Abstract
Introduction
To date, most clinical comparisons of ezetimibe-statin combination therapy versus statin monotherapy have relied entirely on surrogate variables. In this systematic review, we study the efficacy and safety of ezetimibe-statin combination therapy in comparison to statin monotherapy in terms of the prevention of cardiovascular events in hyperlipidemic patients with atherosclerosis and/or diabetes mellitus.
Method
This review is based on a systematic literature search (1995 to July 2015) in PubMed, the Excerpta Medica Database (EMBASE), the Cochrane Library, and the ClinicalTrials.gov registry.
Results
Nine randomized, controlled trials with data from a total of 19 461 patients were included. Ezetimibe-statin combination therapy was associated with a lower risk of cardiovascular events than statin monotherapy: 33% of the patients treated with ezetimibe and a statin, and 35% of those treated with a statin alone, had a cardiovascular event within seven years (number needed to treat [NNT]: 50 over 7 years). Combination therapy was also significantly more effective in preventing a composite endpoint consisting of death due to cardiovascular disease, nonfatal myocardial infarction, unstable angina pectoris, coronary revascularization, and nonfatal stroke (hazard ratio [HR] 0.94, 95% confidence interval [0,89; 0,99]; p = 0.016). Diabetic patients benefited from combination therapy rather than monotherapy with respect to cardiovascular morbidity (HR 0.87 [0.78; 0.94]). On the other hand, the addition of ezetimibe to statin therapy did not lessen either cardiovascular or overall mortality. Serious undesired events occurred in 38% of the patients taking ezetimibe and a statin and in 39% of the patients taking a statin alone (relative risk 1.09 [0.77; 1.55]).
Conclusion
In high-risk patients with an acute coronary syndrome, combination therapy with ezetimibe and a statin lowered the risk of cardiovascular events in comparison to statin monotherapy. The risk of dying or suffering an adverse drug effect was similar in the two treatment groups.
Coronary heart disease (CHD) and its acute manifestations, such as myocardial infarction, are the leading causes of death in Europe (1). Patients with overt CHD and/or with diabetes mellitus are at an increased risk of cardiovascular events and dying from CHD. Lifestyle interventions, such as regular exercise, a healthy diet, weight loss, and smoking cessation, reduce the risk of adverse cardiovascular events (2– 4). Controlling other risk factors, such as diabetes, arterial hypertension and hyperlipidemia, with pharmacotherapy also contributes to risk reduction (5).
Today, statins are the treatment of choice for the prevention of cardiovascular disease in patients with increased cholesterol levels or a generally increased risk of CHD (6); their ability to lower cholesterol and their protective effect against CHD have been demonstrated in numerous studies (7– 9). The selective cholesterol absorption inhibitor ezetimibe has been available as a statin alternative for more than a decade. Ezetimibe is approved in combination with a statin when the target cholesterol levels are not attained with statin treatment alone (10). To date, studies comparing the advantages and disadvantages of ezetimibe-statin combination therapy with statin treatment alone have generally focused on surrogate parameters, such as the reduction of low-density lipoprotein (LDL) cholesterol levels (11– 16). Numerous studies demonstrated a cholesterol-lowering effect (12, 14, 16). However, based on these data alone the benefits of ezetimibe-statin combination therapy cannot be assessed conclusively since it remains controversial whether there is a causal relationship between the lowering of LDL cholesterol levels and the reduction in cardiovascular events (17).
The aim of this study was to evaluate the efficacy and safety of ezetimibe-statin combination therapy for the prevention of cardiovascular events in patients with hyperlipidemia and overt atherosclerosis and/or diabetes mellitus, in comparison with statin treatment alone. This research question is part of a systematic review registered in the PROSPERO database, an international database of prospectively registered systematic reviews in health and social care (18).
Methods
Literature search and selection
We conducted a systematic literature search of PubMed, Excerpta Medica Database (EMBASE) and the Cochrane Library for the period 1995 to July 2015, using combinations of pertinent keywords and, where possible, medical subject headings (MeSH) (eTable 1). In addition, the ClinicalTrials.gov registry as well as reference lists were searched to identify pertinent studies.
eTable 1.
| a) PubMed search strategy (9 September 2014) | ||
| Search | Terms | Hits |
| #1 | ezetimib*[tw] OR ezetrol[tw] OR inegy[tw] OR vytorin[tw] OR zetia[tw] | 1943 |
| #2 | 163222–33–1[rn] | 1249 |
| #3 | SCH 58235[tw] OR SCH58235[tw] | 20 |
| #4 | (#1 OR #2 OR #3) | 1944 |
| #5 | „atherosclerosis“[MeSH] OR atherosclero*[tiab] | 116899 |
| #6 | „diabetes mellitus“[MeSH] OR diabetes[tiab] | 445031 |
| #7 | „hypercholesterolemia“[MeSH] OR hypercholesterol* [tiab] | 37936 |
| #8 | sitosterol* [tw] | 4326 |
| #9 | phytosterol* [tw] | 3184 |
| #10 | „cholesterol“[MeSH] OR cholesterol[tiab] OR ldl[tiab] | 241224 |
| #11 | low[tiab] AND lipoprotein* [tiab] | 64098 |
| #12 | #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 | 748618 |
| #13 | (#4 AND #12) | 1644 |
| #14 | systematic[sb] | 222746 |
| #15 | „randomized controlled trial“[publication type] OR randomized controlled trial[tiab] OR randomised controlled trial[tiab] OR „single blind method“[MeSH] OR „double blind method“[MeSH] OR „random allocation“ [MeSH] | 477053 |
| #16 | (#13 AND (#14 OR #15)) | 366 |
| #17 | „animals“[MeSH] NOT „humans“[MeSH] | 3924347 |
| #18 | (#16 NOT #17) | 362 |
| #19 | (#18 AND 1995:2014[dp]) | 362 |
| b) Cochrane Library search strategy (4 September 2014) | ||
| Search | Terms | Hits |
| #1 | ezetimib* or ezetrol or inegy or „SCH 58235“ or SCH58235 or vytorin or zetia in other reviews, trials, methods studies, technology assessments and economic evaluations | 516 |
| #2 | (ezetimib* or ezetrol or inegy or „SCH 58235“ or SCH58235 or vytorin or zetia):ti,ab in Cochrane Reviews (reviews and protocols) | 3 |
| #3 | #1 or #2 | 519 |
| #4 | [mh atherosclerosis] or atherosclero*:ti,ab,kw | 5308 |
| #5 | [mh „diabetes mellitus“] or diabetes:ti,ab,kw | 27208 |
| #6 | [mh hypercholesterolemia] or hypercholesterol*em*:ti,ab,kw | 4583 |
| #7 | sitosterol*em*:ti,ab,kw | 7 |
| #8 | phytosterol*em*:ti,ab,kw | 2 |
| #9 | [mh cholesterol] or (cholesterol or ldl):ti,ab,kw or (low near/3 lipoprotein*):ti,ab,kw | 19763 |
| #10 | {or #4–#9} | 46874 |
| #11 | #3 and #10 [or #4–#9] | 449 |
| #12 | #11 publication year from 1995 to 2014 | 449 |
| #13 | [mh animals] not [mh humans] | 5673 |
| #14 | #12 not #13 | 446 |
| c) EMBASE search strategy (9 September 2014) | ||
| Search | Terms | Hits |
| #1 | ’ezetimibe’/exp OR ezetimib*:ab,ti | 6185 |
| #2 | ezetrol:ab,ti OR inegy:ab,ti OR vytorin:ab,ti OR zetia:ab,ti | 155 |
| #3 | ’163222 33 1’:rn | 5238 |
| #4 | ’sch 58235’:ab,ti OR sch58235:ab,ti | 19 |
| #5 | #1 OR #2 OR #3 OR #4 | 6202 |
| #6 | ’atherosclerosis’/exp OR atherosclero*:ab,ti | 204392 |
| #7 | ’diabetes mellitus’/exp OR diabetes:ab,ti | 698376 |
| #8 | ’hypercholesterolemia’/exp OR hypercholesterol*:ab,ti | 62397 |
| #9 | sitosterol*em* | 339 |
| #10 | phytosterol*em* | 92 |
| #11 | ’cholesterol’/exp OR cholesterol:ab,ti OR ldl:ab,ti | 317237 |
| #12 | (low NEAR/6 lipoprotein*):ab,ti | 65326 |
| #13 | #6 OR #7 OR #8 OR #9OR #10 OR #11 OR #12 | 1103174 |
| #14 | #5 AND #13 | 5151 |
| #15 | ’systematic review’/exp OR ’meta analysis’/exp OR ’systematic review’:ab,ti OR (meta NEXT/1 analy*):ab,ti OR metaanaly*:ab,ti OR (review:it AND systematic:ab,ti) OR (systematic:ab,ti AND (bibliographic:ab,ti OR literature:ab,ti OR review:ab,ti OR reviewed:ab,ti OR reviews:ab,ti)) OR ’research synthesis’:ab,ti OR ’research integration’:ab,ti OR ’evidence synthesis’:ab,ti OR (comprehensive*:ab,ti AND (bibliographic:ab,ti OR literature:ab,ti)) | 224209 |
| #16 | ’randomized controlled trial’/exp OR (randomi?ed NEXT/1 ’controlled trial’):ab,ti OR ’double blind procedure’/exp OR ’single blind procedure’/exp OR ’triple blind procedure’/exp OR ’randomization’/exp OR (allocat* NEAR/2 random*):ab,ti | 461090 |
| #17 | #15 OR #16 | 664729 |
