Abstract
Zingiber officinale Roscoe has been widely used as a folk medicine to treat various diseases, including cancer. This study aims to re-examine the therapeutic potential of co-administration of natural products and cancer chemotherapeutics. Candidate material for this project, α-zingiberene, was extracted from Zingiber officinale Roscoe, and α-zingiberene makes up 35.02 ± 0.30% of its total essential oil. α-Zingiberene showed low IC50 values, 60.6 ± 3.6, 46.2 ± 0.6, 172.0 ± 6.6, 80.3 ± 6.6 (μg/mL) in HeLa, SiHa, MCF-7 and HL-60 cells each. These values are a little bit higher than IC50 values of general essential oil in those cells. The treatment of α-zingiberene produced nucleosomal DNA fragmentation in SiHa cells, and the percentage of sub-diploid cells increased in a concentration-dependent manner in SiHa cells, hallmark features of apoptosis. Mitochondrial cytochrome c activation and an in vitro caspase-3 activity assay demonstrated that the activation of caspases accompanies the apoptotic effect of α-zingiberene, which mediates cell death. These results suggest that the apoptotic effect of α-zingiberene on SiHa cells may converge caspase-3 activation through the release of mitochondrial cytochrome c into cytoplasm. It is considered that anti-proliferative effect of α-zingiberene is a result of apoptotic effects, and α-zingiberene is worth furthermore study to develop it as cancer chemotherapeutics.
Keywords: Cytotoxic activity, α-Zingiberene, Anti-proliferation, General essential oil, Cervix cancer cells
INTRODUCTION
Aromatic plant is a very precious source for developing new drugs. Plant essential oil, its components and second metabolite have many applications in folk medicine. For example, it has been used increasingly for the purpose of aromatherapy based on its various properties, such as anti-oxidative, anti-inflammatory, anti-bacterial and anti-cancer activities (1–3). Zingiber officinale Roscoe (Zingiberaceae) has been used in folk medicine for treating pain, inflammation, arthritis, urinary infections, female diseases and gastrointestinal disorders (4). Ginger is a common name of Zingiber officinale Roscoe. Most of the studies on the evaluation of ginger products for disease control potential have been centered around crude extracts on various physiological activity. Ginger essential oil (GEO) has been demonstrated to have anti-inflammatory, anti-nociceptive, and immunemodulatory effects in animal models (5,6). Regarding the anti-inflammatory action of GEO, its inhibitory actions on leukocyte-endothelial cell interactions in the microcirculatory network have been demonstrated (7).
Since 2000, there have been a very few research regarding cancer chemotherapeutic activity of Zingiber officinale Roscoe. A report found that [6]-gingerol and [6]-paradol exerted inhibitory effects on the viability and DNA synthesis of human promyelocytic leukemia (HL-60) cells (8). Another report showed that several ginger constituents, including some diarylheptanoids and gingerol-related compounds, had significant cytotoxic and apoptotic activities against human promyelocytic leukemia cells (9). In addition, the phenolic alkanone 6-gingerol was found to affect the viability of cancer cells only slightly, while 6-shogaol had a significant inhibitory effect by damaging microtubules and inducing mitotic arrest (10). Though α-zingiberene has been reported to be the major constituent of Zingiber officinale Roscoe, even the latest studies with advanced analysis techniques (11–15) provided nearly no mechanistic evidence for its medicinal activity. The aim of this study is to provide a basic insight on the chemopreventive potential of GEO and its component by in vitro research.
MATERIALS AND METHODS
Isolation of ginger essential oil and α-zingiberene
Fresh rhizomes of Zingiber officinale Roscoe were purchased from Omni Herb Co. (Daegu, Korea), which was established by the group of Korean oriental M.D. in the year of 1975. It was identified that the Zingiber officinale Roscoe we used in this study was harvested in Korea. Ginger essential oil (GEO) was extracted by conventional steam distillation using a clevenger-type apparatus for 3 hr. The essential oil was kept at 4°C in dark glass vials, and then used in tests. The oil was quantitatively and qualitatively analyzed using gas chromatography (GC) with flame ionization detector (Shimadzu GC-17A) and GC with mass spectrometry detector (QP 5050A) operating at 70 eV and with a mass range of 40~400 amu. Compounds were tentatively identified by comparing experimentally obtained linear temperature programmed retention indices with those reported in the literature. Mass spectra of the essential oil compounds were compared with the ones reported in the Wiley mass spectra library (sixth edition) as an aid to identification (16).
