Supplemental digital content is available in the text.
Background
Multiple modifications of the porcine genome are required to prevent rejection after pig-to-primate xenotransplantation. Here, we produced pigs with a knockout of the α1,3-galactosyltransferase gene (GGTA1-KO) combined with transgenic expression of the human anti-apoptotic/anti-inflammatory molecules heme oxygenase-1 and A20, and investigated their xenoprotective properties.
Methods
The GGTA1-KO/human heme oxygenase-1 (hHO-1)/human A20 (hA20) transgenic pigs were produced in a stepwise approach using zinc finger nuclease vectors targeting the GGTA1 gene and a Sleeping Beauty vector coding for hA20. Two piglets were analyzed by quantitative reverse-transcription polymerase chain reaction, flow cytometry, and sequencing. The biological function of the genetic modifications was tested in a 51Chromium release assay and by ex vivo kidney perfusions with human blood.
Results
Disruption of the GGTA1 gene by deletion of few basepairs was demonstrated in GGTA1-KO/hHO-1/hA20 transgenic pigs. The hHO-1 and hA20 mRNA expression was confirmed by quantitative reverse-transcription polymerase chain reaction. Ex vivo perfusion of 2 transgenic kidneys was feasible for the maximum experimental time of 240 minutes without symptoms of rejection.
Conclusions
Results indicate that GGTA1-KO/hHO-1/hA20 transgenic pigs are a promising model to alleviate rejection and ischemia-reperfusion damage in porcine xenografts and could serve as a background for further genetic modifications toward the production of a donor pig that is clinically relevant for xenotransplantation.
The hyperacute rejection (HAR) is the first immunological barrier occurring after pig-to-primate xenotransplantation. It can be reliably prevented by expressing human complement regulatory genes1-3 and/or by knocking out the α1,3-galactosyltransferase gene (GGTA1) in pigs.4 Current research efforts focus on the acute vascular rejection (AVR) which can be initiated by anti–non-Gal antibody-binding, followed by complement and endothelial activation, finally leading to microvascular thrombosis and graft rejection. Molecular incompatibilities between primate coagulation factors and porcine anticoagulant regulators are considered another causative factor in coagulation dysregulation after pig-to-primate xenotransplantation.5 Ischemia-reperfusion injury (IRI) is also an important factor in xenotransplantation, and the ischemic tissue is damaged after reperfusion by reactive oxygen species. The complement system, proinflammatory changes of the endothelium, and neutrophils are critically involved in this cascade of deteriorating events. Strategies to overcome AVR and IRI include the expression of human anticoagulant, anti-apoptotic, and/or anti-inflammatory transgenes on porcine tissues and organs.
Previously, we had produced a human heme oxygenase-1 (hHO-1) transgenic pig line and demonstrated significant protection of hHO-1 transgenic porcine aortic endothelial cells against TNF-α–mediated apoptosis and prolonged survival of hHO-1 transgenic kidneys in ex vivo xenoperfusion experiments with human blood.6 The anti-apoptotic and anti-inflammatory properties of hHO-1 are mediated by catabolizing cytotoxic free heme to biliverdin, carbon monoxide (CO), and free iron. Biliverdin is reduced to bilirubin, which acts as a potent antioxidant.7 The CO provides anti-apoptotic and anti-inflammatory effects via activation of the p38 MAPK pathway.8 Free iron upregulates ferritin which protects cells from oxidative damage.9 This renders hHO-1 promising for attenuating the detrimental effects during xenograft rejection.
Human TNF-α–induced protein 3, also known as human A20 (hA20), is a cytoplasmic zinc-finger protein that prevents inflammation by blockade of NF-κB activation10 and inhibition of TNF-α–mediated apoptosis.11 We had shown that transgenic hA20 expression in pigs also provided immune modulatory effects in porcine aortic endothelial cells by rendering them less susceptible to CD95(Fas)L-mediated cell death.12 Because of previous experience,12 we decided to construct a new hA20 expression vector based on the Sleeping Beauty (SB) transposon system13 to ensure high ubiquitous expression of the transgene. The SB transposons are less susceptible to rearrangements and not affected by reverse transcriptase-induced mutations,14 usually leading to stable gene expression profiles.
Here, we report the production and characterisation of pigs that express cytoprotective hHO-1 and hA20 transgenes on a α1,3-galactosyltransferase knockout (GGTA1-KO) background. The GGTA1 gene was disrupted by targeting exon 9 of the GGTA1 gene coding for the catalytic domain of the α1,3 galactosyltransferase with zinc-finger nucleases (ZFN) as recently described.15 These genetic modifications were combined in 1 porcine genotype to evaluate if the triple genetic modifications would result in a better control of xenograft rejection. We studied the biological function of the transgenes in vitro in a 51Chromium (51Cr) release assay and performed ex vivo kidney xenoperfusions with human AB-pooled blood to investigate its protective effects on IRI.
MATERIALS AND METHODS
Animals
The animals used in this study were German Landrace pigs from the experimental herd of the Institute of Farm Animal Genetics, Mariensee. Animal experiments were conducted in accordance with the German Animal Welfare Act and had been approved by the local authority (Lower Saxony State Office for Consumer Protection and Food Safety, LAVES, TVA: 33.14-42502-04-12/0891).
Design of hA20 Expression Vector
The cDNA of the hA20 gene (kindly provided by Rudi Beyaert, LMBP 3778) was expressed by the 9.7-kb vector pSB-CAG-hA20-IRES-neo, based on the hyperactive SB transposon system13 (kindly provided by Dr. Zoltán Ivics, Paul-Ehrlich-Institut, Langen, Germany). The transposable element consists of the cytomegalovirus early enhancer / chicken β actin promoter (CAGGS) followed by an E-tag coupled upstream to the hA20 cDNA. A neomycin resistance cassette was linked via IRES to the hA20 sequence to allow antibiotic selection. Inverted terminal repeat sequences flank the integration unit which was cloned from the pCAGGS/hA20 vector12 into the SB vector via SpeI and XhoI restriction sites.
