Abstract
The growth factor heregulin (HRG), a ligand of ErbB3 and ErbB4 receptors, contributes to breast cancer development and the promotion of metastatic disease, and its expression in breast tumors has been associated with poor clinical outcome and resistance to therapy. In this study, we found that breast cancer cells exposed to sustained HRG treatment show markedly enhanced Rac1 activation and migratory activity in response to the CXCR4 ligand SDF-1/CXCL12, effects mediated by P-Rex1, a Rac-guanine nucleotide exchange factor (GEF) aberrantly expressed in breast cancer. Notably, HRG treatment upregulates surface expression levels of CXCR4, a G protein-coupled receptor (GPCR) implicated in breast cancer metastasis and an indicator of poor prognosis in breast cancer patients. A detailed mechanistic analysis revealed that CXCR4 upregulation and sensitization of the Rac response/motility by HRG are mediated by the transcription factor hypoxia-inducible factor 1α (HIF-1α) via ErbB3 and independently of ErbB4. HRG caused prominent induction in the nuclear expression of HIF-1α, which transcriptionally activates the CXCR4 gene via binding to a responsive element located in positions −1376 to −1372 in the CXCR4 promoter, as revealed by mutagenesis analysis and chromatin immunoprecipitation (ChIP). Our results uncovered a novel function for ErbB3 in enhancing breast cancer cell motility and sensitization of the P-Rex1/Rac1 pathway through HIF-1α-mediated transcriptional induction of CXCR4.
INTRODUCTION
ErbB receptors are known to play key roles in cell proliferation, survival, and motility and have been widely implicated in the initiation and progression of cancer. Members of this family of transmembrane tyrosine kinases include epidermal growth factor receptors (EGFR) (ErbB1/HER1), ErbB2/HER2, ErbB3/HER3, and ErbB4/HER4. Ligands with distinctive affinities for ErbB receptors promote their homo- and heterodimerization, leading to stimulation of intrinsic tyrosine kinase activity; recruitment of adaptors and effectors to autophosphorylated tyrosine sites; and activation of key signaling cascades, namely, the phosphatidylinositol-3 kinase (PI3K)/Akt, extracellular signal regulated kinase (ERK), and protein kinase C (PKC) pathways (1–4). Dysregulation of the ErbB signaling pathway is a common alteration in human cancer, and it occurs largely as a consequence of gain-of-function mutations (e.g., EGFR); gene amplification (e.g., ErbB2); and/or overexpression of ErbB ligands, such as EGF and transforming growth factor alpha (TGFα) (EGFR ligands) and heregulin-1/neuregulin-1 (HRG) (ErbB3/ErbB4 ligand) (5–10).
ErbB3 has been shown to be crucially important in breast cancer progression. This receptor is catalytically inactive, and hence, its signaling capacity depends entirely on dimerization with other catalytically competent ErbB partners. ErbB2, the only orphan member of the ErbB receptor family, is the preferred dimerization partner for ErbB3, and the ErbB2/ErbB3 heterodimer, which signals preferentially through PI3K, is regarded as a major oncogenic unit in ErbB2-overexpressing mammary tumors (1, 7, 8, 11, 12). ErbB3 expression in invasive human breast carcinomas has been associated with reduced patient survival (13). Enhanced production of HRG, which could be induced by oncogenic inputs, such as phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PI3KCA) mutations, occurs in a significant proportion of breast tumors, including ErbB2-low tumors (6, 14–17). Notably, transgenic overexpression of HRG in mouse mammary glands leads to the development of adenocarcinomas (18). Studies using MCF-7 breast cancer cells ectopically overexpressing HRG, a model that mimics the scenario observed in human tumors, established prominent roles for the growth factor in motility and invasion. Furthermore, HRG promotes the secretion of matrix metalloproteases and confers metastatic properties on MCF-7 cells when inoculated into nude mice (10, 19–22). Enhanced HRG/ErbB3 signaling has also been implicated in resistance to anticancer agents, including antiestrogens, ErbB tyrosine kinase inhibitors, and taxanes, and adaptive responses leading to drug resistance involve reprogramming of the kinome through reactivation of an HRG/ErbB3 axis (23–29). Consistent with the critical role of ErbB3 activation in breast cancer and other cancers, several targeted approaches designed to block HRG/ErbB3 are currently under clinical evaluation (30–32). Despite the recognized complexities of ErbB4 signaling and controversies regarding its role in cancers, this HRG receptor has been also implicated in breast tumorigenesis (33, 34). An understanding of the network of HRG-ErbB3/4 effectors implicated in cancer progression should afford novel therapeutic options for the treatment of breast cancer or other neoplasias.
Previously, we reported that treatment of breast cancer cells with HRG triggers a motile response that is mediated by the activation of Rac1 (35, 36), a GTPase widely implicated in actin cytoskeleton reorganization, migration, and metastatic dissemination (37). Like most members of the Rho/Rac small G protein family, Rac1 is a molecular switch that cycles between inactive (GDP-bound) and active (GTP-bound) states. Guanine nucleotide exchange factors (GEFs) promote GTP loading, thereby activating Rac1, whereas GTPase-activating proteins (GAPs) stimulate GTP hydrolysis by enhancing intrinsic GTPase activity, thus rendering the small G protein in the inactive state (38, 39). We have previously identified P-Rex1 as a main Rac-GEF responsible for Rac1 activation in response to ErbB ligands in breast cancer cells. P-Rex1 is aberrantly upregulated in human luminal breast tumors and cell lines, possibly through a mechanism that involves demethylation of the PREX1 gene promoter (40–42). P-Rex1 is dually activated by the PI3K product PIP3, and Gβγ subunits released upon G protein-coupled receptor (GPCR) activation. HRG and other ErbB ligands translocate P-Rex1 to the plasma membrane in a PI3K-dependent manner, leading to its activation. The requirement for P-Rex1 in HRG-induced Rac1 activation, ruffle formation, and motility, as well as its role in mammary tumorigenesis, has been unambiguously established by means of RNA interference (RNAi)-mediated loss of function approaches (36, 40, 43, 44). Consistent with the established requirement for Gβγ subunits in P-Rex1 activation, the GEF is also an effector of GPCRs (45). Recently, we and others reported that stimulation of the GPCR CXCR4 by its ligand, chemokine stromal-derived factor 1 (SDF-1/CXCL12), induces P-Rex1-dependent Rac1 activation and motility (40, 46, 47), which is consistent with the well-established role of the SDF-1/CXCR4 pathway in metastatic dissemination (48, 49). Indeed, CXCR4 has been functionally linked to breast cancer cell motility, invasion, and metastasis and is considered a marker of poor patient prognosis (50–55). CXCR4 is also essential for ErbB2-mediated tumor metastasis, and coexpression with ErbB2 and EGFR is predictive of a poor prognosis in breast cancer patients (56, 57), arguing for potential functional interrelationships between CXCR4 and ErbB receptors.
In this study, we aimed to investigate the effect of sustained HRG activation on CXCR4 signaling in breast cancer cells. Our analysis revealed a novel functional link whereby ErbB3 activation upregulates CXCR4 levels, which functionally translated into an enhanced motile response and Rac1 activation via P-Rex1. A comprehensive mechanistic analysis of this cross talk revealed a central role for the transcription factor hypoxia-inducible factor 1α (HIF-1α) as a mediator of HRG-induced CXCR4 upregulation and sensitization of Rac1 responses in breast cancer cells.
MATERIALS AND METHODS
Materials.
Heregulin β1 and SDF-1α were purchased from R&D Systems (Minneapolis, MN). The HIF-1α inhibitor SC205346 was obtained from EMD/Calbiochem (Gibbstown, NJ).
Cell lines.
MCF-7, BT-474, and MDA-MB-361 human breast cancer cell lines were obtained from the ATCC and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 10 μg/ml insulin, 100 U/ml penicillin, and 100 μg/ml streptomycin. MCF-10A cells were obtained from the ATCC and cultured in DMEM–F-12 medium supplemented with 5% horse serum, 20 ng/ml EGF, 0.5 μg/ml hydrocortisone, 100 ng/ml cholera toxin, 10 μg/ml insulin, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Western blot analysis.
Cells were lysed in a buffer containing 2% SDS, 62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 5% 2-mercaptoethanol, and 0.002% bromophenol blue, and extracts were subjected to SDS-polyacrylamide gel electrophoresis (PAGE), as described previously (58). For Western blots, the following antibodies were used: anti-Rac1 clone 23A8 (Upstate Biotechnology, Lake Placid, NY); anti-ErbB3, anti-ErbB4, anti-phospho-Akt (Ser473), and anti-phospho-Erk1/2 (Thr202/Tyr204) (Cell Signaling Technology, Beverly, MA); antivinculin and anti-P-Rex1 (Sigma, St. Louis, MO); and anti-HIF-1α (BD Biosciences, San Jose, CA). Bands were visualized by enhanced chemiluminescence (ECL). Images were captured using a FujiFilm LAS-3000 system and analyzed with the LAS-2000 software. Densitometric analysis of the bands was carried out using NIH ImageJ software.
RNAi.
For transient depletion of ErbB3, ErbB4, HIF-1α, and P-Rex1, we used ON-TARGETplus RNAi pools from Dharmacon (Lafayette, CO). ON-TARGETplus nontargeting pool (catalog number D-001810-0-05) was used as a control. Small interfering RNAs (siRNAs) were transfected with Lipofectamine RNAi/Max (Invitrogen-Life Technologies, Grand Island, NY). After 24 h, the cells were serum starved and used for the indicated experiments.
Rac1-GTP pulldown assays.
Cells were serum starved for 24 h and then stimulated with HRG (10 ng/ml; 16 h). HRG was then removed by extensive washing with serum-free medium. At the indicated times (0 to 8 h), Rac1-GTP levels were determined using a pulldown assay, as previously described (59). Briefly, the cells were lysed in a buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 5 mM β-glycerophosphate, 1 mM dithiothreitol (DTT), protease inhibitors, and 10 μg/ml of glutathione S-transferase (GST)–PBD (the Rac/Cdc42 binding domain of Pak1). The lysates were cleared by centrifugation (10 min at 4°C; 13,000 × g) and incubated with glutathione-Sepharose 4B beads (GE Healthcare, Mickleton, NJ) for 45 min at 4°C. After centrifugation, the beads were washed twice with the pulldown buffer and run on SDS-PAGE gels. Rac1 was detected by Western blotting using an anti-Rac1 antibody.
