Abstract
Background
A few patients, after receiving solid organ transplantation, return to performing various sports and competitions; however, at present, data no study had evaluated the effects of endurance cycling races on their renal function.
Methods
Race times and short form (36) health survey questionnaires of 10 kidney transplant recipients (KTR) and 8 liver transplant recipients (LTR) transplanted recipients involved in a road cycling race (130 km) were compared with 35 healthy control subjects (HCS), also taking laboratory blood and urine tests the day before the race, at the end of the race, and 18 to 24 hours after competing.
Results
The 3 groups showed similar race times (KTR, 5 hours 59 minutes ± 0 hours 39 minutes; LTR, 6 hours 20 minutes ± 1 hour 11 minutes; HCS, 5 hours 40 minutes ± 1 hour 28 minutes), similar short form (36) health survey scores, and similar trend of laboratory parameters which returned to baseline after 18 to 24 hours. After the race, there was an increase in creatinine (0.24 mg/dL; effect size [ES] = 0.78; P < 0.001), urea (22 mg/dL; ES = 1.42; P < 0.001), and a decrease of estimated glomerular filtration rate (−17 mL/min; ES = 0.85; P < 0.001). The increase of blood uric acid was more remarkable in HCS and KTR (2.3 mg/dL; ES = 1.39; P < 0.001). The KTR showed an increase of microalbuminuria (167.4 mg/L; ES = 1.20; P < 0.001) and proteinuria (175 mg/mL; ES = 0.97; P < 0.001) similar to LTR (microalbuminuria: 176.0 mg/L; ES = 1.26; P < 0.001; proteinuria: 213 mg/mL; ES = 1.18; P < 0.001), with high individual variability. The HCS had a nonsignificant increase of microalbuminuria (4.4 mg/L; ES = 0.03; P = 0.338) and proteinuria (59 mg/mL; ES = 0.33; P = 0.084).
Conclusions
Selected and well-trained KTR and LTR patients can participate to an endurance cycling race showing final race times and temporary modifications of kidney function similar to those of HCS group, despite some differences related to baseline clinical conditions and pharmacological therapies. Patients involved in this study represent the upper limit of performance currently available for transplant recipients and cannot be considered representative of the entire transplanted population.
Patients with solid transplanted organs are characterized by high risk of cardiovascular disease1,2 and metabolic syndrome associated to chronic inflammation at subclinic status and sarcopenia.3,4 These medical conditions are mainly caused by the side effects of the immunosuppressive therapy, on a sedentary lifestyle,5,6 and by the chronic disease that led to transplantation.
Several studies have shown the positive effects of physical exercise in preventing primary and secondary cardiovascular diseases.7,8 However, the world of transplantations is still far from a regular and practical application of physical activity and sport as an opportunity to improve the health conditions of transplanted patients, and to counteract the side effects of the immunosuppressive therapy with the exercise. It is a fact that there is a lack of awareness related to this topic and a widespread hesitation of the patients and their families. Moreover, there is no consensus among transplant professionals about the need for, or the recommended extent of exercise after transplantation.9
On the other hand, it is well known that there are some patients returning to sports activities at amateur or professional level after a successful transplantation. The analysis of these experiences10,11 allows to give original information concerning the usefulness of physical activity and sport in transplanted patients, opening the question if a resumed or a newly started sporting activity could be dangerous for the safety of the graft.
The aim of this paper is to contribute to answering this question, studying the kidney function in a group of transplanted patients involved in a long-distance road cycling race.
MATERIALS AND METHODS
Subjects
In recent years, a small group of transplanted patients out of the about 12 000 participants has taken part in the Nove Colli road cycling race. Among these, we selected male subjects, aged between 18 and 80 years, kidney or liver transplanted at least 1 year before, who usually practice cycling and voluntarily participate in the race. Recruitment was possible through the help of Associazione Nazionale Emodializzati, Dialisi e Trapianto Sport, an association involved in promoting the participation of transplanted patients in sports events.
Eighteen transplanted patients divided in 10 kidney transplant recipients (KTR) (mean ± SD age, 50 ± 6 years; weight, 73 ± 6 kg; height, 1.74 ± 0.04 m; BMI, 24.2 ± 2.6 kg/m2; time from transplant, 9.5 ± 6.5 years; length of dialysis treatment before transplantation 22 ± 18 months, range 3-59), and 8 liver transplant recipients (LTR) (mean ± SD age, 57 ± 13 years; weight, 72 ± 3 kg; height, 1.76 ± 0.01 m; BMI, 23.1 ± 1.1 kg/m2; time from transplant, 9.0 ± 4.4 years) were recruited.
