Abstract
Proneural proteins of the class I/II basic Helix Loop Helix (bHLH) family are highly conserved transcription factors. Class I bHLH proteins are expressed in a broad number of tissues during development, while class II bHLH protein expression is more tissue restricted. Our understanding of the function of class I/II bHLH transcription factors in both invertebrate and vertebrate neurobiology is largely focused on their function as regulators of neurogenesis. Here, we show that the class I bHLH proteins Daughterless and Tcf4 are expressed in postmitotic neurons in Drosophila melanogaster and mice, respectively, where they function to restrict neurite branching and synapse formation. Our data indicates that Daughterless performs this function in part by restricting the expression of the cell adhesion molecule Neurexin. This suggests a role for these proteins outside of their established roles in neurogenesis.
Keywords: Daughterless, TCF4, NMJ, proneural, bHLH, Pitt-Hopkins, schizophrenia
Graphical Abstract

Introduction
Proneural proteins of the class I/II basic Helix Loop Helix (bHLH) family are transcription factors that are highly conserved from invertebrates to humans (reviewed in Guillemot, 2007; Powell and Jarman, 2008). Class I bHLH proteins are expressed in a broad number of tissues during development, while class II bHLH protein expression is more tissue restricted. Generally, class I bHLH proteins heterodimerize with class II bHLH proteins to activate gene expression (Powell and Jarman, 2008). However, class I bHLH proteins have also been reported to homodimerize (Cabrera and Alonso, 1991), and restrict (Lim et al., 2008) or activate (Tanaka-Matakatsu et al., 2014) gene expression. Class I bHLH proteins also form heterodimers with class V bHLH proteins of the Inhibitor of Differentiation (ID) family. This I/V interaction inhibits class I bHLH DNA binding and functions as a dominant negative interaction to compete against binding of type I factors with type II factors, thus preventing gene expression (Massari and Murre, 2000; Quednow et al., 2014).
Our current understanding of the function of class I/II bHLH transcription factors in both invertebrate and vertebrate neurobiology is largely focused on their function as master regulators of embryonic neurogenesis (Powell and Jarman, 2008). A wealth of literature shows that the expression of class I/II bHLH proteins is both necessary and sufficient to initiate programs that lead to the differentiation of neural stem/progenitor cells (NSCs/NPCs) (Guillemot, 2007). However, we are also beginning to appreciate the roles that class II bHLH proteins play in terminally differentiated cells. For example, in Drosophila, the class II bHLH protein Atonal functions to promote postmitotic neuron migration, axon guidance and arborization in the Dorsal Cluster neurons in the brain (Hassan et al., 2000). In mammals, the Atonal homolog Atoh1/Math1 is essential for migration of postmitotic retrotrapezoid nucleus neurons required for proper respiration (Huang et al., 2012). However, to date, there is no published finding on the function of any class I bHLH protein in postmitotic neurons in either vertebrates or invertebrates.
Mutations in Transcription Factor 4 (TCF4, a human class I bHLH protein) have been reliably identified in genome-wide association studies as a susceptibility risk factor for schizophrenia (Consortium, 2011; Stefansson et al., 2009; Steinberg et al., 2011), and have also been associated with Pitt-Hopkins syndrome (Amiel et al., 2007; Brockschmidt et al., 2007; Sepp et al., 2012; Zweier et al., 2007). It is unknown whether these diseases are due to defects in neurogenesis, in mature differentiated cells, or both. Literature suggests that disrupted neurogenesis and/or neural differentiation may be partly responsible. For example, Tcf4 is expressed widely in the nervous system during mouse embryonic development and is also expressed in germinal layers (dentate gyrus, subventricular zone, rostral migratory stream) of the postnatal mouse brain (Flora et al., 2007). Tcf4 homozygous mutant mice are viable with normal overall brain morphology, but only live for 1–6 days (Flora et al., 2007).
These diseases and neurocognitive disorders may also be a result of dysfunction in postmitotic/differentiated neurons. To further understand the contributions of type I bHLH proteins in postmitotic neurons, we analyzed the effect of altering daughterless (da, the only type I bHLH protein in Drosophila (Caudy et al., 1988)) and Tcf4 using two genetically tractable model systems, Drosophila melanogaster and Mus musculus.
In this study, we show that Da is expressed in the postmitotic motor neurons of the fly neuromuscular junction (NMJ) where it functions to restrict axonal arborization in these postmitotic cells. Our data indicates that Da performs this function by restricting the expression of neurexin. Similarly, we show that Tcf4 is also expressed in mouse brain postmitotic neurons, and that mouse Tcf4 also functions to restrict neurite branching/synaptogenesis and neurexin expression.
Experimental Procedures
Multilabeled Immunohistochemistry
The larval filet preparations and mouse brains were fixed with 4% paraformaldehyde. Then the tissue sections were immunostained with various antibodies for multiplex fluorescence evaluation. Additional details are found in the Supplemental Experimental Procedures.
Bioinformatic Analysis
To compare the gene sets, a collection of MATLAB scripts were written that utilized network programming and file operation concepts in addition to statistics. The ChIP-chip data for Da (MacArthur et al., 2009) and microarray data from SH-SY5Y cells for TCF4 (Forrest et al., 2013) were utilized. Additional details are found in the Supplemental Experimental Procedures.
Larval electrophysiological recording and behavioral analysis
Drosophila third instar larvae were prepared for two-electrode voltage clamp recordings. Segmental nerves were stimulated with a 1 Hz 10 V pulse. Recordings were digitized and analyzed by dividing the evoked excitatory junctional current (eEJC) amplitude area with the miniature excitatory junctional current (mEJC) area. All larval behavior was digitally recorded using a Sony DCR-SR47 Handycam with Carl Zeiss optics. Additional details are found in the Supplemental Experimental Procedures.
Microinjection of lentivirus in adult mouse brain
Four Tcf4 siRNA expression lentiviral vectors from Applied Biological Materials Inc were packaged in HEK293T cells. The lentiviruses were concentrated and purified by ultracentrifugation. Adult mice were anesthetized with avertin and mounted on a stereotaxic frame. The subventricular zone (SVZ) was targeted by stereotactic coordinates (from Bregma) at anterior-posterior (AP) +1.20 mm, medial-lateral (ML) ± 0.75 mm and dorsal-ventral (DV) −2.4 mm. One microliter of lentivirus was injected using a digital Micromanipulator. Additional details are found in the Supplemental Experimental Procedures.
Chromatin immunoprecipitation (ChIP) assay
Adult mouse NSCs/NPCs were cultured in matrigel-coated 10-cm culture dish with proliferation media for 3 days and then differentiation media for 3 days. Additional details are found in the Supplemental Experimental Procedures.
