Abstract
Propolis is a multicomponent, active, complex resinous substance collected by honeybees from a variety of plant sources. We have studied the effect of propolis on neurite outgrowth of SH-SY5Y human neuroblastoma cells induced to differentiate by all-trans-retinoic acid (RA). Propolis, at a concentration of 3 μg/mL, had no significant effect on the viability of differentiating SH-SY5Y cells. However, the neurite outgrowth of the differentiating SH-SY5Y cells treated with propolis (0.3~3 μg/mL) for 48 hr was significantly inhibited in a dose-dependent manner. Treatment of RA-stimulated differentiating SH-SY5Y cells with 0.3 to 3 μg/mL propolis resulted in decreased level of transglutaminase and 43-kDa growth-associated protein (GAP-43) in a dose-dependent manner. The results indicate that propolis is able to inhibit neurite outgrowth of differentiating SH-SY5Y cells.
Keywords: Propolis, SH-SY5Y neuroblastoma cell, Neurite outgrowth, TGase, GAP-43
INTRODUCTION
Propolis is made from a sticky substance that honeybees produce by mixing their own waxes with resinous sap obtained from certain trees and other flowering plants. Propolis has been popularly used in folk medicine. The putative therapeutic properties of propolis could be related to its anti-bacterial (1), anti-inflammatory (2), anti-oxidative (3,4), and tumoricidal activities (5,6). Although propolis contains more than 300 different chemical compounds, phenolic acids and esters, polyphenols such as flavonoids and phenolic acids/ester are relatively popular constituents (7). It has been well demonstrated that propolis has a distinct pronounced cytotoxic and anticarcinogenic effect in tumor cell models (8–10).
Established cell lines derived from nervous system tissue have proven to be powerful tools for elucidating cellular and molecular mechanisms of nervous system development and function. They also have been used to understand the action mechanism of neurotoxic chemicals. As neurons showing characteristic morphology with many cell processes have the ability of extending neurite, neurite outgrowth in cultured neurons is considered one indication of neuroregenerative potential (11,12). The human SH-SY5Y neuroblastoma cell line has the capability of undergoing neuronal maturation. It can be differentiated into neuron-like cells with neurites in response to all-trans-retinoic acid (RA) stimulation (13). Therefore, it has been used as in vitro cell model for studying the mechanisms of neuronal differentiation and neurotoxicity (14,15). Neuronal differentiation of SH-SY5Y cells in response to RA is coupled with increased expression and activation of transglutaminase (TGase) (16). It was demonstrated that TGase is required for the promotion of neurite outgrowth (17). Growth-associated protein 43 (GAP-43), a nervous tissue-specific cytoplasmic protein, has been termed a ‘growth’ or ‘plasticity’ protein because it is expressed at high levels in neuronal growth cones during development and axonal regeneration (18).
In the present study, we investigated the effect of propolis on RA-induced differentiation of SH-SY5Y human neuroblastoma cell line. We found that propolis inhibited the neurite outgrowth and the expression of TGase protein of differentiating SH-SY5Y neuroblastoma cells.
MATERIALS AND METHODS
Propolis
Crude propolis was collected from the region of Yongin (Gyeonggi, Korea), and kept desiccated in a refrigerator (−20°C) before being processed. Ethanol propolis extract (EEP) was prepared by the following procedure. Briefly, frozen propolis was powdered in a blender, placed in 95% ethanol solution and incubated at room temperature for 3 months. The ethanol extract was filtered through Whatman No. 2 paper. The filtered ethanol extract (1 mL) was evaporated to dryness, yielding 200 mg. Propolis was dissolved in 95% ethanol solution.
Cell culture
SH-SY5Y human neuroblastoma cells were purchased from Korean Cell Line Bank (Seoul, Korea). Cells were maintained in complete culture medium consisting of Ham’s F12 and Eagle’s minimum essential medium (Invitrogen Co., Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone, Logan, Utah, USA) plus 100 units/mL penicillin, 100 μg/mL streptomycin, and nonessential amino acids in a humidified incubator with 5% CO2 and 95% air at 37°C. The medium was refreshed every 2 days.
