Abstract
Background
Desensitization with IVIG and rituximab allows acceptable graft survival in sensitized kidney transplant recipients with preexisting donor-specific antibodies (DSAs) and a positive crossmatch. There is little published data reporting the durability of DSA removal in kidney transplant recipients treated with IVIG and rituximab.
Methods
We conducted a 3-year prospective DSA monitoring study in living donor kidney recipients with preexisting DSA to assess the durability of DSA removal after a perioperative protocol of IVIG and rituximab. All recipients had flow crossmatch titers less than 1:32. Data were analyzed using linear mixed effects models and Kaplan-Meier survival methods.
Results
The longitudinal database comprised 210 mean fluorescence intensity (MFI) determinations. Forty-two DSAs were identified in 29 patients. Pretreatment MFI averaged 4715 ± 3962 (range, 947-20 129). At 1 month posttransplant, 18 patients (62%) had a complete response (MFI < 1000) and an additional 9 patients (31%) had a partial response (MFI reduced but >1000). There was a 46% reduction (P < 0.001) in DSA MFI at 1 month posttransplant that was sustained throughout the 3-year follow-up period and was observed for both class I and II DSAs regardless of pretreatment MFI levels. With a mean posttransplant follow-up of 1048 ± 574 days, 3-year patient and graft survivals were 95% and 90%. Four patients (14%) had acute rejection between days 125 and 560.
Conclusions
Desensitization with IVIG and rituximab results in early and sustained DSA removal over a 3-year posttransplant period in living donor kidney transplant recipients with pretransplant DSA and a positive crossmatch, excellent patient and graft survivals and a low incidence of acute rejection.
Previous studies have shown a correlation between preexisting donor specific antibodies (DSAs) at the time of kidney transplantation and hyperacute rejection, antibody-mediated rejection (AMR), and high rates of graft loss.1-4 Historically, a positive crossmatch was considered a contraindication to kidney transplantation. Fourteen percent of current waitlisted patients are sensitized limiting their access to crossmatch negative deceased or living donors.5 Previous groups have developed desensitization protocols resulting in acceptable kidney graft survival in patients with pre-transplant DSA and a positive crossmatch6-9 and these have been shown to offer a survival advantage and to be cost effective as compared to remaining on dialysis.9-11
Previous work from our group described prolonged rejection-free graft survival in sensitized living donor kidney transplant recipients with preexisting DSA and a positive crossmatch when rituximab was added to an induction regimen of high-dose IVIG and thymoglobulin or alemtuzumab.12 While our preliminary data indicated a 50% reduction in DSA mean fluorescence intensity (MFI) 1 month posttransplant,13 the long-term durability of DSA removal remained to be determined.
We currently report the results of a single-center, prospective study of DSA monitoring for three years posttransplant in living donor kidney transplant recipients with preexisting DSA and a positive crossmatch treated with IVIG/alemtuzumab/rituximab. Our aims were to determine the magnitude, temporal trajectory, and durability of antibody removal with this desensitization protocol.
MATERIALS AND METHODS
Study Population
This prospective study included 29 consecutive ABO compatible sensitized patients who underwent living donor kidney transplantation at our institution from November 2009 to September 2014. Selection criteria for desensitization at our center includes no other living donor with a negative crossmatch, no paired donor exchange, and a T- and/or B-cell flow crossmatch titer less than 1:32. All recipients had at least 1 preexisting DSA with an MFI of 947 or more and a negative CDC T-cell and B-cell final crossmatch performed after the initial dose of IVIG. Informed consent was obtained. The study protocol was approved by the Vanderbilt University Institutional Review Board (IRB 121415).
Immunosuppression Protocol
We used a modified version of the high dose IVIG/rituximab desensitization protocol previously described by Jordan and Vo8,10 Patients received 2 doses of IVIG (Gamunex-C, Talecris) 2 g/kg, the first dose was given 5 days before transplantation and a second dose was given 7 days after transplantation. A single dose of rituximab 375 mg/m2 intravenously (IV) was given one day after transplantation. All patients received induction with methylprednisolone 500 mg IV and alemtuzumab 30 mg IV begun intra-operatively. Posttransplant immunosuppression consisted of a two day methylprednisolone taper (day 1, 250 mg; day 2, 125 mg) followed by prednisone 20 mg beginning day 3 tapered to 5 mg daily by three months, tacrolimus (target trough level 7-10 ng/ml), and mycophenolate mofetil (initial dose 1000 mg twice daily).
Antibody Detection Assays
Determination of Donor-Specific Antibody (DSA)
Solid phase microarray bead technology (Luminex) using single antigen beads (One Lambda-Thermo Fisher) was used to identify DSA pre-transplant, both before treatment with the first dose of IVIG, and posttransplant at one, six, nine, and 12 months, and every 6 months thereafter, up to 36 months posttransplantation. All sera were pre-treated with dithiothreitol or EDTA before testing in order to reduce interference from IgM and/or complement. For HLA-A, −B, and –DR antibodies, MFI greater than 2000 was considered positive with 1000–2000 considered borderline positive. The cutoff for Cw antibody was 5000 MFI and the cutoff for DQ was 2500. Antibody specificity was confirmed with multiple sera and at least two different antibody platforms (e.g. Luminex and Flow).
Flow and Cytotoxic Crossmatch
A three-color flow crossmatch was used in this study. The median channel shift (MCS) from the negative control was calculated (Becton Dickinson FacsCalibur on 1024 scale). The cutoff for a positive reaction is 40 MCS for T cells, 60 MCS for Class I antibody on B cells, and 100 MCS for Class II antibody on B cells. An auto-crossmatch was performed with each crossmatch. Interpretation was based on a significant shift in the allo-crossmatch when compared with the auto-crossmatch. Pronase was not used to treat donor cells before transplant. A standard CDC crossmatch was also performed on all patients. The cytotoxic crossmatch was considered compatible if negative with dithiothreitol-treated serum. Only the CDC crossmatch was required to be negative after IVIG desensitization before proceeding to transplant.
C1q Assay
In order to determine whether DSA was complement fixing, C1q single antigen bead was measured both pre-transplant before treatment with IVIG and posttransplant in those patients that developed a de novo DSA (dnDSA) during the 3-year monitoring period. The C1q assay utilizes the same bead panel that is used with the Luminex single antigen assay but with an anti-C1q conjugate instead of an anti-IgG conjugate. A positive C1q assay confirms the ability of the antibody to fix complement. Our lab cut-off for a positive C1q assay is >500.
