Abstract
Background
Acute lung injury (ALI) is a life-threatening hypoxemic respiratory disorder with high incidence and mortality. ALI usually manifests as widespread inflammation and lung fibrosis with the accumulation of pro-inflammatory and pro-fibrotic factors and collagen. Thymic stromal lymphopoietin (TSLP) has a significant role in regulation of inflammation but little is known about its roles in lung fibrosis or ALI. This study aimed to define the role and possible regulatory mechanism of TSLP in lung fibrosis.
Material/Methods
We cultured human lung fibroblast MRC-5 cells and overexpressed or inhibited TSLP by the vector or small interfering RNA transfection. Then, the pro-fibrotic factors skeletal muscle actin alpha (α-SMA) and collagen I, and the 4 mitogen-activated protein kinases (MAPKs) – MAPK7, p38, extracellular signal-regulated kinase 1 (ERK1), and c-Jun N-terminal kinase 1 (JNK1) – were detected by Western blot.
Results
Results showed that TSLP promoted the production of α-SMA and collagen I (P<0.001), suggesting that it can accelerate MRC-5 cell fibrosis. It also activated the expression of MAPK7, p-p38, p-ERK1, and p-JNK1, but the total MAPK7, p-38, ERK1, and JNK1 protein levels were mostly unchanged, indicating the activated MAPK pathways that might contribute to the promotion of cell fibrosis.
Conclusions
This study shows the pro-fibrotic role of TSLP in MRC-5 cells, suggesting TSLP is a potential therapeutic target for treating lung fibrosis in ALI. It possibly functions via activating MAPKs. These findings add to our understanding of the mechanism of fibrosis.
MeSH Keywords: Acute Lung Injury; Mitogen-Activated Protein Kinases; Thymic Factor, Circulating
Background
Acute lung injury (ALI) is a life-threatening hypoxemic respiratory failure showing bilateral infiltrates in the absence of left atrial hypertension [1], which develops to acute respiratory distress syndrome in the severe stage [2,3]. Patients with ALI frequently have sepsis and multiple organ failure at the same time. Causes of ALI are divided into direct and indirect injuries, including sepsis, pneumonia, trauma, aspiration, multiple blood transfusions, and drug abuse or overdose [1,4–6]. Therapeutic methods benefit from the improvement of mechanical ventilation, and positive-pressure ventilation supplemented with oxygen and positive end-expiratory pressure is necessary for almost all ALI patients [7]. The efficacy and safety of lung recruitment maneuvers and individualized treatment are still under discussion.
Acute and widespread inflammation and tissue fibrosis play vital roles in the development of ALI. Polymorphonuclear neutrophils in the injured lung cannot be eliminated by alveolar macrophages in time, thus producing excess inflammatory factors, including interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) [8]. Other pro-inflammatory factors, such as Toll-like receptor 2 (TLR2), interleukin 6 (IL-6), IL-17, and IL-23, all of which are potential therapeutic targets for treating ALI, also accelerate inflammation and fibrosis and thus aggravate ALI [9]. In addition, lung fibrosis is promoted in the early phase of ALI, manifesting as increased collagen fiber content [10].
Thymic stromal lymphopoietin (TSLP) is an epithelial cell-derived cytokine, mainly expressed in skin, gut, lung, and thymus, that plays pivotal roles in regulating T helper 2-mediated inflammation [11]. TSLP and its receptor TSLPR mediate pro-inflammatory cytokines and chemokines release, thus contributing to airway inflammation [12]. Inhibition of mitogen-activated protein kinases (MAPKs) suppresses the IL-1β-induced production of TSLP in endometrioma stromal cells [13], indicating the association between TSLP and MAPK pathways. However, little is known about the regulatory mechanism of TSLP in pulmonary fibrosis of ALI. In this study we aimed to discover the function of TSLP and the potential mechanism in mediating cell fibrosis in ALI. Human lung fibroblast MRC-5 cells were used and TSLP expression was altered to detect fibrosis indicators, including α-SMA and collagen I. We also analyzed the activation of major MAPKs to discover the possible mechanism of TSLP in cell fibrosis. Our study results add to the evidence of a connection between TSLP and lung cell fibrosis, and provide a potential target for treating lung fibrosis in ALI.
