Background
Extremely few reports have been published on experience with multiple combined pancreas-kidney re-transplantation including long-term results. We here analyze our experience with two patients following successful combined pancreas fourth-kidney third and pancreas third-kidney second transplantation.
Methods
Patient and graft survival as well as graft function and major complications were recorded. Patient 1 (women, 47 years) underwent combined pancreas fourth-kidney third transplantation after previous removal of the first and second renal and the second pancreatic grafts. Patient 2 (men, 51 years) underwent combined pancreas third-kidney second transplantation after nephrectomy of the first renal graft. Immunosuppression consisted of induction with alemtuzumab and maintenance with tacrolimus, mycophenolate mofetil/mycophenolic acid and steroids.
Results
After a follow-up of 44 and 49 months, respectively, both patients are doing well with stable graft function. Leukopenia, thrombocytopenia, bacterial sepsis, and chronic hepatitis C as major complications were controllable.
Conclusions
Multiple pancreas-retransplantations combined with simultaneous renal transplantation are feasible. Meticulous immunosuppression, careful monitoring, and excellent patient adherence are of crucial importance.
Further to previously published experiences in pancreas retransplantation, Rudolph et al recently reported acceptable 1-year graft survival rates in repeat pancreas retransplantation in select candidates.1–7
Which patients are suitable for the challenge of multiple pancreas retransplantation?
We recently reported a superior 5-year pancreatic graft survival with 68.8% in simultaneous pancreas-kidney (SPK) transplants as compared to 62.5% in pancreas after kidney (PAK) transplants.8 Rudolph et al reported on pancreas retransplantation, identifying higher rates of rejection for PAK than that for SPK.5
To confirm suitable criteria for repeat pancreas retransplantation, we here report the detailed course in 1 case each of combined pancreas fourth-kidney third and pancreas third-kidney second transplantation with good long-term outcome.
PATIENTS AND METHODS
Patient 1
A 47-year-old type I diabetic woman underwent a combined pancreas fourth-kidney third transplantation (donor age, 16 years) after having lost 2 renal as well as 2 pancreas grafts due to chronic rejection and 1 pancreas graft due to early technical failure. The intervals between the first, second, third, and fourth pancreas transplantations were 13, 12, and 10 years, respectively. The time between the first and second and the third renal transplantations was 18 and 12 years, respectively. The first and second previous renal grafts and the second pancreatic grafts were retrieved before retransplantation. Their histological analysis identified a severe arteriosclerosis and arteriolosclerosis indicating diabetic nephropathy plus chronic pyelonephritis in the first renal graft and a chronic vasculopathy plus chronic pyelonephritis in the second kidney graft. The cause of the first pancreatic graft loss was most likely rejection, but no biopsy was performed, and this remains speculative. In the retrieved (second) pancreatic graft, an arterial thrombosis with parenchyma necrosis was histologically verified.
Via a transperitoneal access, the fourth pancreas graft was implanted in the right small pelvis with an arterial anastomosis (Y graft of the donor mesenteric and splenic arteries) to the recipient right external iliac artery and a venous anastomosis (donor portal vein) to the recipient inferior vena cava. The exocrine drainage of the pancreas was established through a duodenojejunostomy. The third kidney graft was implanted in the left side of the small pelvis with an arterial and venous anastomosis to the external iliac vessels and ureteral anastomosis according to the technique described by Lich-Gregoir. Detailed data about the preceding medical history and surgical technique are given in Table 1.
TABLE 1.
