Abstract
Eryngium foetidum Linn. leaves (EF) are widely used in Thailand and many countries throughout Asia as a culinary seasoning and a traditional medicine. However, adverse effect of high dose consumption in long duration has not been evaluated. The aim of this study was to investigate chronic toxicity of EF in mice. Thirty-two ICR male mice were divided into 4 groups of 8 mice each. The mice were fed AIN-76 rodent diet, or AIN-76 rodent diet supplemented with ground freeze-dried EF at 0.8%, 1.6% and 3.2% that is equivalent to approximately 35, 73 and 155 times that of human consumption, respectively, at 97.5 percentile for a period of 24 weeks. At the end of experiment, the mice were euthanized and blood samples were collected for hematological and biochemical evaluations. Necropsy was performed while visceral organs such as lung, liver, kidneys, spleen etc. were collected, weighed and histopathologically examined. Blood urea nitrogen (BUN) results of mice in 1.6% and 3.2% EF diet groups were significantly higher than the BUN of control group. No significant difference was noted in other biochemical and hematological properties between the treatment groups and control; all results were within normal range. Histopathology of almost all visceral organs showed no significant changes. However, tubulonephrosis and chronic interstitial nephritis were observed in the groups treated with 1.6% and 3.2% EF diet. Body weight was reduced significantly at week 12 to week 20 when compared to the control group while relative kidney weights were significantly increased. In conclusion, the consumption of EF in diet at high doses illustrated the adverse effect on some biochemical parameters and histopathology in mice. Our findings suggested that EF daily consumption for 24 weeks, at higher doses than the 0.8% EF diet (35 times of human consumption), might cause adverse effect on kidney function in mice.
Keywords: Eryngium foetidum Linn., Chronic toxicity, Kidney, Mice, AIN-76 diet
INTRODUCTION
Eryngium foetidum Linn. (EF) is a plant in family Apiaceae, native to Central and Latin America and the West Indies (1,2). EF, also known as “Phakchi-farang”, is widely used in Thailand and many countries in Asia as a culinary seasoning in habitual diet. The National Bureau of Agricultural Commodity and Food Standard Survey “Food Consumption Data of Thailand in 2006” showed that the serving size of fresh edible EF for consumers was 0.48 g/kg BW/day. Acceptable Daily Intake (ADI) of EF that equivalent to approximately less than 10.12 g (fresh EF) and 0.58 g (freeze-dried EF) did not show adverse effect in human weight 60 kg. It has been used as a traditional medicine for treatment of various diseases such as cold, influenza, hypertension, constipation, diarrhea and malaria (3). The component of EF depends on the geographic location of cultivation area (3,4). Phytochemical component of EF contains various compounds such as flavonoids, tannins, saponin and several triterpenoids (5). Their antioxidant compounds consist of vitamin C, vitamin E, carotenoid, xanthophil and phenolic compound (6). Moreover, the major components are α-cholesterol, brassicasterol, campesterol, stigmasterol and the minor components such as cholesterol, β-sitosterol, Δ5-avenasterol Δ5–24-stigmastadienol and Δ7-avenasterol are found (7).
Previous studies showed that EF possesses various health benefits such as anti-inflammatory and antioxidant properties in vitro and in vivo (5,7,8). From the previous study, reduced inflammatory responses were reported in mice treated with EF after 12-O-tetradecanoylphorbol acetate induction (7). EF also exhibited the highest inhibitory activity against MCF-7 human mammary carcinoma cell (9). Recently, it was reported that EF had anti-clastogenic activity in mouse erythrocyte (10). However, consumption at high dose for long term and its toxic effect have never been reported. Therefore, EF should be studied scientifically for its toxicity in order to establish suitable dose for human consumption in the form of supplement or herbal products.
MATERIALS AND METHODS
Chemicals
For preparation of animal diet, AIN-76 semi-purified diet, sodium caseinate (ECCO 2300) the product of Vichi Enterprise. Co., Ltd., New York, USA. DL-methionine and vitamin mix were the products of Fluka, Sigma Aldrich Co., Ltd. (CAT No: 59-51-8). Mineral mix (M.W. = 253.3, 500 g; CAT No: 101384; Lot No: 2200 K) and choline bitartrate were purchased from MP Biomedical LLC, Solon, California, USA. Cellulose (SOLKA-FLOC® 200 FCC) was the product from FS&D (St. Louis, Missouri, USA). Vitamin K1 was the product of DSM Nutritional Products Ltd. (Sisseln, Switzerland). Most of the common ingredients used in the study such as corn flour, sucrose and corn oil were obtained locally.