| #18 | #14 AND #17 | 736 |
| #19 | ’animal’/exp NOT ’human’/exp | 4362176 |
| #20 | #18 NOT #19 | 735 |
| #21 | #20 AND [1995–2014]/py | 735 |
| #22 | #21 AND [embase]/lim NOT [medline]/lim | 197 |
| d) ClinicalTrials.gov search strategy (9 September 2014) | ||
| Search | Terms | Hits |
| #1 | interventional studies | ezetimib* OR ezetrol OR inegy OR vytorin OR zetia | 219 |
| e) PubMed search strategy – Update search (6 July 2015) | ||
| Search | Terms | Hits |
| #1 | ezetimib* [tw] OR ezetrol[tw] OR inegy[tw] OR vytorin[tw] OR zetia[tw] | 2146 |
| #2 | 163222–33–1[rn] | 1331 |
| #3 | SCH 58235[tw] OR SCH58235[tw] | 20 |
| #4 | (#1 OR #2 OR #3) | 2147 |
| #5 | „atherosclerosis“[MeSH] OR atherosclero* [tiab] | 123052 |
| #6 | „diabetes mellitus“[MeSH] OR diabetes[tiab] | 470624 |
| #7 | „hypercholesterolemia“[MeSH] OR hypercholesterol* [tiab] | 39111 |
| #8 | sitosterol* [tw] | 4546 |
| #9 | phytosterol* [tw] | 3383 |
| #10 | „cholesterol“[MeSH] OR cholesterol[tiab] OR ldl[tiab] | 250229 |
| #11 | low[tiab] AND lipoprotein* [tiab] | 67123 |
| #12 | (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) | 786117 |
| #13 | (#4 AND #12) | 1798 |
| #14 | systematic[sb] | 254021 |
| #15 | „randomized controlled trial“[publication type] OR randomized controlled trial[tiab] OR randomised controlled trial[tiab] OR „single blind method“[MeSH] OR „double blind method“[MeSH] OR „random allocation“[MeSH] | 500987 |
| #16 | (#13 AND (#14 OR #15)) | 402 |
| #17 | „animals“[MeSH] NOT „humans“[MeSH] | 4017863 |
| #18 | (#16 NOT #17) | 398 |
| #19 | (#18 AND 2014:2015[dp]) | 46 |
| f) Cochrane Library search strategy – Update search (6 July 2015) | ||
| Search | Terms | Hits |
| #1 | ezetimib* or ezetrol or inegy or „SCH 58235“ or SCH58235 or vytorin or zetia in other reviews, trials, methods studies, technology assessments and economic evaluations | 644 |
| #2 | (ezetimib* or ezetrol or inegy or „SCH 58235“ or SCH58235 or vytorin or zetia):ti,ab in Cochrane Reviews (reviews and protocols) | 3 |
| #3 | #1 or #2 | 647 |
| #4 | [mh atherosclerosis] or atherosclero*:ti,ab,kw | 6005 |
| #5 | [mh „diabetes mellitus“] or diabetes:ti,ab,kw | 32921 |
| #6 | [mh hypercholesterolemia] or hypercholesterol*em*:ti,ab,kw | 5003 |
| #7 | sitosterol*em*:ti,ab,kw | 7 |
| #8 | phytosterol*em*:ti,ab,kw | 4 |
| #9 | [mh cholesterol] or (cholesterol or ldl):ti,ab,kw or (low near/3 lipoprotein*):ti,ab,kw | 22266 |
| #10 | {or #4–#9} | 54638 |
| #11 | #3 and #10 | 569 |
| #12 | #11 publication year from 2014 to 2015 | 70 |
| g) EMBASE search strategy – Update search (6 July 2015) | ||
| Search | Terms | Hits |
| #1 | ’ezetimibe’/exp OR ezetimib*:ab,ti | 6185 |
| #2 | ezetrol:ab,ti OR inegy:ab,ti OR vytorin:ab,ti OR zetia:ab,ti | 155 |
| #3 | ’163222 33 1’:rn | 5238 |
| #4 | ’SCH 58235’:ab,ti OR SCH58235:ab,ti | 19 |
| #5 | #1 OR #2 OR #3 OR #4 | 6202 |
| #6 | ’atherosclerosis’/exp OR atherosclero*:ab,ti | 204392 |
| #7 | ’diabetes mellitus’/exp OR diabetes:ab,ti | 698376 |
| #8 | ’hypercholesterolemia’/exp OR hypercholesterol*:ab,ti | 62397 |
| #9 | sitosterol*em* | 339 |
| #10 | phytosterol*em* | 92 |
| #11 | ’cholesterol’/exp OR cholesterol:ab,ti OR ldl:ab,ti | 317237 |
| #12 | (low NEAR/6 lipoprotein*):ab,ti | 65326 |
| #13 | #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 | 1103174 |
| #14 | #5 AND #13 | 5151 |
| #15 | ’systematic review’/exp OR ’meta analysis’/exp OR ’systematic review’:ab,ti OR (meta NEXT/1 analy*):ab,ti OR metaanaly*:ab,ti OR (review:it AND systematic:ab,ti) OR (systematic:ab,ti AND (bibliographic:ab,ti OR literature:ab,ti OR review:ab,ti OR reviewed:ab,ti OR reviews:ab,ti)) OR ’research synthesis’:ab,ti OR ’research integration’:ab,ti OR ’evidence synthesis’:ab,ti OR (comprehensive*:ab,ti AND (bibliographic:ab,ti OR literature:ab,ti)) | 224209 |
| #16 | ’randomized controlled trial’/exp OR (randomi?ed NEXT/1 ’controlled trial’):ab,ti OR ’double blind procedure’/exp OR ’single blind procedure’/exp OR ’triple blind procedure’/exp OR ’randomization’/exp OR (allocat* NEAR/2 random*):ab,ti | 461090 |
| #17 | #15 OR #16 | 664729 |
| #18 | #14 AND #17 | 736 |
| #19 | ’animal’/exp NOT ’human’/exp | 4362176 |
| #20 | #18 NOT #19 | 803 |
| #21 | #20 AND [2014–2015]/py | 92 |
| #22 | #21 NOT [medline]/lim | 51 |
| h) ClinicalTrials.gov search strategy – Update search (6 July 2015) | ||
| Search | Terms | Hits |
| #1 | ezetimib* OR ezetrol OR inegy OR vytorin OR zetia | interventional studies | updated on or after 09/01/2014 | 133 |
The database searches were performed between 4 and 9 September 2014. The detailed search strategies are shown in the eTables 1a-d. The PubMed search identified 362 articles, the Cochrane Library search 446, the EMBASE search 197, and the clinicaltrials.gov search 219. The results of these update searches conducted in July 2015 are shown in the eTables 1e-h.
The selection of abstracts and full-text articles was carried out in two consecutive steps, each performed independently by two persons. In case of disagreement, a third person was called in. The selection criteria were defined a priori:
Population: patients of any age with hyperlipidemia and overt atherosclerosis and/or diabetes mellitus
Intervention: ezetimibe-statin combination therapy
Control intervention: statin monotherapy
Endpoints (date of data collection at least 6 months after randomization): cardiovascular morbidity, cardiovascular mortality, all-cause mortality, quality of life, adverse events
Study design: randomized controlled trials (RCTs).
Risk of bias and quality of evidence
The Cochrane Collaboration’s tool for assessing risk of bias (19) was used to judge the risk of bias in the included RCTs. Two persons independently assessed the risk of selection bias, performance bias, detection bias, attrition bias, and reporting bias. The risk of bias was summarized and assessed as follows (eTable 2):
eTable 2. Authors’ judgement of risk of bias for the included studies.
| Author (year) | Adequate method of randomization | Concealment of treatment sequence ensured | Treatment groups comparable after randomization | Blinding of participants | Blinding of endpoint assessors | Identical treatment except for intervention under investigation | Endpoint determined at the same point in time | Drop-out rate <20% | Differentialdrop-out rate between study groups <15% | ITT analysis | Determined endpoint actually reported | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arimura et al. (2012) (20) |
unclear | unclear | yes | unclear | unclear | yes | no | yes | yes | no | yes | high |
| Dagli et al. (2007) (21) |
unclear | unclear | yes | yes | yes | yes | yes | unclear | unclear | yes | unclear | unclear |
| Feldman et al. (2004) (22) |
unclear | unclear | yes | yes | unclear | yes | yes | yes | yes | yes | unclear | unclear |
| Gaudiani et al. (2005) (23) |
unclear | unclear | yes | yes | unclear | yes | yes | yes | yes | yes | unclear | unclear |
| IMPROVE-IT Studie (2014) (27, 28) |
yes | yes | yes | yes | yes*1 | yes | no | no*2 | yes | yes | yes | low |
| Masuda et al. (2015) (29) |
yes | yes | unclear*3 | no | no | yes | yes | no*4 | yes | yes*5 | yes | high |
| Meaney et al. (2009 (24) |
unclear | unclear | unclear | no | yes | yes | yes | no*6 | no | unclear | yes | high |
| Nakamura et al. (2012) (25) |
yes | yes | yes | unclear | yes | unclear*7 | yes | yes | yes | no | yes | unclear |
| West et al. (2011) (26) |
yes | unclear | yes | yes | yes | unclear | yes | no | yes | no | yes | unclear |
ITT, Intention to Treat
*1Endpoints were determined by an independent Clinical Events Committee, except for revascularization.
*2Of the 18’144 randomized subjects, 42% discontinued the intake of the medication early.
*3Study participants in the statin monotherapy group were older than those in the ezetimibe-statin combination therapy group. The ezetimibe-statin combination therapy group included more smokers and patients with diabetes mellitus.
*4Of the 51 subjects, 11 discontinued the study.
*5ITT analysis, at least for the analysis of the adverse events
*6Of the 90 study participants, 26 discontinued the study early (drop-out rate: 29%).
*7Individual treatment for coronary heart disease (CHD)
High risk of bias: The study had methodological shortcomings, making a distortion of results highly likely.
Unclear risk of bias: For one or more components, the risk of bias was unclear.
Low risk of bias: The risk of distortion was judged as low for all components.
