Abstract
Background
Hepatitis C virus (HCV) infection, as a major cause of chronic hepatic diseases, is always accompanied with an abnormality of lipid metabolism. The aim of this study was to investigate the pathogenic role of free fatty acids (FFA) in human HCV infection.
Material/Methods
Peripheral blood lipid indexes among HCV patients with different viral loads (199 samples) and healthy donors (80 samples) were detected by clinical biochemistry tests. HCV replication and the expression of growth arrest and DNA-damage-inducible gene 45-α (GADD45α) in Huh7 cells and clinical samples were quantified by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting. Lipid accumulation in Huh7 cells was detected by immunofluorescence.
Results
In this study, we found that FFA showed a significant positive correlation with viral load in peripheral blood of HCV patients, but not total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), or low-density lipoprotein cholesterol (LDL-C). GADD45α expression in HCV patients dramatically decreased with the increase of viral load. In Huh7 cells, FFA treatment significantly enhanced HCV replication. HCV infection inhibited GADD45α expression, and this effect was further enhanced with the presence of FFA treatment. Ectopic expression of GADD45α in HCV-infected Huh7 cells markedly inhibited the absorption of FFA and HCV replication. However, FFA significantly elevated GADD45α expression without HCV infection.
Conclusions
These results demonstrated that HCV down-regulates GADD45α expression to enhance FFA absorption and thus facilitate its replication. GADD45α is an essential mediator for the pathogenesis of HCV infection. Thus, our study provides potential clues in the search for novel therapeutics and fatty lipid control options for HCV patients.
MeSH Keywords: Fatty Acids, Nonesterified; Hepatitis C Antibodies; Lipid Accumulation Product
Background
Human hepatitis C virus (HCV) infects approximately 170 million persons and adds about 3 million new infections each year worldwide [1,2]. In China, a recent survey showed that total HCV infection rate was 0.4% in the human population in six regions during 2006–2008 [3]. Among HCV-exposed individuals, up to 60–85% will develop chronic infection, which can ultimately lead to hepatic steatosis, fibrosis, cirrhosis, hepatocellular carcinoma, and even death [4]. Due to the high prevalence of HCV chronic infection and its relation to liver diseases, studies in recent years have confirmed that HCV hijacks cellular lipid metabolism to facilitate its replication [5–7]. HCV core protein induces lipid accumulation in hepatocytes by inhibiting microsomal triglyceride transfer protein (MTP) activity [8]; HCV enhances the production of reactive oxygen species (ROS), which results in the peroxidation of membrane lipids, and coopts the very low density lipoprotein (VLDL) secretion pathway for viral maturation or secretion [9]. In addition, HCV also promotes its own replication and inhibits the antiviral effects of IFN-α via inducing the de novo synthesis of fatty acids and impairs fatty acid oxidation [10–12]. Importantly, inhibitors of fatty acid biosynthetic pathways have been effectively used to inhibit HCV replication [13,14]. These research results suggest a strong contribution of lipid metabolism to HCV replication in hepatocytes.
Altered serum lipid profiles are common histologic features of chronic infection with HCV [15]. Chronic HCV patients exhibit low levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) compared with uninfected controls [16]. Free fatty acids (FFA), mainly composed of palmitic and oleic acids, are the major components of liver triglycerides that overflow to hepatocytes and cause hepatic steatosis [17]. Recently, it was found that FFA promote HCV RNA replication in HCV replicon cells and attenuate the antiviral response of IFN-α in a cell culture model [12]. More importantly, FFA treatment also induces an increased amount of intracellular ROS in Huh7 cells [18]; enhanced ROS production has been thought to injure liver cells and cause alanine transaminase (ALT) secretion. Most patients with chronic HCV have elevated or fluctuating levels of serum ALT [19,20], and the presence of elevated ALT levels in HCV patients is frequently used as a guideline for commencing treatment [21,22].
Genome-wide expression analysis found that FFA treatment induced a relatively high level of GADD45α mRNA expression [23]. GADD45α is a member of the GADD45 family, which mainly serves as a tumor suppressor with the abilities to regulate cell proliferation, DNA repair, and apoptosis [24–26]. Overexpression of GADD45α leads to growth suppression in numerous cell types; however, when GADD45α is depleted or repressed, cells show uncontrolled proliferation [27]. As for the role of GADD45α in HCV infection, several studies have found that the HCV non-structural 5A (NS5A) protein depends on p53 to down-regulate GADD45α expression and subsequently triggers abnormal cellular proliferation in an HCV-infected cell model [28,29]. Furthermore, patients with HCV-infected hepatocellular carcinoma also exhibit a low level of GADD45α expression in tumor tissues [30,31]. However, HCV proteins including core, NS3, NS5A, and HCV subgenomic replicon induce ROS production in human hepatoma cells [32], and enhanced ROS production has been found to have the ability to stimulate GADD45α expression via activating the JNK/p53 pathway [33]. Thus, the interaction between HCV and GADD45α is complicated. In the present study, we investigated the role of FFA and GADD45α in HCV infection and found that HCV increases FFA absorption and promotes its own replication via down-regulating GADD45α expression.
