Introduction
Antiviral therapy for recurrent hepatitis C in liver transplant recipients has been associated with low efficacy, poor tolerability, and drug-drug interactions. Recent approval of various hepatitis C direct-acting antivirals has resulted in improvement of these parameters. We evaluated the efficacy and safety of 12 week all-oral interferon- and ribavirin-free therapy with sofosbuvir and simeprevir.
Methods
Thirty-two genotype 1 liver transplant recipients with recurrent hepatitis C infection were retrospectively analyzed. All patients received 12 weeks of sofosbuvir 400 mg and simeprevir 150 mg orally daily. The primary endpoint was sustained virologic response 12 weeks after treatment.
Results
Sustained virologic response 12 weeks after treatment was achieved in 30 of 32 (94%; 95% confidence interval, 79-99%) patients. All patients enjoyed on-treatment virological response. Both patients who relapsed were cirrhotic, previously treated with Q80K polymorphism. Significant improvements in alkaline phosphatase, albumin, alanine aminotransferase levels, and platelets were seen at 12-week post therapy. Treatment was well tolerated. No grade 3 or 4 adverse events were noted. Headache and fatigue were the most common complaints.
Conclusion
Combination of sofosbuvir and simeprevir for 12 weeks resulted in 94% sustained virological response-12 rates in patients with hepatitis C genotype 1 and was well tolerated.
Chronic hepatitis C infection (CHC) is the leading cause for orthotopic liver transplantation (OLT) in the United States.1 Recurrence of CHC is almost universal among patients who are viremic at the time of OLT. Cirrhosis of the liver allograft develops in 30% of patients within 5 years after OLT2 and is the leading cause of graft loss among OLT patients with CHC. The rate of liver decompensation among cirrhotic patients with recurrent CHC after liver transplant is more rapid, and survival is shortened compared with immunocompetent CHC patients.3 However, eradication of hepatitis C virus (HCV) after liver transplant improves allograft and patient survivals.3,4
Previously, antiviral therapy in posttransplant setting with interferon or pegylated-interferon (Peg-IFN) and ribavirin was challenging, including low rates of sustained virological response (SVR), poor tolerability, and high rates of treatment discontinuation due to adverse events.5 Addition of protease inhibitors, boceprevir or telaprevir, resulted in improved SVR rates. However, poor tolerability, drug-drug interaction with tacrolimus and cyclosporine, as well as high rates of adverse events (including deaths) limited the use of these agents in the posttransplant setting.6-9
Sofosbuvir is a potent pan-genotypic inhibitor of the HCV NS5B polymerase inhibitor which is administered once daily. A multicenter study recently reported sofosbuvir and ribavirin combination therapy for 24 weeks in patients with compensated recurrent CHC after OLT with 70% of patients achieving SVR. Adverse events were uncommon but anemia was reported in 20% of patients.10
Simeprevir is a once daily HCV NS3/4A protease inhibitor which was first approved for use in combination with Peg-IFN and ribavirin to treat genotype 1 CHC patients.11-13 The combination of simeprevir and sofosbuvir, with or without ribavirin administered for 12 or 24 weeks, has been shown to be efficacious and well tolerated in immunocompetent CHC genotype 1 patients.14 The SVR12 was observed in 92% to 94% of patients, even in those with characteristics historically associated with low treatment success.14 The SVR rates were similar irrespective of duration of treatment or coadministration of ribavirin. As a result of these findings, the vast majority of prescriptions for simeprevir are written for the simeprevir/sofosbuvir combination15 and the U.S. Food and Drug Administration approved this regimen for use in immunocompetent CHC genotype 1 patients in November 2014.16
Based on the promising results of combination simeprevir/sofosbuvir therapy in immunocompetent CHC patients, we conducted this retrospective study to evaluate the efficacy and safety of a 12-week course of combination oral simeprevir and sofosbuvir in liver transplant recipients with recurrent genotype 1, hepatitis C infection.