Pathologies leading to the transplant in the group of KTR were: glomerulonephritis (n = 3), nephroangiosclerosis (n = 2), polycystic kidney disease (n = 2), end-stage kidney disease (n = 2); and in the LTR group: cirrhosis HCV-related (n = 4), HBV-related (n = 2), primary sclerosing cholangitis (n = 1), liver damage from medications (n = 1). Regarding the daily immunosuppressive therapy, in KTR group, 6 patients were assuming tacrolimus (trough level, 5.6 ± 1.6 μ/g per liter), 4 cyclosporine (trough level, 130 ± 35 ng/mL); in 5 patients, calcineurin inhibitors were associated with steroid therapy (methylprednisolone 4 ± 2 mg), in 6 patients with inhibitors of DNA synthesis (mycophenolic acid, 720 ± 290 mg). In 4 LTR patients, the immunosuppressive therapy consisted in Everolimus (trough level, 3.8 ± 0.8 ng/mL) and in the other 4 patients in tacrolimus (trough level, 4.6 ± 1.1 μ/g per liter) associated in 2 case with steroids (methylprednisolone, 4 mg) and in 2 cases to inhibitors of DNA synthesis (mycophenolic acid, 720 mg).
All patients had good blood pressure control (BP <140/80 mmHg). Seven patients were treated with angiotensin converting enzyme (ACE) inhibitors (5 KTR, 2 LTR), 7 used β-blockers (6 KTRs, 1 LTR). No patient was treated with hypoglicemic therapy or erythropoietin.
Thirty-five matching men (age, 50 ± 10 years; weight, 74 ± 9 kg; height, 1.75 ± 0.06 m; BMI, 24.1 ± 2.3 kg/m2) who usually practice cycling and voluntarily participate in the short route of the same race were recruited as healthy control subjects (HCS), with the help of the organization of the race, through a newsletter and e-mail sent to all the participants. They reported no significant clinical problem, with BP values within the normal range (BP < 140/80 mm Hg). One subject was taking regular treatment with ACE inhibitors and another one occasionally took steroids.
The Race
“Nove Colli” is a long-distance cycling road race that takes place in Romagna (Forlì-Cesena and Rimini, Italy), with starting grid and finish line placed at sea level in Cesenatico. It is one of the most prestigious events of this kind, one of the oldest (held since 1971), and probably one of the most known not only in Italy for this type of competitions. The race is open only to leisure and amateur cyclists who must submit a valid medical certificate of fitness for road cycling competitions. The maximum number of race entries is 12 000. Participants were divided into 7 departure grids at 3-minute intervals from each other. Before starting, participants can choose between a short route (130 km) and a long route (200 km). The transplanted patients chose the short route, as 63% of the participants that were considered in the present study as HCS. The characteristics of the route were: length, 130 km; total uphill gradient, 1 871 m; uphill riding, 50 km over 4 hills; downhill riding, 46 km; flat terrain, 34 km; maximum riding time allowed, 7.5 hours.
Protocol
The study was divided into different control phases:
-
(i)
a baseline overall medical assessment of all the subjects, with collection of anamnestic data, information on drug therapy, measurement of resting heart rate and BP, assessment of the subjective perception of the health-related quality of life with the short form (36) health survey (SF-36) questionnaire, as well as with an ad hoc questionnaire investigating the level of training carried out throughout the year and in preparation for the race.
-
(ii)
Collection of venous blood and urine samples at time 1 (T1) (the day before the race), time 2 (T2) (immediately after crossing the finish line), and time 3 (T3) (18-24 hours after competing).
-
(iii)
In addition, at T2 the amount of fluid ingestion (mL) during the race was recorded by direct question and marked in a register.
-
(iv)
Athletes observation at T2 (post) and 3 (18-24 hours) included the assessment of possible adverse events during or after the race. The investigators used a specific case report form to record the results of the clinical assessment.
Laboratory Methods
The testing laboratory of S. Orsola Hospital (Bologna) carried out the blood chemistry and urinalysis assessments.
We collected 30 mL of venous blood (whole blood) from the antecubital vein and 30 mL of urine from all participants in the study for each time. Blood sampling was done in a sitting position and fasting at T1 and T3. Blood and urine samples were stored at room temperature and subsequently transported to the laboratory analysis with suitable transport.
