Abstract
Calpain is an intracellular Ca2+-regulated protease system whose substrates include proteins involved in proliferation, survival, migration, invasion, and sensitivity to therapeutic drugs. Genetic disruption of calpain attenuated the tumorigenic potential of breast cancer cells and hypersensitized cells to 17AAG, an inhibitor of the molecular chaperone HSP90. Calpain-1 or -2 overexpression rendered cells resistant to 17AAG, whereas downregulation or inhibition of calpain-1/2 led to increased cell death in multiple breast cancer cell lines, including models of HER2+ (SKBR3) and triple-negative basal-cell-like (MDA-MB-231) breast cancer. In an MDA-MB-231 orthotopic xenograft model, calpain knockdown or 17AAG treatment independently attenuated tumor growth and metastasis, while the combination was most effective. Calpain knockdown was associated with increased 17AAG-induced degradation of the HSP90 clients cyclin D1 and AKT and multidrug resistance protein 2, which correlated with increased expression of antimitogenic p27KIP1 and proapoptotic BIM proteins. Like other therapeutics, 17AAG can be effluxed by specific ABC transporters. Calpain expression positively correlated with the expression of P glycoprotein in mouse embryonic fibroblasts. Importantly, we show that calpain affects ABC transporter function and efflux of clinically relevant doxorubicin. These observations provide a compelling rationale for exploring the combination of calpain inhibition with new or existing cancer therapeutics.
INTRODUCTION
Calpains are a family of intracellular calcium-dependent proteases. Of the 15 mammalian isoforms, calpain-1 and calpain-2 are ubiquitously expressed and have been most extensively studied (reviewed in reference 1). They are heterodimers consisting of isoform-specific catalytic subunits encoded by CAPN1 and CAPN2, respectively, and a common regulatory subunit encoded by CAPNS1. Heterodimerization is essential for both stability and enzymatic activity; hence, genetic disruption of CAPNS1 results in loss of both calpain-1 and -2 activities (2, 3).
Elevated calpain expression or activity has been associated with multiple cancer types, including colon, liver, lung, prostate, ovarian, and breast cancers (reviewed in reference 4). Mechanistic understanding of calpain's involvement in cancer is confounded by its broad range of substrates, including signaling or structural proteins that regulate diverse cellular functions such as mitogenesis, migration, invasion, and death (1). Biomarker studies suggest a positive correlation between elevated calpain expression and a poor clinical outcome in breast cancer (5–7).
Paradoxically, calpain is associated with prodeath (8, 9) or prosurvival (9–11), depending upon the physiologic context and specific challenges cells are exposed to. For example, calpain-mediated cleavage of PP2A supports activation of AKT and a prosurvival function in mouse embryonic fibroblasts (MEFs) (10), and in breast cancer cells and xenograft mouse models, knockdown of CAPN2 was associated with attenuated tumor growth and deregulation of the phosphatidylinositol 3-kinase (PI3K)–AKT–FoxO signaling axis (12). In contrast, prodeath functions for calpain are suggested by resistance to specific apoptotic challenges in calpain-deficient neuronal cells (8). Thus, therapeutic benefits of calpain inhibition in cancer will depend upon the specific challenges faced by cancer cells.
HSP90 is a molecular chaperone with >200 client proteins, including proteins involved in oncogenesis, such as HER2, AKT, cRAF, and cyclin D1 (13). Tumor cells are thought to hijack HSP90 function to stabilize both native and mutated oncogenic signaling molecules, protecting them from degradation and promoting tumorigenesis (14). Many HSP90 inhibitors are undergoing study for their potential use as cancer therapy (15). The first HSP90 inhibitor to enter clinical trials was 17AAG (tanespimycin), a less toxic, more soluble analog of geldanamycin. By binding in the ATP-binding pocket of HSP90, 17AAG prevents ATP hydrolysis and releases HSP90 clients for subsequent degradation (15). The antiproliferative and antitumor effects of 17AAG are well established across different tumor cell lines (16, 17). Mechanistically, 17AAG induces both cell cycle arrest and apoptosis through its ability to inhibit HSP90 functions (16).
HSP90 inhibitors, including 17AAG, may have limited use as monotherapies because of acquired resistance mediated in part by the expression of one or more ABC transporters, including multidrug resistance protein 1 (MRP1; ABCC1) and P glycoprotein (Pgp; ABCB1) (18, 19). However, several clinical trials have demonstrated additive or synergistic effects when 17AAG is used in combination with existing targeted agents or chemotherapies, including trastuzumab (20), taxanes (21), and cisplatin (22).
Relationships between calpain and HSP90 include reports that calpain cleaves HSP90 (23) or regulates its expression (24); others suggest that HSP90 regulates calpain activity by competing with the endogenous calpain inhibitor calpastatin for binding and regulation of calpain localization and access to specific substrates (25). The interplay between HSP90 and calpain and their overlapping clients and substrates provides a rationale for targeting both proteins. Here we show that disruption of calpain is associated with increased sensitivity to 17AAG in cultured cells and an in vivo model of metastatic breast cancer.
MATERIALS AND METHODS
Cell culture and reagents.
Wild-type (capns1+/+), knockout (capns1−/−), and rescued (capns1−/−/Δcapns1) MEFs (3) and MDA-MB-231 cells were maintained in Dulbecco's modified Eagle's medium (Sigma)–10% fetal bovine serum (FBS; Sigma)–1% l-glutamine (Gibco)–1% antibiotic-antimycotic (AA; Gibco). The Δcapns1 mutant lentiviral rescue vector encodes a truncated CAPNS1 protein that lacks the amino-terminal glycine-rich domain (3). SKBR3 cells were cultured in 5A McCoy (Sigma)–10% FBS–1% AA. To generate cell lines with depleted calpain-1 and calpain-2 expression, MDA-MB-231 and SKBR3 cells were transduced with a control lentivirus (pLKO.1) or a lentivirus expressing one of two different short hairpin RNA (shRNA) sequences targeting the transcript encoding the CAPNS1 small regulatory subunit (shRNA–Capns1-1, shRNA–Capns1-2) (Open Biosystems catalog no. RMS4533-NM_001749). To generate calpain-overexpressing cell lines, MDA-MB-231 cells were transduced with a vector control (pWPXLD) or a lentivirus encoding wild-type CAPN1, wild-type CAPN2, or protease-inactive CAPN2 (CAPN2-C105S). 17AAG was purchased from LC Laboratories (Cedarlane). 5-Fluorouracil (5-FU), cisplatin, and doxorubicin were generously provided by Xiaolong Yang (Queen's University). Senju Pharmaceuticals Co., Ltd., generously provided the calpain inhibitor SNJ1945.
Cell viability, migration, and invasion assays.
For cell viability assays, cells were plated in triplicate at 2 × 104/well of 24-well plates. The next day, increasing concentrations of 17AAG, 5-FU, doxorubicin, and cisplatin were added to the wells. After 48 h of drug treatment, cell viability was quantified by trypan blue exclusion. Data are presented as the average percentage of dead cells ± the standard deviation (SD). Experiments were performed at least three times. Fifty percent lethal doses (LD50s) were calculated by using CompuSyn software. For cell migration assays, 1 × 105 serum-starved MDA-MB-231 cells were plated in the upper chamber of Transwells (BD Falcon) in the absence or presence of 10 or 25 μM 17AAG, while the bottom chamber contained complete drug-free medium. After 8 h, nonmigrated cells were removed from the top of the insert with a cotton swab and migrated cells were fixed in ice-cold 10% methanol and stained in 0.1% crystal violet–20% methanol. The migrated cells in five random fields were counted with a 10× objective. Data are expressed as the mean ± the SD. Similarly, for cell invasion, 1 × 105 cells were plated in Transwells coated with growth factor-depleted Matrigel (BD, VWR) and allowed to invade for 24 h. Experiments were performed three times.
In vivo tumor growth and metastatic studies.
Green fluorescent protein (GFP)-expressing MDA-MB-231 cells transduced with control or shRNA–Capns1-1 lentivirus (1 × 106 cells in 25 μl of phosphate-buffered saline [PBS]/25 μl of Matrigel [BD, VWR]) were injected into the mammary fat pads of BalbC-Rag2−/−/IL2Rγc−/− mice. Seven days after engraftment, 75 mg/kg 17AAG or the vehicle (10% dimethyl sulfoxide–0.05% Tween 80–PBS) was injected intraperitoneally daily 5 days/week. Tumor volumes were assessed by caliper measurement. After 28 days, tumors were excised and mice were allowed to recover. Ten days later, mice were euthanized for lung dissection. GFP-expressing metastatic nodules were imaged with a Hamamatsu B/W ORCA-ER digital camera with a 420/20-nm excitation filter and a 520/20-nm emission filter. Mice were housed in the animal care facility, and procedures were carried out according to guidelines specified by the Canadian Council on Animal Care, with the approval of the institutional animal care committee.