Material and Methods
Cell culture and transfection
Human lung fibroblast MRC-5 cells (ATCC, Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Carlsbad, CA), and maintained in a humidified atmosphere with 5% CO2 at 37°C.
Cells were seeded in 6-well plates and divided into 4 groups: Control, si-Control, TSLP, and si-TSLP. Transfection was performed in serum-free medium using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Cells in the si-Control and si-TSLP groups were transfected with the negative control for siRNA transfection and the TSLP-specific siRNA designed by RiboBio (Guangzhou, China). For TSLP overexpression in the TSLP group, the coding sequence of TSLP was cloned into pcDNA3.1 vector (TranSheepBio, Shanghai, China) and the vector (50 μM) was transfected into the cells. After the transfection, cells were recovered in complete medium and incubated at 37°C for 48 h before sampling.
Real-time quantitative PCR (qPCR)
Cells of the 4 groups were collected and lysed in TRIzol (Invitrogen) for RNA extraction. The DNA contamination was removed using DNaseI (Invitrogen) and detected by agarose gel electrophoresis. The quantity and quality of RNAs were examined by NanoDrop 2000 (Thermo Scientific, Carlsbad, CA), and 1 μg RNAs of each sample was applied to reverse transcription into complementary DNAs (cDNAs) using the PrimeScript 1st Strand cDNA Synthesis Kit (TaKaRa, Dalian, China). qPCR was conducted on a LightCycler 480 platform with 20-ng cDNAs and the specific primers for human TSLP (Fw: 5′-TAT GAG TGG GAC CAA AAG TAC CG-3′ and Rv: 5′-ACG CCA CAA TCC TTG TAA TTG TG-3′) in each reaction. Data were analyzed using 2−ΔΔCt method with GAPDH (Fw: 5′-GAA GGT GAA GGT CGG AGT C-3′ and Rv: 5′-GAA GAT GGT GAT GGG ATT TG-3′) as an endogenous reference.
Western blot
Protein samples of the cells were extracted by lysing the cells with radio immunoprecipitation assay lysis buffer (Beyotime, Shanghai, China). The protein samples were quantified using the BCA Protein Assay Kit (Beyotime) and the same amount of samples were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Protein bands on the gel were transferred to a polyvinylidene fluoride membrane and blocked in 5% skim milk for 2 h at room temperature and then the blot was incubated in the specific primary antibodies for TSLP (ab47943), skeletal muscle actin (α-SMA, ab5694), collagen I (ab6308), MAPK7 (ab40809), extracellular signal-regulated kinase 1 (ERK1, ab180163), phospho-ERK1 (p-ERK1, ab24157), p38 (ab7952), p-p38 (ab178867), c-Jun N-terminal kinase 1 (JNK1, ab199380), and p-JNK1 (ab47337), purchased from Abcam (Cambridge, UK), overnight at 4°C. β-actin (ab189073) was used as an endogenous reference. After washing, the blot was incubated in horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Positive signals were developed using the ECL Plus Western Blotting Substrate (Thermo Scientific). Band densities for each sample were analyzed using ImageJ 1.49 software (National Institutes of Health, Bethesda, MD).
Statistical analysis
All experiments were repeated in triplicate and results are indicated as the mean ± standard deviation. P values were calculated by t test in SPSS (IBM, New York, USA) and differences were considered significant at P<0.05.
