Supplemental digital content is available in the text.
Background
Naturally acquired immune regulation amongst family members can result in mutual regulation between living related renal transplant donor and recipients. Pretransplant bidirectional regulation predisposed to superior renal allograft outcome in a CAMPATH-1H protocol. We tested whether Rhesus macaques, a large animal model of choice for preclinical transplant studies, share these immunoregulatory properties.
Methods
Antigen-specific linked suppression was measured by trans vivo delayed-type hypersensitivity [tvDTH] response. Neutralizing antibodies to regulatory cytokines, IL-10, TGF-β, and IL-35 were coinjected to ascertain the role of these cytokines in the regulatory response.
Results
Peripheral blood mononuclear cells (PBMC) of 116 Rhesus macaques in 50 families and 78 human subjects in 25 families were analyzed. Suppression of the recall response of 25% or greater was detected in 30 of 51 (59%) monkeys, and 25 of 36 (69%) human subjects when PBMC were coinjected with antigens of the mother, containing the noninherited maternal antigens. In 33% of Rhesus and 32% of human subjects, linked suppression was also seen when PBMC from the mother was assayed with antigens from offspring. Bidirectional regulation was also seen between greater than 50% of the major histocompatibility complex (MHC)-identical full siblings; subcellular antigens caused significant linked suppression in 7 of 10 (Rhesus) and 8 of 15 (human) cases, indicating the importance of familial minor H antigens. The lowest incidence of regulation was seen in MHC-1 haplotype mismatched siblings in both species. Linked suppression was most effectively reversed by antibodies that neutralized TGFβ1, and the 2 subunits of IL-35 (Ebi3 and IL12p35).
Conclusions
Rhesus macaques provide a suitable model for analyzing the impact of bidirectional regulation in living related donor-recipient pairs.
Clonal deletion, anergy, and immunoregulation comprise the 3 major mechanisms by which transplant tolerance induction and maintenance are thought to occur.1 In the case of transplants between family members, all 3 mechanisms may be in play from the moment the transplant is placed, due to microchimerism-based preconditioning of host and donor.2 Recent analysis of the immunoregulation aspect of this preconditioning has indicated its potential clinical relevance. Indeed, pretransplant “natural” alloantigen-specific immune regulation predisposed to superior graft outcome in both mice and humans.3,4 Surprisingly, we found that the optimal condition for success of a living donor kidney transplant in a depletional (CAMPATH-1H) protocol was not simply antidonor regulation, but “bidirectional” (recipient antidonor, but also donor antirecipient) regulation. The data suggested that transplant success in these heavily lymphodepleted patients might have resulted from mutual recognition by CAMPATH-1H–resistant recipient and tissue-resident donor T regulatory (Treg) cells of alloantigens expressed by the donor or recipient, respectively. In fact, when regulation was only unidirectional, for example, host-to-donor only, with no reciprocal regulation on the donor side, there was a very high incidence of class I and class II DSA and early rejection.4 Although the exact nature of the primary antigen exposure required to educate the host and donor Treg cells to these foreign antigens is unknown, one likely source is a previous encounter with antigens expressed by naturally acquired microchimeric cells.5,6 In living-related individuals, such antigens may include: (a) noninherited maternal antigens (NIMA) in offspring, (b) inherited paternal antigens (IPA) expressed by fetus-derived cells in the mothers, or (c) transmaternally derived antigens, expressed by cells from an elder sibling, for example, cells that persist in the mother from a previous pregnancy.7,8
The Rhesus macaque is a clinically relevant model for the study of transplantation, and although the MHC of Rhesus is highly complex,9 colonies have been bred in such a way as to permit selection of live-related donor-recipient pairs with MHC identity or defined MHC single haplotype mismatches.9–11 We previously published an extensive analysis of posttransplant immune regulation in Rhesus monkeys using the trans vivo delayed-type hypersensitivity (tvDTH) assay, with PBMC obtained at various timepoints after kidney allotransplantation and immunosuppression withdrawal.12 From this study, we learned that (a) biopsy rejection status was correlated with a tvDTH response to donor antigen (dAg), (b) allograft acceptors had low anti-dAg tvDTH responses, (c) tvDTH responses of the acceptor monkeys became strongly positive with addition to the mouse footpad injections of anti-TGFβ1 but not anti-IL10 antibodies, (d) transplant acceptor monkeys had latent TGFβ1+ CD4 T cell infiltrates in their kidney allograft biopsies, and (e) coinjection of dAg, recall Ag (tetanus toxoid [TT]), and monkey PBMC in the footpad of CB17.scid mice led to a pronounced (50-75%) linked suppression of the TT response in allograft acceptors, but not in rejector monkeys.12 The transplant donors and recipients in this study of tolerance induction were all unrelated. Interestingly, a single Mamu-DR match was strongly associated in the depletional protocol used, with long-term metastable tolerance, and the development of both antidonor tvDTH linked suppression in PBMC, and maintenance of surface CD4+TGFβLAP+ cells in the allograft.12
Given this previous experience with depletional trials of tolerance in humans and monkeys, and the recent mixed successes of clinical trials of transplant tolerance induction combining leukocyte depletion with mixed chimerism in living related donor-recipient pairs,13–15 several laboratories have become interested in finding appropriate donor-recipient pairings to optimize results of tolerance trials in kidney allografts. However, although there were published reports on pretransplant alloreactivity predicting posttransplant outcome in trials of renal transplant tolerance induction in unrelated monkeys,16,17 we know little about pretransplant immune regulation that might exist between related monkeys. So we wished to answer the question: does naturally acquired antigen-specific regulation exist in the Rhesus macaque, and is it therefore a suitable model in which to test prospectively whether pretransplant unidirectional or bidirectional regulation would predispose to better allograft survival?
Previous work in mouse models identified IL-10 and TGFβ1 produced by indirect pathway CD4+ Treg cells as important cytokines in sustaining regulatory tolerance to NIMA before and after a fully allogeneic NIMA-expressing heart transplantation.3,18 The discovery in 2007 of the IL-12 family cytokine IL-35 and its prominence in peripheral immune regulation and tumor-induced suppression in mice19,20 suggested that an important aspect of immune regulation may have been previously overlooked. Indeed, in men with prostate cancer, we recently found CD8+CTLA4+ regulatory T cells that suppressed immune responses to the tumor antigen prostate acid phosphatase via IL-35, with no contribution from either IL-10 or TGFβ1.21 Although IL-35 has been shown to play an important role in suppressing immune responses to tumors in both mouse models19,20 and in humans,21,22 the role of the IL-35 in the function of natural allospecific regulatory T cells is currently unknown.