Propolis treatment
For differentiation studies, the cells were plated in 24-well plates at a density of 1 × 105 cells/mL. After incubation for 2 days, 10 μM all-trans-RA was added to the medium for 2 days. Cells treated with RA showed significant neurite outgrowth. In the case of chemical treatment of cells undergoing differentiation, the SH-SY5Y cells were plated in the same way. After incubation for 2 days, 10 μM RA and various concentrations of propolis were treated simultaneously.
Cell viability
The methylthiazoltetrazolium (MTT) assay was performed to measure cytotoxicity of propolis on differentiating SH-SY5Y cells. Cells were plated in 24-well plates at a density of 1 × 105 cells/mL. After incubation for 24 hr, the medium was refreshed, and propolis (0.3 and 3 μg/mL) was administered for 2 days in the presence or absence of 10 μM RA. Following exposure to propolis, the cells were incubated for 4 hr with 0.5 mg/mL MTT at 37°C. The reaction was stopped by removing the medium and adding acid isopropanol. The absorbance was measured at 540 nm of wavelength using a microplate reader (Bio-Rad Laoratories, Hercules, CA, USA).
Cell morphological analysis
SH-SY5Y cells were plated in 24-well plates at a density of 1 × 105 cells/mL. After incubation for 24 hr, the medium was refreshed, and 10 μM RA and various concentrations of propolis were added simultaneously. After incubation for 2 days, the cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 3 min, washed with PBS, stained with Coomassie Brilliant Blue, and washed with PBS. The morphological changes in the cells were observed under a phase-contrast microscope. Those cells whose cell body diameters longer than twice of the diameter of cell body were considered as neurite-bearing cells (19). The percentage of the cells with neurites in a particular culture was determined by counting at least 200 cells in each sample.
Western blot analysis
For Western blot analysis, SH-SY5Y cells were plated in 100-mm plates at a density of 1 × 105 cells/mL (10 mL). After incubation for 24 hr, the medium was refreshed and incubated in the presence and absence of RA and propolis. After incubation for indicated times, the cells were washed with ice-cold PBS and lysed with lysis buffer (50 mM 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 5 mM EDTA) containing protease inhibitor cocktail (Sigma-Aldrich Korea Ltd., Yongin, Korea) at 4°C for 30 min. Total proteins were obtained after centrifugation at 10,000 ×g for 30 min at 4°C. The protein concentrations were determined by Bradford assay (Pierce, Rockford, Illinois). All proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel and electrophoretically transferred to polyvinyl difluoride nitrocellulose membrane (Millipore Co., Bedford, MA, USA). After blocking at room temperature for 90 min in Tris-buffered saline (TBS)-Tween containing 5% skim milk, the membranes were incubated with primary antibodies of anti-TGase (Santa Crus, CA, USA) and anti-GAP-43 (Santa Crus), followed by incubation with horseradish peroxidase-conjugated secondary antibody (Zymed, San Francisco, CA, USA). After washing 5 times with TBS-Tween, the bands were detected with a standard ECL detection system (LabFrontier Co., Seoul, Korea).
Statistical analysis
Results were expressed as mean values ± standard error (mean ± S.E.). Statistical analysis was performed by Student’s t-test. A level of p < 0.05 was considered to be significant.
RESULT
Propolis inhibits neurite outgrowth of differentiating SH-SY5Y neuroblastoma cells
The composition of propolis depends on the vegetation of the collection area (20). The estimation of total flavonoid contents in the ethanol extracts of our propolis was done by colorimetric method. We used quercetin as a standard compound. The total flavonoid content of our propolis sample was 10.31 ± 0.84 g per 100 g ethanol extract.