The blood samples were centrifuged for 5 minutes in a bench-top centrifuge 3 500 rpm (rcf: 2465 xg) at 20 to 22 °C immediately after their arrival in the laboratory.
The following laboratory tests (ADVIA 2120; Hematology System, Siemens, Erlangen, Germany) were carried out on the samples: complete blood count with flow cytometry and light-scattering, hemoglobin (g/dL) with optical colorimetric method, leukocytes (103/mm3) with peroxidase and basophils methods (cytochemical reactions), and lymphocytes (%) with cytochemical reactions.
Renal function parameters: creatinine (mg/dL) with Jaffè method, estimated glomerular filtration rate (eGFR) with Modification of Diet in Renal Diseases formula (isotope dilution mass spectrometry method, mass spectrometry isotope dilution calibrated), urea (mg/dL) with urease and glutamate dehydrogenase, uric acid (mg/dL) with enzymatic colorimetric test.
Urinalysis: urine specific gravity (densimetry), urine sediment (optical microscope), microalbuminuria (mg/L) (nephelometry), and proteinuria (mg/1000 mL) (turbidimetry).
The reports of the analysis were sent privately to each participant.
Organizational Aspects
The study team included a physician and a nurse for collection of medical history and blood tests, and a researcher involved in the distribution and collection of questionnaires.
The SF-36 questionnaire and an ad hoc questionnaire investigating the amount of training performed during the year, and general information as well, have been administered before taking the first blood sample on the day before the race.
The study was conducted in the respect of well-acknowledged ethical standards and national/international legislation. This trial was carried out in compliance with International Conference on Harmonisation Guidelines for Good Clinical Practice, with the Declaration of Helsinki and with national rules regarding clinical trial management.
The participants were informed about the nature of the research, assurances of anonymity, and their right to withdraw at any time. Written informed consent was obtained by the participants before inclusion, according to the procedures approved by the Ethical Committee of the Italian Institute of Health.
STATISTICAL ANALYSIS
Unless otherwise indicated, the data are presented as the mean ± standard deviation. After verifying the assumptions of normality and homoscedasticity, 1-way analysis of variance for independent samples were used to compare the mean values for the SF-36 questionnaire, quantity of training questionnaire, and the fluid ingestion during the competition between KTR, LTR, and HCS. Significance was set at P less than 0.05. When appropriate, post hoc Bonferroni tests have been used for pairwise comparisons. Linear mixed models were used to test the effect of group and sampling time on blood and urinary parameters. Fixed effects were included for group, time, and their interaction, and a random intercept and effect of time were included for individual cyclists. Models of best fit were selected using a stepwise selection method. The HCS group and the pre-race time point were set as reference values for group and time, respectively. When significantly different from 0 (P < 0.05), raw coefficients were reported for fixed effects (and their interaction) with 95% confidence intervals, whereas standardized coefficients were reported as the effect size (ES). Statistical analyses were carried out using the software R, version 3.0.3.
RESULTS
The race started at 6.30 am of May 18, 2014. Weather conditions were good, with a temperature of 12.6°C at the start and of 18.9°C at 12 noon.
All the participants completed the route without adverse events. There was no significant difference (P > 0.41) in the average time race between the 3 groups (5 hours 59 minutes ± 0 hour 39 minutes in KTR, 6 hours 20 minutes ± 1 hour 11 minutes in LTR, 5 hours 40 minutes ± 1 hours 28 minutes in HCS).
No significant differences were found in the quantity of training expressed as amount of training workouts per week (3.1 ± 0.6 KTR, 3.6 ± 1.8 LTR, 2.8 ± 1.1 HCS; P = 0.21) and hours of training for each session (2.6 ± 0.8 KTR, 2.8 ± 0.7 LTR, 2.8 ± 0.8 HCS; P = 0.77).
With respect to hydration status, no significant differences (P = 0.10) were found in the amount of fluid ingestion during the race (KTR, 2000 ± 500 mL; LTR, 1833 ± 764 mL; HCS, 2533 ± 1067 mL).
