Abstract
Altering the fuel source from petroleum-based ultra-low sulfur diesel to biodiesel and its blends is considered by many to be a sustainable choice for controlling exposures to particulate material. As the exhaust of biodiesel/diesel blends is composed of a combination of combustion products of polycyclic aromatic hydrocarbons and fatty acid methyl esters, we hypothesize that 50% biodiesel/diesel blend (BD50) exposure could induce harmful outcomes because of its ability to trigger oxidative damage. Here, adverse effects were compared in murine male reproductive organs after pharyngeal aspiration with particles generated by engine fueled with BD50 or neat petroleum diesel (D100). When compared with D100, exposure to BD50 significantly altered sperm integrity, including concentration, motility, and morphological abnormalities, as well as increasing testosterone levels in testes during the time course postexposure. Serum level of luteinizing hormone was significantly depleted only after BD50 exposure. Moreover, we observed that exposure to BD50 significantly increased sperm DNA fragmentation and the upregulation of inflammatory cytokines in the serum and testes on Day 7 postexposure when compared with D100. Histological evaluation of testes sections from BD50 exposure indicated more noticeable interstitial edema, degenerating spermatocytes, and dystrophic seminiferous tubules with arrested spermatogenesis. Significant differences in the level of oxidative stress assessed by accumulation of lipid peroxidation products and depletion of glutathione were detected on exposure to respirable BD50 and D100. Taken together, these results indicate that exposure of mice to inhalable BD50 caused more pronounced adverse effects on male reproductive function than diesel.
Keywords: pulmonary exposure, biodiesel particles, oxidative stress, male reproduction, sperm quality, DNA fragmentation
INTRODUCTION
The use of biodiesel (BD) or its blends with petroleum diesel (D100) is contemplated to be a justifiable approach to reduce occupational and environmental exposures to particulate matter (PM). Exposure to diesel exhaust in humans has been shown to cause a number of adverse health outcomes, including pulmonary, cardiovascular diseases, and cancer [Tokiwa and Ohnishi, 1986; Watkinson et al., 1998; Holgate et al., 2003a; Garshick et al., 2004; Mills et al., 2005, 2007; Rivero et al., 2005; Nemmar et al., 2007, 2009; Tornqvist et al., 2007; Peretz et al., 2008; Pronk et al., 2009; Sawyer et al., 2010; Hazari et al., 2011; Silverman et al., 2012]. Diesel exhaust particulates (DEPs) have also been reported to cause the disruption of male reproductive function. Prior studies have shown that DEP exposure disturbed spermatogenesis, resulting in reduction of daily sperm production and motility, increased morphological sperm abnormalities, and ultrastructural changes in Leydig cells in mice [Yoshida et al., 1999; Yoshida and Takeda, 2004; Izawa et al., 2008; Li et al., 2012]. In male rats, the regulation of testicular function was altered resulting in elevation of serum testosterone and reduction of luteinizing hormone (LH) and sperm production after DEP exposure [Watanabe and Oonuki, 1999; Tsukue et al., 2001, 2002; Izawa et al., 2007; Li et al., 2007, 2009b; Ramdhan et al., 2009].
On the other hand, most BD fuels are produced by the transesterification of vegetable oils generating fatty acid alkyl esters [Knothe et al., 2010]. The major components of these oils are monounsaturated oleic acid, polyunsaturated linoleic acid, and linolenic acid, which are prone to oxidation on combustion forming a variety of peroxides and secondary oxidation products [Song et al., 2000; Knothe, 2005]. Moreover, exposure to BD was found to increase the particle-bound volatile organic fraction of PM and carbonyl emissions [Purcell et al., 1996; Liu et al., 2009], inducing a rise in cellular toxicity [Liu et al., 2009]. Recent studies demonstrate that BD or its blends are more mutagenic and increase formation of multinucleated cells when compared with ultralow sulfur diesel fuel (ULSD) [Ackland et al., 2007; Bunger et al., 2007; Krahl et al., 2009; Kisin et al., 2013]. Brito et al. [2010] demonstrated that exposure to diesel, neat BD, and 50% biodiesel/diesel blend (BD50) induces acute lung inflammation. Recently, we reported that pharyngeal aspiration or inhalation exposure to BD exhaust causes pronounced pulmonary damage accompanied by a robust inflammatory response and severe oxidative stress when compared with diesel exhaust nanoparticles [Shvedova et al., 2013; Yanamala et al., 2013].
Diesel engine emissions from the use of diesel, or its blends with biodiesel, are highly complex mixtures of aerosols and gases. Most of the particles from these engine exhausts are of nanoscale and potentially have hundreds of chemicals absorbed onto their surfaces, including known and suspected mutagens and carcinogens, for example, polycyclic aromatic hydrocarbons (PAH) and nitrated PAH (nPAH) [Mermelstein et al., 1981; Ohe, 1984; Rivedal et al., 2003; Bünger et al., 2012]. Recently, it has been shown that human exposure to PAHs causes alterations in male sperm quality, including morphology, concentration, and vitality, as well as DNA damage, thus affecting male reproductive function [Gaspari et al., 2003; Meeker et al., 2007; Han et al., 2011; Jeng et al., 2013].
To address whether exposure to respirable BD adversely affects spermatogenesis, we compared the effects of BD50 and neat petroleum diesel in male C57BL6 mice. We found that pulmonary exposure to BD50 significantly altered sperm concentration, motility, and morphology. Moreover, BD50 caused upregulation of inflammatory cytokines, increase in testicular testosterone, and reduction of serum LH. Testicular histopathology revealed interstitial edema, clustering of the dystrophic seminiferous tubules with arrested spermatogenesis, and the presence of degenerating spermatocytes. Additionally, we observed that exposure to inhalable BD50 caused severe oxidative stress and DNA damage in mouse male reproductive organs. Here, for the first time, we demonstrate that exposure to respirable BD50 generates a pronounced toxicity to the male reproductive system when compared with diesel.
MATERIALS AND METHODS
Animals
Specific pathogen-free adult male C57BL/6 mice (7–8 weeks) were supplied by Jackson Laboratories (Bar Harbor, ME) and weighed 20.0±1.9 g when used. Animals were housed one mouse per cage receiving filtered high-efficiency particulate air in the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International-accredited National Institute of Occupational and Safety Health (NIOSH) animal facility. All animals were acclimated in the animal facility under controlled temperature and humidity for 1 week prior to use. Beta Chips (Northeastern Products Corp., Warrensburg, NY) were used for bedding and changed weekly. Animals were supplied with water and certified chow 7913 (Harlan Teklad, Indianapolis, IN) ad libitum, in accordance with the guidelines and policy set forth by the Institute of Laboratory Animal Resources, National Research Council. All experimental procedures were conducted in accordance with a protocol approved by the NIOSH Institutional Animal Care and Use Committee.
General Experimental Design
To assess reproductive toxicity, mice were treated by repeated pharyngeal aspiration with suspensions of BD50 or D100 exhaust particles (15 µg/mouse/day of total carbon) in United States Pharmacopeia (USP) sterile water (Hospira, Lake Forest, IL). The corresponding control mice were administered USP sterile water. Mice were exposed twice a week for two consecutive weeks with a cumulative dose of 60 µg per mouse of total carbon. Specifically, human equivalent workplace exposure to a deposited cumulative dose of 60 µg of total carbon can be achieved in ~88 working weeks at allowable exposure concentration limits defined by MSHA (160 µg/m3 of total carbon). Calculations were performed using the formula published previously by our group [Yanamala et al., 2013]. Animals were weighed and sacrificed on Days 7 and 28 following their last exposure. Blood samples were collected and kept at room temperature for 1.5 hr to allow for clotting. The samples were then centrifuged at 1,700g for 15 min at 4°C, and serum was separated and stored at −80° C until assayed for cytokine responses and LH level. Testes were removed and weighed. The right testis was used for histologic examination, whereas the left one was processed for testosterone measurements, cytokines responses, and biomarkers of oxidative stress. The epididymis were excised and trimmed carefully of excess tissue and fat and immediately weighed. The right epididymis was processed for semen analysis (sperm density, motility, and morphology evaluation), whereas the left epididymis was used for sperm DNA fragmentation screening.
Exhaust/Emission Generation and Diesel Particulate Matter Samples Collection System
The diesel particulate matter (DPM) samples were collected at the diesel laboratory at the NIOSH Office of Mine Safety and Health Research. A single batch of neat corn-based fatty acid methyl ester (FAME) BD was acquired from Peter Cremer (Cincinnati, OH, NEXSOL™ BD-100), and a single batch of petroleum-based ULSD fuel was acquired from a local supplier. The BD50 blend was prepared at the site. The fractions of BD and ULSD were determined volumetrically. The samples of BD50 and D100 particulates were collected from the exhaust of a mechanically controlled, naturally aspirated directly injected Isuzu C240 (Isuzu Motors Limited, Tokyo, Japan) diesel engine equipped with a diesel oxidation catalytic converter (DOC, Lubrizol, New Market, ON). The engine was exercised over four steady-state operating conditions [Yanamala et al., 2013]. The exhaust particles originating from the four different loads were collected and combined to perform the study.
A high-volume sampling system was developed to advance the methods of collecting representative samples of diesel particulates. This system allows for collecting nanosized and ultrafine DPM aerosols in liquid media, therefore preserving the sampled aerosol to the highest possible level of physical and chemical characteristics. The method for the DPM sample collection system has been described in detail by Yanamala et al. [2013]. The samples collected for each of the fuels were combined and further concentrated using a rotating evaporator (Eppendorf Vacufuge, Hamburg, Germany). The particle suspensions were sonicated using a Vibra Cell (Sonics & Materials, Newtown, CT) before administering them to animals.