Disagreements were resolved by discussion. In addition, the quality of evidence was assessed across endpoints using the approach of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group (30). Where good-quality studies were available, the evidence was considered to be associated with a low risk of bias. Evidence was assessed as being consistent if the effect sizes were similar across the individual studies and pointed in the same direction. Evidence was classed as direct when it demonstrated a direct relationship between the intervention and the health-relevant endpoint and the results of the study were applicable to the target population. It was classed as precise when the results showed a low degree of uncertainty. Finally, the quality of evidence was classed as high, moderate, low, or very low. If the quality is high, the authors are very confident that the true effect is close to the effect estimate. In contrast, if the quality is very low, the authors assume that the true effect is likely to be significantly different from the effect estimate (30).
Synthesis of evidence
We performed meta-analyses of comparable studies with the same endpoint. In all meta-analyses, binary endpoints were evaluated and the risk ratio (RR) as well as the corresponding 95% confidence interval (CI) with random effects was calculated using the Mantel-Haenszel method (31). The extent of statistical heterogeneity was determined by I2 (32). All meta-analyses were performed using Review Manager 5.3, a Cochrane Collaboration software (tech.cochrane.org/revman/download). Due to the limited number of studies available, no funnel plots could be used to estimate the publication bias. If it was not possible to perform a meta-analysis, a descriptive summary of the results of the single study was produced. The effect estimates reported in the studies were discussed. If no relative effect estimates were provided, we calculated the risk ratio with corresponding 95% CI.
Results
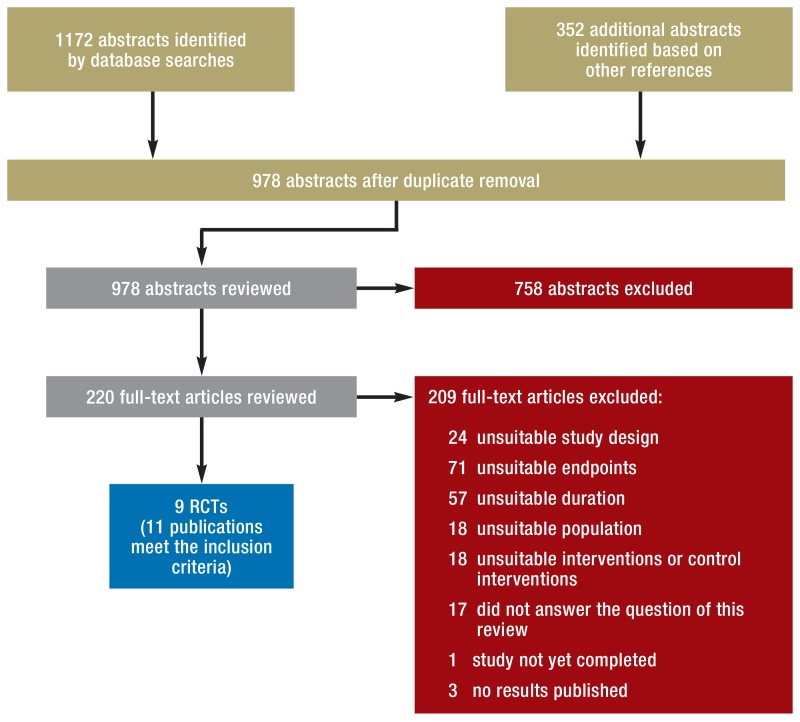
Altogether, our search identified 978 abstracts. Of these, 220 were regarded as potentially relevant, included as full-text articles and reviewed. Nine RCTs (11 publications) met the inclusion criteria (20– 29, 33). In the eFigure, the flow of the literature selection process is depicted and the reasons for exclusion of a full-text article are listed.
eFigure.
Process of literature selection
RCT, randomized controlled trial
The RCTs included in our systematic review contained data of altogether 19 461 adult patients (20– 29, 33). One study was classed as having a low risk of bias (27, 28, 33), five an unclear risk of bias (21– 23, 25, 26), and three a high risk of bias (20, 24, 29). Five RCTs had a double-blind design (21– 23, 26– 28) and two an open-label design (24, 29). In two further studies, no information about blinding was provided (20, 25). Four RCTs were sponsored by a pharmaceutical company (22– 24, 27, 28, 33), two (25, 26) were supported by national funding bodies, and three provided no information about funding (20, 21, 29). Study durations ranged from 6 to 84 months. In all studies, ezetimibe was administered in the approved dose of 10 mg/day in combination with a statin and compared with statin monotherapy. Information about the type and dosage of the statins used as well as other study characteristics is provided in the Table and, in greater detail, in eTable 3.
Table. Duration, dosage and results of the included studies.
| Author / study (year), study duration | Intervention (n) vs. control intervention (n); run-in / wash-out phase | Relevant Endpoints | Results | |||
|---|---|---|---|---|---|---|
| Arimura et al. (2012) (20) 6–8 months |
1. Ezetimibe 10 mg/day + Atorvastatin 10 mg/day (25) 2. Atorvastatin 10 mg/day (25) |
Cardiovascular morbidity a) non-fatal myocardial infarction b) target lesion revascularization c) target vein revascularization d) non-target vein revascularization e) stent thrombosis |
a) E + A: 0/25 (0%)*1; A: 0/25 (0%)*1 b) E + A: 2/25 (8%)*1 ; A: 2/25 (8%)*1 c) E + A: 2/25 (8%)*1 ; A: 3/25 (12%)*1 d) E + A: 1/25 (4%)*1; A: 0/25 (0%)*1 e) E + A: 0/25 (0%)*1 ; A: 0/25 (0%)*1 |
|||
| Cardiovascular morality | E+A: 1/25 (4%)*1; A: 0/25 (0%)*1; RR: 3.12; 95% CI: [0.12; 80.39]*2 |
|||||
| Dagli et al. (2007) (21) 6 months |
1. Ezetimibe 10 mg/day + Pravastatin 10 mg/day (50) 2. Pravastatin 40 mg/day (50) |
Adverse events | E + P | P | p value | |
| Number of AEs | 6/50 (12%) | 3/50 (6%) | >0.05 | |||
| Discontinuation due to AE | 0/50 (0%) | 0/50 (0%) | not reported | |||
| Feldman et al. (2004) (22)) 6 months (23 weeks) |
1. Ezetimibe 10 mg + Simvastatin 10 mg/day (251)) 2. Ezetimibe 10 mg + Simvastatin 20 mg/day (109)) 3. Ezetimibe 10 mg + Simvastatin 40 mg/day (97)) 4. Simvastatin 20 mg/day (253) |
Adverse events | E + S 10/10 mg | E + S 10/20 mg | E + S 10/40 mg | S 20 mg |
| Number of AEs | 140/251 (56%) | 74/109 (68%) | 63/97 (65%) | 168/253 (66%) | ||
| Number of SAEs | 20/251 (8%) | 3/109 (3%) | 4/97 (4%) | 12/253 (5%) | ||
| Discontinuation due to AE | 11/251 (4%) | 7/109 (6%) | 5/97 (5%) | 14/253 (6%) | ||
| Rhabdomyolysis | 0/251 (0%) | 0/109 (0%) | 0/97 (0%) | 0/253 (0%) | ||
| Hepatitis, hepatotoxicity | 0/251 (0%) | 0/109 (0%) | 0/97 (0%) | 0/253 (0%) | ||
| Gaudiani et al. (2005) (23)) 6 months |
1. Ezetimibe 10 mg/day + Simvastatin 20 mg/day open-label (104)) 2. Simvastatin 40 mg/day (20 mg of it open-label) (110) |
All-cause mortality | E + S: 0/104 (0%); S: 0/110 (0%) | |||
| Adverse events | E + S | S | ||||
| Number of SAEs | 5/104 (5%) | 1/110 (10%) | ||||
| Discontinuation due to AE | 2/104 (2%) | 5/110 (5%) | ||||
| Anemia | 1/104 (1%) | 4/110 (4%) | ||||
| Edema | 5/104 (5%) | 5/110 (5%) | ||||
| Weight gain | 1/104 (1%) | 0/110 (0%) | ||||
| Myopathy | 0/104 (0%) | 0/110 (0%) | ||||
| IMPROVE-IT study (2014)) (27), 28))) 84 months |
1. Ezetimibe 10 mg/day + Simvastatin 40 mg/day (6% increased dose of simvastatin to 80 mg/day, because LDL >79) (9067)) 2. Simvastatin 40 mg/day (27% increased dose of simvastatin to 80 mg/day, because LDL >79) (9077) |
Cardiovascular morbidity) a) Composite endpoint (cardiovascular death, non-fatal MI, unstable AP with hospitalization, coronary revascularization, non-fatal stroke)) b) MI) c) coronary revascularization ≥ 30 days) d) Hospitalization for unstable AP |
a) E + S: 2572/9067 (33%); S: 2742/9077 (35%);) HR after 7 years: 0.94; [0.89; 0.99];) p = 0.016; NNT: 50 after 7 years) b) E + S: 977/9067 (13%); S: 1118/9077 (15%);) HR after 7 years: 0.87 [0.80; 0.95]; p = 0.002) c) E + S: 1871/9067 (24%); S: 1962/9077 (26%);) HR after 7 years: 0.96; [0.85; 1.33]; p = 0.18) d) E + S: 156/9067 (2%); S: 148/9077 (2%);) HR after 7 years: 1.06 [0.85; 1.33]; p = 0.6 |
|||
| Cardiovascular mortality | E + S: 537/9067 (7%); S: 538/9077 (7%);) HR after 7 years: 1 [0.89; 1.13]; p = 1 |
|||||
| All-cause mortality | E + S: 1215/9067 (15%); S: 1231/9077 (15%);) HR after 7 years: 0.99 [0.91; 1.07]; p = 0.78 |
|||||
| Adverse events | E + S | S | p-value not adj. | |||
| Number of AEs | 5486/9067 (61%) | 5472/9077 (60%) | p-value not reported | |||
| Number of SAEs | 3640/9067 (40%) | 3649/9077 (40%) | p-value not reported | |||
| Cancer | 748/9067 (10%) | 732/9077 (10%) | p = 0.57 | |||
| Cholecystectomy | 133/9067 (2%) | 134/9077 (2%) | p = 0.96 | |||
| Gallbladder-related AEs | 281/9067 (3%) | 321/9077 (4%) | p = 0.10 | |||
| Rhabdomyolysis | 13/9067 (0.1%) | 18/9077 (0.2%) | p = 0.37 | |||
| Myopathy | 15/9067 (0.2%) | 10/9077 (0.1%) | p = 0.32 | |||
| Masuda et al. (2015) (29)) 6 months |
1. Ezetimibe 10 mg/day + Rosuvastatin 5 mg/day (26)) 2. Rosuvastatin 5 mg/day (25) |
Cardiovascular morbidity) a) MI) b) coronary revascularization |
c) E + R: 0/26 (0%); R: 0/25 (0%)) d) E + R: 1/26 (4%); R: 1/25 (4%) |
|||
| All-cause mortality | E + R: 0/26 (0%); R: 0/25 (0%) | |||||