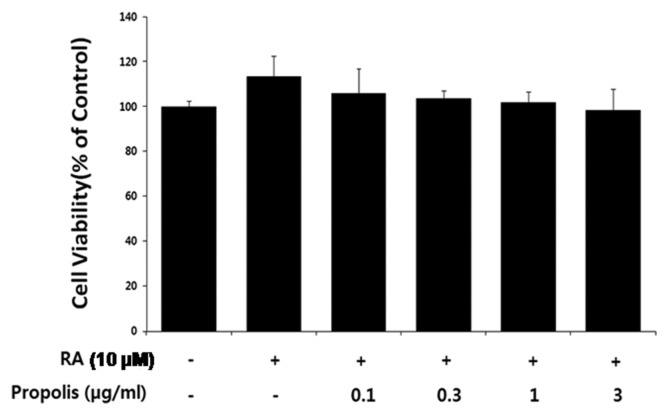
To investigate the effect of propolis on differentiation of SH-SY5Y human neuroblastoma cells, we first determined the suitable subacute propolis concentrations. Cells were treated with propolis (0.3 and 3 μg/mL) for 2 days in the presence of 10 μM RA. Following exposure to propolis, viability of SH-SY5Y cells was determined by the MTT assay. The percentage of viable cells was calculated by defining the viability of cells without RA and propolis treatment as 100%. Data are expressed as the mean ± standard error obtained from 4 independent experiments. As shown in Fig. 1, up to 3 μg/mL of propolis had no significant effect on the viability of differentiating SH-SY5Y neuroblastoma cells.
Fig. 1.
Effect of propolis on the cell viability of differentiating SH-SY5Y cells. The cells were treated with propolis for 48 hr in the presence of RA (10 μM). Then, the viability of SH-SY5Y cells was determined by MTT assay. The percentage of viable cells was calculated by defining the viability of cells without RA and propolis treatment as 100%. Data are expressed as the mean ± standard error obtained from four independent experiments.
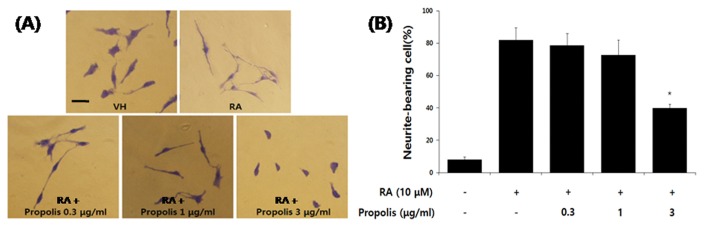
The effects of propolis on the differentiation of SH-SY5Y cells induced by RA was initially assessed by examining the effect on neurite outgrowth. Propolis was used at various concentrations up to 3 μg/mL, which had no significant effect on the viability of differentiating SH-SY5Y cells. Although propolis had no significant effect on the cell viability at concentrations (< 3 μg/mL), the neurite outgrowth of differentiating SH-SY5Y cells at concentrations of 0.3 to 3 μg/mL propolis was significantly inhibited in a dose-dependent manner (Fig. 2).
Fig. 2.
Effects of propolis on neurite outgrowth in differentiating SH-SY5Y neuroblastoma cells. (A) The cells were induced to differentiate for 48 hr with RA (10 μM) in the presence or absence of propolis (0.3~3 μg/mL). Cells were fixed in 4% paraformaldehyde and stained with Coomassie Brilliant Blue (CBB). Shown are images of typical fields of cells viewed by inverted light microscope. (B) Neurite outgrowth was quantified by counting the number of cells exhibiting neurites that were two times longer than the cell body diameter in length. The proportion of cells with neurites was expressed as a percentage of the total number of cells. Approximately 300 cells were counted in each sample. The data are expressed as the mean ± standard error of four independent experiments. *p<0.05 when compared to RA-treated cells. Scale bar = 100 μm.