Cell migration.
After serum starvation (24 h), the cells were stimulated with HRG (10 ng/ml; 16 h), harvested with 1 mM EDTA, and suspended in 0.1% BSA-DMEM. Cells (3 × 104 cells/well) were seeded in the upper compartment of a Boyden chamber (NeuroProbe, Gaithersburg, MD). A 12-μm-pore-size polycarbonate filter (NeuroProbe) coated overnight with type IV collagen in cold phosphate-buffered saline (PBS) was used to separate the upper and lower compartments. In the lower chamber, we used 0.1% BSA-DMEM with or without SDF-1 (100 ng/ml). After 16 h of incubation at 37°C, the nonmigrating cells on the upper side of the membrane were wiped off the surface. Migrating cells on the lower side of the membrane were fixed, stained with Diff Quik stain set (Dade Behring-Siemens, Malvern, PA), and counted by contrast microscopy in 5 independent fields. In some experiments, 10 μg/ml CXCR4 blocking antibody (MAB170/12G5; R&D Systems) or a control isotype antibody was added to the top chamber.
HIF-1α immunocytochemistry.
Cells on coverslides were serum starved for 24 h, stimulated with HRG (10 ng/ml) for different times, and fixed with 4% formaldehyde in PBS. For staining, we used an anti-HIF1α antibody (BD Biosciences; 1:150 dilution; 90-min incubation), followed by a Cy3-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch, West Grove, PA; 1:1,500; 90-min incubation). The cells were counterstained with DAPI (4′,6′-diamidino-2-phenylindole; 1 μg/ml; 20 min). The slides were mounted using Vectashield, and the cells were visualized with a Nikon TE2000-U fluorescence microscope.
FACS analysis.
Cells were harvested with 1 mM EDTA, suspended in ice-cold staining buffer (1% BSA in PBS), and stained with an anti-CXCR4 antibody (1:200) (BD Biosciences) or isotype control antibody for 60 min at 4°C. Samples were analyzed on a FACScan flow cytometer (BD Biosciences).
Generation of a CXCR4 promoter luciferase reporter and mutants.
The CXCR4 gene promoter (bp −2625 to −71) was amplified by PCR from human genomic DNA obtained from MCF-7 cells using Platinum Taq DNA polymerase high fidelity (Invitrogen) and the following primers: 5′-TGGAATTTCAGATGTGGATGAACC (forward) and 5′-CTGCCGCAGCCAACAAACTGAAGT (reverse). The PCR product was cloned into the TOPO-TA pCR2.1 cloning vector (Invitrogen), digested with KpnI and XhoI restriction enzymes, and subcloned into the pGL3-Basic luciferase reporter vector (Promega, Madison, WI). All the constructs were confirmed by DNA sequencing.
Mutations in putative hypoxia-responsive element (HRE) sites in the CXCR4 promoter reporter (GCGTG, in the HRE consensus core, to ATACA) were introduced with the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). The following primers were used for PCR: for HRE-1 (bp −1376 to −1372), we used 5′-GGGTCCGTGTCGCGACGTGTATGCCTCGGTCCCAG (forward) and 5′-CTGGGACCGAGGCATACACGTCGCGACACGGACCC (reverse); for HRE-2 (bp −950 to −946), we used 5′-CAACGCCTAGAACAGTATACAGCACGCAGTTCGTCC (forward) and 5′-GGACGAACTGCGTGCTGTATACTGTTCTAGGCGTTG (reverse); for HRE-3 (bp −128 to −124), we used 5′-GCGCCGCGCTCGGAATACATTTTTATAAAAGTCCG (forward) and 5′-CGGACTTATAAAAATGTATTCCGAGCGCGGCGC (reverse). All the mutant constructs were confirmed by DNA sequencing.
Luciferase promoter studies.
Cells in 12-well plates were cotransfected with 450 ng of the CXCR4 reporter constructs or empty vector and 50 ng of the Renilla luciferase expression vector pRL-TK (Promega), using Lipofectamine 2000 (Invitrogen). After 24 h, the cells were serum starved for 24 h and treated with 10 ng/ml HRG or vehicle for 12 h. The cells were then lysed with passive lysis buffer (Promega), and luciferase activity was determined in cell extracts using the Dual-Luciferase reporter assay system (Promega). Each experiment was performed in triplicate. The results were normalized to Renilla luciferase activity and expressed as relative luciferase units (RLU).
ChIP assay.
The chromatin immunoprecipitation (ChIP) assay was performed as described previously (60, 61). Briefly, 2 × 106 cells were fixed in 1% formaldehyde for 10 min to cross-link DNA with associated proteins. The reaction was terminated by the addition of 125 mM glycine. After washing, the cells were resuspended in a cell lysis buffer {5 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 8.0, 85 mM KCl, 0.5% NP-40, and protease inhibitors} and incubated on ice for 10 min. Nuclear pellets were isolated by centrifugation (4,000 rpm; 5 min) and sonicated (10 s; 10 times). The DNA was fragmented in a range of 200 to 1,000 bp. Ten nanograms of chromatin obtained after sonication was diluted in ChIP buffer (16.7 mM Tris-HCl, pH 8.1, 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, and 167 mM NaCl) and incubated overnight at 4°C with 2 μg of anti-HIF-1α antibody (AF1935; R&D Systems) or normal goat IgG (AB108C; R&D Systems), followed by 1 h of incubation with protein A/protein G (50% each)-agarose beads previously blocked with salmon sperm DNA (Stratagene). After centrifugation (800 rpm; 1 min), 20 μl of the supernatant was saved as an input control. The beads were sequentially washed with a low-salt immune complex wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, and 150 mM NaCl), a high-salt immune complex wash buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl, pH 8.1, and 500 mM NaCl), an LiCl immune complex wash buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, and 10 mM Tris-HCl, pH 8.1), and TE buffer (10 mM Tris-HCl, pH 8.1, 1 mM EDTA, pH 8.0). Protein-DNA complexes were eluted in a buffer containing 1% SDS and 0.1 M NaHCO3. Cross-linking was reversed by incubation with 200 mM NaCl (65°C overnight), followed by incubation with 40 mM Tris-HCl, pH 6.5, 10 mM EDTA, and 20 μg of proteinase K (45°C; 2 h). DNA was then extracted with the QIAquick PCR purification kit (Qiagen) and analyzed by PCR using Ampli-Taq Gold DNA polymerase (Invitrogen). As primers for ChIP analysis (HRE-1 site), we used 5′-GAGTGCAGTCTGGGCAATCC (forward) and 5′ CGGGCGTCTTCCACGATTTTG (reverse). As a positive control, we used primers designed against a known HRE site in the VEGFA promoter (62): 5′ CCTCAGTTCCCTGGCAACATCTG (forward) and 5′-GAAGAATTTGGCACCAAGTTTGT (reverse). As a negative control, we used primers designed against the promoter region (bp −1151 to −1002) of the β-actin gene (ACTB): 5′-CCCTCCTCCTCTTCCTCAAT (forward) and 5′-AAAGGCAACTTTCGGAACGG (reverse).
Statistical analysis.
For statistical analysis of data, we used analysis of variance (ANOVA) and Bonferroni's multiple-comparison test, using GraphPad Prism software.
RESULTS
HRG sensitizes breast cancer cells to SDF-1-induced Rac1 activation and motility.
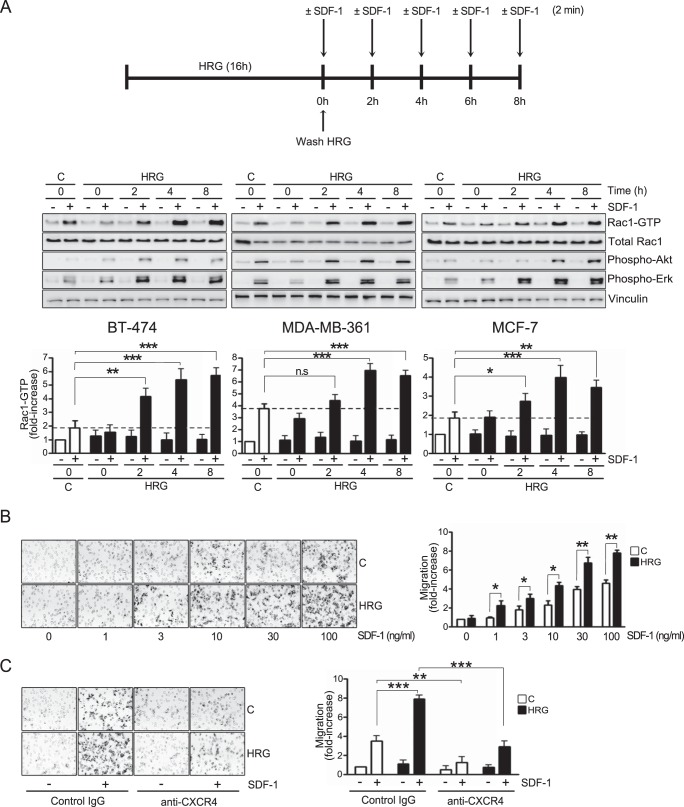
The chemokine SDF-1/CXCL12 and its receptor, CXCR4, have been widely implicated in the progression of breast cancer and other malignancies. CXCR4 expression in breast cancer is associated with aggressiveness and a poor prognosis (48–55). SDF-1 activates the Rac1 small GTPase to promote breast cancer cell motility (40). Since elevated production of the ErbB ligand HRG occurs in a significant proportion of breast tumors (5, 6, 15, 17), we decided to examine the effect of persistent HRG treatment on CXCR4-mediated Rac1 activation and motility in breast cancer cells. To this end, breast cancer cells (BT-474, MDA-MB-361, and MCF-7) were subjected to prolonged treatment with HRG (10 ng/ml; 16 h). Following extensive washing to remove the growth factor, Rac1-GTP levels in response to SDF-1 (100 ng/ml; 2 min) were determined at different times (0 to 8 h) after HRG treatment using a pulldown assay (36, 59). In control (vehicle-treated) BT-474, MDA-MB-361, and MCF-7 cells, SDF-1-activated Rac1 (Fig. 1A, top and middle). Densitometric analysis revealed a 2- to 3.5-fold elevation in Rac1-GTP levels by SDF-1, depending on the cell line (Fig. 1A, bottom). Notably, upon sustained HRG treatment, activation of Rac1 by SDF-1 was much higher in all three cell lines examined. For example, 4 to 8 h after HRG treatment, Rac1 activation by SDF-1 in BT-4T4 cells was ∼4 times higher than that observed in control cells. This sensitization in signaling activation as a consequence of HRG treatment was also evident for established downstream CXCR4 effectors, namely, Akt and Erk (Fig. 1A, top and middle).