We therefore sought to test these hypotheses: (1) that antigen-specific, naturally acquired immune regulation in the Rhesus macaque exists, and that its familial pattern is similar to that in humans; (2) that such preexisting allospecific regulation means that mutual (bidirectional) regulation can exist naturally between select pairs of related Rhesus monkeys, as in humans; and (3) that IL-35 plays a critical role in such regulation in both species.
MATERIALS AND METHODS
Source of PBMC
Peripheral blood was obtained by venipuncture from 116 healthy Rhesus Macaques housed either at the Wisconsin National Primate Research Center (Madison, WI) or at Alphagenesis (Yemassee, South Carolina, NIAID sponsored colony), according to Research Animal Resources Committee–approved protocols. All of the monkeys were entered into screening for bidirectional regulation with 1 or more family members (n = 79 pairings). Peripheral blood was also obtained by venipuncture from 78 human subjects after internal review board-approved written informed consent; again, all participants were entered with 1 or more family member. Human subjects included prospective kidney transplant recipients with end-stage renal disease and their healthy donors (n = 22 pairs, 44 total subjects) and 34 additional healthy family members. A subset of the human subjects was screened for bidirectional regulation (n = 39 pairings). All PBMC were isolated by Ficoll-Hypaque separation (Lymphocyte Separation Media, Cellgro) and ammonium chloride-potassium buffer lysis of interface cells to remove erythrocytes as previously described.12,23
Test for Linked Suppression by tvDTH Assay
The trans-vivo delayed type hypersensitivity (tvDTH) assay has been described extensively elsewhere.23–25 Antigen-specific linked suppression was measured by injecting PBMC into the footpads of CB17.SCID mice along with recall antigen (TT + diphtheria toxoid [TT/D] or Epstein-Barr virus) with or without soluble antigen from related family members (lysates of PBMC). The soluble antigen was prepared by sonication of cells, 12 × 106 in 100 μL, followed by 16,000×g centrifugation to remove large cell fragments.23 Linked suppression was calculated as the % inhibition of the footpad swelling response to recall antigen in the presence of subcellular antigen, as compared with the response to recall antigen alone, using the formula:

In the pretransplant setting, we will consider reductions of 25% or greater to be evidence of significant alloregulation in 1 direction. For bidirectional regulation, patient-donor related pairs in whom the combined regulation score (A vs B, plus B vs A) was 66% or greater had significantly better outcomes in a depletional kidney transplant protocol than pairs in which the combined regulation score was less than 66%.4 The same standard for bidirectional regulation was applied in the present study: that is, only those pairs with linked suppression values of at least 25%, and a combined regulation score of 66% or greater were considered bidirectional regulators.
To determine the cytokines involved in regulation, neutralizing antibodies to “classical” immunoregulatory cytokines (TGFβ, IL-10) or to subunits of IL-35 (IL-12α, Ebi3) were included in the assay, as described elsewhere.21,26
Sources of Anticytokine Antibody
Neutralizing antibody to TGFβ1 (rabbit IgG), human IL-10 (goat IgG), IL-12α/p35 (mouse IgG1) as well as control rabbit and goat IgG were obtained from R&D Systems (Minneapolis, MN). Neutralizing antibody to IL-10 (rat IgG1, JES3-9D7, cross-reactive with human and rhesus, LEAF purified reagent) and control Rat IgG1 were obtained from BioLegend (San Diego, CA) and control mouse IgG1, κ control was obtained from BD Biosciences (San Jose, CA). Anti-Ebi3 was a gift from Dr. Dario Vignali.
Statistical Analysis
Comparisons of regulation magnitude between groups of monkey or human subjects were performed using the analysis of variance test with Bonferroni posttest comparison between groups. For cytokine neutralization experiments, repeated measures analysis of variance was used, with Bonferroni posttest comparison between individual groups.
RESULTS
Patterns of Immune Regulation in Rhesus Monkey Families
We tested a total of 116 Rhesus macaques for regulation toward antigens of immediate family members (166 total tests). As shown in Figure 1, normal healthy Rhesus monkeys do not regulate their recall responses in the presence of self antigens prepared from autologous cell sonicates. However, they frequently regulated their tvDTH response to the recall antigen TT in the presence of antigen preparations made from maternal cells, that is, containing NIMA. Of the 51 monkeys tested, 30, or 59% had regulation to NIMA (defined as 25% inhibition of a recall antigen response), whereas 17, or 33%, had strong (≥50%) regulation.
FIGURE 1.
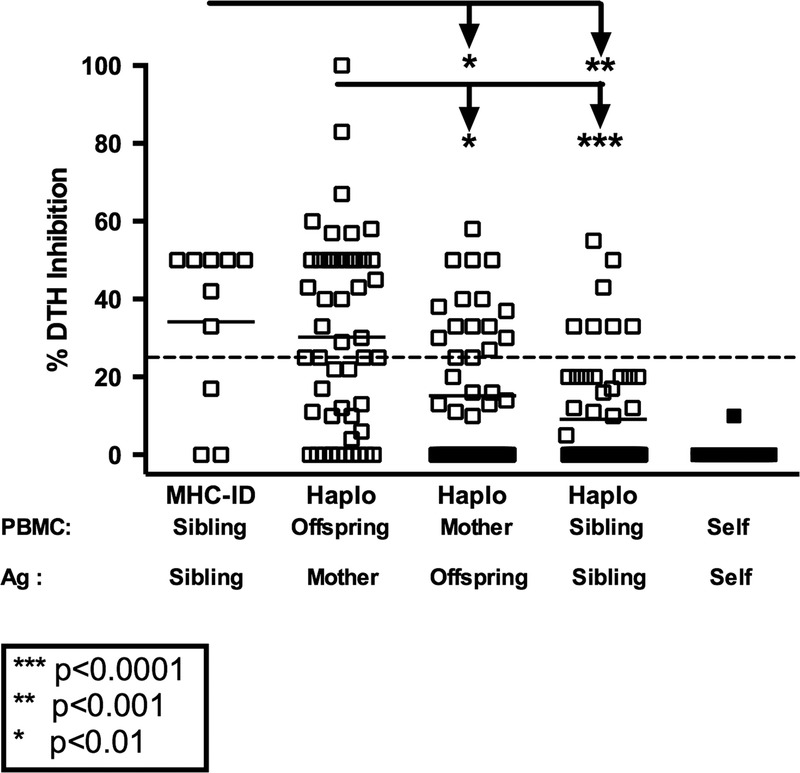
Regulation pattern in Rhesus, by relationship. PBMC from individual Rhesus (n = 116) within a family were tested in the tvDTH assay for regulation using antigen (cell lysate) from all available family members (n = 166 total tests). The % inhibition of the recall response in the presence of the familial antigen is plotted with responses grouped based on the familial relationship. Dashed line represents 25% DTH inhibition. MHC-ID siblings showed the highest level of regulation and this was significantly higher than that see between 1 haplo-mismatched siblings (**P ≤ 0.001) or maternal responses to offspring (*P < 0.01). Offspring responding to maternal antigen show the next highest level of regulation and this was significantly higher than the mother's response to offspring antigen (*P < 0.01) and 1 haplo mismatched sibling responses to each other (***P < 0.0001).