Complete Blood Count
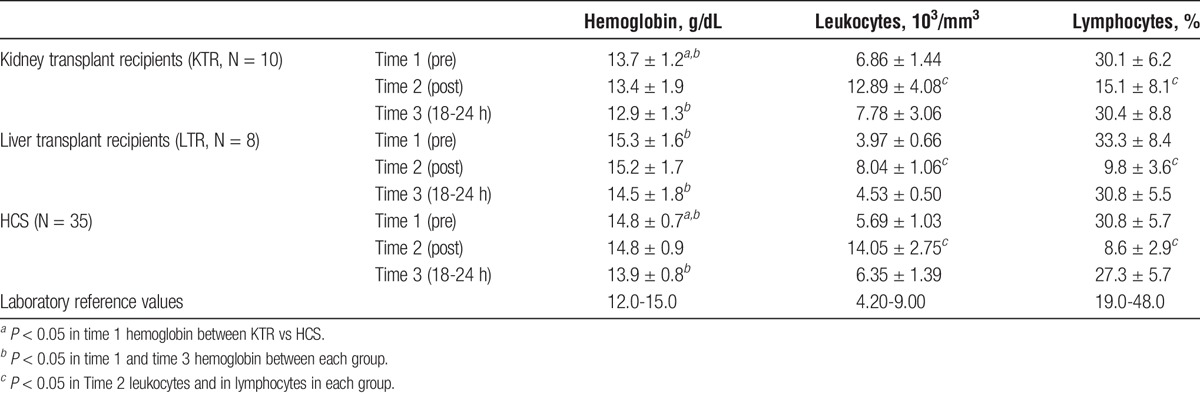
The value of hemoglobin before the race was significantly lower in KTR versus HCS, with a difference of 1 mg/dL (ES = 0.73; P = 0.009); the difference between LTR and HCS was not significant. The 3 groups showed the same trend of the hemoglobin values after performing physical effort. The level was unchanged at T2, whereas there was a statistically significant decrease at T3, the level being 0.9 mg/dL (ES = 0.67; P < 0.001) lower versus baseline. However, the data remained within the normal range, and the observed change had no clinical significance. Leukocytes showed a high variability. When compared with HCS, baseline values were higher in KTR versus HCS (difference of 1.17 × 103/mm3; ES, 0.29; P = 0.004) and lower in LTR (difference of 1.72 × 103/mm3; ES, 0.42; P < 0.001). The trend after the race was similar for the 3 groups. We observed a significant increase of leukocytes at T2 in HCS (difference of 8.36 × 103/mm3; ES, 2.06; P < 0.001); the changes were less remarkable in TR, and in particular in LTR (LTR −4.29 × 103/mm3 vs HCS; ES, 1.05; P < 0.001; KTR −2.32 103/mm3 vs HCS; ES, 0.57; P = 0.0023). At T3, there is a general and uniform trend to return to baseline values, even though the leukocyte count was on average slightly higher (0.66 × 103/mm3; ES, 0.16; P = 0.005).
As for the percentage of lymphocytes, there were no significant differences at T1 between HCS, KTR, and LTR. The general trend at T2 and T3 was similar in all the groups. At T2, there was a marked decrease both in HCS and in LTR (−22.2%; ES, 2.00; P < 0.001); this change was less evident in KTR (ES, 0.65; P < 0.001). At T3, there was a trend back to the baseline values in all the groups. In general, the percentage of lymphocytes remained slightly lower in HCS and LTR (difference of 3.5%; ES, 0.31; P < 0.001). Descriptive data are shown in Table 1.
TABLE 1.
Complete blood count (Mean ± SD) at 3 different times of analysis: before the race (Time 1), after (Time 2), and the day after the race (Time 3)
Renal Function
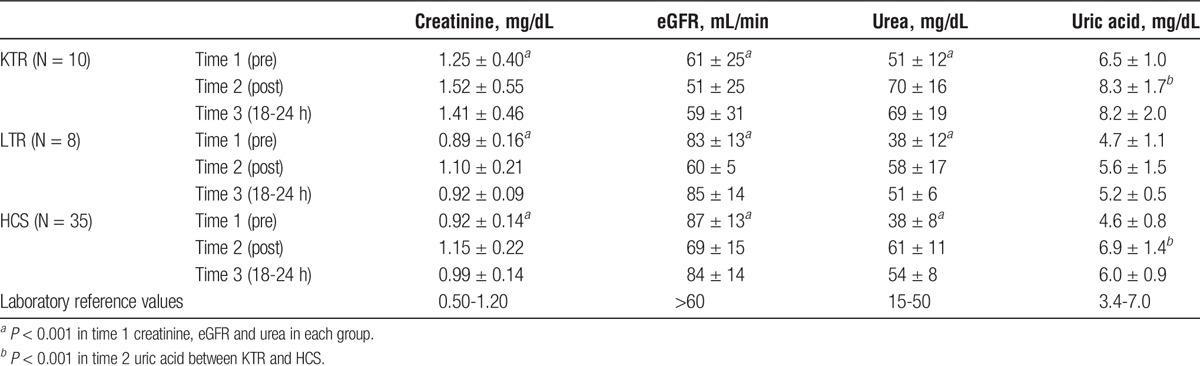
Both the HCS and the LTR showed renal function tests in the normal range at T1. The KTR group had values of creatinine and urea slightly higher than normal, and significantly higher versus HCS and LTR (P < 0.001 for both variables). The changes at T2 were similar in the 3 groups, with a significant increase of creatinine (0.24 mg/dL; ES, 0.78; P < 0.001) and urea (22 mg/dL; ES, 1.42; P < 0.001) and a significant decrease of eGFR (−17 mL/min; ES, 0.85; P < 0.001).