All doses of BD50 and D100 exhaust particles were standardized based on the amount of total carbon analyzed by NIOSH Method 5040 [NMAM, 2003; Birch, 2004] as mentioned previously [Yanamala et al., 2013]. Additionally, the hydrodynamic diameter of BD50 and D100 particulates (216 and 312 nm, respectively) was determined by dynamic light scattering [Yanamala et al., 2013].
Particulate Aspiration
Mouse pharyngeal aspiration was used for particulate administration. Briefly, after anesthesia with a mixture of ketamine (Phoenix Pharmaceutical Inc, St. Joseph, MO) and xylazine (62.5 and 2.5 mg/kg subcutaneous in the abdominal area; Phoenix Pharmaceutical Inc, St. Joseph, MO), the mouse was placed on a board in a near vertical position, and the animal’s tongue was extended with lined forceps. A suspension of BD50 or D100 exhaust particles (15 µg/mouse/day of total carbon, twice a week, for 2 weeks) was placed posterior in the throat, and the tongue held until the suspension was aspirated into the lungs. All particles were sterilized prior to administration. All mice from the control, BD50-, and D100-treated groups survived this exposure procedure and exhibited no negative behavioral or health outcomes.
Levels of Oxidative Stress Markers
Oxidative damage in the testis following pulmonary exposure to BD50 or D100 was evaluated by the presence of hydroxynonenalhistidine (HNE-His) protein adduct and glutathione (GSH) level. HNE-His adducts, lipid peroxidation end products, were measured in tissue homogenates by ELISA using an OxiSelect™ HNE-His adduct kit (Cell Biolabs, San Diego, CA). The quantity of HNE-His adducts in protein samples were determined by comparing its absorbance with that of a known HNE-BSA standard curve.
GSH concentration in testis homogenates was determined using ThioGlo −1 (Covalent Associates Inc., Corvallis, OR), a maleimide reagent, which produces highly fluorescent adducts on its reaction with SHA groups [Shvedova et al., 2005]. Glutathione content was estimated by an immediate fluorescence response registered on the addition of ThioGlo™ 1 to the testis homogenate. A standard curve was established by the addition of GSH (0.04–4.0 µM) to 100 mM phosphate buffer (PBS, pH 7.4) containing 10 µM ThioGlo −1. The Synergy H1 hybrid multimode microplate reader (BioTek Instruments, Winooski, VT) was used for the assay of fluorescence using excitation at 388 nm and emission at 500 nm. The data obtained were exported and analyzed using Gen5™ data analysis software (BioTek Instruments).
Epididymal Sperm Concentration and Motility
The total sperm cell counts and motility were assessed as described previously by Polyzos et al. [2009]. The caudal right epididymis was minced into 2 ml prewarmed M16 medium (Sigma-Aldrich, St. Louis, MO) and incubated at 37°C in 5% CO2 for 5 min to allow sperm to disperse. Sperm concentration and motility were evaluated from the same sperm cell suspension using an improved Neubauer hemocytometer chamber (Hausser Scientific, Horsham, PA). Ten microliter of the warm sperm cell suspension was loaded into the hemocytometer and immediately examined under microscope. About 300–400 cells per slide were tracked for motility assessment by scoring the number of all motile and nonmotile sperm in the same field.
Epididymal Sperm Morphology
Sperm morphology was assessed as described previously by Pereira et al. [1981]. Briefly, 10 µl of Eosin Y (Leica Microsystems, Buffalo Grove, IL) was mixed with 90 µl of sperm suspension obtained from the right epididymis as described in the preceding paragraph and incubated for 30 min at room temperature. A drop of sperm suspension (5 µl) was smeared on the slide, air dried, and mounted with low-viscosity mounting medium. Sperm morphology was examined on bright field microscopy (Olympus Provis, B&B Microscopes, Pittsburgh, PA), and spermatozoa were classified as follows: sperm cells with normal morphology and cells presenting abnormalities in head, neck/mid-piece, and tail. At least 500 sperm cells were recorded for each slide under 1,000 × magnification.
Sperm DNA Fragmentation Screening
Sperm chromatin integrity was assessed by the sperm chromatin structure assay (SCSA). This test provides detection of damaged DNA and altered proteins in sperm nuclei via flow cytometry of acridine orange stained sperm [Evenson and Melamed, 1983]. Cell suspension from the left caudal epididymis was prepared in M16 medium (as described above) and incubated for 5 min at 37°C in 5% CO2. After incubation, the sperm cells were filtered through a 70-µm sterile cell strainer to remove the tissues and other debris and to form a more uniform cell suspension. Next, the sperm cell suspension was centrifuged for 3 min at 2,000 rcf (37°C), the supernatant was removed, and the cells were resuspended in 100 µl of M16 medium. The concentrated cell suspensions were immediately frozen and stored in liquid nitrogen until shipping to SCSA Diagnostics (Brookings, SD) for evaluation of cells with DNA fragmentation. The DNA fragmentation index value indicates the percent of sperm cells containing DNA damage.
Histopathology of the Testis
Testes were removed and fixed with 10% buffered formaldehyde. The testes were embedded in paraffin and sectioned at a thickness of 5 µm on an HM 320 rotary microtone (Carl Zeiss, Thornwood, NY). Prepared sections were stained with hematoxylin and eosin (H&E), and histopathological evaluation was performed by certified pathologist. Sample identification was coded to ensure unbiased evaluation.
Preparation of Tissue Homogenates
The left testis was homogenized with a tissue tearer (model 985–370, Biospec Products, Racine, WI) in cold PBS (pH, 7.4) for 2 min on ice. The homogenate suspensions were aliquoted and frozen at −80° C until processed.
Measurement of Cytokines and Chemokines
Proinflammatory cytokines, IL-1α, IL-1β, IL-6, and TNF-α, in the serum and testes homogenates from mice exposed to BD50 and D100 exhaust particulates were analyzed using a Bio-Plex system (Bio-Rad, Hercules, CA). The concentrations were calculated using Bio-Plex Manager 6.1 software (Bio-Rad) based on standard curves.
Testosterone Level in the Testes
The concentration of testosterone in the testes homogenate was determined by applying the competitive enzyme immunoassay technique using a BlueGene Testosterone ELISA kit (Antibodies-Online, Atlanta, GA). The optical density was determined using a Synergy™ H1 hybrid multimode microplate reader (BioTek Instruments). Using Gen5™ data analysis software (BioTek Instruments), the concentration of testosterone in the samples was determined.
Luteinizing Hormone Concentration in the Serum
The concentration of LH was determined using an ELISA kit from BlueGene Biotech (Shanghai, China). The optical density was determined using a Synergy H1 hybrid multimode microplate reader (BioTek Instruments, Winooski, VT). Using Gen5™ data analysis software (BioTek Instruments, Winooski, VT), the sample concentrations were calculated. Each sample was run in duplicate.
Protein Assay
Measurements of protein in tissue homogenates from mouse testis were run using a Bio-Rad protein assay kit (Richmond, CA).
Statistical Analysis
Results were compared by one-way ANOVA using all the pairwise multiple comparison procedures (Holm-Sidak method). All results are presented as mean + SEM. P values of less than 0.05 were considered to indicate statistical significant.
RESULTS
Proinflammatory Cytokine in Serum Following BD50 or D100 Exposures
The release of cytokines was used as a marker of proinflammatory responses in the serum of mice exposed to BD50 and D100 exhaust (Table I). IL-1α, IL-1β, and TNF-α in the serum were uniquely upregulated only after exposure to BD50 (Day 7 post-treatment). The elevation of TNF-1α was found to be significantly stronger in BD50 when compared with that of D100. The level of IL-6 was found to be significantly elevated after exposure to either BD50 or D100, with a stronger response from BD50. Overall, the serum levels of the proinflammatory cytokines following BD50 exposure were higher when compared with D100.
TABLE I.
Differential Responses in Serum Cytokines on Day 7 Post Repeated Exposure to Diesel (D100) or Biodiesel Blend (BD50) Exhaust Particulates
| pg/ml | Control | D100 | BD50 |
|---|---|---|---|
| IL-1α | 17.4 ± 1.3 | 19.9 ± 1.4 | 21.0 ± 1.1a |
| IL-1β | 153.2 ± 24.6 | 213.8 ± 19.7 | 220.8 ± 14.4a |
| IL-6 | 21.2 ± 1.7 | 27.8 ± 2.9a | 33.4 ± 3.0a |
| TNF-α | 295.5 ± 16.6 | 354.3 ± 31.5 | 470.4 ± 37.0ab |
P<0.05, vs control.
P<0.05, vs D100 exposure.