Approximately one third of the time (16/48 tests), Rhesus dams displayed regulation toward soluble antigens from an offspring. This regulation, toward IPA,27 was generally not as strong as NIMA-specific regulation, with only 4 of 48 (8%) mothers in such tests regulating at the 50% or greater level. The incidence of anti-IPA regulation by mothers was significantly (*P < 0.01) lower than the incidence of anti-NIMA regulation by their offspring (Figure 1).
Among monkey siblings, we also found evidence of natural regulation. For example, in 7 of 10 monkeys tested, the PBMC showed regulation toward lysates prepared from cells of MHC-identical full siblings, with 5 of 10 individuals showing a 50% or greater level of inhibition toward the non-MHC antigen differences. In contrast, when tested with subcellular antigens prepared from cells of a 1 haplo-MHC mismatched full sibling, only 7 of 57 (13%) monkeys showed regulation, and only 2 [4%] had 50% or greater inhibition of recall response. The mean % inhibition of recall response was significantly (**P < 0.001) different between the 2 sibling groups.
Patterns of Immune Regulation in Human Families
The results in Rhesus are consistent with those previously reported in a much smaller patient sample—that is, regulation in kidney transplant patients, and their living donors was strongest in sons and daughters, toward NIMA, and in HLA-identical siblings, toward familial non-MHC antigens.4 That study was performed with an ethnically homogeneous group of human subjects (n = 29; 95% white). To address this possible source of bias, we have begun to include African American and Hispanic subjects (n = 13 and 6, respectively) in our ongoing studies. As shown in Figure 2, the pattern of immune regulation in human families (n = 113 tests of PBMC from 79 individuals) remained remarkably similar to that seen in the Rhesus macaques. None of the responders tested (0/6) were found to regulate their recall responses in the presence of self-antigens prepared from autologous cell sonicates. The highest incidence of regulation (≥25% inhibition of DTH) was found when offspring PBMC were tested with maternal antigen (n = 24/36 or 66% of those tested); with strong (≥50% inhibition) regulation detected in 17 of 36 or 47% of the offspring tested.
FIGURE 2.
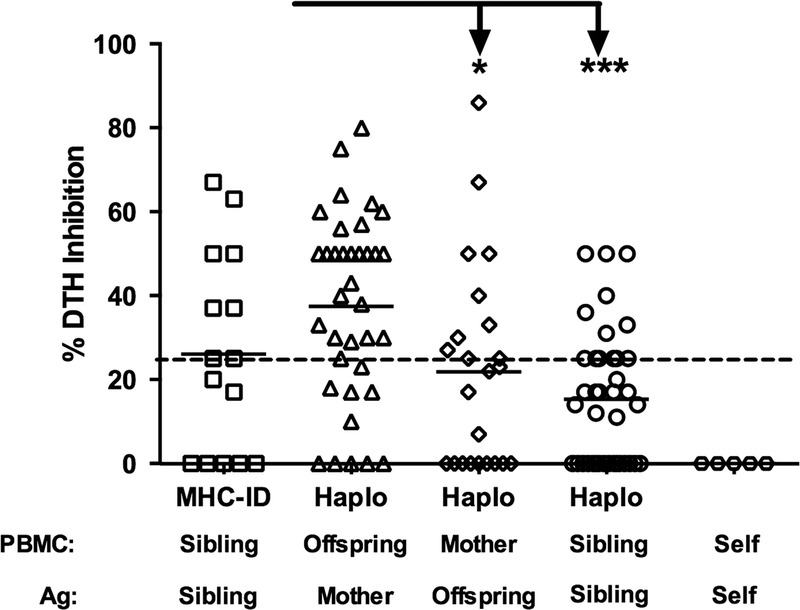
Regulation pattern in humans, by relationship. PBMC (n = 78) from individual human subjects within a family were tested in the tvDTH assay for regulation using antigen (cell lysate) from all available family members (n = 113 total tests). Dashed line represents 25% DTH inhibition. The % inhibition of the recall response in the presence of the familial antigen is plotted with responses grouped based on the familial relationship. Offspring responding to maternal antigen show the highest level of regulation and this was significantly higher than the mother's response to offspring antigen (*P < 0.01) and one haplo mismatched sibling responses to each other (***P < 0.001).
As expected, the majority of the mothers did not regulate to the IPAs found in soluble Ag preparations of offspring cells. The mean % inhibition score of maternal anti-IPA responses was also significantly less than that of the anti-NIMA response (Figure 2: 22 ± 24 % vs 37 ± 22%; P = 0.01). However, 10 of 23 mothers had regulation values to a specific offspring's antigen of 25% or greater, and 4 scored at 50% or greater inhibition, suggesting that at least some of her offspring's IPAs can stimulate the mother's PBMC to regulate.