Doxorubicin localization.
Cells were plated on coverslips in six-well plates, allowed to grow overnight, and then incubated with 3.5 μM doxorubicin for 1 h, fixed, incubated with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) for 15 min, and examined by confocal microscopy. Average nuclear and cytoplasmic intensities were quantified with Metamorph software.
Immunoblotting and antibodies.
Cells or tumors were lysed in radioimmunoprecipitation assay lysis buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 0.1% SDS, and 0.5% sodium deoxycholate supplemented with protease inhibitors) and quantified with the Pierce bicinchoninic acid protein assay kit (Thermo Scientific). Both live and dead drug-treated cells were collected. To isolate membrane fractions, cells were spun at 240 × g for 5 min at 4°C and cell pellets were resuspended in 1 ml of lysis buffer (10 mM Tris [pH 7.6], 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, protease inhibitors) and kept on ice for 10 min. Lysates were homogenized with a Tenbroeck homogenizer (>80 vigorous strokes) and clarified by centrifugation (380 × g, 4°C, 15 min). Membrane fractions were separated by ultracentrifugation (100,000 × g, 30 min) in high-speed Eppendorf tubes (Beckman) and resuspended in 50 μl of a sucrose solution (250 mM sucrose, 50 mM Tris, pH 7.6). The protein concentration was determined as described above. Rabbit polyclonal anti-calpain-2 (CAPN2 and CAPNS1) and anti-RasGAP antibodies were raised against bacterially expressed rat calpain-2 and human RasGAP, respectively. Pgp, MRP1, MRP2, and Na+/K+-ATPase were generously provided by Susan Cole. Antibodies against CAPN2 and CAPNS1 were kindly provided by Jiro Takano and Takaomi Saido (RIKEN Brain Science Institute). Commercial antibodies were as follows: extracellular signal-regulated kinase 1/2 (ERK1/2), phosphorylated ERK1/2 (pERK1/2), AKT, and tubulin from Cell Signaling; cyclin D1 and CAPN1 from Santa Cruz; p27 from BD Transduction Laboratories; and HSP90 from Stressgen. Protein levels were assessed by densitometry with ImageJ software.
RESULTS
Calpain expression mediates sensitivity to the HSP90 inhibitor 17AAG in vitro.
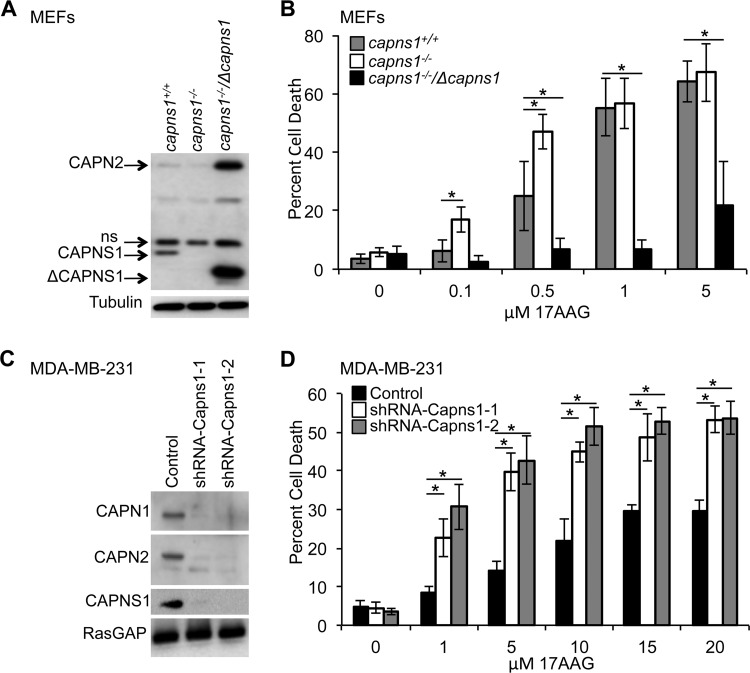
To explore the relationship between calpain expression and sensitivity to the HSP90 inhibitor 17AAG in vitro, we challenged capns1+/+ and capns1−/− MEFs (3) with increasing concentrations of the drug and assessed cell viability. capns1−/− MEFs were more sensitive to 17AAG treatment at low concentrations, with 17.04% ± 4.48% and 47.05% ± 6.02% cell death at 0.1 and 0.5 μM 17AAG, respectively (LD50, 0.98 μM), compared to 6.17% ± 3.64% (P = 0.0010) and 24.91% ± 11.88% (P = 0.0044) cell death in capns1+/+ MEFs (LD50, 1.77 μM) (Fig. 1B). When calpain expression was restored to capns1−/− MEFs by transduction with a retrovirus expressing a truncated CAPNS1 protein, the cells became significantly more resistant to 17AAG (LD50, 17.7 μM), with only minimal cell death at higher concentrations of 17AAG (6.98% ± 3.12% [P = 0.007] and 6.57% ± 3.15% [P = 4.1 × 10−7] at 0.5 and 1 μM, respectively, compared to the wild-type control). This increased resistance to 17AGG correlated with increased levels of both the exogenously expressed truncated CAPNS1 subunit and the endogenous CAPN2 catalytic subunit (Fig. 1A). In contrast, lower levels of CAPN2 were noted in capns1−/− cells than in capns1+/+ cells, consistent with CAPNS1 being required for the stability and activity of CAPN2 and CAPN1 catalytic subunits.
FIG 1.
Calpain expression correlates with sensitivity to 17AAG in vitro. (A) Immunoblot analysis with anti-calpain-2 antibody detects CAPN2 and CAPNS1 in wild-type (capns1+/+), CAPNS1 null (capns1−/−), or CAPNS1 null MEFs in which calpain is rescued by exogenous expression of a truncated ΔCAPNS1 protein (capns1−/−/Δcapns1). ns, nonspecific band. A tubulin blot served as a loading control. (B) Assays of the death of the cells described in panel A after treatment with a range of 17AAG concentrations for 48 h. LD50s: capns1+/+ MEFs, 1.77 μM; capns1−/− MEFs, 0.98 μM; capns1−/−/Δcapns1 MEFs, 17.7 μM (P ≤ 0.0001). (C) Immunoblot analysis of CAPN1, CAPN2, and CAPNS1 in MDA-MB-231 cells infected with a control lentivirus or a lentivirus expressing an shRNA construct targeting CAPNS1 (shRNA–capns1-1 or shRNA–Capns1-2). Blotting for RasGAP served as a loading control. (D) Analysis of the death of the 17AAG-treated MDA-MB-231 cells described for panel C. Data are mean ± SD. LD50s: control, 94.1 μM; shRNA–Capns1-1, 16.17 μM; shRNA–Capns1-2, 11.70 μM (P ≤ 0.0001). *, P ≤ 0.01 by Student's t test (relative to capns1+/+ [B] or the control [D]). One-way ANOVA was used to detect significant differences between LD50s.
17AAG has been reported to suppress growth and induce apoptosis in cultured breast cancer cells (26) and to improve breast cancer patient outcomes (20). This encouraged us to explore the effect of calpain disruption on 17AGG sensitivity in breast cancer cell lines. Calpain knockdown in the triple-negative breast cancer cell line MDA-MB-231 was associated with increased sensitivity to 17AAG (Fig. 1D). LD50s were significantly decreased in cells with lower calpain expression (15.3 and 10.9 μM in shRNA–Capns1-1 and shRNA–Capns1-2 cells, respectively) than in wild-type control cells (94.1 μM). Knockdown of calpain was achieved with two independent shRNAs directed against Capns1 and was associated with reduced levels of the targeted small subunit (CAPNS1), as well as the catalytic subunits of both calpain-1 and calpain-2 (CAPN1 and CAPN2, respectively) (Fig. 1C). This observation was reproduced in the HER2+ breast cancer cell line SKBR3 (see Fig. S1 in the supplemental material), highlighting the importance of calpain in 17AAG sensitivity across different cellular contexts.
Calpain proteolytic activity is required for mediating resistance to 17AAG.