Oxidative Stress Markers
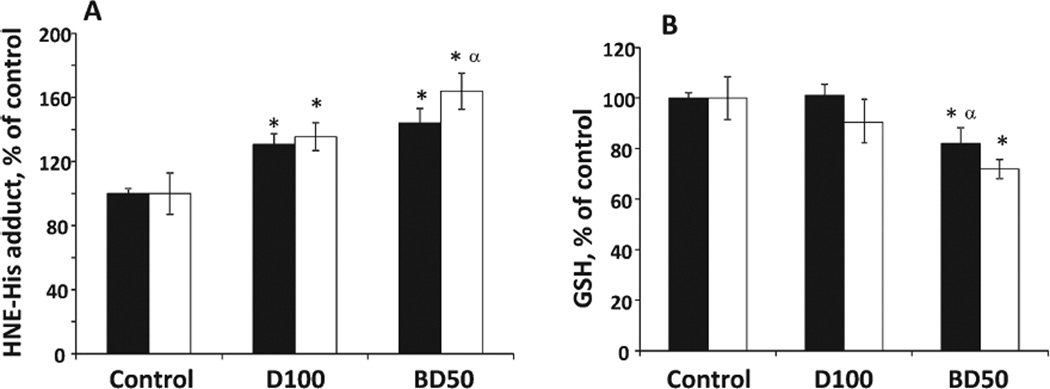
The level of oxidative damage in the testes caused by BD50 or D100 exhaust PM was assessed by lipid peroxidation products and GSH (Fig. 1). Exposure to BD50 resulted in significant 44 and 64% accumulation of lipid peroxidation products, measured as HNE-His adducts, over control throughout the time course of 7 and 28 days post-treatment, respectively (Fig. 1A). Similarly, D100 caused significant 30 and 35% increases of HNE-His adducts observed on Days 7 and 28 postexposure, respectively. However, accumulation of lipid peroxidation products was significantly greater on exposure to BD50 when compared with D100 on Day 28 post-treatment. Time course of GSH depletion in the testes of mice exposed to BD50 revealed significant 18 and 28% decreases from control samples at 7 and 28 days postaspiration, respectively (Fig. 1B). No significant changes in the level of GSH were found after exposure to D100. Additionally, alterations in the level of GSH were significantly different on Day 7 postexposure to DB50 and D100.
Fig. 1.
The degree of oxidative stress evaluated by HNE-His adduct (A) and GSH (B) in the testes of C57BL6 mice on Days 7 and 28 after repeated exposure to D100 or BD50 exhaust particles. Mice were exposed via pharyngeal aspiration (15 µg/mouse/day of total carbon, twice a week for 2 weeks). Black columns: 7 days after last repeated exposure to BD50 or D100 exhaust particulate; and clear columns: 28 days after last repeated exposure to BD50 or D100 exhaust particulate. Data are presented as percent of control. Means ± SE (n = 10 mice per group). Significantly different from *controls (P < 0.05) and αD100 exhaust particulate-exposed group (P < 0.05).
Epididymis Sperm Characteristics
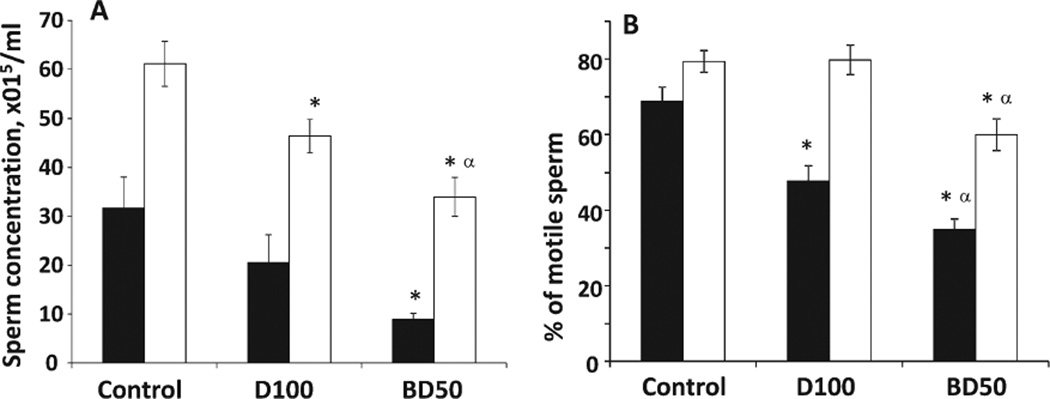
To characterize the impact of BD50 or D100 treatment on the male reproductive system, epididymis sperm concentration (Fig. 2A), sperm motility (Fig. 2B), and morphological abnormalities (Fig. 3) were analyzed on Days 7 and 28 after pharyngeal aspiration.
Fig. 2.
Concentration (A) and motility (B) of epididymal sperm from C57BL6 mice on days 7 and 28 after repeated exposure to D100 or BD50 combustion exhaust particles. Mice were exposed to exhaust particles via pharyngeal aspiration (15 µg/mouse/day of total carbon, twice a week for 2 weeks). Black columns: 7 days after last repeated exposure to BD50 or D100 exhaust particulate; and clear columns: 28 days after last repeated exposure to BD50 or D100 exhaust particulate. Means ± SE (n = 10 mice per group). Significantly different from *controls (P < 0.05) and αD100 exhaust particulate-exposed group (P < 0.05).
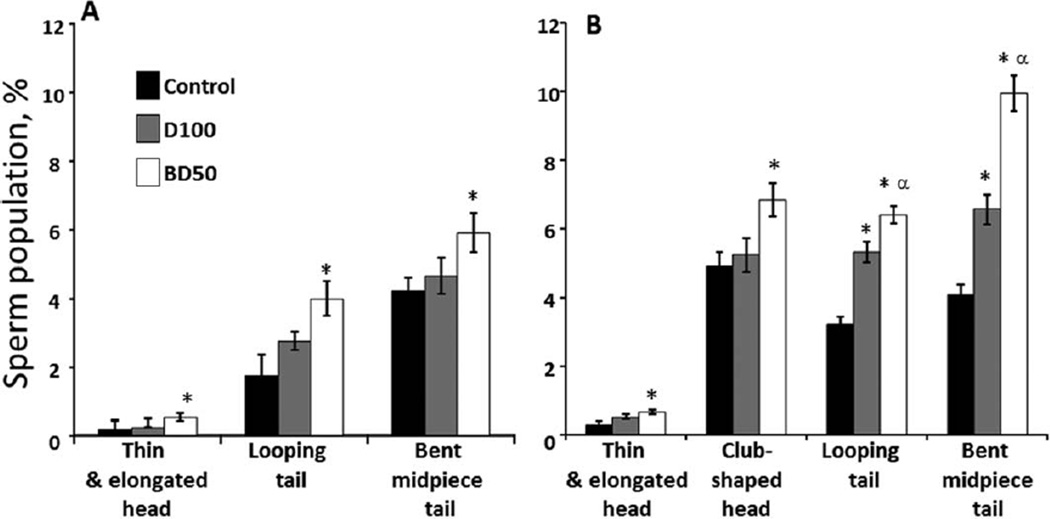
Fig. 3.
Morphological abnormalities of epididymal sperm from C57BL6 mice on Days 7 and 28 postrepeated exposure to D100 or BD50 combustion exhaust particles. (A) Day 7 and (B) Day 28 postexposure to combustion exhaust particles. Mice were exposed to exhaust particles via pharyngeal aspiration (15 µg/mouse/day of total carbon, twice a week for 2 weeks). Black columns: control mice; gray columns: mice exposed to D100 exhaust particulate; and clear columns: mice exposed to BD50 exhaust. Data presented as percent of abnormal cells population when compared with total number of sperm. Means ± SE (n = 10 mice per group). Significantly different from *controls (P < 0.05) and αD100 exhaust particulate-exposed group (P < 0.05).
We found that BD50 exposure induced a significant 72% and 44% decrease in epididymis sperm concentration in contrast to the control on Days 7 and 28 postexposure, respectively (Fig. 2A). Exposure to D100 resulted in a significant reduction of sperm concentration by 24% on Day 28 only.
Similarly, BD50 exhaust significantly diminished sperm motility by 34% and 19% when compared with the control on Days 7 and 28 postexposure, respectively (Fig. 2B), whereas D100 decreased the number of motile cells by 21% when compared with the control on Day 7 postexposure only.
Sperm morphological examination demonstrated a significant 3.4-, 1.7-, and 1.4-fold increase in the number of abnormal thin/elongated heads, looping, and bent mid-piece tails versus control on Day 7 post-BD50 exposure, respectively (Fig. 3A). No significant changes were found on Day 7 after exposure to D100 exhaust. More pronounced morphological abnormalities were found on Day 28 postexposure in both D100- and BD50-treated mice (Fig. 3B). Thus, significant 2.2- and 1.4-fold increase in the number of abnormal thin/elongated and club-shaped heads, respectively, over control were detected only after exposure to BD50. Additionally, BD50 induced significant twofold and 2.4-fold rises over the control in the number of looping and bent mid-piece tails, whereas D100 exposure increased up to 1.65- and 1.6-fold when compared with control mice, respectively.
Interestingly, effect of BD50 induced significantly stronger changes in sperm concentration and morphological abnormalities only on Day 28 postexposure, whereas alterations in motility of the sperm were more pronounced throughout the time course of the experiment when compared with D100.
Testicular Cytokines Following BD50 or D100 Exhaust Exposures
The release of proinflammatory cytokines in the testes of mice exposed to BD50 and D100 exhaust are shown in Table II. IL-1α, IL-1β, and TNF-α were upregulated after exposure to BD50 or D100 (Day 7 post-treatment). The elevation of TNF-1α was found to be significantly stronger in BD50 when compared with that of D100. The levels of IL-6 in both groups were not different from the control.
TABLE II.