When PBMC were tested with soluble Ag from their HLA identically matched sibling (minor antigen mismatch only), the result was not uniformly regulatory, as had been reported previously.4 Regulation was seen in 8 of 15 or 53% of cases of responses to antigen prepared from an HLA-identical sibling. Of these, 4 of 8 had regulatory responses to non-MHC antigens sufficient to cause strong (≥50%) regulation. The mean % regulation among HLA-identical siblings was not significantly different (P = 0.12; Figure 2) from that found in HLA-1-haplotype mismatched siblings. The difference approached but did not reach significance (P = 0.08) when African American, and Hispanic subjects were excluded from the analysis (data not shown), indicating that regulation between HLA identically matched white subjects was less robust in this cohort as compared to the previous one.4
Analysis of Immune Responses Between Pairs of Related Subjects
Our previous study of renal transplant living donors and recipients implicated that mutual or bidirectional regulation between the donor and recipient was associated with significantly better graft survival than if only 1 member of the pair had strong regulation to the other.4 We therefore analyzed the Rhesus and human families in this study to determine if the same patterns of regulation were apparent in both these populations. Figures 3 and 4 give example patterns of regulation within human and monkey families. Figure 3 shows the raw data on which the calculation of % inhibition was based. In panel A, bidirectional regulation was seen in a case of 2 HLA-identical siblings. Both had strong (40-50 × 10−4 in) net swelling responses to recall antigen, and both reduced their recall response by 50% (40 → 20, and 50 → 25) when antigen from their sibling was coinjected with the TT/D, panel B) shows a mother-daughter pair in which unidirectional regulation was observed. Both showed strong recall tvDTH responses. The daughter regulated her recall response by 50% (60 → 30 ) in the presence of the maternal antigen, but the mother did not reciprocate: in the presence of the daughter's antigen, the TT/D response remained high, with only a slight(7%; 70 → 65) reduction in swelling. Panel C shows a nonregulating pair of HLA-1 haplotype MM sisters. In 1 direction, the net swelling response to TT/D actually increased slightly (17%; 50 → 60), whereas in the opposite direction, there was a decrease of the same amount (17%; 60 → 50) regulation. Note that the net swelling response to the alloantigen alone in all cases was 10 × 10−4 in or less.
FIGURE 3.
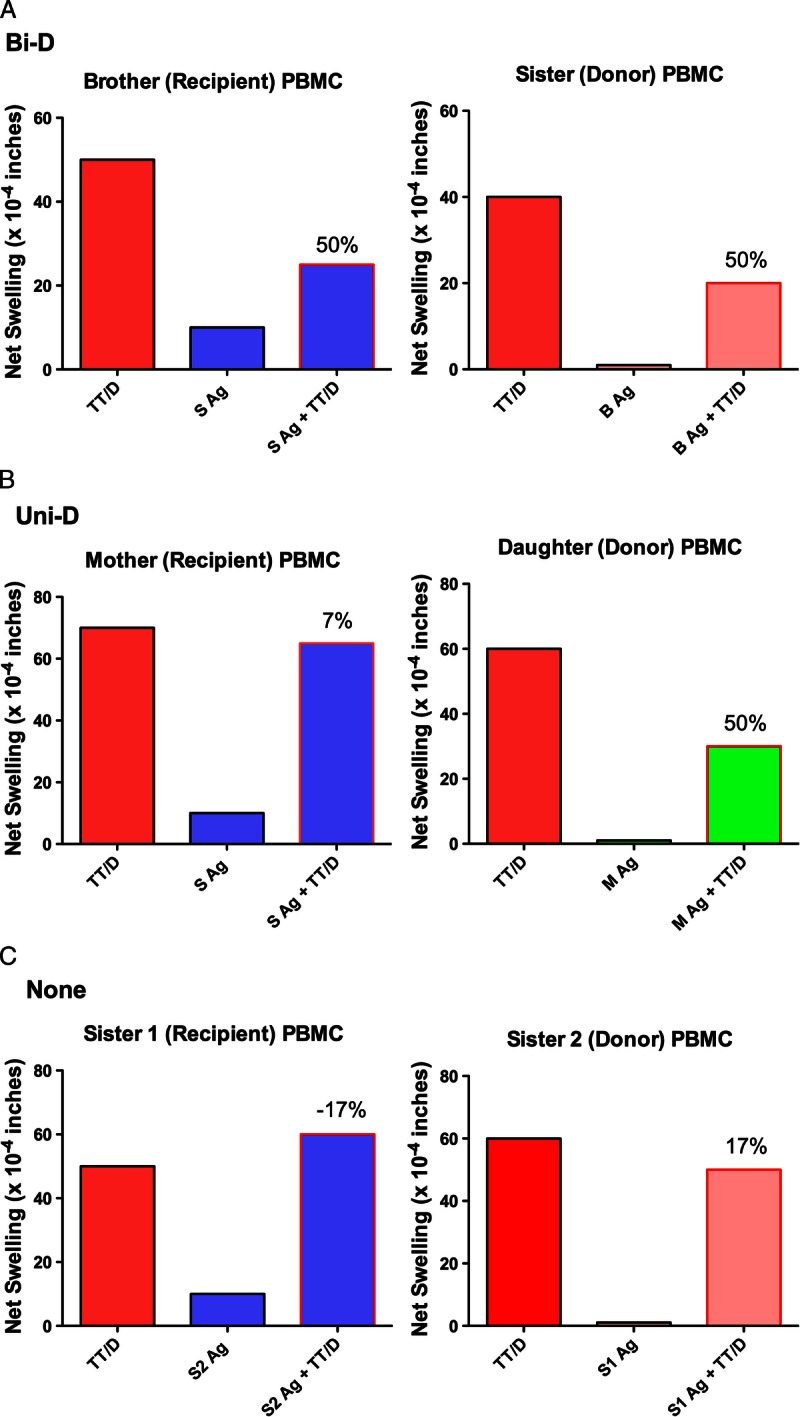
Examples of 3 patterns of immune regulation measured by trans vivo DTH. Representative tvDTH data of 3 patterns of immune regulation within a family. A, Bidirectional regulation, FTx02 HLA-identical siblings (both HLA-A2,32; B7,27; DR1,15). B, Unidirectional regulation, FTx 06 mother-offspring pair maternal HLA type: A1,2; B8,62; DR17,4; daughter HLA type: A1,2;B62,35; DR1,4. C, No regulation, FTx09 HLA-one-haplo mismatch siblings. Sister 1 HLA type: A1,2; B8,-; DR17,-. Sister 2 HLA type A1,3; B8,-; DR17.
FIGURE 4.