FIG 1.
HRG sensitizes breast cancer cells to SDF-1-induced Rac1 activation and motility. (A) BT-474, MDA-MB-361, and MCF-7 cells were incubated for 16 h with either HRG (10 ng/ml) or vehicle (C [control]). After washing, the cells were treated at different times (0 to 8 h) with SDF-1 (100 ng/ml; 2 min), and Rac1-GTP levels were determined using a pulldown assay. Phospho-Akt, phospho-Erk, and total Rac1 levels were determined by Western blotting in cell lysates. (Top and middle) Representative experiments. (Bottom) Densitometric values of Rac1-GTP levels (means and standard errors of the mean [SEM], normalized to total Rac1) expressed as fold increase relative to control cells. (B) Motility in Boyden chambers in response to SDF-1 (0 to 100 ng/ml; 16 h). Experiments were carried out in MCF-7 cells subjected to treatment (16 h) with either HRG (10 ng/ml) or vehicle (control). (Left) Representative images. (Right) Quantification of migrating cells by contrast microscopy in 5 independent fields. The results are expressed as means and standard deviations (SD) of triplicate measurements. Two additional experiments gave similar results. (C) MCF-7 cells (HRG or vehicle treated) were analyzed for cell motility in response to SDF-1 (100 ng/ml; 16 h) in the presence of either an anti-CXCR4 antibody or an isotype control (IgG) antibody (10 μg/ml). (Left) Representative images. (Right) Quantification of migrating cells by contrast microscopy in 5 independent fields. The results are expressed as means and SD of triplicate measurements. Two additional experiments gave similar results. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As Rac1 is a crucial mediator of cell motility (37), we next examined if prolonged HRG treatment could also influence the migration of breast cancer cells in response to CXCR4 stimulation. Serum-starved MCF-7 cells were incubated with HRG (10 ng/ml) or vehicle (control) for 16 h. After extensive washing to remove the HRG, the cells were seeded in a Boyden chamber, and cell migration in response to SDF-1 (1 to 100 ng/ml; 16 h) was examined. SDF-1 caused a concentration-dependent activation of MCF-7 cell motility. Consistent with the effect observed for Rac1 activation, a significant potentiation in the promigratory effect of SDF-1 could be seen in breast cancer cells subjected to HRG treatment (Fig. 1B). As expected, migration induced by SDF-1 was impaired by addition of an anti-CXCR4 blocking antibody in both control and HRG-treated cells but was not affected by control IgG (Fig. 1C). Taken together, these results indicate that prolonged HRG treatment causes significant sensitization of CXCR4-driven motility and signaling.
P-Rex1 mediates SDF-1α-induced Rac1 activation and migration in breast cancer cells.
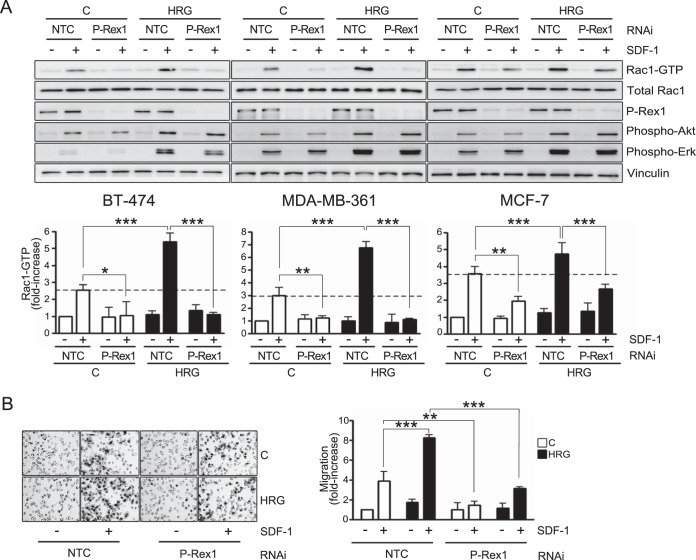
We previously reported that P-Rex1, a PI3K- and Gβγ-dependent Rac-GEF, is aberrantly overexpressed in breast cancer cell lines and tumors of luminal origin. This Rac-GEF mediates Rac1 activation and motility in response to growth factors (40, 42). To assess a potential role of P-Rex1 in Rac1 activation by SDF-1 and in the sensitizing effect of HRG, BT-474, MDA-MB-361, and MCF-7 cells were subjected to P-Rex1 RNAi silencing, which led to >90% reduction in P-Rex1 levels in all cases. An unrelated sequence was used as a nontarget control (NTC). As shown in Fig. 2A (top), P-Rex1 RNAi inhibited Rac1 activation induced by SDF-1. Densitometric analysis showed that in BT-474 and MDA-MB-361 cells, P-Rex1 silencing completely inhibited Rac1 activation in both control and HRG-treated cells, indicating that it is the only Rac-GEF mediating the SDF-1 response in these cell lines. In MCF-7 cells, the effect of P-Rex1 RNAi was partial (Fig. 2A, bottom), arguing that another Rac-GEF(s), in addition to P-Rex1, contributes to Rac1 activation by SDF-1 in the cell line. The similar degrees of inhibition by P-Rex1 RNAi in vehicle- and HRG-treated cells (54% and 57% inhibition, respectively) suggest a lack of compensatory mechanisms by other Rac-GEFs.
FIG 2.
P-Rex1 mediates SDF-1-induced activation of Rac1 and motility in breast cancer cells. (A) BT-474, MDA-MB-361, and MCF-7 cells were subjected to either P-Rex1 or NTC RNAi and 48 h later incubated for 16 h with either HRG (10 ng/ml) or vehicle (C). Four h after HRG removal, the cells were treated with SDF-1 (100 ng/ml; 2 min), and Rac1-GTP levels were determined using a pulldown assay. Phospho-Akt, phospho-Erk, total Rac1, and P-Rex1 levels were determined by Western blotting in cell lysates. (Top) Representative experiments. (Bottom) Densitometric values of Rac1-GTP levels (means and SEM, normalized to total Rac1) expressed as fold increase relative to vehicle-treated NTC cells (minus SDF-1). (B) MCF-7 cells subjected to either P-Rex1 or NTC RNAi were treated for 16 h with either HRG (10 ng/ml) or vehicle (C). Motility in response to SDF-1 (100 ng/ml; 16 h) was determined using a Boyden chamber. (Left) Representative images. (Right) Quantification of migrating cells by contrast microscopy in 5 independent fields. The results are expressed as means and SD of triplicate measurements. Two additional experiments gave similar results. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As with the Rac response, P-Rex1 RNAi also caused a substantial inhibition of MCF-7 cell migration in both vehicle- and HRG-treated cells (Fig. 2b). These results indicate that P-Rex1 is an essential mediator of Rac1 activation and motility induced by SDF-1 in breast cancer cells.
HRG upregulates CXCR4 surface expression in breast cancer cells.
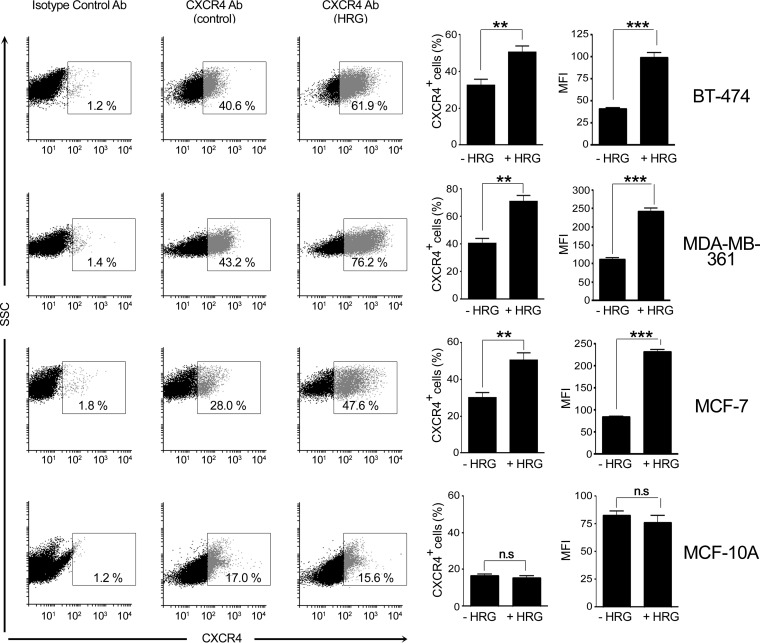
We speculated that the enhanced SDF-1 response in HRG-treated cells might involve changes in CXCR4 expression. To test this hypothesis, we determined CXCR4 levels using flow cytometry. BT-474, MDA-MB-361, and MCF-7 cells were treated with either HRG (16 h; 10 ng/ml) or vehicle, collected, and incubated with either an anti-CXCR4 antibody or an isotope control antibody. As the cells were not permeabilized, this approach would measure only surface CXCR4 receptors. We observed that HRG treatment caused a significant increase in CXCR4 surface expression in all three cell lines. Changes were observed both in the number of CXCR4-positive cells and in the mean fluorescence intensity (Fig. 3), which reflects an increase in the number of surface CXCR4 receptors per cell. On the other hand, HRG failed to induce CXCR4 expression in normal MCF-10A cells. These results suggest that sensitization of breast cancer cells to SDF-1 by sustained HRG treatment may be related to elevated availability of CXCR4 to ligand.
FIG 3.
HRG upregulates CXCR4 surface expression in breast cancer cells. BT-474, MDA-MB-361, and MCF-7 breast cancer cells or normal MCF-10A cells were treated for 16 h with either HRG (10 ng/ml) or vehicle (control). Cells were collected and incubated with either anti-CXCR4 or isotype control (IgG) antibody, as described in Materials and Methods. CXCR4 expression was determined by flow cytometry. (Left) Representative dot plots for CXCR4 surface expression. (Middle) Percentages of CXCR4-positive cells. (Right) Mean fluorescence intensities (MFI) for CXCR4 expression. The results are expressed as means and SEM of the results of 3 individual experiments. **, P < 0.01; ***, P < 0.001; n.s, not significant.
HRG sensitization of the CXCR4/P-Rex1/Rac1 axis is mediated by HIF-1α.
