Abstract
The potassium homeostatic system is very tightly regulated. Recent studies have shed light on the sensing and molecular mechanisms responsible for this tight control. In addition to classic feedback regulation mediated by a rise in extracellular fluid (ECF) [K+], there is evidence for a feedforward mechanism: Dietary K+ intake is sensed in the gut, and an unidentified gut factor is activated to stimulate renal K+ excretion. This pathway may explain renal and extrarenal responses to altered K+ intake that occur independently of changes in ECF [K+]. Mechanisms for conserving ECF K+ during fasting or K+ deprivation have been described: Kidney NADPH oxidase activation initiates a cascade that provokes the retraction of K+ channels from the cell membrane, and muscle becomes resistant to insulin stimulation of cellular K+ uptake. How these mechanisms are triggered by K+ deprivation remains unclear. Cellular AMP kinase–dependent protein kinase activity provokes the acute transfer of K+ from the ECF to the ICF, which may be important in exercise or ischemia. These recent advances may shed light on the beneficial effects of a high-K+ diet for the cardiovascular system.
Keywords: dietary potassium, potassium adaptation, potassium excretion, kidney, muscle, insulin
INTRODUCTION
Homeostatic control of extracellular fluid (ECF) K+ concentration is critical for normal function of nerve and muscle cells because ECF [K+] is a major determinant of membrane potential (1, 2). In addition, a diet rich in K+ lowers blood pressure, blunts the negative effects of dietary NaCl, and reduces the risk of kidney stones and bone loss (3). In mammals, the ECF [K+] ranges between 3.8 and 5 mM, whereas the intracellular fluid (ICF) [K+] is 120–140 mM (4, 5). Among major electrolytes, K+ has the highest ratio of daily intake to extracellular pool size (i.e., turnover), that is, the most significant homeostatic challenge. To meet this challenge, the K+ homeostatic system is very efficient at clearing plasma K+ after a K+-containing meal and is very efficient at maintaining ECF [K+] during fasting or K+-poor diet, as evidenced by minimal changes in plasma [K+] during these challenges. The components of the K+ homeostatic system are not yet fully understood but include the sensing of K+ intake, the regulation of K+ distribution between the ECF and the ICF, and the regulation of K+ excretion (Figure 1). This review focuses on the significant recent progress in understanding the individual components of the K+ homeostasis system and how they are linked.
Figure 1.
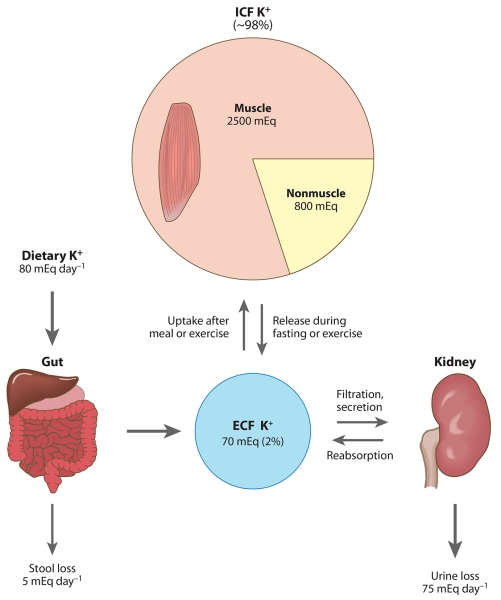
A schematic diagram illustrating daily K+ fluxes into and out of the extracellular fluid (ECF) pool in an average person. Approximately 98% of the body’s K+ is located in the intracellular fluid (ICF), mainly in muscle, and only approximately 2% is located in the ECF. The ECF pool is regulated by input from the gut, output via the kidney and stools, and redistribution between the ECF and the ICF.
SENSING K+ INTAKE: FEEDFORWARD VERSUS FEEDBACK CONTROL OF EXTRACELLULAR FLUID [K+]
Extracellular K+ Homeostasis: General Considerations
Extracellular K+ homeostasis relies on the maintenance of total body K+ content as well as a regulated distribution of K+ between a very large intracellular pool (>90% of total body K+) and a much smaller extracellular pool (~2% total body K+). Total body K+ content is maintained by continuously matching K+ excretion to dietary K+ intake. This task is accomplished by the kidneys, which are responsible for approximately 90% of K+ excretion (6, 7). Thus, the kidneys play a predominant role in the maintenance of day-to-day K+ balance. In concert with renal excretion, rapid shifts in K+ from the ECF to the large ICF pool of K+ are necessary to buffer ECF K+. The importance of the concerted actions of kidney and muscle is best illustrated by considering what happens during a K+-containing meal and during fasting. Total ECF K+ is only ~70 mEq K+, and ingesting a meal containing K+-rich foods such as fruit and meal may add another 70 mEq K+ to the bloodstream within a short time of its ingestion, which would double the ECF [K+] if there were not rapid adjustments to either transfer the K+ to the ICF compartment or excrete it. In fact, after a meal there is little change in ECF K+ because postprandial insulin stimulates muscle and liver to actively take up both glucose and the K+ not excreted in the short term by the kidneys (8). After the meal, muscle activity releases K+ into the ECF K+, and somewhat mysteriously, the kidneys excrete the amount consumed in the meal. In the animal kingdom, maintaining ECF K+ between meals is critically important because fasting can be prolonged, e.g., nine weeks in gray whales and elephant seals (9). During fasting or consumption of a low-K+ diet, the kidney reduces K+ excretion to near zero by reabsorbing more and secreting less K+ (6, 10). Yet, K+ loss in the stools and sweat persists and challenges the small pool of ECF K+. To balance the discrepancy between K+ input and output over time, muscle exhibits an altruistic specialization to donate ICF [K+] to the ECF (4, 11, 12). In these settings, the sensing of K+ status and appropriate adjustments are critical to maintain a ratio of ECF to ICF [K+] compatible with normal nerve and muscle cell excitability. Simply put, the adjustment may be regulated by feedback or feedforward control of ECF K+.
Feedback Control of K+ Homeostasis
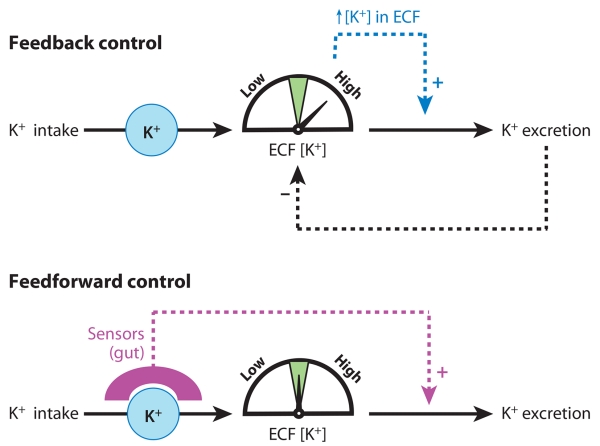
It has long been appreciated that an increase in ECF [K+] leads to an increase in K+ secretion in the collecting ducts mediated by direct stimulation of renal Na,K-ATPase, increased tubular flow, and an increase in aldosterone secretion. All three factors directly increase Na+ reabsorption via epithelial sodium channels (ENaCs), increasing the driving force for K+ secretion (13-15). The regulation of aldosterone synthesis by K+ has been recently reviewed (16). Conversely, a decrease in plasma [K+], mediated by either decreased intake or increased excretion, decreases K+ secretion by reducing ECF K+ stimulation of Na,K-ATPase activity, tubular flow, and aldosterone; these reductions decrease Na+ reabsorption via ENaC, which depresses the driving force for K+ secretion (7). In addition, decreasing plasma K+ increases expression of H,K-ATPases located in the distal nephron that effect active K+ reabsorption (7). Changes in renal K+ excretion help to normalize extracellular [K+], an example of classic feedback control, as illustrated in Figure 2.
Figure 2.
Schematic diagram illustrating control of extracellular fluid (ECF) [K+] via feedback versus feedforward mechanisms. (Top) In feedback control, a rise in ECF [K+] is the signal that initiates stimulation of K+ excretion by the kidney. The increased excretion brings ECF [K+] back toward the normal value. This process depends on the error signal of elevated ECF [K+] and stops when ECF [K+] is returned to the control range. (Bottom) In feedforward control, a local increase in [K+] in the gut is sensed during K+ intake and initiates stimulation of K+ excretion by the kidney independently of (i.e., before) a rise in ECF [K+], which helps to prevent a rise in ECF [K+].
Feedforward Control of K+ Homeostasis
Rabinowitz and colleagues challenged the traditional view of feedback control of ECF K+ two decades ago (17, 18). They confirmed that plasma K+ stimulates renal K+ excretion but that this response requires an elevation of plasma K+ above its normal range (19, 20). This group also provided evidence that aldosterone stimulates renal K+ excretion only at supraphysiological levels, with little effect within the physiological range of K+ (17, 20). In studies in sheep (21), meal intake over 1 h produced a pronounced kaliuresis in the absence of a change in plasma aldosterone concentration. Plasma [K+] was, indeed, increased after a K+-containing meal, but the magnitude of the increase (0.5 mEq L−1) was too small, when reproduced by intravenous K+ infusion, to account for the meal-induced kaliuresis (20). Rabinowitz concluded that meal-induced increases in renal K+ excretion cannot be explained by changes in plasma K+ or aldosterone concentration. To explain the meal-induced kaliuresis, he proposed a kaliuretic reflex arising from sensors in the splanchnic bed (i.e., gut, portal circulation, and/or liver). According to this proposal, renal K+ excretion is increased, without (or before) increases in extracellular [K+], by a mechanism controlled by sensing of K+ intake (i.e., sensing of local increase in [K+] in splanchnic areas during K+ intake). Thus, a new concept of feedforward control of K+ homeostasis arose (Figure 2).
Feedback versus Feedforward Control: Teleological Considerations
Most homeostatic regulation in the body is under feedback control because it offers robust control (22). However, this type of control can be slow to respond to an external disturbance and inevitably mandates an error signal, a significant disturbance of the system. In contrast, feedforward control may allow a quick control of output function in anticipation of a rise of the signal. This type of control may add speed or accuracy to the control (at the expense of robustness). A combination of both feedback and feedforward control mechanisms may provide both robustness and accuracy (or speed) of the regulation.