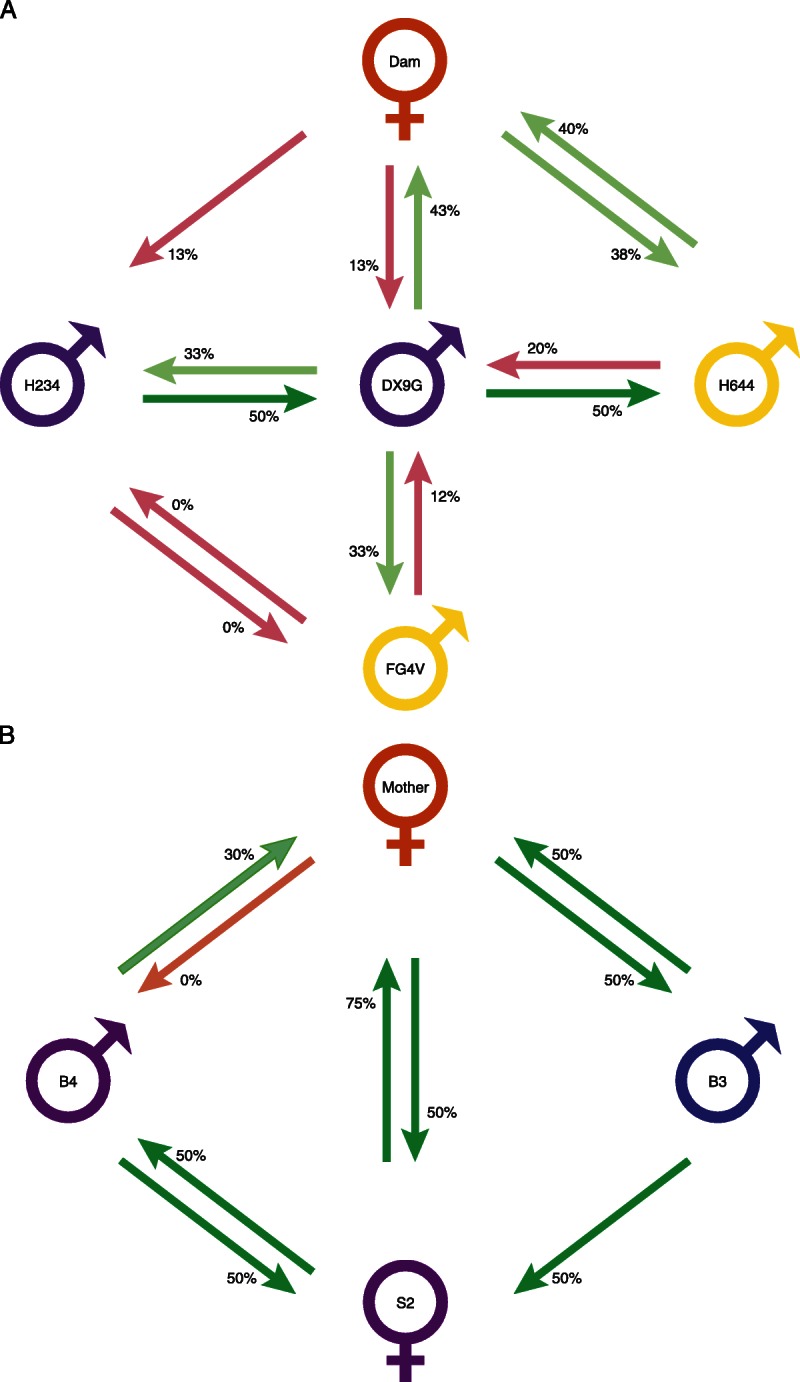
Diagrammatic representation of regulation relationship between individuals in two families. The tvDTH-linked suppression response of PBMC from each individual to the antigen lysate from the family member is represented by a green arrows (regulation ≥ 25%) or pink arrow (regulation < 25%) with base of arrow by the PBMC donor and arrowhead pointing to antigen source. Individuals with dual green arrows between them are bidirectional pairs, where as those with a single or two pink arrows are uniregulatory or nonregulatory pairs. A, Rhesus Macaque family NIAID 8:H234 and DX9G are MHC-ID siblings (Mamu A002a, A018a; B001c, B002); Dam MHC type is A018a, A004; B002, B048; FG4V MHC is A002a, A018a; B012a, B002 and H644 is A002, A018a, B002 and B012b. B, human family FTX02. HLA type of Mother (green) is A2, 11; B7, 51; DR1, 4; HLA-Type of both the second oldest daughter (S2) and that of youngest male sibling (B4) is the same : A2, 32; B7, 27; DR1, 15; thus the differences in the response of the mother to these 2 HLA-identical offspring must be due to minor H differences between the two. The HLA type of the male sibling (B3) is A1, 11; B8, 51; Dr4, 17; and is identical to S2- A2, 32; B7, 27; DR1, 15. The letters M, B, and S represent mother, brother, and sister; the numbers represent the offspring birth order.
Figure 4 represents diagrammatically the regulation patterns in a family of Rhesus macaque (panel A, NIAID family 8) and humans (panel B, FTx02). In both families, multiple offspring were screened with antigens from the mother (or Dam) as well as with full siblings. Both families also have at least 1 pair of MHC identically matched individuals (purple symbols); in the Rhesus family, the other siblings are 1 haplotype mismatched to the identical siblings (yellow symbols), whereas in the human family, the other sibling is a complete mismatch to the identical siblings (blue symbol). In the Rhesus family, there were no instances of sensitization at the level of cellular immunity: that is, no response to subcellular antigen alone exceeded 10 × 10−4 in, and most were zero (data not shown). As illustrated in Figure 4A, there were 2 cases of bidirectional regulation within this family: the dam-offspring (H644) pair, as well as the MHC-identical pair DX9G and H234. Other pairings resulted in either unidirectional (DX9G-FG4V and DX9G-H644, both 1-haplotype MHC mismatched pairs, Dam and offspring [DX9G] pair) or nonregulation (FG4V-H234). In 1 pair, DX9G-H644, the combined % regulation value was 70%, but the individual values for regulation (20%, 50%) did not reach the minimum standard of 25% or greater on each side to qualify as bidirectional under our standard definition. Finally, the maternal responses to MHC identical sibs DX9G and H234 were similar, with minimal (12.5%) regulation toward each.
In a human family, presented in panel B, there were several interesting findings: (1) the HLA identical pair B4-S2 clearly shows bidirectional regulation; (2) although all 3 siblings regulated to NIMA (arrows toward the mother), there was variability—that is, brother B3 and sister S2 regulated strongly (50 and 75%), whereas brother B4 was a relatively weak regulator (30%); (3) the responses of the mother to each offspring's antigens were almost a mirror image of their anti-NIMA responses, that is, she regulated strongly (50%) to B3 and S2, but not at all to B4. In fact, she also had a baseline response to B4's antigens, net swelling of 20 × 10−4 in (data not shown) which suggests some degree of sensitization to B4's IPAs. Because B4 and S2 were HLA-identical, the IPA to which the mother is sensitized in 1 case, and those to which her PBMC are highly regulatory in the other case, must both be non-HLA/minor H antigens. Of the 4 pairs analyzed in both directions in this family, the regulation pattern was: 3 bidirectional regulators (B4-S2, M-S2, and M-B3), and 1 case of unidirectional regulation (M-B4).