Eryngium foetidum L. leaves (EF) preparation
EF were purchased from 2 central distributors in Pathum Thanee and Nakhon Pathom Provinces, Thailand. Freeze-dried EF was prepared as previously study that reported about clastogenicity of EF that administration to mice received both direct-acting, mitomycin C (MMC), and indirect acting, 7, 12-dimethylbenz(a) anthracene (DMBA) clastogens. The study using ICR mice that mice were fed AIN-76 semipurified diet supplemented with ground freeze dried EF at 0.8%, 1.6% and 3.2% for 2 weeks. The results shown no clastogenic effect and mice were treated with diets mixwell with EF decreased the number of micronucleated peripheral reticulocytes in all the MMC-treated groups in a dose dependent manner (10). Briefly, edible parts of EF was cleaned with tap and deionized water, drained and air dried, slightly crushed to small pieces, quick freezed with liquid nitrogen, then lyophilized, ground, packed and stored at −20°C until analysis.
Animal and diet preparation
Thirty-two ICR male mice, 3 weeks old, weighing 23 ± 2 g were purchased from the National Laboratory Animal Center (NLAC), Mahidol University. The animals were housed in strict hygienic conventional animal room of Section of Animal Laboratory, National Cancer Institute (NCI), Thailand. The mice were maintained at 23 ± 2°C with 50 ± 10% humidity and 12 hrlight/dark cycles and housed in filter-top plastic cages according to the Institute Care Guidelines approved by the Animal Ethic Committee of NCI (AE. No. 241/2554). During experimental period, the animals were closely daily observed for general behavioral change and clinical signs of their toxicity.
Chronic toxicity study
In acclimatization period for 1 week, the mice were given modified AIN-76 diet (basal diet) and water ad libitum. After that, the mice were randomly assigned to four groups of eight mice each. Group 1 was fed on basal AIN-76 diet and served as the control. Groups 2, 3 and 4 were experimental groups and fed on basal AIN-76 diets supplemented with ground freeze-dried EF in three concentration levels which were 0.8%, 1.6% and 3.2%, respectively, and water ad libitum for 24 weeks. During the experimental period, the mice were observed for clinical signs, morbidity or mortality daily. Body weight was weekly recorded and diet consumption was daily monitored over 24 weeks. At the end of the experiment, the mice were sacrificed and blood was collected for determining hematological and biochemical values. Visceral organs such as brain, heart, lung, liver, stomach, small intestine, adrenal gland, lymph node, salivary gland and reproductive organ were collected and weighed to determine relative organ weight (g/100 g BW of mouse). After that they were fixed in 10% neutral buffered formalin for conventional histopathological examinations.
Hematological analysis was performed using a fully automated hematology analyzer (CELL-DYN®3700, Abbott Laboratories, Santa Clara, CA, USA). Hematological parameters and morphologic characteristics were examined, i.e. complete blood count (CBC) such as red blood cells (RBC), hemoglobin (Hb), hematocrit (Hct), white blood cells (WBC), % of neutrophils, band, eosinophils, lymphocytes and monocytes, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelets count (PLT). Biochemical examinations were performed using an automated chemistry analyzer (Modular P800, Roche Diagnostics, New York, USA. Serial No. 2017-10). Biochemical parameters such as blood urea nitrogen (BUN), creatinine (CREA), uric acid (UA), cholesterol (CHOL), total protein (TP), albumin (ALB), globulin (GLOB), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), calcium (Ca), phosphorus (P) and magnesium (Mg) were evaluated.
Statistical analysis
Data were expressed as mean ± standard error of the mean (S.E.M.) and analyzed by the analysis of variance ANOVA test. Significant difference between the control and experimental groups was made using the Two Independent sample by Mann-Whitney U test. For histopathological examination, Fisher’s Exact Probability test was applied. A probability level of less than 5% (p-value < 0.05) was considered significant.