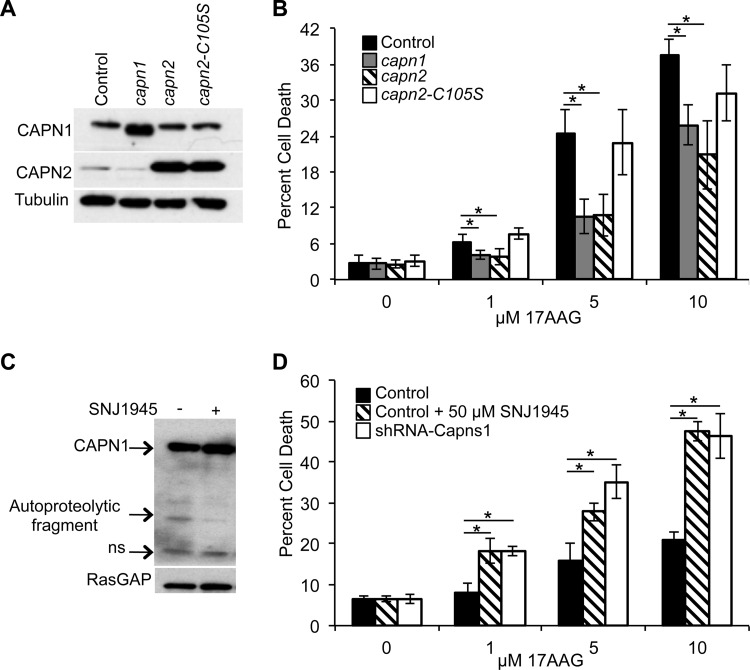
To determine if calpain protease activity is important for mediating resistance to 17AAG, we transduced MDA-MB-231 cells with lentiviruses expressing recombinant CAPN1, CAPN2, or protease-dead CAPN2 (Fig. 2A), where the active-site cysteine is mutated to serine (C105S) (27), and analyzed their sensitivity to 17AAG (Fig. 2B). Cells overexpressing either active CAPN1 (LD50, 43.9 μM) or CAPN2 (LD50, 58.4 μM) were more resistant to 17AAG treatment. Specifically, at 5 μM 17AAG, there was a >50% reduction in cell death: 10.60% ± 2.92% cell death in CAPN1-expressing cells (P = 0.004) and 10.73% ± 3.43% cell death in CAPN2-expressing cells (P = 8.2 × 10−5), compared to 24.50% ± 4.03% cell death in control cells (LD50, 16.7 μM). In contrast, cells expressing protease-dead CAPN2 (LD50, 27.2 μM) behaved similarly to control cells, with 22.95% ± 5.02% cell death (P = 0.59) at 5 μM 17AAG. The calpain inhibitor SNJ1945 (28), which was shown to inhibit calpain-1 autoproteolytic activity (Fig. 2C), also enhanced 17AAG-induced cell death in MDA-MB-231 cells (Fig. 2D). MDA-MB-231 cells treated with 50 μM SNJ1945 and increasing concentrations of 17AAG (LD50, 16.9 μM) were, on average, >1.8-fold more sensitive (P = 2.33 × 10−6 at 5 μM) than control cells treated with 17AAG alone (LD50, 166 μM). Convincingly, 17AAG-induced cell death in control cells treated with SNJ1945 was similar to that in cells transduced with shRNA targeting capns1 (P = 0.631 at 5 μM; LD50, 13.3 μM). These results argue that loss of calpain activity increases sensitivity to HSP90 inhibition.
FIG 2.
Calpain activity is essential for mediating resistance to 17AAG. (A) Immunoblot analysis of MDA-MB-231 cells infected with a lentivirus expressing the vector alone (control), CAPN1, CAPN2, or protease-inactive mutant CAPN2 (CAPN2-C105S). (B) Assays of the death of the MDA-MB-231 cells described in panel A treated for 48 h with the concentrations of 17AAG indicated. Data are mean ± SD. LD50s: control, 16.7 μM; capn1, 43.9 μM; capn2, 58.4 μM; capn2-C105S, 27.2 μM (P = 0.0063). (C) Immunoblot analysis of CAPN1 in MDA-MB-231 cells treated with 50 μM SNJ1945 for 24 h. RasGAP blot assays were used as a loading control. An autoproteolytic fragment of CAPN1 and a nonspecific (ns) band are indicated. (D) Assays of the death of MDA-MB-231 control, calpain knockdown (shRNA-Capns1), or control cells treated with 50 μM SNJ1945 and challenged with increasing concentrations of 17AAG for 48 h. Data are mean ± SD. LD50s: control, 166 μM; shRNA-Capns1, 13.3 μM; control plus SNJ1945, 16.9 μM. Student's t tests were performed. *, P ≤ 0.01 relative to the control. One-way ANOVA was used to detect significant differences between LD50s.
Calpain knockdown and HSP90 inhibition suppress cell migration and invasion.
Although calpain (29, 30) and HSP90 (31) have been independently implicated in promoting cell migration and invasion, cooperative effects have not been described. 17AAG alone suppressed MBA-MB-231 cell migration by 20% (P = 4.47 × 10−4) and 17% (P = 4.44 × 10−4) at 10 and 25 μM, respectively (Fig. 3A; see Fig. S2 in the supplemental material). Calpain knockdown alone suppressed cell migration by 49% (P = 1.31 × 10−8) (Fig. 3A; see Fig. S2). A 17AAG challenge of calpain knockdown cells did not result in further inhibition of cell migration (P = 0.07 and P = 0.45 at 10 and 25 μM, respectively). In contrast, cell invasion was more effectively suppressed by 17AAG treatment combined with calpain knockdown (Fig. 3B; see Fig. S2). 17AAG alone suppressed invasion by 36% (P = 3.53 × 10−7) or 48% (P = 1.35 × 10−8) at 5 or 10 μM, respectively, while calpain knockdown alone was associated with a 67% reduction (P = 2.93 × 10−10). A 17AAG challenge of calpain knockdown cells was associated with a 42% (P = 5.63 × 10−4) or 75% (P = 4.55 × 10−10) reduction in invasion at 5 or 10 μM, respectively, and the combined suppressive effect of calpain knockdown and 17AAG at 5 or 10 μM on invasion was 81% (P = 7.71 × 10−11) or 92% (P = 2.73 × 10−15), respectively. This suggests that combined inhibition of calpain and HSP90 might attenuate cancer metastasis.
FIG 3.
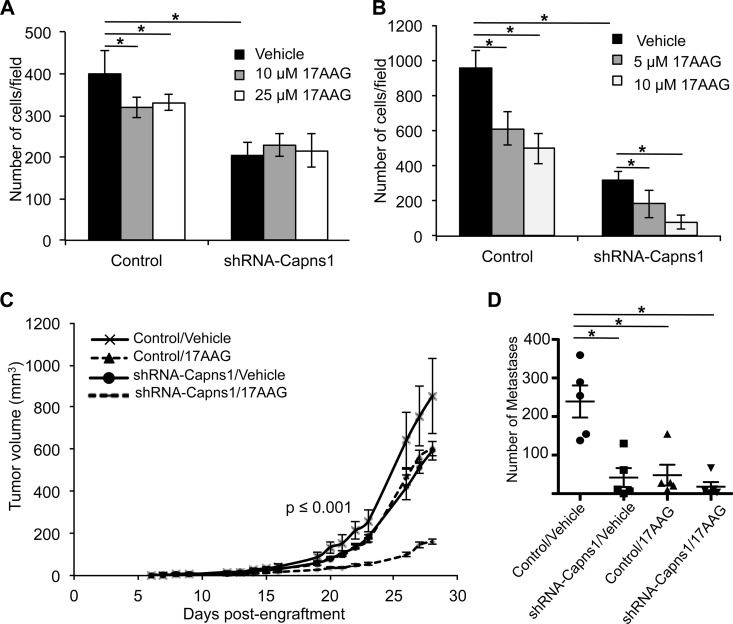
Calpain knockdown enhances sensitivity to 17AAG in vitro and in vivo. (A and B) MDA-MB-231 cells transduced with a control lentivirus or a lentivirus expressing shRNA-Capns1 were subjected to transwell migration (A) or Matrigel invasion (B) assays for 8 or 24 h in the presence of the vehicle or the concentrations of 17AAG indicated, respectively. Bar graphs show the average number of cells in five random fields ± the SD. (C) MDA-MB-231 cells transduced with a control or shRNA-Capns1-expressing lentivirus were injected into the mammary glands of Rag2γ−/−/IL2Rγc−/− mice and treated with the vehicle or 17AAG. Curves represent mean tumor volumes ± the standard error of the mean for five mice in each of the cohorts indicated. Single-factor ANOVA of the k value (growth rate; P = 7.4 × 10−4) or doubling time (P = 6.36 × 10−4) indicated significant differences between all of the cohorts except the 17AAG and calpain knockdown cohorts. (D) Average numbers of lung metastases in each mouse ± SD are indicated. *, significantly different cohorts (P ≤ 0.01).
17AAG and calpain knockdown combine to effectively suppress tumorigenesis.