Results
TSLP promotes α-SMA and collagen I
To analyze the function of TSLP, we performed overexpression and inhibition of TSLP using its specific overexpression vector or siRNA, respectively. qPCR and Western blot analyses were conducted to verify the successful transfection of the vector and siRNA. The TSLP mRNA level in the si-Control group was only slightly changed compared to the Control group (P<0.05), while that of the TSLP group was obviously up-regulated and the si-TSLP group was significantly down-regulated (P<0.001, Figure 1A). The TSLP protein levels showed similar changing patterns, with no obvious change existing between the Control group and the si-Control group (P<0.05), but significant up-regulation and down-regulation were found in the TSLP group and the si-TSLP group (P<0.001, Figure 1B). These results show the effectiveness of TSLP overexpression and inhibition, and the cells were thus used for further analysis.
Figure 1.
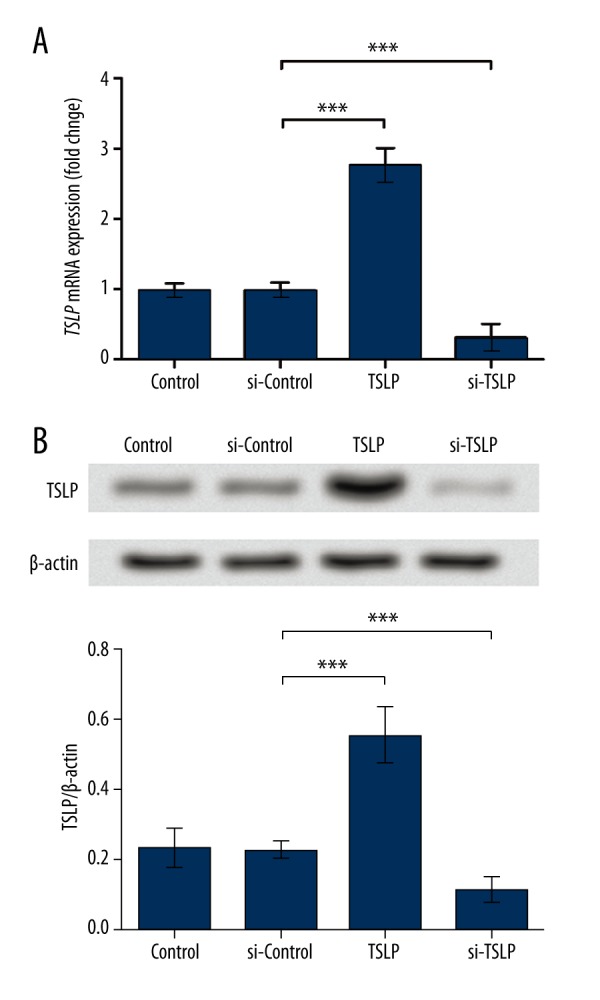
Transfection induces the overexpression and inhibition of TSLP. (A) TSLP mRNA level was up-regulated in the TSLP group, and down-regulated in the si-TSLP group. (B) TSLP protein level was up-regulated in the TSLP group and down-regulated in the si-TSLP group. TSLP levels in the si-Control group were almost unchanged. GAPDH and β-actin were used as the endogenous references for qPCR and Western blot, respectively. *** P<0.001. TSLP – thymic stromal lymphopoietin. Control – cells without transfection. si-Control – cells transfected with the control siRNA. TSLP – cells transfected with TSLP overexpression vector. si-TSLP – cells transfected with TSLP-specific siRNA.