IL-35 Is Required for NIMA- and IPA-Specific Regulation in Both Rhesus and Human PBMC
We next investigated the mechanism of tvDTH regulation in monkey and human PBMC (Figure 5). We selected living-related pairs that showed a high degree of linked suppression (50-60%). These included HLA-identical sib-sib, son-to-mother (NIMA) and mother-to-son (IPA) pairs. The TT-specific recall responses of both monkey and human (n = 3 subjects each) were in the range of 40 × 10−4 in. Neutralizing antibodies to regulatory cytokines, IL-10, TGFβ, and IL-35 were included in the regulation tvDTH assay to determine which, if any were critical for the bystander regulation response. Antibodies to IL-10 caused a slight, but not significant increase in the average swelling response over the controls (TT/D + Ag, and TT/D + Ag + control IgG). Antibodies to TGFβ1 caused a partial, but significant reversal of linked suppression. Antibodies to either subunit of IL-35 (Ebi3, IL12p35—data not shown) or a combination of antibodies to both subunits (αIL-35) resulted in the complete reversal of linked suppression caused by familial antigens (NIMA, IPA, and minor H; P ≤ 0.0001). Importantly, antibodies directed to the IL12 p40 subunit yielded no change in linked suppression as compared to suppressed swelling value of antigen plus TT ± control Ab (data not shown).
FIGURE 5.
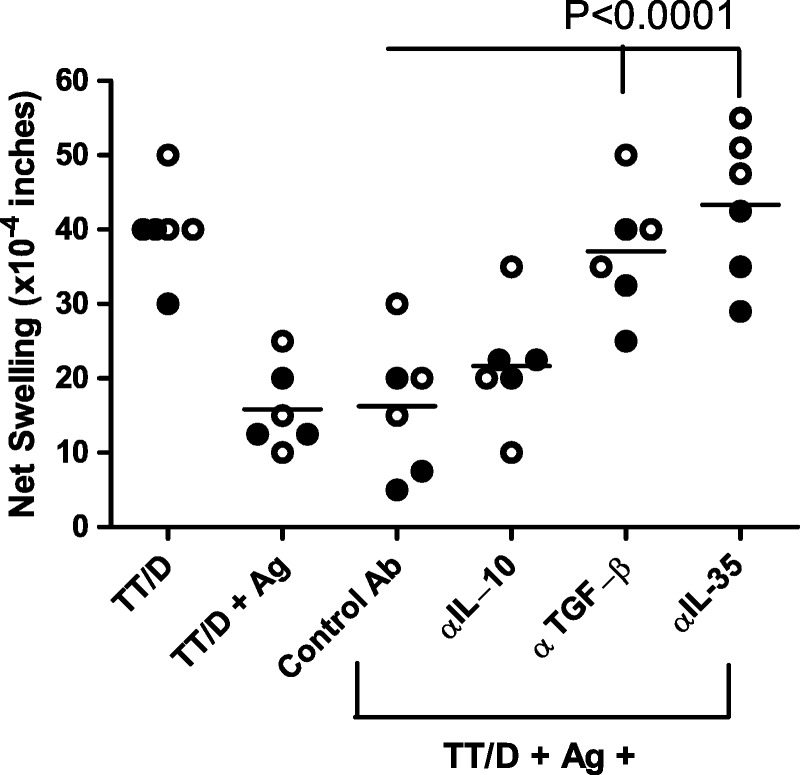
Neutralization of regulatory cytokine impact on linked suppression. PBMC from 3 Rhesus (filled symbols) and 3 humans (open symbols) were tested in the tvDTH-linked suppression assay with neutralizing antibodies to inhibitory cytokines to determine the role, if any of these cytokines on the linked suppression responses. PBMC were injected with TT/D (positive control) or TT/D with familial antigen (Ag) known to induce at least a 50% linked suppression response. Reversal of the linked suppression in the presence of control Ab (IgG matching species and subtype to neutralizing Ab) or antibodies to various inhibitory cytokines (anti-IL-10, anti-TGFβ, anti-IL-35, which is a combination of anti-p35 [IL12] and anti-Ebi3 Abs) was measured. Neither control IgG nor neutralization of IL-10 significantly reversed the linked suppression, whereas neutralization of either TGFβ or IL-35 significantly (P < 0.0001) reversed the linked suppression, resulting in a net swelling that was not different than that with TT/D + PBMC alone.
DISCUSSION
Our goal was to determine if the outbred Rhesus macaque, a major model NHP species in preclinical studies of organ transplant tolerance induction, has the same pattern of intrafamilial regulation as that seen in human transplant donor-recipient pairs.4 The results of tvDTH linked suppression assays, previously used to analyze posttransplant regulation in unrelated Rhesus monkeys,12 here show clearly that this is true. The pattern of intrafamilial linked suppression of tvDTH responses to recall antigen was very similar between monkey (Figure 1) and human (Figure 2) blood donors. Antigens of the mother (NIMA) were the most consistent source for induction of linked suppression of footpad swelling. This was not due to any particularly suppressive quality of female antigens, since when we combined all 67 pairs of monkey siblings (ID and HAPLO MM), female antigen caused a mean of 9.7% inhibition, male antigen 15% inhibition (P = 0.5) (Haynes, L., unpublished observations). Alternatively, it may be due to NIMA-specific Treg cells.5
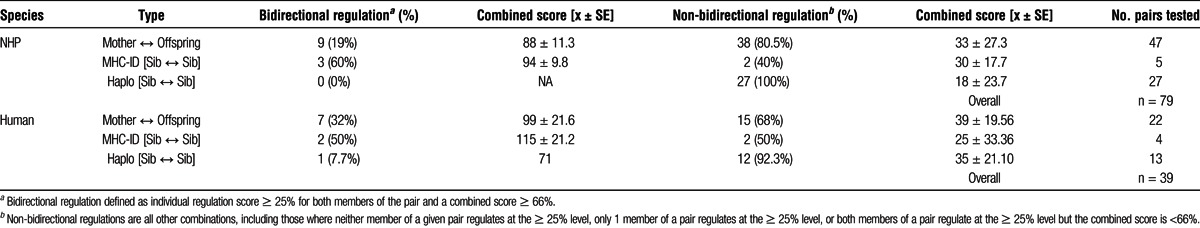
Antigens of MHC-identical siblings, identified by extended MHC haplotype analysis, were the next most consistent inducer of linked suppression in monkey PBMC (the latter result in monkeys is in agreement with the strong regulation found previously in HLA-identical siblings before live-donor kidney transplantation,4 and the more variable but often bidirectional regulation found in the present study group [Table 1]), followed by inherited antigens of the father (IPA) recognized by maternal cells when exposed to the antigens of son or daughter. The lowest incidence of immune regulation was found in response to antigen prepared from an MHC 1 haplotype MM sibling.