Recent Evidence Supporting Feedforward Control of K+ Homeostasis
The studies of Morita and colleagues (23, 24) supported the idea of K+ sensing or feedforward control of K+ homeostasis. After intraportal delivery of KCl to anesthetized rats, these investigators observed increases in hepatic afferent nerve activity (HANA) and urinary K+ excretion, the latter of which occurred in the absence of increases in plasma [K+]. Bumetanide attenuated the effect of KCl on HANA, suggesting that the Na+-K+-2Cl− cotransporter is involved in the sensing of portal [K+]. This research group suggested that the Na+-K+-2Cl− cotransporter serves as both a Na+ and a K+ sensor in the portal vein and is downregulated by high Na+ and K+ intake (25). One caveat in this group’s design, however, is that the hepatoportal area may have been exposed to very high [K+] and/or hypertonicity because KCl and/or NaCl solutions were injected into the portal vein as a bolus over a very short period of time in the experimental design. If so, membrane depolarization or liver shrinkage could have provoked the increase in HANA (26).
Additional evidence for feedforward regulation comes from our recent studies demonstrating that when dietary K+ intake was reduced to 33% of the control level for two weeks, there was no change in plasma [K+] or [aldosterone], yet there was a significant decrease in renal K+ excretion along with a decrease in (insulin-stimulated) extrarenal cellular K+ uptake (27, 28). We also routinely observed a decrease in (insulin-stimulated) extrarenal cellular K+ uptake in rats fasted overnight before assay of K+ uptake, compared with fed rats, with little if any decrease in plasma [K+] (C.S. Choi, J. Youn & A. McDonough, unpublished). These studies demonstrate that, analogous to the response to increased K+ intake, the body is able to sense reduced K+ intake in the absence of even minor changes in extracellular [K+] and, via unidentified signaling connection(s), both decreases renal K+ excretion to match output to input as well as decreases insulin-stimulated cellular K+ uptake to prevent a postprandial drop in plasma [K+]. These results support the notion that K+ intake is sensed independently of circulating [K+] and support the operation of a feedforward control mechanism that is capable of close control of ECF [K+].
Evidence that a Gut Factor Is Activated During Dietary K+ Intake
Motivated by the evidence reviewed above, we directly tested the hypothesis that K+ intake is sensed by putative K+ sensors in the hepatic portal vein (29). We assayed renal K+ excretion as a biomarker of sensitivity to this putative signal. In our first attempts, we infused K+ for 2 h into the stomach, the hepatic portal vein, or a systemic vein of fasted rats. Plasma [K+] and renal K+ excretion profiles were indistinguishable between the three infusion groups, which seemed to provide evidence against portal or gut sensing of K+ intake. Next, we fed the rats a K+-free meal during the K+ infusions to more closely mimic the reality of K+ ingestion with a meal. In contrast to the fasting state, there was a dramatic effect of K+ infusion route on the plasma [K+] profile during K+ infusion combined with a K+-free meal. Intraportal and systemic infusions similarly increased plasma [K+], whereas intragastric K+ infusion did not significantly increase plasma [K+]. However, renal K+ excretion was similarly increased in all three K+ infusion groups fed the K+-deficient meal. That is, the ratio of change in K+ excretion to change in plasma [K+] (the renal efficiency) was markedly increased when the intragastric K+ infusion was combined with a meal, the normal condition of dietary K+ intake. Plasma [K+] did not rise during the intragastric K+ infusion because the meal either slowed the rate of K+ absorption into the blood or increased K+ cellular uptake or both. In any case, adding the meal to the K+ infusion revealed an increase in renal K+ secretion in the absence of the rise in plasma [K+] that was seen when K+ was infused in the unfed state. These data provide evidence for a gut, rather than a hepatoportal, factor that plays a role in K+ homeostasis by a feedforward mechanism that senses K+ intake during a meal and enhances renal clearance of plasma K+. This response would, theoretically, minimize increases in plasma K+ concentration during or after a meal.
Postprandial Changes in Hormones that Contribute to Feedforward Control of K+ Homeostasis
After a meal, insulin secretion by the pancreatic β-cells is stimulated by meal nutrients such as glucose and amino acids, and the rise in insulin then plays a critical role in the cellular disposal of the ingested nutrients. Because insulin also stimulates cellular K+ uptake, meal ingestion also leads to a rapid transfer of K+ from the ECF to the ICF of insulin-sensitive cells, buffering a postprandial increase in [K+]. In fact, intravenous K+ infusion results in a lower plasma [K+] profile in rats coincidentally fed a meal compared with fasted rats (29, 30). In addition, raising blood glucose alone with an oral load enhances extrarenal disposal of a K+ load by stimulating insulin secretion (31, 32). Oral glucose can even lower serum [K+] in patients with end-stage renal disease; such patients have negligible renal K+ excretion, demonstrating the importance of endogenous insulin secretion to prevent hyperkalemia in this group by transferring K+ from the ECF to the ICF (33). In both normal and pathological conditions, the consumption of K+ along with a meal containing nutrients that stimulate insulin release leads to the activation of plasma K+ clearance independent of plasma [K+]—evidence of feedforward control of extracellular K+ homeostasis by insulin.
Glucagon has also been implicated in feedforward control of ECF [K+] after a protein-rich (and thus also K+-rich) meal. Intraportal, but not intrarenal, infusion of a physiological dose of glucagon produces significant increases in renal blood flow and glomerular filtration rate (GFR), suggesting the existence of a hepatorenal axis (34). Earlier studies had determined that glucagon infusion also increases the excretion of inorganic ions, including K+ (35). Because cyclic AMP (cAMP), the intracellular second messenger of glucagon in the liver, is rapidly released from hepatocytes into the plasma after glucagon infusion (36) and because the kidney can take up and secrete cyclic nucleotides, Bankir and colleagues (37-39) postulated that extracellular circulating cAMP is the link between glucagon’s action in the liver and the subsequent renal hemodynamic and ion transport effects. These researchers’ results demonstrated that the renal increase in GFR after glucagon infusion depends on both glucagon and a rise in plasma cAMP and that cAMP alone can account for the increase in ion excretion. Specifically related to K+, the focus of this review, Bankir and colleagues’ results demonstrated that a 100-min infusion of glucagon roughly doubled the excretion of K+ and lowered plasma [K+] (37). Importantly, they also calculated that the increased K+ excretion was not just a function of increased tubular flow rate (secondary to the increase in GFR). Rather, there was a parallel increase in the transtubular K+ gradient (the ratio of urine-to-plasma [K+] divided by the ratio of urine-to-plasma osmolality), with the increases in K+ excretion suggesting a specific effect on a K+ secretion pathway(s). The effect of glucagon on K+ excretion (but not those on urine output) was fully reversed 40 min after glucagon infusion was terminated (37). These effects of dietary protein to increase GFR and K+ secretion is evidence for another layer of feedforward control of renal K+ excretion to buffer a rise in plasma [K+] after a meal.
Putting It All Together: Feedforward Plus Feedback Control Mechanisms Control Plasma K+
The studies summarized above provide evidence for three distinct layers of feedforward regulation aimed to acutely buffer ECF [K+] after a K+-containing meal, all independent of a rise in ECF [K+]: (a) Insulin release rapidly stimulates cellular K+ uptake into extrarenal, insulin-responsive tissues; (b) glucagon, at least in part owing to cAMP released from the liver, rapidly increases renal K+ excretion after a protein-rich meal; and (c) an unidentified gut factor senses K+ ingestion, rapidly stimulates renal K+ excretion, and potentially stimulates extrarenal K+ uptake. If plasma [K+] increases despite these layers of control, feedbackregulation is activated: Plasma K+ can increase K+ excretion, as discussed above, and aldosterone synthesis is stimulated, which increases K+ secretion secondary to activation of ENaC (16). Aldosterone acts only after a finite lag period, not well suited for rapid control, but can chronically increase K+ secretion until [K+] is normalized. The close physiological control of ECF [K+] is evidence of the efficacy of the combination of feedback with feedforward control.
Regulation of Renal Function by the Gastrointestinal Tract: New Directions
That the three layers of feedforward regulation (insulin, glucagon, gut factor) are activated by ingestion of K+ with a meal raises the possibility of additional layers of regulation of K+ homeostasis by the gut. The regulation of renal function by the gastrointestinal (GI) tract was very recently reviewed (26). Relevant to K+ status, uroguanylin, produced in the small intestines, is stimulated by an oral Na+ load or a high-salt diet and is both natriuretic and kaliuretic without changing GFR or renal blood flow (40). This suggests a fourth layer of K+ regulation after a salt-containing meal. However, when the effects of uroguanylin were studied directly in principal cells of the isolated mouse collecting duct (the cells responsible for K+ secretion in this region), researchers found that the signaling pathway is cGMP independent and that uroguanylin activates a G protein–coupled receptor that increases arachidonic acid concentration via phospholipase A2 activation, a pathway known to inhibit apical ROMK (renal outer medulla potassium) channels in these cells (41, 42). Thus, inhibition of ROMK apparently does not explain the uroguanylin-mediated kaliuresis. Another potential layer of GI regulation of K+ homeostasis is on the horizon now that it has been discovered that taste channels and receptors, analogous to those found in the tongue, are expressed in entero-endocrine cells of the intestine and respond to bitter, sweet, and umami by releasing signaling substances that can act on target tissues throughout the body (43, 44). Whether dietary K+ can alter gating of these receptors or channels in a manner that would alter renal K+ excretion or the distribution of K+ between ICF and ECF remains to be investigated.
MOLECULAR MECHANISMS OF RENAL K+ ADAPTATION
When dietary K+ intake is chronically increased or decreased, the kidneys respond by appropriately increasing or decreasing K+ excretion, respectively. This so-called K+ adaptation is critical for chronic K+ balance and involves adaptations all along the nephron. Fine and final control of K+ excretion is routinely assigned to the distal portion of the nephron, especially the collecting ducts. This region contains two cell types that play distinct roles in K+ homeostasis and adaptation: (a) Principal cells can secrete K+, and (b) intercalated cells (in the medullary collecting duct) can reabsorb K+. It has been well established that high K+ intake increases K+ secretion in principal cells and that low K+ intake suppresses K+ secretion. The topic of chronic dietary K+ regulation of renal K+ transport has been reviewed recently (7, 45-47), and additional progress has been made more recently. In this section we review the signaling mechanisms known to connect a change in K+ intake to a homeostatic change in K+ excretion and the gaps in our knowledge that still remain.