| Adverse events | E + R | R | ||||
| Discontinuation due to AE | 2/26 (8%) | 1/25 (4%) | ||||
| Rhabdomyolysis | 0/26 (0%) | 0/25 (0%) | ||||
| Myalgia | 0/26 (0%) | 1/25 (4%) | ||||
| Skin rash | 1/26 (4%) | 0/25 (0%) | ||||
| Meaney et al. (2009) (24)) 12 months |
1. Ezetimibe + Simvastatin (10/20 mg/day): Starting month 2, the dose was increased to 10/40 mg/day, if the therapeutic goal had not been reached (30).) 2. Pravastatin 40 mg/day; starting month 2, 10 mg ezetimibe, if the therapeutic goal had not been reached (30).) 3. Simvastatin 40 mg /day: Starting month 2, the dose was increased to 80 mg, if the therapeutic goal had not been reached (30). |
Adverse events | E + S | P + possibly E | S | |
| Discontinuation due to rash | 1/30 (3%) | 0/30 (0%) | 0/30 (0%) | |||
| Discontinuation due to myalgia | 1/30 (3%) | 0/30 (0%) | 0/30 (0%) | |||
| Discontinuation due to CPK increase | 0/30 (0%) | 0/30 (0%) | 3/30 (10%) | |||
| Nakamura et al. (2012) (25)6 months | 1. Ezetimibe 10 mg/day + statin (individual dose) (32)2. Statin (doubling of previous dose) (31) | Adverse events | E + St | St | ||
| Discontinuation due to AE | 3/32 (9%) | 3/31 (10%) | ||||
| Stroke | 1/32 (3%) | 0/31 (0%) | ||||
| Rash | 1/32 (3%) | 0/31 (0%) | ||||
| West et al. (2011) (26) 24 months |
1. Ezetimibe 10 mg/day + Simvastatin 40 mg/day (22) 2. Simvastatin 40 mg/day (22) |
Cardiovascular morbidity MACE (= CV death, MI, stroke, transient ischemic attack) |
E + S: 4/22 (18%); S: 2/22 (10%); RR: 2.22 [0.36; 13.62]*2 |
|||
| Adverse events | E + S | S | ||||
| Myalgia | 0/22 (0%) | 1/22 (5%) | ||||
A, atorvastatin; adj., adjusted; AE, adverse event; AP, angina pectoris; CPK, creatine phosphokinase; E, ezetimibe; HR, Hazard Ratio; CI, confidence interval; LDL, low-density lipoprotein; MACE, major adverse cardiovascular event;
MI, myocardial infarction; n, number of subjects; NNT, number needed to treat; p, p-value; P, pravastatin; R, rosuvastatin; RR, Risk Ratio; SAE, serious adverse event; S, Simvastatin; St, statin
*1self-calculated for intention-to-treat (ITT) analysis; in the article, a per-protocol analysis was performed.
*2Risk Ratio (self-calculated)
eTable 3. Characteristics and results of the included studies.
| Author / study (year), study design, pharmaceutical sponsor, country, duration | Population (n), age (SD / MinMax), gender, baseline cholesterol levels, other characteristics | Intervention (n) vs. control intervention (n), run-in / wash-out phase | Relevant endpoints | Results | Risk of bias | |||
|---|---|---|---|---|---|---|---|---|
| Arimura et al. (2012), RCT (monocentric, unclear whether blinded), sponsor: not specified, Japan[20] 6–8 months medication; starting on the day of stent placement; follow-up: 6–8 months (253 ±77 days) |
Adult patients with stable angina pectoris and dyslipidemia (defined as: LDL-C⋛140mg/dL, triglycerides ⋛150mg/dL, or HDL-C <40mg/dl), with a stent (metal or drug-eluting), without familial hypercholesterolemia (50) 6 patients dropped out of the study (3 each group); the following information is based on the 44 remaining patients: Age: E + A: 69 J (± 9), A: 69 J (± 8) Sex distribution: E + A: 68% m, 32% f A: 73% m, 27% f Baseline cholesterol: not specified Proportion of patients with diabetes : not specified |
1. Ezetimibe 10 mg/day + Atorvastatin 10 mg/day (25) 2. Atorvastatin 10 mg/day (25) Run-in/wash-out phase: not specified |
Cardiovascular morbidity a) non-fatal myocardial infarction b) target lesion revascularization c) target vein revascularization ) non-target vein revascularization e) stent thrombosis |
a) E + A: 0/25 (0%)*1 A: 0/25 (0%)*1 b) E + A: 2/25 (8%)*1 A: 2/25 (8%)*1 c) E + A: 2/25 (8%)*1 A: 3/25 (12%)*1 d) E + A: 1/25 (4%)*1 A: 0/25 (0%)*1 e) E + A: 0/25 (0%)*1 A: 0/25 (0%)*1 |
high | |||
| Cardiovascular mortality | E + A: 1/25 (4%)*1A: 0/25 (0%)*1 RR: 3,12; 95% CI: [0.12; 80.39]*2 | |||||||
| All-cause mortality | not specified | |||||||
| Quality of life | not specified | |||||||
| Adverse events | not specified | |||||||
| Dagli et al. (2007), RCT (single-center, double-blind), sponsor: not specified, Tukey (21) 6 months |
Adult patients with primary hyperlipidemia and CHD or known CHD risk or 10-year CHD risk <20%, ldl 210–370mg/dl after 10-week wash-out phase; patients unstable ap or mi were excluded (100) Age: E + P: 53 yrs (± 12); P: 57 yrs (± 11) Sex distribution: E + P: 46% m, 54% f P: 52% m, 48% f Baseline cholesterol (mg/dL) E + P (total): 250.9 ± 51.8; DL: 158,1 ± 47,5 P (total): 231.1 ± 83,5; LDL: 165,7 ± 29,7 Proportion of patients with diabetes: 0% |
1. Ezetimibe 10 mg/day + Pravastatin 10 mg/day (50) 2. Pravastatin 40 mg/day (50) Wash-out phase: 4–12 weeks before randomization |
Cardiovascular morbidity | not specified | unclear | |||
| Cardiovascular mortality | not specified | |||||||
| All-cause mortality | not specified | |||||||
| Quality of life | not specified | |||||||
| Adverse events | E + P | P | p-value | |||||
| Number of AEs | 6/50 (12%) |
3/50 (6%) |
p >0.05 | |||||
| Number of SAEs | not specified | |||||||
| Discontinuation due to AE | 0/50 (0%) |
0/50 (0%) |
not reported | |||||
| Feldman et al. (2004), RCT (multicenter, double-blind), Merck/Schering Plough Pharmaceuticals, USA (22)6 months (23 weeks) | Adult patients with CHD or CHD risk equivalent according to the NCEP ATP III guideline*3 and LDL cholesterol ⋛130 mg/dL and TG ≤ 350 mg/dL (710) Age: E + S 10/10 mg: 61 yrs (± 10) E + S 10/20 mg: 64 yrs (± 10) E + S 10/40 mg: 62 yrs (± 10) S 20 mg: 62 yrs (± 10) Sex distribution: E + S 10/10 mg: 69% m, 31% E + S 10/20 mg: 54% m, 46% f E + S 10/40 mg: 62% m, 38% f S 20 mg: 62% m, 38% f Baseline cholesterol (mg/dL): E + S 10/10 mg (total): 247.6 ± 38.0; LDL: 165.1 ± 34.3 E + S 10/20 mg (total): 248.8 ± 37.9; LDL: 167.3 ± 33.0 E + S 10/40 mg (total): 252.3 ± 43.7; LDL: 170.5 ± 40.6 S 20 mg (total): 256.7 ± 46.8; LDL: 173.8 ± 44.7 Proportion of patients with diabetes : E + S 10/10 mg: 51% E + S 10/20 mg: 55% E + S 10/40 mg: 42% S 20 mg: 45% |
1.Ezetimibe 10 mg + Simvastatin 10 mg/day (251) 2. Ezetimibe 10 mg + Simvastatin 20 mg/day (109) 3. Ezetimibe 10 mg + Simvastatin 40 mg/day (97) 4. Simvastatin 20 mg/day (253) Every 6 weeks after randomization, the simvastatin dose was increased to max. 80mg/day, as long as an LDL level of 100mg/dL was not attained. Simvastatin was open-label, blinding was only performed for ezetimibe Run-in phase: 4 weeks placebo |
Cardiovascular morbidity | not specified | unclear | |||
| Cardiovascular mortality | not specified | |||||||
| All-cause mortality | not specified | |||||||
| Quality of life | not specified | |||||||
| Adverse events | E + S 10/10 mg | E + S 10/20 mg | E + S 10/40 mg | S 20 mg | ||||
| Number of AEs | 140/251 (56%) |
74/109 (68%) |
63/97 (65%) |
168/253 (66%) |
||||
| Number of SAEs | 20/251 (8%) |
3/109 (3%) |
4/97 (4%) |
12/253 (5%) |
||||
| Discontinuation due to AE | 11/251 (4%) |
7/109 (6%) |
5/97 (5%) |
14/253 (6%) |
||||
| Rhabdomyolysis | 0/251 (0%) |
0/109 (0%) |
0/97 (0%) |
0/253 (0%) |
||||
| Hepatitis, hepatotoxicity | 0/251 (0%) |
0/109 (0%) |
0/97 (0%) |
0/253 (0%) |
||||
| Gaudiani et al. (2005), RCT (multicenter, double-blind), Merck/Schering- Plough Pharmaceuticals, USA (23) 6 months |
Adult patients with hypercholesterolemia and type-2 diabetes mellitus (for 3 months with stable thiazolidinedione treatment) (excluded, if MI within last 3 months or familial hypercholesterolemia) (214) Age: E + S: 58 yrs (35–80) S: 58 yrs (37–78) Sex distribution: E + S: 60% m, 40% f S: 56% m, 44% f Baseline cholesterol levels (mg/dL)*4 : E + S (total): 173.3 ± 40.5; LDL: 94.6 ± 28.8 S (total): 169 ± 29.6; LDL: 92.3 ± 24.5 Proportion of patients with a theroscklerosis: not specified |
1. Ezetimibe 10 mg/day + Simvastatin 20 mg/day „open-label“ (104) 2. Simvastatin 40 mg/day (20 mg of this open-label) (110) Run-in phase: 6-week open-label Simvastatin 20mg/day |