RESULTS
No significant changes in general behavior or other major physiological activities of the mice were found. However, the mice receiving high dose of EF at 3.2% (Group 4) exhibited some clinical signs such as aggressiveness, rough fur, increased urination, disagreeable odor of urine and feces. In addition, fecal color changed into dark green when compared with the control group.
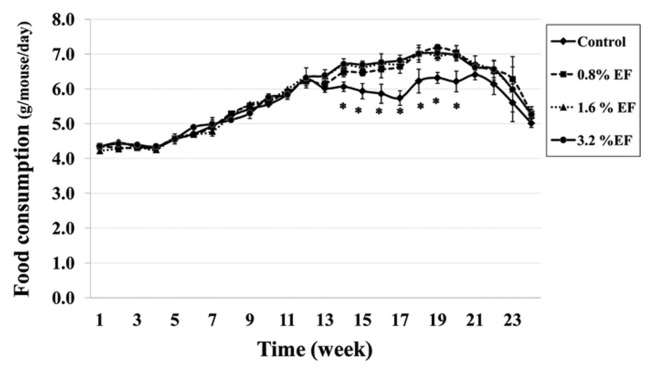
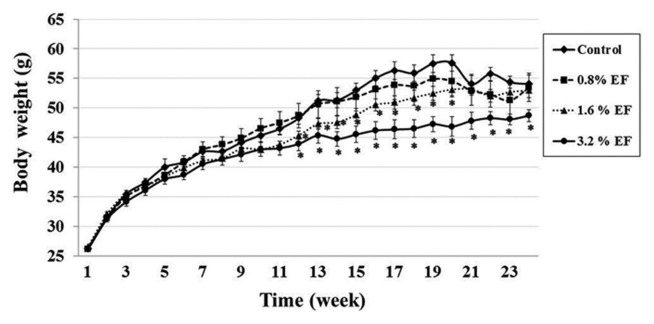
Mean food consumption (g/mouse/day) of the control group (Group 1) and the experimental groups (Groups 2, 3, 4) were 5.48 ± 0.05, 5.77 ± 0.05, 5.77 ± 0.05 and 5.80 ± 0.05 g/mouse/day, respectively. Interestingly, the mean food consumption significantly decreased in the experimental groups treated with EF at week 14 to week 20 when compared with the control group (Fig. 1). Body weights of the mice treated with 1.6% EF (Group 3) were significantly reduced at week 12 to week 20. While the 3.2% EF treated group (Group 4) showed a significant decrease in their body weights at week 12 until the end of the experiment (Fig. 2). There was no difference in the relative organ weights between the treatment and control groups except the kidney. The right kidney weights of mice in Groups 3 and 4 were significantly higher than those of the control group, whereas the left kidney weights of mice only in Group 4 significantly higher than those of the control group (Table 1).
Fig. 1.
Food consumption of mice receiving EF for 24 weeks. *Significantly different from the control group at p<0.05. Values are mean ± SE.
Fig. 2.
Body weight of mice receiving EF for 24 weeks. *Significantly different from the control group at p < 0.05. Values are mean ± SE.
Table 1.
Relative organ weights (g/100 g BW) and body weights (g) of mice receiving diet with and without EF for 24 weeks
| Organs | Control | Dose of EF in AIN-76 diet | ||
|---|---|---|---|---|
|
|
|
|||
| AIN-76 diet | 0.8% | 1.6% | 3.2% | |
| Brain | 0.835 ± 0.03 | 0.884 ± 0.04 | 0.892 ± 0.03 | 0.908 ± 0.05 |
| Heart | 0.426 ± 0.02 | 0.443 ± 0.02 | 0.492 ± 0.04 | 0.455 ± 0.01 |
| Kidney, left | 0.555 ± 0.02 | 0.621 ± 0.04 | 0.647 ± 0.03 | 0.684 ± 0.02* |
| Kidney, right | 0.549 ± 0.02 | 0.639 ± 0.04 | 0.675 ± 0.04* | 0.694 ± 0.01* |
| Liver | 4.838 ± 0.02 | 4.629 ± 0.13 | 4.512 ± 0.21 | 4.460 ± 0.11 |
| Lung | 0.653 ± 0.05 | 0.669 ± 0.05 | 0.622 ± 0.04 | 0.695 ± 0.04 |
| Spleen | 0.248 ± 0.02 | 0.258 ± 0.03 | 0.272 ± 0.02 | 0.277 ± 0.02 |
| Testis | 0.448 ± 0.03 | 0.478 ± 0.04 | 0.487 ± 0.03 | 0.497 ± 0.02 |
|
| ||||
| Body weight (1st wk ) | 26.28 ± 0.36 | 26.31 ± 0.29 | 26.58 ± 0.20 | 25.58 ± 0.21 |
| Body weight (24st wk ) | 55.68 ± 1.82 | 52.33 ± 2.19 | 52.31 ± 1.44 | 49.64 ± 1.23* |
| Weight gain | 29.40 ± 1.46 | 26.02 ± 1.90 | 24.73 ± 1.24 | 24.06 ± 1.02* |
Significantly different from the control group at p < 0.05. Values are mean ± SE.
