Abstract
Background
Classical Hodgkin lymphoma (cHL) is characterized by sparse malignant Hodgkin and Reed-Sternberg cells dispersed in an inflammatory microenvironment. Immune evasion of malignant cells is partially due to the existence of a subpopulation of immunosuppressive regulatory T cells (Treg). The aim of this study was to analyze T cell composition in cHL with special emphasis on Treg in regard to Epstein-Barr virus (EBV) status, subtype, and patient age.
Material/Methods
The study included 102 patients with cHL diagnosed during a 12-year period. EBV status of cHL was assessed immunohistochemically using antibodies directed to the EBV- encoded LMP1. To define T lymphocyte populations, slides were double-stained with FOXP3 for Treg, and CD4 or CD8 for T cells. In each case the number of single- and/or double-positive cells was counted on an image analyzer in 10 high-power fields. Statistical analysis was performed and differences were considered significant at P<0.05.
Results
EBV-positive status of cHL was confirmed in 30 (29%) cases, mainly in patients older than 54 years and in mixed cellularity subtype. In EBV-positive cHL, higher numbers of CD8+ cells were found. In cHL with positive EBV status, more FOXP3+ Treg were found, as well as higher numbers of FOXP3+CD4+ Treg compared with EBV-negative cHL. The number of CD4+ cells decreased with age. The frequency of FOXP3+CD8+ Treg was variable, without a statistically significant association with age or EBV status.
Conclusions
EBV status has an impact on composition of T cell populations in the cHL microenvironment.
MeSH Keywords: Herpesvirus 4, Human; Hodgkin Disease; T-Lymphocytes, Regulatory
Background
Hodgkin lymphoma (HL) is one of the most common lymphomas in the Western world: it accounts for 30% of all lymphomas and 15% of all malignancies in young adults [1,2]. On the basis of difference in the morphology, phenotype of the lymphoma cells, and the composition of inflammatory infiltrate, HL is divided into classical HL (cHL), subtyped into nodular sclerosis (NS), mixed cellularity (MC), lymphocyte rich (LR), and lymphocyte depleted (LD); and nodular lymphocyte predominant HL (NLPHL), which accounts for only 5% of HL [3,4]. In industrialized countries HL presents with a bimodal age distribution, with a rise in incidence in young adults (20–34 years) and in elderly people (55–75 years). In developing countries, the first peak occurs earlier, in children and teenagers. Also, an inverse correlation between incidence of HL and socioeconomic status was observed [5–7]. A prerequisite for the diagnosis of HL is malignant mononuclear Hodgkin cells and multinuclear Reed-Sternberg cells (HRS), which represent only 1–10% of the total tumor mass and reside in abundant inflammatory infiltrate. The origin of HRS cell has long been an enigma due to the global loss of B cell phenotype and aberrant expression of markers characteristic of T cells or dendritic cells [3,4]. In 1994 Kuppers discovered that HRS are derived from pre-apoptotic germinal center B cells that have acquired disadvantageous “crippling” mutations, and in normal circumstances would have undergone apoptosis [8–10]. In less than 1% of cases, HRS is of T cell origin [11]. The pathogenesis of HL is only partially understood and includes genetic and environmental factors [2 6]. Genetic lesions frequently involve members of the NFκB family and the Jak-Stat signaling pathway, pointing to their central role in the pathogenesis, but multiple signaling pathways exist and cooperate [3,4]. Epstein-Barr virus (EBV) can be found in HRS in 40% of cHL in the Western world, and in 90–100% in developing countries, such as those in Africa and Latin America [5–7]. EBV-positive HL is more frequent in children, older adult males, and mixed cellularity subtype of cHL [6]. Older individuals with EBV-positive HL have a worse prognosis compared to young adults with EBV-positive HL, who have longer disease-free survival (DFS) [6,12]. In cHL, EBV expresses a limited set of latent viral genes: EBNA1, LMP1, LMP2A, EBER1, and EBER2 [13]. The tumor microenvironment of cHL is composed of mixtures of lymphocytes, plasma cells, macrophages, eosinophils, and mastocytes [14,15]. In the last decade, regulatory T cells (Treg) have been studied extensively in non-tumor and tumor pathology [16]. Treg are developmentally and functionally distinct T cell subpopulations engaged in sustaining immunological self-tolerance and homeostasis [17]. FOXP3, a forkhead winged-helix transcriptional factor, is considered as a lineage-specific marker for Treg necessary for their development and function, as the majority of Treg are FOXP3-positive [17–22]. In the tumor microenvironment, Treg exert their suppressive activity on effector T cells (Teff), fostering immune privilege for tumor cells [21–25]. Unlike the majority of solid tumors, in which high numbers of Treg are connected to poor outcome, in HL it is associated with favorable outcome [24,26–28]. Treg have been studied in HL in the context of EBV status, but without identifying Treg subsets [26,29–31]. The aim of our study was to analyze T cell composition in cHL with special emphasis on Treg and Treg subtypes in regard to EBV status, cHL subtype, and patient age.