History, Comorbidities, Perioperative and Surgical Data, Donor Data, Immunosuppression, Concomitant Medication, Actual Laboratory Values
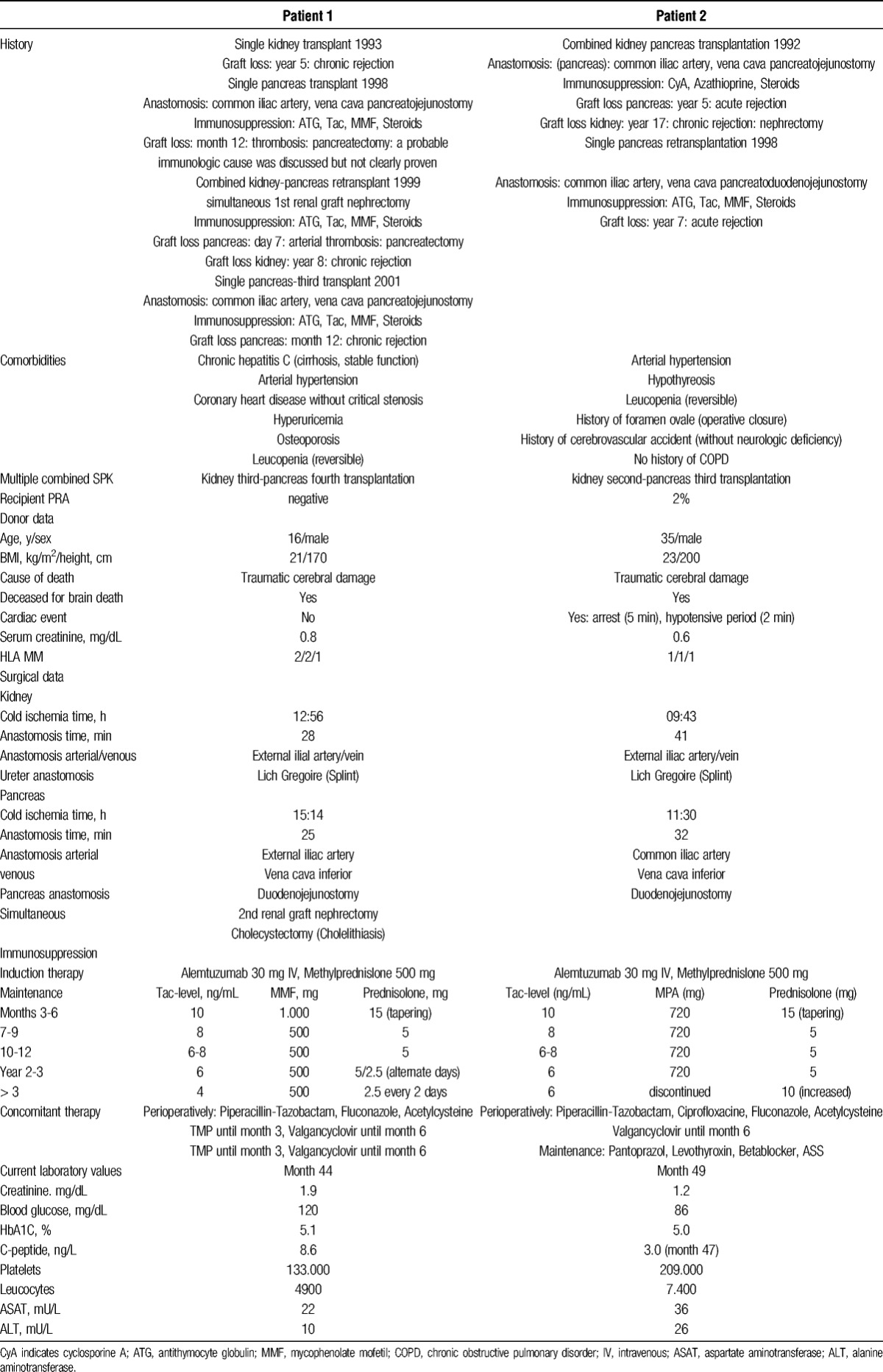
Immunosuppression consisted of alemtuzumab, tacrolimus (Tac), mycophenolate mofetil, and steroids gradually tapered to 2.5 mg (Table 1). This low-dosed triple immunosuppression was adapted to the elastography-proven high-grade hepatitis C fibrosis (median stiffness 19.6 kPa at month 15 after transplantation) with stable liver function.
The concomitant medication, including infectious prophylaxis with trimethoprim-sulfametoxazole and valgancyclovir, is depicted in Table 1.
Patient 2
A 51-year-old type I diabetic man underwent a combined pancreas third-kidney second transplantation (donor age, 35 years) after immunological loss of all his previous kidney and pancreas grafts. The renal graft was removed at another center, and the cause of graft loss according to the histological workup was chronic rejection. The time intervals between the first and second and the third pancreas transplantations were 18 and 12 years, respectively. The time between first and the second renal transplantations was 18 months. The third pancreas was implanted in the right small pelvis with an arterial anastomosis (Y graft of the donor mesenteric and splenic arteries) to the recipient right common iliac artery and a venous anastomosis (donor portal vein) to the recipient vena cava inferior. The exocrine drainage was established through a duodenojejunostomy. The second renal graft was positioned in the left iliac side and anastomosed to the left external iliac vessels. The ureter was implanted according to the method of Lich-Gregoir.