MATERIALS AND METHODS
Patients
This is a retrospective analysis of 35 consecutive adult liver transplant recipients with recurrent genotype 1, HCV infection who were treated with combination simeprevir and sofosbuvir at Keck Hospital, University of Southern California. Recurrent hepatitis C infection was defined by detectable HCV RNA in the serum and histological evidence of hepatitis in the new allograft. Before initiating antiviral therapy, patients were required to have been on a stable immunosuppression regimen without acute cellular rejection and without any changes to the dosage of immunosuppression in the preceding 6 months. Patients whose glomerular filtration rate was less than 35 mL/minute were not eligible to receive treatment. Patients with decompensated cirrhosis (Child-Pugh score ≥ 8) were excluded. This study was approved by the institutional review board of the University of Southern California.
Three patients met exclusion criteria, 2 as a result of episodes of acute cellular rejection within 6 months and 1 due to decompensated liver cirrhosis. Thirty-two patients had received sofosbuvir 400 mg and simeprevir 150 mg orally daily for 12 weeks. No ribavirin was used. During treatment, patients were examined, and blood work (comprehensive metabolic panel, hematologic panel, immunosuppression level, and HCV RNA level) was obtained at weeks 4, 8, and 12 during therapy and 12 weeks after discontinuation of treatment (SVR12). The HCV RNA NS3 gene sequence analysis was initially performed in genotype 1a patients to assess for the Q80K polymorphism because efficacy of simeprevir given in conjunction with peg-interferon and ribavirin diminishes with this preexisting polymorphism.11-13 However, this practice was discontinued after it was shown that Q80K may not be relevant in patients receiving simeprevir and sofosbuvir.14
Efficacy and Safety Assessment
Primary endpoint was the proportion of patients achieving undetectable HCV RNA in serum, 12 weeks after completing therapy. Serum HCV RNA was analyzed by using the Roche Cobas Ampliprep/TaqMan version 2.0 real-time polymerase chase reaction system (Roche Molecular Systems, Inc., Branchburg, NJ). The lower limit of quantification was 15 IU/ml. Safety data were collected during treatment and at each visit. These data were graded in severity from 1 to 4. Grades 1 and 2 adverse events included mild to moderate symptoms with local or noninvasive intervention. Grades 3 and 4 adverse events were considered severe; or medically significant ranging from hospitalizations to life-threatening conditions.17
Patients were advised to avoid excessive sun exposure due to photosensitivity effects of simeprevir. All data, including unscheduled clinic visits, hospitalizations, changes in physical examination, and laboratory parameters, were recorded.
Statistical Analysis
Baseline characteristics, laboratory values, and adverse events were described with frequencies (percentages) or medians (range). Laboratory values were compared by treatment status (pretreatment, end of therapy, 12 weeks after therapy). The Wilcoxon signed rank test was used to compare differences for statistical significance, as appropriate. Analyses were performed using R 3.1.2 (R Development Core Team, Vienna, Austria).
RESULTS
Patient Demographics
Thirty-two consecutive liver transplant recipients with genotype 1 recurrent hepatitis C infection were initiated on combination simeprevir and sofosbuvir therapy between April and August 2014. Baseline demographic and clinical characteristics are shown in Table 1. Most patients were men and of Hispanic ethnicity. Median time since OLT was 48 months. Fifty-six percent of patients had previous therapy with pegylated interferon and ribavirin of which 22% were post-OLT treatment failures. None of the patients had been treated with either boceprevir or telaprevir previously. Sixty-nine percent of patients were infected with genotype 1a and median HCV RNA at treatment initiation was 6.58 log10 IU/mL. METAVIR fibrosis score F3/4 was present in 7 (22%) patients.
TABLE 1.
Baseline characteristics of study population (n = 32)
Treatment Efficacy
The SVR12 was achieved in 30 of 32 (94%; 95% confidence interval, 79-99%) patients. The HCV RNA became undetectable in 26 of 32 (81%) patients at week 4 (rapid virological response) and in all patients by end of therapy. There were no on-treatment virological failures or breakthroughs. Four of 6 patients with detectable HCV RNA at week 4 achieved SVR12. Among patients with F3-F4 fibrosis level, SVR12 was 75%. The effect of SVR12 on individual liver tests is shown in Figure 1. Improvements were seen in alkaline phosphatase, albumin, alanine aminotransferase levels, and platelet counts for both groups of patients. The HCV RNA NS3 gene sequence analysis was performed in 17 of 22 (77%) genotype 1a patients. Q80K polymorphism at baseline was detected in 14 of 17 (82%) genotype 1a patients that were tested.