Production of GGTA1-KO/hHO-1/hA20 Donor Pigs
Porcine ear fibroblasts from a well-characterized hHO-1 transgenic pig6 were transfected with a pair of ZFN-based plasmids targeting exon 9 of the porcine GGTA1 gene by electroporation (Gene PulserXcell unit, Bio-Rad). Gal-negative cells were enriched by counterselection with streptavidin-conjugated magnetic beads (Dynabeads, Life Technologies) in a magnetic field15 and served as donor cells for somatic cell nuclear transfer (SCNT). Fibroblasts from a GGTA1-KO/hHO-1 fetus were electroporated with the pSB-CAG-hA20-IRES-neo vector and the SB transposase 100× plasmid (molar ratio, 10:1). After antibiotic selection with 800 μg G418/mL medium for 14 days, cell colonies were screened for genomic integration of hA20 by polymerase chain reaction (PCR) and used for SCNT. One fetus from day 25 post conceptionem was used for recloning.
Transgenic Expression Analysis by Real-Time PCR
The mRNA expression of hHO-1 and hA20 was analyzed in GGTA1-KO/hHO-1/hA20 transgenic fetuses and pigs by quantitative reverse-transcription (RT-q)PCR as previously described.6 In brief, 0.6 μg of total RNA was treated with DNAse I (RNase-free, 1 U/μL, Biozym Scientific GmbH, Germany) before incubation with 0.8 μL 25 mM EDTA (Roth, Germany) solution. Reverse transcription (RT) was run for 10 minutes at 25°C, 60 minutes at 42°C, and 5 minutes at 99°C. Two microliters of 1:5 diluted RT mix were added to the real-time PCR mix. The real-time PCR was performed in the ABI 7500 Fast Real-Time System (Life Technologies) for 10 minutes at 95°C, 40 cycles of 95°C for 15 seconds, followed by 60°C for 1 minute (for primers used see SDC, Materials and Methods). For quantification, threshold cycle values were fitted to the standard curves using the Sequence Detection Software 1.4. Normalization factors were calculated with the Excel based software geNorm (http://medgen.ugent.be/~jvdesomp/genorm/). The specificity of the PCR product size was confirmed by electrophoresis on a 3.5% agarose gel.
Flow Cytometry Analysis
Absence of Gal epitopes on ear fibroblasts from the GGTA1-KO/hHO-1/hA20 transgenic pigs was investigated by flow cytometry.15 Approximately 1 × 106 fibroblasts were incubated with Fluorescein isothiocyanate-conjugated Isolectin-B4 (3 μg/mL cell suspension; isolated from Griffonia simplicifolia; Enzo Life Sciences) for 5 minutes at 37°C. After centrifugation, the cell pellet was resuspended in 1 mL phosphate-buffered saline. Fluorescence was measured in a Gallios Flow Cytometer (Beckman Coulter). The emitted signal was collected at wavelength range of 505 to 545 nm, converted by a logarithmic amplifier, and processed using company-owned software. Fibroblasts from a GGTA1-KO pig, and wild-type (WT) fibroblasts incubated with or without lectin served as controls.
Sequencing of GGTA1 Gene Locus in Cloned Pigs
The success of genetic modification of the targeted region within the GGTA1 gene was confirmed by allele-specific sequencing of the GGTA1 gene. The PCR products of the ZFN target site within the GGTA1 gene were purified with the Invisorb Fragment CleanUp Kit (STRATEC Molecular, Germany) and ligated with the commercial pGEM-T Easy Vector (Promega). The ligation product was transformed into E. coli XL10-Gold ultracompetent cells (Stratagene). Colonies were picked the following day for control PCR and were sent for sequencing with T7/SP6 primers.
Assay for Complement-Mediated Lysis of Porcine Fibroblasts
The 51Cr release assays were performed to study the susceptibility of GGTA1-KO/hHO-1/hA20 cells and controls to lysis by human antibody/complement. Porcine fibroblasts (3 × 106) were labelled with 100 μCi of sodium [51Cr]-chromate (GE Healthcare, Buckinghamshire, Great Britain; 1 Ci = 37 GBq) and plated at 1 × 104 cells/well in microtiter plates. After 18 hours, dead fibroblasts were removed by “dumping.” Cells were incubated with increasing concentrations of pooled complement preserved normal human serum (Dunn Labortechnik, Asbach, Germany). After 4 hours, 25 μL of the cell supernatant were removed, and the amount of radioactivity was measured in a Microbeta scintillation counter (Wallac, Turku, Finland). The mean counts per minute obtained in triplicate cultures was used for all calculations. The spontaneous release of 51Cr was determined by incubating the target cells with medium alone, whereas the maximum release was determined by incubating in 2% Triton X-100. Specific lysis was calculated as follows: % specific lysis = (experimental 51Cr release − spontaneous 51Cr release) / (maximum 51Cr release − spontaneous 51Cr release) × 100.