The detailed data on the patient's medical history preceding retransplantion and surgical details are given in Table 1.
Immunosuppression consisted of alemtuzumab 30 mg, Tac, steroids and cautiously reduced mycophenolic acid (MPA) regarding leukopenia (Table 1).
A clinically diagnosed acute rejection (increase in serum creatinine, fever) was reversed by pulsed methylprednisolone (1750 mg in total over post-transplant days 15-18).
Prednisolone was increased in the third year due to idiopathic thrombopenia that was reversed with rituximab, romiplastin, and a steroid bolus.
The concomitant therapy including infectious prophylaxis is listed in Table 1. Regarding leukopenia, trimethoprim-sulfametoxazole was avoided, and the valgancyclovir dose reduced. The pulmonary situation was cautiously monitored by chest X-ray with consistent respiratory exercises controlled by scheduled physiotherapist visits in the perioperative period.
Before listing for combined retransplantantion, both patients were precisely evaluated including infectiological and oncological screening. They were seen by a dentist, otolaryngologist, gynecologist, and urologist. Viral screening included CMV and EBV. Furthermore, they underwent gastroscopy, colonoscopy, abdominal, and pelvic ultrasound, including Doppler sonography of the pelvic and peripheral vessels, ophthalmological (split lamp), and neurological investigation. Finally, spirometry, exercise stress test, echocardiography, and coronary angiography were performed to evaluate the perioperative risk.
The posttransplant routine controls included regular laboratory tests of renal and pancreatic function (including C-peptide serum levels), blood cell count, liver function tests, daily glycemia self-controls, 3 to 6 monthly ophthalmological and ultrasound check-ups, yearly check-up visits including chest X-ray, electrocardiogram, echocardiography.
RESULTS
Patient 1
The postoperative course of the pancreatic graft was free of any immunological or vascular complications and characterized by normoglycemia without exogenous insulin. Renal function was altered by delayed graft function requiring three dialyses with complete recovery within the first month.
Notable complications were a postoperative hematoma requiring relaparotomy on day 1 after transplantation, CMV replication without tissue invasion (positive CMV-PCR in week 4) treated by valgancyclovir for 6 months and right hip necrosis (probably related to osteoporosis and perimenopausal age) occurring in month 36 with subsequent implantation of endoprosthesis.
Currently, at month 44, the patient is in good general condition with normal renal and pancreatic function. Liver function is stable (Table 1).
Patient 2
Regarding the pancreatic graft, no immunological or vascular complications occurred. The patient did not need any exogenous insulin during the entire hospital stay. The postoperative course of the kidney showed good initial function. However, it was complicated by a reversible acute rejection in the first month after transplantation.
In the long-term course, 3 major complications occurred: CMV replication without tissue invasion (positive CMV PCR in week 4), treated with valgancyclovir for 6 months, a Staphylococcus-driven bacterial sepsis treated with antibiotics based on susceptibility testing in month 4, and idiopathic thrombocytopenia in month 32, reversed by rituximab, steroids, romiplostim, and discontinuation of MPA.
Currently, at month 49, the patient is in good general health with stable renal and pancreatic graft function and showing normal platelet and leukocyte counts (Table 1).
Because of his hematologic risk situation, he is on dual immunosuppression with Tac and steroids after discontinuation of MPA.
Neither of the 2 patients developed malignancies or significant secondary diabetic complications, such as critical peripheral angiopathy/retinopathy.
DISCUSSION
Several centres do not perform repeat pancreas retransplants. Increased risks for graft loss of the second pancreatic graft due to technical failures and acute rejection are considered a strong deterrent.1–3,5,7 In fact, reports on acceptable results in pancreas retransplantation highlight patient selection as a crucial prerequisite.1–6 Data on pancreas retransplantation recently reported by Rudolph et al confirm their acceptable 1-year graft survival of 75.8% in highly selected candidates, while noting a decreased 5-year actuarial graft survival for second and third transplants, as compared to primary transplants, and a higher rejection rate for PAK than for SPK.1 Öllinger et al retrospectively identified a superior 5-year pancreatic graft survival (68.8%) in SPK first transplants as compared to 62.5% in PAK first graft recipients.