Part of GEO was subjected to flash chromatography on SiO2 gel soaked with AgNO3 (10%) column (63 cm × 5 cm i.d.) chromatography eluted with CH2Cl2-acetone in proportions of 99 : 1, 95 : 5 and 90 : 10 (200 mL for each eluent) to afford 30 fractions which were individually analyzed using GC-FID and then pooled into nine groups (1 to 9). Fraction 6 was composed of pure α-zingiberene. 1H NMR spectra of α-zingiberene was recorded on a Jeol 300 MHz instrument using deuterated chloroform as solvent and tetramethylsilane as internal standard. 13C NMR was performed on a Jeol (75 MHz) instrument.
Cell lines
HeLa (cervix cancer cell), SiHa (cervix cancer cell), MCF-7 (breast cancer cell) and HL60 cell (leukemia) lines were obtained from Korean Cell Line Bank (KCLB). The HeLa and MCF-7 cells were cultured in Minimal Essential Medium, and SiHa cells in Dulbecco’s Modified Eagle’s Medium, and HL60 cells in RPMI medium with 10% fetal bovine serum in a water-saturated atmosphere of 95% O2 and 5% CO2 at 37°C.
Cell proliferation assay
This is based on the conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to MTT-formazan by mitochondrial enzymes as previously described (17). HeLa, SiHa, MCF-7 and HL60 cells were seeded at a density of 7 × 104, 5 × 104, 4 × 104, and 7 × 105 cells per well each in 24-well plates and incubated for 24 hr. GEO, α-zingiberene and cisplatin (Sigma, St. Louis, MO, USA) was dissolved in PBS and added to the culture media at concentrations of 0~200 μg/mL range, and the cells were incubated for 24 hr. 120 μL of stock MTT (Sigma, St. Louis, MO, USA) solution was added into each well under the dark condition, and plates were incubated at 37°C for 24 hr. After centrifugation, 1 mL of the diluted DMSO with ethylalcohol (1:1) was added, which was performed to dissolve formazan. After shaking for 10 min at room temperature, 100 μL of each solution was transferred to 96-well plates, and the absorbance value of each well was read at 540 nm using ELISA reader (Model 550 Microplate Reader, Bio-Rad, Hercules, California, USA).
Quantitative analysis of fragment DNA
SiHa cells were incubated in growth medium for 4 hr with 1 μCi/mL [3H]-thymidine (Amersham-Pharmacia Biotech., London, UK). Then the cells were washed twice with PBS and incubated for 24 hr after treatment of α-zingiberene. The cells were washed and lysed with lysis buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA, 0.2% Triton X-100) (18). Low and high molecular weight DNA were separated by centrifugation and the amount of [3H]-thymidine of each supernatant was determined by liquid scintillation counter (Beckmann, Danvers, MA, USA). The percent change of DNA fragments was calculated as follows: % Fragments = [cpm of small DNA/(cpm of small DNA + cpm of large DNA) × 100].
Flow Cytometry analysis
After treatment with α-zingiberene for 24 hr, SiHa cells were washed with cold PBS and resuspended in PBS. DNA contents of cells were measured using a DNA staining kit (Cyle Test Plus DNA Reagent Kit, Becton Dickinson, Heidelberg, Germany). Propidium iodide (PI)-stained nuclear fractions were obtained by following the kit protocol. Data were acquired using Cell Quest Software with a FACS calibur (Becton Dickison) flow cytometry system using 20,000 cells per analysis. Cell cycle distributions were calculated using Mod Fit LT 2.0 software (Verity Software House, Topsham, ME, USA).
Preparation of cytosolic extracts and immunoblotting
After treatment of α-zingiberene for 24 hr, the cells were collected and resuspended in 500 μL of extraction buffer (50 mM Pipes-KOH, 220 mM mannitol, 68 mM sucrose, pH 7.4, 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, and protease inhibitors). After 30 min incubation on ice, cells were homogenized using a glass dounce and a tight pestle (50 strokes). Cell homogenates were centrifuged and 10 μL of protein was loaded on 15% SDS-polyacrylamid gels (19). Mitochondrial cytochrome c was detected with anti-cytochrome c monoclonal antibody (Phar Mingen).