Ex Vivo Kidney Perfusion
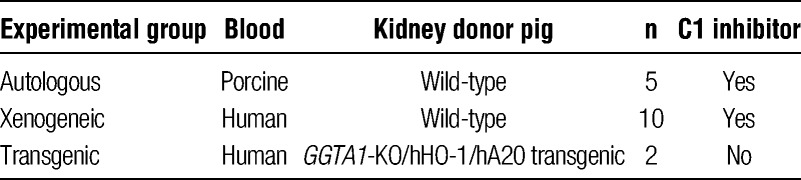
Kidneys from WT or GGTA1-KO/hHO-1/hA20 transgenic pigs were perfused with human (xenogeneic) or porcine blood (autologous, WT) in a well-established ex vivo perfusion circuit.6,16 The perfusion experiments with WT kidneys were not run in parallel with the triple transgenic organs, but taken from previous studies.6,16 Perfusion of WT organs consistently results in symptoms of a hyperacute rejection. To save experimental costs and to comply with animal welfare, we decided not to run control experiments in parallel to the triple transgenic organs. Briefly, pigs were sacrificed at a weight of 25 to 42 kg for kidney explantation. Kidneys were connected to the perfusion circuit and were perfused with organ-weight matched (350-900 mL) heparinized (1 IU/mL, Ratiopharm GmbH, Germany) porcine or human blood diluted with Tyrode's solution (Sigma-Aldrich) and Ringer lactate (B. Braun Melsungen, Germany) to a hematocrit of 24%. Human blood was freshly withdrawn and pooled from 2 healthy AB-positive donors per experiment from a pool of 30 available donors. C1 inhibitor (Berinert, a gift from CSL Behring, Germany, final concentration 1 IU/mL) was added to the perfusate of all WT kidneys, but not to the GGTA1-KO/hHO-1/hA20 transgenic kidneys (Table 1). Heparin was added to the blood and coating of the tubing was used to avoid blood clotting on the surfaces and to prevent xeno-specific activation of human blood coagulation. A double head roller pump (Stoeckert Instrumente GmbH, Germany) ensured physiological perfusion conditions (mean arterial pressure [MAP], 70-140 mm Hg; flow, 50–130 mL/min) of the circuit. Blood gas tension and temperature were kept constant (pH 7.4 p(O2) >200 mm Hg, p(CO2) 20-35 mm Hg, 37°C) using a heated neonatal diffusion membrane oxygenator (3381 Minimax Medtronic, Germany). The renovascular resistance (RVR) was monitored throughout the whole perfusion period. It was calculated by the following formula: RVR (mm Hg/mL per minute) = (MAP/flow). Rejection was defined as a 3-fold increase in RVR above baseline. Survival time of the kidneys was defined as the time from the start of perfusion to rejection or end of experiment after 240 minutes. The resistance index (RI) was calculated as: RI [mm Hg/mL per min/g] = (MAP/flow)/kidney weight. Blood samples were taken before and at regular timepoints during the perfusion for analyses of thrombocyte counts, fibrinogen, antithrombin activity, thrombin-antithrombin (TAT) complex, and D-dimers. Fibrinogen concentrations were determined by the Clauss method (Siemens, Germany), antithrombin activity was analyzed by a chromogenic anti-Xa assay (Siemens), D-dimer levels were measured by a latex particle-based immunoassay (Roche, Germany), and TAT by an enzyme-linked immunosorbent assay (Haemochrom Diagnostica, Germany).
TABLE 1.
Experimental setup for kidney perfusion
RESULTS
Generation of GGTA1-KO/hHO-1/hA20 Transgenic Pigs
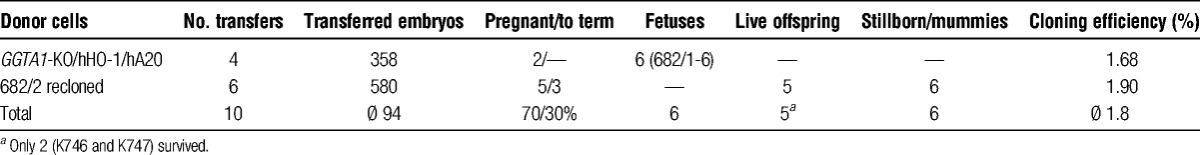
Eleven fetuses were obtained from 1 recipient after transfer of 88 GGTA1-KO/hHO-1 embryos. Fibroblasts isolated from 1 GGTA1-KO/hHO-1 transgenic fetus were cotransfected with the pSB-CAG-hA20-IRES-neo vector and the SB transposase 100× plasmid and served as donor cells for SCNT. One of 4 recipients became pregnant and was sacrificed on day 25 after transfer. Six GGTA1-KO/hHO-1/hA20 transgenic fetuses were obtained, of which 1 fetus (682/2) was used for recloning. A total of 580 triple transgenic reconstructed embryos were transferred to 6 recipients. Three recipients gave birth to 6 stillborn and 5 liveborn piglets with low birth weights between 0.5 and 0.9 kg. Three piglets died within 24 hours after birth, 2 pigs (K746 and K747) survived and developed normally (Table 2). All piglets were confirmed as GGTA1-KO/hHO-1/hA20-transgenic by PCR (data not shown).
TABLE 2.
Overview of SCNT experiments
Transgene Expression in Offspring
The RT-qPCR analysis revealed that both transgenes were expressed in all fibroblast samples from GGTA1-KO/hHO-1/hA20 transgenic offspring. The mean hHO-1 mRNA expression level varied among piglets and was increased approximately 1.7-fold in 8 of 11 live and stillborn GGTA1-KO/hHO-1/hA20 transgenic piglets compared to 3 fetuses (Figure 1A). Expression of hA20 was compared to a pig (K271) originating from our previous hA20 line.12 The hA20 mRNA levels were increased 37-fold in GGTA1-KO/hHO-1/hA20 transgenic fetuses and 27-fold in pigs recloned from these fetuses (Figure 1B). The WT-controls showed neither hHO-1 nor hA20 expression.
FIGURE 1.