Differential Responses in Testicular Cytokines on Day 7 Post Repeated Exposure to Diesel (D100) or Biodiesel (BD50) Exhaust Particulates
| pg/mg protein | Control | D100 | BD50 |
|---|---|---|---|
| IL-1α | 0.9 ± 0.0 | 1.3 ± 0.1a | 1.3 ± 0.0a |
| IL-1β | 8.8 ± 0.7 | 13.2 ± 0.9a | 12.3 ± 0.4a |
| IL-6 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.0 |
| TNF-α | 18.7 ± 1.2 | 23.2 ± 0.9a | 25.6 ± 0.6ab |
P<0.05, vs control.
P<0.05, vs D100 exposure.
Testicular Testosterone Level
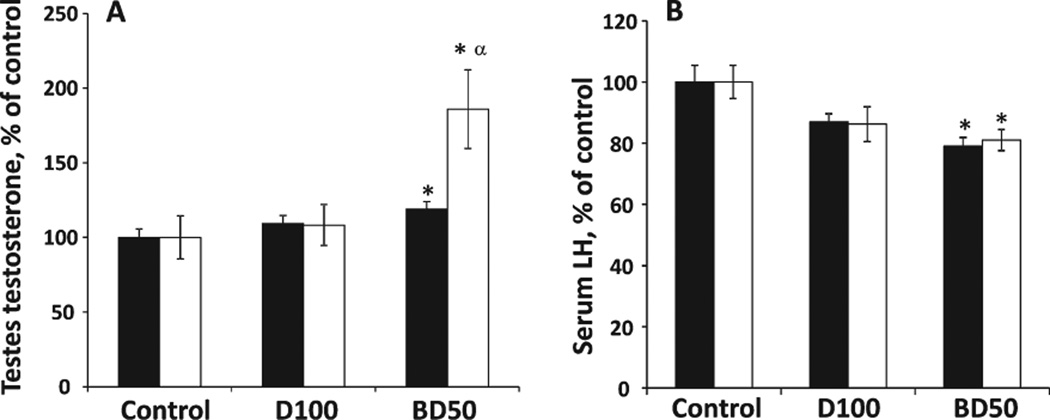
The testosterone concentration in the testes homogenates was measured after pharyngeal aspiration of BD50 or D100 exhaust particulates on Days 7 and 28 postexposure (Fig. 4A). A significant 19% augmentation in testicular testosterone level was found in the BD50 group on Day 7 postexposure, which progressed further (86% over control) by Day 28. No significant changes were found after exposure to D100. Moreover, the elevation of testosterone concentration was found to be significantly stronger in BD50 when compared with that of D100.
Fig. 4.
Testicular testosterone (A) and serum LH (B) levels in C57BL6 mice on Days 7 and 28 after repeated exposure to D100 or BD50 exhaust particulate. Mice were exposed to exhaust particles via pharyngeal aspiration (15 µg/mouse/day of total carbon, twice a week for 2 weeks). Black columns: 7 days after last repeated exposure to BD50 or D100 exhaust particulate; and clear columns: 28 days after last repeated exposure to BD50 or D100 exhaust particulate. Data presented as percent of control. Means ± SE (n = 10 mice per group). Significantly different from *controls (P < 0.05) and αD100 exhaust particulate-exposed group (P < 0.05).
Serum Luteinizing Hormone Concentration
We investigated the serum concentration of LH in mice on Days 7 and 28 postexposure with BD50 or D100 exhaust particulate. Serum concentrations of LH in the BD50 exposure group were significantly decreased by 21% and 19% on Days 7 and 28 post-treatment, respectively, when compared with the control group (Fig. 4B). Exposure to D100 exhaust particulate did not induce significant depletion of LH in serum when compared with the control group. The results from BD50 exposure were not significantly different when compared with D100.
Histological Evaluation
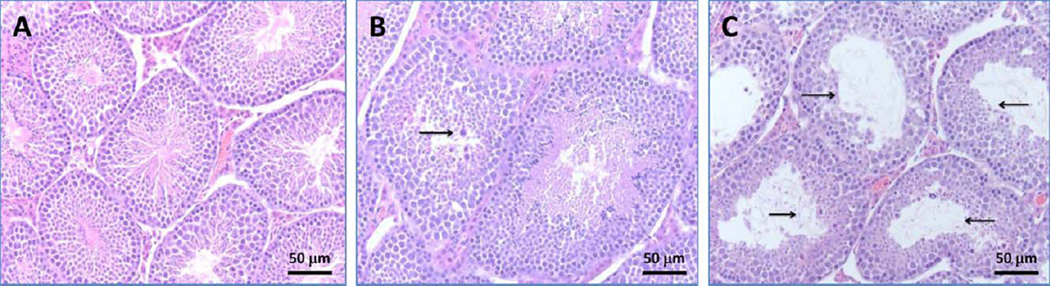
Formalin-fixed, paraffin-wax embedded testes sections stained with H&E were examined microscopically. Normal architecture of seminiferous tubules and orderly spermatogenesis were detected in the testes from control mice on Days 7 and 28 postexposure. Additionally, no histopathological changes were observed in Leydig or Sertoli cells (Fig. 5A). Sections of D100 and BD50 groups on Day 7 postexposure showed no significant pathologic alterations. However, on Day 28 postexposure, sections of D100 group showed interstitial edema and occasional dystrophic seminiferous tubules with arrested spermatogenesis and the presence of degenerating spermatocytes (Fig. 5B). These histologic changes were even more prominent in BD50 group, where dystrophic seminiferous tubules were clustering, especially in subcapsular areas (Fig. 5C).
Fig. 5.
Light micrographs of H&E stained sections from testes of mice 28 days after pharyngeal aspiration of D100 or BD50 (cumulative dose 60 mg per mouse): (A) control mice, (B) mice exposed to D100, and (C) mice exposed to BD50. Sections of D100 group showed interstitial edema and occasional dystrophic seminiferous tubules with arrested spermatogenesis and the presence of degenerating spermatocytes (arrows). These histologic changes were more prominent in BD50 group, where dystrophic seminiferous tubules were clustering, especially in subcapsular areas (arrows).
DNA Damage
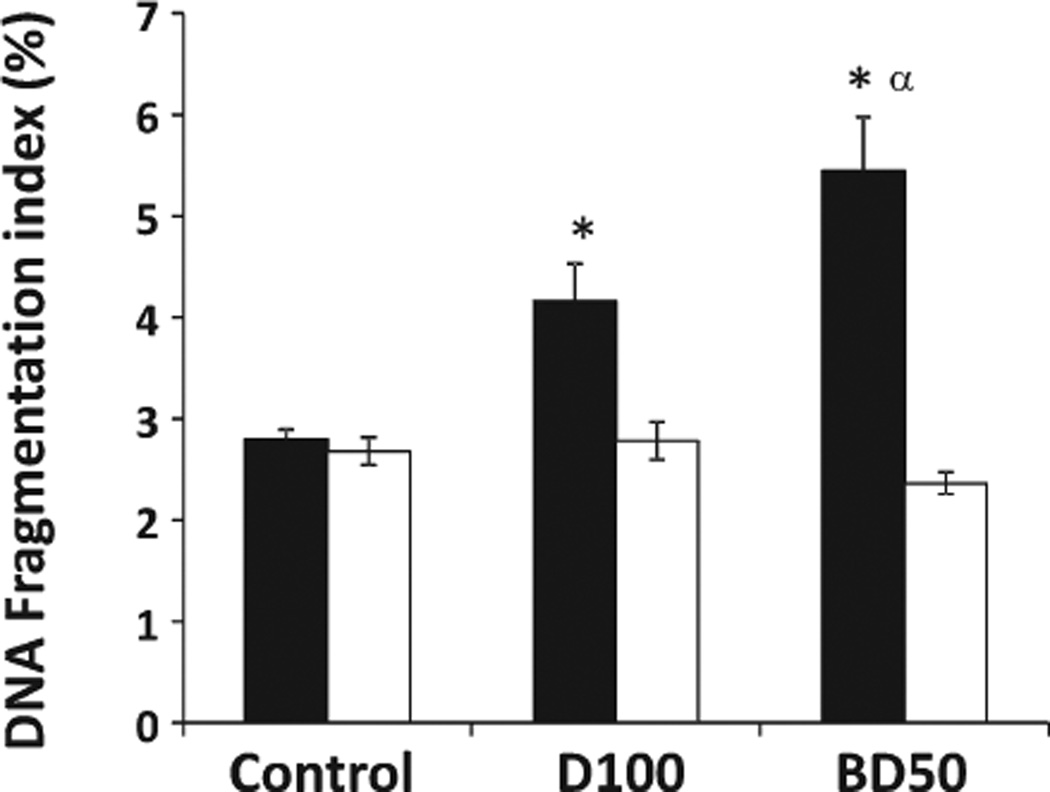
The sperm chromatin structure abnormalities were measured in the cells derived from the caudal epididymis of mice on Days 7 and 28 postexposure to BD50 or D100 using SCSA. The levels of DNA fragmentation were significantly increased by 1.5- and 1.9-fold in the samples from D100- and BD50-exposed mice, respectively, only on Day 7 post-treatments (Fig. 6). Furthermore, the level of DNA fragmentation was found to be significantly stronger in BD50 when compared with that of D100. No changes in DNA fragmentation were found on Day 28 postexposure in either the BD50 or the D100 samples.
Fig. 6.