Caspase-3 assay
After treatment of α-zingiberene for 24 hr, SiHa cells were harvested, washed twice with ice-cold PBS, and resuspended in lysis buffer (10 mM HEPES, pH 7.4, 2 mM Ethylenediaminetetraacetic acid (EDTA), 0.1% 3-((3-cholamidopropyl dimethylammonio)-1-propane-sulfonate) (CHAPS), 5 mM DL-Dithiothreitol (DTT), 1 mM Phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL aprotinin, 20 μg/mL leupeptin). The rest of the protocol followed the manufacturer’s instruction (Bio-Fad Lab., Hercules, CA, USA). The fluorescence was measured in a microplate reader (BIO-TEK Instruments, Winooski, VT, USA) using 360 nm excitation and 530 nm mission. Data were expressed fold-induction of caspase-3 activity compared to that of control cells.
Statistical analysis
The data were processed using (Graph Pad Software, CA, USA), and the statistical parameters, mean value, and SEM (standard error of mean) were calculated and compared among the groups. Statistical significance was determined using ANOVA (*p < 0.05, **p < 0.01).
RESULTS
Isolation of ginger essential oil and α-zingiberene
The main constituents as detected by GC/MS analysis was α-zingiberene which constituted 35.02 ± 0.30% of general essential oil (GEO), ar-curcumene (15.27 ± 0.85%), β-sesquiphellandrene (12.32 ± 0.43%) and others are minor components less than 5% each.
Inhibition of cell viability by ginger essential oil or α-zingiberene
The general essential oil as well as its one component, purified α-zingiberene were evaluated for its anticancer activity in vitro, initially against cervix cancer cell line SiHa (Table 1). The obtained results indicated that GEO was cytotoxic against this cell line with an IC50 value of 38.6 μg/mL. Further, the cytotoxic potential of GEO and α-zingiberene were evaluated against different human tumor cell line and positive control assay was carried out with cisplatin. The GEO displayed activity against all cell lines tested with IC50 values ranging from 38.6 to 82.0 μg/mL, being SiHa the most sensitive cell line to the GEO, with an IC50 of 38.6 μg/mL. Otherwise, the purified α-zingiberene from the essential oil showed a cytotoxic activity against HeLa, SiHa, MCF-7 and HL60 cell lines, ranging from 46.2 to 172.0 μg/mL.
Table 1.
IC50 (μg/mL) values of α-zingiberene to general essential oil (GEO) from Zingiber officinale Roscoe. and control (cisplatin) against cell lines
| Sample | HeLa | SiHa | MCF-7 | HL60 |
|---|---|---|---|---|
| GEO | 46.0 ± 0.9 | 38.6 ± 0.87 | 82.6 ± 3.2 | 39.1 ± 4.2 |
| α-Zingiberene | 60.6 ± 3.6 | 46.2 ± 0.6 | 172.0 ± 6.6 | 80.3 ± 6.6 |
| Cisplatin (control) | 28.2 ± 2.6 | 56.2 ± 3.8 | 31.2 ± 2.2 | 31.1 ± 1.8 |
Concentration dependent cytotoxic effect of α-zingiberene
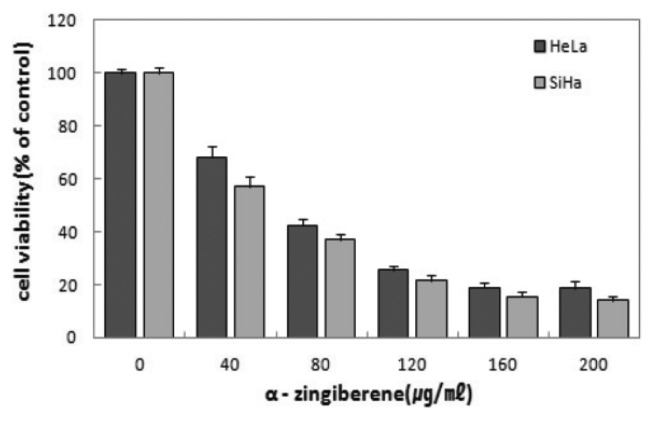
Dose dependent cytotoxic effect of α-zingiberene on two cervix cancer cell lines were evaluated. The viability was inhibited > 81.5% in HeLa cells exposed to 200 μg/mL of α-zingiberene, and the viability of SiHa was inhibited 86%, exposed to 200 μg/mL of α-zingiberene. Cell viabilities of two cervix cancer cells were inhibited in a concentration dependent manner (Fig. 1).