Material and Methods
Huh7 cell line culture and materials
The Huh7 cell line was acquired from the Basic Medicine School of Wuhan University and was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Hyclone) containing 10% fetal bovine serum (BCS) (Hyclone), 1% penicillin, and 1% streptomycin (Beyotime, China). HCVcc-JFH-1 was provided by the laboratory of Wen-Zhe Ho (Wuhan University, Wuhan). The HCV RNA polymerase chain reaction (PCR) commercial kit was from Daan Gene Co. Ltd., Guangzhou, China; FFA detection kits, HDL-C kit, and LDL-C kit were purchased from Sekisui Medical Technology (Japan). The TC, triglyceride (TG), and alanine aminotransferase (ALT) test kit was from Beijing Lab Biotech Co. (Beijing, China). Oleate (No.0-7501) and palmitate (No.P-0500) were from Sigma. Oleate was mixed with palmitate in a 2:1 ratio to form the FFA mixture, as described by others [12,34].
Participants
All HCV-positive patients were enrolled from the Department of Infectious Diseases of Zhongnan Hospital of Wuhan University from 2011 to 2013. Informed consent was obtained from each subject before inclusion in this study, and this study was approved by the hospital’s ethics committee. Serum samples from 199 untreated HCV patients were collected before therapy. The diagnosis of chronic hepatitis was based on the criteria from the Hepatitis C Prevention Guide, which was created by the Parasitic Epidemiology branch of The Chinese Medical Association Society of Liver Diseases (http://www.heporg.com). The age range of the patients was 15 to 81 years; the average age was 47 years. Among them, 114 were male, and 85 were female. A total of 80 healthy subjects without liver diseases, cerebrovascular diseases, and dyslipidemia were enrolled as a control group, who passed radiographic examination and serological detection in the physical examination center of the same hospital. The age range of the control group was 18 to 72 years; the average age was 43.7 years. Among them, 45 were male and 35 were female.
Serum index detection
Serum samples of fasting venous blood from the 199 HCV patients were collected before treatment. Fasting venous blood from the control group also was collected. All blood samples were centrifuged for 10 min at 3000 rpm/min, and the collected serum was tested or stored at −20°C if the testing was not timely; serum must be tested within a week. First, HCV-RNA virus load was detected according to the manufacturer’s protocol. Based on the result, the patients were divided into low (5.0×102 ≤HCV-RNA copy, <103 copies/mL, n=82), medium (103 ≤HCV-RNA copy, ≤105 copies/mL, n=61) and high (HCV-RNA copies >105 copies/mL, n=56) virus load groups according to the criteria outlined in the Hepatitis C Prevention Guide by the Parasitic Epidemiology branch of The Chinese Medical Association Society of Liver Diseases. Then the levels of serum FFA, ALT, TC, TG, HDL-C, and LDL-C were measured in both the patient and control groups. HCV-RNA viral loads were detected by the fluorescence quantitative real-time polymerase chain reaction (qRT-PCR) (ABI 7300 fluorescent quantitative PCR analyzer); levels of serum FFA, ALT, TC, and TG were measured by an enzymatic method; and HDL-C and LDL-C were measured by a direct method (Beckman 5400 automatic biochemical analyzer). To ensure the accuracy and reproducibility of test results, all above tests were controlled by the hospital internal quality control department and assessed by Ministry of Health Clinical Laboratory Center external quality assessment.
Human liver tissues
Tumor tissues from liver cancer patients coinciding with chronic HCV infection were examined. Similar tissues from uninfected patients with liver cancer were used as controls.
RNA quantification by fluorescence qRT-PCR
HCV RNA was quantified by fluorescence qRT-PCR. The HCV RNA from HCV-infected Huh7 cells was extracted using the TRIzol reagent (Invitrogen, Carlsbad, California, USA). The HCV primers, forward (5′-AGCCATGGCGTTAGTATGAGTGTC-3′) and reverse (5′-ACAAGGCCTTTCGCAACCCAA-3′), were designed based on the HCV sequence (GenBank accession no. M67463) that was complementary to a sequence within the 5′ untranslated region of the HCV genome, according to a previous method [35]. RNA was quantified using RT-PCR with the SYBR Green Real-Time PCR Master Mix kit (Toyobo). GAPDH was the endogenous control gene, forward primer (5′-ACCACAGTCCATGCCATCAC-3′) and reverse primer (5′-TCCACCACCCTGT TGCTGTA-3′).
siRNA and overexpression assays
siRNA inhibition of GADD45α was purchased from Qiagen (Milano, Italia): Hs_GADD45α _5_HP validated siRNA (NM_001924). Huh7 cells were transfected with 5 nM double-stranded siRNA using HiPerfect transfection reagent (Qiagen) according to the manufacturer’s protocol. GADD45α depletion was evaluated through Western blot. Full length of GADD45α cDNA (GenBank accession No. M60974) was amplified from human normal liver tissue and then subcloned in-frame into pcDNA3.1 to generate the recombinant GADD45α eukaryotic expression plasmid forward primer (5′-CGTAAGCTTCCTGCAGTTTGCAATATG-3′) and reverse primer (5′-GCTAGATATCGATGC CATCACCGTTCAG-3′). Huh7 cells were transfected with 2 μg of total DNA of pcDNA3.1-GADD45α using Lipofectamine 2000 (Invitrogen). Expression of GADD45α was identified by Western blotting 48 h later.