DNA damage of epididymal sperm from C57BL6 mice on Days 7 and 28 postrepeated exposure to D100 or BD50 exhaust particles. Mice were exposed to exhaust particles via pharyngeal aspiration (15 µg/ mouse/day of total carbon, twice a week for 2 weeks). Black columns: 7 days after last repeated exposure to BD50 or D100 exhaust particulate; and clear columns: 28 days after last repeated exposure to BD50 or D100 exhaust particulate. Data presented as percent of control. Means ± SE (n = 10 mice per group). Significantly different from *controls (P < 0.05) and αD100 exhaust particulate-exposed group (P < 0.05).
DISCUSSION
Oxidative stress has been implicated in numerous diseases such as cancer, connective tissue disorders, aging, infection, inflammation, acquired immunodeficiency syndrome, and male infertility [Agarwal and Said, 2005; Benedetti et al., 2012; Guerriero et al., 2014]. Seminal reactive oxygen species (ROS) were increased in a large proportion of infertile men, demonstrating that oxidative stress may be a major cause of male infertility [Pasqualotto et al., 2001; Agarwal and Said, 2005]. Oxidative stress results from the imbalance between production of the ROS and the protective effect of the antioxidant system responsible for their neutralization and removal. An excess of ROS causes a pathological reaction resulting in cell and tissue damage.
Spermatozoa are the most susceptible to the harmful effects of ROS due to the large amounts of unsaturated fatty acids that can be oxidized (lipid peroxidation) in their cell membrane. The lipid peroxidation process leads to a loss of membrane integrity and an increase in its permeability, inactivation of cellular enzymes, and structural DNA damage. The overall consequence is reduced sperm count and activity, decreased motility, abnormal morphology, and impaired function [Walczak–Jedrzejowska et al., 2013].
As biodiesel/diesel blend exhaust is composed of a combination of PAHs and FAMEs combustion products, we hypothesized that BD50 exhaust particulate could induce adverse effects on male reproductive function because of its ability to cause oxidative damage. As previously reported, DEPs disrupt male reproductive function resulting in reduction of daily sperm production and motility, increased morphological sperm abnormalities, ultrastructural changes in Leydig cells, elevation of serum testosterone, and reduction of LH level [Watanabe and Oonuki, 1999; Yoshida et al., 1999; Tsukue et al., 2001, 2002; Yoshida and Takeda, 2004; Izawa et al., 2007, 2008; Li et al., 2007, 2009b, 2012; Ramdhan et al., 2009]. Human exposure to PAHs causes modification to male sperm quality, including morphology, concentration, and vitality, as well as causing DNA damage in sperm, thus affecting male reproductive function [Gaspari et al., 2003; Meeker et al., 2007; Han et al., 2011; Jeng et al., 2013]. Some PAHs, such as benzo(a)pyrene, fluoranthene, or benzo(ghi)perylene (chemically reactive and nonvolatile), are emitted at greater levels in biodiesel than diesel exhaust [Kado et al., 1996] and are well-known endocrine disrupters [Raychoudhury and Kubinski, 2003]. Nitro-PAHs, such as 4-nitro-3-phenylphenol, have been shown to induce lipid peroxidation and decrease both superoxide dismutase and glutathione peroxidase (GSH-Px) activity [Mi et al., 2010].
Recent publications demonstrate that biodiesel and its blend (BD50) particles promote more pronounced cardiovascular alterations, as well as pulmonary and systemic inflammation, than diesel [Brito et al., 2010]. Moreover, Shvedova et al. [2013] and Yanamala et al. [2013] showed that pharyngeal aspiration or inhalation exposure to BD exhaust causes pulmonary damage accompanied by a robust inflammatory responses and severe oxidative stress. These studies suggest that BD aerosols might produce greater adverse effects when compared with D100 exposure. Furthermore, very recent publications, including our own, demonstrate that biodiesel or its blends are more mutagenic and increase formation of multinucleated cells when compared with ultralow sulfur diesel fuel [Ackland et al., 2007; Bunger et al., 2007; Krahl et al., 2009; Kisin et al., 2013]. These effects are most likely attributed to the greater levels of PAHs and nPAHs in the exhaust, which are potent mutagens and carcinogens [Tokiwa and Ohnishi, 1986]. To the best of our knowledge, the current study is the first to report adverse effects of biodiesel or its blends exhaust on male reproductive function.
The major components of BD fuels are monounsaturated oleic, polyunsaturated linoleic, and linolenic acids that are prone to oxidation on combustion, causing the formation of peroxides and a variety of secondary oxidation products [Song et al., 2000; Knothe, 2005]. On the other hand, reactive metabolites of PAHs can be oxidized, enter redox cycles, and increase the formation of ROS [Farmer et al., 2003]. Oxidative stress is known to play a crucial role in the etiology of defective sperm function via a mechanism involving the induction of peroxidative damage to the plasma membrane [Aitken et al., 2014]. An increase in intracellular ROS levels has been shown to damage tissue and cells. Similarly, in earlier studies [Shvedova et al., 2013; Yanamala et al., 2013], we found greater oxidative stress after exposure to BD50 than D100 exhaust. The susceptibility of epididymal spermatozoa to the existing oxidative stress observed in the testes is not unexpected due to the fact that spermatozoa are uniquely rich in polyunsaturated fatty acids [Guerriero et al., 2014]. The latter are susceptible to free radical attack generating lipid peroxidation chain reactions that culminate in electrophilic lipid aldehydes such as 4-HNE or acrolein. These products of lipid peroxidation are also capable of triggering ROS generation by sperm mitochondria [Aitken et al., 2012], thus showing that oxidative stress in spermatozoa is a self-propagating cycle that, once initiated, will inevitably lead to oxidative damage, a loss of functionality, and cell death [Aitken et al., 2014]. In the current study, greater accumulation of lipid peroxidation products (HNE-His adduct) and depletion of a major antioxidant, GSH, were found in the testes of BD50 exhaust-exposed mice when compared with D100 (Fig. 1). This oxidant/antioxidant imbalance found in the BD50-exposed mice correlated well with the impairment of sperm characteristics demonstrated (Figs. 2, 3, and Fig. 7). A pronounced reduction of sperm motility and amplification of morphological abnormalities on pulmonary exposure to BD50 exhaust particulates when compared with D100 was found throughout the postexposure time course. This is also in agreement with previous studies [Saleh and Agarwal, 2002; El-Demerdash et al., 2004; Aitken and Curry, 2011; Aitken et al., 2012], showing that increased sperm membrane lipid peroxidation inhibits sperm progress motility, increases the percent of total sperm abnormalities, and causes a decrease in the fertilizing potential of sperm (Fig. 7). Experiments involving exposure of mammalian spermatozoa to a variety of ROS using a xanthine oxidase ROS-generating system demonstrate the vulnerability of sperm motility to oxidative stress and identify hydrogen peroxide as the most cytotoxic metabolite [Awda et al., 2009; Martinez-Pastor et al., 2009]. Moreover, it has been shown that H2O2 inhibits activity of the glucose-6-phosphate dehydrogenase (G6PD) leading to a decrease in the availability of NADPH and accumulation of oxidized/reduced glutathione, thus reducing antioxidant defense properties of the spermatozoa [Griveau et al., 1995]. Furthermore, GSH deficiency is involved in the instability of the mid-piece of sperm and the shape of the sperm resulting in defective motility [Garrido et al., 2004]. In keeping with this, we found GSH depletion and augmentation of the instability of the mid-piece and the shape of the sperm in the BD50 exhaust-exposed samples (Figs. 1, 3, and 7).
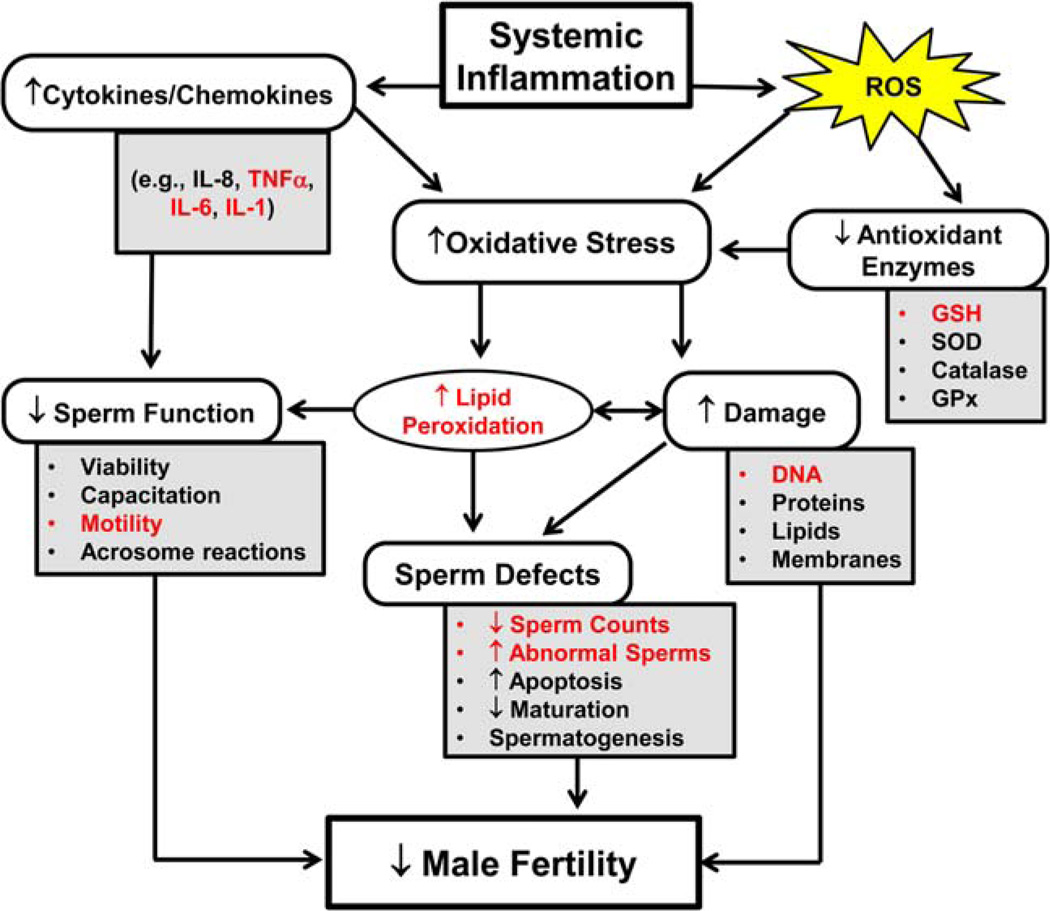
Fig. 7.