FIGURE 1.
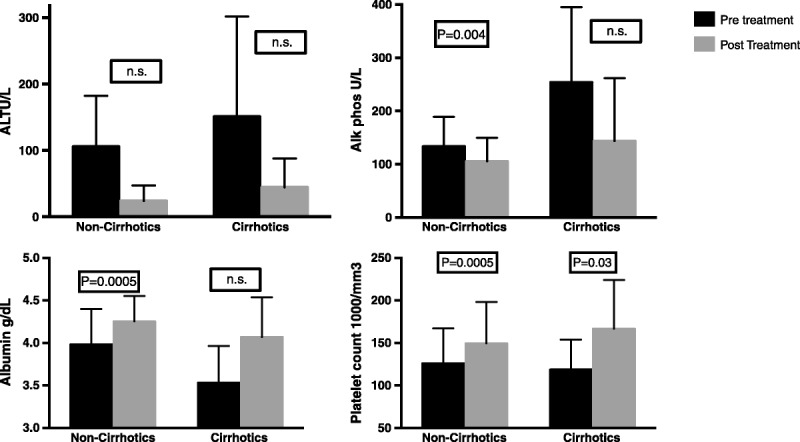
The SVR12 associated improvements in levels of ALT, alkaline phosphatase, albumin, and platelets (median and range) in patients with F0-F2 fibrosis and F3-F4 fibrosis. Statistical analysis performed by Wilcoxon signed rank test. ALT indicates alanine aminotransferase.
Two patients relapsed 1-month after discontinuation of therapy. Both patients (age, 57 and 61 years) had compensated cirrhosis, previously unsuccessfully treated after OLT, who were infected with genotype 1a with the Q80K mutation. Unlike the patients who responded, aminotransferases remained abnormal while on therapy and rebounded to higher levels after virological relapse in both patients. Alanine aminotransferase values ranged from 40 to 70 IU/L during therapy and were noted to be 129 and 124 IU/L at SVR12.
Safety
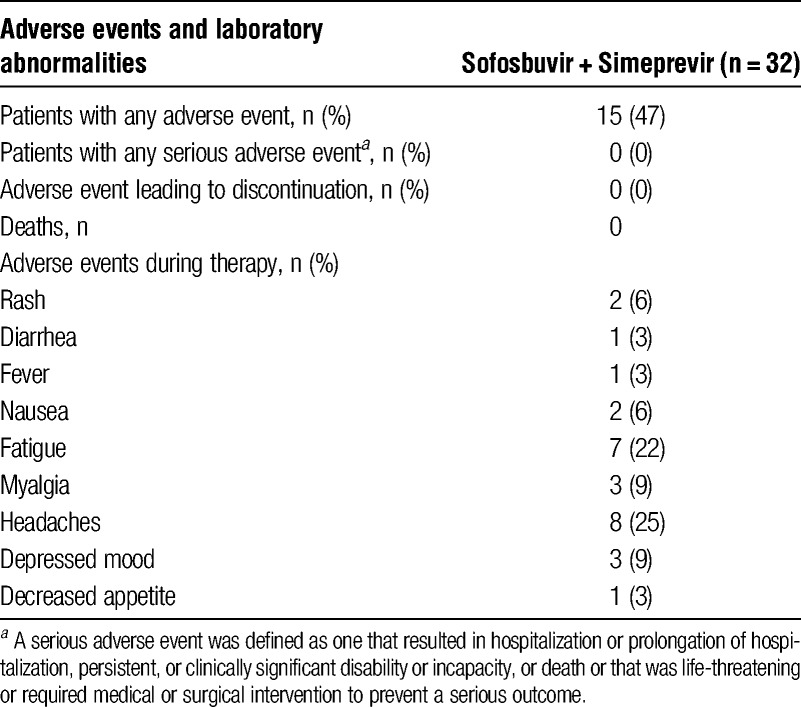
All patients completed 12 weeks of therapy without interruption. There were no grade 3 or 4 adverse events. Grade 1 and 2 adverse events were reported by 47% of patients (Table 2). Headaches (25%) and fatigue (22%) were the more common side effects. Mild skin rash were reported by 6% of patients. These did not require any therapy. Median bilirubin levels at baseline and at 4 weeks remained the same. Transient rise in serum bilirubin level (1.6 mg/dL increase) was observed in 3 patients during the first month of therapy that returned to baseline levels subsequently. No changes in white blood cell or red blood cells were observed, and no significant change in tacrolimus or cyclosporine trough levels was noted during therapy.