Western blotting
Huh7 cells (2×105) were cultured in a 24-well plate to 80–85% confluence and then co-cultured with different concentrations of FFA with or without HCV. Cells were washed with phosphate-buffered saline (PBS) after 4 h, and then fresh DMEM containing FFA was added and cells were cultured for another 72 h. HCV-NS3 protein expression was detected by Western blot using NS3 mAb (8G-2, Abcam); GADD45α antibody was from Santa Cruz Biotechnology (Santa Cruz, California, USA). Total cell extracts were prepared at 4ºC in 135 mM NaCl, 20 mM Tris-HCl pH 7.5, 1 mM CaCl2, and 1% Triton X-100 in the presence of phosphatase and protease inhibitors. Protein concentrations were measured colorimetrically with a BCA Protein Assay kit (Pierce) and then subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
ROS level detection
Huh7 cells were transfected with pcDNA3.1-GADD45α or siRNA, and then treated with HCV with FFA for 72 hours. Cell samples were incubated with 5 M redox sensitive dye DCF-DA (Beyotime, Shanghai, China) for 30 minutes at 37°C. Reactive oxygen species-mediated oxidation of the fluorescent compound DCF was measured by excitation at 488 nm and an emission wavelength of 525 nm using a flow cytometer (BD Accuri C6).
Immunofluorescence
Infected cells were grown on glass coverslips, washed twice with PBS supplemented with 1% BSA, and fixed in 4% paraformaldehyde for 30 min at room temperature. Cells were then washed three times. For staining lipid droplets, Bodipy-493/503 1 μg/mL (Thermo Fisher Scientific, Inc.) was used; the nuclei were counterstained by Hochest 33258.
Statistical analysis
SPSS 19.0 was used for clinical data analysis, and results from cellular studies were analyzed by GraphPad Prism 5.0. Quantitative data are indicated by mean ±SD. Differences among different groups were tested by one-way analysis of variance (ANOVA) followed by the Neuman-Keuls post hoc test, and a Spearman correlation analysis was performed to examine factors related to HCV-RNA viral load. A p value of <0.05 was considered statistically significant.
Results
FFA concentration positively correlates with HCV viral load in peripheral blood of HCV patients
HCV-positive patients were divided into different groups according to HCV RNA load in peripheral blood, as shown in Table I. The levels of FFA in the low, medium, and high viral load groups were significantly higher than the level in the control group (p<0.05, p<0.01, and p<0.01). Concentrations of TC, TG, HDL-C, and LDL-C did not exhibit a consistent trend with HCV RNA load, although TG decreased with HCV RNA load, but there was no marked difference on the whole. We also evaluated the hepatocellular injury index, ALT in peripheral blood. ALT is the principal reference standard biomarker to diagnose liver dysfunction [36]. Our data revealed that HCV significantly increased the degree of hepatocellular injury with the increase in HCV viral load (Table 1).
Table 1.
Comparison of liver function index and lipid-related indicators in each group (mean ±SD).
| HCV-RNA copy group | No. | FFA (μmol/L) | TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | ALT (U/L) |
|---|---|---|---|---|---|---|---|
| Health control | 80 | 132.14±7.62 | 5.2±1.15 | 1.49±0.66 | 1.19±0.22 | 3.26±1.12 | 27.75±1.74 |
| High | 56 | 651.33±92.543,5 | 4.58±1.032,5 | 1.29±0.772,5 | 1.35±0.442,5 | 2.77±0.581,5 | 85.64±5.751,4 |
| Medium | 61 | 590.99±39.793 | 4.32±1.171 | 1.34±1.132 | 1.2±0.502 | 2.5±0.643 | 55.67±3.471 |
| Low | 82 | 301.7±49.311 | 4.41±1.092 | 1.37±1.112 | 1.25±0.452 | 2.56±0.613 | 41.45±5.511 |
| F value | 123.5 | 2.89 | 1.91 | 0.91 | 6.03 | 109.8 | |
| P value | <0.01 | >0.05 | >0.05 | >0.05 | >0.05 | <0.01 |
Compared with control group, p<0.05;
Compared with control group, p>0.05;
Compared with control group, p<0.01;
Compared with groups of copy <105, p<0.05;
Compared with groups of copy <105, p>0.05;
Compared with groups of copy <105, p<0.01.
The Spearman correlation analysis of levels of serum FFA with other indicators in patient groups is summarized in Table 2. Levels of serum FFA were positively correlated with HCV viral load and serum ALT concentration (p<0.05). Levels of serum FFA were highly correlated with serum TG (p<0.01); although they were not correlated with serum TC, HDL-C, and LDL-C (p>0.05), this may be due to the fact that FFA are metabolic intermediates of TG [37].
Table 2.
Correlation analysis of HCV RNA virus load and levels of serum lipid-related indicators in patient groups.
| Groups | P value | r value | Groups | P value | r value |
|---|---|---|---|---|---|
| FFA and RNA copy | 0.041 | 0.76 | FFA and TG | 0.002 | 0.42 |
| FFA and ALT | 0.031 | 0.68 | FFA and HDL-C | 0.12 | −0.18 |
| FFA and TC | 0.94 | 0.19 | FFA and LCL-C | 0.15 | 0.16 |
p<0.05;
p<0.01.