Material and Methods
This retrospective case-control study included patients diagnosed with cHL in a 12-year period (1997–2009) at the Department of Pathology, Forensic Medicine, and Cytology, Clinical Hospital Center Split, Croatia. Paraffin blocks of tumor tissue were retrieved from the Department’s archive, and were reviewed and classified according to WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues, 2008. All samples were taken prior the initiation of treatment (cytostatic or irradiation). Inclusion criteria were: sufficient amount of tumor tissue in paraffin block, as well as all the necessary patient data (age, sex, and HL subtype). All cases that did not fulfill the criteria were excluded. A total of 102 cases were included. Approval for the study was obtained from the Hospital Ethics Committee (code 2181-147-08-01/01-M.J.).
Paraffin-embedded tissue samples were cut into slices 3-μm-thick, deparaffinized, and dehydrated. For antigen retrieval, slides were incubated for 20 min in TRIS/EDTA buffer, pH 9 (Dako, Denmark). Endogenous peroxidase activity was blocked by adding 200 μl of 3% H2O2. After rinsing in phosphate-buffered saline (PBD), monoclonal antibodies were applied. EBV status was assessed using LMP-1 mouse monoclonal antibody (clone CS1-4, Dako, Denmark), and results were interpreted based on brown membranous staining in HRS cells. For double-immunohistochemistry staining, the EnVisionTM G/2 Double stain System and Rabbit/Mouse DAB+Permanent Red, Dako, Denmark) were used according to the manufacturer’s instructions.
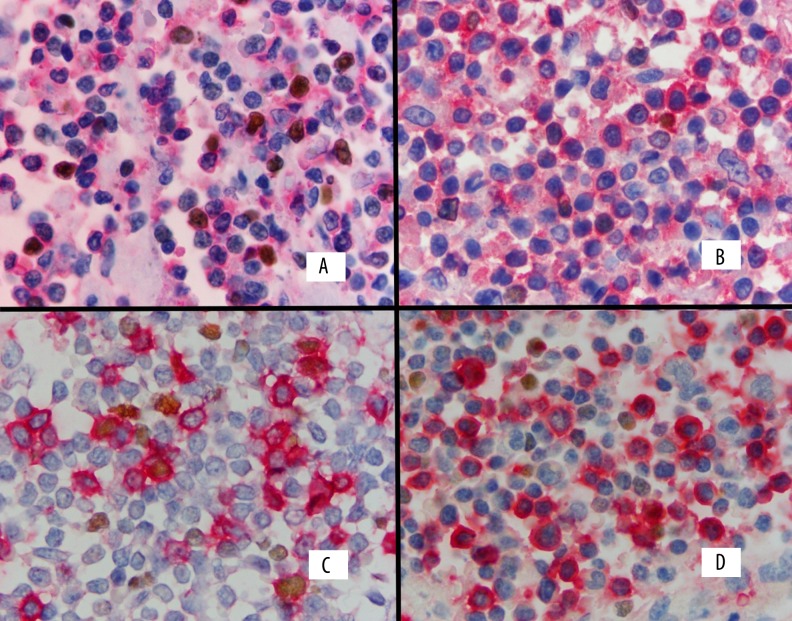
Monoclonal mouse antibody against FOXP3 (236A/E7, Santa Cruz Biotechnology, Inc.) was applied first (1:50), incubated overnight in a humidity-controlled chamber, and then the staining process was finished in a Dako Autostainer using the standard manufacturer’s protocol. Consecutively, monoclonal antibody CD4 (clone 4 B12, Novocastra, UK, 1:100) or monoclonal antibody CD8 (clone DK35, Dako, Denmark 1:100) was applied, incubated for 1 h, and the procedure was finished using the standard manufacturer’s protocol. DAB was used as a chromogen to identify FOXP3 and Permanent Red was used to identify CD4 or CD8. Positive results were identified as brown nuclear staining of FOXP3 and red membranous staining for CD4 or CD8 (Figure 1). Slides were analyzed with an Olympus BX41 microscope at 1000×, photographed with an Olympus digital camera, and analyzed with the Analysis program (Soft Imaging System analysis program). The number of FOXP3 cells, CD4, CD8, and dual-positive FOXP3+CD4+ and FOXP3+CD8+ cells were counted in 10 random fields and expressed as total cell number.