RESULTS
Daughterless is expressed in postmitotic neurons and functions to restrict axon arborization at the Drosophila NMJ
To investigate the role of da in postmitotic neurons, we determined whether Da protein was expressed in differentiated neural cells. We focused on the ventral nerve cord (VNC) of flies (Figure 1) and observed extensive Da protein expression by immunohistochemistry in most cells (Figures 1A–C) with an established Da antibody (Cronmiller and Cummings, 1993). We used the Gal4/UAS system to express a membrane tagged GFP with the elav-Gal4 driver to exclusively identify postmitotic neurons at this stage of development (Robinow and White, 1988, 1991; Yao and White, 1994) (Figures 1A, 1B). We observed significant expression of Da in the nuclei of multiple postmitotic neurons (Figures 1A–1C; S1A–S1C). In order to validate the specificity of Da expression in postmitotic neurons, we used RNA interference (RNAi) to knock down Da expression in postmitotic neurons by driving a short hairpin targeting da under UAS control (UAS-daRNAi1) with the elav-Gal4 driver that incorporates Dicer (UAS: Dcr2;;elav-Gal4). We observed an approximate 58% reduction in Da protein expression in the nuclei of elav positive VNC neurons in Da knockdown animals compared to WT controls (Figure 1G, inset in Figures 1A–1F). We further validated the efficiency of the knockdown by RT-qPCR analysis, and observed a roughly 54% decrease in da mRNA levels from whole 3rd instar larval brains compared to outcrossed controls (Figures 1H; S1G). For further validation of expression and knockdown, we utilized OK371-Gal4 as a second driver to express these transgenes in postmitotic glutamatergic motor neurons (Figure S1). We again observed a significant amount of Da protein expression within postmitotic glutamatergic motor neurons marked with this driver in 3rd instar larval VNCs (Figures S1A–S1C). Knocking down Da in these neurons led to an approximate 37% decrease in Da protein expression in situ (Figures S1D–S1G). Finally, to verify protein knockdown in whole brain tissue, we performed Western blot analysis from 3rd instar larval brains with altered Da expression in postmitotic neurons (using the elav-Gal4 driver). We observed a significant decrease in Da protein when Da was knocked down (Figures 1I–1J), and an increase in Da protein when overexpressed compared to controls (Figures 1I–1J). Taken together, these data suggest that Da is present in postmitotic, glutamatergic neurons in 3rd instar larval VNCs, and that the elav-Gal4 and OK371-Gal4 drivers significantly knock down Da protein expression in situ with differential efficiency.
Figure 1. Daughterless is expressed in postmitotic neurons of the VNC.

Confocal images of third instar VNC labeled with α-Da (red), DAPI (blue), and membrane bound GFP (green). Membrane bound GFP expressed by elav-GAL4 labels postmitotic neurons. (A–C) Wild type VNC. Arrows mark postmitotic neuron highlighted in inset. (D–F) VNC where Da expression is knocked down. Arrows mark postmitotic neuron highlighted in inset. (G) Intensity of Da immunofluorescence within GFP+ neurons normalized to Da immunofluorescence in GFP− neurons. (H) Percentage change of da mRNA levels in genotypes listed relative to UAS: Dcr2;;elav-Gal4 outcrossed control. (I) Western blot analysis of Da protein expression from third instar larval brains in control, overexpression, and knockdown genotypes. (J) Optical density quantification of Western blot relative to actin controls in each genotype. Error bars represent standard error. Scale bar is 10 μm.
In Drosophila, the motor neurons controlling the larval neuromuscular junction have their soma in the VNC of 3rd instar larval brains, and project axons out of the VNC that synapse with the musculature in the larval body wall. As Da is abundantly expressed in third instar postmitotic neurons throughout the VNC, we used the well-defined innervation of the third instar Drosophila NMJ muscle 6/7 of abdominal segment 3 to analyze the effects of Da alteration on synaptic development. We used RNAi to knock down Da protein expression and a UAS:da transgenic construct to overexpress Da protein in postmitotic motor neurons (elav-Gal4) and glutamatergic neurons (OK371-Gal4). We observed a significant increase in the number of synaptic boutons when we knocked down Da expression with both drivers compared to control NMJs (Figures 2A–2B, Table S1). There was a stronger effect with the UAS: Dcr2;;elav-Gal4 driver compared to the OK371-Gal4 driver, which is consistent with the observed efficiency of Da knockdown (Figures 1, S1). To verify the knockdown specificity, we performed three separate experiments. First, we simultaneously knocked down and overexpressed Da. With both drivers we observed a significant rescue in the number of boutons (Figure 2B, Table S1). Second, we used a separate independent RNAi construct to knock down Da, and again observed a significant increase in boutons compared to outcrossed controls (Table S1). Third, we expressed a nonspecific luciferase RNAi in postmitotic neurons and found no significant difference between luciferase RNAi and controls (Table S1). Conversely, when we overexpressed Da in postmitotic motor neurons we observed a significant decrease in total bouton number (Figures 2A–2B, Table S1).
Figure 2. Altered Da expression affects the number of synaptic boutons.
(A) Confocal images of third instar larval NMJs, muscles 6 and 7, labeled with α-HRP (white). Control, Da knockdown, and Da overexpression are labeled. (B) Quantification of the total number of boutons and 1s/1b subtype of bouton. Genotypes indicated. Responder corresponds to UAS mediated expression. Gal4 Driver corresponds to elav-Gal4 (Pan-Neural) or OK371-Gal4 (Glutamatergic). (C) Quantification of total number of branches and tertiary subtype branches. Error bars denote standard error in all cases. * indicates p<0.05. ** indicates p<0.01. *** indicates p<0.01. Scale bar is 10 μm.
Mature wild-type boutons are morphologically divergent consisting of 1s (small) and 1b (big) boutons. There was a significant increase in the number of both 1s and 1b boutons when we knocked down Da compared to controls (Figure 2B). Again, we observed rescue with Da (Figure 2B, Table S1) and obtained consistent data with a second independent RNAi line (Table S1). Conversely, we observed a significant decrease in both 1s and 1b when we overexpressed Da (Figure 2B, Table S1). Thus, Da affects both 1s and 1b boutons similarly.
Examination of the branch number in each genotype revealed a significant increase in overall axonal branching (Figures 2A, 2C), including the tertiary (Figures 2A, 2C) in motor neurons when we knocked down Da. We again observed rescue with Da (Figure 2C, Table S1) and similar results with an independent RNAi line (Table S1). Conversely, there was a significant decrease in overall (Figures 2A, 2C, Table S1) and tertiary axonal branching (Figures 2A, 2C, Table S1) when Da was overexpressed. Taken together, these data suggest that Da functions in postmitotic neurons to restrain axonal branching and synaptic growth of the NMJ.