Outcome Measures
The primary outcome measures were the change and temporal trajectory of DSA MFI post-treatment over time. A complete response to desensitization was defined as a DSA MFI of less than 1000 on the 1 month posttransplant serum sample (less than 5000 for Cw and less than 2500 for DQ). A partial response was defined as a decrease in DSA from baseline but greater than 1000 (5000 for Cw and 2500 for DQ). Secondary outcome measures were patient and graft survival and the incidence of acute rejection.
Diagnosis and Treatment of Rejection
Allograft biopsies were performed for cause if elevated serum creatinine or allograft rejection was suspected clinically. Protocol biopsies are not performed as part of standard of care at our center and were not performed in this study. Acute rejection was defined by the Cooperative Clinical Trials in Transplantation criteria14 with humoral rejection having C4d immunostaining and/or DSA. Acute cellular rejection (ACR) was treated with a five day course of methylprednisolone 500 mg IV daily followed by thymoglobulin 1 mg/kg for 7 to 14 days if no improvement with steroids alone. Antibody mediated rejection was treated with plasmapheresis for five consecutive days followed by a single dose each of IVIG two grams/kg and rituximab 375 mg/m2.
Statistical Analysis
Data were analyzed at the individual antibody and patient levels. In order to address circumstances where kidney transplant recipients had more than one unique pre-treatment DSA, patient-level analyses of change and temporal trajectories were conducted for each patient’s: 1) average/only pre-treatment DSA MFI value and 2) their highest/only pre-treatment DSA MFI value. Paired t-tests were used to test individual DSA-level MFI changes between pre-treatment and posttransplant MFI values. Linear mixed effect models were used to evaluate patient-level temporal trajectories of the highest and average DSA MFI and whether the trajectory of the highest/only pre-treatment DSA differed on the basis of whether it was class I or class II. This approach utilized all available data for each patient resulting in 210 individual MFI determinations and a sample size of 133 highest or average MFI values for each mixed effects model. Actuarial patient and graft survival were determined using Kaplan-Meier methods. Unless noted, summary data are reported as mean ± standard deviation. Data were analyzed using IBM SPSS statistical software (version 22, Armonk, NY).
RESULTS
Baseline Characteristics
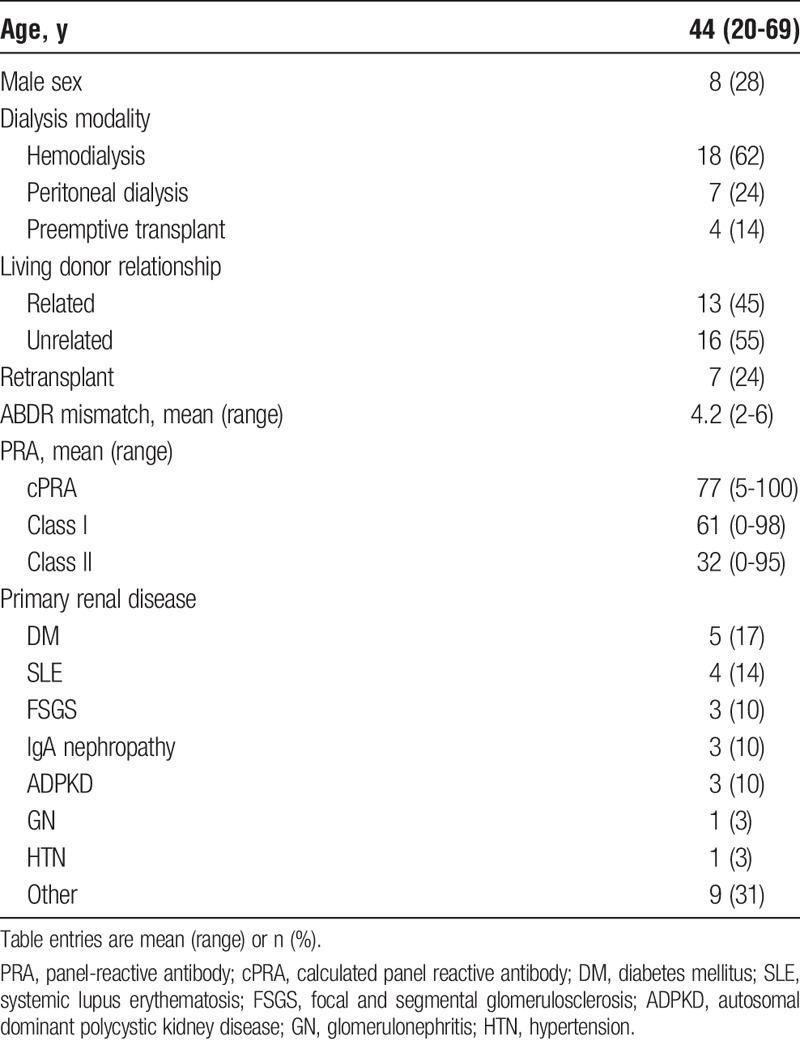
Recipient demographic and clinical characteristics are summarized in Table 1. The mean patient age was 44 (range 20-69) years, 21 (72%) were women, 7 (24%) had prior failed transplants, the mean HLA mismatch was 4.2 (range 2-6), and the mean panel-reactive antibody was 61% class I and 32% class II.
TABLE 1.
Patient characteristics (n = 29)
Recipient immunologic characteristics are summarized in Table 2. Before the first dose of IVIG, there were 42 preexisting DSAs with MFI greater or equal to 947 identified in 29 patients (range 1-5 DSA/patient). Ten patients (34%) had more than one DSA. Twenty-one patients had DSA to class I alone, 6 to class II alone, and 2 to both class I and class II. Using the highest MFI for those with more than one DSA, the mean MFI pre-treatment was 5584 ± 4351 (range 947-20,129).
TABLE 2.