Figure 1.
Double immunostaining for FOXP3 and CD4 and CD8 expression in classical Hodgkin lymphoma. FOXP3 expression is shown as nuclear brown staining and CD4 or CD8 expression as membranous red staining. Double-positive cells show combined nuclear brown and membranous red staining. (A) Numerous double-positive FOXP3/CD4 cells and single-positive CD4 cells. (B) Rare double-positive FOXP3/CD4 cells and numerous single-positive CD4 cells. (C) Some double-positive FOXP3/CD8 cells with single-positive CD8 cells. (D) No double-positive FOXP3/CD8 cell and some single-positive CD8 cells.
For statistical analysis, the Mann-Whitney test, log-rank test, and χ2 test were used. Results were considered significant at P<0.05.
Results
Of the 102 patients, 53 (52%) were males and 49 (48%) were females. Median age was 36 years (range, 5–87); 24.5% patients were younger than 26.5 years, 26.5% were 26.5–36 years, 25.5% were 36.1–54 years, and 23.5% were >54 years old. According to the expression of LMP1 in malignant cells, EBV status was negative in 72 (71%) patients and was positive in 30 (29%) patients. Subtypes were: 62% nodular sclerosis (NS), 24.5% mixed cellularity (MC), and 13.5% lymphocyte-rich (LR) and lymphocyte-depleted (LD) subtypes together. The absolute numbers of FOXP3+, CD4+, CD8+, FOXP3+CD4+, and FOXP3+CD8+ cells in the microenvironment, counted in 10 high-power magnification fields, are shown in Table 1.
Table 1.
Distribution of T cell phenotypes in microenvironment of classic Hodgkin lymphoma on 10 high power magnification fields.
| Phenotype | Median (min-max) N |
|---|---|
| FOXP3+ | 536 (17–2368) |
| CD4+ | 2920 (708–5318) |
| FOXP3+CD4+ | 414 (16–2355) |
| CD8+ | 656 (33–2899) |
| FOXP3+CD8+ | 4 (0–431) |
There was a statistically significant correlation between EBV status and patient age (χ2=43.9; P<0.001). EBV-positive HL was 13 times more frequent in patients older than 54 years than in younger patients (p<0.001). Most of the EBV-positive HL were of MC subtype (P=0.001). EBV-positive HL also had higher numbers of FOXP3+ (χ2=6.8; P=0.009) and CD8+ (χ2=4.27; P=0.029) cells. Twice as many FOXP3+CD4+ cells were found in EBV-positive compared with EBV-negative cases (χ2=6.8 P=0.009). EBV-negative cases contained more CD4+ cells (χ2=7.31; P=0.007) (Table 2). Correlation of cHL subtypes showed that NS and MC contained more FOXP3+ (p=0.014) and FOXP3+CD4+ cells compared to LD and LR subtypes (χ2=21.890, p=0.004) (data not shown).