The increase of blood uric acid was more remarkable in HCS and KTR (2.3 mg/dL; ES, 1.39; P < 0.001). At T3, all groups showed a consistent trend to return to baseline values, but the urea and uric acid values remained higher than the average. Descriptive data are shown in Table 2.
TABLE 2.
Renal function (Mean ± SD) at 3 different times of analysis: before the race (Time 1), after (Time 2), and the day after the race (Time 3)
Urinalysis
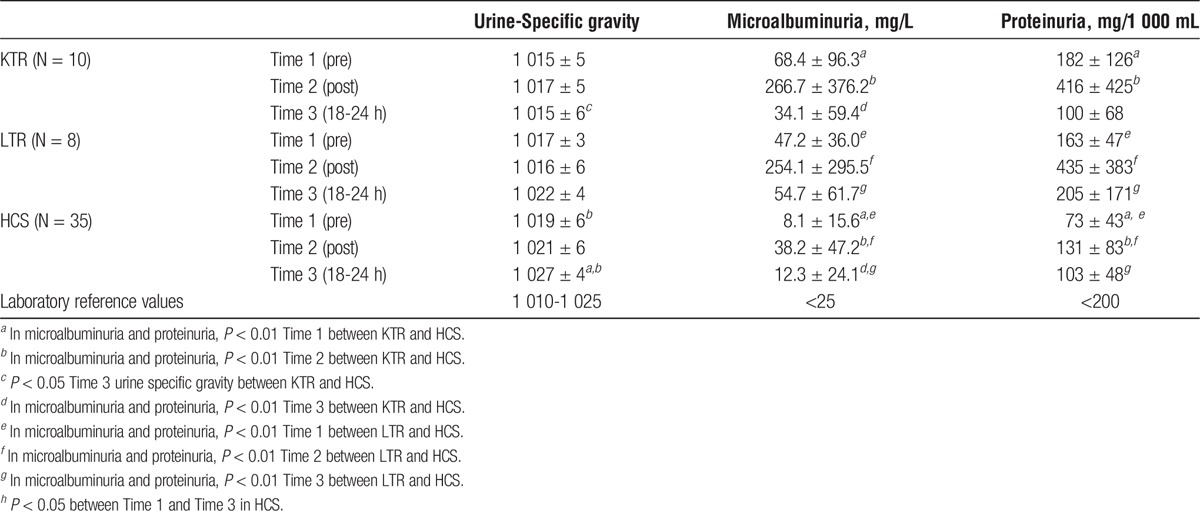
The baseline values of urine specific gravity were lower in KTR (−5; ES = 0.68) and in LTR (−2; ES = 0.28), vs HCS. At T2, the values were practically stable in all the groups.
There was a significantly different trend in the following period: at T3, we observed a significant increase in HCS (9; ES, 1.30; P < 0.001); this increase was present also in LTR, even though less remarkable and at the border of significance (−4; ES, 0.57; P = 0.063). In KTR, the values did not show changes versus baseline.
We did not observe cases of significant changes of urine sediment after the race. At T2 and T3, there was a microhematuria (20-40 red blood cells per field) in 10% of KTR, 12% of LTR, and 14% of HCS.
In KTR and LTR, the baseline values of microalbuminuria and proteinuria were slightly higher than normal. The trend of the 2 parameters was similar in the 3 groups at the different observation times. In particular, at T2, we observed in KTR, a significant increase of microalbuminuria (167.4 mg/L; ES, 1.20; P < 0.001) and proteinuria (175 mg/mL; ES, 0.97; P < 0.001). Similar increase were observed in LTR (microalbuminuria: 176.0 mg/L; ES = 1.26; P < 0.001; proteinuria: 213 mg/mL; ES = 1.18; P < 0.001), with a high individual variability.