GADD45α expression is inhibited in liver cancer tissues from HCV-infected patients
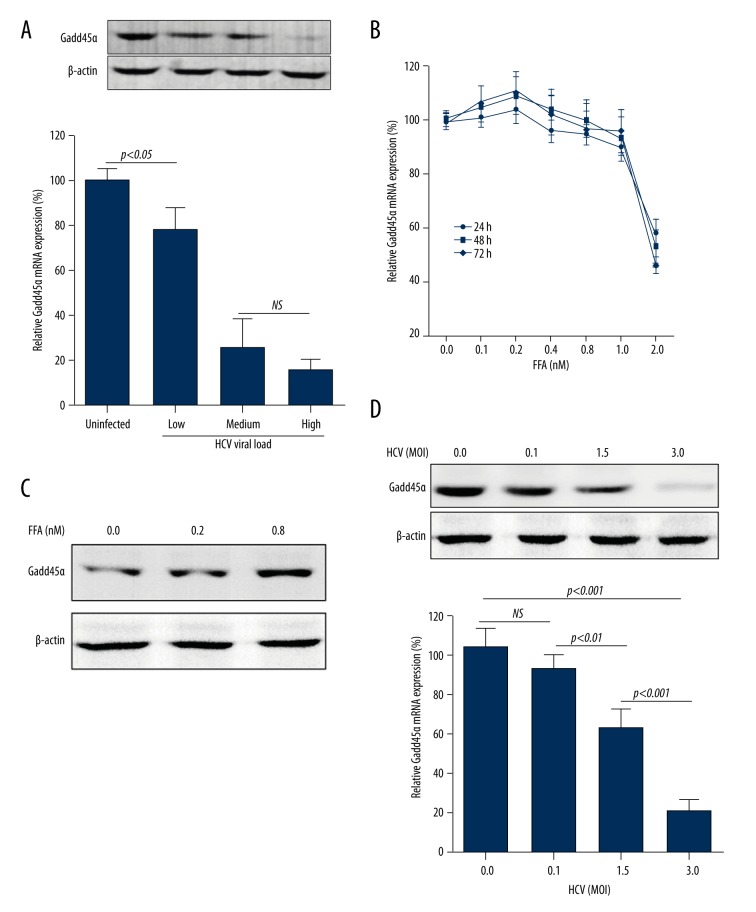
We measured GADD45α expression in tumor tissues from liver cancer patients with chronic HCV infection. Uninfected liver cancer patients were used as controls. GADD45α protein expression and mRNA level in tumor tissues were significantly decreased with the increase of serum viral load (Figure 1A).
Figure 1.
The role of FFA and HCV in GADD45α expression. (A) GADD45α protein expression and mRNA level were detected in tumor tissues from liver cancer patients coinciding with chronic HCV infection. Low, medium, and high mean different serum viral loads. (B) CCK-8 assay showing the effect of different concentrations of FFA (up to 2 mM) on cell viability of Huh7 cells. Cell viability was expressed as percentage of untreated cells. Huh7 cells (2×105) were cultured in a 24-well plate to 80%–85% confluence and then treated with different concentrations of FFA (C) or infected with HCV (D) for 72 hours. mRNA level of GADD45α was quantified by RT-PCR, and protein expression was detected by Western blotting. Results are shown as mean ±SD of three independent experiments.
FFA promote GADD45α expression
We first evaluated the cell cytotoxic activity of FFA in Huh7 cells. FFA didn’t exhibit an obvious adverse effect on cellular viability until 2 mM (Figure 1B), which greatly exceeded the range of concentration of FFA in HCV patients (<0.8 mM). This result hints that FFA alone (at the concentration in HCV patients) may not be enough to impact normal cell function. We then identified the influence of FFA on GADD45α expression; FFA significantly promoted GADD45α expression in Huh7 cells (Figure 1C).
HCV infection down-regulates GADD45α expression
However, in chronic HCV patients, serum concentration showed a contrary change with GADD45α expression, and HCV NS5A protein has been showed to down-regulate GADD45α expression and subsequently trigger aberrant cellular proliferation [29]. The expression of the GADD45α protein and mRNA level were next examined in HCV-infected Huh7 cells. Data revealed that GADD45α expression was reduced in a multiplicity of infection (MOI)-dependent manner (Figure 1D).