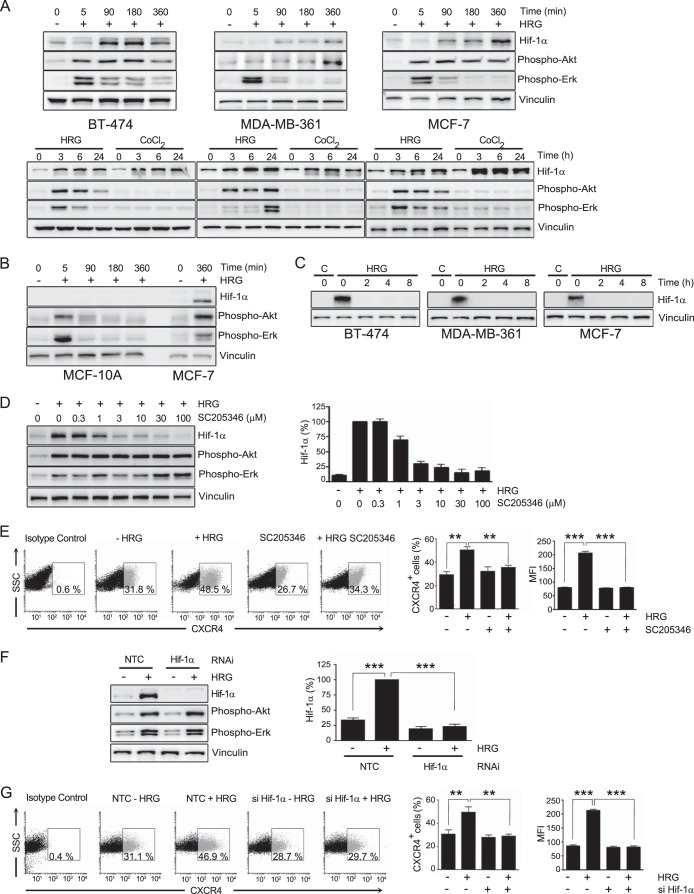
To further investigate the mechanisms involved in the potentiation of CXCR4 responses by HRG in breast cancer cells, we turned our attention to HIF-1α, a member of the HIF family of transcription factors. HIF-1α is known to be induced by hypoxia, leading to transcriptional activation of genes implicated in various aspects of tumor progression, namely, proliferation, migration, invasion, angiogenesis, and metabolic adaptation (63, 64); however, it can also be induced by growth factors, including ErbB ligands (65, 66). We found that HRG caused strong time-dependent upregulation of HIF-1α expression in BT-474, MDA-361, and MCF-7 cells. As a positive control, we used CoCl2, an agent that causes HIF-1α stabilization (66). HIF-1α induction was readily observed 90 min after HRG treatment (Fig. 4A, top) and was sustained for at least 24 h (Fig. 4A, bottom). On the other hand, HRG did not induce HIF-1α expression in MCF-10A cells (Fig. 4B). Consistent with its short half-life, HIF-1α expression in breast cancer cells returned to basal levels 2 h after removing HRG from the medium (Fig. 4C).
FIG 4.
HIF-1α mediates CXCR4 induction by HRG in breast cancer cells. (A) Induction of HIF-1α by HRG. BT-474, MDA-MB-361, and MCF-7 breast cancer cells were incubated with HRG (10 ng/ml) for different times. HIF-1α, phospho-Akt, and phospho-Erk were determined by Western blotting in cell lysates. (Top) Short-term incubation with HRG. (Bottom) Long-term incubation with HRG. As a positive control for HIF-1α induction, CoCl2 (100 μM) was used. (B) HRG (10 ng/ml) does not induce HIF-1α in MCF-10A cells. (C) Breast cancer cells were incubated with HRG (10 ng/ml; 16 h) or vehicle (control). After washing, cells were lysed at different times (0 to 8 h), and HIF-1α expression was determined by Western blotting. (D) Effect of the HIF-1α inhibitor SC205346 (0.3 to 100 μM, added 1 h before and during HRG treatment) on HIF-1α induction by HRG (10 ng/ml; 6 h) in MCF-7 cells. (Left) Representative experiment. (Right) Densitometric values of HIF-1α expression levels (means and SEM of the results of 3 independent experiments) expressed as percentages relative to cells with HRG treatment. (E) MCF-7 cells were treated for 16 h with either HRG (10 ng/ml) or vehicle in the presence or absence of SC205346 (3 μM), and CXCR4 was determined by flow cytometry. (Left) Representative dot plots for CXCR4 surface expression. (Middle) Percentages of CXCR4-positive cells. (Right) MFI for CXCR4 expression. The results are expressed as means and SEM of the results of 3 individual experiments. (F) MCF-7 cells were subjected to either HIF-1α or NTC RNAi. After 48 h, the cells were treated for 16 h with either HRG (10 ng/ml) or vehicle. The cell lysates were subjected to Western blotting with the indicated antibodies. (Left) Representative experiment. (Right) Densitometric analysis of HIF-1α expression, expressed as percentages relative to cells with HRG treatment. (G) Effect of HIF-1α RNAi on CXCR4 surface expression. MCF-7 cells were treated for 16 h with either HRG (10 ng/ml) or vehicle, and CXCR4 was determined by flow cytometry. (Left) Representative dot plots for CXCR4 surface expression. (Middle) Percentages of CXCR4-positive cells. (Right) Mean fluorescence intensities for CXCR4 expression. The results are expressed as means and SEM of 3 individual experiments. **, P < 0.01; ***, P < 0.001.
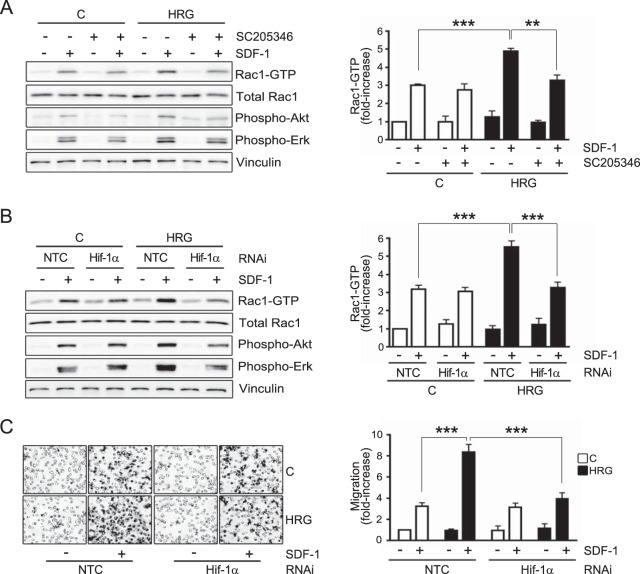
To determine if HIF-1α is implicated in CXCR4 induction by HRG, we first used the HIF-1α inhibitor SC205346 (67). As shown in Fig. 4D, SC205346 inhibited HIF-1α accumulation in response to HRG in a dose-dependent manner. Most remarkably, flow cytometry analysis revealed that SC205346 prevented CXCR4 surface expression upregulation caused by HRG treatment, as it affects the induction in the number of CXCR4-positive cells, as well as in the number of CXCR4 surface receptors per cell (Fig. 4E). As a second approach to establish the involvement of HIF-1α in the HRG effect, we used HIF-1α RNAi. We found that the induction of HIF-1α by HRG was essentially abolished in MCF-7 cells subjected to HIF-1α RNAi, whereas HIF-1α still accumulated in response to HRG in cells transfected with an NTC RNAi duplex (Fig. 4F). Similar to the effect of the HIF-1α inhibitor, RNAi silencing of HIF-1α inhibited CXCR4 induction caused by HRG treatment in MCF-7 cells (Fig. 4G). Therefore, CXCR4 upregulation by HRG was causally associated with the induction of HIF-1α by the growth factor.
Once we established the requirement for HIF-1α in CXCR4 induction by HRG, we reasoned that the enhanced Rac1 activation as a consequence of treatment with the growth factor should also be dependent on HIF-1α. MCF-7 cells subjected to HRG or vehicle (control) treatment were incubated with the HIF-1α inhibitor SC205346 (1 h before and during HRG treatment), and Rac-GTP levels in response to SDF-1 were then determined. Rac1 activation by SDF-1 was not affected by the HIF-1α inhibitor in control cells. On the other hand, in cells subjected to HRG treatment, the sensitization in Rac1 activation was abolished by SC205346 (Fig. 5A). Similar results were observed upon silencing HIF-1α induction using RNAi (Fig. 5B). Consistent with these results, the sensitization in the promotile activity of SDF-1 caused by HRG treatment was lost in cells subjected to HIF-1α RNAi (Fig. 5C). Taken together, these results indicate that the magnitude of the CXCR4-driven motile and signaling responses in breast cancer cells is regulated by HRG-mediated induction of HIF-1α.
FIG 5.
HIF-1α mediates HRG-induced sensitization of Rac1 activation and motility. (A) MCF-7 cells were incubated for 16 h with either HRG (10 ng/ml) or vehicle (control) in the presence of the HIF-1α inhibitor SC205346 (3 μM, added 1 h before and during HRG or vehicle incubation). Four hours after HRG removal, the cells were treated with SDF-1 (100 ng/ml; 2 min), and Rac1-GTP levels were determined using a pulldown assay. (B) Rac1 activation by SDF-1 was determined in MCF-7 cells transfected with either HIF-1α or nontarget control RNAi duplexes. (A and B) (Left) Representative experiments. (Right) Densitometric values of Rac1-GTP levels (means and SEM, normalized to total Rac1; n = 3), expressed as fold increase relative to vehicle-treated NTC cells (minus SDF-1). (C) MCF-7 cells subjected to either HIF-1α or NTC RNAi were treated for 16 h with either HRG (10 ng/ml) or vehicle. Motility in response to SDF-1 (100 ng/ml; 16 h) was determined in a Boyden chamber. Shown is the effect of HIF-1α RNAi on MCF-7 cell motility. (Left) Representative images. (Right) Quantification of migrating cells by contrast microscopy in 5 independent fields. The results are expressed as means and SD of triplicate measurements. Two additional experiments gave similar results. C, control (vehicle). **, P < 0.01; ***, P < 0.001.
HIF-1α and CXCR4 upregulation by HRG is mediated by ErbB3 and independent of ErbB4.