We employed an orthotopic xenograft model to explore the combined effects of calpain knockdown and HSP90 inhibition on mammary tumor growth and metastasis. MDA-MB-231 cells transduced with control or shRNA-Capns1-expressing lentivirus were injected into the mammary fat pads of Rag2−/−/IL2Rγc−/− mice. Tumor-bearing mice were then treated with either the vehicle control or 75 mg/kg 17AAG, and tumor growth was assessed (Fig. 3C). Control tumors in vehicle-treated mice reached 852 ± 177 mm3 after 28 days. Calpain knockdown and 17AAG treatment alone reduced tumor volumes to 592 ± 44 and 604 ± 35 mm3, respectively. However, the combination was more effective than the control (P = 0.005), 17AAG alone (P = 1.3 × 10−5) or calpain knockdown alone (P = 2.4 × 10−6), with tumors reaching only 162 ± 12 mm3. Single-factor analysis of variance (ANOVA) revealed significantly different tumor doubling times (P = 6.36 × 10−4) and growth rates (P = 7.4 × 10−4).
In vitro observations of cell migration and especially invasion suggested that calpain and HSP90 might influence metastasis in this xenograft model. To explore this, tumors were resected by recovery surgery at 28 days postengraftment, and 10 days later, the mice were sacrificed to assess metastasis to the lungs. Calpain knockdown or 17AAG treatment was independently associated with reduced frequencies of lung metastatic lesions (Fig. 3D; see Fig. S2C in the supplemental material) (P = 0.0035 or P = 0.0049, respectively). Combined calpain knockdown and 17AAG treatment was also associated with reduced metastasis relative to control tumors, but this was not significantly different from that seen with calpain knockdown or 17AAG in isolation (P = 0.4096). These xenograft studies show that combined inhibition of calpain and HSP90 has a significant suppressive effect on tumor growth and metastasis.
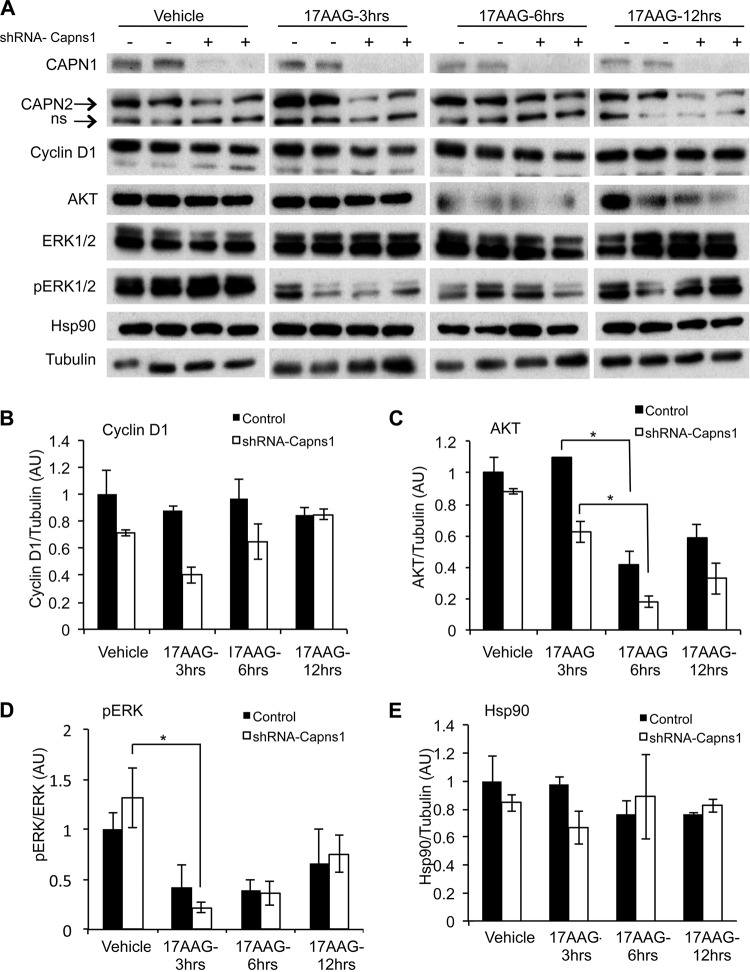
Downregulation of HSP90 clients by 17AGG is enhanced by calpain knockdown.
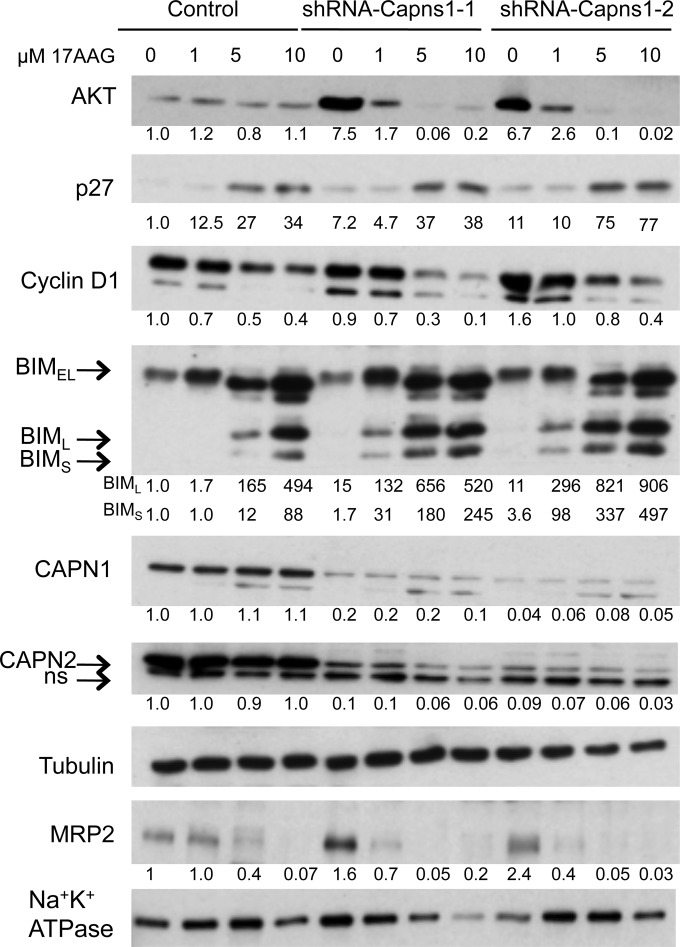
We next explored the molecular basis of the combined effect of targeting calpain and HSP90. In a previous paper, we reported that calpain-2 knockdown suppressed AKT-FoxO3a signaling in a mouse mammary carcinoma model (12). Here, we observed that capns1 knockdown in MDA-MB-231 cells was associated with increased levels of HSP90 client protein AKT (Fig. 4), but interestingly, this appeared to be highly dependent upon HSP90, since 17AAG treatment resulted in rapid AKT loss. In contrast, AKT levels were relatively lower and resistant to a 17AAG challenge in control cells. Although phosphorylation of AKT could not be consistently detected in MDA-MB-231 cells (data not shown), 17AAG treatment was nevertheless associated with changes in FoxO3a targets that would be expected upon AKT signal suppression, namely, increased p27KIP1 and BIM and decreased cyclin D1 (Fig. 4). 17AAG dose responses indicated an accentuated response in calpain knockdown cells with respect to enhanced degradation of cyclin D1 and AKT (see Fig. S3 in the supplemental material), as well as increased levels of the antimitogenic protein p27KIP1 and the BIML and BIMS isoforms of proapoptotic protein BIM. We also observed enhanced degradation of the ABC transporter MRP2 in calpain knockdown MDA-MB-231 cells. Densitometry analysis shows 50 to 75% MRP2 reductions in knockdown cells compared with no reduction in control cells at 1 μM 17AAG and a >95% MRP2 reduction in knockdown cells compared with 60% in control cells at 5 μM 17AAG (Fig. 4; see Fig. S3 in the supplemental material).
FIG 4.
Calpain knockdown enhances 17AGG-induced downregulation of HSP90 clients and upregulation of antiproliferative and proapoptotic proteins in vitro. MDA-MB-231 cells transduced with the control or two Capns1 knockdown lentiviruses (shRNA–Capns1-1, shRNA–Capns1-2) were treated for 48 h with the concentrations of 17AAG indicated. Live and dead cells were pooled and analyzed by immunoblotting for the proteins indicated. Densitometry was performed, and relative expression levels in whole-cell lysates, normalized to tubulin, are reported below the lanes, indicating expression compared to the control at 0 μM 17AAG. MRP2 was normalized to Na+/K+-ATPase in membrane fractions. For statistical analyses of MRP2 expression levels, see Fig. S3 in the supplemental material.