Recipient immunologic characteristics
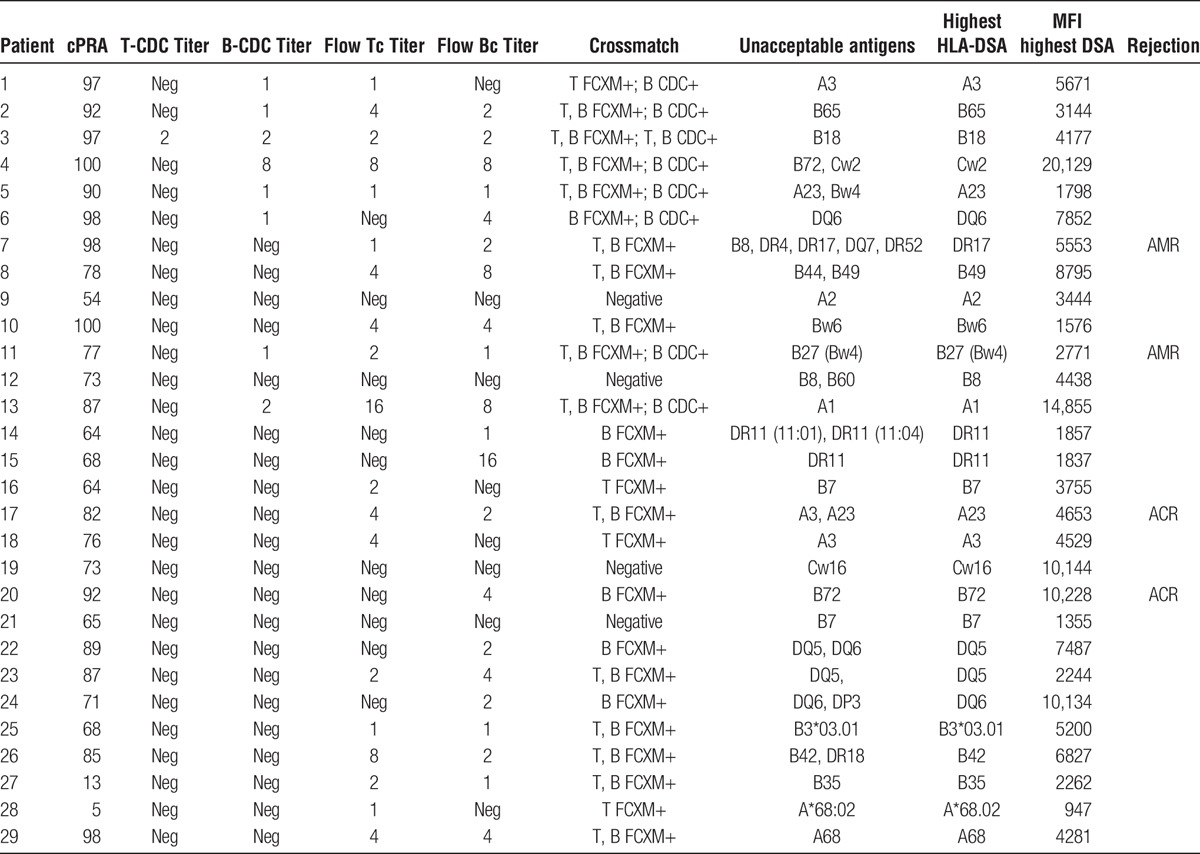
Pre-treatment crossmatch and immunologic data are also summarized in Table 2. Twenty-five patients (86%) had a positive crossmatch, flow or CDC or both, before treatment. While our protocol required a negative CDC crossmatch after the first dose of IVIG to ultimately proceed with transplantation, one patient had both positive CDC T and B cell crossmatches, seven patients had a positive CDC B cell crossmatch, and 17 patients had positive flow T and/or B crossmatches but negative cytotoxic crossmatches before receiving the first dose of IVIG. Four additional patients had preexisting DSA but negative flow and cytotoxic crossmatches pre-IVIG but were included in this desensitization protocol due to high panel-reactive antibody, high DSA-MFI and/or multiple DSAs.
DSA Monitoring
Forty-two preexisting DSAs were identified in 29 kidney transplant patients, 28 to class I and 14 to class II. Using only the highest MFI for those with more than one DSA, the mean MFI pre-treatment was 5584 ± 4351 (range 947-20,129). When DSAs were averaged for patients having more than one DSA, the average/only pre-treatment MFI was 4798 ± 3489 (range 947-14,855). Average percentage reductions in individual DSA MFI values were 46 ± 102% at month one, 31 ± 82% at month three, 64 ± 49% at month six, 80 ± 32% at month 12, and 63 ± 51% at month 36. Paired tests demonstrated that these reductions were statistically significant at each time point (all P ≤ 0.004). After adjusting for multiple comparisons, there was no difference in the percentage reduction in MFI between class I and class II DSAs at any time point. Individual patient-level trajectories of the average and highest pre-treatment DSA MFI values are shown in Figure 1. We found a consistent pattern of early and durable reduction in MFI over time after desensitization with IVIG and rituximab, whether analyzing individual patients’ average/only MFI (Figure 1A) or highest/only MFI (Figure 1B). MFI values over time for the six patients with borderline baseline values (MFI 1000–2000) are shown in Figure 2.
FIGURE 1.
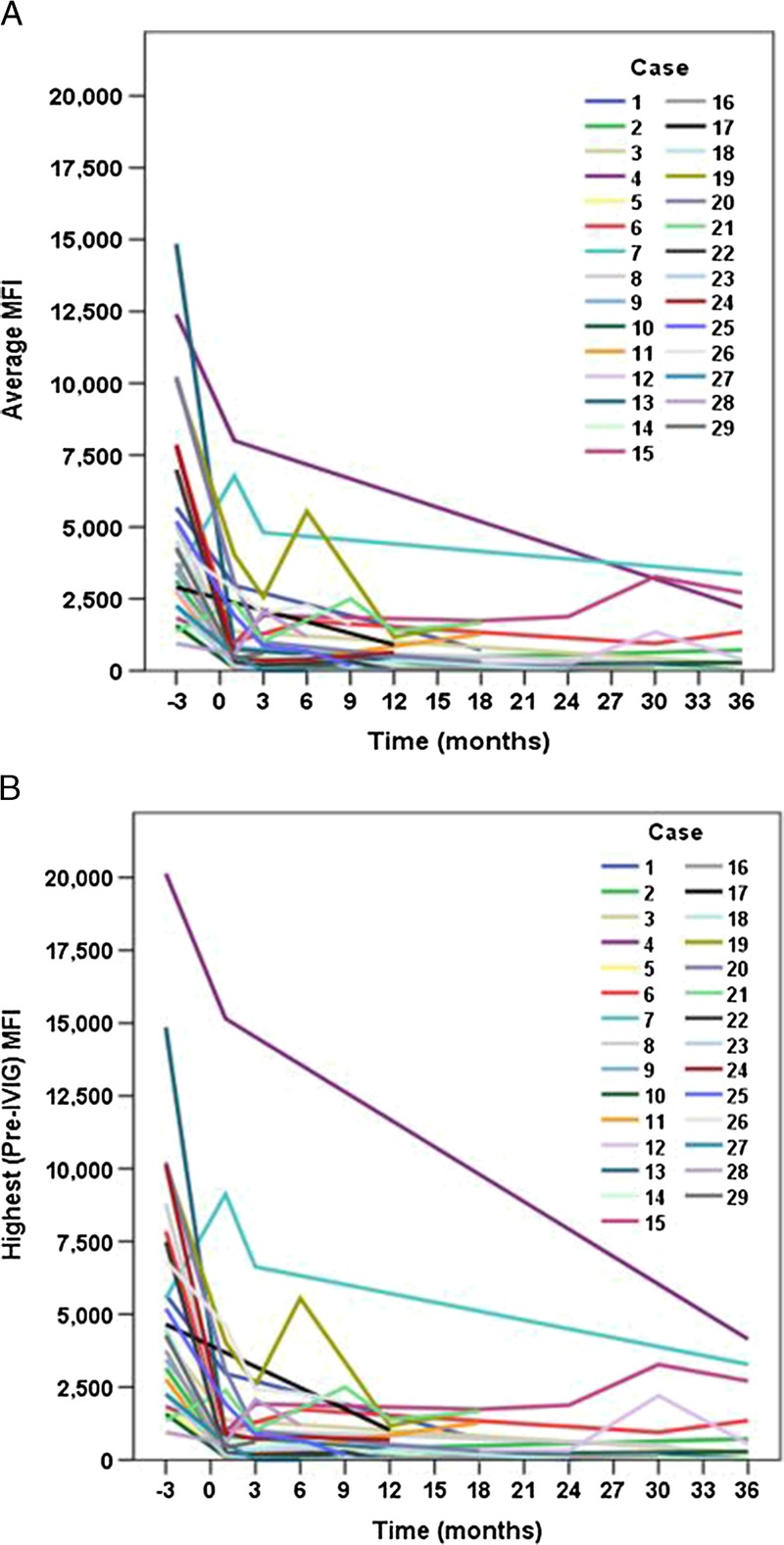
Trajectories of the average/only (panel A) and highest/only (panel B) DSA MFI values over time are shown for each of 29 patients.