HRG is a specific ligand for ErbB3 and ErbB4 receptors. We previously reported that both ErbB receptors become activated in response to HRG in breast cancer cells, as evidenced by receptor autophosphorylation (35). Based on the involvement of ErbB3 in HRG-induced cell motility (35), we speculated that this ErbB receptor mediates the sensitization of SDF-1 responses by HRG. To test this hypothesis, we used RNAi to specifically silence ErbB3 or ErbB4 expression from MCF-7 cells (∼100% depletion, as judged by Western blotting) (Fig. 6A, left). When we assessed the induction of HIF-1α by HRG, we found that silencing ErbB3 abolished this response. On the other hand, ErbB4 RNAi depletion had no effect on HIF-1α induction (Fig. 6A, right). Activation of Akt and Erk by HRG was also impaired upon ErbB3 silencing but was not affected by ErbB4 RNAi depletion (Fig. 6A, left), implying a main role for ErbB3 in driving the activation of these pathways in MCF-7 cells. Reductions in ErbB3 and ErbB4 receptor levels could be noticed as a consequence of HRG treatment, as expected from receptor internalization and degradation in response to the growth factor.
FIG 6.
ErbB3 mediates HRG-induced sensitization of the CXCR4/Rac1 pathway in breast cancer cells. (A) MCF-7 cells were subjected to ErbB3, ErbB4, HIF-1α, or nontarget control RNAi. After 48 h, the cells were incubated for 6 h with either HRG (10 ng/ml) or vehicle (control). The cell lysates were subjected to Western blotting with the indicated antibodies. (Left) Representative experiment. (Right) Densitometric values of HIF-1α expression levels (means and SEM; n = 3) expressed as fold increase relative to cells without HRG treatment. (B) MCF-7 cells subjected to ErbB3, ErbB4, or NTC RNAi were treated for 16 h with either HRG (10 ng/ml) or vehicle (control). CXCR4 expression was determined by flow cytometry. (Left) Representative dot plots for CXCR4 surface expression. (Middle) Percentages of CXCR4-positive cells. (Right) Mean fluorescence intensities for CXCR4 expression. The results are expressed as means and SEM of the results of 3 individual experiments. (C) Effect of ErbB3 or ErbB4 RNAi on Rac1 activation by SDF-1 (100 ng/ml; 2 min) in MCF-7 cells treated with HRG or vehicle. (Left) Representative experiment. (Right) Densitometric values of Rac1-GTP levels (means and SEM, normalized to total Rac1; n = 3) expressed as fold increase relative to control cells, NTC (minus SDF-1). (D) Effect of ErbB3 or ErbB4 RNAi on MCF-7 cell motility in response to SDF-1 (0 to 100 ng/ml; 16 h) as determined with a Boyden chamber. The cells were previously treated for 16 h with either HRG (10 ng/ml) or vehicle (control). (Left) Representative images. (Right) Quantification of migrating cells by contrast microscopy in 5 independent fields. The results are expressed as means and SD of triplicate measurements. Two additional experiments gave similar results. **, P < 0.01; ***, P < 0.001; n.s., not significant.
Given that ErbB3 is the main HRG receptor driving HIF-1α induction, we reasoned that this ErbB receptor drives CXCR4 induction in response to the growth factor. Indeed, flow cytometry analysis showed that ErbB3 RNAi abolished the induction in CXCR4 surface receptors caused by HRG treatment, whereas ErbB4 RNAi had essentially no effect (Fig. 6B). Following the same reasoning, we found that ErbB3 RNAi, but not ErbB4 RNAi, inhibited the sensitizing effect of HRG on SDF-1-induced Rac1 activation (Fig. 6C) and motility (Fig. 6D), thus assigning a fundamental role to ErbB3 in controlling CXCR4 responses in breast cancer cells. As expected, the activation of Rac1 and cell motility by SDF-1 was not affected by silencing ErbB3 or ErbB4 expression in cells that had not been exposed to HRG (vehicle treated).
HRG controls CXCR4 transcriptional activation in breast cancer cells via HIF-1α.
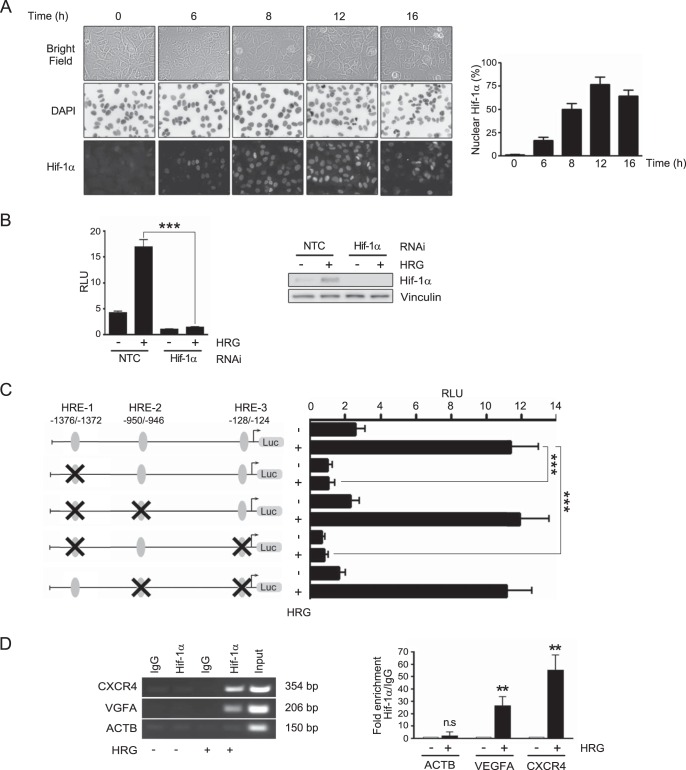
Nuclear accumulation of HIF-1α occurs in response to hypoxia, as well as other stimuli, including growth factors, and is required for the transcriptional activation of genes via binding to HREs in target genes (66). We asked if treating breast cancer cells with HRG leads to elevations in nuclear HIF-1α, which was assessed by immunocytochemistry. Figure 7A shows a significant increase in HIF-1α nuclear staining, which became evident at 6 h after HRG treatment and peaked at ∼12 h.
FIG 7.
Transcriptional activation of the human CXCR4 promoter by HRG is mediated by HIF-1α. (A) MCF-7 cells were treated with HRG (10 ng/ml) for the indicated times, fixed, and subjected to HIF-1α immunocytochemistry. The cells were counterstained with DAPI. (Right) Representative experiment. (Left) Quantification of cells with nuclear HIF-1α staining. (B) MCF-7 cells subjected to either HIF-1α or NTC RNAi were cotransfected with the pGL3-CXCR4 promoter construct and the Renilla luciferase expression vector pRL-TK (for normalization). The cells were treated for 12 h with either HRG (10 ng/ml) or vehicle, and luciferase activity was determined. A Western blot for HIF-1α is shown. (C) Luciferase reporter activities of mutated pGL3-CXCR4 promoter constructs. HRE-1, -2, and -3 sites are indicated with ovals. Mutated sites are marked with an X. (B and C) The data are expressed as means and SEM of three independent experiments. (D) ChIP assay for the HRE-1 site in the CXCR4 promoter in MCF-7 cells. The cells were treated for 12 h with either HRG (10 ng/ml) or vehicle. As a positive control, we used a region encompassing an HRE present in the VEGFA promoter. The ACTB promoter was used as a negative control. The sizes of the expected bands are indicated in each case. (Left) Representative ChIP assay. (Right) Densitometric analysis of HIF-1α binding. The data are presented as fold enrichment (HRG/vehicle) and expressed as means and SEM of the results of 3 independent experiments. **, P < 0.01; ***, P < 0.001.
CXCR4 has been previously defined as a hypoxia-inducible gene in cancer cells and a bona fide HIF-1α effector (68, 69). In order to determine if HRG stimulates transcriptional activation of the CXCR4 gene via HIF-1α, we pursued a gene promoter analysis. A 2.6-kb fragment of the human CXCR4 promoter was cloned by PCR from genomic DNA obtained from MCF-7 cells and subcloned into the pGL3-Basic luciferase reporter vector. The resulting construct was cotransfected, together with the pRL-TK Renilla luciferase expression vector (for normalization), into MCF-7 cells, which were then subjected to treatment with either HRG (10 ng/ml; 12 h) or vehicle and analyzed for luciferase reporter activity. As shown in Fig. 7B, HRG caused significant induction (∼4-fold) in luciferase activity. In order to determine if the induction was caused by HIF-1α, we carried out similar experiments in MCF-7 cells subjected to HIF-1α RNAi silencing (Fig. 4E). Complete inhibition of CXCR4 reporter activation by HRG was observed in cells subjected to HIF-1α RNAi depletion relative to cells transfected with NTC RNAi, thus indicating the involvement of HIF-1α in gene induction by the growth factor (Fig. 7B).
Analysis of the 2.6-kb CXCR4 promoter fragment revealed six sites containing the consensus core HRE sequence RCGTG (where R is G or A) (66). These sites were located in positions −1802 to −1798, −1376 to −1372, −979 to −975, −950 to −946, −944 to −940, and −128 to −124 (the positions are numbered according to the sequence NC_000002.12 in the NCBI database). To narrow down the element(s) in the CXCR4 gene involved in the HRG response, we focused on 3 HRE sequences previously studied in the context of the hypoxic response, located in positions −1376 to −1372 (68), −950 to −946 (referred to as positions −1725 to −1721 in reference 70), and −128 to −124 (referred to as positions −29 to −25 in reference 71). These sites were named HRE-1, HRE-2, and HRE-3, respectively. The HRE sequence GCGTG was mutated, and the resulting mutants were transfected into MCF-7 cells and assessed for transcriptional activity in response to HRG. As shown in Fig. 7C, mutation of the HRE-1 site abolished the induction of luciferase reporter activity caused by HRG. Conversely, mutations in HRE-2 and HRE-3 sites did not appreciably affect CXCR4 promoter activity, suggesting the specific involvement of the HRE-1 site in the HRG response mediated by HIF-1α.