Hematological examination of the mice in Group 4 is shown in Table 2. There was a significantly increase in mean corpuscular hemoglobin concentration (MCHC) when compared with the control group but this alteration was within the reference range reported by the National Laboratory Animal Center (NLAC). For biochemical examination, a significant increase in BUN in Groups 3 and 4 in a dose dependent manner was shown when compared with the control group (Table 3). Furthermore, alkaline phosphatase (ALP) was significantly decreased in Groups 3 and 4 when compared with the control group. CHOL level (mg/dL) in Group 3 (139.88 ± 1.99) and Group 4 (134.75 ± 0.13) significantly decreased when compared with the control group (162.38 ± 2.67). No difference in histopathological examination in the visceral organs of the treated mice was found when compared with the control group except tubulonephrosis, characterized by diffuse hydropic degeneration of renal tubular epithelium, and multifocal chronic interstitial nephritis, characterized by infiltration of lymphocyte and mononuclear cells in interstitium. Splenic hemosiderosis seen by increased hemosiderin-laden macrophages in white pulp and proliferation of gut-associated lymphoid tissue (GALT) invading into submucosa of large intestine were noted. Descriptive microscopic findings of visceral organs and degree of lesions are shown in Table 4.
Table 2.
Hematological values of mice receiving diet containing EF for 24 week
| Parameters | Control | Dose of EF in AIN-76 diet | ||
|---|---|---|---|---|
|
|
|
|||
| AIN-76 diet | 0.8% | 1.6% | 3.2% | |
| Hct (%) | 38.13 ± 1.37 | 37.50 ± 1.04 | 37.00 ± 0.65 | 36.88 ± 0.74 |
| Hb (g/dL) | 13.54 ± 0.42 | 13.06 ± 0.34 | 13.00 ± 0.33 | 13.48 ± 0.35 |
| MCH (pg) | 16.10 ± 0.18 | 15.84 ± 0.14 | 15.64 ± 0.15 | 16.36 ± 0.12 |
| MCHC (g/dL) | 35.04 ± 0.50 | 34.30 ± 0.54 | 35.35 ± 0.25 | 36.38 ± 0.24* |
| MCV (μm 3 ) | 46.03 ± 0.79 | 46.25 ± 0.76 | 44.19 ± 0.51 | 44.90 ± 0.33 |
| RBC (× 106 cells/mm3 ) | 8.36 ± 0.27 | 8.21 ± 0.26 | 8.11 ± 0.22 | 8.05 ± 0.17 |
| RDW (%) | 21.9 ± 0.70 | 21.5 ± 0.70 | 21.7 ± 0.50 | 21.6 ± 0.30 |
|
| ||||
| Band (%) | 3.14 ± 0.38 | 3.40 ± 0.54 | 3.17 ± 0.69 | 3.17 ± 0.47 |
| Eosinophils (%) | 1.50 ± 0.25 | 1.60 ± 0.19 | 1.67 ± 0.41 | 1.33 ± 0.18 |
| Lymphocytes (%) | 65.00 ± 2.60 | 66.00 ± 5.31 | 66.25 ± 2.74 | 67.00 ± 1.58 |
| Monocytes (%) | 3.63 ± 0.82 | 5.00 ± 0.57 | 2.13 ± 0.35 | 4.63 ± 0.82 |
| Neutrophils (%) | 22.63 ± 1.76 | 23.00 ± 1.31 | 23.63 ± 1.28 | 23.88 ± 0.85 |
| WBC (× 103 cells/mm 3 ) | 7.94 ± 0.28 | 7.93 ± 0.47 | 7.96 ± 0.31 | 8.34 ± 0.35 |
|
| ||||
| Platelets count (× 103 cells/mm3) | 1,411.88 ± 114.24 | 1,293.25 ± 117.76 | 1,289.25 ± 120.56 | 1,288.12 ± 33.23 |
Significantly different from the control group at p<0.05. Values are mean±SE.