Fig. 1.
Decreased cell viability by α-zingiberene in HeLa and SiHa cells. After treatment of α-zingiberene for 24 hr, the cell viability was assessed by MTT staining. Results are expressed as the percent change of the control condition in which the cells were grown in the medium without drug. Data points represent the mean values of five replicates with the bars indicating s.e.m.
Effect of α-zingiberene on DNA fragmentation
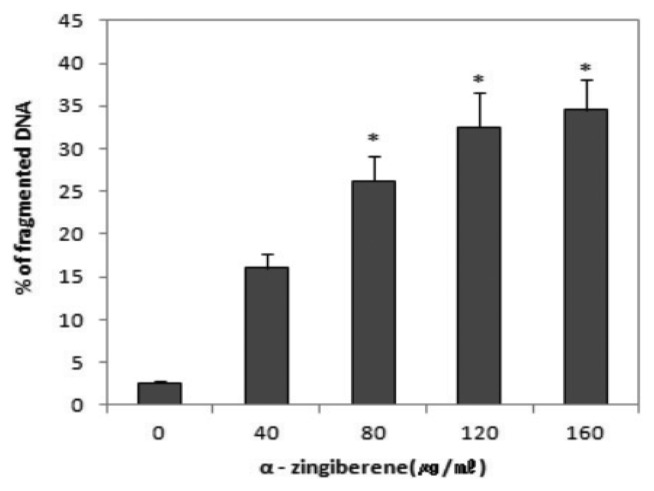
The apoptotic response, as judged by the appearance of a DNA ladder, was examined. A characteristic nucleosomal DNA fragmentation pattern, which is the biochemical hallmark of apoptosis, was quantitatively analysed using [3H]-thymidine incorporation test (Fig. 2). Fragmented DNA was significantly increased ranging from 80~160 μg/mL of α-zingiberene (*p < 0.05).
Fig. 2.
Effect of α-zingiberene on quantitation of DNA fragmentation. DNA fragmentation was analysed by [3H]-thymidine incorporation test, after cells were treated with each concentration of α-zingiberene for 24 hr. Data were presented as percentage of counts per minute (cpm) of fragmented DNA compared to total cpm. Data point represent the mean values of five replicates with bars indicating s.e.m. *p < 0.05 compared to control.
Effect of α-zingiberene on sub-diploid cell population
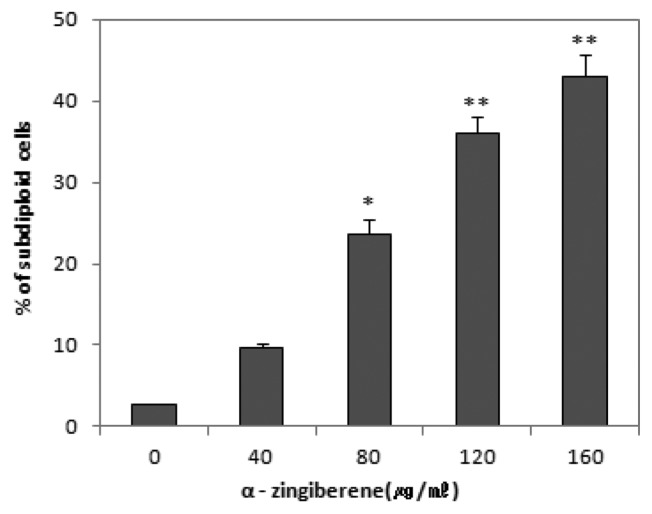
These results that the inhibitory action of α-zingiberene on the proliferation of SiHa cells was caused by the induction of apoptosis were also confirmed by the quantitation of apoptotic sub-diploid cells. As shown in Fig. 3, the percentage of sub-diploid cells was increased to 23.6%, 36.1%, and 43.0 5% by α-zingiberene treatment at concentrations of 80, 120, and 160 μg/mL respectively in SiHa cells. These results suggest that α-zingiberene induces clear apoptosis in SiHa cells in the range of 80~160 μg/mL (*p < 0.05, **p < 0.01).