Table 2.
Correlation of EBV status with clinical and histological parameters in 102 cases of cHL.
| Parameter | LMP1− N=72 (%) |
LMP1+ N=30 (%) |
χ2 | OR (95%CI) | P | |
|---|---|---|---|---|---|---|
| Sex | M | 37 (51) | 16 (53) | 0.032 | 0.925 (0.394–2.17) | 0.585 |
| F | 35 (49) | 14 (47) | ||||
| Age | <54 | 68 (94) | 10 (33) | 43.90 | 3.4 (9.6–12.0) | <0.001 |
| >54.1 | 4 (6) | 20 (67) | ||||
| Subtype | NS | 50 (70) | 13 (43) | 11.28 | 1.465 (0.829–2.59) | 0.189 |
| MC | 11 (15) | 14 (47) | ||||
| LP and LD | 11 (15) | 3 (10) | ||||
| FOXP3+ | <536.5 | 42 (58) | 9 (30) | 6.8 | 3.27 (1.3–8.1) | 0.011 |
| >536.51 | 30 (41) | 21 (70) | ||||
| CD4+ | <3464 | 49 (68) | 28 (93) | 7.31 | 0.674 (0.451–1.006) | 0.053 |
| >3464.1 | 23 (32) | 2 (7) | ||||
| CD8+ | <656.5 | 41 (57) | 10 (33) | 4.72 | 2.64 (1.09–6.45) | 0.032 |
| >656.51 | 31 (43) | 20 (67) | ||||
| FOX+CD4+ | <414.5 | 42 (58) | 9 (30) | 6.8 | 3.27 (1.31–8.1) | 0.011 |
| >414.51 | 30 (42) | 21 (70) |
When correlating age groups with total number of all T cell types, we found that patients younger than 26.5 years had higher numbers of CD4+ cells compared to patients aged 26.5–36 years (χ2=21.890, P=0.009). Distribution of FOXP3+CD8+ cells was so variable that no statistical differences were found. The findings are summarized in Table 3.
Table 3.
Correlation of patient’s age with number of T cells.
| T cells parameter | N | Age (years) | χ2 | P | |||
|---|---|---|---|---|---|---|---|
| <26.5 N=25 (%) |
26.5–36 N=27 (%) |
36.1–54 N=26 (%) |
>54 N=24 (%) |
||||
| FOXP3+ | <227.75 | 7 (28) | 3 (11) | 10 (39) | 5 (21) | 9.650 | 0.380 |
| 227.75–536,5 | 8 (32) | 6 (22) | 7 (27) | 5 (21) | |||
| 536.55–902 | 5 (20) | 8 (30) | 6 (23) | 7 (29) | |||
| >902.1 | 5 (20) | 10 (37) | 3 (11) | 7 (29) | |||
| CD8+ | <293 | 8 (32) | 10 (37) | 4 (15) | 3 (12) | 11.148 | 0.266 |
| 293.1–656.5 | 6 (24) | 7 (26) | 8 (31) | 5 (21) | |||
| 656.5–1056.5 | 3 (12) | 5 (18.5) | 8 (31) | 10 (42) | |||
| >1056.5 | 8 (32) | 5 (18.5) | 6 (23) | 6 (25) | |||
| FOX+CD8+ | 0 | ||||||
| 0.0001–4 | |||||||
| 4001–30.75 | 1 (4) | 1 (4) | 1 (4) | 1.117 | 0.773 | ||
| >30.75 | 24 (96) | 27 (100) | 25 (96) | 23 (96) | |||
| CD4+ | <2363 | 6 (24) | 7 (26) | 4 (15) | 8 (33) | 21.890 | 0.009 |
| 2363.1–2920.5 | 13 (48) | 8 (31) | 5 (21) | ||||
| 2920.5–3464 | 9 (36) | 4 (15) | 6 (23) | 7 (29) | |||
| >3464.1 | 10 (40) | 3 (11) | 8 (31) | 4 (17) | |||
| FOX+CD4+ | <188.75 | 6 (24) | 4 (15) | 9 (35) | 6 (25) | 9.336 | 0.407 |
| 188.75–414.5 | 9 (36) | 5 (18) | 8 (31) | 4 (17) | |||
| 414.5–811.75 | 5 (20) | 8 (30) | 6 (23) | 7 (29) | |||
| >811.75 | 5 (20) | 10 (37) | 3 (11) | 7 (29) | |||
Discussion
Results of our study showed that EBV-positive cHL contains generally higher number of FOXP3+Treg than EBV-negative cHL, and FOXP3+CD4+ Treg are the dominant Treg population. In EBV-negative cHL, higher numbers of non-regulatory CD4+ cells were found. We observed that the number of CD4+ cells decreased with age at some point after age 26 years. Distribution of FOXP3+CD8+ Treg was so variable that no clear associations were found. We found that 29% of cHL were EBV-positive, and most of these patients were older than 54 years and had MC subtype, which is in concordance with previous findings [1]. Our results show that our study population fit the “Western” population pattern, with approximately 30% EBV-positive cHL in immunocompetent patients. Previous studies have shown that the cHL