A schematic representation of several biological effects associated with inflammation, oxidative stress, and male infertility. This figure illustrates how enhanced oxidative stress can cause changes at different levels in the male reproductive system, leading to infertility. The precise mechanisms that are modulated/effected on BD50 particulate exposure in male mice are highlighted in red colored font.
Exposure to BD50, not D100, exhaust enhanced testosterone secretion in testes and decreased LH level in the serum (Fig. 4). In addition, testicular histopathology analysis showed more prominent changes in the seminiferous tubules after exposure to BD50 (Fig. 5). In line with our findings, previous publications reported an increase in testicular concentration of testosterone and induction of degeneration of tubules after exposure to nanoparticlerich diesel exhaust [Li et al., 2012]. These results suggest that nanoparticles affect testosterone biosynthesis and might have a causal role in steroidogenesis in the testis. Testosterone is mainly synthesized in the Leydig cells of the testes, and LH is the major stimulant of testosterone production [Ewing et al., 1983]. However, a high level of testosterone suppresses systemic levels of LH by a negative feedback loop and inhibits the release of gonadotropin-releasing hormone (GnRH) and consequently LH [Shibata et al., 2007]. Additionally, IL-1β, detected in the serum of mice (Table I), also plays an important role in the suppression of GnRH secretion [Tomaszewska-Zaremba and Herman, 2009] and thus altered circulatory LH levels in our study.
Apart from the influence on peroxidation of the cell membrane lipids, ROS can also cause damage to DNA and may lead to apoptosis through DNA fragmentation (Fig. 7). Oxidative stress has been correlated with high frequencies of single and double DNA strand breaks [Aitken and Krausz, 2001]. Kemal Duru et al. [2000] reported that exposure of the sperm to artificially produced ROS increased DNA damage in the form of modification of all bases, production of base-free sites, deletion, DNA crosslinks, and chromosomal rearrangements. High levels of ROS mediate the DNA fragmentation commonly observed in spermatozoa of infertile males [Aitken et al., 1998; Saleh and Agarwal, 2002]. We found significantly increased degree of DNA fragmentation on BD50 exhaust exposure in comparison with D100 on Day 7 post-treatment, indicating a higher population of cells with DNA damage (Figs. 6 and 7). The absence of DNA damage on Day 28 postexposure could be due to induction of nucleotide excision repair. Verhofstad et al. [2010] exposed male mice to benzo(a)pyrene to study DNA adduct kinetics in sperm and testis. The maximum adduct level in sperm was observed at about 1 week postexposure with a subsequent decrease in the following 3 weeks. The use of DNA repair-deficient mice allowed the authors to conclude that DNA damage in spermatogonia relies on nucleotide excision repair for maintaining its genetic integrity [Verhofstad et al., 2010].
Despite the often contradictory results from multiple clinical and experimental studies regarding the effects of cytokines on sperm quantity and quality, various cytokines are involved in the regulation of gonad and sperm function [Fraczek et al., 2012]. Moreover, cytokines may play a crucial role in propagation of oxidative stress in semen, especially during the inflammatory process (Fig. 7). It was reported that the addition of TNF-α, IL-1α, or IL-1β to human spermatozoa resulted in an increase of ROS production by a rise of sperm membrane peroxidation [Buch et al., 1994]. A number of authors observed a relationship between the increased secretion of cytokines in the testes or semen and abnormality or fertilizing ability of sperm [Fraczek et al., 2012]. It was shown that increased levels of TNF-α or IL-1β were related to the quality of semen, such as sperm count, motility, and morphology [Gruschwitz et al., 1996; Sanocka et al., 2003; Al-Azemi et al., 2010]. In agreement with the previous studies, we found that exposure to BD50 or D100 induced upregulation of IL-1α, IL-1β, and TNF-α in the testes of mice with a stronger effect of BD50 (Table II and Fig. 7). Moreover, among the various inflammatory cytokines, TNF-α is the most potent inducer of apoptosis in spermatozoa [Li et al., 2009a]. Furthermore, we found that pulmonary exposure to BD50 induced enhanced systemic inflammation measured by the release of inflammatory mediators in the serum of mice (Table I) when compared with D100. Importantly, accumulation of TNF-α, IL-1α, and IL-1β proinflammatory cytokines was found only in mice exposed to BD exhaust. Additionally, the level of IL-6 was greater in B50 samples. These potent proinflammatory cytokines, especially IL-1β, are likely to play a central pathophysiological role in the inhibition of the neuroendocrine reproductive axis [Tomaszewska-Zaremba and Herman, 2009]. Interestingly, only the IL-1β level was significantly upregulated on Day 28 postexposure in BD50 samples (data not shown). Most upregulated cytokines found in the current study are similar to those reported by Yanamala et al. [2013] and clearly indicate that BD50 particles can potentiate distinct and prolonged systemic inflammatory responses. Several authors suggested an involvement of sperm apoptosis in the impairment of men’s fertility [Fraczek et al., 2012]. Induction of sperm apoptosis could be one of the mechanisms by which proinflammatory cytokines may affect spermatozoa during male reproductive system inflammation (Fig. 7).
Overall, our data suggest that systemic outcomes of pulmonary exposure to respirable exhaust PM, leading to male reproductive toxicity, were more pronounced in BD50 when compared with D100. The chain of pathological events was realized through synergized interactions of oxidative stress and inflammatory response culminating in the impairment of sperm quality, functions, and DNA damage (Fig. 7).
Acknowledgments
Grant sponsor: NIOSH; Grant numbers: 2927ZKCY, OH008282.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. Mention of trade names or commercial products do not constitute endorsement or recommendation for use.
Footnotes
AUTHOR CONTRIBUTIONS
E.R.K. designed the study, completed the experiments, analyzed the data, and prepared the manuscript draft with important intellectual input from N.Y. and A.A.S. N.Y. designed the study, completed the experiments, and analyzed the data. M.T.F. completed the experiments and collected the data. D.W.G. and M.R.S. performed histopathological evaluation. A.D.B. collected the diesel/biodiesel exhaust particles. V.E.K. designed the study. A.A.S. coordinated the study. All authors approved the final manuscript.