Cardiovascular morbidity | not specified | unclear | |||
| Cardiovascular mortality | not specified | |||||||
| All-cause mortality | E + S: 0/104 (0%); S: 0/110 (0%) | |||||||
| Quality of life | not specified | |||||||
| Adverse events | E + S | S | ||||||
| Number of AEs | not specified | |||||||
| Number of SAEs | 5/104 (5%) |
1/110 (10%) |
||||||
| Discontinuation due to AE | 2/104 (2%) |
5/110 (5%) |
||||||
| Anemia | 1/104 (1%) |
4/110 (4%) |
||||||
| Edema | 5/104 (5%) |
5/110 (5%) |
||||||
| Weight gain | 1/104 (1%) |
0/110 (0%) |
||||||
| Myopathy | 0/104 (0%) |
0/110 (0%) |
||||||
| IMPROVE-IT study (2014), RCT (multicenter, double-blind) Merck Sharp & Dohme, USA, Europe, Australia, New Zealand, South America, Israel, Asia, South Africa (27, 28) 84 months |
Adults hospitalized <10 days for acute coronary syndrome (18144): mi (75%) or unstable AP (25%) Age: E + S: 64 yrs (SD: not specified) S: 64 yrs (SD: not specified) Sex distribution: E + S: 75% m, 25% f S: 76% m, 24% f Baseline cholesterol (mg/dL): E + S (total): not specified; LDL: 95 (79, 110) S (total): not specified; LDL: 95 (79, 220) Proportion of patients with diabetes: E + S: 27% S: 27% |
1. Ezetimibe 10 mg/day + Simvastatin 40 mg/day (6% increased simvastatin to 80 mg/day, because LDL >79) (9067) 2. Simvastatin 40mg/day (27% eincreased to 80mg/day, because LDL >79) (9077) Run-in / wash-out phase: not specified |
Cardiovascular morbidity a) Composite endpoint (cardiovascular death, MI, unstable AP requiring hospitalization, coronary revascularization, stroke) b) MI c) Coronary revascularization ⋛ 30 daye d) Hospitalization for unstable AP |
a) E + S: 2572/9067 (33%) S: 2742/9077 (35%) HR after 7 years: 0.94 [0.89; 0.99]; p = 0.016; NNT: 50 Patients with diabetes mellitus had a greater advantage due to ezetimibe than those without diabetes mellitus: Diabetes melltius: HR: 0.86 [0.78; 0.94] kein Diabetes melltius: HR: 0.98 [0.92; 1.04]; Interaktion p = 0.023 b) E + S: 977/9067 (13%); S: 1118/9077 (15%); HR after 7 years: 0.87 [0.80; 0.95]; p = 0.002 c) E + S: 1871/9067 (24%); S: 1962/9077 (26%); HR after 7 years: 0.96 [0.85; 1.33]; p = 0.18 d) E + S: 156/9067 (2%); S: 148/9077 (2%); HR after 7 years: 1.06 [0.85; 1.33]; p = 0.62 |
unclear | |||
| Cardiovascular mortality | E + S: 537/9067 (7%); S: 538/9077 (7%); HR after 7 years: 1 [0.89; 1.13]; p = 1 |
|||||||
| All-cause mortality | E + S: 1215/9067 (15%); S: 1231/9077 (15%); HR after 7 years: 0.99 [0.91; 1.07]; p = 0.78 |
|||||||
| Quality of life | not specified | |||||||
| Adverse events | E + S | S | p-value not adj. | |||||
| Number of AEs | 5486/9067 (61%) |
5472/9077 (60%) |
p not reported | |||||
| Number of SAEs | 3640/9067 (40%) |
3649/9077 (40%) |
p not reported | |||||
| Cancer | 748/9067 (10%) |
732/9077 (10%) |
p = 0.57 | |||||
| Cholecystectomy | 133/9067 (2%) |
134/9077 (2%) |
p = 0.96 | |||||
| Gallbladder-related AEs | 281/9067 (3%) |
321/9077 (4%) |
p = 0.10 | |||||
| Rhabdomyolysis | 13/9067 (0.1%) |
18/9077 (0.2%) |
p = 0.37 | |||||
| Myopathy | 15/9067 (0.2%) |
10/9077 (0.1%) |
p = 0.32 | |||||
| Masuda et al. (2015), (RCT single-center, open-label), sponsor: not specified, Japan (29) 6 months |
Adult patients with clinically stable AP, prior to elective percutaneous coronary intervention, with LDL-C levels of at least 100mg/dl at baseline (51) The following information is based solely on the 44 subjects who underwent intravascular ultrasonography. Age: E + R: 64 yrs (± 8) R: 70 yrs (± 8) Sex distribution: E + R: 91% m, 9% f R: 84% m, 16% f Baseline cholesterol (mg/dL): not specified Proportion of patients with diabetes: E + R: 52% R: 42% |
1. Ezetimibe 10 mg/day + Rosuvastatin 5 mg/day (26) 2. Rosuvastatin 5 mg/day (25) Run-in / wash-out phase: not specified |
Cardiovascular morbidity a) MI b) Coronary revascularization |
c) E + R: 0/26 (0%); R: 0/25 (0%) d) E + R: 1/26 (4%); R: 1/25 (4%) |
high | |||
| Cardiovascular mortality | not specified | |||||||
| All-cause mortality | E + R: 0/26 (0%); R: 0/25 (0%) | |||||||
| Quality of life | not specified | |||||||
| Adverse events | E + R | R | ||||||
| Discontinuation due to AE | 2/26 (8%) |
1/25 (4%) |
||||||
| Rhabdomyolysis | 0/26 (0%) |
0/25 (0%) |
||||||
| Myalgia | 0/26 (0%) |
1/25 (4%) |
||||||
| Drug eruption | 1/26 (4%) |
0/25 (0%) |
||||||
| Number of AEs, SAEs | not specified | |||||||
| Meaney et al. (2009), RCT (single-center, open-label), Merck Sharp & Dohme, Mexiko (24) 12 months |
Adult patients with 10-year risk of cardiovascular mortality ⋛ 20 according to ATP III recommendations. Age: S + E: 58 yrs (± 9); P + (possibly E): 59 yrs (± 7); S: 57 rs (± 8) Sex distribution (conflicting information): S + E: 19 m, 21 f; P + (possibly E): 13 m, 17 f; S: 12 m, 19 f Baseline cholesterol (mg/dL): S + E (total): 216 ± 40; LDL: 131 ± 39 P+ (possibly E) (total): 207 ± 31; LDL: 128 ± 30 S (total): 215 ±38; LDL: 130 ± 33 Proportion of patients with diabetes: S + E: 14/30 (47%); P + (possibly E): 16/30 (53%); S: 15/30 (50%) |
1. Ezetimibe + Simvastatin (10/20 mg/day): Starting month 2, the dose was increased to 10/40 mg/day, if the therapeutic goal was not attained (30). 2. Pravastatin 40 mg/day: Starting month 2, additional 10 mg ezetimibe, if the therapeutic goal was not attained (30). 3. Simvastatin 40 mg /day: Starting month 2, the dose was increased to 80 mg, if the therapeutic goal was not attained (30). Run-in / wash-out phase: not specified |
Cardiovascular morbidity | not specified | high | |||
| Cardiovascular mortality | not specified | |||||||
| All-cause mortality | not specified | |||||||
| Quality of life | not specified | |||||||
| Adverse events | E + S | P + evtl. E | S | |||||
| Discontinuation due to skin rash | 1/30 (3%) |
0/30 (0%) |
0/30 (0%) |
|||||
| Discontinuation due to myalgia | 1/30 (3%) |
0/30 (0%) |
0/30 (0%) |
|||||
| Discontinuation due to CPK increase | 0/30 (0%) |
0/30 (0%) |
3/30 (10%) |
|||||
| Number of AEs, SAE | not specified | |||||||
| Nakamura et al. (2012), RCT (single-center, blinding unclear), no pharmaceutical sponsor, Japan (25) 6 months |
Adult patients with hypercholesterolemia (increased chylomicron remnants ⋛ 5.0mg and LDL ⋛ 100mg/dL + at least 1 organic stenosis of an important coronary artery demonstrated by angiography); all patients had stable CHD (no AP) at rest, no increase in AP attacks during the last year, no acute coronary syndrome within the last 4 weeks) (63) Age: E + St: 61 yrs (± 10); St: 64 yrs (± 9) Sex distribution: E + St: 76% m, 24% f; St: 82% m, 18% f Baseline cholesterol (mg/dL): E + St (total): 193 (182, 221); LDL: 120 (105, 139) St (total): 200 (187, 221); LDL: 121 (107, 141) Proportion of patients with diabetes: E + St: 35%; St: 37% |
1. Ezetimibe 10 mg/day + Statin (individual dose) (32) 2. Statin (doubling of the previous dose) (31) E + statin (statin and average dose) Atorvastatin: 7%, 10 mg Pravastatin: 59%, 12.7 mg Rosuvastatin: 24%, 5.8 mg Pitavastatin: 10%, 1.5 mg Statin: (Statin and average baseline dose) Atorvastatin: 21%, 11.7 mg Pravastatin: 43%, 11.7 mg Rosuvastatin: 32%, 6,1 mg Pitavastatin: 4%, 2 mg Run-in / wash-out phase: not specified |
Cardiovascular morbidity | not specified | unclear | |||
| Cardiovascular mortality | not specified | |||||||
| All-cause mortality | not specified | |||||||
| Quality of life | not specified | |||||||
| Adverse events | E + St | St | ||||||
| Discontinuation due to AE | 3/32 (9%) |
3/31 (10%) |
||||||
| Stroke | 1/32 (3%) |
0/31 (0%) |
||||||
| Skin rash | 1/32 (3%) |
0/31 (0%) |
||||||
| Number of AEs, SAE | not specified | |||||||
| West et al. (2011), RCT (single-center, double-blind), no pharmaceutical sponsor, USA (26) 24 months |
