Background
Mesenchymal stem cells (MSCs) are a valuable cell source in regenerative medicine. Recently, several studies have shown that MSCs can be easily isolated from human amnion. In this study, we investigated the therapeutic effect of transplantation of human amnion-derived MSCs (hAMSCs) in rats with liver fibrosis.
Methods
Liver fibrosis was induced by an intraperitoneal injection of 2 mL/kg of 50% carbon tetrachloride twice a week for 6 weeks. At 3 weeks, hAMSCs (1 × 106 cells) were transplanted intravenously. Rats were sacrificed at 7 weeks, and histological analyses and quantitative reverse-transcription polymerase chain reaction were performed. In vitro experiments were conducted to investigate the effect of hAMSCs on the activation of Kupffer cells.
Results
Transplantation of hAMSCs significantly reduced the fibrotic area, deposition of type-I collagen, the number of α-smooth muscle actin–positive hepatic stellate cells, and CD68-positive Kupffer cells in the livers. messenger RNA expression of α-smooth muscle actin and tissue inhibitor of metalloproteinase-1 was significantly decreased and the expression of matrix metalloproteinase-9 and hepatocyte growth factor was significantly increased in the liver of hAMSC-treated rats. Transplantation of hAMSCs at 3 weeks plus 5 weeks did not have an additive effect. In vitro experiments demonstrated that Kupffer cell activation induced by lipopolysaccharide was significantly decreased by culturing with conditioned medium obtained from hAMSCs.
Conclusions
Transplantation of hAMSCs provided significant improvement in a rat model of liver fibrosis, possibly through the inhibition of Kupffer cell and hepatic stellate cell activation. hAMSCs may be a potential new treatment for liver fibrosis.
Liver cirrhosis is a progressed stage of fibrosis caused by chronic liver injury caused by various factors, such as viral infections, alcohol, drugs, and chemical toxicity.1 The only effective available treatment for end-stage liver cirrhosis is liver transplantation. However, because of the lack of donors, complications, and organ rejection after liver transplantation, alternative treatments are needed.2
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into various lineages, including bone, cartilage, or fat, and are present in adult tissues.3 At present, MSCs have been investigated in regenerative medicine because of their ability to differentiate and their potential to heal damaged tissues by the secretion of various growth factors and anti-inflammatory molecules.4,5 The efficacy of autologous and allogeneic MSC transplantation in patients with liver cirrhosis has recently been reported.6-9
The fetal membrane comprises amnion and chorion, which envelops the developing fetus. Although the human fetal membrane is generally discarded as medical waste after delivery, fetal tissues have been found to be rich sources of MSCs.10-13 We have previously reported that systemic administration of amnion-derived MSCs (AMSCs) improved rats with hindlimb ischemia,14 myocarditis,15,16 glomerulonephritis,17 and ischemia-reperfusion–induced acute kidney injury18 by inducing angiogenesis and anti-inflammatory effects.
In this study, we investigated whether the administration of human AMSCs (hAMSCs) improves carbon tetrachloride (CCl4)–induced liver fibrosis in rats and explored its underlying mechanisms.
MATERIALS AND METHODS
Isolation and Expansion of hAMSCs
The Medical Ethical Committee of Hokkaido University Graduate School of Medicine, Sapporo, Japan approved this work, and all pregnant women gave written informed consent. The human fetal membrane was obtained during caesarean deliveries, and the amnion was manually peeled from the chorion. The hAMSCs were isolated and expanded by digestion with collagenase type III (Worthington Biochemical Corporation, Lakewood, NJ), followed by seeding in uncoated plastic dishes with minimal essential medium (MEM) α (Life Technologies, Carlsbad, LA) supplemented with 10% fetal bovine serum (Life Technologies), 100 U/mL of penicillin, and 100 ng/mL of streptomycin (Wako Pure Chemical Industries, Osaka, Japan). The culture was maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. After 3 to 4 days in culture, the nonadherent cells were removed, and the adherent cells were maintained in culture until they reached 80% confluence. The passage was performed using 0.5% trypsin-ethylenediaminetetraacetic acid (Life Technologies).
Differentiation of hAMSCs Into Adipocytes and Osteocytes
The hAMSCs were seeded onto 6-well plates, and differentiation into adipocytes and osteocytes was induced when the hAMSCs were 80% to 90% confluent. To induce differentiation into adipocytes, hAMSCs were cultured with hMSC adipogenic differentiation medium (Lonza, Basel, Switzerland), according to the manufacturer's instructions. After 3 weeks of differentiation, cells were stained with oil red O (Sigma-Aldrich, St. Louis, MO) to confirm differentiation. To induce differentiation into osteocytes, hAMSCs were cultured in hMSC osteogenic differentiation medium (Lonza), according to the manufacturer's instructions. After 2 weeks of differentiation, cells were stained with Alizarin red S (Sigma-Aldrich) to confirm differentiation.
Flow Cytometry
Cultured hAMSCs were stained using the Human MSC Analysis Kit (BD, Franklin Lakes, NJ), which included fluorescein-isothiocyanate–conjugated antibody against CD90, PerCP-Cy5.5–conjugated antibody against CD105, phycoerythrin-conjugated CD44, and allophycocyanin-conjugated antibody against CD73 as well as a negative cocktail (phycoerythrin-conjugated CD11b, CD19, CD34, CD45, and HLA-DR), according to the manufacturer's instructions. Cells were analyzed by a flow cytometer (FACSCanto II, BD).
Animals
The experimental protocol was approved by the Animal Care and Use Committees of Hokkaido University. Six-week-old male Sprague–Dawley rats were procured from Japan SLC (Hamamatsu, Japan), and 3 rats were housed per cage in a temperature-controlled room (24°C) on a 12-hour/12-hour light/dark cycle. All rats had ad libitum access to standard chow and water.