To further confirm the relevance of the HRE-1 site to the transcriptional activation of the CXCR4 gene promoter by HRG, we assessed HIF-1α binding using a ChIP assay (Fig. 7D). Using primers specifically designed to amplify the HRE-1 site and an anti-HIF-1α antibody for immunoprecipitation, a band of the expected size (354 bp) was obtained in response to HRG treatment. In contrast, no band could be detected when cells were treated with vehicle. Likewise, no signal could be detected when control IgG was used for immunoprecipitation in both HRG- and vehicle-treated cells. As a positive control, we used primers designed for the amplification of a well-characterized HRE site in the VEGFA gene promoter (62), which gave the expected band (206 bp) with the anti-HIF-1α antibody but no band with control IgG. Additionally, we used as a negative control an unrelated sequence in the β-actin gene (ACTB) promoter (bp −1151 to −1002), which, as expected, did not give any band with either an anti-HIF-1α antibody or control IgG. Thus, HIF-1α binds to the HRE-1 site in HRG-treated cells. Altogether, results from luciferase reporter assays and ChIP indicated that the HRE-1 site located in positions −1376 to −1372 in the human CXCR4 promoter is responsible for HIF-1α-mediated transcriptional activation of the gene in response to HRG in breast cancer cells.
DISCUSSION
HRG functions as an effective ligand for ErbB3 and ErbB4 and is highly expressed in approximately half of breast tumors (5, 6, 22, 72). Increasing evidence links HRG/ErbB3 signaling to mammary tumorigenesis and metastasis. In addition, studies have assigned key roles to autocrine HRG-mediated activation of ErbB3/ErbB2 in sustaining tumor growth and conferring resistance to therapeutic agents (15, 17, 25, 26, 28, 72–74). The fact that ErbB3 signals primarily through activation of the PI3K pathway (1, 7, 8) invariably argues for a prominent role of the receptor in shifting the balance toward enhanced cell survival, invasiveness, and the maintenance of the tumor phenotype. Therefore, understanding the mechanisms by which ErbB3 receptors drive their phenotypic responses is of the utmost importance.
In the present study, we identified ErbB3 as a key regulator of CXCR4, a chemokine receptor widely implicated in breast cancer progression and metastasis. Our results clearly show that prolonged HRG treatment induces the expression of CXCR4 in breast cancer cells, leading to enhanced motility signaling via the P-Rex1/Rac1 pathway in response to activation by its ligand, SDF-1. Early studies reported that CXCR4 is upregulated in breast tumors; moreover, CXCR4 expression is enriched in metastatic breast cancer cells and confers invasive properties, and it is considered a marker of poor patient prognosis (50–55). The relevance of the SDF-1/CXCR4 axis in metastasis has also been demonstrated in other cancer types, including prostate, lung, and pancreatic cancers (75). Expression of CXCR4 is controlled by a number of mechanisms, including transcriptional control, posttranslational modifications, and oligomerization, and such mechanisms can be dysregulated in cancer cells. In hypoxic regions of tumors, HIF-1α, a transcription factor that is sensitive to oxygen concentrations, induces the expression of genes linked to growth, survival, and metastasis, including CXCR4 (63, 69). Our results indicate that HIF-1α plays a primary role in CXCR4 induction by HRG in breast cancer cells. HIF-1α has a very short half-life, and protein stability is an important regulator of its expression. Indeed, under reduced oxygen availability, HIF-1α degradation is inhibited and the protein accumulates (63). However, and in agreement with the results of Laughner et al. (65), we did not find any changes in the HIF-1α protein half-life in MCF-7 cells as a consequence of HRG treatment (data not shown). Most notably, oncogenic alterations (such as ErbB2 gain of function), activation of growth factor receptors (such as EGFR and insulin-like growth factor 1 [IGF-1] receptor), and activation of signaling pathways (such as PI3K and ERK/MAPK) increase the rate of HIF-1α synthesis (63). Our results clearly show that HRG treatment stimulates HIF-1α-mediated transcriptional activation of the CXCR4 gene, as demonstrated using luciferase reporter assays. Site-directed mutagenesis analysis of putative HRE sites in the CXCR4 gene promoter and validation with ChIP established that the HRE site in positions −1376 to −1372 (HRE-1) mediates the transcriptional induction of CXCR4 by HRG. This site has been previously shown to be critical for hypoxia/HIF-1α-inducible reporter activity in renal cell carcinoma cells (68). The site in positions −950 to −946 (HRE-2) has been previously identified in ovarian cancer cells as a hypoxia-regulated site that binds HIF-1α (70), but we found it dispensable in the context of HRG stimulation. Likewise, the reported HRE site in positions −128 to −124 (HRE-3) is not implicated in HRG-mediated transcriptional activation of CXCR4. This HIF-1α binding site has been previously characterized as a regulator of acidosis but not hypoxic responsiveness in endothelial cells (71). Whereas we cannot fully exclude the involvement of other putative sites where HIF-1α binds in the CXCR4 gene promoter, the fact that targeted mutation of HRE-1 totally obliterated the transcriptional response induced by HRG suggests that this may be the sole HIF-1α-responsive element responsive to the growth factor. It is conceivable that the HRE is also responsive to other growth factors implicated in breast cancer progression that are known to induce HIF-1α expression, such as EGF, IGF, and TGF-β (66).
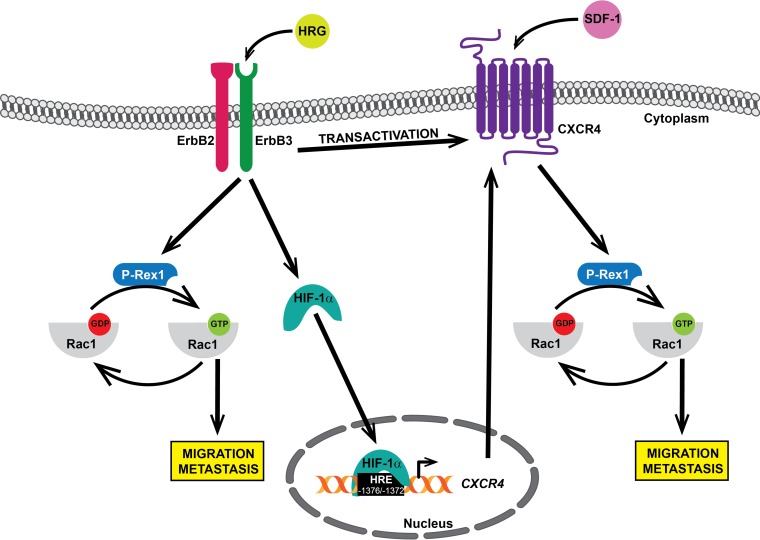
Our studies substantiate the central role of the Rac pathway as a downstream effector of receptors driving breast cancer progression. Rac1 is well known for promoting actin reorganization and conferring a motile phenotype on cancer cells (37). Inhibition of Rac1 function in breast cancer cells impairs the formation of membrane ruffles and lamellipodia by growth factors (including EGF and HRG) and cytokines (such as SDF-1), resulting in reduced cell motility (35, 40, 76). Although constitutively active Rac mutant variants have been described in cancer (77, 78), hyperactivation of Rac1 is primarily the consequence of disproportionate inputs from receptors and their effectors, such as PI3K, which lead to enhanced activation of Rac-GEFs to promote a metastatic phenotype (79). The mechanisms by which tyrosine kinase receptors and GPCRs signal to Rac1 in breast cancer cells are becoming increasingly understood. In luminal breast cancer cells, it became clear that the Rac-GEF P-Rex1 is the main mediator of Rac1 activation in response to stimulation of membrane receptors, including ErbB receptors, IGF-1 receptor, platelet-derived growth factor receptor, and CXCR4 (36, 40, 43, 46, 47, 80). Accordingly, silencing P-Rex1 from breast cancer cells hampers motile responses to GPCR and tyrosine kinase receptor ligands (40). P-Rex1 is activated by a dual mechanism that involves the PI3K product PIP3 and Gβγ subunits released from heterotrimeric Gi proteins (45). In the case of ErbB receptors, the Gβγ component of P-Rex1 activation in luminal breast cancer cells is provided by CXCR4, a Gi-coupled receptor (40). Indeed, we previously reported that HRG stimulation of ErbB3 transactivates CXCR4, as reflected by elevation in tyrosine and serine phosphorylation in this GPCR, as well as rapid recruitment of β-arrestin. This transactivation of CXCR4 occurs in a ligand-independent manner (40). Transactivation of ErbB receptors by SDF-1 stimulation has also been reported (81, 82), suggesting a complex bidirectional interrelationship between specific membrane receptors implicated in tumorigenesis and metastasis. We propose that the induction of CXCR4 by HRG/ErbB3 identified in this study represents another mechanism that takes part in the control of P-Rex1/Rac1 activation. Most notably, HRG treatment sensitizes breast cancer cells to Rac1 activation and motility in response to SDF-1. Therefore, we propose a model in which HRG contributes to breast cancer cell motility by at least three different mechanisms: direct activation of P-Rex1/Rac1 via ErbB3 (40, 43), transactivation of CXCR4 in an SDF-1-independent manner (40), and upregulation of CXCR4 (this study). A model summarizing these mechanisms is depicted in Fig. 8.
FIG 8.
Interplay between ErbB3 and CXCR4 for the activation of Rac1 and breast cancer cell motility. ErbB3 stimulation with HRG activates the P-Rex1/Rac1 pathway through CXCR4-independent and CXCR4-dependent mechanisms.
Lastly, our studies highlight the role of ErbB3 in breast cancer cell motility. HRG binds with high affinity to ErbB3 and ErbB4, and both receptors become activated in response to this ligand. Whereas breast cancer cell motility and Rac1 activation by HRG are sensitive to ErbB3 RNAi silencing, ErbB4 is, on the other hand, dispensable (35). The role of ErbB4 in breast cancer cell motility is less understood, largely due to the complexities arising from the expression of spliced variants and proteolytically cleaved receptors with distinctive intracellular localization. Nonetheless, ErbB4 can mediate migratory responses via alternative mechanisms, such as those involving the transcriptional coactivator YAP and protection of EGFR-induced degradation by the E3 ubiquitin ligase c-Cbl (34, 83). Together with our previous analysis (40), the results from this study unquestionably support a distinctive role for ErbB3 in induction and transactivation of CXCR4 via HIF-1α.
In summary, our study emphasizes the complexities of ErbB signaling in breast cancer cells by establishing a novel link between ErbB3 receptor stimulation and the activation of the P-Rex1/Rac1 pathway via induction of CXCR4, an effect transcriptionally mediated by HIF-1α. In addition to the mechanistic and signaling implications of our studies, it is important to highlight the relevance of these pathways for breast cancer therapy. CXCR4 antagonists inhibit tumorigenic and metastatic phenotypes, including in breast cancer models, and are currently under clinical evaluation for cancer treatment (84). Likewise, inhibitors of the Rac pathway, including P-Rex1 inhibitors, are currently under study as anticancer agents (85–87). Thus, targeting effectors of ErbB receptors represents a potential alternative or complement to ErbB-targeted therapies or other therapeutic modalities to treat breast cancer patients.