Daughterless regulates active zone number and localization, synaptic transmission and locomotion behavior
We next determined whether perturbation of Da in postmitotic neurons affected neuronal function. Each bouton contains several uniformly spaced active zones where presynaptic vesicles are docked and released (Kittel et al., 2006a). These active zones are associated with electron-dense cytomatrices called T-bars (Zhai and Bellen, 2004), which are readily marked by the presynaptic T-bar associated protein Bruchpilot (Brp), a homolog of mammalian active zone protein ELK/CAST (Kittel et al., 2006b). We assessed whether Brp expression was changed in postmitotic neurons when Da expression was altered. We observed a significant increase in the total number of active zones (Figures S2A–S2B) with Da knockdown in postmitotic neurons using UAS: Dcr2;;elav-Gal4. However, there was no significant increase in the active zone number with Da knockdown using OK371-Gal4 (Figure S2B), suggesting that a stronger knockdown is required for this effect in neurons. Similarly, there were no significant differences in active zone density when using either driver to knock down Da (Figure S2C). Further, overexpression of Da did not result in any significant difference in active zone number (Figures S2A–S2B) or density (Figure S2C).
Interestingly, we did observe a mislocalization of Brp in postmitotic axons when we overexpressed Da (arrow and inset in Figure S2A). While Brp puncta are normally restricted to presynaptic boutons in normal postmitotic axons, Brp puncta appear localized in the motor axon proper (arrow and inset in Figure S2A) in postmitotic neurons overexpressing Da. These data suggest that there is no functional defect in Brp biosynthesis in these neurons when Da is overexpressed. Thus, the increase in Brp after Da knockdown is most likely due to the increase in the number of overall boutons, which were higher in animals when elav-Gal4 is used (significant increase in active zones) and lower in animals when OK371-Gal4 is used (no significant increase in active zones).
Given the defects we observed in bouton number, branching, and active zone localization when we altered Da levels in postmitotic neurons, we next analyzed synaptic transmission and larval locomotor behavior in these animals. We analyzed neurotransmission at muscle 6 of abdominal segment 3 or 4 using two-electrode voltage clamp. Overexpression of Da in glutamatergic postmitotic neurons led to a significant decrease in evoked eEJC amplitudes (Figures 3A, 3C) and mEJC frequency (Figures 3B–3C). Although we observed a trend for decreased mEJC amplitudes, this was not statistically significant (p = 0.14, Figure 3C) and there was no significant change in quantal content after Da overexpression (data not shown). These results suggest that the significant decrease in branching and boutons we observed when we overexpressed Da in motor neurons leads to a significant decrease in neurotransmission at these synapses.
Figure 3. Altered Da expression affects synaptic transmission and behavior.
(A) Upper: Representative eEJC amplitudes obtained after stimulation of the presynaptic motor neuron. Lower: Sample traces of spontaneous activity. (B) Sample traces of spontaneous activity for a one min period. (C) Quantification of mEJC amplitudes, mEJC amplitudes, and mEJC frequency. (D) Total number of larval contractions per 30 seconds in different genotypes. Genotypes are indicated. Responder corresponds to UAS mediated expression. Gal4 Driver corresponds to elav-Gal4 (Pan-Neural) or OK371-Gal4 (Glutamatergic). Error bars denote standard error in all cases. * indicates p<0.05. ** indicates p<0.01.
Interestingly, knockdown of Da did not significantly affect neurotransmission compared to outcrossed controls (Figure S2D–F), although we did observe a trend for decreased mEJC frequency (Figure S2D). Taken together, these data suggest that although the NMJ synapse is overelaborated when Da is knocked down in postmitotic motor neurons, these synapses may not be functioning at their full capacity. Therefore, we do not observe any significant increase or hyperactivity in overall synaptic transmission at these synapses. To directly validate this observation, we analyzed behavioral output of the larvae by measuring larval contraction after we knocked down and overexpressed Da in postmitotic neurons. Larval contraction relies on proper glutamatergic neurotransmission and is significantly affected by mutations that affect transmission (Sandstrom, 2004). Thus, altered behavior after knock down Da in postmitotic neurons would be consistent with decreased synaptic transmission overall in the animal. We observed a significant decrease in larval contractions when we both knocked down and overexpressed Da in postmitotic neurons compared to outcrossed control (Figure 3D). Taken together, these data suggest that misexpression of Da leads to decreased larval locomotion behavior.
Da restriction of axonal branching and synapse formation is mediated by Neurexin expression
Da binds DNA at E box (CANNTG) consensus sequences to control transcription (Cabrera and Alonso, 1991; Cronmiller and Cummings, 1993; Massari and Murre, 2000). Therefore, we hypothesized that Da function in postmitotic neurons may be transcriptional. In an initial attempt to generate a tractable list of bona fide Da target genes we could analyze at the larval NMJ, we used published data sets from two large-scale genome-wide analyses involving Drosophila Da and human TCF4 (Figure S3A). From these analyses, we identified 44 candidate genes that were specifically expressed in the fly NMJ. We sorted this list of 44 genes into categories relevant for neurotransmission, microtubule associated proteins, axon guidance, cell adhesion, and vesicle associated proteins to smaller overlapping profiles (Figure S3A).
To validate this approach, we performed RT-qPCR to analyze the relative mRNA levels of the neurexin (nrx-1) transcripts in larval brains when we knocked down and overexpressed Da (Figure S3B). nrx-1 encodes a cell surface adhesion molecule, and is the only functional Neurexin protein in fly neurons (Zeng et al., 2007). We chose nrx-1 because previous literature demonstrated that nrx-1 mutants exhibited significantly fewer boutons at the 6/7 fly larval NMJ while increased Nrx-1 protein expression significantly increased the number of boutons (Li et al., 2007). This is exactly the opposite of what we observe with Da, where decreased Da leads to increased boutons and increased Da leads to decreased boutons (Figure 2). Further, human NRXN1 is a known transcriptional target of TCF4 in HEK293 cells (Forrest et al., 2012), and fly nrx-1 is a direct transcriptional target of Da in Drosophila embryos (MacArthur et al., 2009). Finally, TCF4 is associated with schizophrenia (Consortium, 2011; Stefansson et al., 2009; Steinberg et al., 2011), as is NRXN1 (Rujescu et al., 2009).
We observed a 3.21-fold increase in nrx-1 mRNA in brains where Da was knocked down (UAS: Dcr2;;elav-Gal4, Figure S3B). Conversely, we observed an 86% decrease in nrx-1 mRNA levels in brains where Da was overexpressed (Figure S3B). The Nrx-1 protein is located pre-synaptically, interacts with neuroligin on the post-synaptic membrane at the synapse, and is important for synapse formation, synaptic growth, synaptic transmission and function in Drosophila (Li et al., 2007; Zeng et al., 2007). Because of the importance of Nrx-1 at the pre-synapse specifically, we analyzed the relative expression of the Nrx-1 protein at the axonal terminal boutons in animals with altered Da expression. We observed a significant increase in Nrx-1 immunolocalization in terminal axonal boutons when we knocked down Da using the elav-Gal4 driver (Figures 4B, 4E, 4H, 4K, 4M) compared to outcrossed controls (Figures 4A, 4D, 4G, 4J, 4M). Conversely, we observed a slight decrease in Nrx-1 immunolocalization in axonal terminal boutons when we overexpressed Da (Figures 4C, 4F, 4I, 4L, 4M). To confirm the decrease in Nrx-1 immunostaining, we utilized a nrx-1 GFP reporter construct to analyze the relative nrx-1 mediated GFP fluorescence in the VNC (Figure S3C). We observed a significant increase in this GFP reporter when we knocked down Da using elav-Gal4 (Figures S3D, S3F). Conversely, we observed a slight decrease in GFP reporter when we overexpressed Da (Figures S3E, S3F). These data are consistent with our observations of Nrx-1 protein expression in terminal boutons when Da is altered, and suggest that Da functions as a repressor of nrx-1 transcription in postmitotic neurons.