Induction of Liver Fibrosis and hAMSC Transplantation
Liver fibrosis was induced by an intraperitoneal injection of 2 mL/kg of 50% CCl4 (Wako Pure Chemical Industries) in olive oil twice a week for 6 weeks. In the Control group, rats were injected with olive oil alone (Figure 1). One million hAMSCs suspended in 200 μL of phosphate-buffered saline were intravenously injected through the penile vein after 2 weeks of CCl4 treatment. Two hundred microliters of phosphate-buffered saline was injected in the untreated rats and those treated with CCl4 (Figure 1).
FIGURE 1.
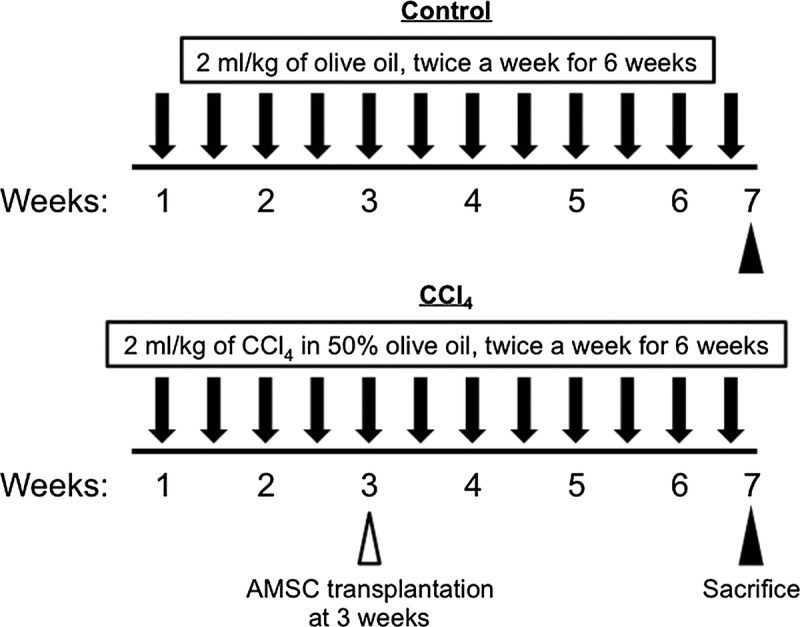
Experimental protocol for CCl4-induced liver fibrosis. Rats received 2 mg/kg of CCl4 in 50% olive oil twice a week for 6 weeks. hAMSCs (1 × 106 cells) were infused intravenously at 3 weeks. All rats were sacrificed at 7 weeks.
Histological Examination
All rats were sacrificed after 6 weeks of CCl4 treatment. The left lobe of the liver was removed, fixed in 40 g/L of formaldehyde saline, embedded in paraffin, and cut into 5-μm sections. Tissue sections were stained with Masson trichrome. Ten random fields on a section from each rat were photographed, and blue-stained areas were calculated from the entire liver cross-sectional area (%, ×20) with a digital image analyzer (WinROOF; Mitani Co., Fukui, Japan).
Immunohistochemical Examination
The tissue sections were stained with anti-rat type I collagen antibody (dilution, 1:100,000; LSL, Tokyo, Japan) for 60 minutes at room temperature. To assess the activation of hepatic stellate cells (HSCs), the tissue sections were stained with anti-rat α-smooth muscle actin (SMA) antibody (1:800, Thermo Scientific, Waltham, MA, USA) for 30 minutes at room temperature. To assess the infiltration of Kupffer cells, the tissue sections were stained with anti-rat CD68 monoclonal antibody (dilution, 1:50; AbD Serotec, Kidlington, United Kingdom) for 40 min at room temperature. Ten random fields on a section from each rat were photographed, and stained areas were calculated from the entire liver cross-sectional area.
RNA Isolation and Quantitative Reverse-Transcription Polymerase Chain Reaction
Total RNA of the rat liver was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany), and 1 μg of the total RNA was reverse-transcribed into cDNA using the QuantiTect Reverse Transcription Kit (Qiagen). Polymerase chain reaction (PCR) amplification was performed using a 25-μL reaction mixture that contained 1 μL of cDNA and 12.5 μL of Platinum SYBR Green PCR Mix (Invitrogen, Carlsbad, CA). β-actin messenger RNA that was amplified from the same samples served as an internal control. After initial denaturation at 95°C for 2 minutes, a 2-step cycle procedure was used (denaturation at 95°C for 15 seconds, annealing and extension at 60°C for 1 minute) for 40 cycles in a 7700 Sequence Detector (Applied Biosystems, Foster City, CA). Gene expression levels were determined using the comparative threshold cycle (ddCt) method with β-actin used as an endogenous control. Data were analyzed with Sequence Detection Systems software (Applied Biosystems). Primer sequences are shown in Table 1.
TABLE 1.
qRT-PCR Primer Sequences

In Vitro Experiments Using Rat Kupffer Cells
Livers were excised from 8-week-old Sprague–Dawley rats, perfused with collagenase and centrifuged twice (90g for 1 minute). The cell fraction in the supernatant was centrifuged again (690g for 5 minutes), and the obtained cell pellet was suspended with serum-free Dulbecco MEM (DMEM, Life Technologies). The cells were then seeded onto a noncoated plate for 45 minutes at room temperature, and the adhesive cells were regarded as Kupffer cells. The next day, cells were treated with 100 ng/mL lipopolysaccharide (LPS) in standard medium or conditioned medium obtained from hAMSCs for 4 hours. A conditioned medium was collected by culturing subconfluent hAMSCs with serum-free MEM α for 48 hours, and standard medium was collected by incubating serum-free MEM α without hAMSCs for 48 hours. Cell number was evaluated by measuring the cellular level of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, indicative of the mitochondrial function of living cells and cell viability, using a CellTiter96 AQueous One Solution Kit (Promega, Madison, WI) and a microplate reader (490 nm, Promega). Concentration of rat TNF-α in the culture media was measured using the Quantikine ELISA Kit (R&D Systems, Minneapolis, MN), following the manufacturer's instructions.
Statistical Analysis
Data are shown as mean ± SEM. Parameters among the groups were compared by 1-way analysis of variance, followed by a Tukey test. Differences were considered significant at P less than 0.05.