microenvironment is enriched in Treg, and their high number could explain immune evasion of HRS [29,30]. HRS cells express ligands CCL17, CCL22, and Galectin 1, which selectively attract CCR4 Treg to the cHL microenvironment [15,32]. HRS cells also express IL21, which upregulates CC chemokine macrophage-inflammatory protein-3α and attracts FOXP3+ Treg [33]. Baumforth et al. found that in EBV-positive cHL, EBNA1 upregulates expression of CCL20 in HRS cells, resulting in the increased migration of FOXP3+ cells to the microenvironment [31]. Although most authors agree that the cHL microenvironment is enriched in Treg, no clear evidence of greater bias toward higher number of Treg in EBV-positive cHL has been found [26,27,29–31]. Results from a study by Assis et al. were somewhat similar to ours. They performed qualitative and quantitative analysis of Treg in 130 cases of cHL in a Brazilian population and found that the presence of EBV correlates with increased number of CD4+CD25+FOXP3+ Treg, but there was no association between Treg and clinical characteristics [34]. Unlike Baumforth et al., we found higher numbers of FOXP3+Treg and FOXP3+CD4+ cells in EBV-positive cHL [31]. This may be because our study was performed with a larger number of patients. CD4+ lymphocytes are the dominant T cells in the cHL microenvironment [35]. We found increased numbers of CD4+ cells in EBV-negative cHL cases. According to Baumforth et al., this could partially be explained by the absence of EBNA1 effect on migration of Treg [31]. Morales et al. recently reported that EBV is responsible for enrichment of the HL microenvironment with regulatory Type 1 cells (Tr1) by increasing gene expression of Tr1-related markers and associated immunosuppressive cytokines, such as interleukin 10. This observation suggests that differences in EBV-positive and EBV-negative cHL Treg pools could be even bigger and include new molecules and regulatory mechanisms [36]. Kyatsu et al. analyzed the T cell microenvironment in HIV-associated HL, which is always an EBV-positive neoplasm. They found that the HIV-associated HL microenvironment contained significantly lower proportions of FOXP3+ Treg and higher proportions of CD8+ cells, compared to control HIV-negative HL patients. Also, in the control group, EBV-positive HL was associated with more FOXP3+ cells and CD8+ cells than in EBV-negative HL, which is in agreement with our results [37]. Although the majority of CD8 cells are directed toward EBV antigens expressing latency III program, LMP1 and LMP2 can also act as immunogens for CD8+ cells [38]. Kyatsu et al. reported that the abundant CD8+ cells present in HIV-associated HL probably are not LMP-specific cytotoxic cells, but rather immature anergic cells [37]. Cosmi et al. considered that increased number of CD8+ in EBV-positive HL in immunocompetent patients do not necessarily reflect antiviral immune response, but perhaps are a part of the total regulatory pool [39]. CD8 regulatory T cells share functional characteristics with CD4+ Treg; they are anergic (do not produce perforin or granzyme) and immunosuppressive (their effectiveness in viral-induced tumors is questionable) [39, 40]. We showed that the number of FOXP3+CD8+ cells in the cHL microenvironment is variable and without statistically significant differences. CD8+ Treg can be FOXP3-, which could mean that the number of CD8+Treg cells was underestimated by FOXP3 expression