Personal immunological and infectious risk, coronary, and peripheral vascular status and more general operative risk factors are important criteria for selecting suitable candidates for multiple retransplants, especially in the light of donor organ shortage. So far, there is a paucity of reports on long-term experiences in third and fourth pancreas retransplantation.
In light of this, we report on our experience with 2 cases of multiple combined pancreas-kidney retransplantation, 1 pancreas fourth-kidney third and 1 pancreas third-kidney second transplantation with good long-term outcome.
Suitability criteria for repeat pancreas retransplantation were excellent adherence and good general clinical condition without any critical coronary or peripheral artery stenosis, with minimal (panel reactive antibodies 2%) or negative grade of immunization, and stable comorbidities and reliable self-control to prevent infections.
Experienced surgeons following standard techniques performed both multiple retransplants. Preceding old grafts were removed for thrombosis, technical reasons, consistent with the experiences of LaMattina and Rudolph.1,5 These graft removals also contributed to creating anatomical/surgical space to position the new organs. The pancreas grafts were positioned via a transperitoneal access in the right iliac side by making an arterial anastomosis to the right external/common iliac artery, respectively; the venous anastomosis was made to the inferior vena cava inferior, and exocrine drainage was established through a duodenojejunostomy. The renal grafts were implanted in the left iliac side according to standard techniques.8
The fact that both study patients had a negative or very low PRA probably leads the potential benefit of explanting previous transplants to be questioned considering the relatively low immunologic risk to be anticipated in subsequent retransplantation. No pancreatic venous thrombosis occurred in either patient. Because of the pancreatic vulnerability to vascular complications and because of the inherently intrapancreatic low microvascular flow and hypercoagulable blood of diabetic persons, patients were started early on maintenance acetylsalicylic acid.9
In addition to the selection of recipients, donor organs must also be carefully selected to minimize risk factors negatively influencing graft and patient survival. In both cases, the young donor age (16 and 35 years) and good recipient vessel quality were probable factors explaining the good primary pancreatic function despite the relatively long cold ischemia time (>11 and >15 hours, respectively). This was also reflected in the low pancreas donor risk index of the 2 transplants, namely, 1.10 and 1.01.10
With regard to efficient induction therapy in repeat SPK retransplants, alemtuzumab as an effective lymphocyte-depleting agent was administered in both patients, who had received antithymocyte globulin preparations with previous transplants.11–16 The maintenance immunosuppression in 1 patient consisted of a low-dosed triple-drug regimen with minimized steroids due to chronic hepatitis C and osteoporosis. The other patient received a dual combination without MPA due to leukopenia and idiopathic thrombopenia.
No pancreatic rejection occurred, indicating an immunological advantage of combined retransplantation as compared to pancreas retransplantation alone (either PAK or PTA) observed by Rudolph et al in their single-center report.1 All infectious complications were treated successfully.
No tumor, peripheral diabetic angiopathy, or retinopathy occurred. This is in accordance with Giannarelli et al,17 who reported an improvement or stabilization of diabetic co-morbidities in the majority of patients following successful pancreas transplantation.
After a follow-up period of 44 and 49 months, respectively, both patients are in good general health with normal renal and pancreatic function.
As a conclusion drawn from this very small but accurately monitored cohort, we would consider repeat re-SPK to be feasible in the following conditions:
Stable physical condition, suitable coronary, and peripheral vascular status, minimal or negative grade of immunization, careful operative technique performed by experienced surgeons, excision of preceding nonfunctioning grafts, early anticoagulation, T cell-depleting induction therapy, cautious patient-specific maintenance immunosuppression, regular long-term follow-up check-ups to ensure excellent long-term patient adherence and medical accuracy.
Footnotes
The authors declare no funding or conflicts of interests.
Trial registry number: AN2014-0265 341/4, 5 Ethics Committee of the Medical University of Innsbruck, Austria (approval: 25-Sep-2014)
C.B. participated in research design, performance of research, data analysis, writing of the article. M.M. participated in performance of the research, data analysis, writing of the article. C.M. participated in performance of the research. T.D. participated in data analysis. M.B. participated in performance of the research. J.P. participated in performance of the research. R.O. participated in performance of the research. D.O. participated in performance of the research. S.S. participated in performance of the research, writing of the article.