RESULTS
Characterization of hAMSCs
To evaluate the multipotency of hAMSCs, we induced differentiation of cultured hAMSCs into adipocytes and osteocytes. The hAMSCs differentiated into adipocytes and osteocytes, as demonstrated by oil red O and Alizarin red S staining, respectively (Figure 2A). Flow cytometry of cultured hAMSCs showed that they expressed CD44, CD73, CD90, and CD105, but not CD11b, CD19, CD34, CD45 or HLA-DR, which is a characteristic expression pattern for MSCs (Figure 2B).19
FIGURE 2.
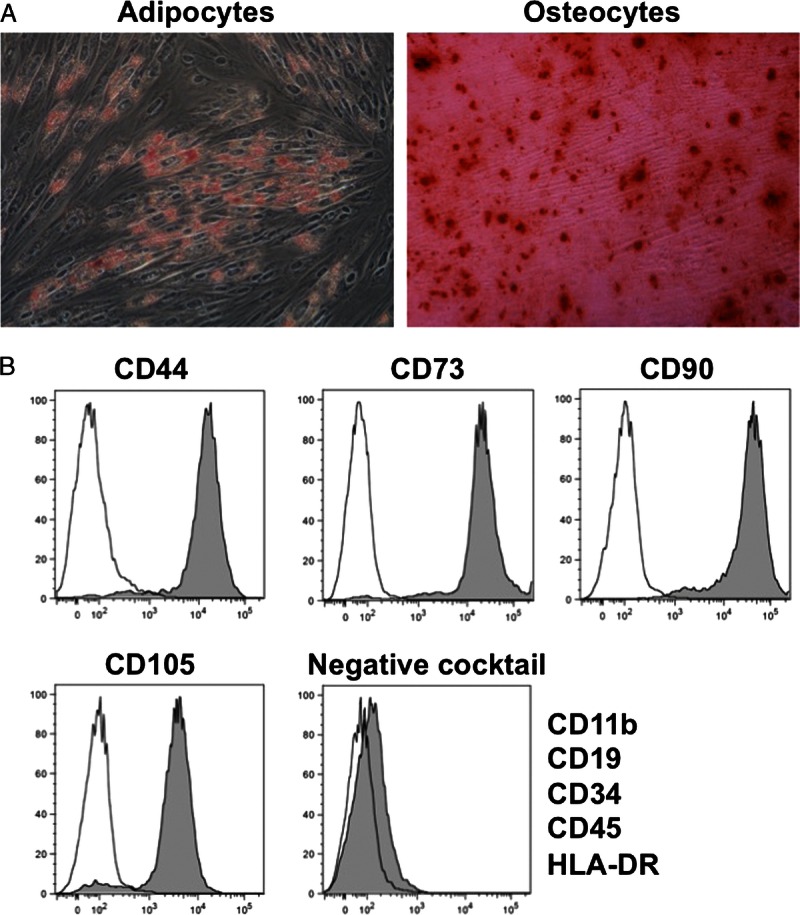
Characterization of cultured hAMSCs. A, Multipotency of hAMSCs. Differentiation into adipocytes was confirmed by the existence of lipid vesicles stained with oil red O (left). Differentiation into osteocytes was confirmed by the existence of mineral nodule deposition stained with Alizarin red S (right). B, Flow cytometry of hAMSCs. The negative cocktail contained antibodies against CD11b, CD19, CD34, CD45, and HLA-DR. Closed areas indicate staining with a specific antibody, whereas open areas represent staining with isotype control antibodies.
Effect of hAMSC Transplantation on Histological Parameters in CCl4-Treated Rats
We started with 6 rats for Control group, and 15 rats for CCl4 group and CCl4 + AMSC group, respectively. In the CCl4 group, 3 rats died at week 6. In the CCl4 + AMSC group, 2 rats died at week 6, and 3 rats died at the day of AMSC transplantation due to the overdose of pentobarbital. Therefore, we analyzed 6 control rats, 12 CCl4 rats, and 10 CCl4 + AMSC rats. In the CCl4 group, severe fibrosis was observed; however, the fibrosis was significantly attenuated by hAMSC transplantation at week 7 (Figure 3A). Consistent with this finding, the upregulated expression of type I collagen by CCl4 treatment was also attenuated by hAMSC transplantation (Figure 3B). The expression of α-SMA, a marker for the activation of HSCs, was significantly increased in the CCl4 group; however, hAMSC transplantation significantly suppressed this increase (Figure 3C). The expression of CD68, a marker for Kupffer cells, was significantly increased in the CCl4 group; however, hAMSC transplantation significantly decreased the infiltration of CD68-positive Kupffer cells (Figure 3D).
FIGURE 3.
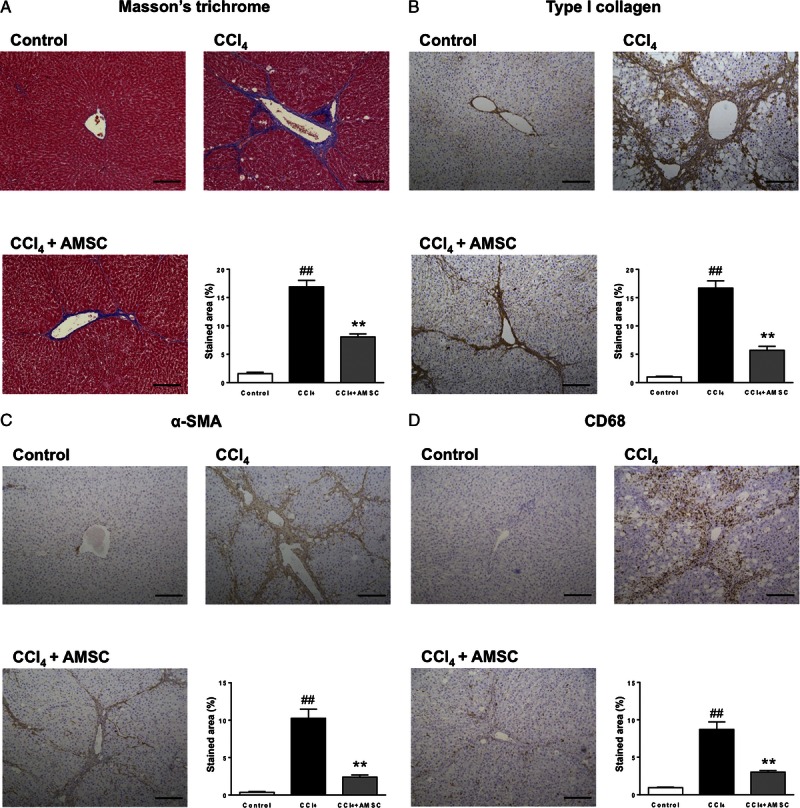
Effect of hAMSC transplantation on histological parameters in CCl4-treated rats (A) Masson’s trichrome staining. Fibrotic area was stained blue and calculated from the entire liver cross-sectional area. Immunohistochemical analyses of (B) Type I collagen, (C) α-SMA and (D) CD68 were performed, and the stained areas were calculated from the entire liver cross-sectional area. Scale bars, 200 μm. The values are reported as the mean ± SEM (n = 6 in control group, n = 12 in CCl4 group, and n = 10 in CCl4 + AMSC group). ##P < 0.01 versus Control group. **P < 0.01 versus CCl4 group.