Renal Responses to High K+ Intake
To secrete K+ from the ECF to the tubular lumen, the cation is pumped into the cell across the basolateral membrane via the Na+ pump and is then transported from the cell to the tubular lumen via apical K+ channels. The K+ channel that secretes K+ under normal flow conditions is a ROMK-like small-conductance K+ (SK) channel originally cloned by Hebert and colleagues (48). In addition, both principal and intercalated cells express big-conductance K+ (BK) channels that have a low open probability and density under normal conditions but increased activity under high-flow conditionsor high-K+ diet [which increases flow to the cortical collecting duct (CCD)], recently reviewed by Sansom and colleagues (49, 50).
During chronic high K+ intake or acute experimental infusion, the following adaptations can occur to increase K+ secretion: (a) An increase in plasma K+ stimulates Na,K-ATPase activity and cellular K+ uptake, which increases the driving force for K+ secretion in the collecting duct cells. (b) Proximal tubule and loop of Henle salt and water absorption is inhibited during an acute K+ load (15, 51, 52), which increases tubule flow rate in the distal nephron. This increase in tubule flow rate can both activate K+ secretion through BK channels (49) and increase the delivery and reabsorption of NaCl in the principal cells, which increases the electrochemical driving force for K+ secretion through ROMK (7). (c) High plasma K+ stimulates aldosterone synthesis and secretion, which, by increasing both Na+ reabsorption via ENaC and Na,K-ATPase, increase the electrochemical gradient for K+ secretion into the lumen and decrease K+ reabsorption in the collecting ducts (7, 47). At the molecular level, there is evidence that ROMK and BK channel are expressed in both apical membranes and intracellular vesicles and that both redistribute to the apical membranes during a chronic high-K+ diet (53, 54), without an apparent increase in total abundance (54, 55).
Many open questions remain in our understanding of what drives the renal response to a K+ challenge (whether it is the normal amount consumed in a meal, a high-K+ diet, or acute infusion). The effects of aldosterone and high plasma K+ on the collecting ducts can directly increase K+ secretion via ROMKs like SK, as described above. However, significant K+ adaptation can occur independently of elevations in aldosterone (56) and with little or no change in plasma [K+] (28, 57). Although we can postulate that a gut factor, glucagon, and uroguanylin are mediators, there is scant evidence that can explain regulation at the level of receptors and transporters. At the subcellular level, signaling studies in the thick ascending limb, a region in which Na+ and volume reabsorption is inhibited during high K+ intake, show that a high-K+ diet is associated with increases in both inducible nitric oxide synthase (iNOS) and nitric oxide (NO) production as well as heme oxygenase 2 and carbon monoxide (CO) production (58, 59). Both NO, via cGMP, and CO stimulate the 70-pS ROMK-related channel in the thick ascending limb apical membrane. The stimulation of the channel increases K+ recycling in the medulla because of the parallel NKCC and is not thought to contribute to kaliuresis. Nonetheless, these studies reveal molecular mechanisms that may also be activated in the collecting duct to increase K+ secretion during high K+ intake.
Renal Responses to K+ Restriction
During K+ deprivation sufficient to lower plasma K+, the following adaptations that decrease K+ excretion occur. (a) GFR is reduced and fractional absorption in the proximal tubule increases, reducing delivery to the distal nephron (60). (b) The abundance of ROMKs is reduced by ~50% (55). (c) The abundance of Na+ transporters (NKCC2, NCC, and ENaC) is reduced by ~50%, which can decrease the electrochemical gradient for K+ secretion secondary to reduced Na+ reabsorption. (d) The abundance of H,K-ATPase is increased, which is likely to facilitate K+ reabsorption in the face of decreased ROMKs (61). (e) Finally, perhaps most important to reduce K+ excretion, ROMK is phosphorylated and retracted from the apical membrane (7, 45, 46).
Wang and colleagues (7, 45, 46) have intensively investigated and recently reviewed the molecular mechanisms connecting intake of a nominally K+-free diet to the redistribution of ROMK out of the apical membrane in the principal cells, thus reducing K+ secretion and excretion. Wang and colleagues provided evidence that low K+ intake for one week initiates the following chain of events: (a) Low K+ stimulates the production of superoxide anions (62), at least in part by activatingNADPH oxidase II (63); (b) superoxide production increases expression of protein tyrosine kinase (PTK) such as c-Src by two to three days (62, 64) and decreases expression of protein phosphatase 2B (65); and (c) PTK phosphorylates ROMK (45), which provokes the redistribution of ROMK out of the apical membranes (53). In support of this chain of events, pretreatment with the reactive oxygen species (ROS) scavenger tempol prevents the increased expression of c-Src, blunts the phosphorylation of ROMK, and blunts the decrease in K+ excretion (62, 63).
In addition, Wang and colleagues also explored the role of mitogen-activated protein kinase (MAPK) in mediating the effect of superoxide anions on ROMK channel activity and found that a one-day low K+ intake increased the phosphorylation of p38 MAPK and extracellular signal–regulated kinase (ERK), both blunted by tempol, and that this was associated with decreased ROMK channel activity independent of and parallel to activation by PTK activity (66). This research group suggests that MAPK-induced inhibition of ROMK activity plays a predominant role in suppressing K+ secretion in the early stage (<24 h) of K+ depletion. Further study supports the hypothesis that ING4 (inhibitor of growth 4), which is stimulated by low K+ intake dependent on superoxide anion production, may mediate the effect of a low-K+ diet on MAPK to depress ROMK channel activity (67). In summary, a low-K+ diet increases superoxide anion production, which, in parallel, increases the expression and activity of (a) PTK and (b) ING4 and MAPK, which in turn independently depress CCD K+ channel activity and K+ secretion (67).
Signals to the Kidney?
The signaling connection between a decrease in K+ intake, a decrease in plasma [K+], and activation of NADPH oxidase has not been explored in detail. As discussed above, at the cellular level there is evidence that a high K+ intake increases NO production (in the thick ascending limb at the least) associated with increased K+ channel activity, whereas low K+ increases superoxide anion production, known to react with and lower the levels of NO (46, 68, 69) associated with decreased K+ channel activity. Angiotensin II (AngII), an important renal hormone, stimulates Na+ reabsorption via stimulating NADPH oxidase, increasing superoxide anion and depressing cellular NO levels (68, 69). AngII suppresses K+ secretion (70), and Wei and colleagues (71) examined whether AngII also inhibits ROMK activity. Their studies confirmed their hypothesis and demonstrated that during a K+-restricted diet (not during a normal-K+ diet) AngII treatment inhibits ROMK channel activity, at least in part by increasing expression of NADPH oxidase and PTK. Whether renin secretion and/or local AngII production are increased during K+ restriction in general remains to be determined.
Chronically reducing K+ intake by two-thirds to 0.33% (normal rat chow is 1% K+) activates renal mechanisms to retain K+ but does not significantly depress plasma [K+] (28), evidence that a change in plasma K+ is not a requisite error signal. Comparing the responses observed in rats fed 0.33% K+ with normal K+ versus responses in rats fed 0 K+ is informative as to the signaling mechanisms driving the homeostatic responses. Rats fed a K+-deficient diet long enough to cause a drop in plasma [K+] show a wide array of changes in transporter protein levels, including a decrease in muscle Na,K-ATPase α2 subunit abundance (72), decreases in renal ROMK (55) and aquaporin 2 (AQP2) (73), increases in renal colonic H,K-ATPase (61), and a fall in muscle K+ stores (4). These changes were not observed when rats were fed a 0.33% K+ diet, even though urinary K+ excretion was reduced 80%, evidence of significant K+ conservation in the absence of an error signal, i.e., a change in plasma [K+] from normal levels. Even when rats were maintained on the 0.33% K+ diet for 30 days, they did not manifest any changes in plasma [K+] or in the abundance of muscle Na,K-ATPase α2 isoform, renal ROMK, H,K-ATPases, or AQP2. These results lead us to believe that the changes observed in response to the K+-deficient diet may be provoked by an actual fall in plasma [K+]. The K+ adaptation in the 0.33%-K+-diet-fed rats may be explained by a marked increase in the expression of the PTK cSrc along with the expected phosphorylation of ROMK, leading to the inactivation of ROMK via its retraction from the cell surface (28). This retraction of ROMK would increase net K+ reabsorption in the CCDs mediated by existing pools of H,K-ATPases.
The findings after a “modest” reduction in K+ intake demonstrate that there are two categories of responses to reduced K+ intake: a first line of defense activated by an undefined signal that provokes renal K+ conservation, and a second line of defense activated when plasma [K+] actually falls, in which ROMK and H,K-ATPase abundance changes occur, consistent with renal K+ conservation. Very important to understanding K+ homeostasis, these findings establish that a drop in plasma [K+] is not a requisite for setting off renal K+ adaptation mediated by ROMK phosphorylation and the decreased distribution of ROMK in the apical membrane. Further study of models of “modest” reductions in K+ intake will be very useful to determine the homeostatic signals that connect intake to output because such models eliminate the set of changes that occur secondarily to a fall in plasma [K+].
The proximal response to chronic K+ deprivation described in the renal cells at this stage of our understanding involves increasing NADPH oxidase abundance and activity. That is, we do not understand the signaling upstream of this point. Whatever the signal, it can likely activate NADPH oxidase and superoxide anion production. Another important gap that should be addressed is to determine whether the acute increase in K+ excretion seen after K+ ingestion, mediated by the putative gut factor, involves a decrease in phosphorylation of ROMK and whether this decrease is mediated by the suppression of NADPH oxidase generation of superoxide anions. This information will be critical to establish the nature and actions of the gut factor.