Statin-naive adults with peripheral arterial occlusive disease (PAOD) (44) (n = 34 remaining in the study) Age: E + S: 62 yrs (± 8); S: 59 yrs (± 10) Sex distribution: S + E: 56% m, 44% f; S: 69% m, 31% f Baseline cholesterol (mg/dL): E + S (total): 189 ± 10; LDL: 118 ± 9 S (total): 194 ± 11; LDL: 118 ± 10 Proportion of patients with diabetes: E + S: 28%; S: 31% |
1. Ezetimibe 10 mg/day + Simvastatin 40 mg/day (22) 2. Simvastatin 40 mg/day (22) 3. Study arm was part of a parallel observational study and thus not reported here |
Cardiovascular morbidity MACE (= death, myocardial infarction, stroke, transient ischemic attack) |
E + S: 4/22 (18%); S: 2/22 (10%); RR 2,22; [0.36; 13,62]*1 |
unclear | |||
| Cardiovascular mortality | not specified | |||||||
| All-cause mortality | not specified | |||||||
| Quality of life | not specified | |||||||
| Adverse events | E + S | S | ||||||
| Myalgia | 0/22 (0%) |
1/22 (5%) |
||||||
| Number of AEs, SAE, Discontinuation due to AE | not specified | |||||||
A, Atorvastatin; adj., adjusted; AE, adverse event; AP, angina pectoris; CHD, coronary heart disease; CI, confidence interval; CPK, creatine phosphokinase; E, ezetimibe; f, female; HDL, high-density lipoprotein; HR, Hazard Ratio; LDL, low-density lipoprotein; m, male; MACE, major adverse cardiovascular event; MinMax, minimum and maximum value; MI, myocardial infarction; n, number of study participants; NCEP ATP, National Cholesterol Education Program Adult Treatment Panel; NNT, number needed to treat; p, p-value; P, Pravastatin; PAOD, peripheral arterial occlusive disease; R, rosuvastatin; RCT, randomized controlled trial; RR, risk ratio; S, simvastatin; SAE, serious adverse event; SD, standard deviation; St, statin; TG, triglyceride; yrs, years
*1self-calculated for ITT analysis; analysis in the article per protocol
*2Risk Ratio self-calculated
*3NCEP ATP III guideline: Patients with CHD or CHD risk equivalent have a target LDL cholesterol levels <100. For patients with 2 or more risk factors, the target LDL cholesterol levels <130. In persons with no risk factors, target LDL cholesterol levels are <160 (34).
*4units converted from mmol/l to mg/dl using an online calculator: www.tellmed.ch/tellmed/tools/diagnostische_scores_berechnungen/umrechnung_von_mg_dl.php
In the following, we will summarize the results by endpoints. First, we will address cardiovascular morbidity, then mortality, and finally adverse events. The quality of evidence of the individual endpoints and the corresponding effect sizes are described in eTable 4.
eTable 4. Quality of evidence based on the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) and results for each endpoint.
| Quality assessment | Number of events and number of patients | Effect | Quality of evidence | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Ezetimibe 10 mg/day + statin 10–80 mg/day | Statin 10–80 mg/day | Risk ratio (95% CI) | |
| Cardiovascular morbidity in high-risk patients after acute coronary syndrome (follow-up: 84 months) | |||||||||
| 1*1 | RCT | not high | not high | not high | not high | 2572/9067 (33%) | 2742/9077 (35%) | HR: 0.94 [0.89; 0.99] | + + + + high |
| Cardiovascular morbidity in hyperlipidemic patients with atherosclerosis without acute coronary syndrome (follow-up: 6–24 months) | |||||||||
| 3*2 | RCT | high*3 | not high | not high | very high*4 | 4/22 (18%) | 2/22 (10%) | RR: 2.22 [0.36; 13.62] | very low + |
| Cardiovascular mortality (follow-up: 6–84 months)) | |||||||||
| 2 | RCT | high*3 | not high | not high | not high | 538/9092 (6%) | 538/9102 (6%) | HR: 1.00 [0.89; 1.12] | moderate + + + |
| All-cause mortality in high-risk patients after acute coronary syndrome (follow-up: 84 months) | |||||||||
| 1 | RCT | not high | not high | not high | not high | 1215/9067 (15%) | 1231/9077 (15%) | HR: 0.99 [0.91; 1.07] | + + + + high |
| All-cause mortality in hyperlipidemic patients with atherosclerosis without acute coronary syndrome | |||||||||
| 1 | RCT | high*3 | not high | not high | very high*4 | 0/104 (0%) | 0/110 (0%) | RR not calculable | very low + |
| Quality of life – no evidence identified | |||||||||
| Number of adverse events (follow-up: 6 months) | |||||||||
| 3 | RCT | not high | not high | not high | high*5 | 5769/9574 (60%) | 5643/9380 (60%) | RR: 0.98 [0.89; 1.07] | moderate + + + |
| Number of serious adverse events (follow-up: 6 months) | |||||||||
| 3 | RCT | not high | not high | not high | very high*4 | 3672/9628 (38%) | 3662/9440 (39%) | RR: 1.09 [0.77; 1.55] | low + + |
| Study discontinuation due to adverse events (follow-up: 6–12 months) | |||||||||
| 6 | RCT | high*6 | not high | not high | high*5 | 32/699 (5%) | 26/499 (5%) | RR: 0.85 [0.51; 1.43] | low + + |
GRADE, Grading of Recommendations Assessment, Development and Evaluation; HR, Hazard Ratio; CI, confidence interval; RCT, randomized controlled trial; RR, risk ratio
*1This study reports both a composite endpoint and individual endpoints (myocardial infarction, revascularization, unstable angina pectoris). Since the composite endpoint was the study’s primary endpoint, it was reported here.
*2Cardiovascular morbidity was reported in three RCTs (in one study as a composite endpoint, in the other studies as single endpoints [myocardial infarction, revascularization, stent thrombosis]). Since the data were not combined in a meta-analysis, the results of the study which evaluated a composite endpoint (death, myocardial infarction, stroke, and transient ischemic attack), are reported here. In the two other studies, the incidence of cardiovascular events was comparably low in both treatment groups.
*3Randomization, concealed assignment, blinding at times unclear
*4The confidence interval contains effect estimates that can indicate both an advantage and a disadvantage for ezetimibe-statin therapy. The number of subjects is very small; thus, the results may be due to random effects and lack of power. Result rates very low.
*5The confidence interval contains effect estimates that can indicate both an advantage and a disadvantage for ezetimibe-statin therapy.
*6Randomization was unclear and no blinding was carried out.
Cardiovascular morbidity
Three RCTs on cardiovascular morbidity evaluated either composite or single endpoints, e.g. myocardial infarction (20, 26– 28, 33). It was not possible to perform a meta-analysis because either the endpoint were too different or no results were available in the studies so that the risk ratios could not be calculated.
Composite endpoint
The IMPROVE-IT study included 18 144 patients presenting with acute coronary syndrome (myocardial infarction, unstable angina pectoris [AP]). The primary composite endpoint comprised cardiovascular death, non-fatal myocardial infarction, unstable AP requiring hospitalization, coronary revascularization, and non-fatal stroke (27, 28, 33). The risk of experiencing one of these cardiovascular events during the 7-year study period was significantly lower in the ezetimibe-statin group compared with the statin group (33% versus 35%; Hazard Radio (HR) 0.94; 95% CI [0.89; 0.99]; p = 0.016). Consequently, 50 patients have to be treated with ezetimibe-statin combination therapy to prevent one recurrence of a cardiovascular event compared with patients treated with statin alone (number needed to treat: 50 in seven years). The primary endpoint difference between the groups was due to differences in coronary revascularization, myocardial infarction and stroke event rates, but not due to mortality (all-cause mortality). Looking selectively at the patients with diabetes mellitus in the IMPROVE-IT study (n = 4899), 40% of these patients in the ezetimibe-statin combination therapy group and 46% in the statin monotherapy group experienced a cardiovascular event (HR 0.87 [0.78; 0.94]). Subjects without diabetes mellitus (n = 13 202) showed a comparable risk for cardiovascular events in both treatment groups (HR 0.98 [0.92; 1.04]) (27, 28, 33).