Propolis decreased the expression levels of TGase and GAP-43 proteins of differentiating SH-SY5Y neuroblastoma cells
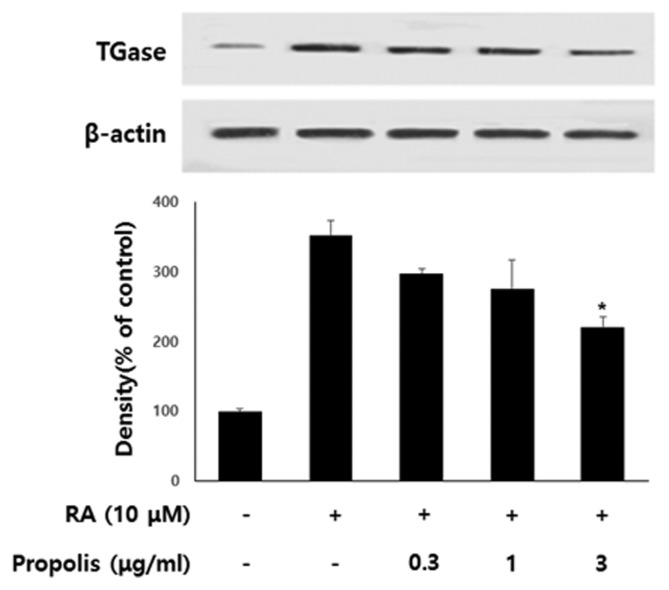
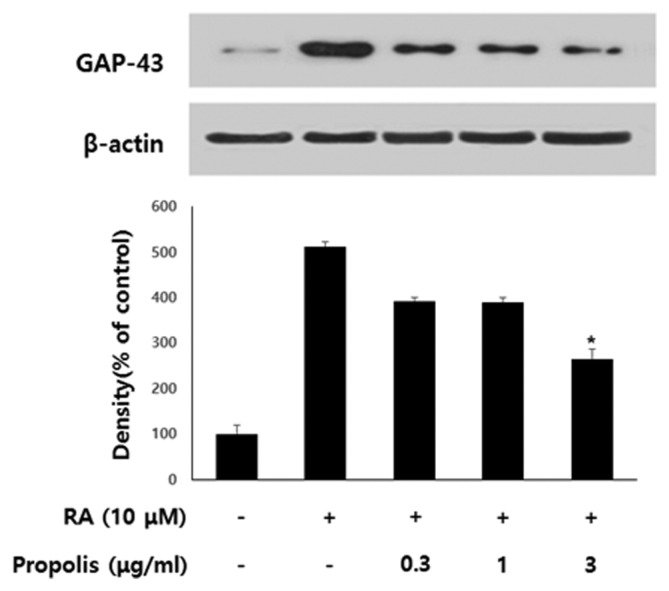
The molecular basis of the inhibitory effects of propolis on neurite outgrowth of SH-SY5Y cells was studied further by probing Western blots of cell extracts with antibodies against TGase. TGase is one of a set of proteins that are strongly up-regulated in differentiating SHSY5Y cells in response to RA, and is known to be necessary for neuronal differentiation of SH-SY5Y cells (21,22). TGase, therefore, is widely used as a common indicator for RA-induced differentiation of neuronal cells. In order to determine the effects of propolis on the expressioin of TGase protein in differentiating SH-SY5Y cells, cells were treated with 10 μM RA and various concentrations of propolis for 48 hr. As shown in Fig. 3, the cell extracts of propolis treated groups exhibited decreased cross-reactivities with TGase antibody when compared to control extracts in a dose-dependent manner. Since GAP-43 is a neural-specific protein that is thought to play a role in neurite outgrowth, GAP-43 is used as a common indicator for RA-induced differentiation of SH-SY5Y cells (23,24). In order to determine the effects of propolis on the expression of GAP-43 protein in differentiating SH-SY5Y cells, the cells were treated with 10 μM RA and various concentrations of propolis for 48 hr. As shown in Fig. 4, propolis-treated cell extracts exhibited the decrease of crossreactivities with the GAP-43 antibody when compared to control extracts in a dose-dependent manner.