Also, the HCS had an increase of both parameters (microalbuminuria: 4.4 mg/L; ES = 0.03; P = 0.338; proteinuria: 59 mg/mL; ES = 0.33; P = 0.084), without statistically significant differences versus baseline. All groups showed a trend to return to baseline at T3.
In KTR, microalbuminuria and proteinuria at T3 were lower than at baseline. Descriptive data are shown in Table 3.
TABLE 3.
Urinalysis (Mean ± SD) at 3 different times of analysis: before the race (Time 1), after (Time 2), and the day after the race (Time 3)
Health-Related Quality Of Life
Table 4 shows the descriptive statistics of SF-36 scales, in transplanted recipients (KTR and LTR) compared to HCS.
TABLE 4.
Descriptive statistics of SF-36 scales
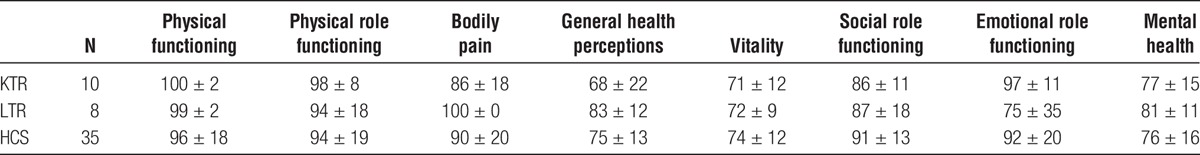
No significant differences were found in any of the SF-36 questionnaire scales between the 3 groups.
DISCUSSION
The data of the present study show that it is possible for a selected group of kidney and liver transplanted patients to participate to a long-distance cycling race with chronometric results superimposable to those of healthy amateur cyclists. These transplanted recipients are characterized by the capacity to express the same amount of training workouts per week and hours of training for each session compared to healthy amateur cyclists, as a prerequisite necessary to take advantage of their performance capacities. Obviously, these patients had no contraindications to participate in a road cycling races; they were checked every 6 months by their transplant center for many years after receiving transplantation, and the immunosuppressive therapies were well tolerated. These patients are also characterized by a subjective perception of personal physical, psychological, and emotional needs comparable to the population of healthy subjects, allowing them to achieve a level of quality of life similar to the general population of active individuals.12,13 In these transplant recipients, this 130-km cycling race induces physiological modifications of kidney function, urinalysis, and complete blood count, similar to that observed in healthy amateur cyclists with a return to baseline values in the recovery period after the end of the competition. Furthermore, all the investigated parameters had a similar trend both in transplanted and healthy subjects, despite some differences related to the baseline clinical conditions.
The KTR showed lower hemoglobin levels, on average, at all times. We can argue that in these patients, the mild decrease of eGFR (61 ± 25 mL/min) may contribute to the development of a state of relative anaemia. A slight decrease of hemoglobin was recorded in all the groups (KTR, LTR, and HCS) after the race, in particular at T3. Among the possible causes, we could recognize an increase of blood volume induced by the endurance physical activity.14 Both groups of transplant recipients showed, like HCS, a significant increase of leukocytes and a decrease of the percentage of lymphocytes immediately after the race. The lower increase of the leukocyte number in transplant recipients versus HCS was observed also in another study,15 and may be a consequence of the immunosuppressive treatment. The significant changes induced by physical stress on the immune system and the inflammation status are a very interesting research field and suggest the opportunity to carry out additional tests (like cytokines).16,17
Considering the parameters of renal function, there were similar changes in the 3 groups after about 6 hours of race cycling, with significant increase of creatinine, urea and uric acid, and decrease of eGFR (Table 2). In all 3 groups, the functional parameters, in particular creatinine and eGFR, showed a trend to baseline values after 18 to 24 hours; the persistence of higher values of urea and uric acid may be the result of a temporary status of hypercatabolism.18 The higher values of creatinine, urea and uric acid, and the lower values of eGFR in KTR observed at T1, T2, and T3 are compatible with the condition of “single kidney.” This may also be the effect of the treatment with calcineurin inhibitors (vasoconstrictor effect on afferent arterioles), which were the basis of immunosuppressive therapy in our KTR.19 This may also be the result of the treatment with ACE inhibitors, used in 5 of 10 KTRs.