Effects of hAMSC Transplantation on Gene Expression in CCl4-Treated Rats
We next examined the expression profile of fibrosis-related genes in the liver. The CCl4 treatment significantly increased the expression of α-SMA, matrix metalloproteinase (MMP)-2, tissue inhibitor of metalloproteinase (TIMP)-1, and TGF-β (Figure 4A, B, D, and E), and the expressions of α-SMA and TIMP-1 were significantly decreased by hAMSC transplantation (Figure 4A and D). The expressions of MMP-9 and hepatocyte growth factor (HGF) were not affected by CCl4 treatment, but significantly increased by hAMSC transplantation (Figure 4C and F). The expressions of VEGF and TGF-α were significantly decreased by CCl4 treatment, but these decreases were not attenuated by hAMSC transplantation (Figure 4G and I). The expressions of EGF and IL-10 were not affected by CCl4 treatment and hAMSC transplantation (Figure 4H and J), respectively.
FIGURE 4.
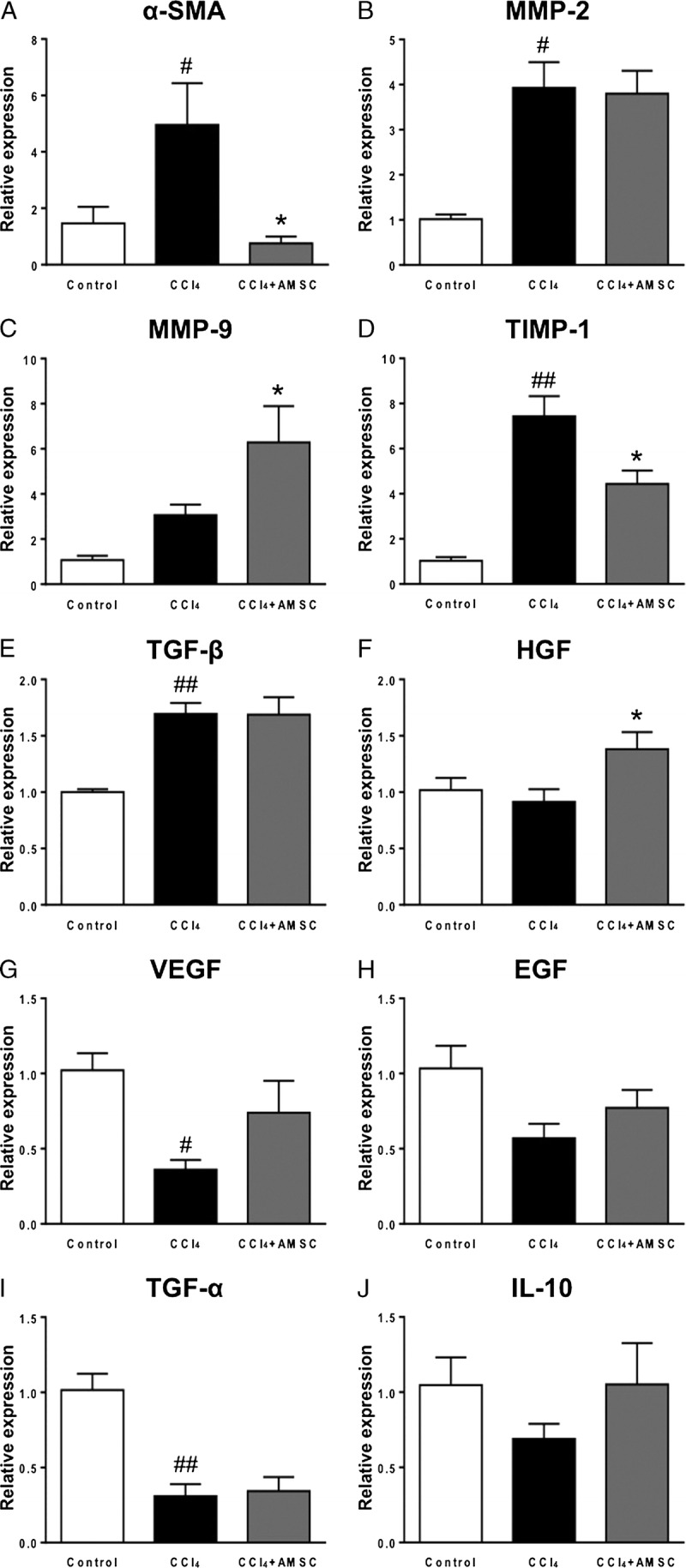
Gene expression analysis of hAMSC transplantation in CCl4-induced liver fibrosis, qRT-PCR for (A) SMA, (B) MMP-2, (C) MMP-9, (D) TIMP-1, (E) transforming growth factor (TGF)-β, (F) HGF, (G) vascular endothelial growth factor (VEGF), (H) epidermal growth factor (EGF), (I) TGF-α, and (J) IL-10. The values are reported as the mean ± SEM of (n = 6 in control group, n = 12 in CCl4 group, and n = 10 in CCl4 + AMSC group). #P < 0.05, ##P < 0.01 versus Control group. *P < 0.05 versus CCl4 group. qRT-PCR indicates quantitative reverse transcription-polymerase chain reaction.