MOLECULAR MECHANISMS OF EXTRARENAL K+ ADAPTATION
Critical Role of Insulin in Acute Regulation of Extracellular K+
After a K+-containing meal, plasma insulin prevents an excessive rise in plasma K+ level by rapidly stimulating cellular K+ uptake via Na+ pumps (74). Without this action of insulin, ingested K+ could produce life-threatening hyperkalemia. DeFronzo and coworkers (8) showed that insulin infusion caused a dose-dependent decline in plasma [K+] and that the majority of the decline in plasma [K+] was due to net K+ uptake by the splanchnic bed (during the first hour) and peripheral tissues (during the second hour). Choi et al. (75) introduced the K-clamp technique for the quantification of insulin’s action on cellular K+ uptake in vivo at constant, basal plasma [K+]. In the K-clamp technique, conscious rats are infused with insulin to stimulate K+ (and glucose) uptake and then, on the basis of immediate determinations of plasma [K+] (and glucose), infused with K+ (and glucose) appropriate to clamp plasma [K+] (and glucose) at basal levels. The amount of K+ infused (Kinf) at steady state is equivalent to the sum of insulin’s effects on cellular K+ uptake and renal K+ excretion. The initial studies showed that physiological concentrations of insulin do not significantly affect renal K+ excretion (27, 75). Therefore, the K-clamp appears to be a good measure of insulin-stimulated cellular K+ uptake (75). This technique has been used to determine that insulin’s action on cellular K+ uptake is subject to control by dietary K+ intake.
Extrarenal Adaptation to Low K+ Intake: Decreased Insulin Sensitivity
When K+ intake is very low, K+ output will exceed K+ input despite renal K+ conservation (via the colon, etc.). When this happens, the large stores of K+ in the muscleICF are tapped to blunt the fall in ECF [K+] (Figure 2). The K+ shift from the ICF to the ECF has been attributed to a fall in muscle Na+ pump levels (4). During dietary K+ deprivation, Na+ pump number decreases by more than 50%, estimated by 3H-ouabain binding, Na,K-ATPase enzymatic activity, and 86Rb uptake (12, 76). At the molecular level, K+ deprivation provokes a specific decrease in the Na+ pump α2 (not α1) catalytic isoform abundance in skeletal muscles (72, 77). This decrease in muscle Na+ pumps theoretically protects against acute hypokalemia during insulin stimulation if an animal consumes a diet containing sugar but low K+. We tested the hypothesis that insulin-stimulated cellular K+ uptake decreases during K+ deprivation in rats as a function of the decrease in muscle α2 abundance. After 10 days of K+ deprivation, plasma [K+] fell to 2.9 mM, Na,K-ATPase activity and α2 subunit levels fell by more than 50%, muscle K+ stores decreased significantly (4), and insulin-mediated K+ clearance to the ICF decreased to less than 10% of control. These findings provide evidence for a relationship between the Na,K-ATPase α2 pool size and insulin-stimulated K+ uptake (75). However, when the same analyses were done after only two days of K+ deprivation, plasma [K+] fell slightly from 4.2 mM to 3.8 mM, there was not yet a significant fall in muscle Na,K-ATPase activity or expression, and insulin-mediated cellular K+ uptake decreased to 20% of control—evidence against a direct relationship between the Na,K-ATPase α2 pool size and insulin-stimulated K+ uptake (75). These data demonstrate that short-term K+ deprivation leads to a significant insulin resistance of cellular K+ uptake (with normal stimulation of glucose uptake) that precedes the decreases in muscle Na,K-ATPase expression, perhaps mediated by Na+ pump inactivation or internalization. Longer K+ deprivation does provoke a more profound decrease in insulin-sensitive cellular K+ uptake that is likely secondary to the decrease in Na,K-ATPase α2 abundance (75). In addition, this study (75) demonstrated that insulin-stimulated cellular K+ uptake, like renal K+ excretion, is profoundly suppressed during K+ deprivation. Thus, K+ adaptation occurs in both the kidneys and extrarenal tissues in response to changes in K+ intake. A correlation between K+ intake and insulin-sensitive K+ clearance is physiologically advantageous because it would, theoretically, blunt acute hypokalemia after a K+-poor, carbohydrate-rich meal.
Differential Regulation of Insulin Action on K+ Uptake versus Glucose Uptake
Of the many homeostatic systems of the body, the K+ and glucose homeostatic systems are unique in that they share acute regulation by insulin. This feature suggests the potential for interactions or cross talk between the two systems. Insulin resistance with respect to glucose metabolism is usually associated with hyperinsulinemia because pancreatic β-cells increase insulin secretion to compensate for insulin resistance (78). The resulting hyperinsulinemic may provoke hypokalemia secondary to stimulating excessive cellular K+ uptake unless insulin’s action on K+ uptake is similarly dampened. Impaired insulin action on K+ fluxes has, indeed, been reported in obesity and diabetes (79, 80), which are both associated with insulin resistance with respect to glucose metabolism. However, insulin-mediated K+ uptake is not altered in uremia, whereas insulin-mediated glucose uptake is markedly impaired (81). We determined the acute effect of insulin on K+ cellular uptake in the high-fat-fed rat, a well-established model of insulin resistance with respect to glucose metabolism (82, 83). We discovered, inadvertently, that rats ate less of the typical 66% high-fat diet such that their dietary K+ intake was reduced to one-third of that consumed by the rats eating a control diet of 1% K+ (27). In this series, both insulin-stimulated K+ uptake as well as insulin-stimulated glucose uptake were suppressed. Supplementing the high-fat diet with enough K+ to match that consumed in the control diet group restored insulin action on K+ uptake to control levels but did not correct insulin-stimulated glucose uptake (27). These data indicate that insulin action on glucose uptake is selectively (i.e., without change in insulin action on K+ uptake) impaired by high-fat feeding when K+ intake is normal. These studies reveal two distinct varieties of insulin resistance: resistance to cellular glucose uptake observed in type 2 diabetes and resistance to cellular K+ uptake observed during K+ deprivation or fasting.
The molecular mechanisms responsible for the differential regulation of glucose versus K+ cellular uptake by insulin have not been examined. Presumably, these pathways involve events that occur after insulin binds to its receptor, which is a common step in the regulation of K+ and glucose uptake. The resistance of muscle cell glucose uptake during insulin resistance is attributed to an impairment of insulin signaling at the level of PI 3-kinase, which affects GLUT4 trafficking (recently reviewed in Reference 84). Insulin has been proposed to activate cellular K+ uptake by activation of Na+ pumps resident in the plasma membrane or by translocation of Na+ pumps from intracellular pools to the plasma membrane, although this issue remains controversial (74, 85, 86). A rather new proposal is that the Na+ pumps can be activated by the translocation of the Na+ pump–associated protein phospholemman (PLM) to the plasma membrane; such translocation has been demonstrated in exercising muscles (87). Whether this PLM translocation also occurs during insulin stimulation remains to be determined. Establishing the connection between K+ deprivation and decreased sensitivity of cell K+ uptake to insulin is an important goal. Testing the hypothesis that the signaling cascade activated in the kidney during K+ deprivation—namely increased activity of NADPH oxidase, superoxide anion production, and PTK activity—affects insulin stimulation of K+ cellular uptake warrants consideration.
In a related line of investigation, we examined insulin-stimulated cellular K+ uptake during chronic dexamethasone treatment. This was an important gap to explore because (a) it has long been known that dexamethasone treatment causes insulin resistance of cellular glucose clearance (88), (b) chronic treatment of patients or experimental animals with glucocorticoids leads to an increase in skeletal muscle Na+ pumps by ~50% (89, 90), and (c) this large increase in muscle Na+ pumps could theoretically provoke an increase in insulin-driven cellular K+ uptake after a meal and acute hypokalemia. Using the K-clamp, we assessed insulin-stimulated K+ and glucose uptake in rats treated with dexamethasone for seven days, sufficient to raise Na,K-ATPase α2 levels by ~50%. Interestingly, the measurements revealed depressed (rather than increased) cellular uptake of both K+ and glucose in response to insulin, implicating a common step in insulin signaling that affects both glucose and K+ uptake (91). These results provide novel evidence for insulin resistance of K+ clearance as well as glucose clearance during glucocorticoid treatment. Although we were originally thinking that cellular clearance of K+ may be higher in steroid-treated animals, the results suggest that the increase in Na,K-ATPase synthesis and abundance may be a compensation to counteract the dexamethasone-induced insulin resistance and that insulin-stimulated cell K+ uptake may be even more suppressed if the Na,K-ATPase pool size is not increased.
Extrarenal Adaptation to High K+ Intake
For more than 50 years, investigators have known that that rats maintained on a high-K+ diet survive an acute K+ load that is lethal to rats maintained on a control diet and that this adaptation is observed even in nephrectomized rats (92). This observation has led to the hypothesis that a major mechanism of the K+ adaptation is extrarenal. In fact, it was later demonstrated that rats adapted to a 10% K+ diet for 10 days exhibited (a) increased skeletal muscle (ouabain-binding) Na+ pump activity and number as well as increased 86Rb uptake during an acute K+ load compared with control rats and (b) increased 86Rb efflux upon termination of the K+ infusion (93). These results demonstratethat skeletal muscle serves as a buffer compartment both during acute high K+ intake, temporarily transferring K+ from the ECF to the ICF until renal excretion can match K+ intake, as well as during chronic K+ deprivation by the net transfer of K+ from the ICF to the ECF.
Rapid Reversal of K+ Conservation State
In the wild it is common for large carnivorous mammals to experience long periods of fasting between large, protein-rich meals. The adaptations that occur to preserve ECF [K+] during prolonged fasts, e.g., reduced renal K+ secretion and reduced insulin-stimulated K+ uptake, can theoretically pose a risk of hyperkalemia when the fast is broken with a high-K+ meal if the K+ conservation mechanisms are not rapidly turned off. Sensing of K+ intake in the gut and activation of a gut factor that communicates to key tissues in a feedforward manner may rapidly turn off the K+ conservation mechanisms and prevent a rise in plasma [K+]. Two relevant studies support this idea. First, Norgaard and colleagues (12) fed rats a K+-deficient diet for three weeks, during which plasma K+ fell to ~2 mM, muscle Na+ pumps (ouabain binding) fell to 30% of control, and muscle K+ stores fell by 40%. Nonetheless, a single day of a normal-K+ diet corrected plasma and muscle K+ stores to control levels. Sadre and colleagues deprived rats of K+ for four weeks and then demonstrated that K+ administration via a gastric tube normalized muscle K+ stores within 24 h but provoked an increase in plasma [K+], which the researchers attributed to a decreased ability of the muscle to absorb the large K+ dose (94). In both of these studies K+ restoration was provided via the GI tract and may have stimulated a gut-derived factor to stimulate cellular K+ uptake and renal K+ excretion. An important question to answer is whether the gut is critical to the homeostatic adjustment to K+ restoration in a K+-depleted animal. This question could be assessed by infusing a K+ load via another route.