Figure 4. Da phenotypes are mediated by Neurexin.
(A–L) Confocal images of third instar larval NMJs, muscle 6 and 7, labeled with α-Nrx1 (red) and α-HRP (green). Control, Da knockdown, and Da overexpression are labeled. (G–L) High magnification of boxed area in (A–C). Arrows indicate boutons where Nrxn1 expression is normally highest. (M) Relative abundance of Nrxn1 immunofluorescence in genotypes indicated in arbitrary units. (N) Total number of boutons, (O) bouton subtypes, and (P) active zones listed for control and experimental genotypes. Magenta indicates Neurexin-1 rescue genotype (UAS: Dcr2; UAS:daRNAi1/UAS:Nrx1RNAi; elav-Gal4). Error bars denote standard error in all cases. * indicates p<0.05. ** indicates p<0.01.
Because we observed increased Nrx-1 immunolocalization and nrx-1 GFP reporter expression in terminal boutons after Da knockdown, we determined if knockdown of Nrx-1 in a background where Da was also knocked down could potentially rescue the increased bouton phenotype we observed. We co-expressed both da and nrx-1 RNAi constructs in postmitotic neurons of the fly NMJ. Knock down of Nrx-1 in cells where Da was also knocked down suppressed the significant increase in boutons (Figure 4N), bouton subtypes (Figure 4O) and active zone numbers (Figure 4P). Taken together, these data suggest that the increased bouton and active zone phenotype in animals where Da was knocked down may be partially mediated by increased nrx-1 expression in fly motor neurons and increased localization of Nrx-1 protein at terminal boutons in these animals.
Da homodimers mediate synapse restriction via repression of Neurexin
Generally, class I bHLH proteins heterodimerize with other bHLH proteins to activate gene expression (Powell and Jarman, 2008). However, class I bHLH proteins have also been reported to homodimerize (Cabrera and Alonso, 1991). Our lab has previously shown that Da can function as both an activator and a repressor on cis-regulatory elements in the atonal gene in the same cells at a different time in development (2 hours later) (Melicharek et al., 2008). Da repression of gene expression has been shown by others as well (Andrade-Zapata and Baonza, 2014). It is hypothesized that an increase in Da homodimers would mediate this repression and mimic Da overexpression phenotypes (Andrade-Zapata and Baonza, 2014; Bhattacharya and Baker, 2011). Therefore, we hypothesized that Da homodimers would mimic the phenotypes we observe with Da overexpression alone. Recently, peptide-linked Da homodimers were created and can be expressed in a tissue specific fashion using Gal4/UAS (Tanaka-Matakatsu et al., 2014). We expressed these homodimers specifically in postmitotic neurons and analyzed their effect on axon arborization at the Drosophila NMJ. Similar to Da overexpression, we observed a significant decrease in total bouton number compared to controls (Figure S4A–S4C), decreased 1s and 1b boutons (Figure S4D), decreased total branch number (Figure S4E), and decreased tertiary branch numbers (Figure S4F). We then analyzed the expression of nrx-1 in situ using the nrx1 GFP reporter construct; however, we did not obtain any viable 3rd instar larvae (0/144) of the appropriate genotype (elav-Gal4;; nrx-1 GFP/UAS:Da-Da), suggesting that we may be decreasing nrx-1 expression to such an extent in the nervous system in this genetic background as to induce nrx-1 mediated loss-of-function lethality. Taken together, these data suggest that Da homodimers mediate (in part) the decreased number of boutons associated with Da overexpression, and suggest a potential mechanism by which Da controls axonal branching and synapse formation in these neurons.
The mammalian Da homolog Tcf4 is expressed in postmitotic neurons and restricts neurite branching
We next determined if this observation was conserved outside of invertebrates. Da, and its mammalian ortholog, Tcf4, share share conserved functions which were recently highlighted by the finding that expression of human TCF4 can rescue loss of function da mutant phenotypes in Drosophila (Tamberg et al., 2015). To explore the postmitotic neuronal function of Tcf4 in mammals, we first tested for specificity of Tcf4 antibody and efficiency of Tcf4 siRNA knockdown in mouse NSCs/NPCs using Western blot and RT-PCR analysis (Figure S5). We observed significant knockdown of both Tcf4 mRNA and Tcf4 protein with multiple siRNAs targeting mouse Tcf4, suggesting that both the Tcf4 antibody and siRNA knockdown for Tcf4 are effective.
To interrogate the function of Tcf4 in mammalian postmitotic neurons, we first evaluated the cellular expression pattern of Tcf4 immunoreactivity in adult mouse brain tissues (Figures 5, S6). We observed that Tcf4 protein is highly expressed in some but not all postmitotic neurons throughout the adult mouse brain, predominantly in the prefrontal cortex and limbic system (i.e. hippocampus, amygdala, data not shown). Double-labeled immunofluorescent confocal imaging analysis showed punctate staining of Tcf4 in the soma and neurites of mature dopaminergic tyrosine hydroxylase (TH) positive neurons (Figure S6A) and glutamatergic (vGlut2) postmitotic neurons (Figure S6C). We observed both nuclear and cytoplasmic localization of Tcf4 (Figures 5, S6), which is consistent with what we observed in postmitotic neurons in the fly. We also observed only nuclear staining in some neurons or only cytoplasmic staining in most neurons (Figures 5, S6). In particular, the majority of Tcf4 immunoreactivity was found in the neuronal cytoplasmic soma and dendrites of adult mouse brains (Figure 5A). Multilabeled immunofluorescent histochemistry validated that Tcf4 expression was located predominantly in MAP2-positive soma and dendrites but rarely colocalized with axonal presynaptic markers synaptophysin, NF-M and vGlut2 (Figures 5, S6). Taken together, these data suggest that Tcf4 is both nuclear and cytoplasmic in mammalian postmitotic neurons and localized mainly to the postsynaptic soma and dendrites.
Figure 5. Tcf4 is expressed in the soma and dendrites of postmitotic neurons in adult mouse brain detected by multilabeled fluorescent immunohistochemistry.
Representative confocal single stack (A–E) and maximized images (F) showing Tcf4 expression in the cytoplasmic soma and dendrites of postmitotic neurons in prefrontal cortex (A) and its complete colocalization (Arrows) with postsynaptic MAP2 (B, D) but absence of colocalization with presynaptic Synaptophysin (C, E). (F) is the maximized image of 15 stacks separated by 0.5 μm thickness.