Table 3.
Biochemical values of mice receiving diet containing EF for 24 week
| Parameters | Control | Dose of EF in AIN-76 diet | ||
|---|---|---|---|---|
|
|
|
|||
| AIN-76 diet | 0.8% | 1.6% | 3.2% | |
| ALB (g/dL) | 3.49 ± 0.08 | 3.47 ± 0.05 | 3.28 ± 0.07 | 3.29 ± 0.07 |
| ALP (U/L) | 51.88 ± 1.38 | 49.88 ± 2.10 | 44.63 ± 2.03* | 42.88 ± 1.89* |
| ALT (U/L) | 18.50 ± 1.35 | 17.88 ± 2.18 | 17.50 ± 1.52 | 18.88 ± 0.77 |
| AST (U/L) | 62.63 ± 4.36 | 64.25 ± 5.90 | 66.38 ± 3.26 | 67.25 ± 2.77 |
| BUN (mg/dL) | 23.30 ± 1.55 | 24.68 ± 1.80 | 28.41 ± 1.4* | 28.51 ± 0.97* |
| CHOL (mg/dL) | 162.38 ± 2.67 | 160.38 ± 2.10 | 139.88 ± 1.99* | 134.75 ± 0.13* |
| CREA (mg/dL) | 0.13 ± 0.00 | 0.13 ± 0.02 | 0.13 ± 0.01 | 0.12 ± 0.01 |
| GLOB (g/dL) | 2.09 ± 0.04 | 2.10 ± 0.06 | 2.21 ± 0.05 | 2.29 ± 0.08 |
| TP (g/dL) | 5.45 ± 0.11 | 5.53 ± 0.04 | 5.53 ± 0.10 | 5.58 ± 0.13 |
| UA (mg/dL) | 2.58 ± 0.19 | 2.59 ± 0.11 | 2.56 ± 0.14 | 2.58 ± 0.10 |
|
| ||||
| Ca (mmol/L) | 10.03 ± 0.09 | 9.95 ± 0.10 | 9.61 ± 0.18 | 9.76 ± 0.21 |
| Mg (mmol/L) | 3.05 ± 0.05 | 3.04 ± 0.12 | 3.01 ± 0.09 | 2.84 ± 0.08 |
| P (mmol/L) | 9.44 ± 0.23 | 9.31 ± 0.30 | 9.56 ± 0.19 | 9.34 ± 0.29 |
Significantly different from the control group at p < 0.05. Values are mean ± SE.
Table 4.
Microscopic histopathological examination of mice receiving ground EF for 24 weeks: data show number of mice that posed abnormality lesions and degree of severities
| Organ | Microscopic findings | Control | Dose of EF in AIN-76 diet | ||
|---|---|---|---|---|---|
|
| |||||
| Basal diet | 0.8% | 1.6% | 3.2% | ||
| Lung | Congestion | 2/8 mild (2) |
3/8 mild (3) |
2/8 mild (2) |
2/8 mild (2) |
| BALT proliferation | 2/8 mild s |
2/8 mild (2) |
3/8 mild (3) |
5/8 mild (5) |
|
|
| |||||
| Liver | Congestion | 3/8 mild (3) |
3/8 mild (3) |
2/8 mild (2) |
2/8 mild (2) |
| Nuclear megalocytosis | 8/8 mild (2), moderate (6) |
8/8 mild (4), moderate (4) |
8/8 mild (6), moderate (2) |
8/8 mild (3), moderate (5) |
|
| Centrilobular hydropic degeneration | 8/8 mild (4), moderate (2), severe (2) |
8/8 moderate (6), severe (2) |
8/8 moderate (4), severe (4) |
8/8 moderate (5), severe (3) |
|
| Centrilobular fatty degeneration | 8/8 mild (3), moderate (4), severe (1) |
8/8 mild (4), moderate (3), severe (1) |
8/8 mild (2), moderate (4), severe (2) |
8/8 mild (3), moderate (3), severe (2) |
|
|
| |||||
| Spleen | Extra medullary hematopoiesis | 5/8 mild (1), moderate (3), severe (1) |
7/8 mild (1), moderate (4), severe (2) |
8/8 mild (2), moderate (3), severe (3) |