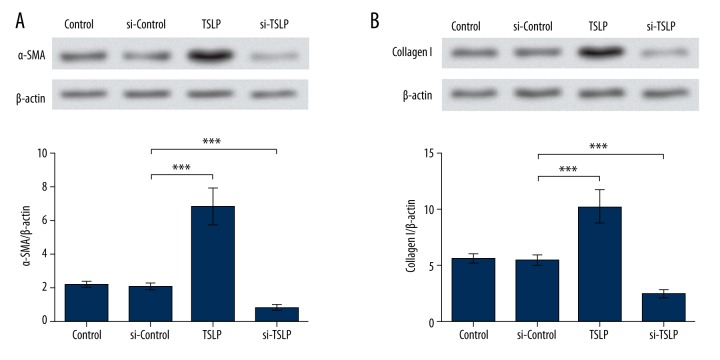
Elevated fibrosis is usually accompanied by increased α-SMA and collagen levels; thus, we tested the protein levels of α-SMA and collagen I to evaluate the cell fibrosis. Their expression levels in the si-Control group were nearly unchanged, but were significantly up-regulated when TSLP was overexpressed by its overexpression vector (P<0.001), and inhibited when TSLP was suppressed (P<0.001, Figure 2A, 2B). These data suggest that TSLP can promote the production of α-SMA and collagen I, which appear to indicate the accelerated fibrosis in MRC-5 cells.
Figure 2.
α-SMA and collagen I were up-regulated by TSLP. (A) Western blot and the corresponding histogram indicate α-SMA was promoted by TSLP overexpression and inhibited by si-TSLP. (B) Western blot and the corresponding histogram indicate Collagen I was promoted by TSLP overexpression and inhibited by si-TSLP. β-actin was used as the endogenous reference for Western blot. *** P<0.001. TSLP – thymic stromal lymphopoietin. Control – cells without transfection. si-Control – cells transfected with the control siRNA. TSLP – cells transfected with TSLP overexpression vector. si-TSLP – cells transfected with TSLP-specific siRNA. α-SMA – skeletal muscle actin alpha.
TSLP activates MAPK pathways
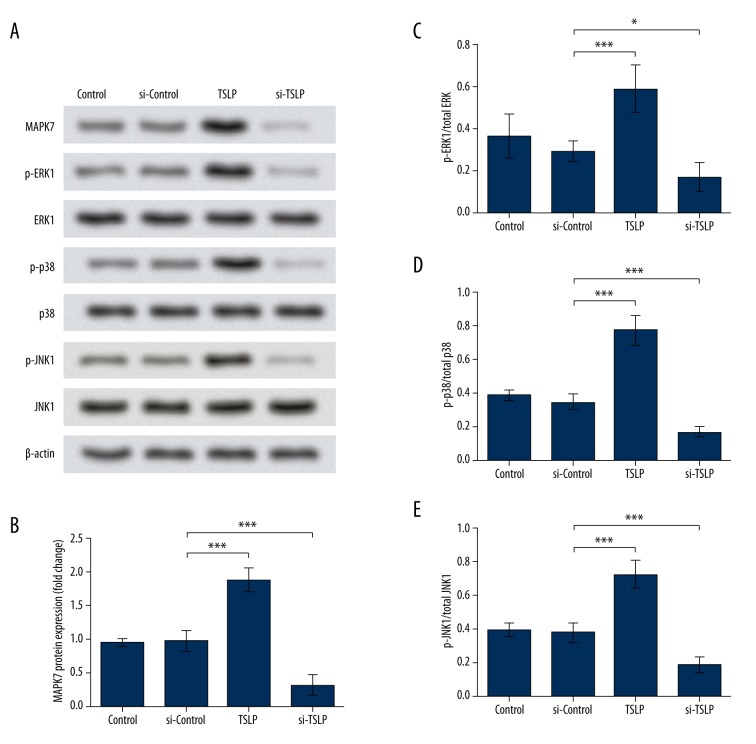
MAPK pathways have been reported to respond to the cytokines induced by stimuli and promote the production of collagen, thus contributing to fibrosis. Therefore, in this study we analyzed the activation of MAPK pathways by detecting the expression of 4 major MAPKs: MAPK7, ERK1, JNK1, and p38. Western blot results showed that the expression of MAPK7 was significantly promoted by TSLP overexpression, and was inhibited in the si-TSLP group (P<0.001, Figure 3A, 3B). Phosphorylated ERK1, p38, and JNK1 were all promoted by TSLP and inhibited when TSLP was inhibited, and their total protein levels were almost unchanged (Figure 3A, 3C–3E), indicating the activation of ERK1, p38, and JNK1 by TSLP. These results suggest that all the major MAPK pathways in mammals are activated by TSLP, which might be the functional mechanism of TSLP in promoting fibrosis in MRC-5 cells.