alone, and other markers are necessary to assess the CD8 Treg “pool” [20]. We found a decreased number of CD4 cells in the cHL microenvironment in individuals older than 26 years, which may reflect a normal process of immune senescence due to decreased thymic output of Teff and Treg cells [40]. We did not find a difference in Treg according to age, which agrees with the results of Dejaco and Fessler, who considered that the number of Treg cells remains the same, but their function is changed [41,42]. In contrast, Raynor reported that aging is associated with increased number of Treg cells, with consequent increased incidence of tumors and autoimmune diseases [43]. Our previous study showed that younger individuals (<35 years old) with EBV-positive cHL have longer disease-free survival [12]. We expected to find that younger individuals with EBV-positive cHL would have higher numbers of FOXP3+ Treg, but we did not find this correlation. The cHL Treg pool is probably highly heterogeneous, comprising FOXP3+ and FOXP3– suppressor cells among CD4 and CD8, as well as other cell types. Other possible mechanisms maybe exist that ensure better outcome in young individuals with EBV-positive cHL.
Conclusions
Epstein-Barr viral status has an impact on the composition of the T cell population in cHL. In cHL with positive EBV status, the microenvironment is more enriched with FOXP3+ Treg, mainly FOXP3+CD4 cells. Aging has an impact on CD4+ numbers, but does not affect the number of Treg cells in cHL.
Acknowledgments
The authors would like to thank Prof. Paul G. Murray (School of Cancer Sciences, University of Birmingham, Birmingham, United Kingdom) for his help and expert advice in the preparation of the manuscript.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: The authors received financial support from the Ministry of Science, Education, and Sports of the Republic of Croatia
References
- 1.Stein H. Hodgkin Lymphoma. In: Swerdlow SH, Campo E, Harris NL, editors. WHO Classification of Tumors of Heamatopoietic and Lymphoid Tissue. 4th ed. Lyon: International Agency for Research on Cancer (IARC); 2008. pp. 322–34. [Google Scholar]
- 2.Salati M, Cesaretti M, Macchia M, et al. Epidemiological overview of hodgkin lymphoma across the Mediterranean Basin. Mediterr J Hematol Infect Dis. 2014;6(1):e2014048. doi: 10.4084/MJHID.2014.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9:15–27. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 4.Bräuninger A, Schmitz R, Bechtel D, et al. Molecular biology of Hodgkin’s and Reed/Sternberg cells in Hodgkin’s lymphoma. Int J Cancer. 2006;118:1853–61. doi: 10.1002/ijc.21716. [DOI] [PubMed] [Google Scholar]
- 5.Dinand V, Arya LS. Epidemiology of childhood Hodgkins disease: is it different in developing countries? Indian Pediatr. 2006;43:141–47. [PubMed] [Google Scholar]
- 6.Gandhi MK, Tellam JT, Khanna R. Epstein-Barr virus-associated Hodgkin’s lymphoma. Br J Haematol. 2004;125:267–81. doi: 10.1111/j.1365-2141.2004.04902.x. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy-Nasser AA, Hanley P, Bollard CM. Hodgkin disease and the role of the immune system. Pediatr Hematol Oncol. 2011;28:176–86. doi: 10.3109/08880018.2011.557261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Küppers R, Rajewsky K, Zhao M, et al. Hodgkin disease: Hodgkin and Reed-Sternberg cells picked from histological sections show clonal immunoglobulin gene rearrangements and appear to be derived from B cells at various stages of development. Proc Natl Acad Sci USA. 1994;91:10962–66. doi: 10.1073/pnas.91.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marafioti T, Hummel M, Foss HD, et al. Hodgkin and Reed-Sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95:1443–50. [PubMed] [Google Scholar]