Fig. 3.
The percentage of sub-diploids cells by flow cytometry analysis after treatment with 0 to 160 μg/mL of α-zingiberene on SiHa cells for 24 hr. Cells were stained with PI, and the number of sub-diploids cells were countered using FACScan flow cytometry. Cells with a subdiploid DNA content (> 5% of G0 content) were considered to be apoptotic. The distribution of cell cycle was analysed with ModiFitLT V 2.0. Data point represents mean values of five replicates, with bars indicating s.e.m. *p < 0.05, **p< 0.01 compared to control.
Cytochrome c release and caspase-3 activation
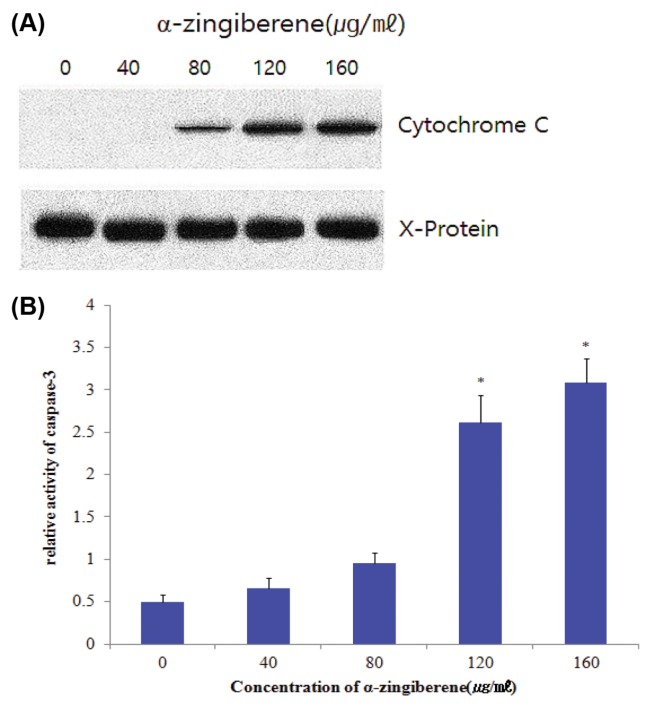
The activation of caspases is regulated by the release of cytochrome c from mitochondria to the cytosol (20,21). The present study showed that cytochrome c release was markedly induced by treatment with α-zingiberene for 24 hr in SiHa cells (Fig. 4A). We confirmed these results using a caspase-3 activity assay. As shown in Fig. 4B, caspase-3 activities were increased by treatment with α-zingiberene 120, 160 μg/mL respectively (*p < 0.05). These results suggest that α-zingiberene induces apoptosis through the release of mitochondrial cytochrome c and subsequently activates caspase-3.
Fig. 4.
Induction of cytochrome c release and caspase-3 activity by α-zingiberene. SiHa cells were treated with each concentration of by α-zingiberene for 24 hr. (A) Mitochondrial cytochrome c was detected by anti-cytochrome c monoclonal antibody. The aggregated cytochrome c, X-protein bends were used to normalize the protein loading. (B) Caspase-3 activity was measured by reading samples in fluorescence microplate reader. Data represents relative activity of caspase-3 after normalization with protein amounts. Data indicate mean values of four replicates, with bars indicating s.e.m. *p < 0.05 compared to control.
DISCUSSION
This study found that GEO mostly consists of α-zingiberene, ar-curcumene and β-sesquiphellandrene. But other recent studies with developed analysis technique show slightly different results. Some reported α-zingiberene as the biggest component (11–15), while other found ar-curcumene was the biggest one (22–25). These differences in the chemical composition of the oil from the same plant part could be due to the environmental, developmental, genetic or some other factors. Yield and composition of oil and oleoresins differ widely with the production conditions (26), variety, cultivars or population (27) and on climatic and soil factors. Besides, some study showed that, as separation error, α-zingiberene transformed to ar-curcumene depending on the fraction because it is thermally labile (22,28).