In vivo, a similar trend emerged, although it was not statistically significant on its own, where xenograft tumors established with calpain knockdown MDA-MB-231 cells displayed more pronounced 17AAG-induced degradation of HSP90 client proteins than in control tumors (Fig. 5). Calpain knockdown tumors displayed increased 17AAG-induced degradation of the HSP90 clients cyclin D1 and AKT (Fig. 5A to C). In control tumors, cyclin D1 levels remained relatively unchanged (P = 0.65) after 17AAG treatment but were reduced by 45% (P = 0.06) after 3 h of 17AAG treatment in calpain knockdown tumors (Fig. 5B). In calpain knockdown tumors, AKT levels were reduced earlier after 17AAG treatment (30% at 3 h [P = 0.08] versus no change [P = 0.47] in control tumors) and displayed greater levels of depletion at 6 and 12 h (Fig. 5C). While the levels of ERK1/2 were not affected, the phosphorylation state was effectively suppressed by 17AAG in control tumors (P = 0.13) and to a greater extent in calpain knockdown tumors (P = 0.05) after 3 h (Fig. 5D). There was no significant change in the levels of HSP90 itself after calpain knockdown or 17AAG treatment (Fig. 5E).
FIG 5.
Calpain knockdown enhances 17AAG-induced downregulation of HSP90 clients in vivo. Mice were engrafted with MDA-MB-231 cells transduced with control or calpain knockdown shRNA-Capns1-expressing lentiviruses. When tumors reached 100 to 200 mm3, mice were treated with the vehicle or 75 mg/kg 17AAG for 3, 6, or 12 h in duplicate. (A) Mice were euthanized, and tumors were excised for immunoblotting analysis of the proteins indicated. (B to E) Densitometric quantitation of cyclin D1 (B), AKT (C), pERK (D), and HSP90 (E) is shown. *, P ≤ 0.05 by Student's t test. AU, arbitrary units.
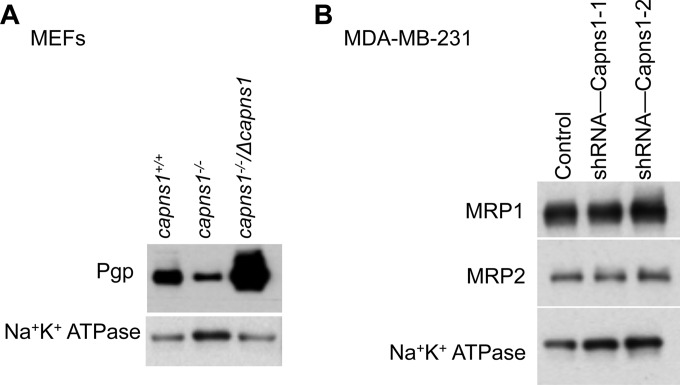
Calpain regulates ABC transporter expression and function.
As with many cancer therapeutics, resistance to 17AAG can result from increased efflux by the ABC transporter Pgp (18) or MRP1 (19). Pgp expression was significantly lower in capns1−/− MEFs than in control capns1+/+ cells and was elevated in capns1−/−/Δcapns1 MEFs (Fig. 6A), which proportionally overexpress calpain (Fig. 1A). Consistent with the reported role of Pgp in effluxing 17AAG (18), sensitivity to this drug in the capns1 MEF panel was proportional to Pgp levels (Fig. 1B). Pgp expression was not detectable in MDA-MB-231 cells (data not shown). However, quantitative reverse transcription-PCR and transcriptome sequencing (data not shown) and immunoblotting analysis of other ABC transporters revealed the expression of MRP1 and MRP2. However, there were no statistically significant differences in the basal levels of MRP1 or MRP2 in MDA-MB-231 cells at either the RNA (data not shown) or the protein level (Fig. 6B).
FIG 6.
Calpain regulates ABC transporter expression and drug sensitivity. (A) Immunoblot analysis of Pgp in membrane fractions of MEFs with the CAPNS1 genotypes indicated. (B) Immunoblot analysis of MRP1 and MRP2 in membrane fractions from control or shRNA-Capns1-transduced MDA-MB-231 cells. Blotting for Na+/K+-ATPase was used to normalize for membrane protein content.
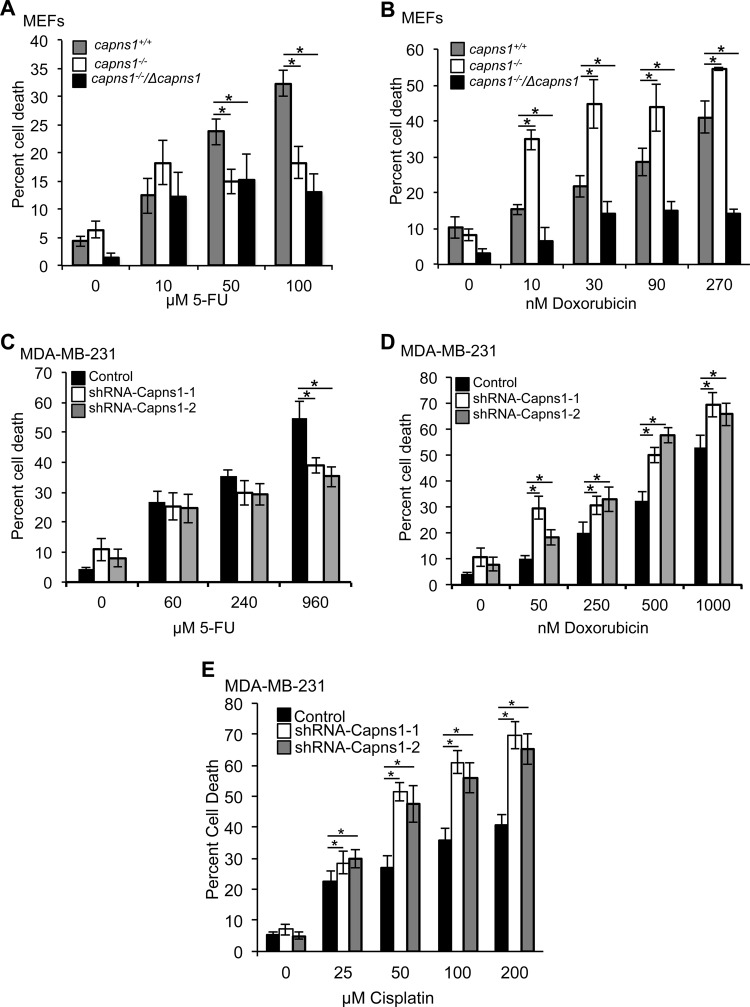
Using clinically relevant drugs and knowledge about which ABC transporters are capable of effluxing them, we explored which ABC transporters calpain might be affecting. In addition to 17AAG, the ABC transporters Pgp, MRP1, and MRP2 can also transport doxorubicin; however, they cannot efflux 5-FU (32). Calpain expression correlated with sensitivity to doxorubicin but not with sensitivity to 5-FU in MEFs (Fig. 7A and B) and in MDA-MB-231 cells (Fig. 7C and D). Although capns1+/+ MEFs were less sensitive to 5-FU than capns1−/− cells were (P = 0.002 at 50 μM), rescuing calpain (capns1−/−/Δcapns1) did not affect 5-FU sensitivity (P = 0.896 compared to capns1−/− cells) (Fig. 7A). Similarly, calpain knockdown in MDA-MB-231 cells was not associated with increased sensitivity to 5-FU; in fact, reduced cell death was apparent in calpain knockdown cells at high concentrations of 5-FU (P = 0.002 for shRNA–Capns1-1, P = 0.0001 for shRNA–Capns1-2) (Fig. 7C). In contrast, calpain expression correlated with sensitivity to doxorubicin in both cell systems. In MEFs, loss of calpain was associated with increased doxorubicin sensitivity and a significant decrease in the LD50 of this drug, a phenotype that was robustly reversed upon rescue with calpain overexpression (Fig. 7B). Calpain knockdown in MDA-MB-231 cells was also associated with increased sensitivity to doxorubicin and reduced LD50s, particularly at lower concentrations (Fig. 7D). In addition, calpain knockdown in MDA-MB-231 cells was associated with increased sensitivity to cisplatin (Fig. 7E), which is known to be effluxed by MRP2 but not MRP1 (33, 34). The LD50s in cells with downregulated calpain were 62.1 and 72.7 μM, compared to 461.8 μM in control cells. These data suggest a regulatory relationship between calpain and MRP2 in MDA-MB-231 cells.
FIG 7.