- 10.Küppers R, Hansmann ML. The Hodgkin and Reed-Sternberg cell. Int J Biochem Cell Biol. 2005;37:511–17. doi: 10.1016/j.biocel.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Tzankov A, Bourgau C, Kaiser A, et al. Rare expression of T-cell markers in classical Hodgkin’s lymphoma. Mod Pathol. 2005;18:1542–49. doi: 10.1038/modpathol.3800473. [DOI] [PubMed] [Google Scholar]
- 12.Glavina-Durdov M, Jakic-Razumovic J, Capkun V, Murray P. Assessment of the prognostic impact of the Epstein-Barr virus-encoded latent membrane protein-1 expression in Hodgkin’s disease. Br J Cancer. 2001;84:1227–34. doi: 10.1054/bjoc.2001.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landais E, Saulquin X, Houssaint E. The human T cell immune response to Epstein-Barr virus. Int J Dev Biol. 2005;49:285–92. doi: 10.1387/ijdb.041947el. [DOI] [PubMed] [Google Scholar]
- 14.Herreros B, Sanchez-Aguilera A, Piris MA. Lymphoma microenvironment: Culprit or innocent? Leukemia. 2008;22:49–58. doi: 10.1038/sj.leu.2404970. [DOI] [PubMed] [Google Scholar]
- 15.Aldinucci D, Gloghini A, Pinto A, et al. The classical Hodgkin’s lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol. 2010;221:248–63. doi: 10.1002/path.2711. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Dhamne C, Chung Y, Alousi AM, et al. Peripheral and thymic foxp3 (+) regulatory T cells in search of origin, distinction, and function. Front Immunol. 2013;4:253. doi: 10.3389/fimmu.2013.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–62. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 20.Povoleri GA, Scottà C, Nova-Lamperti EA, et al. Thymic versus induced regulatory T cells – who regulates the regulators? Front Immunol. 2013;4:169. doi: 10.3389/fimmu.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanchot C, Terme M, Pere H, et al. Tumor-infiltrating regulatory T cells: Phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron. 2013;6:147–57. doi: 10.1007/s12307-012-0122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oleinika K, Nibbs RJ, Graham GJ, Fraser AR. Suppression, subversion and escape: The role of regulatory T cells in cancer progression. Clin Exp Immunol. 2013;171:36–45. doi: 10.1111/j.1365-2249.2012.04657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ondondo B, Jones E, Godkin A, Gallimore A. Home sweet home: the tumor microenvironment as a haven for regulatory T cells. Front Immunol. 2013;4:197. doi: 10.3389/fimmu.2013.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;2:2162–71. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Needham DJ, Lee JX, Beilharz MW. Intra-tumoural regulatory T cells: A potential new target in cancer immunotherapy. Biochem Biophys Res Commun. 2006;343:684–91. doi: 10.1016/j.bbrc.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Tzankov A, Meier C, Hirschmann P, et al. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 27.Alvaro T, Lejeune M, Salvadó MT, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–73. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 28.Kelley TW, Pohlman B, Elson P, Hsi ED. The ratio of FOXP3+ regulatory T cells to granzyme B+ cytotoxic T/NK cells predicts prognosis in classical Hodgkin lymphoma and is independent of bcl-2 and MAL expression. Am J Clin Pathol. 2007;128:958–65. doi: 10.1309/NB3947K383DJ0LQ2. [DOI] [PubMed] [Google Scholar]