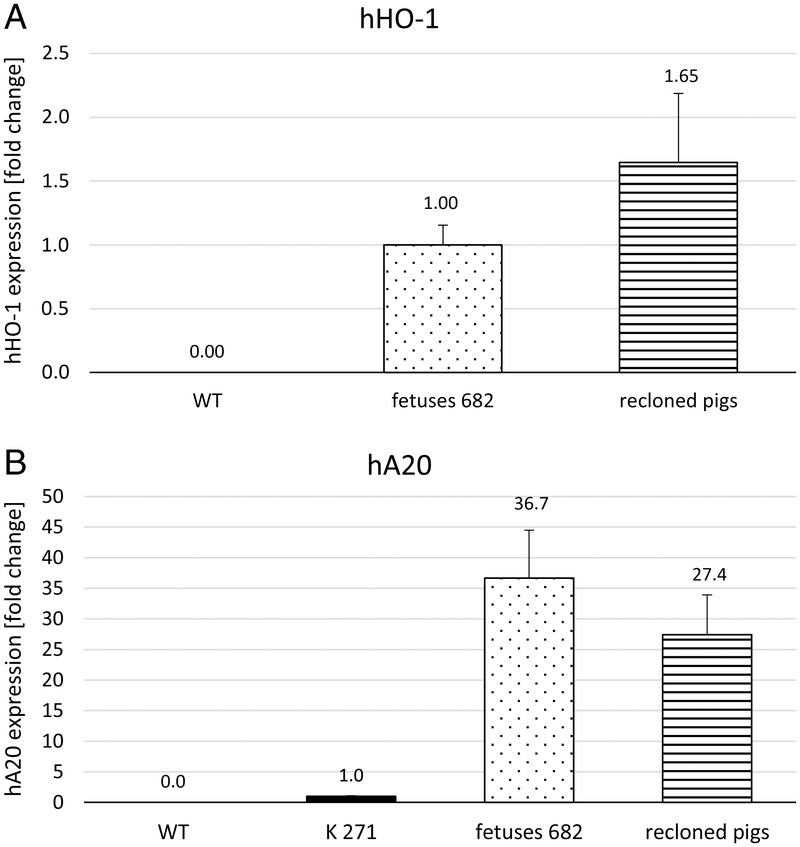
A, HHO-1. B, HA20 transgene expression detected by real-time PCR. A, Expression of hHO-1 mRNA in GGTA1-KO/hHO-1/hA20 transgenic fetuses (n = 3, dotted, set = 1) and live or stillborn recloned pigs (n = 8, striped). Wild-type controls are negative (n = 2). Values from 2 technical replicates per sample. Results are given as -fold change ± standard deviation. B, Expression of hA20 mRNA in clone K271 (bred from previous hA20 pig line, black bar, set = 1) compared to GGTA1-KO/hHO-1/hA20 transgenic fetuses (n = 3; dotted) and live- or stillborn recloned pigs (n = 8, striped). Wild-type controls are negative (n = 2). Values from 2 technical replicates per sample. Results are given as -fold change ± standard deviation.
Analysis of ZFN-Mediated GGTA1-KO
Flow cytometry analysis revealed functional disruption of the GGTA1 gene as demonstrated by absence of Gal epitopes on fibroblasts from pigs K746 and K747. The negative WT control (without GSIB4-lectin) showed no fluorescence, whereas the positive WT control (with GSIB4-lectin) showed massive Gal expression (Figure 2A). Sequencing of genomic DNA from K746 and K747 revealed identical mutations of the GGTA1 gene: a single nucleotide deletion on 1 allele and a dinucleotide deletion on the other allele (Figure 2B).
FIGURE 2.
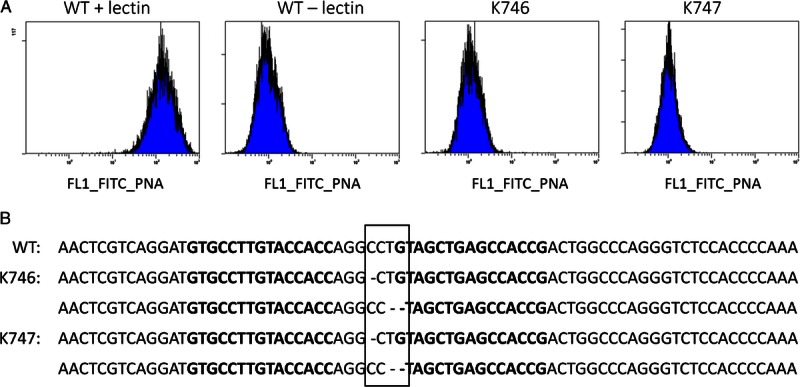
Analysis of ZFN-mediated GGTA1-KO. A, Flow cytometric analysis of fibroblasts from WT controls and both clones (K746 and K747) after incubation with FITC-conjugated isolectin B4 or without (WT negative control). B, Sequencing analysis of the ZFN target region within the GGTA1 gene. The original wild-type sequence is given in the upper line. Both cloned pigs (K746 and K747) carry identical mononucleotide deletion on one and dinucleotide deletion on the other allele (boxed region). Bold letters represent ZFN target sequences.
Decreased Susceptibility of GGTA1-KO/hHO-1/hA20 Fibroblasts to Antibody/Complement-Mediated Lysis
Fibroblasts from WT animals were lysed by increasing concentrations of human antibody/complement. Although % specific lysis was low, a clear-cut dose-response effect could be demonstrated. At the highest antibody/complement concentration (1:5 dilution) with an average of 18.1% (±1.1%) specific lysis was found in fibroblasts from WT pigs. In contrast, specific lysis remained low (5% ± 1.1%) in transgenic cells (Fig. 3).
FIGURE 3.