FIGURE 2.
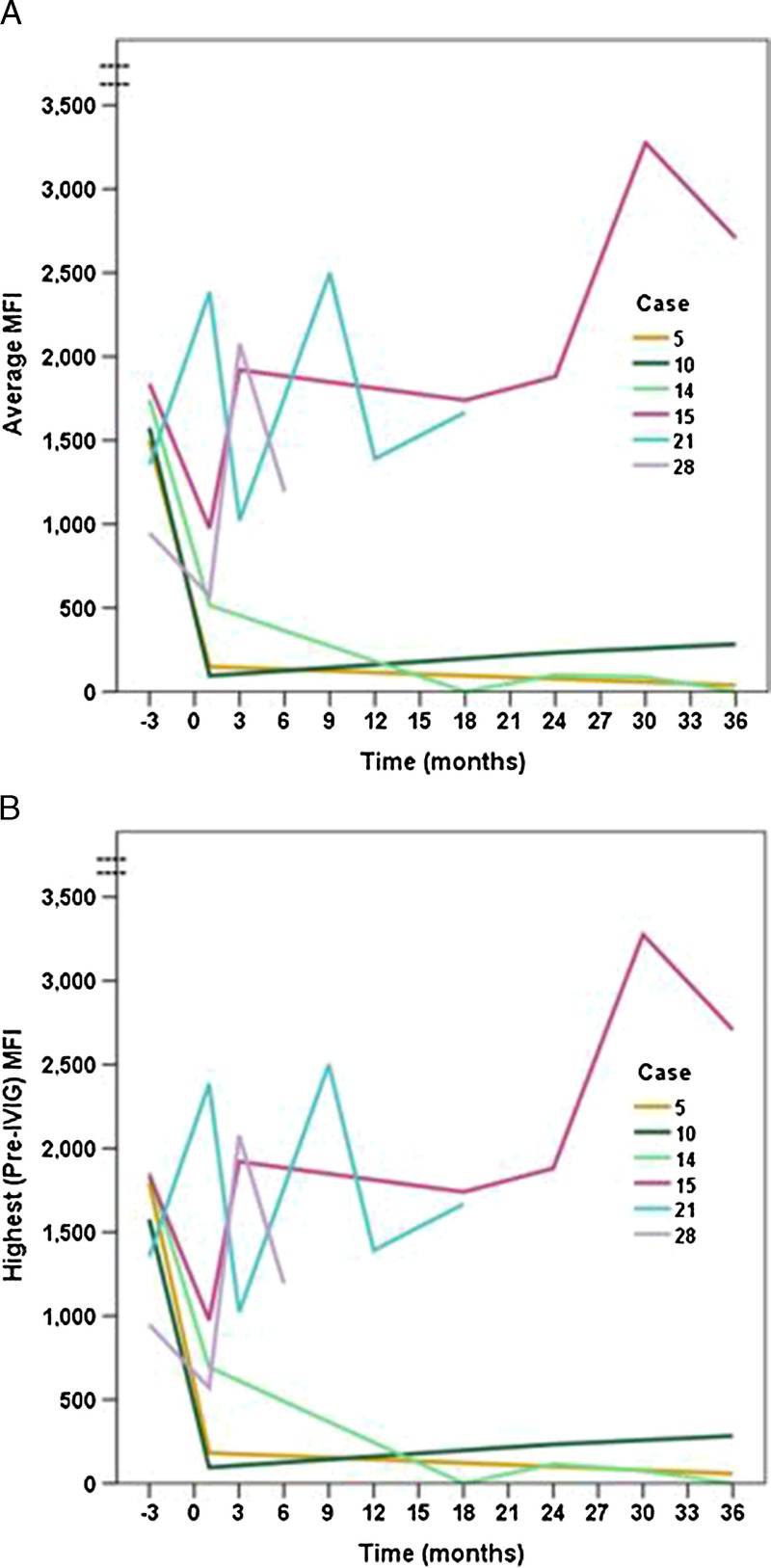
Trajectories of the average/only (panel A) and highest/only (panel B) DSA MFI values over time are shown for 6 patients with baseline values < 2000 (borderline positive).
At one month posttransplant 20 patients (69%) had a complete response to this desensitization protocol with negative DSA and an additional seven patients (24%) had a partial response with MFI reduced from baseline but still positive (MFI ≥ 1000 [>5000 for Cw, >2500 for DQ]). Only two patients (7%) failed to show a reduction in MFI one month posttransplant compared to baseline. Of these 2 patients, 1 patient (7) had 5 preexisting DSA, 3 of which (DR4, DR17, and DR52) were increased from baseline on the 1- and 3-month samples despite desensitization. This patient developed AMR 125 days posttransplant which was successfully treated and currently has a functioning graft with a creatinine of 2.3 mg/dL 4.5 years posttransplant. The second patient (21) had a low level B7 DSA which increased postdesensitization but has done well without rejection with a current creatinine of 1.1 mg/dL almost 2 years posttransplant with DSA MFI remaining in the borderline range. Because of sample size limitations, our analysis does not attempt to identify factors associated with these 2 patients' failure to respond.