- 29.Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 2004;103:1755–62. doi: 10.1182/blood-2003-07-2594. [DOI] [PubMed] [Google Scholar]
- 30.Marshall NA, Culligan DJ, Tighe J, et al. The relationships between Epstein-Barr virus latent membrane protein 1 and regulatory T cells in Hodgkin’s lymphoma. Exp Hematol. 2007;35:596–604. doi: 10.1016/j.exphem.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 31.Baumforth KR, Birgersdotter A, Reynolds GM, et al. Expression of the Epstein-Barr virus-encoded Epstein-Barr virus nuclear antigen 1 in Hodgkin’s lymphoma cells mediates Up-regulation of CCL20 and the migration of regulatory T cells. Am J Pathol. 2008;73:195–204. doi: 10.2353/ajpath.2008.070845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishida T, Ishii T, Inagaki A, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716–22. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 33.Lamprecht B, Kreher S, Anagnostopoulos I, et al. Aberrant expression of the Th2 cytokine IL-21 in Hodgkin lymphoma cells regulates STAT3 signaling and attracts Treg cells via regulation of MIP-3alpha. Blood. 2008;112:3339–47. doi: 10.1182/blood-2008-01-134783. [DOI] [PubMed] [Google Scholar]
- 34.Assis MC, Campos AH, Oliveira JS, et al. Increased expression of CD4+CD25 +FOXP3+ regulatory T cells correlates with Epstein-Barr virus and has no impact on survival in patients with classical Hodgkin lymphoma in Brazil. Med Oncol. 2012;29:3614–19. doi: 10.1007/s12032-012-0299-4. [DOI] [PubMed] [Google Scholar]
- 35.Poppema S, van den Berg A. Interaction between host T cells and Reed-Sternberg cells in Hodgkin lymphomas. Semin Cancer Biol. 2000;10:345–50. doi: 10.1006/scbi.2000.0327. [DOI] [PubMed] [Google Scholar]
- 36.Morales O, Mrizak D, François V, et al. Epstein-Barr virus infection induces an increase of T regulatory type 1 cells in Hodgkin lymphoma patients. Br J Haematol. 2014;166:875–90. doi: 10.1111/bjh.12980. [DOI] [PubMed] [Google Scholar]
- 37.Kiyasu J, Aoki R, Tanaka PY, et al. FOXP3(+) regulatory and TIA-1(+) cytotoxic T lymphocytes in HIV-associated Hodgkin lymphoma. Pathol Int. 2012;62:77–83. doi: 10.1111/j.1440-1827.2011.02754.x. [DOI] [PubMed] [Google Scholar]
- 38.Chapman AL, Rickinson AB, Thomas WA, et al. Epstein-Barr virus-specific cytotoxic T lymphocyte responses in the blood and tumor site of Hodgkin’s disease patients: implications for a T-cell-based therapy. Cancer Res. 2001;61:6219–26. [PubMed] [Google Scholar]
- 39.Cosmi L, Liotta F, Lazzeri E, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–14. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 40.Liotta F, Cosmi L, Romagnani P, et al. Functional features of human CD25+ regulatory thymocytes. Microbes Infect. 2005;7:1017–22. doi: 10.1016/j.micinf.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70:179–89. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 42.Dejaco C, Duftner C, Schirmer M. Are regulatory T-cells linked with aging? Exp Gerontol. 2006;41:339–45. doi: 10.1016/j.exger.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Raynor J, Lages CS, Shehata H, et al. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. 2012;24:482–87. doi: 10.1016/j.coi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]