Clearing Extracellular Fluid K+ Back to the Intracellular Fluid during Exercise and Ischemia
K+ homeostasis can also be significantly disrupted by exercise and/or ischemia independently of changes in K+ intake. During ischemia, ECF [K+] bathing the muscle is significantly elevated because the Na+ pumps are unable to actively clear K+ from the ECF at a rate to match passive leaks. During exercise, [K+] is also elevated around contracting muscles because the rate of K+ leaving the cell (via channels) rises more quickly than the Na+ pump can actively pump K+ back into the cell. Interstitial [K+] in these conditions can rise to 9–10 mM, bathing the exercising muscle (reviewed in detail recently in References 5 and 76). The high ECF [K+] can have life-threatening effects: Above 6.0 mM [K+] there are marked changes in the electrocardiogram, and at higher K+ concentrations lethal arrhythmias may develop (95). When net K+ influx rate rises above net K+ efflux rate, homeostasis is restored. This can, theoretically, be accomplished by increasing active K+ uptake (via Na,K-ATPase) or suppressing K+ efflux (via channels or cotransporters). Clausen and colleagues (76) have provided evidence that the Na+ pump rate is stimulated in exercising muscle by both the rise in ICF Na+ as well as the local release of catecholamine and calcitonin gene-related peptide (CGRP). With chronic exercise training, the number of Na+ pumps increases, which improves K+ homeostasis by increasing Na+ pump capacity (5, 76).
Increasing Na,K-ATPase activity to restore local K+ homeostasis is not feasible in some cases of ischemia or exercise when the tissue becomes ATP depleted. Muscle ATP stores can become depleted and AMP levels increased owing to high ATPase activity during exercise or lack of O2/substrate to make ATP in ischemia. We hypothesized that AMP-dependent protein kinase (AMPK) may play a role in normalizing local K+ gradients (96). AMPK [reviewed recently (97)] acts as a cellular fuel gauge to maintain or restore energy balance by switchingon ATP-generating processes (e.g., cell glucose uptake, fatty acid oxidation) and switching off ATP-consuming processes (e.g., the synthesis of lipid, glycogen, and protein). Mice deficient in muscle and heart AMPK activity exhibit depressed exercise-induced glucose uptake, significant muscle weakness and impaired recovery from fatigue (98), and abnormalities in cardiac electrical conductance (99), all consistent with a problem with local K+ homeostasis.
We specifically tested the hypothesis that infusing conscious rats with the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), to imitate the condition of an elevated AMP:ATP ratio in the ICF, would provoke an increased rate of clearance of K+ from the ICF to the ECF (96). It was previously established that AICAR infusion increased cellular uptake of glucose (100). We found that a 3-h AICAR infusion decreased plasma [K+] from 4.0 mM to 3.3 mM in control rats, from 4.5 mM to 3.8 mM in rats fed a high-K+ diet plus spironolactone, and from 3.2 mM to 2.5 mM in rats fed a K+-deficient diet for one week, all evidence for a pronounced effect of this AMPK activator on K+ homeostasis. There was no evidence for increased renal K+ excretion, consistent with the idea of a net shift of K+ from the ECF to the ICF. To test the hypothesis that the K+ redistributed into muscle, we examined the response in mice lacking AMPK activity in heart and skeletal muscle (101) and found that the K+-lowering response was very significantly blunted compared with control mice (96). We could detect no evidence for activation of Na,K-ATPase activity or translocation of Na,K-ATPase isoforms to the plasma membrane in samples in which translocation of GLUT4 to the membrane was evident (96), indicating that this response is not likely secondary to Na,K-ATPase activation. These results suggest a novel mechanism for redistributing K+ from the ECF to the ICF owing to the activation of AMPK activity with AICAR. The role of AMPK as a cellular fuel gauge can be reconciled with the clearance of K+ that accumulates in the ECF during exercise or ischemia if one postulates that AMPK decreases a K+ efflux pathway in muscle. This action would promote net cellular K+ uptake (by decreasing the ratio of K+ efflux via channels/leaks to K+ influx via ATPases) without increasing ATP consumption. Establishing that a K+ efflux pathway is regulated by AMPK and the molecular identity of the pathway(s) is an important goal because the answers could suggest novel therapeutic strategies to treat acute life-threatening hyperkalemia.
LINKING MECHANISMS OF K+ HOMEOSTASIS TO THE CARDIOVASCULAR BENEFITS OF A K+-RICH DIET
Beneficial effects of high dietary K+ intake on blood pressure, cardiovascular disease, and stroke have been well documented and reviewed (102, 103). Numerous studies have addressed the beneficial effects of K+, but fewer have addressed the molecular mechanisms by which such benefits occur. One hypothesis proposed by Young et al. (103) is that elevation in dietary K+ increases plasma [K+], thereby inhibiting free radical formation, smooth muscle proliferation, and thrombus formation. However, although the relationship between high K+ intake and cardiovascular benefits is very strong, the evidence that the benefit is mediated by elevated ECF K+ is not. First, beneficial effects of K+ supplementation have been observed without an increase in plasma [K+]: Nearly doubling daily K+ intake (by adding 60 mmol/70 kg body weight) for three days did not change serum [K+] in healthy men or women but did diminish platelet reactivity (104). Moreover, in another study of healthy volunteers, KCl or potassium citrate supplementation for six weeks (30 mmol day−1) did not change plasma [K+] but did significantly lower blood pressure (105). Second, clinical studies investigating the relationship between serum [K+] and risk of cardiovascular events have shown a U-shaped association: Both significant hypokalemia and hyperkalemia are riskfactors for cardiovascular events (106, 107). In addition, treating heart failure patients with a combination of spironolactone and ACE inhibitors, which elevates ECF [K+] by decreasing K+ excretion rather than increasing intake, significantly increases hyperkalemia-associated morbidity and mortality (108). These findings of increased risks of cardiovascular events with elevated serum [K+] are at odds with the idea that high dietary K+ intake brings about its beneficial effects by increasing plasma [K+].
Taken together, these considerations suggest that a mechanism other than plasma [K+] must be the mediator of the beneficial effects of dietary K+ intake. This review supports the hypothesis that the process of dietary K+ intake stimulates the mediator of the cardiovascular benefit of a high-K+ diet. Alternatively, we cannot rule out the possibility that a high-K+ diet inhibits the production of a K+-conserving factor that has long-term detrimental effects on the cardiovascular system. Regarding this alternative, a strong candidate for a factor that both promotes K+ conservation during K+ deprivation and has long-term detrimental cardiovascular effects is ROS such as superoxide anions.
The studies of Wang and colleagues (7, 46, 63) have demonstrated that K+ deprivation leads to an increase in NADPH oxidase and superoxide anion production. Such increases activate PTK, key to the retraction of renal K+ channels out of the apical membrane, which reduces K+ secretion and excretion (7, 46, 63). Work from our group has established that this response can occur in the absence of a fall in plasma [K+]: Modest K+ deprivation also increases PTK and ROMK phosphorylation (28). However, consuming a high-K+ diet for one to three days depresses levels of renal PTK and increases ROMK channel activity fourfold (64). Determining the effect of a high-K+ diet or acute K+ intake on renal NADPH oxidase activity and superoxide anion production is an important gap to fill because K+ intake can rapidly increase K+ excretion (29, 57). In addition, determining whether ROS play a role in extrarenal K+ adaptation needs to be investigated.
Young et al. (103) have reviewed the effects of a diet rich in K+ to suppress free radical production and the sequelae of free radicals on the cardiovascular system. More recent studies have provided specifics about the detrimental effects of a low-K+ diet and the beneficial effects of a high-K+ diet for the vasculature. Regarding detrimental effects, carotid arteries from rabbits fed a low-K+ diet for one to three weeks have 100% more superoxide anion production along with an enhanced sensitivity to vasoconstrictors and reduced sensitivity to endothelium-dependent stimuli; these changes can be corrected by treatment with a superoxide dismutase mimetic (109). Two recent studies by Fujita and colleagues (110, 111) in Dahl salt-sensitive (DS) rats fed an 8% NaCl diet demonstrated that the cardiac dysfunction and vascular injury observed in this model were significantly reduced by supplementation with an 8% KCl diet. Specific to superoxide anion production, NADPH oxidase subunits’ expression levels (p22phox, p47phox, and gp91phox) were increased approximately tenfold in arteries from DS rats fed a high-salt diet, ROS generation was increased approximately fourfold, and these levels were returned to baseline in rats supplemented with a high-K+ diet (111). Interestingly, treatment with a high-K+ diet or the ROS scavenger tempol had a rather small effect on the salt-sensitive increase in blood pressure but still attenuated vascular neointimal hyperplasia (111) and improved left ventricular relaxation (110). Although there is emerging agreement that high K+ intake can decrease the expression of NADPH oxidase, decrease superoxide anion production, and reduce cardiovascular injury and dysfunction, still unresolved is the initial connection between the high K+ intake and the change in the NADPH expression. Further experiments will have to be aimed at determining whether K+ ingestion through the gut is a critical link in the pathway. Accomplishing this aim could establish a natural and straight-forward mechanism to improve cardiovascular health.
SUMMARY POINTS.
Additional evidence has been collected for feedforward regulation of K+ homeostasis: Dietary K+ intake is sensed in the gut, and an unidentified gut factor is activated to stimulate renal K+ excretion. This pathway may explain renal and extrarenal responses to altered K+ intake that occur independently of changes in ECF [K+].