7/8 mild (2), moderate (2), Severe (3) |
| Hemosiderosis | 4/8 mild (4) |
7/8* mild (7) |
8/8* mild (4), moderate (4) |
8/8* mild (2), moderate (6) |
|
|
| |||||
| Kidney | Congestion | NRL |
4/8* mild (4) |
4/8* mild (2) |
5/8* mild (5) |
| Tubular regeneration | NRL |
5/8* mild (5) |
6/8* mild (6) |
7/8* mild (7) |
|
| Tubular degeneration | NRL |
3/8* mild (3) |
4/8* mild (2), moderate (2) |
6/8* moderate (6) |
|
| Multifocal interstitial infiltration | NRL |
6/8* mild (6) |
7/8* mild (5), moderate (2) |
8/8* mild (2), moderate (6) |
|
|
| |||||
| Small intestine | GALT proliferation in submucosa | 2/8 mild (2) |
2/8 mild (2) |
3/8 mild (3) |
3/8 mild (3) |
|
| |||||
| Large intestine | GALT proliferation in submucosa | 4/8 mild (4) |
4/8 mild (4) |
8/8* mild (8) |
8/8* mild (8) |
|
| |||||
| Lymph node | Lymphoid hyperplasia | 1/8 mild (1) |
1/8 mild (1) |
1/8 mild (1) |
2/8 mild (2) |
|
| |||||
| Salivary gland | Lymphocyte infilltrative | 1/8 mild (1) |
1/8 mild (1) |
1/8 mild (1) |
2/8 mild (2) |
Significantly different from the control group at p<0.05.
Data are shown in number of mouse with histopathological lesions/total number of mice and severity of histopathological lesion that were evaluated as: mild = lesion 1~2 foci/low power field microscope; moderate = lesion > 3 foci; severe = large clusters or a continuous layer over > 2/3 of the surface.
NRL: No remarkable lesions, BALT: Bronchiole-associated lymphoid tissue, GALT: Gut-associated lymphoid tissue.
DISCUSSION
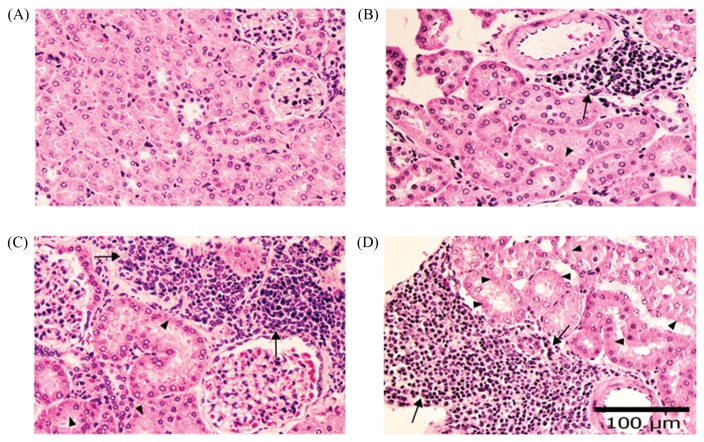
The body weight of mice was significantly decreased conversely to the diet consumption when compared with the dose of EF. Many studies have suggested that dietary fiber aids in decreasing absorption of macronutrients and altering secretion of gut hormones. (11,12). Whereas, consuming diets containing high fiber provides bulk and it is related to lowering of body weights. Moreover, EF contains tannin that might disrupt digestion in mice (6). However, moderate degree of tubulonephrosis and multifocal chronic interstitial nephritis (Fig. 3) were significantly increased in Groups 3 and 4 which affected kidney functions.
Fig. 3.
Histopathology of mice kidney revealed diffuse hydropic tubular degeneration (arrow head) and multifocal chronic interstitial nephritis; infiltration of lymphocytes and mononuclear cell interstitium (arrow). (A) control group and (B-D) treatment groups receiving diet containing ground freeze-dried EF at 0.8%, 1.6% and 3.2% (H&E stain, 40× mag).