In the study by West et al. (n = 44), 18% of patients on ezetimibe-statin combination therapy and 10% on statin monotherapy experienced the composite endpoint (death, myocardial infarction, stroke, and transient ischemic attack; RR 2.22 [0.36; 13.62]) after 24 months (26).
Myocardial infarction
The IMPROVE-IT study demonstrated a lower risk of myocardial infarction for patients on ezetimibe-statin combination therapy. Of the 9067 patients treated with ezetimibe-statin therapy, 13% experienced a myocardial infarction, compared with 15% in the statin monotherapy group (HR 0.87 [0.80–0.95]) (27, 28, 33).
In the studies by Arimura et al. (20) and Masuda et al. (29), none of the 50 and 51 subjects, respectively, experienced a myocardial infarction within 6 to 8 months.
Other endpoints of cardiovascular morbidity
For other single endpoints, such as coronary revascularization (20, 27, 28, 33), unstable angina pectoris (AP) (27, 28, 33) and stent thrombosis (20), no relevant differences were found between the treatment groups (Table).
Cardiovascular mortality
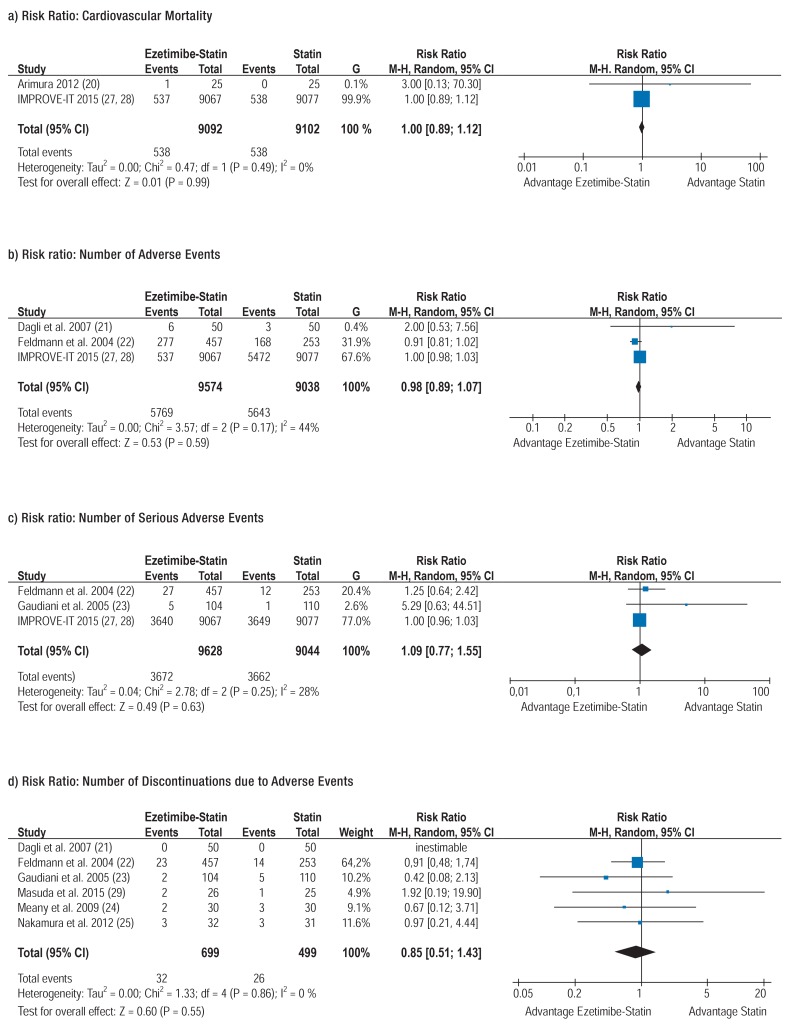
Two of the included studies with altogether 18 194 patients reported cardiovascular mortality (20, 27, 28, 33) and were combined in a meta-analysis. The risk of cardiovascular death was 6% in both treatment groups (RR 1 [0.89; 1.12]) (Figure a).
Figure.
Meta-analyses
CI, confidence interval; M-H, Mantel-Haenszel; Random, random effects model
All-cause mortality
All three studies showed comparable mortality rates in the treatment groups (23, 27– 29, 33). In the IMPROVE-IT study, 15% of patients died in each of the two treatment arms (HR 0.99 [0.91; 1.07]). Thus, ezetimibe as an adjunct to statin treatment did not reduce all-cause mortality (27, 28, 33). In the studies by Gaudiani et al. (23) and Masuda et al. (29), none of the 224 and 51 patients, respectively, died within 6 to 8 months. With no events in either of the two studies, the results could not be aggregated in a meta-analysis.
Adverse events
Adverse drug reactions (ADRs) were operationalized by the following endpoints: number of adverse events (AEs), number of serious adverse events (SAEs) and discontinuation due to adverse events. Evidence synthesis in the form of meta-analyses was possible. For the 3 meta-analyses, between-study heterogeneity was within an acceptable range (I²: 0–44%). Altogether, 7 RCTs reported data on AEs (21– 25, 27– 29, 33). In addition, the most common actual AEs were identified.
Number of adverse events
Adverse events comprise all types of ADRs. In 3 RCTs, the number of all AEs which occurred during the study among the altogether 19 954 patients were documented (21, 22, 27, 28, 33). The meta-analysis revealed that in both the ezetimibe-statin combination therapy group and the statin monotherapy group 60% of patients experienced AEs (RR 0.98 [0.89; 1.07]) (Figure b).
Serious adverse events
Serious adverse events (SAEs) comprise death, life-threatening events and events resulting in hospitalization, congenital anomaly or disability or permanent damage (35). Three RCTs (22, 23, 27, 28, 33) with altogether 18 068 patients reported SAEs which occurred during the studies; these were combined in a meta-analysis. Under ezetimibe-statin combination therapy, 38% of the 9628 patients experienced serious adverse events compared with 39% of the 9440 patients treated with statin monotherapy (RR 1.09 [0.77; 1.55]) (Figure c).
Study discontinuation due to adverse events
Six RCTs with altogether 1198 patients reported on discontinuation due to AEs during periods ranging from 6 to 12 months (21– 25, 29) and were aggregated in a meta-analysis. In both the ezetimibe-statin combination therapy group and the statin monotherapy group, 5% of patients discontinued the study due to AEs (RR 0.85 [0.51; 1.43]) (Figure d).
Actual adverse events
Ezetimibe-simvastatin combination therapy and simvastatin monotherapy had comparable incidence rates of cancer (each 10% within 7 year), cholecystectomies (2%) and gallbladder-related AEs (3–4%) (33). In 2 six-month studies evaluating rosuvastatin and simvastatin, respectively, none of the patients experienced rhabdomyolysis (22, 29). However, in the 84-month study, rhabdomyolysis occurred in 0.1% and 0.2% of the patients treated with ezetimibe-simvastatin combination therapy and simvastatin monotherapy, respectively (33). Myopathies were observed in none of the 224 patients of a six-month study (23) and in 0.2% and 0.1% of the patients with ezetimibe-simvastatin and simvastatin, respectively, in the 84-month study (33). Further information on adverse events, type and dosages of the statins is provided in the Table.
Discussion
Patient-relevant endpoints for the evaluation of the efficacy of ezetimibe-statin combination therapy were reported in 5 of the 9 identified RCTs. From 4 RCTs, only information about adverse drug reactions was obtained.
The evidence showed that patients treated with ezetimibe-statin combination therapy had a lower risk of cardiovascular events compared with those treated with statin monotherapy. However, the absolute difference between the two groups was small (2 percentage points) and due to differences in revascularization, myocardial infarction and stroke. Nevertheless, due to the high relevance of these events for patients, even minor effects are considered to be clinically relevant. Especially patients with diabetes mellitus appear to benefit from ezetimibe-statin combination therapy with respect to cardiovascular morbidity. Even though these conclusions were drawn from an a priori planned and methodologically sound subgroup analysis, they should be interpreted with caution because of the absence of the effects of randomization.
Ezetimibe as an adjunct to statin therapy did not lower cardiovascular mortality and all-cause mortality.
No relevant differences between the groups were found for the rates of adverse events and discontinuation due to adverse events.
Even though 9 RCTs were identified, the results were dominated by the IMPROVE-IT study due to its substantial size (n = 18 144). Here it should be noted that the subjects of the IMPROVE-IT study had low mean lipid levels and experienced an acute coronary syndrome (ACS) event not long before the start of the study. Thus, the results of this review can be applied to post-ACS patients, but not generally to all affected patients with atherosclerosis and/or diabetes mellitus as their risk profile is different. Since the 8 smaller studies were not designed to evaluate cardiovascular endpoints or adverse drug reactions as primary endpoints, data from these studies did not allow to draw reliable conclusions on patients with atherosclerosis and/or diabetes in general. Consequently, this sample was not statistically analyzed to test for significant differences in these endpoints, especially since the event rates were too low to allow the exclusion of random effects.
When interpreting these results, it is important to keep in mind that our meta-analyses were based on pooled data from studies which varied in duration and statin doses used. Furthermore, the baseline plasma cholesterol levels of the included studies were not identical. However, this does not limit the validity of the results obtained, since our focus was on the differences between treatment groups within one study and the groups within a study were comparable in this respect. Other systematic reviews conducted so far have typically been focused on surrogate parameters rather than patient-relevant endpoints. Furthermore, most studies included in these reviews were short, lasting only a few weeks (11–16, 36–38). The authors of a comparable review (17), also focusing on patient-relevant endpoints, arrived at the conclusion that, based on the available evidence, no additional or fewer benefits and no greater or lesser harm can be attributed to ezetimibe-statin combination therapy. However, data from the IMPROVE-IT study, which demonstrated a significant cardiovascular morbidity advantage for ezetimibe-statin combination therapy, were not yet included in their review.