Molecular mechanisms responsible for conserving ECF K+ during fasting or K+ deprivation are emerging: (a) Kidney NADPH oxidase activation initiates a cascade that provokes phosphorylation and retraction of K+ channels from the cell membrane, which decreases K+ excretion, and (b) muscle becomes resistant to postprandial insulin stimulation of cellular K+ uptake while maintaining normal insulin sensitivity of cellular glucose uptake. How these mechanisms are triggered by K+ deprivation remains unclear.
A new link has been established between cellular energy metabolism and ion transport: Cellular AMPK activity provokes acute transfer of K+ from the ECF to the ICF, which may be important during exercise or ischemia.
FUTURE ISSUES.
Although it has been established that renal NADPH oxidase and ROS are increased during diets lacking K+, leading to the phosphorylation and internalization of ROMK, the signaling connection between a decrease in K+ intake and the activation of NADPH oxidase has not been determined. Future experiments could be aimed at determining whether K+ ingestion through the gut is a critical link in the pathway.
Significant increases in K+ excretion can occur independently of elevations in aldosterone and with little or no change in plasma [K+] that may be mediated by a gut factor. Future studies are warranted to establish the nature of the mediator and the underlying molecular mechanisms at the level of the nephrons, receptors, and transporters. This information is critical to determining the identity of the putative gut factor.
Determining the effect of a chronic high-K+ diet or acute K+ intake on renal NADPH oxidase activity and superoxide anion production is an important gap to fill, as are determinations of whether the kaliuresis involves a decrease in ROMK phosphorylation and whether this decrease is mediated by the suppression of NADPH oxidase generation of ROS.
Further study of models of “modest” reductions in K+ intake will be very useful to determine the homeostatic signals that connect intake to output because such models eliminate the set of changes that occur secondarily to a fall in plasma [K+].
Studies in low-K+-fed rats revealed a new variety of insulin resistance to cellular K+ uptake. The molecular mechanisms for this selective insulin sensitivity are not understood. Testing the hypothesis that the signaling cascade activated in kidney during K+ deprivation—namely increased activity of NADPH oxidase, superoxide anion production, and PTK activity—affects the insulin stimulation of K+ cellular uptake warrants consideration.
A fasting animal rapidly turns off K+ conservation mechanisms when breaking the fast with a high-K+ meal, and the mechanisms are not understood. Perhaps the K+ intake via the gut is critical to the homeostatic adjustment to K+ restoration.
Establishing that a K+ efflux pathway is regulated by AMPK and the molecular identity of the pathway(s) is an important goal because the answers could suggest novel therapeutic strategies to treat acute life-threatening hyperkalemia.
Glossary
- ECF
extracellular fluid
- [K+]
potassium concentration
- ICF
intracellular fluid
- ENaC
epithelial sodium channel
- GFR
glomerular filtration rate
- ROMK
renal outer medulla potassium channel
- CCD
cortical collecting duct
- PTK
protein tyrosine kinase
- ROS
reactive oxygen species
- MAPK
mitogen-activated protein kinase
- AMPK
AMP-dependent protein kinase
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Kurtzman NA, Gonzalez J, DeFronzo R, Giebisch G. A patient with hyperkalemia and metabolic acidosis. Am. J. Kidney Dis. 1990;15:333–56. doi: 10.1016/s0272-6386(12)80080-1. [DOI] [PubMed] [Google Scholar]
- 2.Bia MJ, DeFronzo RA. Extrarenal potassium homeostasis. Am. J. Physiol. 1981;240:F257–68. doi: 10.1152/ajprenal.1981.240.4.F257. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine Panel on Dietary Reference Intakes . Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Natl. Acad.; Washington, D.C.: 2004. [Google Scholar]
- 4.Thompson CB, Choi C, Youn JH, McDonough AA. Temporal responses of oxidative vs glycolytic skeletal muscles to K+ deprivation: Na+ pumps and cell cations. Am. J. Physiol. 1999;276:C1411–19. doi: 10.1152/ajpcell.1999.276.6.C1411. [DOI] [PubMed] [Google Scholar]
- 5.Sejersted OM, Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol. Rev. 2000;80:1411–81. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- 6.Giebisch G, Wang W. Potassium transport: from clearance to channels and pumps. Kidney Int. 1996;49:1624–31. doi: 10.1038/ki.1996.236. [DOI] [PubMed] [Google Scholar]
- 7.Wang W. Regulation of renal K transport by dietary K intake. Annu. Rev. Physiol. 2004;66:547–69. doi: 10.1146/annurev.physiol.66.032102.112025. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Felig P, Ferrannini E, Wahren J. Effect of graded doses of insulin on splanchnic and peripheral potassium metabolism in man. Am. J. Physiol. 1980;238:E421–27. doi: 10.1152/ajpendo.1980.238.5.E421. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz RM. Osmoregulation in marine mammals. J. Exp. Biol. 2001;204:1831–44. doi: 10.1242/jeb.204.11.1831. [DOI] [PubMed] [Google Scholar]
- 10.Weiner ID, Wingo CS. Hypokalemia—consequences, causes, and correction. J. Am. Soc. Nephrol. 1997;8:1179–88. doi: 10.1681/ASN.V871179. [DOI] [PubMed] [Google Scholar]
- 11.Heppel LA. The electrolytes of muscle and liver in potassium depleted rats. Am. J. Physiol. 1939;127:385–92. [Google Scholar]
- 12.Norgaard A, Kjeldsen K, Clausen T. Potassium depletion decreases the number of 3H-ouabain binding sites and the active Na-K transport in skeletal muscle. Nature. 1981;293:739–41. doi: 10.1038/293739a0. [DOI] [PubMed] [Google Scholar]
- 13.Giebisch G. Renal potassium transport: mechanisms and regulation. Am. J. Physiol. 1998;274:F817–33. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 14.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am. J. Physiol. Renal Physiol. 2001;280:F786–93. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 15.Field MJ, Stanton BA, Giebisch GH. Differential acute effects of aldosterone, dexamethasone, and hyperkalemia on distal tubular potassium secretion in the rat kidney. J. Clin. Investig. 1984;74:1792–802. doi: 10.1172/JCI111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Mol. Cell. Endocrinol. 2004;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Rabinowitz L. Aldosterone and potassium homeostasis. Kidney Int. 1996;49:1738–42. doi: 10.1038/ki.1996.258. [DOI] [PubMed] [Google Scholar]
- 18.Rabinowitz L. Model of homeostatic regulation of potassium excretion in sheep. Am. J. Physiol. 1988;254:R381–88. doi: 10.1152/ajpregu.1988.254.2.R381. [DOI] [PubMed] [Google Scholar]
- 19.Calo L, Borsatti A, Favaro S, Rabinowitz L. Kaliuresis in normal subjects following oral potassium citrate intake without increased plasma potassium concentration. Nephron. 1995;69:253–58. doi: 10.1159/000188466. [DOI] [PubMed] [Google Scholar]
- 20.Rabinowitz L, Sarason RL, Yamauchi H. Effects of KCl infusion on potassium excretion in sheep. Am. J. Physiol. 1985;249:F263–71. doi: 10.1152/ajprenal.1985.249.2.F263. [DOI] [PubMed] [Google Scholar]
- 21.Rabinowitz L, Green DM, Sarason RL, Yamauchi H. Homeostatic potassium excretion in fed and fasted sheep. Am. J. Physiol. 1988;254:R357–80. doi: 10.1152/ajpregu.1988.254.2.R357. [DOI] [PubMed] [Google Scholar]
- 22.McDonough AA, Thompson CB, Youn JH. Skeletal muscle regulates extracellular potassium. Am. J. Physiol. Renal Physiol. 2002;282:F967–74. doi: 10.1152/ajprenal.00360.2001. [DOI] [PubMed] [Google Scholar]
- 23.Morita H, Fujiki N, Hagiike M, Yamaguchi O, Lee K. Functional evidence for involvement of bumetanide-sensitive Na+K+2Cl− cotransport in the hepatoportal Na+ receptor of the Sprague-Dawley rat. Neurosci. Lett. 1999;264:65–68. doi: 10.1016/s0304-3940(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 24.Morita H, Fujiki N, Miyahara T, Lee K, Tanaka K. Hepatoportal bumetanide-sensitive K+-sensor mechanism controls urinary K+ excretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R1134–39. doi: 10.1152/ajpregu.2000.278.5.R1134. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya Y, Nakashima S, Banno Y, Suzuki Y, Morita H. Effect of high-NaCl or high-KCl diet on hepatic Na+- and K+-receptor sensitivity and NKCC1 expression in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R591–96. doi: 10.1152/ajpregu.00559.2003. [DOI] [PubMed] [Google Scholar]
- 26.Michell AR, Debnam ES, Unwin RJ. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu. Rev. Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330. [DOI] [PubMed] [Google Scholar]
- 27.Choi CS, Lee FN, McDonough AA, Youn JH. Independent regulation of in vivo insulin action on glucose versus K+ uptake by dietary fat and K+ content. Diabetes. 2002;51:915–20. doi: 10.2337/diabetes.51.4.915. [DOI] [PubMed] [Google Scholar]
- 28.Chen P, Guzman JP, Leong PK, Yang LE, Perianayagam A, et al. Modest dietary K+ restriction provokes insulin resistance of cellular K+ uptake and phosphorylation of renal outer medulla K+ channel without fall in plasma K+ concentration. Am. J. Physiol. Cell Physiol. 2006;290:C1355–63. doi: 10.1152/ajpcell.00501.2005. [DOI] [PubMed] [Google Scholar]