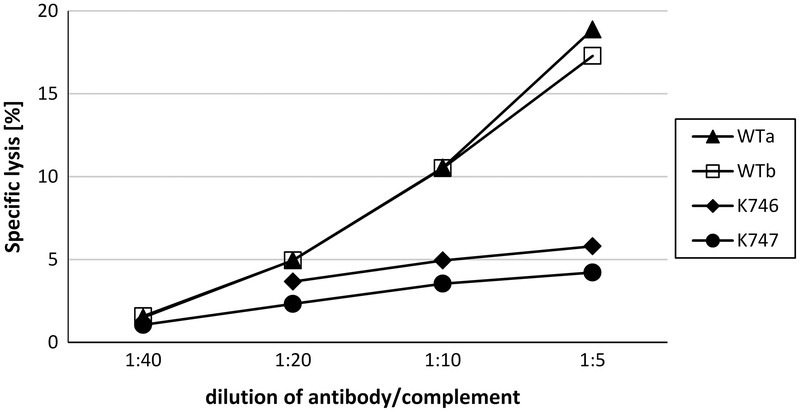
Protection of triple transgenic fibroblasts from antibody/complement-mediated lysis. The 51Cr-labeled fibroblasts from 2 GGTA1-KO/hHO-1/hA20 pigs (K746; K747) and 2 wild-type controls (WTa; WTb) were incubated with increasing concentrations of human antibody/complement. The amount of radioactivity released into the supernatant was determined after 4 hours by measuring an aliquot of 25 μL. The percentage of specific lysis was calculated as described in Material and Methods.
Prolonged Organ Survival in Ex Vivo Kidney Perfusion
Perfusion of kidneys from the 2 GGTA1-KO/hHO-1/hA20 transgenic pigs with human blood was feasible for the maximum duration (240 minutes) of the experiment without addition of the complement inhibitor C1-Inh. The RVR remained constantly low during the perfusion time. Perfusion was similarly successful after autologous perfusion of porcine WT kidneys (n = 5), whereas in xenogeneic perfused WT kidneys (n = 10; human blood) RVR increased dramatically and perfusion had to be terminated after 142 ± 26 minutes, despite addition of a soluble complement inhibitor (C1 inhibitor) (Fig. 4A and B). The maximal RI was 5.1 ± 1.7 in the xenogeneic group, 1.65 ± 0.1 in the autologous group, and 0.7 ± 0.1 in the transgenic group.
FIGURE 4.
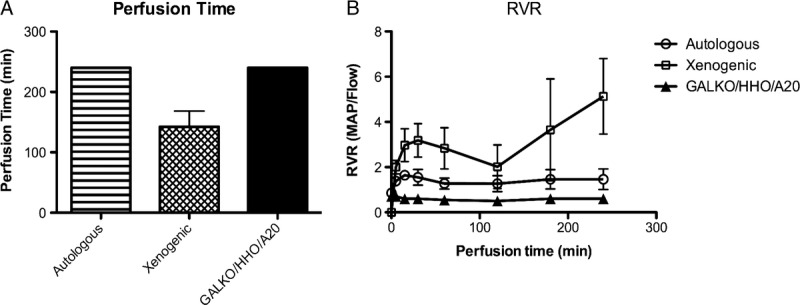
Ex vivo kidney perfusion time and RVR. A, Mean perfusion time is displayed for the three experimental groups: autologous (n = 5, striped bar), xenogeneic (n = 10, squared bar), and GGTA1-KO/hHO-1/hA20 transgenic perfusion group (n = 2, black bar). B, RVR during autologous (n = 5, circle), xenogeneic (n = 10, square), and GGTA1-KO/hHO-1/hA20 transgenic (n = 2, triangle) perfusion over time. Data for WT-perfusions are from Petersen et al6 and Ramackers et al.16
Platelet counts dropped over time in the xenogeneic group, but only slightly decreased in the autologous and the transgenic group, most probably due to shear stress in the roller pump (Fig. 5A). Coagulation parameters changed over time in plasma samples taken from the perfusion circuit at defined time points. Although fibrinogen was consumed in the xenogeneic group, the levels remained stable after an initial slight drop in the GGTA1-KO/hHO-1/hA20 transgenic group, similar to autologous perfusion. Likewise, antithrombin activity dropped in the xenogeneic group, but remained constant over time after an initial decrease in the transgenic group and stable during autologous perfusion (Fig. 5B and C). D-dimer and TAT complex concentrations remained low in the autologous group. In contrast, they were strongly increased in the xenogeneic WT group and also increased in the transgenic group, but to a lesser extent (Fig. 5D and E).
FIGURE 5.