TABLE 1.
Summary of bidirectional, and non-bidirectional regulation status between family membersa
No attempt was made to identify the monkey or human Treg cells responsible for linked suppression in this study. Some of these are clearly minor H antigen-specific. Recent collaborative studies using the tvDTH assay to analyze regulation in normal adults indicated that among the minor H antigens giving rise to preexisting regulation in human subjects were the HA-1 and HA-8, HLA-A2 restricted peptides recognized by CD8 regulatory T cells.8 We have previously reported a remarkable case of 37-year-long kidney transplant tolerance in a female recipient of an HLA-identical, but HA-1H minor antigen- mismatched kidney transplant.26 This case was characterized by HA-1 microchimerism in the recipient's T cells and dendritic cells (DC), as well as low-avidity, TGFβ1-producing CD8 Treg cells in stable equilibrium with high-affinity CD8 CTL, both specific for donor HA-1H. Whether mutual regulation in this donor-recipient pair before the transplant in 1967 had predisposed the recipient to her long-term transplant tolerance is unclear; however, the results reported in the present study as well as previously4 certainly suggest this possibility.
CD4+ regulatory T cells are also likely to be involved in minor H-specific linked suppression within families. For example, nonparous as well as multiparous female subjects were found to regulate HY-derived minor H peptides that can only be recognized in the context of HLA class II, although the tendency to regulate to HY peptides was significantly lower in the mothers of sons,28 perhaps due to an increase in T effector memory cells with similar specificity.
Bidirectional Immune Responses in Organ Transplantation
In the early days of clinical transplantation, when the principal assay used to screen donor-recipient pairs for cell-mediated immunity to alloantigen was the mixed lymphocyte (MLC) test, it was unclear whether the unidirectional MLC, where the donor cells are x-irradiated or otherwise prevented from proliferating was the best predictor of transplant outcomes. In fact, the bidirectional MLC, in which both recipient and donor were cultured together and mutual proliferation was determined, appeared to correlate most reliably with outcome at 1 year after transplantation from cadaver donors.29 This hint of the bidirectional nature of solid organ transplantation was soon abandoned in favor of the idea that transplantation is a unidirectional affair, with passenger leukocytes within the transplant serving merely as a source for disseminating alloantigens in the host, causing immunization.30 Passenger T and B cells did not reappear as significant players in transplant outcome until the work of Starzl on liver transplant in the early 1990s.31,32 The exact role of microchimerism in transplant tolerance remains unresolved; however, the model of donor as well as recipient T cells playing an important part in transplant outcome has been strengthened by subsequent research33,34 (for a recent review see Burlingham and Benichou35).
Technical Issues in the tvDTH Assay: Indirect Versus Semidirect Alloantigen Presentation to T Cells
The tvDTH linked suppression assay is an extremely complex bioassay that detects 1 aspect of infectious tolerance mediated by alloantigen-specific regulatory T cells. It is of interest that the cell lysates used in all the tvDTH assays were prepared by sonication followed by 16,000×g centrifugation to remove large cell fragments, but not exosomes. Such preparations can therefore give rise to both indirect (allo-MHC antigen ingested, processed, and presented as peptides by self APC) and semidirect (e.g., exosomes expressing allo-MHC antigens which are presented as intact molecules by “cross-dressed” self APC) forms of alloreactive T-cell response.36 That both indirect and semidirect pathways are likely being engaged in the tvDTH assay is actually a strength; that is, this assay may accurately reproduce the conditions generated by microchimerism in vivo.
The coexistence of indirect and semidirect allorecognition pathways in the tvDTH assay may help to explain a puzzling finding in the current study. On average, the extent and types of minor H antigen differences between MHC-identical and MHC-1haploMM siblings ought to be similar—yet the manifestation of regulatory activity was consistently lower in the latter group (Figures 1 and 2, and Tables S1 and S2, SDC, http://links.lww.com/TXD/A4 and http://links.lww.com/TXD/A5). In cases of MHC identity, exosomes that may coat the surface of DCs in the responder PBMC will be expected to have either no effect on minor H-specific CD4 and CD8 Treg responses, or might even reinforce such responses because the target minor H peptides may even be represented among those transferred along with intact MHC appearing on the DC surface membrane. In contrast, exosome transfer to DC of an MHC 1haplotype MM sibling,causing allogeneic class I and class II MHC antigen to appear on the APC surface, might activate direct pathway T cells in the recipient, causing release of cytokines and lipid mediators that may trump indirect, minor H-specific immune regulation. The fact that such “cross-dressing” is not an impediment to detection of regulation response in tvDTH when the mother provides the MHC 1 haplotype MM test antigens, may simply reflect the sheer quantity of “NIMA” minor H present that can stimulate regulatory T-cell responses. Experiments are currently underway in our laboratory to test the impact of exosome-free versus exosome- containing antigen preparations on tvDTH-linked suppression to minor H or MHC class I alloantigen.
Possible Mechanisms of Regulation Within Families
The preliminary analysis of the potential mechanism of regulation would suggest that, in both humans and Rhesus macaques, the CD4+ and/or CD8+ Treg cells that mediate linked suppression responses to familial major and minor H alloantigens do so via mainly IL-35, and TGFβ1, with IL-10 playing a minor role. This is a new finding. In previous studies of tolerant patients, only TGFβ1 and IL-10 have been noted to play a role in immune regulation.25,37 The requirement of DC integrins and DC-derived thrombospondin to liberate active from latent TGFβ expressed on the surface of the Treg38 would seem to limit the range of that cytokine to the T effector cells in the immediate vicinity of the DC. No such strict DC dependency would appear to limit the range of IL-35. In addition to the IL-6 receptor gp130 expressed on T cells,39 IL-35 has recently been shown to act through a unique receptor on B cells, heterodimers of IL-27Rα and IL-12Rβ1, to induce regulatory B cells. This may account for the well-established NIMA effect limiting alloantibody production.40 Besides CD4 Treg cells specific for familial antigens as one likely source, antigen-specific CD8+ Treg cells producing IL-35 have been reported to occur in prostate cancer patients21 and may be involved, particularly in minor H antigen-specific regulation, where CD8 Treg cells have been found to play an important role.8
CONCLUSIONS
The implications of our study for modeling live-related donor transplantation are that donor selection in the Rhesus monkey, as in humans, can be done to maximize regulation present naturally within families for the benefit of transplantation tolerance. We are currently developing assays to detecting the production of IL-35 in response to specific alloantigen stimulation. The current results suggest that such an approach, when applied to prospective donor-recipient pairs may hold promise as a way of screening for bidirectional regulation in laboratories where the option of using immunodeficient mice for tvDTH analysis is not available.