Mixed effects models demonstrated that individual patients had statistically significant (P < 0.001) overall reductions in both average and highest MFI over time. Consistent with the DSA-level analyses, patients' average (Figure 3A) and highest MFI values (Figure 3B) were significantly reduced at 1 month posttransplant (P < 0.001), and this reduction was sustained throughout 3 years. The temporal trajectories of the highest pretreatment DSA values did not differ on the basis of whether they were class I or class II (time by DSA class interaction, P = 0.138) (Figure 4).
FIGURE 3.
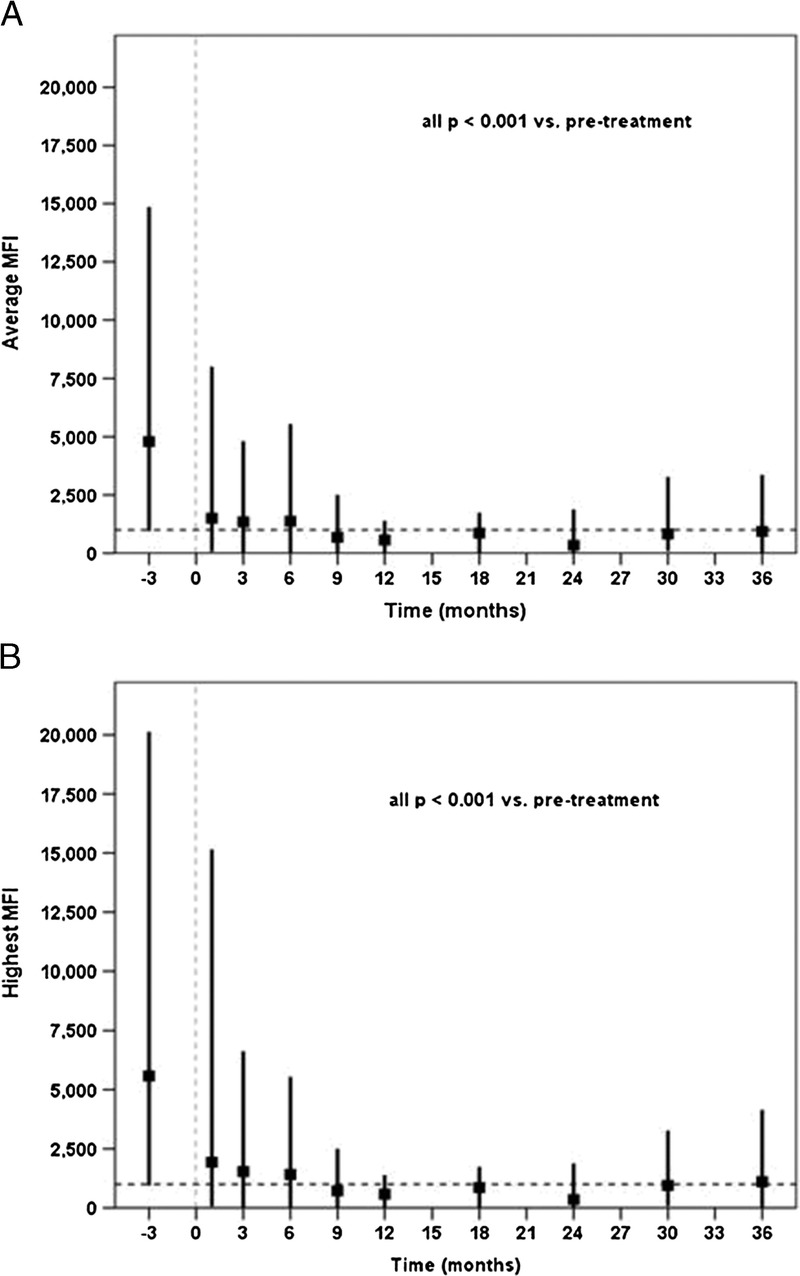
Aggregated average/only (panel A) and highest/only (panel B) DSA MFI values are indicated at each monitoring point. Each vertical line represents the range and mean MFI value at the given monitoring point. Dashed horizontal reference lines represent the value (1000) at which MFI is negligible. The temporal reduction was statistically significant overall and every posttransplant time point is significantly lower than the pretreatment MFI levels.
FIGURE 4.
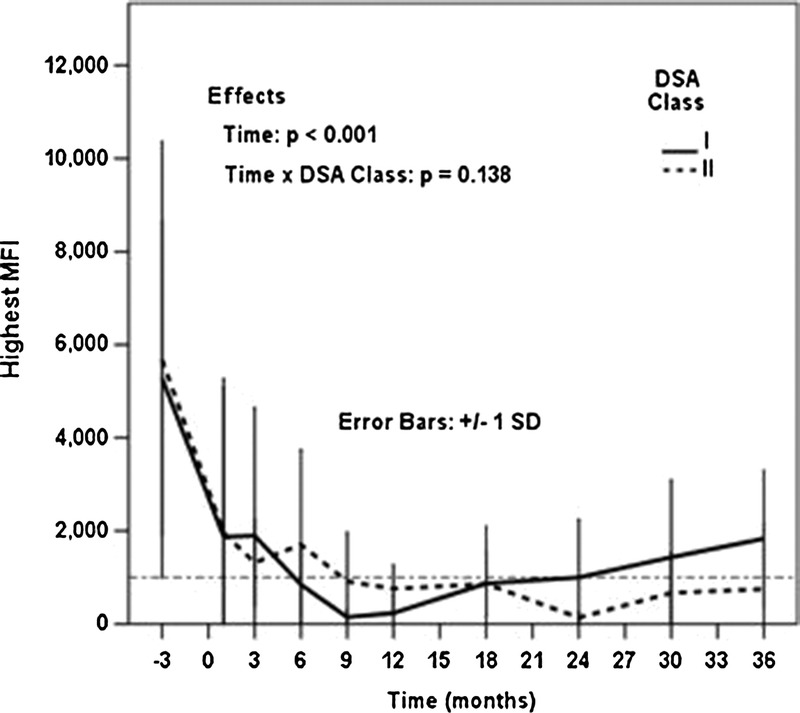
There was no difference in the trajectory of highest/only MFI over time based on whether the DSA was class I or class II.