TABLE 2.
Treatment associated adverse events
DISCUSSION
The availability of several direct-acting antiviral agents without the need for interferon has dramatically altered the landscape of antiviral therapy for CHC. Experiences with these therapies are now being applied in different population of patients, including liver transplant recipients.10,18 Historically, eradication of hepatitis C infection in liver transplant recipients with interferon and ribavirin is associated with improved patient survival and decreased risk of clinical decompensation.3,4 However, SVR was achieved in only about 35% of patients treated with interferon and ribavirin and was associated with numerous side effects, including chronic rejection.19-22 Although improved SVR rates were seen with the addition of telaprevir or boceprevir, this came at a cost of serious side effects and requirement of dose adjustments of immunosuppression agents.6-9
Our study evaluated immunosuppressed patients with genotype 1, advanced fibrosis or cirrhosis, and high viral load, historically a challenging group of patients to treat. A 12-week course of simeprevir and sofosbuvir treatment for liver transplant recipients with recurrent genotype 1 HCV infection resulted in an SVR12 rate of 94%. The virological response to simeprevir and sofosbuvir was very similar to those reported in immunocompetent genotype 1 patients with chronic CHC.14 Among our patients with F3-F4 fibrosis, SVR12 rate was 75%.
A recent multicenter study of liver transplant patients with recurrent genotype 1 hepatitis C treated with sofosbuvir and simeprevir with/without ribavirin for 12 weeks reported a SVR12 rate of 90%.23 Twenty percent of patients used ribavirin, which resulted in more anemia than the nonribavirin group (72% vs 5%). However, the addition of ribavirin did not change SVR12 rates. Like our experience, patients with F3-F4 fibrosis in this multicenter study achieved lower SVR12 (70%).23 The SVR12 rate among immunocompetent genotype 1 patients with cirrhosis was also lower compared to non-cirrhotic patients (86% vs 100%).14 As a result, the FDA has approved a 24-week regimen for patients with liver cirrhosis. Extending therapy from 12 to 24 weeks for liver transplant patients with recurrent HCV who are cirrhotic may be more prudent.
Combination simeprevir and sofosbuvir represents a significant improvement of SVR12 compared to liver transplant patients treated with pegylated interferon and ribavirin (SVR12, 30%),19-22 boceprevir/telaprevir based triple therapy (SVR12, 60%)5,6 or sofosbuvir and ribavirin (SVR12, 70%).10 The efficacy of the combination of ombitasvir, paritaprevir boosted by ritonavir, and dasabuvir with ribavirin for 24 weeks (CORAL-1) was also studied in 34 genotype 1 hepatitis C liver transplant recipients.18 The SVR12 and SVR24 was seen in 33 (97%) patients although only patients with no or mild fibrosis were studied.
The drug-drug interactions of boceprevir or telaprevir with calcineurin inhibitors have been widely reported.6-9 As a result, the use of either of these 2 drugs after liver transplant required dose reduction of tacrolimus and cyclosporine and close monitoring of levels. This was also seen in the regimen used in the CORAL-1 trial that contained paritaprevir (NS3/4A protease-inhibitor) boosted by ritonavir. However, no significant drug-drug interactions were observed in this study including 2 patients that were immunosuppressed with cyclosporine both of whom achieved SVR12. The lack of effect of sofosbuvir and simeprevir on the pharmacokinetics of immunosuppressive agents is a distinct advantage with respect to tolerability and safety in the posttransplant setting.