ACKNOWLEDGMENT
This work was supported by grant CA139120 from the NIH to M.G.K.
REFERENCES
- 1.Olayioye MA, Neve RM, Lane HA, Hynes NE. 2000. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Citri A, Skaria KB, Yarden Y. 2003. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res 284:54–65. doi: 10.1016/S0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- 3.Citri A, Yarden Y. 2006. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7:505–516. [DOI] [PubMed] [Google Scholar]
- 4.Wilson KJ, Gilmore JL, Foley J, Lemmon MA, Riese DJ II. 2009. Functional selectivity of EGF family peptide growth factors: implications for cancer. Pharmacol Ther 122:1–8. doi: 10.1016/j.pharmthera.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suo Z, Risberg B, Karlsson MG, Villman K, Skovlund E, Nesland JM. 2002. The expression of EGFR family ligands in breast carcinomas. Int J Surg Pathol 10:91–99. doi: 10.1177/106689690201000202. [DOI] [PubMed] [Google Scholar]
- 6.Revillion F, Lhotellier V, Hornez L, Bonneterre J, Peyrat JP. 2008. ErbB/HER ligands in human breast cancer, and relationships with their receptors, the bio-pathological features and prognosis. Ann Oncol 19:73–80. [DOI] [PubMed] [Google Scholar]
- 7.Stern DF. 2008. ERBB3/HER3 and ERBB2/HER2 duet in mammary development and breast cancer. J Mammary Gland Biol Neoplasia 13:215–223. doi: 10.1007/s10911-008-9083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hynes NE, MacDonald G. 2009. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Arteaga CL, Engelman JA. 2014. ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25:282–303. doi: 10.1016/j.ccr.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momeny M, Saunus JM, Marturana F, McCart Reed AE, Black D, Sala G, Iacobelli S, Holland JD, Yu D, Da Silva L, Simpson PT, Khanna KK, Chenevix-Trench G, Lakhani SR. 2015. Heregulin-HER3-HER2 signaling promotes matrix metalloproteinase-dependent blood-brain-barrier transendothelial migration of human breast cancer cell lines. Oncotarget 6:3932–3946. doi: 10.18632/oncotarget.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellyer NJ. 2001. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem 276:42153–42161. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- 12.Campbell MR, Moasser MM. 2015. HER targeting in HER2-negative breast cancers: looking for the HER3 positive. Clin Cancer Res 21:2886–2888. doi: 10.1158/1078-0432.CCR-14-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu CG, Masoudi H, Leung S, Voduc DK, Gilks B, Huntsman DG, Wiseman SM. 2010. HER-3 overexpression is prognostic of reduced breast cancer survival: a study of 4046 patients. Ann Surg 251:1107–1116. doi: 10.1097/SLA.0b013e3181dbb77e. [DOI] [PubMed] [Google Scholar]
- 14.Suo Z, Risberg B, Kalsson MG, Willman K, Tierens A, Skovlund E, Nesland JM. 2002. EGFR family expression in breast carcinomas. c-erbB-2 and c-erbB-4 receptors have different effects on survival. J Pathol 196:17–25. doi: 10.1002/path.1003. [DOI] [PubMed] [Google Scholar]
- 15.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL. 2007. Human breast cancer cells selected for resistance to trastuzumab in Vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarty A, Rexer BN, Wang SE, Cook RS, Engelman JA, Arteaga CL. 2010. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene 29:5193–5203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CY, Lin Y, Bratman SV, Feng W, Kuo AH, Scheeren FA, Engreitz JM, Varma S, West RB, Diehn M. 2014. Neuregulin autocrine signaling promotes self-renewal of breast tumor-initiating cells by triggering HER2/HER3 activation. Cancer Res 74:341–352. doi: 10.1158/0008-5472.CAN-13-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinstein EJ, Kitsberg DI, Leder P. 2000. A mouse model for breast cancer induced by amplification and overexpression of the neu promoter and transgene. Mol Med 6:4–16. [PMC free article] [PubMed] [Google Scholar]
- 19.Atlas E, Cardillo M, Mehmi I, Zahedkargaran H, Tang C, Lupu R. 2003. Heregulin is sufficient for the promotion of tumorigenicity and metastasis of breast cancer cells in vivo. Mol Cancer Res 1:165–175. [PubMed] [Google Scholar]
- 20.Kim J, Jeong H, Lee Y, Kim C, Kim H, Kim A. 2013. HRG-beta1-driven ErbB3 signaling induces epithelial-mesenchymal transition in breast cancer cells. BMC Cancer 13:383. doi: 10.1186/1471-2407-13-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asrani K, Keri RA, Galisteo R, Brown SA, Morgan SJ, Ghosh A, Tran NL, Winkles JA. 2013. The HER2- and heregulin beta1 (HRG)-inducible TNFR superfamily member Fn14 promotes HRG-driven breast cancer cell migration, invasion, and MMP9 expression. Mol Cancer Res 11:393–404. doi: 10.1158/1541-7786.MCR-12-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seoane S, Montero JC, Ocana A, Pandiella A. 2016. Breast cancer dissemination promoted by a neuregulin-collagenase 3 signalling node. Oncogene 35:2756–2765. doi: 10.1038/onc.2015.337. [DOI] [PubMed] [Google Scholar]
- 23.Tang CK, Perez C, Grunt T, Waibel C, Cho C, Lupu R. 1996. Involvement of heregulin-beta2 in the acquisition of the hormone-independent phenotype of breast cancer cells. Cancer Res 56:3350–3358. [PubMed] [Google Scholar]
- 24.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, Kosaka T, Holmes AJ, Rogers AM, Cappuzzo F, Mok T, Lee C, Johnson BE, Cantley LC, Janne PA. 2007. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 25.Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M, Fujisaka Y, Philips J, Shimizu T, Maenishi O, Cho Y, Sun J, Destro A, Taira K, Takeda K, Okabe T, Swanson J, Itoh H, Takada M, Lifshits E, Okuno K, Engelman JA, Shivdasani RA, Nishio K, Fukuoka M, Varella-Garcia M, Nakagawa K, Janne PA. 2011. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 3:99ra86. doi: 10.1126/scitranslmed.3002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson TR, Lee DY, Berry L, Shames DS, Settleman J. 2011. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell 20:158–172. doi: 10.1016/j.ccr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Lyu H, Huang J, Liu B. 2014. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer 13:105. doi: 10.1186/1476-4598-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX. 2008. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 29.Dey G, Bharti R, Dhanarajan G, Das S, Dey KK, Kumar BN, Sen R, Mandal M. 2015. Marine lipopeptide Iturin A inhibits Akt mediated GSK3beta and FoxO3a signaling and triggers apoptosis in breast cancer. Sci Rep 5:10316. doi: 10.1038/srep10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai MS, Shamon-Taylor LA, Mehmi I, Tang CK, Lupu R. 2003. Blockage of heregulin expression inhibits tumorigenicity and metastasis of breast cancer. Oncogene 22:761–768. doi: 10.1038/sj.onc.1206130. [DOI] [PubMed] [Google Scholar]
- 31.Kang JC, Poovassery JS, Bansal P, You S, Manjarres IM, Ober RJ, Ward ES. 2014. Engineering multivalent antibodies to target heregulin-induced HER3 signaling in breast cancer cells. MAbs 6:340–353. doi: 10.4161/mabs.27658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaborit N, Lindzen M, Yarden Y. 2016. Emerging anti-cancer antibodies and combination therapies targeting HER3/ERBB3. Hum Vaccin Immunother 12:576–592. doi: 10.1080/21645515.2015.1102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wali VB, Gilmore-Hebert M, Mamillapalli R, Haskins JW, Kurppa KJ, Elenius K, Booth CJ, Stern DF. 2014. Overexpression of ERBB4 JM-a CYT-1 and CYT-2 isoforms in transgenic mice reveals isoform-specific roles in mammary gland development and carcinogenesis. Breast Cancer Res 16:501. doi: 10.1186/s13058-014-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haskins JW, Nguyen DX, Stern DF. 2014. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci Signal 7:ra116. doi: 10.1126/scisignal.2005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang C, Liu Y, Lemmon MA, Kazanietz MG. 2006. Essential role for Rac in heregulin beta1 mitogenic signaling: a mechanism that involves epidermal growth factor receptor and is independent of ErbB4. Mol Cell Biol 26:831–842. doi: 10.1128/MCB.26.3.831-842.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Haber C, Kazanietz MG. 2013. Cucurbitacin I inhibits Rac1 activation in breast cancer cells by a reactive oxygen species-mediated mechanism and independently of Janus tyrosine kinase 2 and P-Rex1. Mol Pharmacol 83:1141–1154. doi: 10.1124/mol.112.084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaffe AB, Hall A. 2005. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 38.Rossman KL, Der CJ, Sondek J. 2005. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 6:167–180. [DOI] [PubMed] [Google Scholar]
- 39.Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. 2012. Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal 24:353–362. doi: 10.1016/j.cellsig.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, Luo J, Benovic JL, Klein-Szanto A, Yagi H, Gutkind JS, Parsons RE, Kazanietz MG. 2010. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell 40:877–892. doi: 10.1016/j.molcel.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Citterio C, Menacho-Marquez M, Garcia-Escudero R, Larive RM, Barreiro O, Sanchez-Madrid F, Paramio JM, Bustelo XR. 2012. The rho exchange factors vav2 and vav3 control a lung metastasis-specific transcriptional program in breast cancer cells. Sci Signal 5:ra71. doi: 10.1126/scisignal.2002962. [DOI] [PubMed] [Google Scholar]
- 42.Barrio-Real L, Benedetti LG, Engel N, Tu Y, Cho S, Sukumar S, Kazanietz MG. 2014. Subtype-specific overexpression of the Rac-GEF P-REX1 in breast cancer is associated with promoter hypomethylation. Breast Cancer Res 16:441. doi: 10.1186/s13058-014-0441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montero JC, Seoane S, Ocana A, Pandiella A. 2011. P-Rex1 participates in Neuregulin-ErbB signal transduction and its expression correlates with patient outcome in breast cancer. Oncogene 30:1059–1071. doi: 10.1038/onc.2010.489. [DOI] [PubMed] [Google Scholar]