To test the hypothesis that Tcf4 also functions to restrict neurite branching in mammalian postmitotic neurons, we used lentiviral transduction to knock down Tcf4 in NSCs/NPCs derived from the SVZ of adult mouse brains. We then differentiated these cells into neurons (co-labeled with Tuj1) and non-neuronal cells, and after 5 days assessed morphological changes of differentiated postmitotic neurons (Figures S7A–S7B). We observed a significant increase in total neurite length (Figure S7C), although the longest neurite length was not significantly affected (Figure S7D). We also observed a significant increase in the total number of neurite branches (Figure S7E). We analyzed the type of neurite branch for each neuron, and found a significant increase in the number of secondary branches (Figure S7F). This is similar to the phenotypes at the Drosophila NMJ, where we observed a significant increase in the number of tertiary branches. Overexpression of Tcf4 in NSCs/NPCs led to significant lethality with decreased GFP expression. Taken together, these data suggest that Tcf4, like its Drosophila ortholog, also functions to restrict neurite branching in postmitotic neurons in vitro.
To validate our in vitro findings for Tcf4 in restricting neurite branching, we used lentiviral transduction to knock down Tcf4 in the postmitotic interneurons of adult mouse olfactory bulb. To ensure that we were analyzing the morphology of mature neurons that were postmitotic, we selected 1 month time-point after lentivirus microinjection and analyzed the morphology of newly born olfactory interneurons that have differentiated and matured from the NPCs and neuroblast cells migrated from the SVZ to the olfactory bulb in adult mouse brains (Geraerts et al., 2006). We utilized this model for multiple reasons. First, lentivirus-mediated stable Tcf4 knockdown does not alter the ability of these labeled neural cells to undergo neurogenesis, or migrate to their proper locations, but could still potentially have an effect on the neurite branching and functional integration of the differentiated postmitotic neurons (Geraerts et al., 2006). Second, the dendrites from these particular neurons form pre-synaptic connections to release neurotransmitter, allowing us to continue to analyze pre-synaptic structures that are also post-mitotic (Egger et al., 2005). However, we cannot completely rule out potential effects on NPCs using this technique.
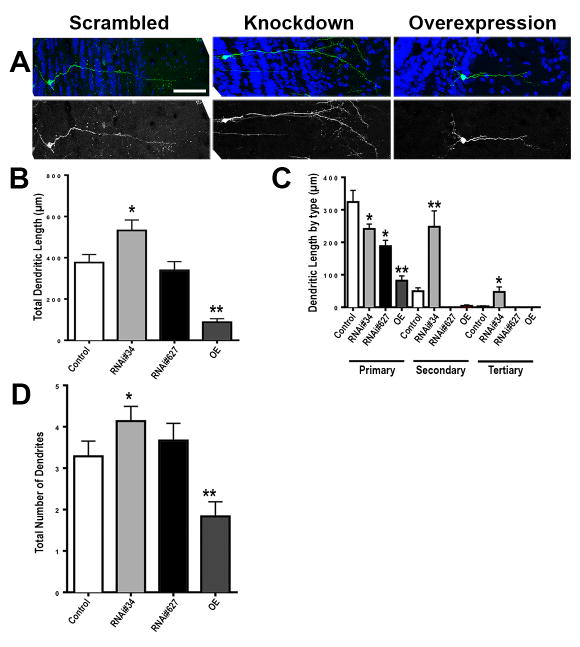
We observed an increase in total dendritic length and dendritic length by type when we knocked down Tcf4 in olfactory interneurons using Tcf4#34 in vivo (Figure 6B), and observed an increase in the total number of dendrites as well (Figure 6D). Though we did not observe an increase in total dendritic length when we knocked down Tcf4 using Tcf4#627, we did observe a significant decrease in dendritic length by type, and a trend for increased total dendritic number. We did not observe any significant difference in the total numbers of dendritic spines in any case (not shown).
Figure 6. TCF4 knockdown promotes but overexpression represses neurite branching in mouse mature olfactory neurons in vivo.
(A) Representative mature olfactory interneurons of the mouse olfactory bulb. (B–D) quantitative analyses of (B) total dendritic length, (C) total dendritic length by type, and (D) total number of dendrites. Nucleus is labeled in blue. GFP is labeled in green. Scrambled control is labeled as control, Tcf4 knockdown is labeled RNAi #, and Tcf4 overexpression is labeled as OE in all cases. Error bars denote standard error in all cases (n=4–6 animals in each case). * indicates p<0.05. ** indicates p<0.01. Scale bar = 50 μm.
Conversely, when we overexpressed Tcf4, we observed a significant decrease in total dendritic length (Figure 6B), dendritic length by type (Figure 6C), and total number of dendrites (Figure 6D). Taken together, these data are consistent with the phenotypes we observe in Drosophila when we decrease and increase Da expression and suggest that Tcf4 also functions to restrict dendritic branching and dendritic length in mammalian postmitotic neurons in vivo.
Neurexin is a direct target of Tcf4 in mammalian postmitotic neurons
In order to determine if Tcf4 also regulates Neurexin (Nrxn1) in mammals, we used lentivirus-mediated siRNA to knock down Tcf4 in differentiated neural cells derived from NSCs/NPCs and analyzed Nrxn1a expression by RT-qPCR using primers specifically targeting Nrxn1a (Treutlein et al., 2014; Ushkaryov et al., 1994), which is homologous to Drosophila nrx-1 (Zeng et al., 2007). We observed a significant increase in Nrxn1a mRNA levels in the differentiated neural cells but a significant decrease in the undifferentiated NSCs/NPCs upon Tcf4 knockdown as compared to scrambled siRNA controls (Figure 7A). We also observed significant increase in Nrxn1b and Nrxn2 mRNA expression but decrease in Nrxn3 mRNA expression after Tcf4 knockdown in differentiated cells (Figures 7A, 7B). However, Nrxn1b and Nrxn2 exhibited slightly increased expression while Nrxn3 showed no significant change after Tcf4 knockdown in the proliferating NSCs/NPCs (Figures 7A, 7B). During the neural cell differentiation process (the scrambled control), the mRNA expression of Nrxn1b, Nrxn2, and Nrxn3 significantly increased but the Nrxn1a expression remained unchanged (Figures 7A, 7B). These data suggest that Tcf4 in postmitotic neural cells suppresses Nrxn1a, Nrxn1b and Nrxn2 but promotes Nrxn3 transcription. In proliferating NSCs/NPCs, Tcf4 is required to maintain Nrxn1a expression but suppresses Nrxn1b and Nrxn2 transcription. These observations are similar to what we observe in Drosophila, with respect to Nrxn1 and Nrxn2, where the expression of these genes increases after Tcf4 knockdown.