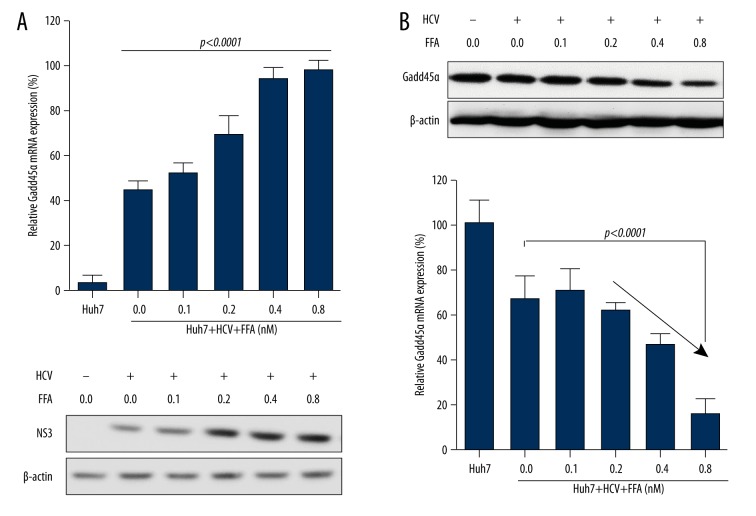
FFA promote HCV replication
To assess the influence of FFA on HCV replication, HCV-infected Huh7 cells (in the condition of same MOI) were stimulated with different doses of FFA. As shown in Figure. 2A, with the increase of FFA concentration, HCV RNA copies and NS3 protein expression were significantly elevated, while protein expression and mRNA levels of GADD45α were markedly decreased (Figure 2B). Thus, these results suggested that FFA promote HCV replication, and then the increased HCV causes the inhibition of GADD45α expression.
Figure 2.
FFA promotes HCV replication in Huh7 cells. Huh7 cells (2×105) were cultured in a 24-well plate to 80%–85% confluence and then co-cultured with different concentrations of FFA with or without HCV (MOI of 1.5) for 72 hours. (A) HCV RNA copies were quantified by RT-PCR, and NS3 protein level was detected by Western blotting. (B) Quantification of the GADD45α protein levels and mRNA levels in Huh7 cells. Results are shown as mean ± SD of three independent experiments.
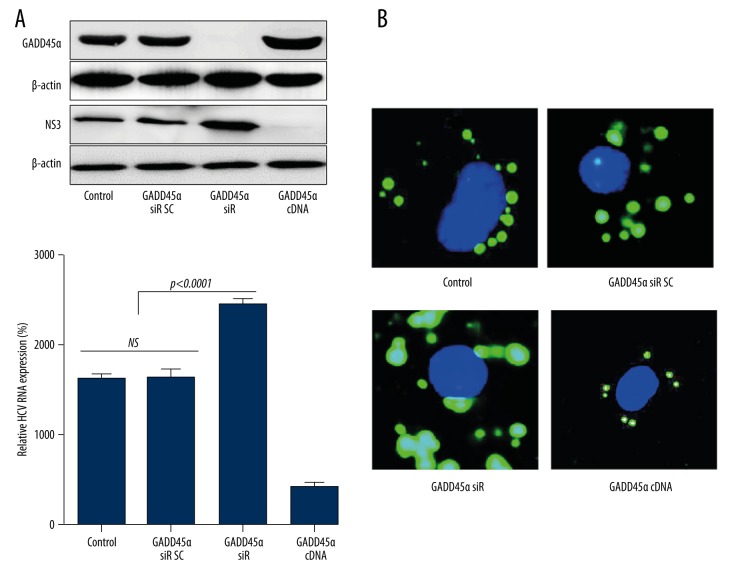
Suppression of GADD45α expression promotes HCV replication and FFA accumulation
To further figure out the correlation of FFA, GADD45α,and HCV infection, HCV replication and FFA accumulation were detected with the presence of GADD45α siRNA or cDNA expressive vector. Huh7 cells were pre-transfected with GADD45α siRNA or pcDNA3.1-GADD45α recombinant eukaryotic expression vector for 48 h, and then infected with HCV and treated by FFA (0.8 mM). Depletion of GADD45α significantly enhanced HCV replication (Figure 3A) and intracellular lipid droplet accumulation (Figure 3B); however, overexpression of GADD45α almost totally reversed this phenomenon.
Figure 3.
GADD45α plays a vital role in regulating FFA-mediated HCV replication. Huh7 cells were transfected with GADD45α siRNA, a negative control siRNA (scramble), or GADD45α over-expressed plasmid. Forty-eight hours later, GADD45α expression was identified (A), and cells were then infected with HCV and simultaneously treated with FFA (0.8 mM). After 72 h, the relative HCV-NS3 protein and RNA expression were detected by Western blotting and RT-PCR (A); intracellular lipid accumulation was detected by Bodipy-493/503 staining and visualized after 400× amplification (B). Results are shown as mean ±SD of three independent experiments.
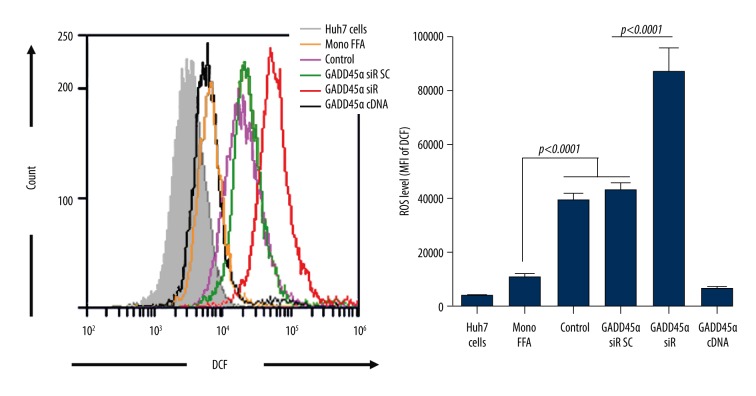
GADD45α decreases ROS production induced by HCV infection
Elevated intracellular ROS production can be induced by FFA treatment and HCV infection, separately, and is usually correlated with an abnormally high level of serum ALT. As we have noticed that ALT is highly elevated in chronic HCV patients, we further evaluated the role of GADD45α in ROS production. As shown in Figure 4, FFA treatment alone caused a minor increase in ROS production; when accompanied with HCV infection, ROS production was greatly raised and further enhanced after inhibition of GADD45α expression. GADD45α overexpression almost totally cleared up the ROS production induced by FFA and HCV infection.