REFERENCES
- Ackland ML, Zou LD, Freestone D, de Waasenburg SV, Michalczyk AA. Diesel exhaust particulate matter induces multinucleate cells and zinc transporter-dependent apoptosis in human airway cells. Immunol Cell Biol. 2007;85:617–622. doi: 10.1038/sj.icb.7100109. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: A clinical approach. BJU Int. 2005;95:503–507. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Saleh RA. Role of oxidants in male infertility: Rationale, significance, and treatment. Urol Clin North Am. 2002;29:817–827. doi: 10.1016/s0094-0143(02)00081-2. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Curry BJ. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14:367–381. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Krausz C. Oxidative stress, DNA damage and the Y chromosome. Reproduction. 2001;122:497–506. doi: 10.1530/rep.0.1220497. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, Irvine DS. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59:1037–1046. doi: 10.1095/biolreprod59.5.1037. [DOI] [PubMed] [Google Scholar]
- Al-Azemi M, Omu FE, Kehinde EO, Anim JT, Oriowo MA, Omu AE. Lithium protects against toxic effects of cadmium in the rat testes. J Assist Reprod Genet. 2010;27:469–476. doi: 10.1007/s10815-010-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, Gibb Z, Mitchell LA, Lambourne SR, Connaughton HS, De Iuliis GN. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol Reprod. 2012;87:110. doi: 10.1095/biolreprod.112.102020. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–38. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awda BJ, Mackenzie-Bell M, Buhr MM. Reactive oxygen species and boar sperm function. Biol Reprod. 2009;81:553–561. doi: 10.1095/biolreprod.109.076471. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Tagliamonte MC, Catalani S, Primiterra M, Canestrari F, De Stefani S, Palini S, Bulletti C. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online. 2012;25:300–306. doi: 10.1016/j.rbmo.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Birch ME. Monitoring of diesel particulate exhaust in the workplace. In: Schlecht PC, Connor O’PF, editors. NIOSH Manual of Analytical Methods (NMAM) 4th. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH); 2004. pp. 229–259. Chapter Q. Publication No. 2003–154. [Google Scholar]
- Brito JM, Belotti L, Toledo AC, Antonangelo L, Silva FS, Alvim DS, Andre PA, Saldiva PHN, Rivero DHRF. Acute cardiovascular and inflammatory toxicity induced by inhalation of diesel and biodiesel exhaust particles. Toxicol Sci. 2010;116:67–78. doi: 10.1093/toxsci/kfq107. [DOI] [PubMed] [Google Scholar]
- Buch JP, Kolon TF, Maulik N, Kreutzer DL, Das DK. Cytokines stimulate lipid membrane peroxidation of human sperm. Fertil Steril. 1994;62:186–188. doi: 10.1016/s0015-0282(16)56838-1. [DOI] [PubMed] [Google Scholar]
- Bunger J, Krahl J, Munack A, Ruschel Y, Schroder O, Emmert B, West G, Muller M, Hallier E, Bruning T. Strong mutagenic effects of diesel engine emissions using vegetable oil as fuel. Arch Toxicol. 2007;81:599–603. doi: 10.1007/s00204-007-0196-3. [DOI] [PubMed] [Google Scholar]
- Bünger J, Krahl J, Schröder O, Schmidt L, Westphal GA. Potential hazards associated with combustion of bio-derived versus petroleum-derived diesel fuel. Crit Rev Toxicol. 2012;42:732–750. doi: 10.3109/10408444.2012.710194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: Protective role of vitamin E and β-carotene. Food Chem Toxicol. 2004;42:1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Evenson DP, Melamed MR. Rapid analysis of normal and abnormal cell types in human semen and testis biopsies by flow cytometry. J Histochem Cytochem. 1983;31:248–253. [PubMed] [Google Scholar]
- Ewing LL, Wing TY, Cochran RC, Kromann N, Zirkin BR. Effect of luteinizing hormone on Leydig cell structure and testosterone secretion. Endocrinology. 1983;112:1763–1769. doi: 10.1210/endo-112-5-1763. [DOI] [PubMed] [Google Scholar]
- Farmer PB, Singh R, Kaur B, Sram RJ, Binkova B, Kalina I, Popov TA, Garte S, Taioli E, Gabelova A, et al. Molecular epidemiology studies of carcinogenic environmental pollutants. Effects of polycyclic aromatic hydrocarbons (PAHs) in environmental pollution on exogenous and oxidative DNA damage. Mutat Res. 2003;544:397–402. doi: 10.1016/j.mrrev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Fraczek M, Czernikiewicz A, Kurpisz M. Cytokines and oxidative stress in the germ line. In: Agarwal A, Aitken RJ, Alvarez JG, editors. Oxidative Stress in Applied Basic Research and Clinical Practice. Studies on Men’s Health and Fertility. New York, NY: Springer Science+Business Media, LLC; 2012. pp. 179–205. [Google Scholar]
- Garrido N, Meseguer M, Alvarez J, Simon C, Pellicer A, Remohi J. Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil Steril. 2004;82(Suppl 3):1059–1066. doi: 10.1016/j.fertnstert.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Garshick E, Laden F, Hart JE, Rosner B, Smith TJ, Dockery DW, Speizer FE. Lung cancer in railroad workers exposed to diesel exhaust. Environ Health Perspect. 2004;12:1539–1543. doi: 10.1289/ehp.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari L, Chang SS, Santella RM, Garte S, Pedotti P, Taioli E. Polycyclic aromatic hydrocarbon-DNA adducts in human sperm as a marker of DNA damage and infertility. Mutat Res. 2003;535:155–160. doi: 10.1016/s1383-5718(02)00297-8. [DOI] [PubMed] [Google Scholar]
- Griveau JF, Dumont E, Renard P, Callegari JP, Le Lannou D. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J Reprod Fertil. 1995;103:17–26. doi: 10.1530/jrf.0.1030017. [DOI] [PubMed] [Google Scholar]
- Gruschwitz MS, Brezinschek R, Brezinschek HP. Cytokine levels in the seminal plasma of infertile males. J Androl. 1996;17:158–163. [PubMed] [Google Scholar]
- Guerriero G, Trocchia S, Abdel-Gawad FK, Ciarci GA. Roles of reactive oxygen species in the spermatogenesis regulation. Front Endocrinol (Lausanne) 2014;22:56. doi: 10.3389/fendo.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Zhou N, Cui Z, Ma M, Li L, Cai M, Li Y, Lin H, Li Y, Ao L, et al. Association between urinary polycyclic aromatic hydrocarbon metabolites and sperm DNA damage: A population study in Chongqing, China. Environ Health Perspect. 2011;119:652–657. doi: 10.1289/ehp.1002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate ST, Sandstrom T, Frew AJ, Stenfors N, Nordenhall C, Salvi S, Blomberg A, Helleday R, Soderberg M. Health effects of acute exposure to air pollution. I. Healthy and asthmatic subjects exposed to diesel exhaust. Res Rep Health Eff Inst. 2003;112:1–30. (discussion 51–67) [PubMed] [Google Scholar]
- Izawa H, Kohara M, Watanabe G, Taya K, Sagai M. Effects of diesel exhaust particles on the male reproductive system in strains of mice with different aryl hydrocarbon receptor responsiveness. J Reprod Dev. 2007;53:1191–1197. doi: 10.1262/jrd.19114. [DOI] [PubMed] [Google Scholar]
- Izawa H, Kohara M, Aizawa K, Suganuma H, Inakuma T, Watanabe G, Taya K, Sagai M. Alleviative effects of quercetin and onion on male reproductive toxicity induced by diesel exhaust particles. Biosci Biotechnol Biochem. 2008;72:1235–1241. doi: 10.1271/bbb.70705. [DOI] [PubMed] [Google Scholar]
- Jeng HA, Pan CH, Chao MR. 1-Hydroxypyrene as a biomarker for assessing the effects of exposure to polycyclic aromatic hydrocarbons on semen quality and sperm DNA integrity. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2013;48:152–158. doi: 10.1080/03601234.2012.716741. [DOI] [PubMed] [Google Scholar]
- Kado NY, Okamoto RA, Kuzmicky PA. Final report for The U.S. Department of Energy and The Renewable Energy Report Library by Department of Environmental Toxicology. Davis: University of California; 1996. Chemical and bioassay analyses of diesel and biodiesel particulate matter: pilot study. [Google Scholar]
- Kemal Duru N, Morshedi M, Oehninger S. Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000;74:1200–1207. doi: 10.1016/s0015-0282(00)01591-0. [DOI] [PubMed] [Google Scholar]
- Kisin E, Shi X, Keane M, Bugarski A, Shvedova AA. Mutagenecity of biodiesel diesel exhaust particles and the effect of engine operating conditions. J Environ Eng Ecol Sci. 2013;2:3. doi: 10.7243/2050-1323-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol. 2005;86:1059–1070. [Google Scholar]
- Knothe G, Krahl J, Van Gerpen JH. The Biodiesel Handbook. 2nd. Urbana, Il: AOCS Press; 2010. [Google Scholar]
- Krahl J, Knothe G, Munack A, Ruschel Y, Schroder O, Hallier E, Westphal G, Bunger J. Comparison of exhaust emissions and their mutagenicity from the combustion of biodiesel, vegetable oil, gas-to-liquid and petrodiesel fuels. Fuel. 2009;88:1064–1069. [Google Scholar]
- Li C, Taneda S, Suzuki AK, Furuta C, Watanabe G, Taya K. Effects of 3-methyl-4-nitrophenol in diesel exhaust particles on the regulation of testicular function in immature male rats. J Androl. 2007;28:252–258. doi: 10.2164/jandrol.106.000802. [DOI] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Lee WM, Cheng CY. Cytokines and junction restructuring events during spermatogenesis in the testis: An emerging concept of regulation. Cytokine Growth Factor Rev. 2009a;20:329–338. doi: 10.1016/j.cytogfr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Taneda S, Taya K, Watanabe G, Li X, Fujitani Y, Ito Y, Nakajima T, Suzuki AK. Effects of inhaled nanoparticlerich diesel exhaust on regulation of testicular function in adult male rats. Inhal Toxicol. 2009b;21:803–811. doi: 10.1080/08958370802524381. [DOI] [PubMed] [Google Scholar]