Figure 7. Endogenous Tcf4 suppresses Neurexin1 (Nrxn1) transcription via direct binding to its promoter in mouse differentiated neural cells.
(A, B) RT-qPCR analysis showing Tcf4 knockdown increased mRNA expression of Nrxn1a, Nrxn1b and Nrxn2 but decreased Nrxn3 in NSCs/NPCs under differentiation condition, whereas Tcf4 knockdown in proliferating NSCs/NPCs decreased Nrxn1a but increased Nrx1b and Nrxn2. Data represent fold changes in the mRNA levels of Nrxns in the indicated group relative to the Scramble siRNA control group in proliferation media. (C–G) Chromatin immunoprecipitation (CHIP)-qPCR assay showing the binding of Tcf4 to various promoter regions of Nrxn1. (C) Diagram illustration of the 2-kb promoter regions of Nrxn1a and Nrxn1b and corresponding regions covered by PCR primers. TSS, transcription start site. The representative end-point PCR product is shown in DNA agarose gel (D, E) and quantitative SYBR Green-based PCR data are shown as fold enrichment relative to IgG control (F) or melting curve analysis due to absence of Ct value in the IgG control (G). NSCs/NPCs from adult mouse brain were cultured in proliferation (E) or differentiation (D) media for 3 days followed by CHIP-qPCR assay. ID2 is used as positive control for the CHIP assay. * p<0.05 and ** p<0.01 indicate statistical significance compared with corresponding Scramble siRNA (A, B) or IgG control (F). ++ p<0.01 indicates a statistical significance compared with corresponding Scramble control in proliferation media (A, B).
Because Da has been previously shown to bind cis-regulatory elements of the nrx-1 locus in Drosophila (MacArthur et al., 2009), we determined if Tcf4 also binds to potential cis-regulatory elements of mammalian Nrxn1. We used ChIP to analyze four areas surrounding the promoters of both the Nrxn1a and Nrxn1b genes (Figure 7C) (Treutlein et al., 2014; Ushkaryov et al., 1994) in postmitotic neurons differentiated from adult mouse NSCs/NPCs. We utilized the ID2 promoter as a positive control for Tcf4 binding (Figure 7D–7F) (Ghosh et al., 2014). We observed a significant enrichment of Tcf4 occupancy to various extent at all four promoter/enhancer regions of the Nrxn1a and Nrxn1b genes compared to IgG controls (Figures 7C–7G). Taken together, these data are consistent with the data observed in Drosophila, and suggest that Tcf4 binds to Nrxn1a and Nrxn1b cis-regulatory elements in postmitotic mammalian neurons to regulate Neurexin transcription.
Discussion
The essential role of the proneural proteins Da/Tcf4 in neurogenesis has been long established (Guillemot, 2007; Powell and Jarman, 2008). Here we show that both Da and Tcf4 appear to function in a similar, unexpected fashion in postmitotic neurons by restricting the number of neurite branches. We show that Da functions to restrict axonal arborization in postmitotic neurons at the fly NMJ by repressing nrx-1 expression. We suggest that Da accomplishes this repression, at least in part, through homodimerization. We further show that the Da homolog, Tcf4, also functions to restrict neurite growth and branching in mouse postmitotic neurons. Tcf4 binds to Nrxn cis-regulatory elements in postmitotic neurons, and knockdown of Tcf4 in postmitotic neurons leads to increased Nrxn mRNA expression.
How Da and Tcf4 accomplishing this restriction of synapses in post-mitotic neurons is currently unknown. This may be accomplished either through altered synaptic pruning of postmitotic axons and dendrites and/or may be accomplished by altering synapse development during circuit formation in these cells. This will require further experimentation to fully resolve. However, our data may indicate that the primary defect in postmitotic neurons is a defect in branching with a secondary defect in presynaptic bouton number. The fact that we do not observe a significant effect in neurotransmission when we knock down Da in postmitotic neurons, as well as the fact that Da overexpression does not alter active zone number would be consistent with a defect that is primarily in branching. However, we do observe a significant effect on larval locomotion behavior when we knock down Da. The disparity between the electrophysiology and behavioral data is likely due to differences in the number of cells involved in each response. While electrophysiology measures only the activity of two segmental motor neurons and a single postsynaptic muscle, larval locomotion requires the cumulative activity of several types of neurons in addition to postsynaptic muscles. The segmental motor neuron activity that induces muscle contraction is activated by presynaptic cholinergic neurons, limited in duration by interneurons, and coordinated between segments by sensory neurons. Therefore, changing the levels of Da in all neurons using elav-Gal4 could affect locomotion by affecting the neurons upstream of segmental motor neurons leading to behavioral phenotypes.
We suggest that Da homodimers mediate nrx-1 expression in postmitotic motor neurons. This does not rule out the possibility, however, that Da may also require heterodimerization partners to function normally in postmitotic neurons. For example, in postmitotic neurons the Id protein promotes neurite elongation by heterodimerizing and suppressing the activities of other neurogenic bHLH transcription factors such as NeuroD (Iavarone and Lasorella, 2006). By knocking down Da/Tcf4 we may phenocopy Id repression, whereby neurogenic bHLH factors fail to heterodimerize and bind to DNA due to de facto increased Id over-expression. This may lead to neurite elongation observed both in vivo and in vitro.
The discovery of Tcf4 in postmitotic neurons opens up additional possibilities for the role this protein may play in the cells and in human diseases associated with TCF4. Common small nucleotide polymorphisms in TCF4 are associated with schizophrenia (Consortium, 2011; Quednow et al., 2014; Shi et al., 2009; Stefansson et al., 2009; Steinberg et al., 2011), while loss of heterozygosity in TCF4 is associated with Pitt-Hopkins syndrome (Amiel et al., 2007; Brockschmidt et al., 2007; Sepp et al., 2012; Zweier et al., 2007). Further understanding TCF4 function in postmitotic neurons could have a profound impact on our understanding of the etiology of these diseases. For example, if TCF4 functions to regulate changes in gene expression required for synaptic pruning or plasticity in mature neurons, then the manipulation of endogenous TCF4 function and/or identification of TCF4 related gene expression in postmitotic neurons in diseases might open up novel therapeutic avenues for treatment of these disorders.