- 29.Lee FN, Oh G, McDonough AA, Youn JH. Evidence for gut factor in K+ homeostasis. Am. J. Physiol. Renal Physiol. 2007;293:F541–47. doi: 10.1152/ajprenal.00427.2006. [DOI] [PubMed] [Google Scholar]
- 30.Jackson CA. Rapid renal potassium adaptation in rats. Am. J. Physiol. 1992;263:F1098–104. doi: 10.1152/ajprenal.1992.263.6.F1098. [DOI] [PubMed] [Google Scholar]
- 31.Allon M, Dansby L, Shanklin N. Glucose modulation of the disposal of an acute potassium load in patients with end-stage renal disease. Am. J. Med. 1993;94:475–82. doi: 10.1016/0002-9343(93)90081-Y. [DOI] [PubMed] [Google Scholar]
- 32.Alvo M, Krsulovic P, Fernandez V, Espinoza AM, Escobar M, Marusic ET. Effect of a simultaneous potassium and carbohydrate load on extrarenal K homeostasis in end-stage renal failure. Nephron. 1989;53:133–37. doi: 10.1159/000185725. [DOI] [PubMed] [Google Scholar]
- 33.Muto S, Sebata K, Watanabe H, Shoji F, Yamamoto Y, et al. Effect of oral glucose administration on serum potassium concentration in hemodialysis patients. Am. J. Kidney Dis. 2005;46:697–705. doi: 10.1053/j.ajkd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Premen AJ. Splanchnic and renal hemodynamic responses to intraportal infusion of glucagon. Am. J. Physiol. 1987;253:F1105–12. doi: 10.1152/ajprenal.1987.253.6.F1105. [DOI] [PubMed] [Google Scholar]
- 35.Pullman TN, Lavender AR, Aho I. Direct effects of glucagon on renal hemodynamics and excretion of inorganic ions. Metabolism. 1967;16:358–73. doi: 10.1016/0026-0495(67)90047-9. [DOI] [PubMed] [Google Scholar]
- 36.Strange RC, Mjos OD. The sources of plasma cyclic AMP: studies in the rat using isoprenaline, nicotinic acid and glucagon. Eur. J. Clin. Investig. 1975;5:147–52. doi: 10.1111/j.1365-2362.1975.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 37.Ahloulay M, Dechaux M, Laborde K, Bankir L. Influence of glucagon on GFR and on urea and electrolyte excretion: direct and indirect effects. Am. J. Physiol. 1995;269:F225–35. doi: 10.1152/ajprenal.1995.269.2.F225. [DOI] [PubMed] [Google Scholar]
- 38.Ahloulay M, Dechaux M, Hassler C, Bouby N, Bankir L. Cyclic AMP is a hepatorenal link influencing natriuresis and contributing to glucagon-induced hyperfiltration in rats. J. Clin. Investig. 1996;98:2251–58. doi: 10.1172/JCI119035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bankir L, Martin H, Dechaux M, Ahloulay M. Plasma cAMP: a hepatorenal link influencing proximal reabsorption and renal hemodynamics? Kidney Int. Suppl. 1997;59:S50–56. [PubMed] [Google Scholar]
- 40.Forte LR, London RM, Freeman RH, Krause WJ. Guanylin peptides: renal actions mediated by cyclic GMP. Am. J. Physiol. Renal Physiol. 2000;278:F180–91. doi: 10.1152/ajprenal.2000.278.2.F180. [DOI] [PubMed] [Google Scholar]
- 41.Sindic A, Schlatter E. Renal electrolyte effects of guanylin and uroguanylin. Curr. Opin. Nephrol. Hypertens. 2007;16:10–15. doi: 10.1097/MNH.0b013e328011cb4a. [DOI] [PubMed] [Google Scholar]
- 42.Sindic A, Velic A, Basoglu C, Hirsch JR, Edemir B, et al. Uroguanylin and guanylin regulate transport of mouse cortical collecting duct independent of guanylate cyclase C. Kidney Int. 2005;68:1008–17. doi: 10.1111/j.1523-1755.2005.00518.x. [DOI] [PubMed] [Google Scholar]
- 43.Sternini C, Anselmi L, Rozengurt E. Enteroendocrine cells: a site of ‘taste’ in gastrointestinal chemosensing. Curr. Opin. Endocrinol. Diabetes Obes. 2008;15:73–78. doi: 10.1097/MED.0b013e3282f43a73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol. Interv. 2008;8:78–81. doi: 10.1124/mi.8.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin DH, Sterling H, Wang WH. The protein tyrosine kinase-dependent pathway mediates the effect of K intake on renal K secretion. Physiology (Bethesda) 2005;20:140–46. doi: 10.1152/physiol.00044.2004. [DOI] [PubMed] [Google Scholar]
- 46.Wang WH. Regulation of ROMK (Kir1.1) channels: new mechanisms and aspects. Am. J. Physiol. Renal Physiol. 2006;290:F14–19. doi: 10.1152/ajprenal.00093.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giebisch GH. A trail of research on potassium. Kidney Int. 2002;62:1498–512. doi: 10.1046/j.1523-1755.2002.t01-2-00644.x. [DOI] [PubMed] [Google Scholar]
- 48.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol. Rev. 2005;85:319–71. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pluznick JL, Sansom SC. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am. J. Physiol. Renal Physiol. 2006;291:F517–29. doi: 10.1152/ajprenal.00118.2006. [DOI] [PubMed] [Google Scholar]
- 50.Grimm PR, Sansom SC. BK channels in the kidney. Curr. Opin. Nephrol. Hypertens. 2007;16:430–36. doi: 10.1097/MNH.0b013e32826fbc7d. [DOI] [PubMed] [Google Scholar]
- 51.Brandis M, Keyes J, Windhager EE. Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am. J. Physiol. 1972;222:421–27. doi: 10.1152/ajplegacy.1972.222.2.421. [DOI] [PubMed] [Google Scholar]
- 52.Sufit CR, Jamison RL. Effect of acute potassium load on reabsorption in Henle’s loop in the rat. Am. J. Physiol. 1983;245:F569–76. doi: 10.1152/ajprenal.1983.245.5.F569. [DOI] [PubMed] [Google Scholar]
- 53.Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am. J. Physiol. Renal Physiol. 2004;286:F881–92. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, et al. Dietary K+ regulates apical membrane expression of maxi-K channels in rabbit cortical collecting duct. Am. J. Physiol. Renal Physiol. 2005;289:F922–32. doi: 10.1152/ajprenal.00057.2005. [DOI] [PubMed] [Google Scholar]
- 55.Mennitt PA, Frindt G, Silver RB, Palmer LG. Potassium restriction downregulates ROMK expression in rat kidney. Am. J. Physiol. Renal Physiol. 2000;278:F916–24. doi: 10.1152/ajprenal.2000.278.6.F916. [DOI] [PubMed] [Google Scholar]
- 56.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J. Gen. Physiol. 1994;104:693–710. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am. J. Physiol. 1999;277:F805–12. doi: 10.1152/ajprenal.1999.277.5.F805. [DOI] [PubMed] [Google Scholar]
- 58.Wang T, Sterling H, Shao WA, Yan Q, Bailey MA, et al. Inhibition of heme oxygenase decreases sodium and fluid absorption in the loop of Henle. Am. J. Physiol. Renal Physiol. 2003;285:F484–90. doi: 10.1152/ajprenal.00135.2003. [DOI] [PubMed] [Google Scholar]
- 59.Gu RM, Wei Y, Jiang HL, Lin DH, Sterling H, et al. K depletion enhances the extracellular Ca2+-induced inhibition of the apical K channels in the mTAL of rat kidney. J. Gen. Physiol. 2002;119:33–44. doi: 10.1085/jgp.119.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walter SJ, Shore AC, Shirley DG. Effect of potassium depletion on renal tubular function in the rat. Clin. Sci. (London) 1988;75:621–28. doi: 10.1042/cs0750621. [DOI] [PubMed] [Google Scholar]
- 61.Silver RB, Soleimani M. H+-K+-ATPases: regulation and role in pathophysiological states. Am. J. Physiol. 1999;276:F799–811. doi: 10.1152/ajprenal.1999.276.6.F799. [DOI] [PubMed] [Google Scholar]
- 62.Babilonia E, Wei Y, Sterling H, Kaminski P, Wolin M, Wang WH. Superoxide anions are involved in mediating the effect of low K intake on c-Src expression and renal K secretion in the cortical collecting duct. J. Biol. Chem. 2005;280:10790–96. doi: 10.1074/jbc.M414610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Babilonia E, Lin D, Zhang Y, Wei Y, Yue P, Wang WH. Role of gp91phox-containing NADPH oxidase in mediating the effect of K restriction on ROMK channels and renal K excretion. J. Am. Soc. Nephrol. 2007;18:2037–45. doi: 10.1681/ASN.2006121333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein tyrosine kinase. Am. J. Physiol. Renal Physiol. 2001;281:F206–12. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y, Lin DH, Wang ZJ, Jin Y, Yang B, Wang WH. K restriction inhibits protein phosphatase 2B (PP2B) and suppression of PP2B decreases ROMK channel activity in the CCD. Am. J. Physiol. Cell Physiol. 2008;294:C765–73. doi: 10.1152/ajpcell.00528.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Babilonia E, Li D, Wang Z, Sun P, Lin DH, et al. Mitogen-activated protein kinases inhibit the ROMK (Kir 1.1)-like small conductance K channels in the cortical collecting duct. J. Am. Soc. Nephrol. 2006;17:2687–96. doi: 10.1681/ASN.2006050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X, Lin DH, Jin Y, Wang KS, Zhang Y, et al. Inhibitor of growth 4 (ING4) is up-regulated by a low K intake and suppresses renal outer medullary K channels (ROMK) by MAPK stimulation. Proc. Natl. Acad. Sci. USA. 2007;104:9517–22. doi: 10.1073/pnas.0703383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R913–35. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 69.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J. Am. Soc. Nephrol. 2007;18:2439–46. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 70.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am. J. Physiol. 1996;271:F143–49. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 71.Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J. Biol. Chem. 2007;282:6455–62. doi: 10.1074/jbc.M607477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson CB, McDonough AA. Skeletal muscle Na,K-ATPase α and β subunit protein levels respond to hypokalemic challenge with isoform and muscle type specificity. J. Biol. Chem. 1996;271:32653–58. doi: 10.1074/jbc.271.51.32653. [DOI] [PubMed] [Google Scholar]