- Li C, Li X, Jigami J, Hasegawa C, Suzuki AK, Zhang Y, Fujitani Y, Nagaoka K, Watanabe G, Taya K. Effect of nanoparticle-rich diesel exhaust on testosterone biosynthesis in adult male mice. Inhal Toxicol. 2012;24:599–608. doi: 10.3109/08958378.2012.702140. [DOI] [PubMed] [Google Scholar]
- Liu YY, Lin TC, Wang YJ, Ho WL. Carbonyl compounds and toxicity assessments of emissions from a diesel engine running on biodiesels. J Air Waste Manag Assoc. 2009;59:163–171. doi: 10.3155/1047-3289.59.2.163. [DOI] [PubMed] [Google Scholar]
- Martinez-Pastor F, Aisen E, Fernandez-Santos MR, Esteso MC, Maroto-Morales A, García-Alvarez O, Garde JJ. Reactive oxygen species generators affect quality parameters and apoptosis markers differently in red deer spermatozoa. Reproduction. 2009;137:225–235. doi: 10.1530/REP-08-0357. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- Mermelstein R, Kiriazides DK, Butler M, McCoy EC, Rosenkranz HS. The extraordinary mutagenicity of nitropyrenes in bacteria. Mutat Res. 1981;89:187–196. doi: 10.1016/0165-1218(81)90236-6. [DOI] [PubMed] [Google Scholar]
- Mi Y, Zhang C, Li C, Taneda S, Watanabe G, Suzuki AK, Taya K. Quercetin attenuates oxidative damage induced by treatment of embryonic chicken spermatogonial cells with 4-nitro-3-phenylphenol in diesel exhaust particles. Biosci Biotechnol Biochem. 2010;74:934–938. doi: 10.1271/bbb.90740. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Al-Maskari S, Ali BH, Al-Amri IS. Cardiovascular and lung inflammatory effects induced by systemically administered diesel exhaust particles in rats. Am J Physiol Lung Cell Mol Physiol. 2007;292:L664–L670. doi: 10.1152/ajplung.00240.2006. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Dhanasekaran S, Yasin J, Ba-Omar H, Fahim MA, Kazzam EE, Ali BH. Evaluation of the direct systemic and cardiopulmonary effects of diesel particles in spontaneously hypertensive rats. Toxicology. 2009;262:50–56. doi: 10.1016/j.tox.2009.05.009. [DOI] [PubMed] [Google Scholar]
- NMAM. NIOSH method 5040 update. In: Schlecht PC, Connor O’PF, editors. NIOSH Manual of Analytical Methods (NMAM) 4th. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH; 2003. pp. 94–113. Third Supplement Publication No. 2003–154. [Google Scholar]
- Ohe T. Mutagenicity of photochemical reaction products of poly-cyclic aromatic hydrocarbons with nitrite. Sci Total Environ. 1984;39:161–175. doi: 10.1016/0048-9697(84)90033-0. [DOI] [PubMed] [Google Scholar]
- Pasqualotto FF, Sharma RK, Kobayashi H, Nelson DR, Thomas AJ, Agarwal A. Oxidative stress in normospermic men undergoing infertility evaluation. J Androl. 2001;22:316–322. [PubMed] [Google Scholar]
- Pereira MA, Sabharwal PS, Gordon L, Wyrobek AJ. The effect of diesel exhaust on sperm-shape abnormalities in mice. Environ Int. 1981;5:459–460. [Google Scholar]
- Peretz AJ, Kaufman D, Trenga CA, Allen J, Carlsten C, Aulet MR, Adar SD, Sullivan JH. Effects of diesel exhaust inhalation on heart rate variability in human volunteers. Environ Res. 2008;107:178–184. doi: 10.1016/j.envres.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos A, Schmid TE, Pina-Guzman B, Quintanilla-Vega B, Marchett FI. Differential sensitivity of male germ cells to mainstream and sidestream tobacco smoke in the mouse. Toxicol Appl Pharmacol. 2009;237:298–305. doi: 10.1016/j.taap.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Pronk A, Coble J, Stewart PA. Occupational exposure to diesel engine exhaust: A literature review. J Expo Sci Environ Epidemiol. 2009;19:443–457. doi: 10.1038/jes.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell DL, McClure BT, McDonald J, Basu HN. Transient testing of soy methyl ester fuels in an indirect injection, compression ignition engine. J Am Oil Chem Soc. 1996;73:381–388. [Google Scholar]
- Ramdhan DH, Ito Y, Yanagiba Y, Yamagishi N, Hayashi Y, Li C, Taneda S, Suzuki AK, Watanabe G, Taya K, et al. Nanoparticle-rich diesel exhaust may disrupt testosterone biosynthesis and metabolism via growth hormone. Toxicol Lett. 2009;191:103–108. doi: 10.1016/j.toxlet.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Raychoudhury SS, Kubinski D. Polycyclic aromatic hydrocarbon-induced cytotoxicity in cultured rat Sertoli cells involves differential apoptotic response. Environ Health Perspect. 2003;111:33–38. doi: 10.1289/ehp.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivedal E, Myhre O, Sanner T, Eide I. Supplemental role of the Ames mutation assay and gap junction intercellular communication in studies of possible carcinogenic compounds from diesel exhaust particles. Arch Toxicol. 2003;77:533–542. doi: 10.1007/s00204-003-0483-6. [DOI] [PubMed] [Google Scholar]
- Rivero DHRF, Soares SRC, Lorenzi G, Saiki M, Godleski JJ, Antonangelo L, Dolhnikoff M, Saldiva PHN. Acute cardiopulmonary alterations induced by fine particulate matter of Sao Paulo, Brazil. Toxicol Sci. 2005;85:898–905. doi: 10.1093/toxsci/kfi137. [DOI] [PubMed] [Google Scholar]
- Saleh RA, Agarwal A. Oxidative stress and male infertility: From research bench to clinical practice. J Androl. 2002;23:737–752. [PubMed] [Google Scholar]
- Sanocka D, Jedrzejczak P, Szumała-Kaekol A, Fraczek M, Kurpisz M. Male genital tract inflammation: The role of selected interleukins in regulation of pro-oxidant and antioxidant enzymatic substances in seminal plasma. J Androl. 2003;24:448–455. doi: 10.1002/j.1939-4640.2003.tb02693.x. [DOI] [PubMed] [Google Scholar]
- Sawyer K, Mundandhara S, Ghio AJ, Madden MC. The effects of ambient particulate matter on human alveolar macrophage oxidative and inflammatory responses. J Toxicol Environ Health A. 2010;73:41–57. doi: 10.1080/15287390903248901. [DOI] [PubMed] [Google Scholar]
- Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2007;19:432–438. doi: 10.1111/j.1365-2826.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Yanamala N, Murray AR, Kisin ER, Khaliullin T, Hatfield MK, Tkach AV, Krantz QT, Nash D, King C, et al. Oxidative stress, inflammatory biomarkers, and toxicity in mouse lung and liver after inhalation exposure to 100% biodiesel or petroleum diesel emissions. J Toxicol Environ Health A. 2013;76:907–921. doi: 10.1080/15287394.2013.825217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D, Samanic CM, Lubin JH, Blair AE, Stewart PA, Vermeulen R, Coble JB, Rothman N, Schleiff PL, Travis WD, et al. The diesel exhaust in miners study: A nested case-control study of lung cancer and diesel exhaust. J Natl Cancer Inst. 2012;104:855–868. doi: 10.1093/jnci/djs034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Fujimoto K, Miyazawa T. Polyunsaturated (n-3) fatty acids susceptible to peroxidation are increased in plasma and tissue lipids of rats fed docosahexaenoic acid-containing oils. J Nutr. 2000;130:3028–3033. doi: 10.1093/jn/130.12.3028. [DOI] [PubMed] [Google Scholar]
- Tokiwa H, Ohnishi Y. Mutagenicity and carcinogenicity of nitroarenes and their sources in the environment. Crit Rev Toxicol. 1986;17:23–60. doi: 10.3109/10408448609037070. [DOI] [PubMed] [Google Scholar]
- Tomaszewska-Zaremba D, Herman A. The role of immunological system in the regulation of gonadoliberin and gonadotropin secretion. Reprod Biol. 2009;9:11–23. doi: 10.1016/s1642-431x(12)60091-6. [DOI] [PubMed] [Google Scholar]
- Tornqvist HN, Mills L, Gonzalez M, Miller MR, Robinson SD, Megson IL, MacNee W, Donaldson K, Soderberg S, Newby DE, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- Tsukue N, Toda N, Tsubone H, Sagai M, Jin WZ, Watanabe G, Taya K, Birumachi J, Suzuki AK. Diesel exhaust (DE) affects the regulation of testicular function in male Fischer 344 rats. J Toxicol Environ Health A. 2001;63:115–126. doi: 10.1080/15287390151126441. [DOI] [PubMed] [Google Scholar]
- Tsukue N, Tsubone H, Suzuki AK. Diesel exhaust affects the abnormal delivery in pregnant mice and the growth of their young. Inhal Toxicol. 2002;14:635–651. doi: 10.1080/08958370290084548. [DOI] [PubMed] [Google Scholar]
- Verhofstad N, van Oostrom CT, van Benthem J, van Schooten FJ, van Steeg H, Godschalk RW. DNA adduct kinetics in reproductive tissues of DNA repair proficient and deficient male mice after oral exposure to benzo(a)pyrene. Environ Mol Mutagen. 2010;51:123–129. doi: 10.1002/em.20516. [DOI] [PubMed] [Google Scholar]
- Walczak-Jedrzejowska R, Karol Wolski J, Slowikowska-Hilczer J. The role of oxidative stress and antioxidants in male fertility. Cent European J Urol. 2013;66:60–67. doi: 10.5173/ceju.2013.01.art19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Oonuki Y. Inhalation of diesel engine exhaust affects spermatogenesis in growing male rats. Environ Health Perspect. 1999;107:539–544. doi: 10.1289/ehp.99107539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkinson WP, Campen MJ, Costa DL. Cardiac arrhythmia induction after exposure to residual oil fly ash particles in a rodent model of pulmonary hypertension. Toxicol Sci. 1998;41:209–216. doi: 10.1006/toxs.1997.2406. [DOI] [PubMed] [Google Scholar]
- Yanamala N, Hatfield MK, Farcas MT, Schwegler-Berry D, Hummer JA, Shurin MR, Birch ME, Gutkin DW, Kisin ER, Kagan VE, et al. Biodiesel versus diesel exposure: Enhanced pulmonary inflammation, oxidative stress, and differential morphological changes in the mouse lung. Toxicol Appl Pharmacol. 2013;272:373–383. doi: 10.1016/j.taap.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Sagai M, Oshio S, Umeda T, Ihara T, Sugamata M, Sugawara I, Takeda K. Exposure to diesel exhaust affects the male reproductive system of mice. Int J Androl. 1999;22:307–315. doi: 10.1046/j.1365-2605.1999.00185.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Takeda K. The effect of diesel exhaust on murine male reproductive function. J Health Sci. 2004;50:210–214. [Google Scholar]