De Novo DSA
Although the primary aim of this study was to determine the durability of preexisting DSA removal after desensitization and kidney transplantation, a total of 11 dnDSAs were identified in 9 patients (2 patients developed 2 dnDSAs each) with a mean of 433 days (range, 30-1114 days) posttransplant. Four dnDSAs were to class I and 7 were to class II. Only 1 of these 9 patients had a rejection episode with 2 dnDSAs detected at the time of biopsy for irreversible AMR associated with nonadherence. Interestingly, detection of dnDSA was transient in some patients because dnDSA was no longer detected on subsequent samples in 4 of 8 patients with a mean of 242 days after the initial appearance of the dnDSA.
C1q Assay
Eight patients (28%) had a positive C1q assay on preexisting DSA. There were 5 patients to class I and 3 patient to class II with a mean MFI of 5756 (range, 1282-20 129). Of these 8 patients, only 1 developed acute rejection compared with 3 rejection episodes in the remaining 21 patients with a negative C1q assay. All patients with a positive C1q assay on preexisting DSA currently have functioning grafts.
Of the 11 dnDSA identified, 2 were C1q-positive, both in the same patient with nonadherence-associated AMR. Of the remaining 9 dnDSA patients, C1q assay was negative for 6 patients and not available for the remaining 3 patients.
There was no relationship between having a positive C1q assay in either preexisting DSA or dnDSA and graft survival (log-rank P = 0.907) or rejection-free survival (log-rank P = 0.684).
Patient and Graft Survivals
With a mean follow-up of 1048 ± 574 (range, 175-1932) days, actuarial 1- and 3-year patient survivals were 100% and 95% and actuarial 1- and 3-year graft survivals were 100% and 90% (Figures 5A and B). One patient died with a functioning graft of 616 days posttransplant due to cardiopulmonary arrest associated with pulmonary embolus and sepsis, and 1 patient lost her allograft at 601 days posttransplant due to AMR associated with medication nonadherence.
FIGURE 5.
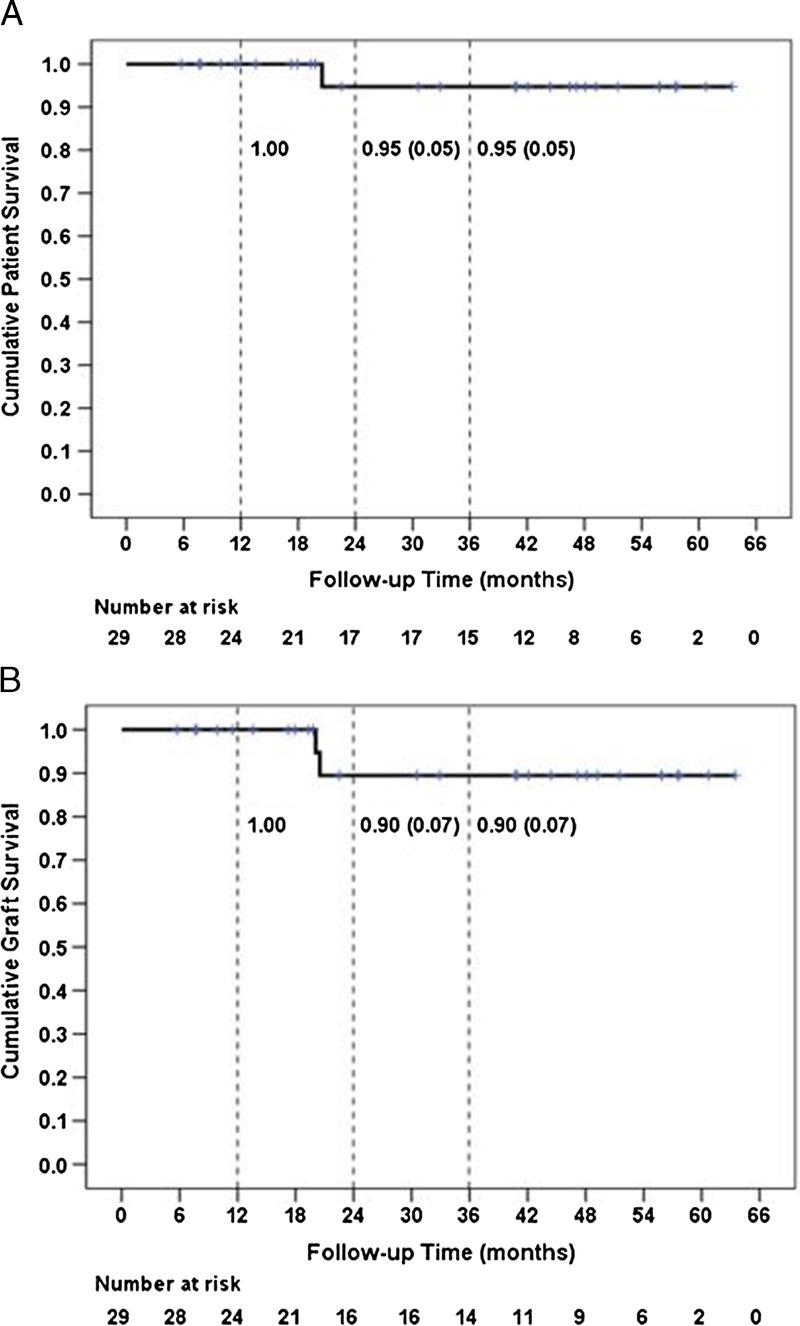
Cumulative patient (panel A) and graft (panel B) survival are depicted for 29 patients. Three-year patient survival was 95% and 3-year graft survival was 90%. The numbers at risk at the beginning of each 6-month interval, which reflect events and censored observations occurring in the preceding interval, are shown below each panel.
The first 5 patients entered into this study have been followed up for at least 5 years since transplant, and all have functioning grafts with a mean serum creatinine of 1.6 (range, 1.0-2.4) mg/dL. Biopsies were done for cause on 3 of these patients. One showed mild chronic allograft nephropathy, one showed moderate chronic allograft nephropathy and calcineurin inhibitor toxicity, and a third showed immune complex glomerulonephritis. There was no evidence on transplant glomerulopathy on these biopsies.
