating and activating the major adaptive immune cells in addition to its basic role in photoperiodism.
Acknowledgements
This research was supported by High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (MAFRA; grant number 113034-3)
References
- [1].Reiter RJ. The melatonin rhythm: both a clock and a calendar[J]. Experientia, 1993, 49(8): 654–664. [DOI] [PubMed] [Google Scholar]
- [2].Pires ML., Benedito-Silva AA, Pinto L, et al. Acute effects of low doses of melatonin on the sleep of young healthy subjects[J]. J Pineal Res, 2001, 31(4): 326–332. [DOI] [PubMed] [Google Scholar]
- [3].Mundey K, Benloucif S, Harsanyi K, et al. Phase-dependent treatment of delayed sleep phase syndrome with melatonin[J]. Sleep, 2005, 28(10): 1271–1278. [DOI] [PubMed] [Google Scholar]
- [4].Korkmaz A, Topal T, Tan DX, et al. Role of melatonin in metabolic regulation[J]. Rev Endocr Metab Disord, 2009, 10(4): 261–270. [DOI] [PubMed] [Google Scholar]
- [5].Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species?[J]. J Pineal Res, 2007, 42(1): 28–42. [DOI] [PubMed] [Google Scholar]
- [6].Peyrot F, Ducrocq C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species[J]. J Pineal Res, 2008, 45(3): 235–246. [DOI] [PubMed] [Google Scholar]
- [7].Jaworek J, Nawrot-Porabka K, Leja-Szpak A, et al. Melatonin as modulator of pancreatic enzyme secretion and pancreatoprotector[J]. J PhysiolPharmacol, 2007, 58(Suppl 6): 65–80. [PubMed] [Google Scholar]
- [8].Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance[J]. Dig Dis Sci, 2002, 47(10): 2336–2348. [DOI] [PubMed] [Google Scholar]
- [9].Fischer TW, Slominski A, Zmijewski MA, et al. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair[J]. Exp Dermatol, 2008, 17(9): 713–730. [DOI] [PubMed] [Google Scholar]
- [10].Zjacić-Rotkvić V, Kavur L, Cigrovski-Berković M. Hormones and aging[J]. Acta Clin Croat, 2010, 49(4): 549–554. [PubMed] [Google Scholar]
- [11].Touitou Y. Human aging and melatonin. Clinical relevance[J]. Exp Gerontol, 2001, 36(7): 1083–1100. [DOI] [PubMed] [Google Scholar]
- [12].Currier NL, Sun LZ, Miller SC. Exogenous melatonin: quantitative enhancement in vivo of cells mediating non-specific immunity[J]. J Neuroimmunol, 2000, 104(2): 101–108. [DOI] [PubMed] [Google Scholar]
- [13].Caroleo MC, Doria G, Nisticò G. Melatonin restores immunodepression in aged and cyclophosphamide-treated mice[J]. Ann N Y Acad Sci, 1994, 719: 343–352. [DOI] [PubMed] [Google Scholar]
- [14].Srinivasan V, Maestroni GJ, Cardinali DP, et al. Melatonin, immune function and aging[J]. Immun Ageing, 2005, 2: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pawelec G, Akbar A, Caruso C, et al. Is immunosenescence infectious?[J]. Trends Immunol, 2004, 25(8): 406–410. [DOI] [PubMed] [Google Scholar]
- [16].Hawkley LC, Cacioppo JT. Stress and the aging immune system[J]. Brain Behav Immun, 2004, 18(2): 114–119. [DOI] [PubMed] [Google Scholar]
- [17].Yeleswaram K, McLaughlin LG, Knipe JO, et al. Pharmacokinetics and oral bioavailability of exogenous melatonin in preclinical animal models and clinical implications[J]. J Pineal Res, 1997, 22(1): 45–51. [DOI] [PubMed] [Google Scholar]
- [18].Sánchez-Hidalgo M, Guerrero Montávez JM, Carrascosa-Salmoral Mdel P, et al. Decreased MT1 and MT2 melatonin receptor expression in extrapineal tissues of the rat during physiological aging[J]. J Pineal Res, 2009, 46(1): 29–35. [DOI] [PubMed] [Google Scholar]
- [19].Stein JV, López-Fraga M, Elustondo FA, et al. APRIL modulates B and T cell immunity[J]. J Clin Invest, 2002, 109(12): 1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Soares A, Govender L, Hughes J, et al. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation[J]. J Immunol Methods, 2010, 362(1-2): 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grayson JM, Zajac AJ, Altman JD, et al. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8 + T cells[J]. J Immunol, 2000, 164(8): 3950–3954. [DOI] [PubMed] [Google Scholar]
- [22].Haynes L, Maue AC. Effects of aging on T cell function[J]. Curr Opin Immunol, 2009, 21(4): 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Deeks SG. HIV infection, inflammation, immunosenescence, and aging[J]. Annu Rev Med, 2011, 62: 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fagnoni FF, Vescovini R, Passeri G, et al. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging[J]. Blood, 2000, 95(9): 2860–2868. [PubMed] [Google Scholar]
- [25].Calvo JR, Rafii-el-Idrissi M, Pozo D, et al. Immunomodulatory role of melatonin: specific binding sites in human and rodent lymphoid cells[J]. J Pineal Res, 1995, 18(3): 119–126. [DOI] [PubMed] [Google Scholar]
- [26].Carrillo-Vico A, García-Pergañeda A, Naji L, et al. Expression of membrane and nuclear melatonin receptor mRNA and protein in the mouse immune system[J]. Cell Mol Life Sci, 2003, 60(10): 2272–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Arias CF, Ballesteros-Tato A, García MI, et al. p21CIP1/WAF1 controls proliferation of activated/memory T cells and affects homeostasis and memory T cell responses[J]. J Immunol, 2007, 178(4): 2296–2306. [DOI] [PubMed] [Google Scholar]
- [28].Eddahri F, Denanglaire S, Bureau F, et al. Interleukin-6/STAT3 signaling regulates the ability of naive T cells to acquire B-cell help capacities[J]. Blood, 2009, 113(11): 2426–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown[J]. J Cell Physiol, 2000, 182(3): 311–322. [DOI] [PubMed] [Google Scholar]
- [30].Poon AM, Liu ZM, Pang CS, et al. Evidence for a direct action of melatonin on the immune system[J]. Biol Signals, 1994, 3(2): 107–117. [DOI] [PubMed] [Google Scholar]