Supplementary Material
Footnotes
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
Supported by: U01 grant AI102456 (to DBK) and by R01 grant AI066219-08 (to WJB).
The authors declare no funding or conflicts of interest.
REFERENCES
- 1. Salisbury EM, Game DS, Lechler RI. Transplantation tolerance. Pediatr Nephrol. 2014; 29: 2263– 2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burlingham WJ, Grailer AP, Heisey DM, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998; 339: 1657– 1664. [DOI] [PubMed] [Google Scholar]
- 3. Dutta P, Dart M, Roenneburg DA, et al. Pretransplant immune-regulation predicts allograft tolerance. Am J Transplant. 2011; 11: 1296– 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jankowska-Gan E, Sheka A, Sollinger HW, et al. Pretransplant immune regulation predicts allograft outcome: bidirectional regulation correlates with excellent renal transplant function in living-related donor-recipient pairs. Transplantation. 2012; 93: 283– 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008; 322: 1562– 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang TT, Chaturvedi V, Ertelt JM, et al. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol. 2014; 192: 4949– 4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dierselhuis MP, Blokland EC, Pool J, et al. Transmaternal cell flow leads to antigen-experienced cord blood. Blood. 2012; 120: 505– 510. [DOI] [PubMed] [Google Scholar]
- 8. van Halteren AG, Jankowska-Gan E, Joosten A, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009; 114: 2263– 2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doxiadis GG, Otting N, de Groot NG, et al. Unprecedented polymorphism of Mhc-DRB region configurations in rhesus macaques. J Immunol. 2000; 164: 3193– 3199. [DOI] [PubMed] [Google Scholar]
- 10. Miller WP, Srinivasan S, Panoskaltsis-Mortari A, et al. GVHD after haploidentical transplantation: a novel, MHC-defined rhesus macaque model identifies CD28- CD8+ T cells as a reservoir of breakthrough T-cell proliferation during costimulation blockade and sirolimus-based immunosuppression. Blood. 2010; 116: 5403– 5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page A, Srinivasan S, Singh K, et al. CD40 blockade combines with CTLA4Ig and sirolimus to produce mixed chimerism in an MHC-defined rhesus macaque transplant model. Am J Transplant. 2012; 12: 115– 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torrealba JR, Katayama M, Fechner JH, Jr, et al. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1 + CD4+ T regulatory cell infiltrates. J Immunol. 2004; 172: 5753– 5764. [DOI] [PubMed] [Google Scholar]
- 13. Scandling JD, Busque S, Shizuru JA, et al. Induced immune tolerance for kidney transplantation. N Engl J Med. 2011; 365: 1359– 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008; 358: 353– 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leventhal J, Abecassis M, Miller J, et al. Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation. 2013; 95: 169– 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nadazdin O, Boskovic S, Murakami T, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011; 3: 86ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamada Y, Boskovic S, Aoyama A, et al. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012; 12: 330– 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molitor-Dart ML, Andrassy J, Kwun J, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007; 179: 6749– 6761. [DOI] [PubMed] [Google Scholar]
- 19. Collison LW, Workman CJ, Kuo TT, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007; 450: 566– 569. [DOI] [PubMed] [Google Scholar]
- 20. Collison LW, Chaturvedi V, Henderson AL, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010; 11: 1093– 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olson BM, Jankowska-Gan E, Becker JT, et al. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J Immunol. 2012; 189: 5590– 5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olson BM, Sullivan JA, Burlingham WJ. Interleukin 35: a key mediator of suppression and the propagation of infectious tolerance. Front Immunol. 2013; 4: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jankowska-Gan E, Hegde S, Burlingham WJ. Trans-vivo delayed type hypersensitivity assay for antigen specific regulation. J Vis Exp. 2013; 75: e4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carrodeguas L, Orosz CG, Waldman WJ, et al. Trans vivo analysis of human delayed-type hypersensitivity reactivity. Hum Immunol. 1999; 60: 640– 651. [DOI] [PubMed] [Google Scholar]
- 25. VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000; 106: 145– 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai J, Lee J, Jankowska-Gan E, et al. Minor H antigen HA-1-specific regulator and effector CD8+ T Cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med. 2004; 199: 1017– 1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci U S A. 2012; 109: 2509– 2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dierselhuis MP, Jankowska-Gan E, Blokland E, et al. HY immune tolerance is common in women without male offspring. PLoS One. 2014; 9: e91274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Opelz G, Terasaki PI. Significance of mixed leukocyte culture testing in cadaver kidney transplantation. Transplantation. 1977; 23: 375– 380. [DOI] [PubMed] [Google Scholar]
- 30. Steinmuller D. Immunization with skin isografts taken from tolerant mice. Science. 1967; 158: 127– 129. [DOI] [PubMed] [Google Scholar]
- 31. Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992; 340: 876– 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993; 17: 1127– 1152. [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson CC, Matzinger P. Immunity or tolerance: opposite outcomes of microchimerism from skin grafts. Nat Med. 2001; 7: 80– 87. [DOI] [PubMed] [Google Scholar]
- 34. Win TS, Rehakova S, Negus MC, et al. Donor CD4 T cells contribute to cardiac allograft vasculopathy by providing help for autoantibody production. Circ Heart Fail. 2009; 2: 361– 369. [DOI] [PubMed] [Google Scholar]
- 35. Burlingham WJ, Benichou G. Bidirectional alloreactivity: a proposed microchimerism-based solution to the NIMA paradox. Chimerism. 2012; 3: 29– 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Breman E, van Miert PP, van der Steen DM, et al. HLA monomers as a tool to monitor indirect allorecognition. Transplantation. 2014; 97: 1119– 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haynes LD, Jankowska-Gan E, Sheka A, et al. Donor-specific indirect pathway analysis reveals a B-cell-independent signature which reflects outcomes in kidney transplant recipients. Am J Transplant. 2012; 12: 640– 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Derks RA, Jankowska-Gan E, Xu Q, et al. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J Immunol. 2007; 179: 3443– 3451. [DOI] [PubMed] [Google Scholar]
- 39. Collison LW, Delgoffe GM, Guy CS, et al. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012; 13: 290– 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Claas FH, Gijbels Y, van Der Velden-de Munck J, et al. Induction of B cell unresponsiveness to noninherited maternal HLA antigens during fetal life. Science. 1988; 241: 1815– 1817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