Effect of Biweekly Transplantation of hAMSCs on Histological Parameters in CCl4-Treated Rats
To evaluate the effect of biweekly transplantation of hAMSCs, hAMSCs were transplanted after 2 and 4 weeks of CCl4 treatment, and evaluated at 7 weeks. We started with 6 rats for Control group, and 13 rats for CCl4 group, CCl4 + AMSC1 group, and CCl4 + AMSC2 group, respectively. In the CCl4 group, 2 rats died at weeks 2 and 7, and 2 rats died at week 3 due to the overdose of pentobarbital. In the CCl4 + AMSC1 group, 1 rat died at week 6, and 3 rats died at the day of AMSC transplantation due to the overdose of pentobarbital. In the CCl4 + AMSC2 group, 1 rat died at week 7, and 1 rat died at the day of AMSC transplantation due to the overdose of pentobarbital. Therefore, we analyzed 6 control rats, 9 CCl4 rats, 9 CCl4 + AMSC1 rats, and 11 CCl4 + AMSC2 rats. Histological examination demonstrated that there is no additive affect by double injection of hAMSCs (Figure 5A-D).
FIGURE 5.
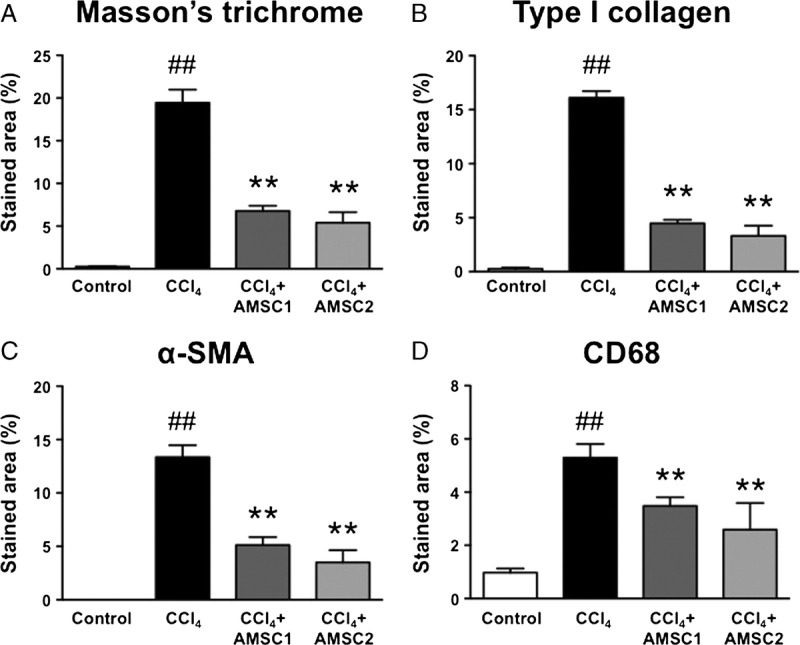
Effect of biweekly transplantation of hAMSCs on histological parameters in CCl4-treated rats, (A) Masson trichrome staining. Fibrotic area was stained blue, and calculated from the entire liver cross-sectional area. Immunohistochemical analyses of (B) type I collagen, (C) SMA, and (D) CD68 were performed, and the stained areas were calculated from the entire liver cross-sectional area. The values were the mean ± SEM (n = 6 in control group, n = 9 in CCl4 group, n = 9 in CCl4 + AMSC1 group, and n = 11 in CCl4 + AMSC2 group). ##P < 0.01 versus Control group. **P < 0.01 versus CCl4 group.
Effect of hAMSC-Conditioned Medium on the Activation of Kupffer Cells In Vitro
To investigate the effect of hAMSC on Kupffer cell activation, Kupffer cells were isolated from the rat liver, stimulated with LPS, and cultured with conditioned medium obtained from hAMSC culture. The LPS treatment did not affect the cell number (Figure 6A), but markedly increased the secretion of TNF-α. Interestingly, exposure to hAMSC-conditioned medium significantly suppressed the activation of isolated Kupffer cells. This finding was consistent across hAMSC conditioned medium obtained from 3 different donors (Figure 6B).
FIGURE 6.