Acute Rejection
Over the follow-up period, 4 patients (14%) had biopsy-proven acute rejection with a mean of 344 ± 178 (range, 125-560) days posttransplant (Table 2). Two of these patients met the criteria for AMR. One patient with AMR (patient 11) was nonadherent and had positive C4d immunostaining and a low-level positive DSA (MFI = 1279) at the time of biopsy. This patient also developed 2 dnDSAs (A31, MFI = 1350 and DR52, MFI = 7921) at the time of AMR. The second patient with AMR had 5 preexisting DSAs, 1 class I (MFI = 1759) and 4 class II (MFI range, 1797-5553), and only 2 of the 5 DSAs were reduced from baseline postdesensitization when measured 1 month posttransplant. All 5 DSAs were still positive at the time of AMR 123 days posttransplant. No dnDSA was detected posttransplant in this patient, and she had a functioning graft at last follow-up of 1384 days posttreatment for AMR.
Two patients had biopsy-proven ACR with negative C4d immunostaining at 325 and 367 days posttransplant, respectively (Table 2). The first patient had a single preexisting DSA with a high MFI pretransplant (MFI = 10 228), and the DSA remained negative posttransplant. She had a functioning graft at last follow-up 212 days posttreatment for ACR. The second patient has 2 preexisting DSAs (MFI = 1157 and 4653), and both DSA remained negative posttransplant. This patient died of sepsis with a functioning graft 249 days after treatment for ACR. Neither of these patients with ACR developed a dnDSA posttransplant.
Infectious Complications
One patient died with a functioning graft due to cardiopulmonary arrest associated with pulmonary emboli and pantoea agglomerans sepsis. There were 3 cases (10%) of polyoma virus nephropathy with serum BK virus, quantitative positive (>500) and SV-40 immunohistochemical staining in tubular cell nuclei on biopsy. All 3 were treated with modification of their immunosuppression and currently have functioning grafts with mean serum creatinine of 2.1 (range, 2-2.2) mg/dL. There was one case of cytomegalovirus disease successfully treated with the most recent creatinine of 0.8 mg/dL.
DISCUSSION
Vo and Jordan6-8,10 pioneered the use of high-dose IVIG to reduce antidonor HLA antibodies and allow successful kidney transplantation. Their initial protocols used IVIG alone,7 whereas subsequent reports showed improved results with the addition of the anti-CD20 agent rituximab.8,15,16 We shortened and modified this protocol for living donor recipients, giving 1 dose of IVIG 5 days before a scheduled transplant and giving a single dose of rituximab and a second dose of IVIG posttransplant.
The presence of preexisting DSA is a predictor of poor posttransplant outcomes.3,16-18 Several studies have also reported that DSA strength specifically correlates with AMR and graft loss,3,17-21 whereas others found no correlation.16 Although desensitization protocols permit acceptable graft survival in patients with preexisting DSA, few studies report prospective data regarding the efficacy and long-term durability of DSA removal with these protocols. Vo et al15 found DSA levels rebounded as early as 1 to 4 weeks posttransplant in 2 of 6 highly sensitized recipients who received IVIG plus placebo but did not rebound out to 12 months in 6 patients treated with IVIG and rituximab. Jackson et al22 also reported a reduction in the incidence and magnitude of HLA antibody rebound out to 12 months with rituximab induction but did not observe an effect on the rate of DSA persistence, AMR, or 5-year allograft survival compared with those desensitized without rituximab. In a study of 31 highly sensitized, waitlisted patients given high-dose IVIG days 1 and 30 and a single dose of rituximab on day 15, Lobashevsky et al23 demonstrated an initial reduction in MFI of greater than 50% but a significant rebound effect by 101 to 200 days for class II and 350 days for class I antibodies. Patients did not receive kidney transplants in this study so the durability of antibody removal may have been limited by the absence of subsequent immunosuppression. Using the protease inhibitor bortezomib, Guthoff et al24 reported that bortezomib alone did not result in a sustained reduction in DSA in sensitized patients awaiting transplant. In a series of living donor kidney transplants that developed dnDSA, Everly et al25 demonstrated complete DSA removal in 18 of 26 patients and a 50% reduction in DSA in an additional 7 recipients treated preemptively with bortezomib but 56% of the patients with a complete response relapsed after a median of 3.8 months. Finally, using bortezomib in combination with plasmapheresis and rituximab, Woodle et al26 recently reported antibody reductions in 86% of patients persisting up to 10 months.
We prospectively monitored preexisting DSA out to 3 years posttransplant after desensitization in living donor kidney transplant recipients. Eighteen of 29 patients (62%) had a complete response and an additional 9 (31%) had a partial response when tested at 1 month posttransplant. This reduction was clinically and statistically significant at each time point studied, and we did not find a significant rebound of DSA MFI levels over 36 months posttransplant. Our study is the first to report such a sustained, durable reduction in DSA in kidney transplant recipients prospectively followed up until 3 years after desensitization with IVIG and rituximab. Moreover, this durable reduction in DSA was observed whether we analyzed highest or mean DSA per patient or stratified whether class I or class II DSA. Although we do not have a control group who received IVIG alone, our data also support the earlier findings of Vo et al,15 suggesting that the addition of rituximab to IVIG prevents early DSA rebound after desensitization.
Preliminary data from our center showed the addition of rituximab resulted in improved 6-month rejection-free graft survival from 50% to 90% compared with historical controls treated with IVIG alone.12 This report extends those early observations in a larger number of patients with longer follow-up and demonstrates excellent patient and graft survival out to 3 years in this high-risk population. The overall incidence of acute rejection (14%) remains acceptable in this high-risk population. Although randomized, controlled trials defining efficacy and optimal dosing regimens are lacking, our data support the use of rituximab in combination with IVIG in DSA-positive, crossmatch-positive kidney transplant recipients.8,15,27 In addition, selection criteria for desensitization with this protocol at our center required T- and/or B-cell flow crossmatch titers less than 1:32 which also may have contributed to the excellent short-term and intermediate-term outcomes.
Although 93% of our patients treated had either a complete or partial response to this desensitization protocol by 1 month posttransplant, 2 patients in our study did not respond. Of these, one went on to develop early AMR 125 days posttransplant which was successfully treated with a course of thymoglobulin, high-dose IVIG, and rituximab. This patient had 5 preexisting DSA with a highest DSA-MFI of 5553 consistent with previous reports that the number or the MFI level of preexisting DSA may be an important prognostic factor.3,22,28,29 Others have noted that an increase in DSA MFI from pretransplant to 2 week posttransplant was indicative of a higher probability of AMR.21 Although the number of nonresponders in our study is limited, our data support the notion that patients with persistently high MFI early posttransplant despite desensitization warrant more aggressive monitoring, such as protocol biopsies or higher levels of immunosuppression.