The absence of changes in specific gravity after the race and during recovery indicates that KTRs have a compromised capacity of urine concentration. This is compatible with a chronic tubular damage by chronic allograft nephropathy and may be partly induced by therapy with calcineurin inhibitors.19 The capacity to concentrate urine was only slightly reduced in LTR. This finding probably depends on the smaller tubular damage due to the different management of the immunosuppressive therapy as suggested by the fact that only 50% of our LTR used calcineurin inhibitors, also with a lower dosage compared with KTR.
It is well known that the intensity of physical exercise may cause reversible albuminuria and proteinuria20 which are considered physiologic findings in the healthy sporting population, even if the underlying physiopathologic mechanism is not yet clearly defined. Different hypotheses include variations of glomerular hemodynamics and a reduced protein absorption at tubular level.21 This study shows that in KTR and in LTR groups, the levels of microalbuminuria and proteinuria were higher at all times versus HCS. In all the 3 groups of subjects considered in the present study, the microalbuminuria and proteinuria resolved after resting. Moreover, the values of these parameters were similar in the 2 groups of transplant recipients. This is partly unexpected, because LTRs have native kidneys that usually do not have organic chronic kidney diseases before transplantation. In our LTR, a long period after the transplant (9 years on average) seems to be compatible with glomerular and tubulointerstitial modifications, despite a good renal function.22 It is also possible to hypothesize a role of immunosuppressive therapy with mechanistic target of rapamycin inhibitors (4 LTR treated of 8)23 even though the large variability of data does not allow definitive conclusions and needs further investigations.
The main limit of this study is the rather small number of patients and the fact that these patients represent the upper limit of performance currently available for transplant recipients. In any way, they cannot be considered representative of the entire transplanted population. However, transplanted patients involved in cycling races are very few, and we recruited all those involved in the race for this study. Moreover, they are far from being homogeneous in terms of age, time from transplantation, pathology leading to transplantation, and immunosuppressive therapy, which represent other limits.
Regarding the kidney function, because of logistic problems, it was not possible to determine the urinary excretion of creatinine during the competition. However, the observed variations of blood parameters were in accordance with the literature after endurance physical effort.24,25
Another limit is the short duration of the follow-up after the race (only 18-24 hours). The possible appearance of adverse effects after several years of cycling (might manifest after 2 to 10 years) should be investigated with further studies.
In conclusion, data collected on transplant recipients who practice sports activities can help us to understand their potential performance capacities, and overall they raise the question whether physical exercise, and eventually sport, can be included among the therapies after receiving solid organ transplantation.
This study showed that selected and well-trained kidney and liver transplant recipients can participate to an endurance cycling race showing performances comparable to those of nontransplanted amateur cyclists. After the race, these transplant recipients showed transient modifications of the kidney function similar to those of healthy amateur cyclists despite some differences related to their baseline clinical conditions and pharmacological therapies.
At present, the results of this study indicate that the transplanted patients can be involved in proper training and endurance competitions, showing no adverse effects after performing sports activities, but they need a careful clinical monitoring and training. In this way, in the long term, it is possible that the positive effects of physical activity will overcome the potential risks of temporary modifications of some parameters of organ function after exercise. Further studies with longer survey periods, and possibly with greater number of patients, are necessary to better understand the impact of endurance training and sports activities on kidney function of transplanted patients.
ACKNOWLEDGMENT
The authors gratefully acknowledge Daniela Storani, Emanuela Grasso, the communication offices of the Italian National Transplant Centre and Novecolli, and the whole team of the “Fausto Coppi” Association which organized the road cycling race for their cooperation in the project.
Footnotes
The authors declare no funding or conflicts of interest.
M.G. has participated in research design, in the writing of the article, and in the performance of the research. R.G.S. has participated in research design, in the writing of the article, and in the performance of the research. T.V. has participated in the writing of the article, in the performance of the research, and in data analysis. Z.M. has participated in the performance of the research. T.A. has participated in the performance of the research. T.P. has participated in the performance of the research. R.E. has participated in the performance of the research. D.M.R. has participated in the writing of the article and in data analysis. T.M. has participated in the performance of the research. D.C. has participated in the writing of the article and in the performance of the research. N.C.A. has participated in research design and in the performance of the research.