Fig. 3.
Effect of propolis on the expression of TGase in differentiating SH-SY5Y cells. The cells were treated with various concentrations of RA (10 μM) and propolis for 48 hr. The Western blot data represents one of three experiments. Densitometric analysis was performed to determine the intensity of TGase bands. Values are normalized to β-actin band and expressed as the percentage of cells treated with retinoic acid (RA) only. Data are mean ± standard error of three independent experiments. *p< 0.05 when compared to RA-treated cells.
Fig. 4.
Effect of propolis on the expression of GAP-43 in differentiating SH-SY5Y cells. The cells were treated with various concentrations of propolis in the presence of 10 μM RA for 48 hr. The Western blot data represents one of three experiments. Densitometric analysis was performed to determine the intensity of GAP-43 bands. Values are normalized to β-actin band and expressed as the percentage of cells treated with retinoic acid (RA) only. Data are mean standard error of three independent experiments. *p< 0.05 when compared to RA-treated cells.
DISCUSSION
Natural products are a promising source for the discovery of new pharmaceuticals. Propolis has been employed extensively since ancient times. Its use continues today as a popular remedy. Propolis presents plenty of biological and pharmacological properties, such as immunomodulatory (25), antitumor (5,6), anti-inflammatory (2), antioxidant (26), antibacterial (1), antiviral (27), antifungal (28), and antiparasite activities (29). However, the effect of propolis on neurosystem remain undefined. It has been suggested that in vitro methods are useful for preliminary investigation of the possible potential of a natural product (30). If such in vitro assays yield positive results, further investigation is necessary to produce data with clinical relevance. Moreover, in vitro and in vivo assays do not always include chemically characterized extracts, and one should take into account that pharmacological variability of preparations is expected (30).
A number of human neuroblastoma cell lines including IMR-32, SH-SY5Y, and SK-N-SH cells have been used to evaluate the effects of toxicants on neurite outgrowth (31). Any changes in neurite outgrowth in differentiating neurons can be used for the elucidation of neuroregenerative potential (32). SH-SY5Y human neuroblastoma cells can be differentiated into neuron-like cells with neurites in response to all-trans-RA stimulation (13). It has been used as an in vitro cell model for studying potential mechanisms of neurotoxicity.
In our experiment, we investigated the effect of propolis on nerve system using SH-SY-5Y human neuroblastoma cell line. Human SH-SY5Y neuroblastoma cells could be induced to differentiate by 10 μM all-trans retinoic acid (RA) treatment. We demonstrated that propolis inhibited neurite outgrowth of differentiating SH-SY5Y neuroblastoma cells. And propolis decreased the expression of TGase and GAP-43 proteins, common markers of differentiating neuroblastoma cells. These results suggest the potential effect of propolis on the differentiation of neuron.
ACKNOWLEDGEMENTS
We would like to thank Dr. Sang Bok Kim (AECL, Canada) for english correction. Funding for this paper was provided by Kyonggi University (2003).
Footnotes
CONFLICT OF INTEREST
Authors declare that there are no conflicts of interest.