The purified α-zingiberene from GEO showed a cytotoxic activity against HeLa, SiHa, MCF-7 and HL60 cell lines, but the IC50 values were higher than those obtained from the GEO, indicating that there may be a synergism or additive activity between α-zingiberene and other substances present in the GEO (Table 1). It is highly desirable to have compounds that cause cancer cell death via apoptosis. Apoptosis eliminates malignant or cancer cells without damaging normal cells and surrounding tissues (29).
Thus, we herein study the apoptotic effect of α-zingiberene, which showed lower IC50 values in HeLa, SiHa, MCF-7 and HL60 cell lines. Fragmented DNA was significantly increased in the ranges of 40~160 μg/mL of α-zingiberene (Fig. 2). Since DNA fragmentation is one of the evidence of apoptosis (30–32), concentration-dependent inhibition of α-zingiberene in two cervix cancer cell line proliferation (Fig. 1) can be the result of apoptosis. SiHa cells treated with α-zingiberene in several concentrations (40~200 μg/mL) showed a concentration-dependent viability decrease with increased sub-diploid DNA production (Fig. 1 and Fig. 3). It is considered that α-zingiberene inhibited cell cycle progression and induced DNA fragmentation. It is reported that increased sub-diploid DNA population is also one of the evidence of apoptosis (33–35). It is also reported that DNA fragmentation reflects sub-diploid (sub-G1) increase and these result apoptosis (36–38). From this research, it is also considered that decreased cell viability of α-zingiberene in SiHa cells is the result of apoptotic effect, not necrotic effect.
There are some reports that caspases were implicated in apoptosis with the discovery that CED-3, the product of a gene required for cell death in the nematode C. elegans, is related to mammalian interleukin 1 B-converting enzyme (39,40). Our studies showed that α-zingiberene treatment to SiHa cells caused a concentration-dependent activation of caspase-3, one of the main executers of the apoptotic process (41–43). This study also showed that activation of caspase-3 is regulated by the release of cytochrome c from mitochondria to the cytosol (Fig. 4). Therefore, this study indicate that the pathway for apoptosis by α-zingiberene exists, in part, due to cytochrome c release and caspase activation, resulting in apoptosis.
Taken together, these findings suggest that α-zingiberene exhibits apoptotic effects, as reflected by the appearance of the, DNA fragmentation, sub-diploid cell increase and caspase 3 activation. Since the safety of this plant for human consumption has been known for many years, and its component α-zingiberene showed apoptotic effects in cervix cancer cell, SiHa, α-zingiberene is considered to be worth furthermore study to develop it as chemotherapeutics.
Supplementary Information
ACKNOWLEDGEMENTS
This work was supported by Dongseo University, “Dongseo Frontier Project” Research Fund of 2015.
REFERENCES
- 1.Fabian D, Sabol M, Domaracká K, Bujnáková D. Essential oils - their antimicrobial activity against Escherichia coli and effect on intestinal cell viability. Toxicol In Vitro. 2006;20:1435–1445. doi: 10.1016/j.tiv.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Barnes J. Quality, efficacy and safety of complementary medicines: fashions, facts and the future. Part I. regulation and quality. Br J Clin Pharmacol. 2003;55:226–233. doi: 10.1046/j.1365-2125.2003.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Shukla Y, Singh M. Cancer preventive properties of ginger: a brief review. Food Chem Toxicol. 2007;45:683–690. doi: 10.1016/j.fct.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Vendruscolo A, Takaki I, Bersani-Amado LE, Dantas JA, Bersani-Amado CA, Cuman RK. Antiinflammatory and antinociceptive activities of Zingiber officinale Roscoe essential oil in experimental animal models. Indian J Pharmacol. 2006;38:58–59. doi: 10.4103/0253-7613.19856. [DOI] [Google Scholar]