Key Messages.
Cardiovascular mortality and all-cause mortality were not reduced by the use of ezetimibe as an adjunct to statin therapy.
Ezetimibe-statin combination therapy reduced the risk of cardiovascular events compared with statin monotherapy.
Especially patients with additional diabetes mellitus should benefit from an ezetimibe-statin combination therapy.
The rates of adverse events were not significantly different between ezetimibe-statin combination therapy and statin monotherapy.
The evidence did not indicate that adding ezetimibe to statin therapy reduces or increases the risk of actual adverse events.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
We would like to extend our special thanks to Evelyn Auer of the Department for Evidence-based Medicine and Clinical Epidemiology for her administrative support and to Irma Klerings, graduate cultural scientist, for conducting the literature search.
Funding
Commissioned by the Medical University of Graz, Institute of Social Medicine and Epidemiology
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.World Health Organization (WHO) Häufigste Todesursachen in Europa: Faktenblatt 2013. www.euro.who.int/__data/assets/pdf_file/0020/185312/Leading-causes-of-death-in-Europe-Fact-Sheet-Ger.pdf?ua=1. (last accessed on 9 September 2015)
- 2.Reddigan JI, Ardern CI, Riddell MC, Kuk JL. Relation of physical activity to cardiovascular disease mortality and the influence of cardiometabolic risk factors. Am J Cardiol. 2011;108:1426–1431. doi: 10.1016/j.amjcard.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Dehghan M, Mente A, Teo KK, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high-risk individuals from 40 countries. Circulation. 2012;126:2705–2712. doi: 10.1161/CIRCULATIONAHA.112.103234. [DOI] [PubMed] [Google Scholar]
- 4.Hurt RD, Weston SA, Ebbert JO, et al. Myocardial infarction and sudden cardiac death in Olmsted County, Minnesota, before and after smoke-free workplace laws. Arch Intern Med. 2012;172:1635–1641. doi: 10.1001/2013.jamainternmed.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.EUROASPIRE II Study Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries. Eur Heart J. 2001;22:554–572. doi: 10.1053/euhj.2001.2610. [DOI] [PubMed] [Google Scholar]
- 6.British Cardiac Society, British Hypertension Society, Diabetes UK. JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart. 2005;91(Suppl 5):v1–v52. doi: 10.1136/hrt.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725–730. doi: 10.1001/archinte.165.7.725. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 9.Taylor F, Huffman MD, Macedo AF, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD004816.pub5. CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fachinformation>Ezetrol ® 10mg Tabletten, Stand 2/2016. www.msd.de/fileadmin/files/fachinformationen/ezetrol.pdf. (last accessed on 9 September 2015) [Google Scholar]
- 11.Angelopoulos J, Krassakopoulos N, Nathanson R, Boukas S, Sampalis JS. Co-administration of ezetimibe and a statin in management of dyslipidemias: a meta-analysis of clinical trials. Arch Med Sci. 2009;5:347–363. [Google Scholar]
- 12.Mikhailidis DP, Sibbring GC, Ballantyne CM, Davies GM, Catapano AL. Meta-analysis of the cholesterol-lowering effect of ezetimibe added to ongoing statin therapy. Curr Med Res Opin. 2007;23:2009–2026. doi: 10.1185/030079907x210507. [DOI] [PubMed] [Google Scholar]
- 13.Charland SL, Malone DC. Prediction of cardiovascular event risk reduction from lipid changes associated with high potency dyslipidemia therapy. Curr Med Res Opin. 2010;26:365–375. doi: 10.1185/03007990903484802. [DOI] [PubMed] [Google Scholar]
- 14.Sharma M, Ansari MT, Abou-Setta AM, et al. Systematic review: comparative effectiveness and harms of combination therapy and monotherapy for dyslipidemia. Ann Intern Med. 2009;151:622–630. doi: 10.7326/0003-4819-151-9-200911030-00144. [DOI] [PubMed] [Google Scholar]
- 15.Ye Y, Zhao X, Zhai G, et al. Effect of high-dose statin versus low-dose statin plus ezetimibe on endothelial function: a meta-analysis of randomized trials. J Cardiovasc Pharmacol Ther. 2012;17:357–365. doi: 10.1177/1074248412449384. [DOI] [PubMed] [Google Scholar]
- 16.Ara R, Tumur I, Pandor A, et al. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Technol Assess. 2008;12 iii, xi-xiii:1–212. doi: 10.3310/hta12210. [DOI] [PubMed] [Google Scholar]
- 17.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG) Ezetimib bei Hypercholesterinämie. Abschlussbericht, Stand: 7/2011. www.iqwig.de/download/A10-02_Abschlussbericht_Ezetimib_bei_Hypercholesterinaemie.pdf. (last accessed on 9 September 2015)
- 18.Nussbaumer B, Mahlknecht P, Glechner A, et al. Efficacy and risk of harms of ezetimibe in hyperlipidemic patients with or without atherosclerosis and/or diabetes mellitus. PROSPERO. 2014 CRD42014013687. crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013687 (last accessed on 2 September 2015) [Google Scholar]
- 19.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 51.0 (updated March 2011) The Cochrane Collaboration. 2011 handbook.cochrane.org (last accessed on 9 September 2015) [Google Scholar]
- 20.Arimura T, Miura S, Ike A, et al. Comparison of the efficacy and safety of statin and statin/ezetimibe therapy after coronary stent implantation in patients with stable angina. J Cardiol. 2012;60:111–118. doi: 10.1016/j.jjcc.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Dagli N, Yavuzkir M, Karaca I. The effects of high dose pravastatin and low dose pravastatin and ezetimibe combination therapy on lipid, glucose metabolism and inflammation. Inflammation. 2007;30:230–235. doi: 10.1007/s10753-007-9041-3. [DOI] [PubMed] [Google Scholar]
- 22.Feldman T, Koren M, Insull W, Jr., et al. Treatment of high-risk patients with ezetimibe plus simvastatin co-administration versus simvastatin alone to attain National Cholesterol Education Program Adult Treatment Panel III low-density lipoprotein cholesterol goals. Am J Cardiol. 2004;93:1481–1486. doi: 10.1016/j.amjcard.2004.02.059. [DOI] [PubMed] [Google Scholar]
- 23.Gaudiani LM, Lewin A, Meneghini L, et al. Efficacy and safety of ezetimibe co-administered with simvastatin in thiazolidinedione-treated type 2 diabetic patients. Diabetes Obes Metab. 2005;7:88–97. doi: 10.1111/j.1463-1326.2004.00420.x. [DOI] [PubMed] [Google Scholar]
- 24.Meaney A, Ceballos G, Asbun J, et al. The VYtorin on carotid intima-media thickness and overall arterial rigidity (VYCTOR) study. J Clin Pharmacol. 2009;49:838–847. doi: 10.1177/0091270009337011. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Hirano M, Kitta Y, et al. A comparison of the efficacy of combined ezetimibe and statin therapy with doubling of statin dose in patients with remnant lipoproteinemia on previous statin therapy. J Cardiol. 2012;60:12–17. doi: 10.1016/j.jjcc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 26.West AM, Anderson JD, Meyer CH, et al. The effect of ezetimibe on peripheral arterial atherosclerosis depends upon statin use at baseline. Atheroscler. 2011;218:156–162. doi: 10.1016/j.atherosclerosis.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blazing M, Giugliano RP, Cannon CP, et al. Evaluating cardiovascular event reduction with ezetimibe as an adjunct to simvastatin in 18,144 patients after acute coronary syndromes: final baseline characteristics of the IMPROVE-IT study population. Am Heart J. 2014;168:205–212e1. doi: 10.1016/j.ahj.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Eng J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 29.Masuda J, Tanigawa T, Yamada T, et al. Effect of combination therapy of ezetimibe and rosuvastatin on regression of coronary atherosclerosis in patients with coronary artery disease. Int Heart J. 2015;56:278–285. doi: 10.1536/ihj.14-311. [DOI] [PubMed] [Google Scholar]
- 30.Falck-Ytter Y, Schünemann H, Guyatt G. AHRQ series commentary 1: rating the evidence in comparative effectiveness reviews. J Clin Epidemiol. 2010;63:474–475. doi: 10.1016/j.jclinepi.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 32.Higgins J, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 33.Merck S, Dohme C. IMPROVE-IT: Examining outcomes in subjects with acute coronary syndrome: vytorin (ezetimibe/simvastatin) vs simvastatin (P04103) clinicaltrials.gov/ct2/show/ NCT00202878. (last accessed on 9 February 2016) [Google Scholar]
- 34.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–#3121. [PubMed] [Google Scholar]
- 35.International Conference on Harmonisation (ICH) Guideline for good clinical practice E6(R1) Current step 4 version. 1996. www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (last accessed on 9 February 2016)
- 36.Bass A, Hinderliter AL, Lee CR. The impact of ezetimibe on endothelial function and other markers of cardiovascular risk. Ann Pharmacother. 2009;43:2021–2030. doi: 10.1345/aph.1M302. [DOI] [PubMed] [Google Scholar]
- 37.Slim H, Thompson PD. Ezetimibe-related myopathy: a systematic review. J Clin Lipidol. 2008;2:328–334. doi: 10.1016/j.jacl.2008.08.430. [DOI] [PubMed] [Google Scholar]
- 38.Pandor A, Ara RM, Tumur I, et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J Int Med. 2009;265:568–580. doi: 10.1111/j.1365-2796.2008.02062.x. [DOI] [PubMed] [Google Scholar]