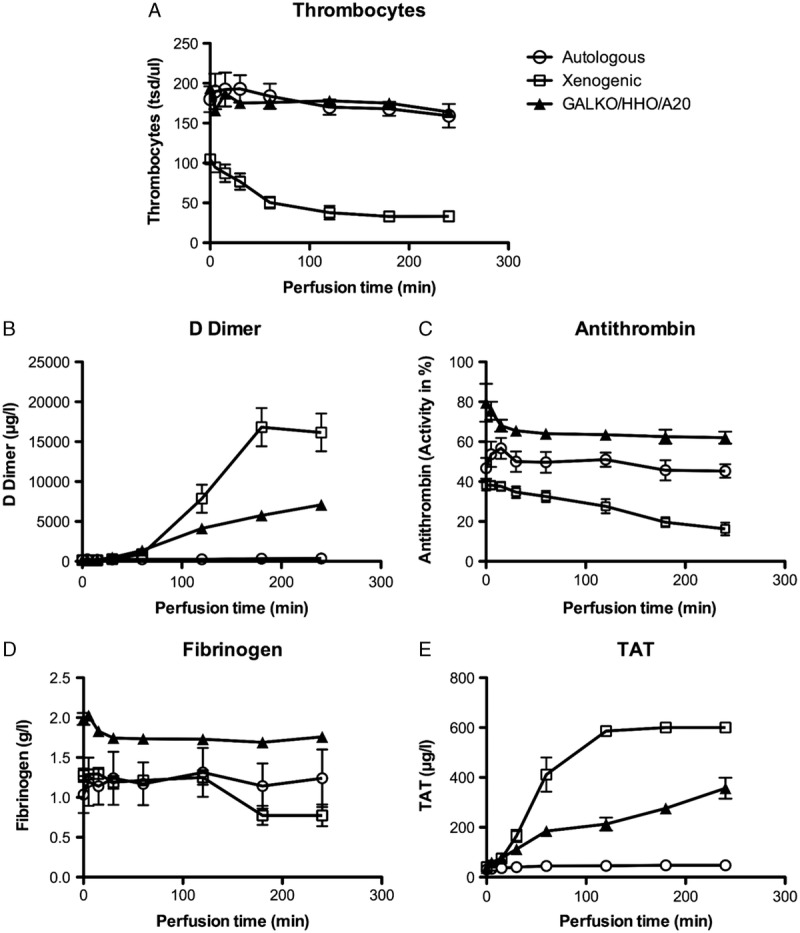
Parameters of coagulation activation during kidney perfusion. A, Thrombocyte counts at defined time points of the perfusion are given for the autologous (circle), xenogeneic (square), and GGTA1-KO/hHO-1/hA20 transgenic perfusion group (triangle). The changes in parameters of coagulation activation in the experimental groups are shown for: (B) fibrinogen, (C) antithrombin activity, (D) D-dimer, and (E) TAT complex. Data for WT-perfusions are from Petersen et al6 and Ramackers et al.16
DISCUSSION
The production of multitransgenic donor pigs for xenotransplantation is considered critical for achieving long-term protection from the immunological rejection cascade associated with porcine-to-primate xenotransplantation, including the HAR, AVR, and cellular rejection. Previously, we reported the production of pigs with various single genetic modifications, including a homozygous ZFN-mediated GGTA1-KO,15 transgenic expression of hA20,12and hHO-1.6 Moreover, we had demonstrated the efficacy of these genetic modifications in protecting porcine organs from rejection by in vitro assays and ex vivo perfusion experiments. Here, we report the successful production and characterization of pigs that combine all 3 genetic modifications to further improve their protective properties against ischemia-reperfusion damage and xenograft rejection.
The successful insertion of these genetic modifications was possible by 2 efficient new molecular technologies, that is, ZFN and the SB transposon system. ZFNs are known to have a ∼10,000-fold higher efficiency than conventional homology-based gene targeting methods for gene disruption and can induce biallelic gene disruption within 1 step.15 Transposon systems, like the hyperactive SB transposon system, have emerged as efficient nonviral method for transgene delivery. The active genomic insertion by the transposase SB100X leads to integration of the transposon, preferably into permissive genomic loci,17 and is thus compatible with high and stable transgene expression. Transgenic porcine cells can easily be used to induce additional genetic changes by these 2 technologies.
The GGTA1-KO/hHO-1/hA20 pigs were produced in a stepwise manner, with a cloning step before adding the next genetic modification. This strategy is promising to arrive at pigs with multiple genetic modifications because it allows selection for the highest expression of each transgene and for normally developing fetuses to avoid problems putatively arising from the cloning process. However, the combined integration of several transgenes into a single permissive locus is preferred to avoid genetic segregation when producing the ultimate donor pig.
Sequencing and flow cytometry analysis revealed that both alleles of the GGTA1 gene were disrupted in the GGTA1-KO/hHO-1/hA20 pigs. Transgenic expression in the GGTA1-KO/hHO-1/hA20 offspring was analyzed by RT-qPCR. HHO-1 mRNA was detected in fibroblasts indicating that the transgene had not been silenced during the stepwise cloning process. Human A20 mRNA expression levels were dramatically increased in the GGTA1-KO/hHO-1/hA20 transgenic offspring compared to our previous hA20 line.12 The most likely explanation for this was the use of the SB-based hA20 transposon vector and the integration site; but also the copy number might have influenced expression levels, as transposons are known to integrate with multiple copy numbers.17 The copy number of the SB vector was not analyzed in the present study.
Detection of the transgenic proteins was complicated due to the highly conserved sequences between the human and porcine protein (protein sequence identity for heme oxygenase-1 [HO-1]: 83%; for A20: 88%), resulting in nonspecific protein bands. These difficulties were encountered already in the analysis of the previous hHO-1 transgenic pig line.6 Western blot analysis for hA20 expression in triple transgenic pigs revealed nonspecific protein bands caused by the cross-reactivity of the antibody (polyclonal rabbit antihuman-A20 antibody, A302-633A, Bethyl Laboratories) with porcine hA20 (Figure S1, SDC, http://links.lww.com/TXD/A6).
The xenoprotective potential of the GGTA1-KO/hHO-1/hA20 transgenic pigs was demonstrated in the 51Cr release assay which revealed significant protection against complement-mediated cytotoxicity similar to a previous GGTA1-KO pig line.15 Antibody-binding to Gal epitopes could be prevented and complement activation was reduced to a minimum which was most likely due to antibody-binding to non-Gal antigens. Current thoughts are that effects of antibody-binding to non-Gal antigens might be controlled by expression of anti-apoptotic and anti-inflammatory transgenes such as hH0-1 and hA20.