Baseline NS3 Q80K mutation results in a 10-fold reduction in in vitro susceptibility of HCV to simeprevir,24 and reduced efficacy of simeprevir when used with Peg-IFN and ribavirin.11-13 Interestingly, both patients in our study who relapsed had Q80K polymorphism. However, the presence of Q80K polymorphism did not decrease the efficacy of simeprevir and sofosbuvir in immunocompetent CHC patients.14 Baseline NS5B resistance-associated variants have been implicated in certain treatment failures treated with sofosbuvir-based regimens.10,25 We did not measure these resistance-associated variants in our study, which may have also played a role in the 2 patients who relapsed. It should be noted that, these 2 patients had many of the other characteristics associated with unfavorable treatment response, including the presence of cirrhosis, previous treatment failure and age older than 45 years. Given our small sample size, we cannot determine the specific variables associated with treatment failure.
We recognize the limitations to this study. Although retrospective in nature, we followed a strict protocol for follow-up and monitoring of laboratory studies. Patients were from a single center, but the characteristics of our patients were representative of liver transplant recipients, including 22% of patients with F3/F4 fibrosis. The sample size was small but comparable to the recently published studies of interferon-free direct-acting antiviral agents in the treatment of recurrent HCV in liver transplant recipients.10,18
In summary, treatment with a 12 week course of all-oral regimen of sofosbuvir and simeprevir in recurrent genotype 1 hepatitis C liver transplant recipients resulted in 94% response. Treatment was well tolerated and did not require dose adjustment of tacrolimus or cyclosporine. Our study is part of a growing number of publications reporting on efficacy of sofosbuvir and simeprevir in posttransplant setting.26,27 Although larger studies are needed to confirm these findings, this regimen should be considered in the growing armamentarium of all-oral therapy for recurrent genotype 1 hepatitis C infection after liver transplantation.
Footnotes
The authors declare no funding or conflicts of interest.
S.K. participated in research design, writing of the article, and data analysis. B.L. participated in research design and data analysis. J.K. participated in research design.M.N. participated in research design. B.K. participated in research design. T.H. participated in research design. Y.E. participated in research design. T.-L.F. participated in research design, writing of the article, and data analysis.
REFERENCES
- 1. Scientific Registry of Transplant Recipients 2012 annual data report. Rockville, MD: Available at: http://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/03_liver_13.pdf. [Google Scholar]
- 2. Prieto M, Berenguer M, Rayón JM, et al. High incidence of allograft cirrhosis in hepatitis C virus genotype 1b infection following transplantation: relationship with rejection episodes. Hepatology. 1999; 29: 250– 256. [DOI] [PubMed] [Google Scholar]
- 3. Berenguer M, Palau A, Aguilera V, et al. Clinical benefits of antiviral therapy in patients with recurrent hepatitis C following liver transplantation. Am J Transplant. 2008; 8: 679– 687. [DOI] [PubMed] [Google Scholar]
- 4. Carrion JA, Navasa M, Garcia-Retortillo M, et al. Efficacy of antiviral therapy on hepatitis C recurrence after liver transplantation: a randomized controlled study. Gastroenterology. 2007; 132: 1746– 1756. [DOI] [PubMed] [Google Scholar]
- 5. Kuo A, Terrault NA. Management of hepatitis C in liver transplant recipients. Am J Transplant. 2006; 6: 449– 458. [DOI] [PubMed] [Google Scholar]
- 6. Burton JR, Jr, O’Leary JG, Verna EC, et al. A US multicenter study of hepatitis C treatment of liver transplant recipients with protease-inhibitor triple therapy. J Hepatol. 2014; 61: 508– 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coilly A, Roche B, Dumortier J, et al. Safety and efficacy of protease inhibitors to treat hepatitis C after liver transplantation: a multicenter experience. J Hepatol. 2014; 60: 78– 86. [DOI] [PubMed] [Google Scholar]