REFERENCES
- 1. Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006; 82: 603– 611. [DOI] [PubMed] [Google Scholar]
- 2. Pilmore H, Dent H, Chang S, et al. Reduction in cardiovascular death after kidney transplantation. Transplantation. 2010; 89: 851– 857. [DOI] [PubMed] [Google Scholar]
- 3. Sharif A. Metabolic syndrome and solid-organ transplantation. Am J Transplant. 2010; 10: 12– 17. [DOI] [PubMed] [Google Scholar]
- 4. Fahal IH. Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant. 2014; 29: 1655– 1665. [DOI] [PubMed] [Google Scholar]
- 5. Painter P, Marcus RL. Assessing physical function and physical activity in patients with CKD. Clin J Am Soc Nephrol. 2013; 8: 861– 872. [DOI] [PubMed] [Google Scholar]
- 6. Mosconi G, Roi GS, Nanni Costa A, et al. Attività fisica nei pazienti con trapianto di rene. G Ital Nefrol. 2011; 28: 174– 187. [PubMed] [Google Scholar]
- 7. Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. 2002; 346: 793– 801. [DOI] [PubMed] [Google Scholar]
- 8. Kohl HW, 3rd, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: global action for public health. Lancet. 2012; 380: 294– 305. [DOI] [PubMed] [Google Scholar]
- 9. Gordon EJ, Prohaska T, Siminoff LA, et al. Needed: tailored exercise regimens for kidney transplant recipients. Am J Kidney Dis. 2005; 45: 769– 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roi GS, Parigino M, Pisoni D, et al. Energy expenditure during a day of sport competitions in kidney transplant recipients. Transplantation. 2010; 90: 1136– 1138. [DOI] [PubMed] [Google Scholar]
- 11. Slapak M. Sport and transplantation. Ann Transpl. 2005; 10: 62– 68. [PubMed] [Google Scholar]
- 12. Mazzoni D, Cicognani E, Mosconi G, et al. Sport activity and health-related quality of life after kidney transplantation. Transplant Proc. 2014; 46: 2231– 2234. [DOI] [PubMed] [Google Scholar]
- 13. Cicognani E, Mazzoni D, Totti V, et al. Health-related quality of life after solid organ transplantation: the role of sport activity. Psychol Health Med. 2014; 22: 1– 8. [DOI] [PubMed] [Google Scholar]
- 14. YH Chiu, Lai JI, Wang SH, et al. Early changes of the anemia phenomenon in male 100-km ultramarathoners. J Chin Med Assoc. 2015; 78: 108– 113. [DOI] [PubMed] [Google Scholar]
- 15. Königsrainer I, Zieker D, Löffler M, et al. Influence of exhaustive exercise on the immune system in solid organ transplant recipients. Exerc Immunol Rev. 2010; 16: 184– 193. [PubMed] [Google Scholar]
- 16. Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013; 4: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gleeson M, Bishop NC, Stensel DJ, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011; 11: 607– 615. [DOI] [PubMed] [Google Scholar]
- 18. Oosthuyse T, Avidon I. Changes in substrate utilisation and protein catabolism during multiday cycling in well-trained cyclists. J Sports Sci. 2015; 33: 507– 517. [DOI] [PubMed] [Google Scholar]
- 19. Pallet N, Djamali A, Legendre C. Challenges in diagnosing acute calcineurin-inhibitor induced nephrotoxicity: from toxicogenomics to emerging biomarkers. Pharmacol Res. 2011; 64: 25– 30. [DOI] [PubMed] [Google Scholar]
- 20. Bellinghieri G, Savica V, Santoro D. Renal alterations during exercise. J Ren Nutr. 2008; 18: 158– 164. [DOI] [PubMed] [Google Scholar]
- 21. Puggina EF, Machado DR, Tourinho Filho H, et al. Half-ironman induces changes in the kidney function of triathletes. An Acad Bras Cienc. 2014; 86: 429– 436. [DOI] [PubMed] [Google Scholar]
- 22. Kim JY, Akalin E, Dikman S, et al. The variable pathology of kidney disease after liver transplantation. Transplantation. 2010; 89: 215– 221. [DOI] [PubMed] [Google Scholar]
- 23. Letavernier E, Legendre C. mTOR inhibitors-induced proteinuria: mechanisms, significance, and management. Transplant Rev (Orlando). 2008; 22: 125– 130. [DOI] [PubMed] [Google Scholar]
- 24. Hewing B, Schattke S, Spethmann S, et al. Cardiac and renal function in a large cohort of amateur marathon runners. Cardiovasc Ultrasound. 2015; 13: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sanchis-Gomar F, Lippi G. Physical activity—an important preanalytical variable. Biochem Med (Zagreb). 2014; 24: 68– 79. [DOI] [PMC free article] [PubMed] [Google Scholar]