The biological function of the genetic modifications was analyzed by ex vivo kidney perfusion experiments with human blood. Ex vivo perfusion of porcine organs has emerged as a valuable tool to evaluate the usefulness of transgenic pig models before nonhuman primate experiments. The GGTA1-KO/hHO-1/hA20 transgenic kidneys were protected against rejection during the ex vivo perfusion, while xenogeneic perfusion of WT kidneys in parallel experiments was rapidly terminated by HAR. This rapid rejection response occurred albeit a moderate concentration (1 IE/mL) of soluble complement inhibitor was used in the WT kidneys to diminish HAR and complement-mediated activation of the endothelium, clearly underlying the strength of the HAR response and the significant amount of protection provided by the genetically modified kidneys. The moderate dose of complement inhibitor did not completely inhibit complement activation,16 instead it prolonged perfusion time compared to nontreated controls18 as shown in our previous perfusion experiments (xenogeneic perfusion time of WT kidney with complement inhibitor: 126 ± 72 min; WT kidney without complement inhibitor: 60 ± 0 minutes). Perfusion of kidneys from single transgenic hHO-1 pigs in the previous study was feasible for 230 ± 25 minutes with supplementary complement inhibitor and 190 ± 80 minutes without.6 In the present study, complement inhibition was not necessary in the GGTA1-KO/hHO-1/hA20 transgenic group and therefore was compatible with studies on xenogeneic coagulation activation. Comparison between our previous and present data show markedly improved survival of GGTA1-KO/hHO 1/hA20 transgenic kidneys over WT kidneys, but also over hHO-1 single transgenic kidney xenogeneic perfusion without complement inhibition. Presumably, the difference between WT and transgenic organs in the present study would have been even greater if no complement inhibitor had been used in WT kidneys.
Consistent with the significantly extended survival time in the perfusion circuit, the protective effects of the genetic modifications were also reflected in various coagulation parameters. Although fibrinogen and antithrombin levels dropped markedly during perfusion in WT organs, these molecules were barely consumed in transgenic kidneys, similar to autologous kidney perfusion. However, moderately increased levels of D-dimer and TAT compared to the autologous group indicated that activation of the coagulation could not be completely prevented. Nevertheless, the levels were considerably lower than those observed in the WT xenogeneic group or in the previous hHO-1 single transgenic group.6 A significant reduction of coagulation activation and platelet consumption was observed although no anticoagulant transgene was expressed in the triple transgenic kidneys. The beneficial effects can be explained by the interaction of IRI and AVR. Reactive oxygen species, which are generated during IRI, usually lead to apoptosis and activation of the endothelium. These features are also found during AVR. Endothelial cell activation promotes upregulation of cytokines, adhesion molecules, and procoagulant molecules like tissue factor (TF) on the endothelial surface.19 The TF forms a complex with coagulation factor VIIa and initiates the extrinsic coagulation cascade, resulting in fibrin clot formation. Upregulated adhesion molecules, such as E-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecule-1, are bound by innate immune cells and allow their transmigration. The innate immune cells produce more proinflammatory cytokines, such as TNF-α, which in turn has shown to upregulate more TF on endothelial cells20 and monocytes.21 Thus, inflammation and coagulation are linked and interact with each other.
HHO-1 and hA20 are well known “xenoprotective genes”19 and can protect tissue and organs from IRI.22,23 The hHO-1–derived CO suppresses endothelial cell apoptosis via activation of the p38 MAPK pathway,24 induces vasodilation, and inhibits platelet aggregation mediated by activation of guanylate cyclase.25 The A20 and HO-1 both block NF-κB activation, which in turn inhibits induction of apoptosis and reduced production of proinflammatory molecules. Thereby, less endothelium is damaged and less TF is expressed and exposed to the recipient's blood.
Although results from this study are preliminary and require substantiation, these results provide important new insight into the combined effects of three genetic modifications on kidney perfusion in a xenoperfusion setting. The comparison with previously perfused hHO-1 kidneys showed that the GGTA1-/hHO-1/hA20 kidneys are advantageous over the hHO-1 single transgenic alternative.6 However, individual effects of each modification cannot be inferred from this study because their interaction with each other is not predictable. It is not clear whether the prolonged perfusion time of the transgenic kidneys in this study was related to the lack of Gal molecules, the additional hA20 expression, or both. So far, no perfusion with GGTA1-KO kidneys alone has been performed.
In conclusion, this is, to the best of our knowledge, the first report on the production of pigs with a combined expression of 2 functional anti-apoptotic and anti-inflammatory transgenes on a GGTA1-KO background. The triple transgenic approach provided significant protection against apoptosis in an in vitro cytotoxicity assay and ischemia-reperfusion damage during ex vivo kidney perfusion, holding great potential for future in vivo experiments. Additional anticoagulant strategies, such as transgenic expression of human thrombomodulin26 or knockdown of porcine TF,27 are required to overcome molecular incompatibilities of anticoagulant pathways to achieve complete inhibition of the coagulation activation. The next step is to test GGTA1-KO/hHO-1/hA20 transgenic pig organs in pig-to-non-human primate xenotransplantation to prove potential clinical relevance.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Zoltán Ivics (Paul-Ehrlich-Institut, Langen, Germany) who kindly provided the Sleeping Beauty transposon system and the SB transposase 100X plasmid. The authors also thank Antje Frenzel and Dr. Sabine Klein for GGTA1-KO analyses. Furthermore, authors thank the staff from the pig facility, Edward Kufeld, Grit Möller, Toni Peker, and Johan Kun, for taking excellent care of the pigs.
Footnotes
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
This project was funded by DFG TR-CRC 127 (Biology of Xenogenic Cells) and ‘REBIRTH’ Cluster of Excellence to H.N.
The authors declare no conflicts of interest.
H.E.A. and B.P. contributed equally to the work.