REFERENCES
- 1. Rudolph EN, Finger EB, Chandolias N, et al. Outcomes of pancreas retransplantation. Transplantation. 2015; 99: 367– 374. [DOI] [PubMed] [Google Scholar]
- 2. Morel P, Schlumpf R, Dunn DL, et al. Pancreas retransplants compared with primary transplants. Transplantation. 1991; 51: 825– 833. [DOI] [PubMed] [Google Scholar]
- 3. Fellmer P, Lanzenberger K, Ulrich F, et al. Complication rate of pancreas retransplantation after simultaneous pancreas-kidney transplantation compared with pancreas after kidney transplantation. Transplant Proc. 2007; 39: 563– 564. [DOI] [PubMed] [Google Scholar]
- 4. Genzini T, Crescentini F, Torricelli FC, et al. Pancreas retransplantation: outcomes of 20 cases. Transplant Proc. 2006; 38: 1937– 1938. [DOI] [PubMed] [Google Scholar]
- 5. LaMattina JC, Sollinger HW, Becker YT, et al. Simultaneous pancreas and kidney (SPK) retransplantation in prior SPK recipients. Clin Transplant. 2012; 26: 495– 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buron F, Thaunat O, Demuylder-Mischler S, et al. Pancreas retransplantation: a second chance for diabetic patients? Transplantation. 2013; 95: 347– 352. [DOI] [PubMed] [Google Scholar]
- 7. Sansalone CV, Maione G, Rossetti O, et al. Pancreas retransplantation: ideal timing and early and late results. Transplant Proc. 2006; 38: 1153– 1155. [DOI] [PubMed] [Google Scholar]
- 8. Öllinger R, Margreiter C, Bösmüller C, Weissenbacher Frank F, et al. Evolution of pancreas transplantation: long-term results and perspectives from a high-volume center. Ann Surg. 2012; 256: 780– 786. [DOI] [PubMed] [Google Scholar]
- 9. Patel SR, Hakim N. Prevention and management of graft thrombosis in pancreatic transplant. Exp Clin Transplant. 2012; 10: 282– 289. [DOI] [PubMed] [Google Scholar]
- 10. Axelrod DA, Sung RS, Meyer KH, et al. Systemic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant. 2010; 10: 837– 845. [DOI] [PubMed] [Google Scholar]
- 11. Bösmüller C, Öllinger R, Sieb M, et al. Tacrolimus monotherapy following alemtuzumab induction in combined kidney-pancreas transplantation: results of a prospective randomized trial. Ann Transplant. 2012; 17: 45– 51. [DOI] [PubMed] [Google Scholar]
- 12. Kaufman DB, Leventhal JR, Gallon LG, et al. Alemtuzumab induction and prednisone-free maintenance immunotherapy in simultaneous pancreas-kidney transplantation comparison with rabbit antithymocyte globulin induction—long-term results. Am J Transplant. 2006; 6: 331– 339. [DOI] [PubMed] [Google Scholar]
- 13. Muthusamy AS, Vaidya AC, Sinha S, et al. Alemtuzumab induction and steroid-free maintenance immunosuppression in pancreas transplantation. Am J Transplant. 2008; 08: 2126– 2131. [DOI] [PubMed] [Google Scholar]
- 14. Farney AC, Doares W, Rogers J, et al. A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation. 2009; 88: 810– 819. [DOI] [PubMed] [Google Scholar]
- 15. Reddy KS, Devarapalli Y, Mazur M, et al. Alemtuzumab with rapid steroid taper in simultaneous kidney and pancreas transplantation: comparison to induction with antithymocyte globulin. Transplant Proc. 2010; 42: 2006– 2008. [DOI] [PubMed] [Google Scholar]
- 16. Uemura T, Ramprasad V, Matsushima K, et al. Single dose of alemtuzumab induction with steroid-free maintenance immunosuppression in pancreas transplantation. Transplantation. 2011; 92: 678– 685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giannarelli R, Coppelli A, Sartini M, et al. Effects of pancreas-kidney transplantation on diabetic retinopathy. Transpl Int. 2005; 18: 619– 622. [DOI] [PubMed] [Google Scholar]