REFERENCES
- 1.Drago B, Mombelli B, De Vecchi E, Fassina MC, Tocalli L, Gismondo MR. In vitro antimicrobial activity of propolis dry extract. J Chemother. 2000;12:390–395. doi: 10.1179/joc.2000.12.5.390. [DOI] [PubMed] [Google Scholar]
- 2.Mizoeva OK, Calder PC. The effect of propolis and its components on eicosanoid production during the inflammatory response. Prostaglandins Leukot Essent Fatty Acids. 1996;55:441–449. doi: 10.1016/S0952-3278(96)90129-5. [DOI] [PubMed] [Google Scholar]
- 3.Krol W, Czuba Z, Scheller S, Gabrys J, Grabiec S, Shani J. Anti-oxidant property of ethanolic extract of propolis (EEP) as evaluated by inhibiting the chemiluminescence oxidation of luminal. Biochem Int. 1990;21:593–597. [PubMed] [Google Scholar]
- 4.Scheller S, Wilczok T, Imielski S, Krol W, Gabrys J, Shani J. Free radical scavenging by ethanol extract of propolis. Int J Radiat Biol. 1990;57:461–465. doi: 10.1080/09553009014552601. [DOI] [PubMed] [Google Scholar]
- 5.Chen SN, Weng MS, Wu CL, Lin JK. Comparison of radical scavenging activity, cytotoxic effects and apoptosis induction in human melanoma cells by Taiwanese propolis from different sources. Evid Based Complement Alternat Med. 2004;1:175–185. doi: 10.1093/ecam/neh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuno T. Isolation and characterization of the tumoricidal substance from Brazilian propolis. Honeybee Sci. 1993;13:49–54. [Google Scholar]
- 7.Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73:S1–S6. doi: 10.1016/S0367-326X(02)00185-5. [DOI] [PubMed] [Google Scholar]
- 8.Scheller S, Krol W, Swiacik J, Owezarek S, Gabrys J, Shani J. Antitumoral property of ethanolic extract of propolis in mice-bearing Ehrlich carcinoma, as compared to bleomycin. Z Naturforsch, C, J Biosci. 1989;44:1063–1065. doi: 10.1515/znc-1989-11-1231. [DOI] [PubMed] [Google Scholar]
- 9.Chiao C, Carothers AM, Grunberger D, Solomon G, Preston AG, Barrett CJ. Apoptosis and altered redox state induced by caffeic acid phenethyl ester (CAPE) in transformed rat fibroblast cells. Cancer Res. 1995;55:3576–3583. [PubMed] [Google Scholar]
- 10.Rao CV, Desai D, Rivenson A, Simi B, Amin S, Reddy BS. Chemoprevention of colon carcinogenesis by phenylethyl-3-methylcaffeate. Cancer Res. 1995;55:2310–2315. [PubMed] [Google Scholar]
- 11.Fournier AE, Takizawa BT, Strittmatte SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itano Y, Nomura Y. 1-Methyl-4-phenylpyridinium ion (MPP+) causes DNA fragmentation and increases Bcl-2 expression in human neuroblastoma, SH-SY5Y cells, through different mechanisms. Brain Res. 1995;704:240–245. doi: 10.1016/0006-8993(95)01120-X. [DOI] [PubMed] [Google Scholar]
- 14.Tosetti P, Taglietti V, Toselli M. Functional changes in potassium conductances of the human neuroblastoma cell line SH-SY5Y during in vitro differentiation. J Neurophysiol. 1998;79:648–658. doi: 10.1152/jn.1998.79.2.648. [DOI] [PubMed] [Google Scholar]
- 15.Uberti D, Piccioni L, Colzi A, Bravi D, Canonico PL, Memo M. Pergolide protects SH-SY5Y cells against neurodegeneration induced by H2O2. Eur J Pharmacol. 2002;434:17–20. doi: 10.1016/S0014-2999(01)01537-0. [DOI] [PubMed] [Google Scholar]
- 16.Singh US, Pan J, Kao YL, Joshi S, Young KL, Baker KM. Tissue transglutaminase mediates activation of RhoA and MAP kinase pathways during retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Biol Chem. 2003;278:391–399. doi: 10.1074/jbc.M206361200. [DOI] [PubMed] [Google Scholar]