In addition to TCF4, variants in NRXN1 predispose to schizophrenia and autism spectrum disorders (Duong et al., 2012; Kirov et al., 2009). Interestingly, when Nrxn1 is specifically knocked out in adult neurons in transgenic mice, these mice exhibit autism-like behaviors including increased self-grooming and deficits in social interactions (Rabaneda et al., 2014), linking loss of Nrxn1 function to adult, postmitotic neurons. Recent studies suggest that TCF4 and members of the Neurexin family may share a common signaling pathway and that Nrxn1 is a transcriptional target of TCF4 (MacArthur et al., 2009). In Drosophila, loss-of-function mutations in nrx-1 lead to fewer boutons, altered active zone apposition, and disrupted synaptic transmission (Li et al., 2007; Zeng et al., 2007). Conversely, overexpression of Nrx-1 lead to increased bouton numbers (Li et al., 2007). In our model, we observe that reduced levels of Da lead to an increase in the levels of Nrx-1 protein, the only Neurexin in Drosophila. In mouse, we found Tcf4 binds to the promoter of both Nrxn1a and Nrxn1b and suppresses Nrxn1a and Nrxn1b expression in the differentiated neural cells. We also observed similar effect in Nrxn2 by Tcf4 knockdown. However, we found that Nrxn3 expression is downregulated by Tcf4 knockdown in differentiated neural cells. This may reflect the distinctive function of Nrxn3 compared to Nrxn1 and Nrxn2 (Aoto et al., 2015). The shared expression patterns and synaptic localization of Nrx-1 in Drosophila and Nrxn1 in mammallian postmitotic neurons, and the highly conserved trans-synaptic function of neurexins at the synapse, suggests that Nrx-1/Nrxn1 may partially mediate Da/TCF4 phenotypes and that this mechanism might be evolutionarily conserved.
In summary, we propose that Da/TCF4 function in postmitotic neurons is important for properly sculpting appropriate neuronal circuitry in the nervous system. Impaired Da/TCF4 function in these neurons can lead to defects in neural circuit formation, synaptic transmission, and behavior. This research opens up the possibility of further exploring how Da/TCF4 affects circuit formation in postmitotic neurons, and may also enhance our understanding of the pathology of human disorders associated with altered TCF4 gene expression.
Supplementary Material
Acknowledgments
We thank M. Akins, V. Auld, W. Du, J. Bethea, H. Bellen, M. Bhat, C. Cronmiller, A. DiAntonio, D. Featherstone, A. Saunders, Y. Wairkar, the Bloomington Stock Center, Vienna Drosophila Stock Center, and the Developmental Studies Hybridoma Bank for fly stocks, antibodies and reagents; Drexel CIC for imaging analysis and assistance, and Marenda lab members for helpful discussion and comments; Fang Li in Hu lab for technical support. This work was sponsored by a grant from the Pitt Hopkins Research Foundation and the Orphan Disease Center at the Perelman School of Medicine at the University of Pennsylvania (to DRM and WH). Work in the Hu laboratory is supported by the National Institute of Health R01MH101041 (to WH). Work in the Marenda lab is supported by the National Science Foundation IOS 1256114 (to DRM).
Footnotes
Author Contributions: Conceptualization, M.D., W.H., D.R.M.; Methodology, M.D., T.Z., Y.Z., W.H., D.R.M; Software, C.S, M.D.; Validation, M.D., T.Z., E.A.W., Y.Z., M.S.; Formal Analysis, M.D., T.Z., Y.Z., F.L., W.H., D.R.M.; Investigation, M.D., T.Z., E.A.W., Y.Z. M.S., T.H., M.N., K.K., F.L.; Writing – Original Draft, M.D., Y.Z., W.H., D.R.M; Writing – Review & Editing, M.D., T.Z., E.A.W., Y.Z., F.L., W.H., D.R.M.; Supervision, W.H., D.R.M.; Project Administration, W.H., D.R.M.; Funding Acquisition, F.L., W.H., D.R.M.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amiel J, Rio M, de Pontual L, Redon R, Malan V, Boddaert N, Plouin P, Carter NP, Lyonnet S, Munnich A, et al. Mutations in TCF4, encoding a class I basic helix-loop-helix transcription factor, are responsible for Pitt-Hopkins syndrome, a severe epileptic encephalopathy associated with autonomic dysfunction. Am J Hum Genet. 2007;80:988–993. doi: 10.1086/515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Zapata I, Baonza A. The bHLH factors extramacrochaetae and daughterless control cell cycle in Drosophila imaginal discs through the transcriptional regulation of the Cdc25 phosphatase string. PLoS Genet. 2014;10:e1004233. doi: 10.1371/journal.pgen.1004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoto J, Foldy C, Ilcus SM, Tabuchi K, Sudhof TC. Distinct circuit-dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat Neurosci. 2015;18:997–1007. doi: 10.1038/nn.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya A, Baker NE. A network of broadly expressed HLH genes regulates tissue-specific cell fates. Cell. 2011;147:881–892. doi: 10.1016/j.cell.2011.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockschmidt A, Todt U, Ryu S, Hoischen A, Landwehr C, Birnbaum S, Frenck W, Radlwimmer B, Lichter P, Engels H, et al. Severe mental retardation with breathing abnormalities (Pitt-Hopkins syndrome) is caused by haploinsufficiency of the neuronal bHLH transcription factor TCF4. Hum Mol Genet. 2007;16:1488–1494. doi: 10.1093/hmg/ddm099. [DOI] [PubMed] [Google Scholar]
- Cabrera CV, Alonso MC. Transcriptional activation by heterodimers of the achaete-scute and daughterless gene products of Drosophila. EMBO J. 1991;10:2965–2973. doi: 10.1002/j.1460-2075.1991.tb07847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy M, Vassin H, Brand M, Tuma R, Jan LY, Jan YN. daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell. 1988;55:1061–1067. doi: 10.1016/0092-8674(88)90250-4. [DOI] [PubMed] [Google Scholar]
- Consortium TSPGWASG. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronmiller C, Cummings CA. The daughterless gene product in Drosophila is a nuclear protein that is broadly expressed throughout the organism during development. Mech Dev. 1993;42:159–169. doi: 10.1016/0925-4773(93)90005-i. [DOI] [PubMed] [Google Scholar]
- Duong L, Klitten LL, Moller RS, Ingason A, Jakobsen KD, Skjodt C, Didriksen M, Hjalgrim H, Werge T, Tommerup N. Mutations in NRXN1 in a family multiply affected with brain disorders: NRXN1 mutations and brain disorders. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2012;159B:354–358. doi: 10.1002/ajmg.b.32036. [DOI] [PubMed] [Google Scholar]
- Egger V, Svoboda K, Mainen ZF. Dendrodendritic synaptic signals in olfactory bulb granule cells: local spine boost and global low-threshold spike. J Neurosci. 2005;25:3521–3530. doi: 10.1523/JNEUROSCI.4746-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora A, Garcia JJ, Thaller C, Zoghbi HY. The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proc Natl Acad Sci U S A. 2007;104:15382–15387. doi: 10.1073/pnas.0707456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest M, Chapman RM, Doyle AM, Tinsley CL, Waite A, Blake DJ. Functional analysis of TCF4 missense mutations that cause Pitt-Hopkins syndrome. Hum Mutat. 2012;33:1676–1686. doi: 10.1002/humu.22160. [DOI] [PubMed] [Google Scholar]