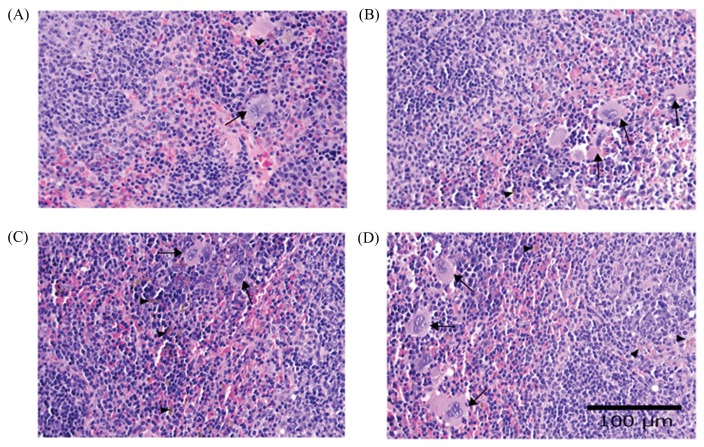
Histopathological lesions in kidney might be caused by toxic metabolites of major constituents in EF after ingestion such as stigmasterol, campesterol and brassicasterol (13). The-metabolite products are excreted via renal tubular epithelium, which might cause xenobiotic-induced cell injury and hydropic degeneration of tubular epithelium and can induce inflammatory cytokine to stimulate lymphocytes and mononuclear cells infiltrated lesion periphery. These constituents can inhibit the absorption of cholesterol and replace a part of cholesterol in cell membrane, i.e. RBC and blood vessel membrane, which make RBC more rigid and fragile (14). Thus, the high level of BUN and relative kidney weight were significantly increased in association with renal lesions. The spleen showed mild, moderate and severe degree grading of extramedullary hematopoiesis in both control group and experimental groups (Fig. 4). However, the mild and moderate degrees were found in normal mice (15), whereas the severe degree of extramedullary hematopoiesis and hemosiderosis occurred in anemic mice because blood morphology of RBC showed anisocytosis and poikilocytosis that possibly to influence excessively increased half-life of RBC. Excessive hemolysis of RBC can occur in spleen, show evidence of increased hemosiderin-laden macrophages and then stimulate production of RBC as seen by increased numbers of megakaryocytes in splenic tissue. Mild degree of proliferation of GALT usually occurs in large intestine, but a significant increase was found only in Groups 3 and 4. This lesion can occur in aging normal mice both in small and large intestines because some antigens in daily diet can induce proliferation of lymphoid cells to stimulate both humoral and cell-mediated immunity (15). Other possible cause of this lesion in our experiment might be because the consumption of high fiber diets provided bulk and relieve constipation (16). This mechanism of fibers causes irritation and induces inflammation of mucosal epithelial cells of large intestine because of defecation. Whereas, low level of ALP may be possibly due to anemia and commonly decreases in aged mice (17). Furthermore, CHOL level in Groups 3 and 4 was shown to significantly decrease when compared with the control group, possibly caused by the high fiber in diet. Other study that support our postulation was by Dreher, who reported that fiber could reduce CHOL level and decreased the body weight of mice (18).
Fig. 4.
Histopathology of mice spleen revealed increased hemosiderosis (arrowhead) and numbers of megakaryocytes (arrow). (A) control group and (B-D) treatment groups receiving diet containing ground freeze-dried EF at 0.8%, 1.6% and 3.2% (H&E stain, 40× mag).
In conclusion, chronic toxicity study of EF demonstrated that EF diet at 0.8% (35 times of human consumption) did not affect hematological and biochemical parameters when compared to the control group. In the groups treated with EF at 73 and 155 times of human consumption, some abnormality signs that led to the significant increase in the serum BUN level were produced, which strongly correlated with kidney histopathological lesion. Consequently, the consumption of Eryngium foetidum leaves at high dose for a long time may cause kidney lesion.
ACKNOWLEDGEMENTS
This work was financially supported by the National Research Council of Thailand (NRCT). The authors would like to thank the DSM Nutritional Products Ltd., Thailand for kindly providing vitamin K1.