- 6.Carrasco FR, Schmidt G, Romero AL, Sartoretto JL, Caparroz-Assef SM, Bersani-Amado CA, Cuman RK. Immunomodulatory activity of Zingiber officinale Roscoe, Salvia officinalis L. and Syzygium aromaticum L. essential oils; evidence for humor- and cell-mediated responses. J Pharm Pharmacol. 2009;61:961–967. doi: 10.1211/jpp.61.07.0017. [DOI] [PubMed] [Google Scholar]
- 7.Nogueira de Melo GA, Grespan R, Fonseca JP, Farinha TO, da Silva EL, Romero AL, Bersani-Amado CA, Cuman RK. Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. J Nat Med. 2011;65:241–246. doi: 10.1007/s11418-010-0479-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee E, Surh YJ. Induction of apoptosis in HL-60 cells by pungent vanilloids, [6]-gingerol and [6]-paradol. Cancer Lett. 1998;134:163–168. doi: 10.1016/S0304-3835(98)00253-5. [DOI] [PubMed] [Google Scholar]
- 9.Wei QY, Ma JP, Cai YJ, Yang L, Liu ZL. Cytotoxic and apoptopic activities of diarylheptanoids and gingerol-related compounds from the rhizomes of Chinese ginger. J Ethnopharmacol. 2005;102:177–184. doi: 10.1016/j.jep.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Ishiguro K, Ando T, Maeda O, Ohmiya N, Niwa Y, Kadomatsu K, Goto H. Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem Biophys Res Commun. 2007;362:218–223. doi: 10.1016/j.bbrc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Norajit K, Laohakunjit N, Kerdchoechuen O. Antibacterial effect of Zingiberaceae essential oils. Molecules. 2007;23:2047–2060. doi: 10.3390/12082047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Moon HI, Cho SB, Kim SK. Composition and immunotoxicity activity of essential oils from leaves of Zingiber officinale Roscoe against Aedes aegypti L. Immunopharmacol Immunotoxicol. 2011;33:201–204. doi: 10.3109/08923973.2010.495393. [DOI] [PubMed] [Google Scholar]
- 13.Sasidharan I, Venugopal VV, Menon AN. Essential oil composition of two unique ginger (Zingiber officinale Roscoe) cultivars from Sikkim. Nat Prod Res. 2012;26:1759–1764. doi: 10.1080/14786419.2011.571215. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto-Ribeiro MM, Grespan R, Kohiyama CY, Ferreira FD, Mossini SA, Silva EL, Filho BA, Mikcha JM, Machinski M. Effect of Zingiber officinale essential oil on Fusarium verticillioides and fumonisin production. Food Chem. 2013;141:3147–3152. doi: 10.1016/j.foodchem.2013.05.144. [DOI] [PubMed] [Google Scholar]
- 15.Khrimian A, Shirali S, Guzman F. Absolute configurations of zingiberenols isolated from Ginger (Zingiber officinale) rhizomes. J Nat Prod. 2015;78:3071–3074. doi: 10.1021/acs.jnatprod.5b00638. [DOI] [PubMed] [Google Scholar]
- 16.Adams RP. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured Publishing Corporation; Carol Stream: 2001. pp. 456–460. [Google Scholar]
- 17.Ji M, Choi J, Lee J, Lee Y. Induction of apoptosis by ar-turmerone on various cell lines. Int J Mol Med. 2004;14:253–256. [PubMed] [Google Scholar]
- 18.Mohammad AM, Razieh Y, Mohammad HS. The cytotoxic and anti-proliferative effects of 3-hydrogenkwadaphin in K562 and Jurkat cells is reduced by guanosine. J Biochem Mol Biol. 2005;38:391–398. doi: 10.5483/BMBRep.2005.38.4.391. [DOI] [PubMed] [Google Scholar]
- 19.Finucanne DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-XL. J Biol Chem. 1999;274:2225–2233. doi: 10.1074/jbc.274.4.2225. [DOI] [PubMed] [Google Scholar]
- 20.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria : a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal M, Walia S, Dhingra S, Khambay BP. Insect growth inhibition, antifeedant and antifungal activity of compounds isolated/derived from Zingiber officinale Roscoe (ginger) rhizomes. Pest Manag Sci. 2001;57:289–300. doi: 10.1002/ps.263. [DOI] [PubMed] [Google Scholar]
- 23.Nogueira de Melo GA, Grespan R, Fonseca JP, Farinha TO, da Silva EL, Romero AL, Bersani-Amado CA, Cuman RK. Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. J Nat Med. 2011;65:241–246. doi: 10.1007/s11418-010-0479-5. [DOI] [PubMed] [Google Scholar]