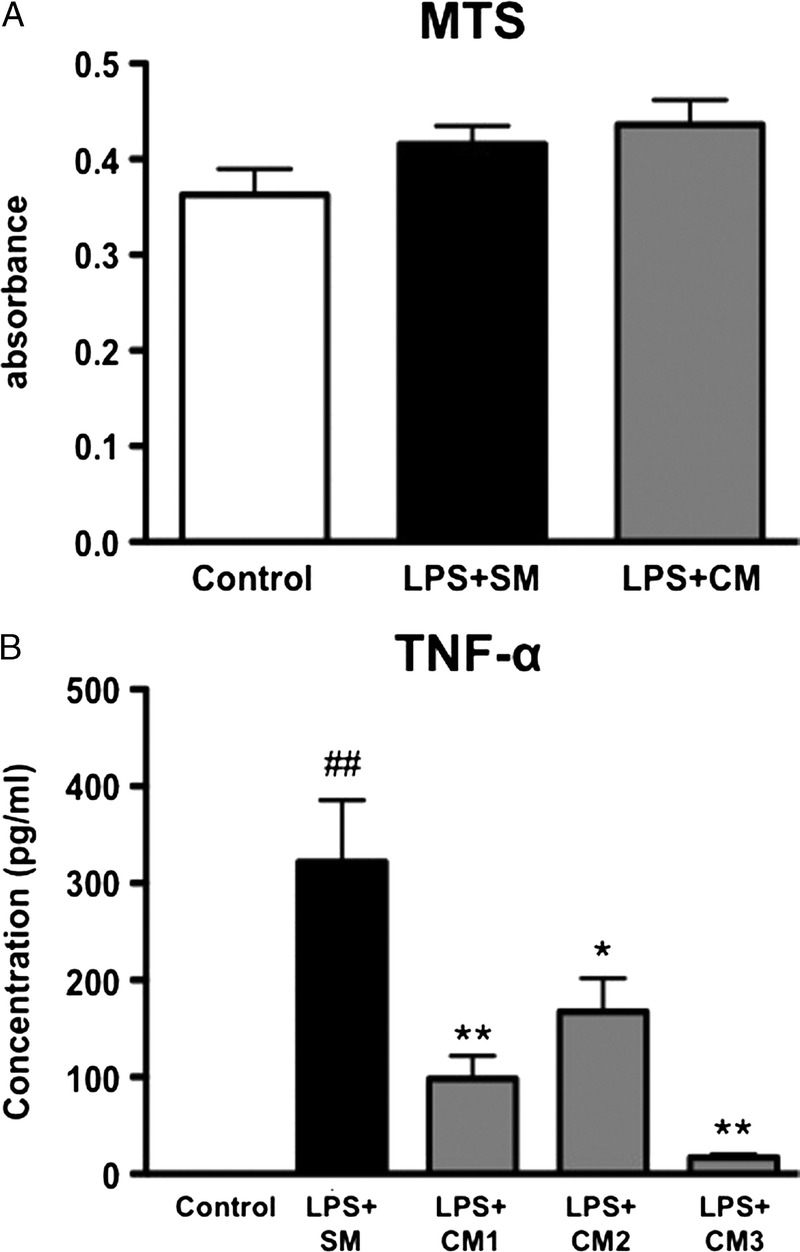
Effect of hAMSC-CM on the activation of Kupffer cells in vitro, (A) Primary Kupffer cells were treated with 100 ng/ml of LPS in standard medium (SM) or hAMSC-CM for 4 h. The number of cells was evaluated by MTS assay. (B) Primary Kupffer cells were treated with 100 ng/ml of LPS in standard medium (SM) or hAMSC-CM for 4 h. CM was obtained from hAMSCs of 3 different donors (CM1, CM2, and CM3). Secretion of TNF-α from the Kupffer cells was measured by ELISA. The values were the mean ± SEM. ##P < 0.01 versus Control. *P < 0.05, **P < 0.01 versus LPS + SM. ELISA indicates enzyme-linked immunosorbent assay; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; CM, conditioned medium.
DISCUSSION
In this study, we investigated the therapeutic potential of hAMSCs in rats with liver fibrosis and found that (1) hAMSC transplantation ameliorated liver fibrosis; (2) hAMSC transplantation suppressed the activation of HSCs; (3) hAMSC transplantation suppressed the infiltration of Kupffer cells; and (4) hAMSC-conditioned medium downregulated the activation of Kupffer cells.
The therapeutic effect of MSCs derived from the bone marrow, adipose tissue, and umbilical cord has been recently reported in a liver fibrosis model.20-35 Although each experimental protocol is different in terms of the duration of CCl4 administration (4 to 12 weeks) and the number of MSCs transplanted (5 × 105 to 3 × 107 cells/rat), it appeared that MSCs suppressed the fibrosis of liver in most reports. In the present study, liver fibrosis was induced by the administration of CCl4 for 6 weeks, and hAMSCs (1 × 106 cells/rat) were transplanted at 3 weeks. Recently, the use of hAMSCs in a liver fibrosis in mice has been reported.36 This group showed that hAMSC transplantation suppressed the activation of HSCs, decreased hepatocyte apoptosis, and promoted liver regeneration. Accordingly, our study showed that hAMSCs suppressed the activation of HSCs as demonstrated by immunohistochemical examination and quantitative reverse transcription-PCR. In addition, we demonstrated that hAMSC transplantation suppressed the infiltration of Kupffer cells. Furthermore, in vitro experiments demonstrated that hAMSC-conditioned medium suppressed the activation of Kupffer cells, which are known to modulate inflammation in the development of liver fibrosis.37 Various studies have shown that Kupffer cells act as an integral factor in hepatocyte apoptosis, inflammation, and fibrosis.38-40 In addition, we have very recently demonstrated that AMSC-conditioned medium suppressed the nuclear translocation of NF-κB, but not the phosphorylation of IκB in macrophages.41 Therefore, the anti-inflammatory effect of AMSCs to Kupffer cells/macrophages could contribute to the anti-fibrotic effect. Conversely, HSCs have been identified as an important cellular source of extracellular matrix (ECM) in liver fibrosis.42 Activated HSCs undergo a phenotypic transdifferentiation to myofibroblasts expressing α-SMA and produce a numerous ECM molecules, such as collagen.43 In this study, we investigated the effect of hAMSC transplantation on HSCs in CCl4-treated rats, which showed that hAMSCs decreased HSC activation, as indicated by the decreased expression of α-SMA.
The TIMP-1 expression has been shown to be increased in the development of liver fibrosis both in murine models and human samples.44 In addition, it has been demonstrated that TIMP-1 significantly attenuated spontaneous resolution of liver fibrosis by the combination of a net reduction of MMP activity and suppression of apoptosis in HSCs.45 We found that the expression of TIMP-1 was significantly decreased and MMP-9 was further increased by hAMSC transplantation. The other possible explanation for the fibrotic resolution is hepatic regeneration. The HGF plays an essential part in the development and regeneration of the liver and shows anti-apoptotic activity in hepatocytes.46 We found that the expression of HGF was significantly increased by hAMSC transplantation. Recently, it has been reported that IL-10 production by infused bone marrow cells is a key negative regulator of the liver fibrosis.47 However, the expression of IL-10 was not significantly increased by hAMSC transplantation in our study. Therefore, hAMSC transplantation may ameliorate liver fibrosis not only by suppressing the infiltration of Kupffer cells and HSC activation but also by inducing MMP-9, inhibition of Timp1 as well as induction of HGF. However, because the upregulation of HGF expression by hAMSC transplantation was relatively small, contribution of HGF induction in the improvement of liver fibrosis might be small.