- 8. Saab S, Manne V, Bau S, et al. Boceprevir in liver transplant recipients. Liver Int. 2015; 35: 192– 197. [DOI] [PubMed] [Google Scholar]
- 9. Burton JR, Jr, Everson GT. Management of the transplant recipient with chronic hepatitis C. Clin Liver Dis. 2013; 17: 73– 91. [DOI] [PubMed] [Google Scholar]
- 10. Charlton M, Gane E, Manns MP, et al. Sofosbuvir and Ribavirin for Treatment of Compensated Recurrent Hepatitis C Virus Infection After Liver Transplantation. Gastroenterology. 2015; 148: 108– 117. [DOI] [PubMed] [Google Scholar]
- 11. Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naïve patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomized, double blind, placebo-controlled trial. Lancet. 2014; 384: 403– 413. [DOI] [PubMed] [Google Scholar]
- 12. Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naïve patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomized, double blind, placebo-controlled phase 3 trial. Lancet. 2014; 384: 414– 426. [DOI] [PubMed] [Google Scholar]
- 13. Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014; 146: 1669– 1679. [DOI] [PubMed] [Google Scholar]
- 14. Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomized study. Lancet. 2014; 384: 1756– 1765. [DOI] [PubMed] [Google Scholar]
- 15. Jensen DM, O’Leary JG, Pockros PJ, et al. Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: real-world experience in a diverse, longitudinal observational cohort (abstract). Hepatology. 2014; 60( S1): 55A. [Google Scholar]
- 16.Food and Drug Administration. Available at: http://mb.cision.com/Main/652/9675365/308904.pdf.
- 17.Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. U.S. Department of Health and Human Services. National Institutes of Health; 2009.
- 18. Kwo PY, Mantry PS, Coakley E, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014; 18: 2375– 2382. [DOI] [PubMed] [Google Scholar]
- 19. Samuel D, Bizollon T, Feray C, et al. Interferon-alpha 2b plus ribavirin in patients with chronic hepatitis C after liver transplantation: a randomized study. Gastroenterology. 2003; 124: 642– 650. [DOI] [PubMed] [Google Scholar]
- 20. Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol. 2008; 49: 274– 287. [DOI] [PubMed] [Google Scholar]
- 21. Calmus Y, Duvoux C, Pageaux G, et al. Treatment of recurrent HCV infection following liver transplantation: results of a multicenter, randomized, versus placebo, trial of ribavirin alone as maintenance therapy after one year of PegIFN a-2a plus ribavirin. J Hepatol. 2012; 57: 564– 571. [DOI] [PubMed] [Google Scholar]
- 22. Wang CS, Ko HH, Yoshida EM, et al. Interferon-based combination anti-viral therapy for hepatitis C virus after liver transplantation: a review and quantitative analysis. Am J Transplant. 2006; 6: 1586– 1599. [DOI] [PubMed] [Google Scholar]
- 23. Pungpapong S, Aqel B, Leise M, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology. 2015; 61: 1880– 1886. [DOI] [PubMed] [Google Scholar]
- 24. Lenz O, Verbinnen T, Fevery B, et al. Virology analyses of HCV isolates from genotype 1-infected patients treated with simeprevir plus peginterferon/ribavirin in Phase IIb/III studies. J Hepatol. 2015; 62: 1008– 1014. [DOI] [PubMed] [Google Scholar]
- 25. Curry MP, Forns X, Chung RT, et al. Sofosbuvir and ribavirin prevent recurrence of HCV infection after liver transplantation: an open-label study. Gastroenterology. 2015; 148: 100– 107. [DOI] [PubMed] [Google Scholar]
- 26. Gutierrez JA, Carrion AF, Avalos D, et al. Sofosbuvir and simeprevir for treatment of hepatitis C virus infection in liver transplant recipients. Liver Transpl. 2015; 21: 823– 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saab S, Greenberg A, Li E, et al. Sofosbuvir and simeprevir is effective for recurrent hepatitis C in liver transplant recipients. Liver Int. 2015. [DOI] [PubMed] [Google Scholar]