Calpain regulates doxorubicin sensitivity and efflux. (A to D) MEFs with the CAPNS1 genotypes indicated (A, B) or MDA-MB-231 cells transduced with the control or shRNA-Capns1-expressing lentiviruses indicated (C, D) were treated with 5-FU (A, C) or doxorubicin (B, D) for 48 h, and cell death was quantified. Doxorubicin LD50s: capns1+/+ MEFs, 261 nM; capns1−/− MEFs, 59.1 nM; capns1−/−/Δcapns1 MEFs, 1,248 nM (P ≤ 0.0001); control MDA-MB-231 cells, 1,152 nM; shRNA–Capns1-1 MDA-MB-231 cells, 421 nM; shRNA–Capns1-2 MDA-MB-231 cells, 428 nM (P ≤ 0.001). (E) Analysis of the death of the cisplatin-treated MDA-MB-231 cells described above. LD50s: control, 461.8 μM; shRNA–Capns1-1, 62.1 μM; shRNA–Capns1-2, 72.7 μM (P = 0.011). Student's t tests were performed. *, P ≤ 0.01 compared to capns1+/+ (A, B) or the control (C to E). One-way ANOVA was used to assess differences between LD50s.
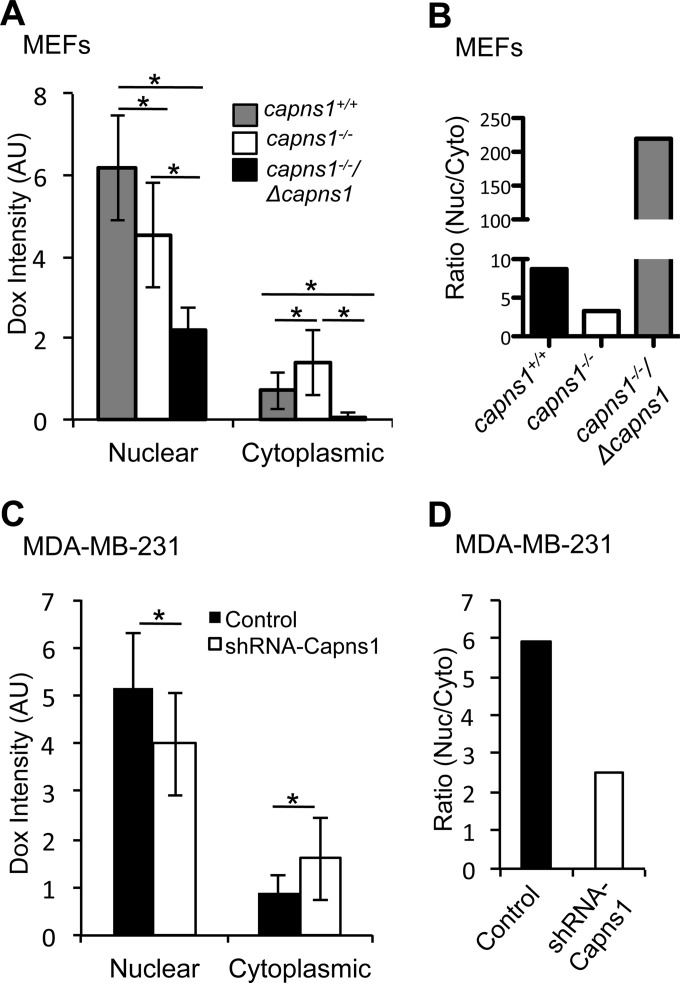
Doxorubicin, like 17AAG, is a substrate for several ABC transporters, including Pgp, MRP1, and MRP2. The inherent fluorescent property of doxorubicin was used as an indirect method to assess ABC transporter function by correlating calpain expression and the intracellular localization of this therapeutic drug. Calpain expression was found to be associated with a significantly altered subcellular localization of doxorubicin in both MEFs and MDA-MB-231 cells (Fig. 8; see Fig. S4 in the supplemental material). Specifically, calpain disruption correlated with an increased cytoplasmic localization of doxorubicin (Fig. 8A and C). Indeed, when calpain expression was rescued in capns1−/− MEFs with overexpression of ΔCAPNS1, doxorubicin localization in the cytoplasm was completely lost (Fig. 8A). Interestingly, nuclear and total fluorescence intensities did not precisely correlate with calpain expression (Fig. 8A and C; see Fig. S3 in the supplemental material); however, the ratio of nuclear/cytoplasmic drug localization showed a consistent positive correlation with calpain expression (Fig. 8B and D). In summary, calpain disruption was associated with an increase in cytoplasmic doxorubicin and a reduced nuclear/cytoplasmic doxorubicin ratio, and this correlated with increased sensitivity to doxorubicin (Fig. 7) and 17AAG (Fig. 1). These results suggest that calpain enhances the cytoplasmic efflux of specific therapeutics by regulating the function of specific ABC transporters.
FIG 8.
Calpain affects ABC transporter function. Subcellular doxorubicin distribution was assessed by confocal microscopy of MEFs (A, B) and MDA-MB-231 cells (C, D) of the genotypes indicated. Cells were treated with 3.5 μM doxorubicin for 1 h, fixed, and stained with DAPI. Nuclear and cytoplasmic doxorubicin fluorescence intensities (A, C) and nuclear/cytoplasmic ratios of doxorubicin fluorescence intensities (B, D) in >30 cells were calculated. Data are plotted as mean ± SD. *, P ≤ 0.05. AU, arbitrary units.
DISCUSSION
We show here for the first time that calpain is positively correlated with resistance to the HSP90 inhibitor 17AAG. This was demonstrated in vitro with MEFs (Fig. 1) and two distinct human breast cancer cell lines, MDA-MB-231 (Fig. 1) and SKBR3 (see Fig. S1 in the supplemental material). In vivo, calpain knockdown combined with 17AAG suppressed tumorigenesis more effectively than either alone (Fig. 3). Our observations suggest that calpain and HSP90 cooperatively contribute to survival, mitogenic signaling, and drug resistance through effects on HSP90 clients, including cyclin D, AKT, and members of the ABC transporter family. This argues that calpain inhibition could enhance the clinical efficacy of HSP90 inhibitors and specific chemotherapeutics.
Previous studies have shown that calpain disruption compromises the AKT signaling axis, resulting in upregulation of the antimitogenic protein p27KIP1 and the proapoptotic protein BIM (10, 12). This effect was amplified by 17AAG, suggesting that calpain contributes to the stabilization of HSP90 client proteins. AKT and cyclin D1 were more sensitive to 17AAG in calpain knockdown cells or tumors (Fig. 4 and 5), and this correlated with upregulation of p27KIP1 and BIM, two proteins that are negatively regulated by AKT (Fig. 3 and 4). Thus, calpain and HSP90 may collaboratively promote tumorigenesis through PI3K-AKT signaling.
Other studies have suggested that calpain may be a useful therapeutic target by increasing cell death after a therapeutic challenge. Calpain expression predicts the response to trastuzumab or cisplatin in breast or ovarian cancer patients, respectively (7, 11, 35). We observed a significant reduction in xenograft tumor growth when 17AAG treatment was combined with calpain knockdown, as well as decreased metastasis. Cell migration and metastasis are two essential processes of the metastatic cascade. HSP90 (31) and calpain (29, 30) have previously been shown to positively regulate these processes. Inhibition of HSP90 or calpain knockdown was associated with decreased in vitro migration and invasion (Fig. 3A and B). Calpain knockdown and 17AAG combined in vitro to more effectively inhibit invasion (Fig. 3B). While the combination was not significantly more effective at suppressing metastasis in vivo, this is likely because each modality was so effective in isolation (Fig. 3D). Clients of HSP90 which are also substrates of calpain might mediate these effects. Cortactin expression has been associated with metastasis (36), and its cleavage by calpain contributes to cell migration (29). Similarly, calpain-mediated cleavage of FAK enhances cell adhesion and cell motility (30). A recent study identified enhanced proteolysis of ezrin, vimentin, and fibronectin in a highly metastatic cell line compared to that in a nonmetastatic counterpart (37). Some of these proteins can be specifically cleaved by calpain, including ezrin (38) and vimentin (39). Ezrin has recently been implicated in promoting the metastatic potential of breast cancer cells by recruiting calpain to focal adhesions to promote their turnover during migration (40). Calpain can also modulate invasion by regulating matrix metalloproteinase 2 (MMP-2) expression (41). Importantly, several of these calpain substrates are also HSP90 clients, including vimentin, MMP-2, and FAK (42, 43). Thus, combined inhibition of calpain and HSP90 may yield additive or synergistic effects on cell death, migration, invasion, tumor growth, and metastasis.