- 24.Bayala B, Bassole IH, Gnoula C, Nebie R, Yonli A, Morel L, Figueredo G, Nikiema JB, Lobaccaro JM, Simpore J. Chemical composition, antioxidant, antiinflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE. 2014;9:e92122. doi: 10.1371/journal.pone.0092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Höferl M, Stoilova I, Wanner J, Schmidt E, Jirovetz L, Trifonova D, Stanchev V, Krastanov A. Composition and comprehensive antioxidant activity of ginger (Zingiber officinale) essential oil from Ecuador. Nat Prod Commun. 2015;10:1085–1090. [PubMed] [Google Scholar]
- 26.Blair J, Aichinger T, Hackal G, Hueber K, Dachler M. Essential oil content and composition in commercially available dill cultivars in comparison to caraway. Ind Crops Prod. 2001;14:229–239. doi: 10.1016/S0926-6690(01)00088-7. [DOI] [Google Scholar]
- 27.Galambosi B, Peura P. Agrobotanical features and oil content of wild and cultivated forms of caraway (Carum carvi L) J Essent Oil Res. 1996;8:389–397. doi: 10.1080/10412905.1996.9700646. [DOI] [Google Scholar]
- 28.Bartley JP, Foley P. Supercritical fluid extraction of australian-grown ginger (Zingiber officinale) J Sci Food Agric. 1994;66:365–371. doi: 10.1002/jsfa.2740660314. [DOI] [Google Scholar]
- 29.Chen W, Lu Y, Gao M, Wu J, Wang A, Shi R. Anti-angiogenesis effect of essential oil from Curcuma zedoaria in vitro and in vivo. J Ethnopharmacol. 2011;133:220–226. doi: 10.1016/j.jep.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 30.Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab. 1996;16:186–194. doi: 10.1097/00004647-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Ioannou YA, Chen FW. Quantitation of DNA fragmentation in apoptosis. Nucleic Acids Res. 1996;24:992–993. doi: 10.1093/nar/24.5.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins JA, Schandi CA, Young KK, Vesely J, Willingham MC. Major DNA fragmentation is a late event in apoptosis. J Histochem Cytochem. 1997;45:923–934. doi: 10.1177/002215549704500702. [DOI] [PubMed] [Google Scholar]
- 33.Chen S, Dobrovolsky VN, Liu F, Wu Y, Zhang Z, Mei N, Guo L. The role of autophagy in usnic acid-induced toxicity in hepatic cells. Toxicol Sci. 2014;142:33–44. doi: 10.1093/toxsci/kfu154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubio V, Calvino E, Garcia-Perez A, Herraez A, Diez JC. Human acute promyelocytic leukemia NB4 cells are sensitive to esculetin through induction of an apoptotic mechanism. Chem Biol Interact. 2014;220:129–139. doi: 10.1016/j.cbi.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Semisch A, Ohle J, Witt B, Hartwig A. Cytotoxicity and genotoxicity of nano - and microparticulate copper oxide: role of solubility and intracellular bioavailability. Part Fibre Toxicol. 2014;11:10. doi: 10.1186/1743-8977-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun LK, Yoshii Y, Hyodo A, Tsurushima H, Saito A, Harakuni T, Li YP, Kariva K, Nozaki M, Morine N. Apoptotic effect in the glioma cells induced by specific protein extracted from Okinawa Habu (Trimeresurus flavoviridis) venom in relation to oxidative stress. Toxicol In Vitro. 2003;17:169–177. doi: 10.1016/S0887-2333(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 37.Komada Y, Zhang XL, Zhou YW, Ido M, Azumam E. Apoptotic cell death of human T lymphoblastoid cells induced by arginine deiminase. Int J Hematol. 1997;65:129–141. doi: 10.1016/S0925-5710(96)00538-5. [DOI] [PubMed] [Google Scholar]
- 38.Zong B, Ma Y, Fu D, Zhang C. Induction of apoptosis in osteosarcoma s180 cells by polysaccharide from Dictyophora indusiata. Cell Biochem Funct. 2013;31:719–723. doi: 10.1002/cbf.2961. [DOI] [PubMed] [Google Scholar]
- 39.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–1012. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 40.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 42.Decaudin D, Marzo I, Brenner C, Kroemer G. Mitochondria in chemotherapy-induced apoptosis: a prospective novel target of cancer therapy. Int J Oncol. 1998;12:141–152. [PubMed] [Google Scholar]
- 43.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.