In this study, we investigated the efficacy of biweekly transplantation to see if there was an additive effect. In this model, there is no additive effect by double injection of hAMSCs. Considering that liver fibrosis in this model progresses over the course of weeks, it is possible that hAMSC transplantation every couple of months would be sufficient in the clinical setting. A future clinical trial should investigate the interval of hAMSC transplantation for the treatment of liver fibrosis.
Recently, several clinical studies using human MSCs in liver fibrosis have been reported.8,9,48-51 Autologous bone marrow MSC infusion therapy has been reported to be safe and effective for patients with liver failure caused by hepatitis B49 and hepatitis C.50 They showed that MSC transplantation contributed to the improvement in serum albumin levels, total bilirubin, prothrombin time, and model for end-stage liver disease score49 or in the Child score, model for end-stage liver disease score, fatigue score, and performance status50 without severe adverse effects. In addition, umbilical cord–derived MSC therapy has been reported to improve serum albumin levels and total serum bilirubin levels and to reduce ascites in patients with decompensated liver cirrhosis.8 Other clinical trials using human MSCs in liver fibrosis are being conducted in several countries (http://www.clinicaltrials.gov). Recent reports have suggested similar efficacy of AMSCs for several other diseases.52-54 It has been demonstrated that intravenous infusion of hAMSCs ameliorates inflammation and fibrosis in the lung induced by bleomycin in mice.54 They tested the efficacy of AMSCs, bone marrow MSCs, and human amniotic epithelial cells, and concluded that AMSC transplantation was more effective in reducing lung injury. In addition, very recently, a first-in-human pilot study using fetal membrane-derived MSCs has been conducted to treat nine patients with steroid-refractory acute graft-versus-host disease. The fetal membrane-derived MSCs appeared safe for intravenous infusion to most patients, and the overall response rate in severe refractory acute graft-versus-host disease appeared to be similar to the rate observed while using bone marrow-derived MSCs.53 This is encouraging because it is rather invasive to aspirate bone marrow from donors. It is non-invasive to obtain amnion because it is discarded as a medical waste, and AMSCs are originated from fetus, but not mother. In addition, we can use younger cells than MSCs from other tissues, such as bone marrow or adipose tissue. In addition, we have recently demonstrated that AMSCs produce a large amount of prostaglandin E2, one of the key modulators of inflammation.55 Therefore, AMSC transplantation would be more pertinent for patients with liver fibrosis that may require repeated cell therapy for a long time.
In conclusion, human AMSC transplantation ameliorated fibrosis in a rat model of liver fibrosis, possibly through the suppression of Kupffer cell infiltration and activity of HSCs. Considering that the fetal membrane is generally discarded as medical waste and can be obtained without an invasive procedure, AMSC transplantation should be considered as a therapeutic strategy for the treatment of liver cirrhosis.
Footnotes
This work was supported by a Grant in Aid for Translational Research Network Program from Ministry of Education, Culture, Sports, Science and Technology Japan.
The authors declare no conflicts of interest.
K.K. participated in the performance of the research, writing of the article, and data analysis. H.H., M.F., A.K., R.H., R.O., T.Y., and K.Y. participated in the performance of the research and writing of the article. S.O. participated in the research design, the performance of the research, and writing of the article. H.T. and N.S. participated in the research design and writing of the article.
REFERENCES
- 1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014; 383: 1749. [DOI] [PubMed] [Google Scholar]
- 2. Esquivel CO. Liver transplantation: where we are and where we are heading. Transplant Proc. 2010; 42: 610. [DOI] [PubMed] [Google Scholar]
- 3. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284: 143. [DOI] [PubMed] [Google Scholar]
- 4. Meirelles Lda S, Fontes AM, Covas DT, et al. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009; 20: 419. [DOI] [PubMed] [Google Scholar]
- 5. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008; 8: 726. [DOI] [PubMed] [Google Scholar]
- 6. Wang L, Li J, Liu H, et al. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013; 28 (Suppl 1): 85. [DOI] [PubMed] [Google Scholar]
- 7. Mohamadnejad M, Alimoghaddam K, Bagheri M, et al. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013; 33: 1490. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012; 27 (Suppl 2): 112. [DOI] [PubMed] [Google Scholar]
- 9. Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007; 10: 459. [PubMed] [Google Scholar]
- 10. In't Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003; 88: 845. [PubMed] [Google Scholar]
- 11. Hu Y, Liao L, Wang Q, et al. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J Lab Clin Med. 2003; 141: 342. [DOI] [PubMed] [Google Scholar]
- 12. In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004; 22: 1338. [DOI] [PubMed] [Google Scholar]
- 13. Alviano F, Fossati V, Marchionni C, et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishikane S, Ohnishi S, Yamahara K, et al. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells. 2008; 26: 2625. [DOI] [PubMed] [Google Scholar]
- 15. Ishikane S, Yamahara K, Sada M, et al. Allogeneic administration of fetal membrane-derived mesenchymal stem cells attenuates acute myocarditis in rats. J Mol Cell Cardiol. 2010; 49: 753. [DOI] [PubMed] [Google Scholar]
- 16. Ohshima M, Yamahara K, Ishikane S, et al. Systemic transplantation of allogenic fetal membrane-derived mesenchymal stem cells suppresses Th1 and Th17 T cell responses in experimental autoimmune myocarditis. J Mol Cell Cardiol. 2012; 53: 420. [DOI] [PubMed] [Google Scholar]
- 17. Tsuda H, Yamahara K, Ishikane S, et al. Allogenic fetal membrane-derived mesenchymal stem cells contribute to renal repair in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2010; 299: F1004. [DOI] [PubMed] [Google Scholar]