Another mechanism by which calpain might enhance the efficacy of specific therapeutics may involve the regulation of ABC transporters that efflux these drugs. Calpain expression was positively associated with steady-state Pgp expression at the cell membrane in MEFs (Fig. 6A), and calpain knockdown in MDA-MB-231 cells correlated with enhanced degradation of MRP2 in response to 17AAG treatment (Fig. 4). One early study suggested that calpain inhibitors increased ubiquitination of Pgp at the cell membrane, and in vitro proteolysis experiments showed that calpain can cleave Pgp (44). If and how calpain cleavage might stabilize Pgp at the cell membrane is currently unknown, and our data did not reveal evidence of this. Alternatively, calpain may affect the transcription of PGP; for example, the transcription factor NF-κB can induce the transcription of PGP in response to vinblastine (45–47) and calpain can activate NF-κB by cleaving and degrading its inhibitor IκB (45, 47). Pgp expression may also be promoted by PI3K-AKT signaling (48), which we and others have shown can be regulated by calpain (10, 12). Although steady-state levels of MRP1 and MRP2 are not affected by loss of calpain (Fig. 6), calpain knockdown in MDA-MB-231 cells was associated with 17AAG-induced loss of MRP2 (Fig. 4), suggesting complex roles for calpain in the regulation of its expression. Calpain knockdown was also associated with increased sensitivity to cisplatin (Fig. 4), which is effluxed by MRP2 but not MRP1 (33, 34).
Although the precise mechanism of calpain-mediated effects of ABC transporters has yet to be determined, we show that aberrant calpain expression modulates ABC transporter function (Fig. 8; see Fig. S4 in the supplemental material). Interestingly, geldanamycins are both substrates and inhibitors of some ABC transporters, including Pgp (18). Pgp and MRP1 are implicated in the transport of 17AAG (18, 19) and doxorubicin (32); the effect of MRP2 on 17AAG is unknown. Our data show that selected ABC transporters, specifically, Pgp in MEFs and MRP2 in MDA-MB-231 cells, have altered functions upon the loss of calpain, and this resulted in the increased cytotoxicity of drugs that are effluxed by these ABC transporters in calpain-deficient cells.
Differences in ABC transporter expression, function, and regulatory interactions with calpain may contribute to the paradoxical observation that calpain can have either prosurvival or prodeath functions, depending upon the cellular context. In our study, calpain was associated with sensitivity to the HSP90 inhibitor 17AAG in three different cell systems. Although a single study suggests that loss of calpain results in increased sensitivity to the antimetabolite 5-FU in gastric cancer cells (49), we observed the opposite in MDA-MB-231 cells (Fig. 7C). Such apparently paradoxical findings are commonly found in the literature relating to calpain. For example, MEFs that lack calpain are more sensitive to tumor necrosis factor alpha and staurosporine but significantly more resistant to UV exposure, etoposide, camptothecin, puromycin, and H2O2 challenges (9). The ABC transporters responsible for transporting different compounds and how they are modulated by calpain activity remain important areas of study.
In conclusion, we have shown that calpain expression correlates with sensitivity to the HSP90 inhibitor 17AAG, as well as doxorubicin and cisplatin, in multiple cell models, and calpain knockdown combined with 17AAG more effectively reduces tumor growth and metastasis in vivo. We provide evidence that calpain regulates sensitivity to clinically relevant therapeutics in part through effects on the expression and stability of specific ABC transporters, including Pgp and MRP2, in a cell type- and cell context-dependent manner. This argues that calpain inhibitors could enhance the efficacy of specific cancer therapeutics.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Jeffery Mewburn and Matthew Gordon for technical assistance, Changnian Shi for statistical analysis, and Susan Cole for helpful discussions.
This work was supported by a grant from the Canadian Institutes of Health Research to P.A.G. and fellowship awards from the Canadian Breast Cancer Foundation and the Terry Fox Transdisciplinary Training Program to S.G.
The funders had no role in the study design, data collection and interpretation, or the decision to submit the work for publication.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01062-15.
REFERENCES
- 1.Goll DE, Thompson VF, Li H, Wei W, Cong J. 2003. The calpain system. Physiol Rev 83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 2.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. 2000. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol 20:4474–4481. doi: 10.1128/MCB.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA. 2006. Conditional disruption of ubiquitous calpains in the mouse. Genesis 44:297–303. doi: 10.1002/dvg.20216. [DOI] [PubMed] [Google Scholar]
- 4.Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. 2011. The calpain system and cancer. Nat Rev Cancer 11:364–374. doi: 10.1038/nrc3050. [DOI] [PubMed] [Google Scholar]
- 5.Storr SJ, Lee KW, Woolston CM, Safuan S, Green AR, Macmillan RD, Benhasouna A, Parr T, Ellis IO, Martin SG. 2012. Calpain system protein expression in basal-like and triple-negative invasive breast cancer. Ann Oncol 23:2289–2296. doi: 10.1093/annonc/mds176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storr SJ, Mohammed RA, Woolston CM, Green AR, Parr T, Spiteri I, Caldas C, Ball GR, Ellis IO, Martin SG. 2011. Calpastatin is associated with lymphovascular invasion in breast cancer. Breast 20:413–418. doi: 10.1016/j.breast.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Storr SJ, Woolston CM, Barros FF, Green AR, Shehata M, Chan SY, Ellis IO, Martin SG. 2011. Calpain-1 expression is associated with relapse-free survival in breast cancer patients treated with trastuzumab following adjuvant chemotherapy. Int J Cancer 129:1773–1780. doi: 10.1002/ijc.25832. [DOI] [PubMed] [Google Scholar]
- 8.Amini M, Ma CL, Farazifard R, Zhu G, Zhang Y, Vanderluit J, Zoltewicz JS, Hage F, Savitt JM, Lagace DC, Slack RS, Beique JC, Baudry M, Greer PA, Bergeron R, Park DS. 2013. Conditional disruption of calpain in the CNS alters dendrite morphology, impairs LTP, and promotes neuronal survival following injury. J Neurosci 33:5773–5784. doi: 10.1523/JNEUROSCI.4247-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan Y, Wu C, De Veyra T, Greer PA. 2006. Ubiquitous calpains promote both apoptosis and survival signals in response to different cell death stimuli. J Biol Chem 281:17689–17698. doi: 10.1074/jbc.M601978200. [DOI] [PubMed] [Google Scholar]
- 10.Bertoli C, Copetti T, Lam EW, Demarchi F, Schneider C. 2009. Calpain small-1 modulates Akt/FoxO3A signaling and apoptosis through PP2A. Oncogene 28:721–733. doi: 10.1038/onc.2008.425. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni S, Reddy KB, Esteva FJ, Moore HC, Budd GT, Tubbs RR. 2010. Calpain regulates sensitivity to trastuzumab and survival in HER2-positive breast cancer. Oncogene 29:1339–1350. doi: 10.1038/onc.2009.422. [DOI] [PubMed] [Google Scholar]
- 12.Ho WC, Pikor L, Gao Y, Elliott BE, Greer PA. 2012. Calpain 2 regulates Akt-FoxO-p27(Kip1) protein signaling pathway in mammary carcinoma. J Biol Chem 287:15458–15465. doi: 10.1074/jbc.M112.349308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beliakoff J, Whitesell L. 2004. Hsp90: an emerging target for breast cancer therapy. Anticancer Drugs 15:651–662. doi: 10.1097/01.cad.0000136876.11928.be. [DOI] [PubMed] [Google Scholar]
- 14.Trepel J, Mollapour M, Giaccone G, Neckers L. 2010. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidera K, Patsavoudi E. 2014. HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov 9:1–20. [PubMed] [Google Scholar]
- 16.Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. 2001. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res 61:4003–4009. [PubMed] [Google Scholar]
- 17.Rodrigues LM, Chung YL, Al Saffar NM, Sharp SY, Jackson LE, Banerji U, Stubbs M, Leach MO, Griffiths JR, Workman P. 2012. Effects of HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG) on NEU/HER2 overexpressing mammary tumours in MMTV-NEU-NT mice monitored by magnetic resonance spectroscopy. BMC Res Notes 5:250. doi: 10.1186/1756-0500-5-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Blower PE, Liu R, Dai Z, Pham AN, Moon H, Fang J, Sadee W. 2007. Chemogenomic analysis identifies geldanamycins as substrates and inhibitors of ABCB1. Pharm Res 24:1702–1712. doi: 10.1007/s11095-007-9300-x. [DOI] [PubMed] [Google Scholar]