Figure 4.
GADD45α inhibits intracellular ROS production of HCV-infected Huh7 cells. Huh7 cells were transfected with GADD45α siRNA, a negative control siRNA (scramble), or GADD45α over-expressed plasmid. Forty-eight hours later, levels of intracellular ROS were analyzed by flow cytometry as described in Material and Methods.
To the best of our knowledge, this is the first report that reveals the correlation of FFA and GADD45α in HCV infection. FFA promote HCV replication, and enhanced HCV replication in turn accelerates FFA accumulation and ROS production of host cells via repressing GADD45α expression.
Discussion
HCV causes chronic liver diseases in 75–85% of the infected individuals; the outcomes include hepatic steatosis and even hepatocellular carcinoma [38]. Regarding the mechanisms involved in HCV-induced liver-related diseases, HCV-induced imbalance in lipid homeostasis seems to be the main cause. HCV infection is associated with enhanced lipogenesis, and reduced secretion and accumulation of lipids in host cells [39,40]. Despite great advances that have been made in our knowledge of HCV interaction with host lipid metabolism, the detailed mechanisms still need to be clarified.
In our study, chronic HCV patients were divided into three groups according to serum HCV viral load, namely, high, medium, and low. Then, blood lipid indexes for HCV patients with different viral loads and healthy donors were detected. We found that levels of FFA in all groups of HCV patients were significantly higher than the level in the healthy control group (p<0.01, p<0.01, and p<0.05; Table 1). And importantly, serum FFA level was positively correlated with the level of enzymatic activity of serum ALT and HCV viral load (Table 2). ALT is a kind of enzyme in liver cells that could reflect the degree of liver injury or inflammation to a certain extent [36], which indicated that serum FFA had a close relation with hepatocyte injury and HCV infection.
FFA, which are mainly composed of palmitic and oleic acids, are the main lipid metabolites that are taken up by the liver [41]. Hepatocytes are able to accumulate FFA, and the increased fat content (until 1.2 mM) is not associated with a significant impairment of the cell integrity, in line with clinical and in vivo experimental data [42]. Similar results were also observed in our study: an FFA concentration less than 0.8 mM (the highest level of serum FFA in our experimental populations) exhibited no detectable influence on cellular viability of hepatocytes (Figure 1B, Table 1). In natural conditions, genome-wide expression analysis has identified that FFA induce intracellular ROS production, and then enhanced ROS activates GADD45α expression to induce cell apoptosis [18,23,43]. GADD45α is a tumor suppressor and plays an important role in the control of cell cycle checkpoint, cell apoptosis, the DNA repair process, and signaling transduction [44,45]. Importantly, GADD45α is down-regulated in HCV-infected patients with hepatocellular carcinoma [30,31]. Cellular studies also found that HCV NS5A protein down-regulates GADD45α expression and subsequently triggers abnormal cellular proliferation in an HCV-infected cell model [29]. We thus collected primary tumor cells from operative tissues of liver cancer patients with HCV infection, and indeed found that GADD45α expression was down-regulated compared with that in uninfected liver cancer patients and was negatively correlated with serum viral load (Figure 1A). So, serum FFA level showed a contrary tendency with GADD45α expression in chronic HCV patients, similar to the results observed in type 2 diabetes mellitus (T2DM), in which increased serum FFA levels showed a negative correlation with GADD45α expression in peripheral blood mononuclear cells from T2DM patients [46].
Next, we took advantage of the HCV cell culture model to study the role FFA and GADD45α play in HCV infection. In Huh7 cells, FFA sole treatment induced GADD45α expression in a dose-dependent manner, and HCV infection without FFA treatment significantly decreased GADD45α expression in a MOI-dependent manner, similar to the influence of HCV NS5A protein on GADD45α expression [29]. When Huh7 cells were simultaneously infected with HCV and treated with FFA, we found that FFA promoted HCV replication and decreased GADD45α expression in a dose-dependent manner (Figure 2). Furthermore, we noticed that interfering GADD45α expression in HCV-infected cells resulted in multiple effects: increased HCV replication, and enhanced FFA accumulation and ROS production in Huh7 cells. Thus, HCV enhances cellular FFA absorption and then facilitates its own replication via down-regulating GADD45α expression.
Conclusions
We clarified for the first time that serum level of FFA closely correlates with HCV infection and that GADD45α down-regulation is absolutely required for FFA-induced HCV replication. Our results demonstrated that fatty lipid control may provide important clues for the developing of novel therapeutic targets for HCV infection.
Footnotes
Conflicts of interest
The authors have no financial conflicts of interest.