H.E.A. participated in the performance of research, writing of the article, and data analysis. B.P. participated in the performance of research, writing of the article, data analysis, and research design. W.R. participated in the performance of experiments and writing of the article. S.P., D.H., J.H.-Q., A.L.-H., P.H., M.Z., W.B., and S.B. participated in the performance of experiments. R.S. participated in the performance of experiments, data analysis, and writing of the article. M.W. participated in the performance of experiments and data analysis. H.N. participated in the research design, performance of experiments, writing of the article.
REFERENCES
- 1. Diamond LE, Quinn CM, Martin MJ, et al. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation. 2001; 71: 132– 142. [DOI] [PubMed] [Google Scholar]
- 2. Langford GA, Yannoutsos N, Cozzi E, et al. Production of pigs transgenic for human decay accelerating factor. Transplant Proc. 1994; 26: 1400– 1401. [PubMed] [Google Scholar]
- 3. Fodor WL, Williams BL, Matis LA, et al. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci U S A. 1994; 91: 11153– 11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Phelps CJ, Koike C, Vaught TD, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003; 299: 411– 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwase H, Ezzelarab MB, Ekser B, et al. The role of platelets in coagulation dysfunction in xenotransplantation, and therapeutic options. Xenotransplantation. 2014; 21: 201– 220. [DOI] [PubMed] [Google Scholar]
- 6. Petersen B, Ramackers W, Lucas-Hahn A, et al. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation. 2011; 18: 355– 368. [DOI] [PubMed] [Google Scholar]
- 7. Tenhunen R, Ross ME, Marver HS, et al. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 1970; 9: 298– 303. [DOI] [PubMed] [Google Scholar]
- 8. Ryter SW, Choi AM. Carbon monoxide: present and future indications for a medical gas. Korean J Intern Med. 2013; 28: 123– 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balla G, Jacob HS, Balla J, et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992; 267: 18148– 18153. [PubMed] [Google Scholar]
- 10. Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc Natl Acad Sci U S A. 1996; 93: 6721– 6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaattela M, Mouritzen H, Elling F, et al. A20 zinc finger protein inhibits TNF and IL-1 signaling. J Immunol. 1996; 156: 1166– 1173. [PubMed] [Google Scholar]
- 12. Oropeza M, Petersen B, Carnwath JW, et al. Transgenic expression of the human A20 gene in cloned pigs provides protection against apoptotic and inflammatory stimuli. Xenotransplantation. 2009; 16: 522– 534. [DOI] [PubMed] [Google Scholar]
- 13. Zayed H, Izsvak Z, Walisko O, et al. Development of hyperactive sleeping beauty transposon vectors by mutational analysis. Mol Ther. 2004; 9: 292– 304. [DOI] [PubMed] [Google Scholar]
- 14. Izsvak Z, Chuah MK, Vandendriessche T, et al. Efficient stable gene transfer into human cells by the Sleeping Beauty transposon vectors. Methods. 2009; 49: 287– 297. [DOI] [PubMed] [Google Scholar]
- 15. Hauschild J, Petersen B, Santiago Y, et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A. 2011; 108: 12013– 12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramackers W, Friedrich L, Tiede A, et al. Effects of pharmacological intervention on coagulopathy and organ function in xenoperfused kidneys. Xenotransplantation. 2008; 15: 46– 55. [DOI] [PubMed] [Google Scholar]
- 17. Garrels W, Mates L, Holler S, et al. Germline transgenic pigs by Sleeping Beauty transposition in porcine zygotes and targeted integration in the pig genome. PLoS One. 2011; 6: e23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiane AE, Videm V, Johansen HT, et al. C1-inhibitor attenuates hyperacute rejection and inhibits complement, leukocyte and platelet activation in an ex vivo pig-to-human perfusion model. Immunopharmacology. 1999; 42: 231– 243. [DOI] [PubMed] [Google Scholar]
- 19. Bach FH, Ferran C, Hechenleitner P, et al. Accommodation of vascularized xenografts: expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nat Med. 1997; 3: 196– 204. [DOI] [PubMed] [Google Scholar]
- 20. Kirchhofer D, Tschopp TB, Hadvary P, et al. Endothelial cells stimulated with tumor necrosis factor-alpha express varying amounts of tissue factor resulting in inhomogenous fibrin deposition in a native blood flow system. Effects of thrombin inhibitors. J Clin Invest. 1994; 93: 2073– 2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Macey MG, Wolf SI, Wheeler-Jones CP, et al. Expression of blood coagulation factors on monocytes after exposure to TNF-treated endothelium in a novel whole blood model of arterial flow. J Immunol Methods. 2009; 350: 133– 141. [DOI] [PubMed] [Google Scholar]
- 22. Lutz J, Luong le A, Strobl M, et al. The A20 gene protects kidneys from ischaemia/reperfusion injury by suppressing pro-inflammatory activation. J Mol Med (Berl). 2008; 86: 1329– 1339. [DOI] [PubMed] [Google Scholar]
- 23. Yet SF, Tian R, Layne MD, et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001; 89: 168– 173. [DOI] [PubMed] [Google Scholar]
- 24. Brouard S, Otterbein LE, Anrather J, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000; 192: 1015– 1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987; 32: 497– 504. [PubMed] [Google Scholar]
- 26. Petersen B, Ramackers W, Tiede A, et al. Pigs transgenic for human thrombomodulin have elevated production of activated protein C. Xenotransplantation. 2009; 16: 486– 495. [DOI] [PubMed] [Google Scholar]
- 27. Ahrens HE, Petersen B, Herrmann D, et al. siRNA mediated knockdown of tissue factor expression in pigs for xenotransplantation. Am J Transplant. 2015; 15: 1407– 1414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