- 18. Tsuda H, Yamahara K, Otani K, et al. Transplantation of allogenic fetal membrane-derived mesenchymal stem cells protect against ischemia-reperfusion-induced acute kidney injury. Cell Transplant. 2013; 23: 889. [DOI] [PubMed] [Google Scholar]
- 19. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006; 8: 315. [DOI] [PubMed] [Google Scholar]
- 20. Usunier B, Benderitter M, Tamarat R, et al. Management of fibrosis: the mesenchymal stromal cells breakthrough. Stem Cells Int. 2014; 2014: 340257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nasir GA, Mohsin S, Khan M, et al. Mesenchymal stem cells and Interleukin-6 attenuate liver fibrosis in mice. J Transl Med. 2013; 11: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013; 22: 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Lian F, Li J, et al. Adipose derived mesenchymal stem cells transplantation via portal vein improves microcirculation and ameliorates liver fibrosis induced by CCl4 in rats. J Transl Med. 2012; 10: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ali G, Mohsin S, Khan M, et al. Nitric oxide augments mesenchymal stem cell ability to repair liver fibrosis. J Transl Med. 2012; 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiao H, Tong Y, Han H, et al. A novel therapeutic regimen for hepatic fibrosis using the combination of mesenchymal stem cells and baicalin. Pharmazie. 2011; 66: 37. [PubMed] [Google Scholar]
- 26. Pan RL, Wang P, Xiang LX, et al. Delta-like 1 serves as a new target and contributor to liver fibrosis down-regulated by mesenchymal stem cell transplantation. J Biol Chem. 2011; 286: 12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rabani V, Shahsavani M, Gharavi M, et al. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int. 2010; 34: 601. [DOI] [PubMed] [Google Scholar]
- 28. Tsai PC, Fu TW, Chen YM, et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009; 15: 484. [DOI] [PubMed] [Google Scholar]
- 29. Hardjo M, Miyazaki M, Sakaguchi M, et al. Suppression of carbon tetrachloride-induced liver fibrosis by transplantation of a clonal mesenchymal stem cell line derived from rat bone marrow. Cell Transplant. 2009; 18: 89. [DOI] [PubMed] [Google Scholar]
- 30. Chang YJ, Liu JW, Lin PC, et al. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 2009; 85: 517. [DOI] [PubMed] [Google Scholar]
- 31. Abdel Aziz MT, Atta HM, Mahfouz S, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin Biochem. 2007; 40: 893. [DOI] [PubMed] [Google Scholar]
- 32. Oyagi S, Hirose M, Kojima M, et al. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006; 44: 742. [DOI] [PubMed] [Google Scholar]
- 33. Zhao DC, Lei JX, Chen R, et al. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005; 11: 3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sakaida I, Terai S, Yamamoto N, et al. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004; 40: 1304. [DOI] [PubMed] [Google Scholar]
- 35. Fang B, Shi M, Liao L, et al. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004; 78: 83. [DOI] [PubMed] [Google Scholar]
- 36. Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One. 2011; 6: e16789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014; 60: 1090. [DOI] [PubMed] [Google Scholar]
- 38. Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014; 59: 2034. [DOI] [PubMed] [Google Scholar]
- 39. Ramachandran P, Iredale JP. Macrophages: central regulators of hepatic fibrogenesis and fibrosis resolution. J Hepatol. 2012; 56: 1417. [DOI] [PubMed] [Google Scholar]
- 40. Heymann F, Trautwein C, Tacke F. Monocytes and macrophages as cellular targets in liver fibrosis. Inflamm Allergy Drug Targets. 2009; 8: 307. [DOI] [PubMed] [Google Scholar]
- 41. Onishi R, Ohnishi S, Higashi R, et al. Human amnion-derived mesenchymal stem cell transplantation ameliorates dextran sulphate sodium-induced severe colitis in rats. Cell Transplant (in press). [DOI] [PubMed] [Google Scholar]
- 42. Muhanna N, Abu Tair L, Doron S, et al. Amelioration of hepatic fibrosis by NK cell activation. Gut. 2011; 60: 90. [DOI] [PubMed] [Google Scholar]
- 43. Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005; 115: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshiji H, Kuriyama S, Miyamoto Y, et al. Tissue inhibitor of metalloproteinases-1 promotes liver fibrosis development in a transgenic mouse model. Hepatology. 2000; 32: 1248. [DOI] [PubMed] [Google Scholar]
- 45. Yoshiji H, Kuriyama S, Yoshii J, et al. Tissue inhibitor of metalloproteinases-1 attenuates spontaneous liver fibrosis resolution in the transgenic mouse. Hepatology. 2002; 36: 850. [DOI] [PubMed] [Google Scholar]
- 46. Ueki T, Kaneda Y, Tsutsui H, et al. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999; 5: 226. [DOI] [PubMed] [Google Scholar]
- 47. Suh YG, Kim JK, Byun JS, et al. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology. 2012; 56: 1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sharma RR, Pollock K, Hubel A, et al. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014; 54: 1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peng L, Xie DY, Lin BL, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011; 54: 820. [DOI] [PubMed] [Google Scholar]
- 50. Amer ME, El-Sayed SZ, El-Kheir WA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011; 23: 936. [DOI] [PubMed] [Google Scholar]
- 51. Kharaziha P, Hellstrom PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009; 21: 1199. [DOI] [PubMed] [Google Scholar]
- 52. Insausti CL, Blanquer M, Garcia-Hernandez AM, et al. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014; 7: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ringden O, Erkers T, Nava S, et al. Fetal membrane cells for treatment of steroid-refractory acute graft-versus-host disease. Stem Cells. 2013; 31: 592. [DOI] [PubMed] [Google Scholar]
- 54. Moodley Y, Vaghjiani V, Chan J, et al. Anti-inflammatory effects of adult stem cells in sustained lung injury: a comparative study. PLoS One. 2013; 8: e69299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamahara K, Harada K, Ohshima M, et al. Comparison of angiogenic, cytoprotective, and immunosuppressive properties of human amnion- and chorion-derived mesenchymal stem cells. PLoS One. 2014; 2: e88319. [DOI] [PMC free article] [PubMed] [Google Scholar]