Source of support: The present study was supported by Natural Science Foundation of Hubei Province Grant 2012FFB04411
References
- 1.Janssen HL, Reesink HW, Lawitz EJ, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 2.Smirnova OA, Ivanova ON, Bartosch B, et al. Hepatitis C virus NS5A protein triggers oxidative stress by inducing NADPH oxidases 1 and 4 and cytochrome P450 2E1. Oxid Med Cell Longev. 2016;2016:8341937. doi: 10.1155/2016/8341937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piao HX, Yang AT, Sun YM, et al. Increasing newly diagnosed rate and changing risk factors of HCV in Yanbian Prefecture, a high endemic area in China. PloS One. 2014;9(1):e86190. doi: 10.1371/journal.pone.0086190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu D, Yi Z, Xin-bo S, et al. Investigation for the Relationship between HCV- RNA level and Hepatic Insufficiency. Chin J Lab Diagn. 2011;15(3):481–83. [Google Scholar]
- 5.Chang ML. Metabolic alterations and hepatitis C: From bench to bedside. World J Gastroenterol. 2016;22(4):1461–76. doi: 10.3748/wjg.v22.i4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamamoto S, Fukuhara T, Ono C, et al. Lipoprotein receptors redundantly participate in entry of hepatitis C virus. PLoS Pathog. 2016;12(5):e1005610. doi: 10.1371/journal.ppat.1005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21(1):33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HC, Ma HC, Yang CH, Lo SY. Production and pathogenicity of hepatitis C virus core gene products. WorldJ Gastroenterol. 2014;20(23):7104–22. doi: 10.3748/wjg.v20.i23.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roingeard P. Hepatitis C virus diversity and hepatic steatosis. J Viral Hepat. 2013;20(2):77–84. doi: 10.1111/jvh.12035. [DOI] [PubMed] [Google Scholar]
- 10.Yang W, Hood BL, Chadwick SL, et al. Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology. 2008;48(5):1396–403. doi: 10.1002/hep.22508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyoshi H, Moriya K, Tsutsumi T, et al. Pathogenesis of lipid metabolism disorder in hepatitis C: Polyunsaturated fatty acids counteract lipid alterations induced by the core protein. J Hepatol. 2011;54(3):432–38. doi: 10.1016/j.jhep.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Gunduz F, Aboulnasr FM, Chandra PK, et al. Free fatty acids induce ER stress and block antiviral activity of interferon alpha against hepatitis C virus in cell culture. Virol J. 2012;9:143. doi: 10.1186/1743-422X-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oslob JD, Johnson RJ, Cai H, et al. Imidazopyridine-based fatty acid synthase inhibitors that show anti-HCV activity and in vivo target modulation. ACS Med Chem Lett. 2013;4(1):113–17. doi: 10.1021/ml300335r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villareal VA, Rodgers MA, Costello DA, Yang PL. Targeting host lipid synthesis and metabolism to inhibit dengue and hepatitis C viruses. Antiviral Res. 2015;124:110–21. doi: 10.1016/j.antiviral.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khattab MA, Eslam M, Aly MM, et al. Serum lipids and chronic hepatitis C genotype 4: interaction and significance. Ann Hepatol. 2012;11(1):37–46. [PubMed] [Google Scholar]
- 16.Alavian SM, Miri SM, Tabatabaei SV, et al. Lipid profiles and hepatitis C viral markers in HCV-infected thalassemic patients. Gut Liver. 2011;5(3):348–55. doi: 10.5009/gnl.2011.5.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larter CZ, Yeh MM, Haigh WG, et al. Hepatic free fatty acids accumulate in experimental steatohepatitis: role of adaptive pathways. J Hepatol. 2008;48(4):638–47. doi: 10.1016/j.jhep.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Chavez-Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:20. doi: 10.1186/1471-230X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Zhao P, Guo H, et al. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis C. Mediators Inflamm. 2012;2012:819636. doi: 10.1155/2012/819636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MS, Lin HS, Chung CM, et al. Serum aminotransferase ratio is independently correlated with hepatosteatosis in patients with HCV: A cross-sectional observational study. BMJ Open. 2015;5(9):e008797. doi: 10.1136/bmjopen-2015-008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39(4):1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 22.Dhumeaux D, Marcellin P, Lerebours E. Treatment of hepatitis C. The 2002 French consensus. Gut. 2003;52(12):1784–87. doi: 10.1136/gut.52.12.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das SK, Mondal AK, Elbein SC. Distinct gene expression profiles characterize cellular responses to palmitate and oleate. J Lipid Res. 2010;51(8):2121–31. doi: 10.1194/jlr.M004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosemary Siafakas A, Richardson DR. Growth arrest and DNA damage-45 alpha (GADD45alpha) Int J Biochem Cell Biol. 2009;41(5):986–89. doi: 10.1016/j.biocel.