REFERENCES
- 1.Adams CD. Flowering plants of Jamaica. University of the West Indies; Mona: 1972. [Google Scholar]
- 2.Ekpong B. Seed development and umble order contribution on eryngo (Eryngium foetidum L.) seed yield and quality [dissertation] [Bangkok]: Kasetsart University; 2005. [Google Scholar]
- 3.Paul JHA, Seaforth CE, Tikasingh T. Eryngium foetidum L.: A review. Fitoterapia. 2011;82:302–308. doi: 10.1016/j.fitote.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Cardozo E, Rubio M, Rojas LB, Alfredo U. Composition of the essential oil from the leaves of Eryngium foetidum L. from the Venezuelan Andes. J Essent Oil Res. 2004;16:33–34. doi: 10.1080/10412905.2004.9698645. [DOI] [Google Scholar]
- 5.Mekhora C, Muangnoi C, Chingsuwanrote P, Dawilai S, Svasti S, Chasri K, Tuntipopipat S. Eryngium foetidum suppresses inflammatory mediators produced by macrophages. Asian Pac J Cancer Prev. 2012;13:653–664. doi: 10.7314/APJCP.2012.13.2.653. [DOI] [PubMed] [Google Scholar]
- 6.Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005;92:491–497. doi: 10.1016/j.foodchem.2004.07.035. [DOI] [Google Scholar]
- 7.García MD, Sáenz MT, Gómez MA, Fernández MA. Topical anti-inflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models. Phytother Res. 1999;13:78–80. doi: 10.1002/(SICI)1099-1573(199902)13:1<78::AID-PTR384>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 8.Bonikowski R, Kula J, Bujacz A, Wajs-Bonikowska A, Zakłos-Szyda M, Wysocki S. Hydroindene-derived chiral synthons from carotol and their cytotoxicity. Tetrahedron Asymmetry. 2012;23:1038–1045. doi: 10.1016/j.tetasy.2012.07.005. [DOI] [Google Scholar]
- 9.Kawaree R. Chemical component variation, cytotoxicity, antioxidant and stability of volatile oils from Thai medicinal plants [dissertation] [Chiang Mai]: Chiang Mai University; 2007. [Google Scholar]
- 10.Promkum C, Butryee C, Tuntipopipat S, Kupradinun P. Anticlastogenic effect of Eryngium foetidum L. assessed by erythrocyte micronucleus assay. Asian Pac J Cancer Prev. 2012;13:3343–3347. doi: 10.7314/APJCP.2012.13.7.3343. [DOI] [PubMed] [Google Scholar]
- 11.Sartorelli DS, Franco LJ, Cardoso MA. High intake of fruits and vegetables predicts weight loss in Brazilian overweight adults. Nutr Res. 2008;28:233–238. doi: 10.1016/j.nutres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21:411–418. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Chivapat S, Sincharoenpokai P, Suppajariyawat P, Rungsipipat A, Phattarapornchaiwat S, Chantarateptawan V. Safety evaluations of ethanolic extract of moringa oleifera lam seed in experimental animals. Thai J Vet Med. 2012;42:343–352. [Google Scholar]
- 14.Ratnayake WM, L’Abbe MR, Mueller R, Hayward S, Plouffe L, Hollywood R, Trick K. Vegetable oils high in phytosterols make erythrocytes less deformable and shorten the life span of stroke-prone spontaneously hypertensive rats. J Nutr. 2000;130:1166–1178. doi: 10.1093/jn/130.5.1166. [DOI] [PubMed] [Google Scholar]
- 15.Peckham JC. In: Animal Histopathology in CRC Handbook of Toxicology. Derelanko MJ, Hollinger MA, editors. CRC Press; New York: 2002. pp. 485–514. [Google Scholar]
- 16.Marlett JA, McBurney MI, Slavin JL. Position of the American Dietetic Association: health implications of dietary fiber. J Am Diet Assoc. 2002;102:993–1000. doi: 10.1016/S0002-8223(02)90228-2. [DOI] [PubMed] [Google Scholar]
- 17.Porte RS, Kaplan JL. The Merck Manual of Diagnosis and Therapy. 19th edition. Planet Friendly Publishing; USA: 2011. [Google Scholar]
- 18.Dreher ML. In: Dietary Fiber Overview in Handbook of Dietary Fiber. Cho SS, editor. CRC Press; India: 2001. [Google Scholar]