- 73.Amlal H, Krane CM, Chen Q, Soleimani M. Early polyuria and urinary concentrating defect in potassium deprivation. Am. J. Physiol. Renal Physiol. 2000;279:F655–63. doi: 10.1152/ajprenal.2000.279.4.F655. [DOI] [PubMed] [Google Scholar]
- 74.McKenna MJ, Gissel H, Clausen T. Effects of electrical stimulation and insulin on Na+-K+-ATPase ([3H]ouabain binding) in rat skeletal muscle. J. Physiol. 2003;547:567–80. doi: 10.1113/jphysiol.2003.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Choi CS, Thompson CB, Leong PK, McDonough AA, Youn JH. Short-term K+ deprivation provokes insulin resistance of cellular K+ uptake revealed with the K+ clamp. Am. J. Physiol. Renal Physiol. 2001;280:F95–102. doi: 10.1152/ajprenal.2001.280.1.F95. [DOI] [PubMed] [Google Scholar]
- 76.Clausen T. Na+-K+pump regulation and skeletal muscle contractility. Physiol. Rev. 2003;83:1269–324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- 77.Azuma KK, Hensley CB, Putnam DS, McDonough AA. Hypokalemia decreases Na+-K+-ATPase α2-but not α1-isoform abundance in heart, muscle, and brain. Am. J. Physiol. 1991;260:C958–64. doi: 10.1152/ajpcell.1991.260.5.C958. [DOI] [PubMed] [Google Scholar]
- 78.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of β-cell function: the hyperbolic correction. Diabetes. 2002;51(Suppl. 1):S212–20. doi: 10.2337/diabetes.51.2007.s212. [DOI] [PubMed] [Google Scholar]
- 79.Arslanian S, Austin A. Impaired insulin mediated potassium uptake in adolescents with IDDM. Biochem. Med. Metab. Biol. 1991;46:364–72. doi: 10.1016/0885-4505(91)90084-x. [DOI] [PubMed] [Google Scholar]
- 80.DeFronzo RA. Obesity is associated with impaired insulin-mediated potassium uptake. Metabolism. 1988;37:105–8. doi: 10.1016/s0026-0495(98)90001-4. [DOI] [PubMed] [Google Scholar]
- 81.Alvestrand A, Wahren J, Smith D, DeFronzo RA. Insulin-mediated potassium uptake is normal in uremic and healthy subjects. Am. J. Physiol. 1984;246:E174–80. doi: 10.1152/ajpendo.1984.246.2.E174. [DOI] [PubMed] [Google Scholar]
- 82.Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am. J. Physiol. 1986;251:E576–83. doi: 10.1152/ajpendo.1986.251.5.E576. [DOI] [PubMed] [Google Scholar]
- 83.Kim JK, Wi JK, Youn JH. Metabolic impairment precedes insulin resistance in skeletal muscle during high-fat feeding in rats. Diabetes. 1996;45:651–58. doi: 10.2337/diab.45.5.651. [DOI] [PubMed] [Google Scholar]
- 84.Graham TE, Kahn BB. Tissue-specific alterations of glucose transport and molecular mechanisms of intertissue communication in obesity and type 2 diabetes. Horm. Metab. Res. 2007;39:717–21. doi: 10.1055/s-2007-985879. [DOI] [PubMed] [Google Scholar]
- 85.Benziane B, Chibalin AV. Skeletal muscle sodium pump regulation: a translocation paradigm. Am. J. Physiol. Endocrinol. Metab. 2008;295:E553–58. doi: 10.1152/ajpendo.90261.2008. [DOI] [PubMed] [Google Scholar]
- 86.Kristensen M, Rasmussen MK, Juel C. Na+-K+ pump location and translocation during muscle contraction in rat skeletal muscle. Pflüg. Arch. 2008;456:979–89. doi: 10.1007/s00424-008-0449-x. [DOI] [PubMed] [Google Scholar]
- 87.Rasmussen MK, Kristensen M, Juel C. Exercise-induced regulation of phospholemman (FXYD1) in rat skeletal muscle: implications for Na+/K+-ATPase activity. Acta Physiol. (Oxf.) 2008;194:67–79. doi: 10.1111/j.1748-1716.2008.01857.x. [DOI] [PubMed] [Google Scholar]
- 88.Weinstein SP, Paquin T, Pritsker A, Haber RS. Glucocorticoid-induced insulin resistance: Dexamethasone inhibits the activation of glucose transport in rat skeletal muscle by both insulin- and noninsulin-related stimuli. Diabetes. 1995;44:441–45. doi: 10.2337/diab.44.4.441. [DOI] [PubMed] [Google Scholar]
- 89.Dorup I, Clausen T. Effects of adrenal steroids on the concentration of Na+-K+ pumps in rat skeletal muscle. J. Endocrinol. 1997;152:49–57. doi: 10.1677/joe.0.1520049. [DOI] [PubMed] [Google Scholar]
- 90.Thompson CB, Dorup I, Ahn J, Leong PK, McDonough AA. Glucocorticoids increase sodium pump α2- and β1-subunit abundance and mRNA in rat skeletal muscle. Am. J. Physiol. Cell Physiol. 2001;280:C509–16. doi: 10.1152/ajpcell.2001.280.3.C509. [DOI] [PubMed] [Google Scholar]
- 91.Rhee MS, Perianayagam A, Chen P, Youn JH, McDonough AA. Dexamethasone treatment causes resistance to insulin-stimulated cellular potassium uptake in the rat. Am. J. Physiol. Cell Physiol. 2004;287:C1229–37. doi: 10.1152/ajpcell.00111.2004. [DOI] [PubMed] [Google Scholar]
- 92.Alexander EA, Levinsky NG. An extrarenal mechanism of potassium adaptation. J. Clin. Investig. 1968;47:740–48. doi: 10.1172/JCI105769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blachley JD, Crider BP, Johnson JH. Extrarenal potassium adaptation: role of skeletal muscle. Am. J. Physiol. 1986;251:F313–18. doi: 10.1152/ajprenal.1986.251.2.F313. [DOI] [PubMed] [Google Scholar]
- 94.Sadre M, Sheng HP, Fiorotto M, Nichols BL. Electrolyte composition changes of chronically K-depleted rats after K loading. J. Appl. Physiol. 1987;63:765–69. doi: 10.1152/jappl.1987.63.2.765. [DOI] [PubMed] [Google Scholar]
- 95.Terkildsen JRCE, Smith NP. The balance between inactivation and activation of the Na+-K+ pump underlies the triphasic accumulation of extracellular K+ ions during myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H3036–45. doi: 10.1152/ajpheart.00771.2007. [DOI] [PubMed] [Google Scholar]
- 96.Zheng D, Perianayagam A, Lee DH, Brannan MD, Yang LE, et al. AMPK activation with AICAR provokes an acute fall in plasma [K+] Am. J. Physiol. Cell Physiol. 2008;294:C126–35. doi: 10.1152/ajpcell.00464.2007. [DOI] [PubMed] [Google Scholar]
- 97.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 98.Mu J, Barton ER, Birnbaum MJ. Selective suppression of AMP-activated protein kinase in skeletal muscle: update on ‘lazy mice’. Biochem. Soc. Trans. 2003;31:236–41. doi: 10.1042/bst0310236. [DOI] [PubMed] [Google Scholar]
- 99.Gollob MH, Seger JJ, Gollob TN, Tapscott T, Gonzales O, et al. Novel PRKAG2 mutation responsible for the genetic syndrome of ventricular preexcitation and conduction system disease with childhood onset and absence of cardiac hypertrophy. Circulation. 2001;104:3030–33. doi: 10.1161/hc5001.102111. [DOI] [PubMed] [Google Scholar]
- 100.Bergeron R, Previs SF, Cline GW, Perret P, Russell RR, 3rd, et al. Effect of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside infusion on in vivo glucose and lipid metabolism in lean and obese Zucker rats. Diabetes. 2001;50:1076–82. doi: 10.2337/diabetes.50.5.1076. [DOI] [PubMed] [Google Scholar]
- 101.Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell. 2001;7:1085–94. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- 102.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. doi: 10.1161/01.HYP.0000202568.01167.B6. [DOI] [PubMed] [Google Scholar]
- 103.Young DB, Lin H, McCabe RD. Potassium’s cardiovascular protective mechanisms. Am. J. Physiol. 1995;268:R825–37. doi: 10.1152/ajpregu.1995.268.4.R825. [DOI] [PubMed] [Google Scholar]
- 104.Kimura M, Lu X, Skurnick J, Awad G, Bogden J, et al. Potassium chloride supplementation diminishes platelet reactivity in humans. Hypertension. 2004;44:969–73. doi: 10.1161/01.HYP.0000147660.58694.6f. [DOI] [PubMed] [Google Scholar]
- 105.Braschi A, Naismith DJ. The effect of a dietary supplement of potassium chloride or potassium citrate on blood pressure in predominantly normotensive volunteers. Br. J. Nutr. 2008;99:1284–92. doi: 10.1017/S0007114507864853. [DOI] [PubMed] [Google Scholar]
- 106.Macdonald JE, Struthers AD. What is the optimal serum potassium level in cardiovascular patients? J. Am. Coll. Cardiol. 2004;43:155–61. doi: 10.1016/j.jacc.2003.06.021. [DOI] [PubMed] [Google Scholar]
- 107.Cohen HW, Madhavan S, Alderman MH. High and low serum potassium associated with cardiovascular events in diuretic-treated patients. J. Hypertens. 2001;19:1315–23. doi: 10.1097/00004872-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 108.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N. Engl. J. Med. 2004;351:543–51. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 109.Yang BC, Li DY, Weng YF, Lynch J, Wingo CS, Mehta JL. Increased superoxide anion generation and altered vasoreactivity in rabbits on low-potassium diet. Am. J. Physiol. 1998;274:H1955–61. doi: 10.1152/ajpheart.1998.274.6.H1955. [DOI] [PubMed] [Google Scholar]
- 110.Matsui H, Shimosawa T, Uetake Y, Wang H, Ogura S, et al. Protective effect of potassium against the hypertensive cardiac dysfunction: association with reactive oxygen species reduction. Hypertension. 2006;48:225–31. doi: 10.1161/01.HYP.0000232617.48372.cb. [DOI] [PubMed] [Google Scholar]
- 111.Kido M, Ando K, Onozato ML, Tojo A, Yoshikawa M, et al. Protective effect of dietary potassium against vascular injury in salt-sensitive hypertension. Hypertension. 2008;51:225–31. doi: 10.1161/HYPERTENSIONAHA.107.098251. [DOI] [PubMed] [Google Scholar]