- 19.Pham AN, Wang J, Fang J, Gao X, Zhang Y, Blower PE, Sadee W, Huang Y. 2009. Pharmacogenomics approach reveals MRP1 (ABCC1)-mediated resistance to geldanamycins. Pharm Res 26:936–945. doi: 10.1007/s11095-008-9796-8. [DOI] [PubMed] [Google Scholar]
- 20.Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME, Sugarman S, Ma W, Patil S, Norton L, Hannah AL, Hudis C. 2011. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res 17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 21.Iyer G, Morris MJ, Rathkopf D, Slovin SF, Steers M, Larson SM, Schwartz LH, Curley T, DeLaCruz A, Ye Q, Heller G, Egorin MJ, Ivy SP, Rosen N, Scher HI, Solit DB. 2012. A phase I trial of docetaxel and pulse-dose 17-allylamino-17-demethoxygeldanamycin in adult patients with solid tumors. Cancer Chemother Pharmacol 69:1089–1097. doi: 10.1007/s00280-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hubbard J, Erlichman C, Toft DO, Qin R, Stensgard BA, Felten S, Ten Eyck C, Batzel G, Ivy SP, Haluska P. 2011. Phase I study of 17-allylamino-17 demethoxygeldanamycin, gemcitabine and/or cisplatin in patients with refractory solid tumors. Invest New Drugs 29:473–480. doi: 10.1007/s10637-009-9381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minami Y, Kimura Y, Kawasaki H, Suzuki K, Yahara I. 1994. The carboxy-terminal region of mammalian HSP90 is required for its dimerization and function in vivo. Mol Cell Biol 14:1459–1464. doi: 10.1128/MCB.14.2.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Luo R, Jiang R, Meng X, Wu X, Zhang S, Hua W. 2013. The role of the Hsp90/Akt pathway in myocardial calpain-induced caspase-3 activation and apoptosis during sepsis. BMC Cardiovasc Disord 13:8. doi: 10.1186/1471-2261-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Averna M, De Tullio R, Pedrazzi M, Bavestrello M, Pellegrini M, Salamino F, Pontremoli S, Melloni E. 2015. Interaction between calpain-1 and HSP90: new insights into the regulation of localization and activity of the protease. PLoS One 10:e0116738. doi: 10.1371/journal.pone.0116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Münster PN, Marchion DC, Basso AD, Rosen N. 2002. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3′-kinase-AKT-dependent pathway. Cancer Res 62:3132–3137. [PubMed] [Google Scholar]
- 27.Elce JS, Hegadorn C, Gauthier S, Vince JW, Davies PL. 1995. Recombinant calpain II: improved expression systems and production of a C105A active-site mutant for crystallography. Protein Eng 8:843–848. doi: 10.1093/protein/8.8.843. [DOI] [PubMed] [Google Scholar]
- 28.Bains M, Cebak JE, Gilmer LK, Barnes CC, Thompson SN, Geddes JW, Hall ED. 2013. Pharmacological analysis of the cortical neuronal cytoskeletal protective efficacy of the calpain inhibitor SNJ-1945 in a mouse traumatic brain injury model. J Neurochem 125:125–132. doi: 10.1111/jnc.12118. [DOI] [PubMed] [Google Scholar]
- 29.Perrin BJ, Amann KJ, Huttenlocher A. 2006. Proteolysis of cortactin by calpain regulates membrane protrusion during cell migration. Mol Biol Cell 17:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sawhney RS, Cookson MM, Omar Y, Hauser J, Brattain MG. 2006. Integrin alpha2-mediated ERK and calpain activation play a critical role in cell adhesion and motility via focal adhesion kinase signaling: identification of a novel signaling pathway. J Biol Chem 281:8497–8510. doi: 10.1074/jbc.M600787200. [DOI] [PubMed] [Google Scholar]
- 31.Taiyab A, Rao Ch M. 2011. HSP90 modulates actin dynamics: inhibition of HSP90 leads to decreased cell motility and impairs invasion. Biochim Biophys Acta 1813:213–221. doi: 10.1016/j.bbamcr.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. 2006. Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 33.Deeley RG, Westlake C, Cole SP. 2006. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 34.Koike K, Kawabe T, Tanaka T, Toh S, Uchiumi T, Wada M, Akiyama S, Ono M, Kuwano M. 1997. A canalicular multispecific organic anion transporter (cMOAT) antisense cDNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res 57:5475–5479. [PubMed] [Google Scholar]
- 35.Storr SJ, Safuan S, Woolston CM, Abdel-Fatah T, Deen S, Chan SY, Martin SG. 2012. Calpain-2 expression is associated with response to platinum based chemotherapy, progression-free and overall survival in ovarian cancer. J Cell Mol Med 16:2422–2428. doi: 10.1111/j.1582-4934.2012.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo ML, Shen XM, Zhang Y, Wei F, Xu X, Cai Y, Zhang X, Sun YT, Zhan Q M, Wu M, Wang MR. 2006. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res 66:11690–11699. doi: 10.1158/0008-5472.CAN-06-1484. [DOI] [PubMed] [Google Scholar]
- 37.Shen C, Yu Y, Li H, Yan G, Liu M, Shen H, Yang P. 2012. Global profiling of proteolytically modified proteins in human metastatic hepatocellular carcinoma cell lines reveals CAPN2 centered network. Proteomics 12:1917–1927. doi: 10.1002/pmic.201200027. [DOI] [PubMed] [Google Scholar]
- 38.Shcherbina A, Bretscher A, Kenney DM, Remold-O'Donnell E. 1999. Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain. FEBS Lett 443:31–36. doi: 10.1016/S0014-5793(98)01674-3. [DOI] [PubMed] [Google Scholar]
- 39.Kwak HI, Kang H, Dave JM, Mendoza EA, Su SC, Maxwell SA, Bayless KJ. 2012. Calpain-mediated vimentin cleavage occurs upstream of MT1-MMP membrane translocation to facilitate endothelial sprout initiation. Angiogenesis 15:287–303. doi: 10.1007/s10456-012-9262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoskin V, Szeto A, Ghaffari A, Greer PA, Cote GP, Elliott BE. 2015. Ezrin regulates focal adhesion and invadopodia dynamics by altering calpain activity to promote breast cancer cell invasion. Mol Biol Cell 26:3464–3479. doi: 10.1091/mbc.E14-12-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng PC, Chen X, Zhu HW, Zheng W, Mao LH, Lin C, Liu JN, Zheng M. 2014. Capn4 is a marker of poor clinical outcomes and promotes nasopharyngeal carcinoma metastasis via nuclear factor-κB-induced matrix metalloproteinase 2 expression. Cancer Sci 105:630–638. doi: 10.1111/cas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Faingold D, Filho VB, Fernandes B, Jagan L, de Barros AM Jr, Orellana ME, Antecka E, and Burnier MN Jr. 2014. Expression of focal adhesion kinase in uveal melanoma and the effects of Hsp90 inhibition by 17-AAG. Pathol Res Pract 210:739–745. doi: 10.1016/j.prp.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 43.Nagaraju GP, Long TE, Park W, Landry JC, Taliaferro-Smith L, Farris AB, Diaz R, El-Rayes BF. 2015. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol Carcinog 54:1147–1158. doi: 10.1002/mc.22185. [DOI] [PubMed] [Google Scholar]
- 44.Ohkawa K, Asakura T, Takada K, Sawai T, Hashizume Y, Okawa Y, Yanaihara N. 1999. Calpain inhibitor causes accumulation of ubiquitinated P-glycoprotein at the cell surface: possible role of calpain in P-glycoprotein turnover. Int J Oncol 15:677–686. [DOI] [PubMed] [Google Scholar]
- 45.Chen F, Demers LM, Vallyathan V, Lu Y, Castranova V, Shi X. 2000. Impairment of NF-kappaB activation and modulation of gene expression by calpastatin. Am J Physiol Cell Physiol 279:C709–C716. [DOI] [PubMed] [Google Scholar]
- 46.Chen Q, Bian Y, Zeng S. 2014. Involvement of AP-1 and NF-kappaB in the up-regulation of P-gp in vinblastine resistant Caco-2 cells. Drug Metab Pharmacokinet 29:223–226. doi: 10.2133/dmpk.DMPK-13-SH-068. [DOI] [PubMed] [Google Scholar]
- 47.Shumway SD, Maki M, Miyamoto S. 1999. The PEST domain of IkappaBalpha is necessary and sufficient for in vitro degradation by mu-calpain. J Biol Chem 274:30874–30881. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- 48.Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, Mills GB, Unate H. 2002. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kappaB signaling. Oncogene 21:1945–1954. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 49.Nabeya Y, Suzuki T, Furuya A, Koide N, Ohkoshi M, Takiguchi M, Ochiai T, Matsubara H, Hiwasa T. 2011. Calpain regulates thymidylate synthase-5-fluoro-dUMP complex levels associated with response to 5-fluorouracil in gastric cancer cells. Cancer Sci 102:1509–1515. doi: 10.1111/j.1349-7006.2011.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.