- Forrest MP, Waite AJ, Martin-Rendon E, Blake DJ. Knockdown of human TCF4 affects multiple signaling pathways involved in cell survival, epithelial to mesenchymal transition and neuronal differentiation. PLoS One. 2013;8:e73169. doi: 10.1371/journal.pone.0073169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraerts M, Eggermont K, Hernandez-Acosta P, Garcia-Verdugo JM, Baekelandt V, Debyser Z. Lentiviral vectors mediate efficient and stable gene transfer in adult neural stem cells in vivo. Hum Gene Ther. 2006;17:635–650. doi: 10.1089/hum.2006.17.635. [DOI] [PubMed] [Google Scholar]
- Ghosh HS, Ceribelli M, Matos I, Lazarovici A, Bussemaker HJ, Lasorella A, Hiebert SW, Liu K, Staudt LM, Reizis B. ETO family protein Mtg16 regulates the balance of dendritic cell subsets by repressing Id2. J Exp Med. 2014;211:1623–1635. doi: 10.1084/jem.20132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot F. Spatial and temporal specification of neural fates by transcription factor codes. Development. 2007;134:3771–3780. doi: 10.1242/dev.006379. [DOI] [PubMed] [Google Scholar]
- Hassan BA, Bermingham NA, He Y, Sun Y, Jan YN, Zoghbi HY, Bellen HJ. atonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron. 2000;25:549–561. doi: 10.1016/s0896-6273(00)81059-4. [DOI] [PubMed] [Google Scholar]
- Huang WH, Tupal S, Huang TW, Ward CS, Neul JL, Klisch TJ, Gray PA, Zoghbi HY. Atoh1 governs the migration of postmitotic neurons that shape respiratory effectiveness at birth and chemoresponsiveness in adulthood. Neuron. 2012;75:799–809. doi: 10.1016/j.neuron.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavarone A, Lasorella A. ID proteins as targets in cancer and tools in neurobiology. Trends Mol Med. 2006;12:588–594. doi: 10.1016/j.molmed.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Kirov G, Grozeva D, Norton N, Ivanov D, Mantripragada KK, Holmans P, Craddock N, Owen MJ, et al. International Schizophrenia C, Wellcome Trust Case Control C. Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Human molecular genetics. 2009;18:1497–1503. doi: 10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Hallermann S, Thomsen S, Wichmann C, Sigrist SJ, Heckmann M. Active zone assembly and synaptic release. Biochemical Society transactions. 2006a;34:939–941. doi: 10.1042/BST0340939. [DOI] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006b;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Li J, Ashley J, Budnik V, Bhat MA. Crucial role of Drosophila neurexin in proper active zone apposition to postsynaptic densities, synaptic growth, and synaptic transmission. Neuron. 2007;55:741–755. doi: 10.1016/j.neuron.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Jafar-Nejad H, Hsu YC, Choi KW. Novel function of the class I bHLH protein Daughterless in the negative regulation of proneural gene expression in the Drosophila eye. EMBO Rep. 2008 doi: 10.1038/embor.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur S, Li XY, Li J, Brown JB, Chu HC, Zeng L, Grondona BP, Hechmer A, Simirenko L, Keranen SV, et al. Developmental roles of 21 Drosophila transcription factors are determined by quantitative differences in binding to an overlapping set of thousands of genomic regions. Genome biology. 2009;10:R80. doi: 10.1186/gb-2009-10-7-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melicharek D, Shah A, DiStefano G, Gangemi AJ, Orapallo A, Vrailas-Mortimer AD, Marenda DR. Identification of Novel Regulators of Atonal Expression in the Developing Drosophila Retina. Genetics. 2008;180:2095–2110. doi: 10.1534/genetics.108.093302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell LM, Jarman AP. Context dependence of proneural bHLH proteins. Curr Opin Genet Dev. 2008;18:411–417. doi: 10.1016/j.gde.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Brzozka MM, Rossner MJ. Transcription factor 4 (TCF4) and schizophrenia: integrating the animal and the human perspective. Cell Mol Life Sci. 2014 doi: 10.1007/s00018-013-1553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaneda LG, Robles-Lanuza E, Nieto-Gonzalez JL, Scholl FG. Neurexin dysfunction in adult neurons results in autistic-like behavior in mice. Cell reports. 2014;8:338–346. doi: 10.1016/j.celrep.2014.06.022. [DOI] [PubMed] [Google Scholar]
- Robinow S, White K. The locus elav of Drosophila melanogaster is expressed in neurons at all developmental stages. Dev Biol. 1988;126:294–303. doi: 10.1016/0012-1606(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietilainen OP, Barnes MR, Toulopoulou T, Picchioni M, Vassos E, Ettinger U, Bramon E, et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet. 2009;18:988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom DJ. Isoflurane depresses glutamate release by reducing neuronal excitability at the Drosophila neuromuscular junction. J Physiol. 2004;558:489–502. doi: 10.1113/jphysiol.2004.065748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp M, Pruunsild P, Timmusk T. Pitt-Hopkins syndrome-associated mutations in TCF4 lead to variable impairment of the transcription factor function ranging from hypomorphic to dominant-negative effects. Hum Mol Genet. 2012;21:2873–2888. doi: 10.1093/hmg/dds112. [DOI] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietilainen OP, Mors O, Mortensen PB, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S, de Jong S, Andreassen OA, Werge T, Borglum AD, Mors O, Mortensen PB, Gustafsson O, Costas J, et al. Irish Schizophrenia Genomics C. Common variants at VRK2 and TCF4 conferring risk of schizophrenia. Hum Mol Genet. 2011;20:4076–4081. doi: 10.1093/hmg/ddr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamberg L, Sepp M, Timmusk T, Palgi M. Introducing Pitt-Hopkins syndrome-associated mutations of TCF4 to Drosophila daughterless. Biol Open. 2015 doi: 10.1242/bio.014696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka-Matakatsu M, Miller J, Borger D, Tang WJ, Du W. Daughterless homodimer synergizes with Eyeless to induce Atonal expression and retinal neuron differentiation. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutlein B, Gokce O, Quake SR, Sudhof TC. Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing. Proc Natl Acad Sci U S A. 2014;111:E1291–1299. doi: 10.1073/pnas.1403244111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov YA, Hata Y, Ichtchenko K, Moomaw C, Afendis S, Slaughter CA, Sudhof TC. Conserved domain structure of beta-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. The Journal of biological chemistry. 1994;269:11987–11992. [PubMed] [Google Scholar]
- Yao KM, White K. Neural specificity of elav expression: defining a Drosophila promoter for directing expression to the nervous system. J Neurochem. 1994;63:41–51. doi: 10.1046/j.1471-4159.1994.63010041.x. [DOI] [PubMed] [Google Scholar]
- Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W. Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS letters. 2007;581:2509–2516. doi: 10.1016/j.febslet.2007.04.068. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 2004;19:262–270. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]
- Zweier C, Peippo MM, Hoyer J, Sousa S, Bottani A, Clayton-Smith J, Reardon W, Saraiva J, Cabral A, Gohring I, et al. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome) Am J Hum Genet. 2007;80:994–1001. doi: 10.1086/515583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.