Figure 3.
Major MAPKs were activated by TSLP. (A) Western blot shows protein levels of MAPK7, p-ERK1, p-p38, and p-JNK1 were up-regulated by TSLP overexpression and down-regulated by si-TSLP. ERK1, p-38, and JNK1 levels remained unchanged. (B) Histogram indicates the band density of MAPK7. (C–E) Histograms indicate the band densities of p-ERK1, p-p38, or p-JNK1 compared to those of ERK1, p38, or JNK1. β-actin was used as the endogenous reference for Western blot. * P<0.05. *** P<0.001. TSLP – thymic stromal lymphopoietin. Control – cells without transfection. si-Control – cells transfected with the control siRNA. TSLP – cells transfected with TSLP overexpression vector. si-TSLP – cells transfected with TSLP-specific siRNA. MAPK7 – mitogen-activated protein kinase 7. ERK1 – extracellular signal-regulated kinase 1. p38 – mitogen-activated protein kinase 14. JNK1 – c-Jun N-terminal kinase 1.
Discussion
The roles and regulation mechanisms of TSLP in lung fibrosis in ALI have not been previously defined. In this study, we tried to determine them by overexpressing and inhibiting TSLP in human lung fibroblast MRC-5 cells and detecting the expression changes of some key factors. The protein levels of α-SMA and collagen I are up-regulated by TSLP. Further analysis indicates that the major MAPKs are activated by TSLP overexpression.
α-SMA is expressed at higher levels in the epithelial cells and periductular collagen fibers of biliary atresia-induced hepatic fibrosis [14], and its inhibition attenuates mouse lung fibrosis [15]. Moreover, a hallmark of fibrosis is the accumulation of fibrillary collagens, especially collagen I. Therefore, α-SMA and collagen I are widely used as indicators of fibrosis, including lung fibrosis [16–18]. In this study, we used these 2 factors to assess the progression of fibrosis in MRC-5. Our findings show that TSLP overexpression significantly up-regulated α-SMA and collagen I levels, both of which were inhibited when TSLP was suppressed. Based on the indicated roles of α-SMA and collagen I in fibrosis, these results suggest that the changes in degree of fibrosis are modulated by TSLP; therefore, TSLP appears to promote MRC-5 cell fibrosis, suggesting its roles in accelerating lung fibrosis in ALI.
MAPKs are serine-threonine kinases that participate in the mediation of various cellular activities [19,20]. They are mainly divided into 4 subgroups: ERK1/2 MAPK, JNK MAPK, p38 MAPK, and ERK5 MAPK (MAPK7). Other MAPKs, like ERK3/4 and ERK7/8, are poorly understood [20–22]. MAPKs have been reported to be associated with ALI and other pulmonary diseases [23]. Inhibiting MAPKs can attenuate lipopolysaccharide-induced ALI [24,25]. With this information, we attempted to analyze the relationship between TSLP and MAPKs in lung fibrosis. Results showed that the expression levels of MAPK7 and the proportion of phosphorylated ERK1, JNK1, and p-38 were all up-regulated by TSLP overexpression, indicating TSLP can activate MAPK pathways in MRC-5 cells. In addition, MAPKs contribute to the progression of fibrosis, as proven in cardiomyopathy [26] and peritoneal fibrosis [27]. Moreover, inhibiting p38 MAPK may attenuate the inflammation and fibrosis of lung diseases [28]. Therefore, it was reasonable to deduce that the activation of MAPK pathways by TSLP might be a potential mechanism of TSLP-induced MRC-5 cell fibrosis. However, the relationship between TSLP and MAPK pathways is complicated, and studies have found that activation of MAPKs can increase TSLP expression [29]. The inter-activation between TSLP and MAPKs might help to amplify the signals during ALI, but this needs further analysis.