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Tront JS, Huang Y, Fornace AJ, Jr, et al. Gadd45a functions as a promoter or suppressor of breast cancer dependent on the oncogenic stress. Cancer Res. 2010;70(23):9671–81. doi: 10.1158/0008-5472.CAN-10-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang F, Zhang W, Li D, Zhan Q. Gadd45a suppresses tumor angiogenesis via inhibition of the mTOR/STAT3 protein pathway. J Biol Chem. 2013;288(9):6552–60. doi: 10.1074/jbc.M112.418335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang P, Ni F, Deng RQ, et al. MiR-362-5p promotes the malignancy of chronic myelocytic leukaemia via down-regulation of GADD45alpha. Mol Cancer. 2015;14(1):190. doi: 10.1186/s12943-015-0465-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Cheng D, Jiang YF, Xiao XQ, Gong GZ. [Hepatitis C virus strain JFH1 down-regulates expression of growth arrest and DNA damage-inducible gene 45a in human hepatoma Huh7.5.1 cells]. Zhonghua Gan Zang Bing Za Zhi. 2012;20(11):807–10. doi: 10.3760/cma.j.issn.1007-3418.2012.11.002. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 29.Cheng D, Zhao L, Zhang L, et al. p53 controls hepatitis C virus non-structural protein 5A-mediated downregulation of GADD45alpha expression via the NF-kappaB and PI3K-Akt pathways. J Gen Virol. 2013;94(Pt 2):326–35. doi: 10.1099/vir.0.046052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gramantieri L, Chieco P, Giovannini C, et al. GADD45-alpha expression in cirrhosis and hepatocellular carcinoma: relationship with DNA repair and proliferation. Hum Pathol. 2005;36(11):1154–62. doi: 10.1016/j.humpath.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Wurmbach E, Chen YB, Khitrov G, et al. Genome-wide molecular profiles of HCV-induced dysplasia and hepatocellular carcinoma. Hepatology. 2007;45(4):938–47. doi: 10.1002/hep.21622. [DOI] [PubMed] [Google Scholar]
- 32.Presser LD, Haskett A, Waris G. Hepatitis C virus-induced furin and thrombospondin-1 activate TGF-beta1: role of TGF-beta1 in HCV replication. Virology. 2011;412(2):284–96. doi: 10.1016/j.virol.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi HJ, Kang KS, Fukui M, Zhu BT. Critical role of the JNK-p53-GADD45alpha apoptotic cascade in mediating oxidative cytotoxicity in hippocampal neurons. Br J Pharmacol. 2011;162(1):175–92. doi: 10.1111/j.1476-5381.2010.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricchi M, Odoardi MR, Carulli L, et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J Gastroenterol Hepatol. 2009;24(5):830–40. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Ali MA, Shi Y, et al. Specifically binding of L-ficolin to N-glycans of HCV envelope glycoproteins E1 and E2 leads to complement activation. Cell Mol Immunol. 2009;6(4):235–44. doi: 10.1038/cmi.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozer JS, Chetty R, Kenna G, et al. Enhancing the utility of alanine aminotransferase as a reference standard biomarker for drug-induced liver injury. Regul Toxicol Pharmacol. 2010;56(3):237–46. doi: 10.1016/j.yrtph.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Kim SP, Catalano KJ, Hsu IR, et al. Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2007;292(6):E1590–98. doi: 10.1152/ajpendo.00669.2006. [DOI] [PubMed] [Google Scholar]
- 38.Fu Y, Wang Y, Xia W, et al. New trends of HCV infection in China revealed by genetic analysis of viral sequences determined from first-time volunteer blood donors. J Viral Hepat. 2011;18(1):42–52. doi: 10.1111/j.1365-2893.2010.01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bose SK, Kim H, Meyer K, et al. Forkhead box transcription factor regulation and lipid accumulation by hepatitis C virus. J Virol. 2014;88(8):4195–203. doi: 10.1128/JVI.03327-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heaton NS, Randall G. Multifaceted roles for lipids in viral infection. Trends Microbiol. 2011;19(7):368–75. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Prog Lipid Res. 2009;48(1):1–26. doi: 10.1016/j.plipres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Chavez-Tapia NC, Rosso N, Tiribelli C. In vitro models for the study of non-alcoholic fatty liver disease. Curr Med Chem. 2011;18(7):1079–84. doi: 10.2174/092986711794940842. [DOI] [PubMed] [Google Scholar]
- 43.Kang HS, Ock J, Lee HJ, et al. Early growth response protein 1 upregulation and nuclear translocation by 2′-benzoyloxycinnamaldehyde induces prostate cancer cell death. Cancer Lett. 2013;329(2):217–27. doi: 10.1016/j.canlet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Tamura RE, Paccez JD, Duncan KC, et al. GADD45alpha and gamma interaction with CDK11p58 regulates SPDEF protein stability and SPDEF-mediated effects on cancer cell migration. Oncotarget. 2016;7(12):13865–79. doi: 10.18632/oncotarget.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zerbini LF, de Vasconcellos JF, Czibere A, et al. JunD-mediated repression of GADD45alpha and gamma regulates escape from cell death in prostate cancer. Cell Cycle. 2011;10(15):2583–91. doi: 10.4161/cc.10.15.16057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manoel-Caetano FS, Xavier DJ, Evangelista AF, et al. Gene expression profiles displayed by peripheral blood mononuclear cells from patients with type 2 diabetes mellitus focusing on biological processes implicated on the pathogenesis of the disease. Gene. 2012;511(2):151–60. doi: 10.1016/j.gene.2012.09.090. [DOI] [PubMed] [Google Scholar]