- 17.Tucholski J, Lesort M, Johnson GV. Tissue transglutaminase is essential for neurite outgrowth in human neuroblastoma SHSY5Y cells. Neuroscience. 2001;102:481–491. doi: 10.1016/S0306-4522(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 18.Kosik KS, Orecchio LD, Bruns GA, Benowitz LI, MacDonald GP, Cox DR, Neve RL. Human GAP-43: its deduced amino acid sequence and chromosomal localization in mouse and human. Neuron. 1988;1:127–132. doi: 10.1016/0896-6273(88)90196-1. [DOI] [PubMed] [Google Scholar]
- 19.Keilbaugh SA, Prusoff WH, Simpson MV. The PC12 cell as a model for studies of the mechanism of induction of peripheral neuropathy by anti-HIV-1 dideoxynucleoside analogs. Biochem Pharmacol. 1991;42:R5–R8. doi: 10.1016/0006-2952(91)90672-R. [DOI] [PubMed] [Google Scholar]
- 20.Bankova VS, De Castro SL, Marucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. doi: 10.1051/apido:2000102. [DOI] [Google Scholar]
- 21.Tucholski J, Lesort M, Johnson GV. Tissue transglutaminase is essential for neurite outgrowth in human neuroblastoma SH-SY5Y cells. Neuroscience. 2001;102:481–491. doi: 10.1016/S0306-4522(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Lesort M, Guttmann RP, Johnson GV. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J Biol Chem. 1998;273:2288–2295. doi: 10.1074/jbc.273.4.2288. [DOI] [PubMed] [Google Scholar]
- 23.Sidell N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982;68:589–596. [PubMed] [Google Scholar]
- 24.Påhlman S, Ruusala AI, Abrahamsson L, Mattsson ME, Esscher T. Retinoic acid-induced differentiation of cultured human neuroblastoma cells: a comparison with phorbolester-induced differentiation. Cell Differ. 1984;14:135–144. doi: 10.1016/0045-6039(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 25.Sforcin JM, Kaneno R, Funari SRC. Absence of seasonal effect on the immunomodulatory action of Brazilian propolis on natural killer activity. J Venom Anim Toxins. 2002;8:19–29. doi: 10.1590/S0104-79302002000100003. [DOI] [Google Scholar]
- 26.Chen YJ, Huang AC, Chang HH, Liao HF, Jiang CM, Lai LY, Chan JT, Chen YY, Chiang J. Caffeic acid phenethyl ester, an antioxidant from propolis, protects peripheral blood mononuclear cells of competitive cyclists against hyperthermal stress. J Food Sci. 2009;74:H162–H167. doi: 10.1111/j.1750-3841.2009.01199.x. [DOI] [PubMed] [Google Scholar]
- 27.Gekker G, Hu S, Spivak M, Lokensgard JR, Peterson PK. Anti-HIV-1 activity of propolis in CD4(+) lymphocyte and microglial cell cultures. J Ethnopharmacol. 2005;102:158–163. doi: 10.1016/j.jep.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 28.Sforcin JM, Fernandes A, Jr, Lopes CAM, Funari SRC, Bankova V. Seasonal effect of Brazilian propolis on Candida albicans and Candida tropicalis. J Venom Anim Toxins. 2001;7:139–144. doi: 10.1590/S0104-79302001000100009. [DOI] [Google Scholar]
- 29.Freitas SF, Shinohara L, Sforcin JM, Guimaraes S. In vitro effects of propolis on Giardia duodenalis trophozoites. Phytomedicine. 2006;13:170–175. doi: 10.1016/j.phymed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Heinrich M, Modarai M, Kortenkamp A. Herbal extracts used for upper respiratory tract infections: are there clinically relevant interactions with the cytochrome P450 enzyme system? Planta Med. 2008;74:657–660. doi: 10.1055/s-2008-1034292. [DOI] [PubMed] [Google Scholar]
- 31.Edsjö A, Holmquist L, Påhlman S. Neuroblastoma as an experimental model for neuronal differentiation and hypoxia-induced tumor cell dedifferentiation. Semin Cancer Biol. 2007;17:248–256. doi: 10.1016/j.semcancer.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Fournier AE, Takizawa BT, Strittmatte SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]