TSLP inhibition resulted in the down-regulation of α-SMA and collagen I, as well as the less-activated MAPKs, suggesting TSLP is a potential therapeutic target for treating lung fibrosis in ALI. TSLP has been shown to contribute to skin fibrosis in atopic dermatitis [30] and diffuse cutaneous systemic sclerosis [31]. Importantly, a previous study proved that inhibition of TSLP is beneficial in suppressing lung fibrosis; nevertheless, it is achieved through regulating the type I receptor of TGF-β and the downstream SMAD family member 2/3 and JNK, rather than the other MAPKs [32]. It is possible that TSLP regulates various pathways in modulating fibrosis in ALI. It would be of great value if these regulatory pathways were defined in detail and confirmed in further studies.
Conclusions
This study indicates that TSLP may promote the fibrosis of MRC-5 cells and activate the MAPK pathways, which is a possible mechanism of TSLP in promoting MRC-5 cell fibrosis. Thus, TSLP is a potential therapeutic target for treating lung fibrosis in ALI. Our study will help to define the molecular regulation of fibrosis, but verification in further studies is necessary.
Footnotes
Conflicts of interest
No conflicts of interest exist.
Source of support: Departmental sources
References
- 1.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: Definition and review. Critical Care Medicine. 2005;33:721–26. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 2.Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–79. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Stringer KA, Serkova NJ, Karnovsky A, et al. Metabolic consequences of sepsis-induced acute lung injury revealed by plasma (1)H-nuclear magnetic resonance quantitative metabolomics and computational analysis. Am J Physiol Lung Cell Mol Physiol. 2011;300:L4–11. doi: 10.1152/ajplung.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marik PE, Corwin HL. Acute lung injury following blood transfusion: expanding the definition. Crit Care Med. 2008;36:3080–84. doi: 10.1097/CCM.0b013e31818c3801. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwenhuizen L, de Groot PG, Grutters JC, Biesma DH. A review of pulmonary coagulopathy in acute lung injury, acute respiratory distress syndrome and pneumonia. Eur J Haematol. 2009;82:413–25. doi: 10.1111/j.1600-0609.2009.01238.x. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–64. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 8.Antonini JM, Murthy GGK, Brain JD. Responses to welding fumes: Lung injury, inflammation, and the release of tumor necrosis factor-α and interleukin-1β. Exp Lung Res. 1997;23(3):205–27. doi: 10.3109/01902149709087368. [DOI] [PubMed] [Google Scholar]
- 9.Liu HZ, Yang HZ, Mi S, et al. Toll like receptor 2 mediates bleomycin-induced acute lung injury, inflammation and fibrosis in mice. Acta Pharmaceutica Sinica. 2010;45:976–86. [PubMed] [Google Scholar]
- 10.Rocco PR, Negri EM, Kurtz PM, et al. Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am J Respir Crit Care Med. 2001;164:1067–71. doi: 10.1164/ajrccm.164.6.2007062. [DOI] [PubMed] [Google Scholar]
- 11.He R, Geha RS. Thymic stromal lymphopoietin. Ann NY Acad Sci. 2010;1183:13–24. [Google Scholar]
- 12.Naresh SR, Lianyu S, Ali S, et al. American Thoracic Society, editor. A65. Gene Expression, Phenotype, and Function in Airway Smooth Muscle: New Insights. 2010. Expression and function of TSLP receptor (TSLPR) in human airway smooth muscle activation: Role of STAT3 and MAPK pathways; pp. A2120–A2120. [Google Scholar]
- 13.Urata Y, Osuga Y, Izumi G, et al. Interleukin-1 stimulates the secretion of thymic stromal lymphopoietin (TSLP) from endometrioma stromal cells: Possible involvement of TSLP in endometriosis. Hum Reprod. 2012;27:3028–35. doi: 10.1093/humrep/des291. [DOI] [PubMed] [Google Scholar]