- 44.Dillon LM, Bean JR, Yang W, Shee K, Symonds LK, Balko JM, McDonald WH, Liu S, Gonzalez-Angulo AM, Mills GB, Arteaga CL, Miller TW. 2015. P-REX1 creates a positive feedback loop to activate growth factor receptor, PI3K/AKT and MEK/ERK signaling in breast cancer. Oncogene 34:3968–3976. doi: 10.1038/onc.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch HC. 2015. Regulation and function of P-Rex family Rac-GEFs. Small GTPases 6:49–70. doi: 10.4161/21541248.2014.973770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, Scofield MA, Dowd FJ, Lin MF, Tu Y. 2009. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene 28:1853–1863. doi: 10.1038/onc.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carretero-Ortega J, Walsh CT, Hernandez-Garcia R, Reyes-Cruz G, Brown JH, Vazquez-Prado J. 2010. Phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 (P-Rex-1), a guanine nucleotide exchange factor for Rac, mediates angiogenic responses to stromal cell-derived factor-1/chemokine stromal cell derived factor-1 (SDF-1/CXCL-12) linked to Rac activation, endothelial cell migration, and in vitro angiogenesis. Mol Pharmacol 77:435–442. doi: 10.1124/mol.109.060400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zlotnik A, Burkhardt AM, Homey B. 2011. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol 11:597–606. doi: 10.1038/nri3049. [DOI] [PubMed] [Google Scholar]
- 49.Ray P, Stacer AC, Fenner J, Cavnar SP, Meguiar K, Brown M, Luker KE, Luker GD. 2015. CXCL12-gamma in primary tumors drives breast cancer metastasis. Oncogene 34:2043–2051. doi: 10.1038/onc.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Stamatoyannopoulos G, Song CZ. 2003. Down-regulation of CXCR4 by inducible small interfering RNA inhibits breast cancer cell invasion in vitro. Cancer Res 63:4801–4804. [PubMed] [Google Scholar]
- 51.Helbig G, Christopherson KW II, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. 2003. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 52.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. 2004. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res 64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 53.Liang Z, Wu H, Reddy S, Zhu A, Wang S, Blevins D, Yoon Y, Zhang Y, Shim H. 2007. Blockade of invasion and metastasis of breast cancer cells via targeting CXCR4 with an artificial microRNA. Biochem Biophys Res Commun 363:542–546. doi: 10.1016/j.bbrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Xu TP, Shen H, Liu LX, Shu YQ. 2013. The impact of chemokine receptor CXCR4 on breast cancer prognosis: a meta-analysis. Cancer Epidemiol 37:725–731. doi: 10.1016/j.canep.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 55.Okuyama Kishima M, de Oliveira CE, Banin-Hirata BK, Losi-Guembarovski R, Brajao de Oliveira K, Amarante MK, Watanabe MA. 2015. Immunohistochemical expression of CXCR4 on breast cancer and its clinical significance. Anal Cell Pathol 2015:891020. doi: 10.1155/2015/891020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. 2004. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Ji R, Li J, Gu Q, Zhao X, Sun T, Wang J, Li J, Du Q, Sun B. 2010. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res 29:16. doi: 10.1186/1756-9966-29-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Burstin VA, Xiao L, Kazanietz MG. 2010. Bryostatin 1 inhibits phorbol ester-induced apoptosis in prostate cancer cells by differentially modulating protein kinase C (PKC) delta translocation and preventing PKCdelta-mediated release of tumor necrosis factor-alpha. Mol Pharmacol 78:325–332. doi: 10.1124/mol.110.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caloca MJ, Wang H, Kazanietz MG. 2003. Characterization of the Rac-GAP (Rac-GTPase-activating protein) activity of beta2-chimaerin, a ‘non-protein kinase C’ phorbol ester receptor. Biochem J 375:313–321. doi: 10.1042/bj20030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deliard S, Zhao J, Xia Q, Grant SF. 19 April 2013. Generation of high quality chromatin immunoprecipitation DNA template for high-throughput sequencing (ChIP-seq). J Vis Exp doi: 10.3791/50286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Gutierrez-Uzquiza A, Garg R, Barrio-Real L, Abera MB, Lopez-Haber C, Rosemblit C, Lu H, Abba M, Kazanietz MG. 2014. Transcriptional regulation of oncogenic protein kinase C (PKC) by STAT1 and Sp1 proteins. J Biol Chem 289:19823–19838. doi: 10.1074/jbc.M114.548446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu P, Kodadek T. 2007. Dynamics of the hypoxia-inducible factor-1-vascular endothelial growth factor promoter complex. J Biol Chem 282:35035–35045. doi: 10.1074/jbc.M707557200. [DOI] [PubMed] [Google Scholar]
- 63.Semenza GL. 2003. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 64.Majmundar AJ, Wong WJ, Simon MC. 2010. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. 2001. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wenger RH, Stiehl DP, Camenisch G. 2005. Integration of oxygen signaling at the consensus HRE. Sci STKE 2005:re12. [DOI] [PubMed] [Google Scholar]
- 67.Lee K, Lee JH, Boovanahalli SK, Jin Y, Lee M, Jin X, Kim JH, Hong YS, Lee JJ. 2007. (Aryloxyacetylamino)benzoic acid analogues: a new class of hypoxia-inducible factor-1 inhibitors. J Med Chem 50:1675–1684. doi: 10.1021/jm0610292. [DOI] [PubMed] [Google Scholar]
- 68.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. 2003. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 69.Busillo JM, Benovic JL. 2007. Regulation of CXCR4 signaling. Biochim Biophys Acta 1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. 2003. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Melchionna R, Romani M, Ambrosino V, D'Arcangelo D, Cencioni C, Porcelli D, Toietta G, Truffa S, Gaetano C, Mangoni A, Pozzoli O, Cappuzzello C, Capogrossi MC, Napolitano M. 2010. Role of HIF-1alpha in proton-mediated CXCR4 down-regulation in endothelial cells. Cardiovasc Res 86:293–301. doi: 10.1093/cvr/cvp393. [DOI] [PubMed] [Google Scholar]
- 72.de Alava E, Ocana A, Abad M, Montero JC, Esparis-Ogando A, Rodriguez CA, Otero AP, Hernandez T, Cruz JJ, Pandiella A. 2007. Neuregulin expression modulates clinical response to trastuzumab in patients with metastatic breast cancer. J Clin Oncol 25:2656–2663. doi: 10.1200/JCO.2006.08.6850. [DOI] [PubMed] [Google Scholar]
- 73.Sheng Q, Liu X, Fleming E, Yuan K, Piao H, Chen J, Moustafa Z, Thomas RK, Greulich H, Schinzel A, Zaghlul S, Batt D, Ettenberg S, Meyerson M, Schoeberl B, Kung AL, Hahn WC, Drapkin R, Livingston DM, Liu JF. 2010. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell 17:298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato Y, Yashiro M, Takakura N. 2013. Heregulin induces resistance to lapatinib-mediated growth inhibition of HER2-amplified cancer cells. Cancer Sci 104:1618–1625. doi: 10.1111/cas.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. 2010. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev 29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang C, Liu Y, Leskow FC, Weaver VM, Kazanietz MG. 2005. Rac-GAP-dependent inhibition of breast cancer cell proliferation by {beta}2-chimerin. J Biol Chem 280:24363–24370. doi: 10.1074/jbc.M411629200. [DOI] [PubMed] [Google Scholar]
- 77.Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Harbeck N, Schmitt M, Lengyel E. 2000. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 19:3013–3020. doi: 10.1038/sj.onc.1203621. [DOI] [PubMed] [Google Scholar]
- 78.Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, Ariyan S, Narayan D, Dutton-Regester K, Capatana A, Holman EC, Bosenberg M, Sznol M, Kluger HM, Brash DE, Stern DF, Materin MA, Lo RS, Mane S, Ma S, Kidd KK, Hayward NK, Lifton RP, Schlessinger J, Boggon TJ, Halaban R. 2012. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barrio-Real L, Kazanietz MG. 2012. Rho GEFs and cancer: linking gene expression and metastatic dissemination. Sci Signal 5:pe43. doi: 10.1126/scisignal.2003543. [DOI] [PubMed] [Google Scholar]
- 80.Campbell MR, Amin D, Moasser MM. 2010. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res 16:1373–1383. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Porcile C, Bajetto A, Barbieri F, Barbero S, Bonavia R, Biglieri M, Pirani P, Florio T, Schettini G. 2005. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res 308:241–253. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 82.Cabioglu N, Summy J, Miller C, Parikh NU, Sahin AA, Tuzlali S, Pumiglia K, Gallick GE, Price JE. 2005. CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activation. Cancer Res 65:6493–6497. doi: 10.1158/0008-5472.CAN-04-1303. [DOI] [PubMed] [Google Scholar]
- 83.Kiuchi T, Ortiz-Zapater E, Monypenny J, Matthews DR, Nguyen LK, Barbeau J, Coban O, Lawler K, Burford B, Rolfe DJ, de Rinaldis E, Dafou D, Simpson MA, Woodman N, Pinder S, Gillett CE, Devauges V, Poland SP, Fruhwirth G, Marra P, Boersma YL, Pluckthun A, Gullick WJ, Yarden Y, Santis G, Winn M, Kholodenko BN, Martin-Fernandez ML, Parker P, Tutt A, Ameer-Beg SM, Ng T. 2014. The ErbB4 CYT2 variant protects EGFR from ligand-induced degradation to enhance cancer cell motility. Sci Signal 7:ra78. doi: 10.1126/scisignal.2005157. [DOI] [PubMed] [Google Scholar]
- 84.Weitzenfeld P, Ben-Baruch A. 2014. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett 352:36–53. doi: 10.1016/j.canlet.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 85.Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ. 2008. Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol 439:111–129. doi: 10.1016/S0076-6879(07)00409-0. [DOI] [PubMed] [Google Scholar]
- 86.Dharmawardhane S, Hernandez E, Vlaar C. 2013. Development of EHop-016: a small molecule inhibitor of Rac. Enzymes 33:117–146. doi: 10.1016/B978-0-12-416749-0.00006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cardama GA, Comin MJ, Hornos L, Gonzalez N, Defelipe L, Turjanski AG, Alonso DF, Gomez DE, Menna PL. 2014. Preclinical development of novel Rac1-GEF signaling inhibitors using a rational design approach in highly aggressive breast cancer cell lines. Anticancer Agents Med Chem 14:840–851. doi: 10.2174/18715206113136660334. [DOI] [PMC free article] [PubMed] [Google Scholar]