- 14.Fang L, Zhan S, Huang C, et al. TRPM7 channel regulates PDGF-BB-induced proliferation of hepatic stellate cells via PI3K and ERK pathways. Toxicol Appl Pharmacol. 2013;272:713–25. doi: 10.1016/j.taap.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Ju W, Zhihong Y, Zhiyou Z, et al. Inhibition of alpha-SMA by the ectodomain of FGFR2c attenuates lung fibrosis. Mol Med. 2012;18:992–1002. doi: 10.2119/molmed.2011.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamashita M, Yamauchi K, Chiba R, et al. The definition of fibrogenic processes in fibroblastic foci of idiopathic pulmonary fibrosis based on morphometric quantification of extracellular matrices. Hum Pathol. 2009;40:1278–87. doi: 10.1016/j.humpath.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Amara N, Goven D, Prost F, et al. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax. 2010;65:733–38. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li FZ, Cai PC, Song LJ, et al. Crosstalk between calpain activation and TGF-beta1 augments collagen-I synthesis in pulmonary fibrosis. Biochim Biophys Acta. 2015;1852:1796–804. doi: 10.1016/j.bbadis.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Torii S, Yamamoto T, Tsuchiya Y, Nishida E. ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci. 2006;97:697–702. doi: 10.1111/j.1349-7006.2006.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 21.Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–86. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim EK, Choi E-J. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Chopra P, Kanoje V, Semwal A, Ray A. Therapeutic potential of inhaled p38 mitogen-activated protein kinase inhibitors for inflammatory pulmonary diseases. Expert Opin Invest Drugs. 2008;17:1411–25. doi: 10.1517/13543784.17.10.1411. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Feng G, Wang GL, Liu GJ. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol. 2008;584:159–65. doi: 10.1016/j.ejphar.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Yeh CH, Yang JJ, Yang ML, et al. Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-kappaB pathway. Free Radic Biol Med. 2014;69:249–57. doi: 10.1016/j.freeradbiomed.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Muchir A, Wu W, Worman HJ. Mitogen-activated protein kinase inhibitor regulation of heart function and fibrosis in cardiomyopathy caused by lamin A/C gene mutation. Trends Cardiovasc Med. 2010;20:217–21. doi: 10.1016/j.tcm.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokubo S, Sakai N, Furuichi K, et al. Activation of p38 mitogen-activated protein kinase promotes peritoneal fibrosis by regulating fibrocytes. Perit Dial Int. 2012;32:10–19. doi: 10.3747/pdi.2010.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma JY, Medicherla S, Kerr I, et al. Selective p38α mitogen-activated protein kinase inhibitor attenuates lung inflammation and fibrosis in IL-13 transgenic mouse model of asthma. J Asthma Allergy. 2008;1:31–44. doi: 10.2147/jaa.s4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryu WI, Lee H, Kim JH, et al. IL-33 induces Egr-1-dependent TSLP expression via the MAPK pathways in human keratinocytes. Exp Dermatol. 2015 doi: 10.1111/exd.12788. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Oh MH, Oh SY, Yu J, et al. IL-13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. J Immunol. 2011;186:7232–42. doi: 10.4049/jimmunol.1100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christmann RB, Mathes A, Affandi AJ, et al. Thymic stromal lymphopoietin is up-regulated in the skin of patients with systemic sclerosis and induces profibrotic genes and intracellular signaling that overlap with those induced by interleukin-13 and transforming growth factor beta. Arthritis Rheum. 2013;65:1335–46. doi: 10.1002/art.37859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung TJ, Liu SF, Liu GZ, et al. shRNA for thymic stromal lymphopoietin: A novel therapeutic approach for pulmonary fibrosis. J Cell Sci Ther. 2013;4:144–52. [Google Scholar]