On the other hand, several patients with a partial response had persistent DSA with excellent allograft function without AMR during the 3-year follow-up period. This suggests caution in interpreting or aggressively treating all patients based on DSA-MFI alone as measured by Luminex after desensitization with IVIG and rituximab. Low DSA levels may persist without allograft injury and some DSA, either preexisting or de novo, may not fix complement or cause graft injury.16,30-32 Although all 4 patients in our study with rejection had a positive B flow crossmatch before transplant and this subset of DSA positive, B flow crossmatch-positive patients may require increased immunosuppression or heightened surveillance. However, the limited number of events in the 3-year study precluded correlating pretransplant crossmatch data or DSA characteristics including C1q binding with subsequent clinical outcomes. Further study is needed to elucidate the pathogenic role of specific types and levels of DSA following desensitization protocols to tailor treatment recommendations to those specific DSA associated with AMR, chronic rejection, and/or reduced graft survival.
Strengths of this study are its single-center design that afforded a uniform desensitization protocol, posttransplant immunosuppression, prospective DSA monitoring, and long-term patient follow-up. The primary limitation of this study is the small sample size. The limited number of events, such as AMR and graft loss, also precluded multivariable analyses of factors related to adverse events. In this cohort, C1q assay did not help to characterize which HLA antibodies correlated with adverse clinical outcomes. Other methods to help prospectively identify detrimental HLA antibodies, such as characterizing subclasses of immunoglobulin or HLA epitope matching, were not done. A larger, multicenter study is needed to confirm the durability of DSA removal after desensitization with IVIG and rituximab and to identify specific DSAs that do not respond to desensitization or that portend AMR or a poor prognosis posttransplant.
In conclusion, this prospective study of DSA monitoring in desensitized living donor kidney transplant recipients shows a highly significant early reduction in DSA which was sustained up to 3 years posttransplant, excellent 1- and 3- year patient and graft survivals, and a low incidence of AMR. We found this early and sustained reduction in DSA held true for both class I and class II DSA. Further study is required to identify specific DSA characteristics associated with poor outcomes despite desensitization.
Footnotes
The authors declare no conflicts of interest.
This work was supported in part by a grant from the Vanderbilt Transplant Center (D.S.).
This work was presented in part at the 2014 World Transplant Congress, San Francisco, CA.
D.S. participated in research design, performance of the research, data analysis, and writing of the article. I.D.F. participated in data analysis and writing of the article. D.C. participated in performance of the research, data analysis, and writing of the article. H.S. participated in research design, data analysis, and writing of the article.
Published online 11 January 2016.
REFERENCES
- 1. Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. http://www.ncbi.nlm.nih.gov/pubmed/4886455. N Engl J Med. 1969; 280: 735– 739. [DOI] [PubMed] [Google Scholar]
- 2. Gloor JM, Winters JL, Cornell LD, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. http://www.ncbi.nlm.nih.gov/pubmed/20121740. Am J Transplant. 2010; 10: 582– 589. [DOI] [PubMed] [Google Scholar]
- 3. Lefaucheur C, Loupy A, Hill GS. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. http://www.ncbi.nlm.nih.gov/pubmed/20634297. J Am Soc Nephrol. 2010; 21: 1398– 1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohan S, Palanisamy A, Tsapepas D, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. http://www.ncbi.nlm.nih.gov/pubmed/23160511. J Am Soc Nephrol. 2012; 23: 2061– 2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.http://srtr.transplant.hrsa.gov/annual_reports/2012/pdf/01_kidney_13.pdf.
- 6. Jordan SC, Vo A, Bunnapradist S, et al. Intravenous immune globulin treatment inhibits crossmatch positivity and allow for successful transplantation of incompatible organs in living-don and cadaver recipients. http://www.ncbi.nlm.nih.gov/pubmed/12973100. Transplantation. 2003; 76: 631– 636. [DOI] [PubMed] [Google Scholar]
- 7. Jordan SC, Tyan D, Stablein D, et al. Evaluation of intravenous immunoglobulin as an agent to low allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. http://www.ncbi.nlm.nih.gov/pubmed/15579530. J Am Soc Nephrol. 2004; 15: 3256– 3262. [DOI] [PubMed] [Google Scholar]
- 8. Vo A, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. http://www.ncbi.nlm.nih.gov/pubmed/18635429. N Engl J Med. 2008; 359: 242– 251. [DOI] [PubMed] [Google Scholar]
- 9. Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipient and survival. http://www.ncbi.nlm.nih.gov/pubmed/21793744. N Engl J Med. 2011; 365: 318– 326. [DOI] [PubMed] [Google Scholar]
- 10. Vo AA, Petrozzino J, Yeung K, et al. Efficacy, outcomes, and cost-effectiveness of desensitization using IVIG and rituximab. http://www.ncbi.nlm.nih.gov/pubmed/23511212. Transplantation. 2013; 95: 852– 858. [DOI] [PubMed] [Google Scholar]
- 11. Orandi BJ, Garonzik-Wang JM, Massie AB, et al. Quantifying the risk of incompatible kidney transplantation: A multicenter study. http://www.ncbi.nlm.nih.gov/pubmed/24913913. Am J Transplant. 2014; 14: 1573– 1580. [DOI] [PubMed] [Google Scholar]
- 12. Umanath K, Shaffer D, Feurer I, et al. Rituximab significantly improves rejection free survival when added to high dose IVIG in patients with positive crossmatch undergoing kidney transplantation. (abstract) Am J Transplant. 2012; 12(Suppl 3): 228. [Google Scholar]
- 13. Shaffer D, Crowe D, Feurer I, et al. IVIG/Rituximab is associated with reduced DSA MFI and prolonged rejection free survival in kidney transplant recipients with positive crossmatch. (abstract) Am J Transplant. 2012; 12(Suppl 3): 232. [Google Scholar]
- 14. Colvin RB, Cohen AH, Saiontz C, et al. Evaluation of pathologic criteria for acute renal allograft rejection: reproducibility, sensitivity, and clinical correlation. http://www.ncbi.nlm.nih.gov/pubmed/9402096. J Am Soc Nephrol. 1997; 8: 1930– 1941. [DOI] [PubMed] [Google Scholar]
- 15. Vo AA, Choi J, Cisneros K, et al. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. http://www.ncbi.nlm.nih.gov/pubmed/24770617. Transplantation. 2014; 98: 312– 319. [DOI] [PubMed] [Google Scholar]
- 16. Amico P, Honger G, Mayr M, et al. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. http://www.ncbi.nlm.nih.gov/pubmed/19502960. Transplantation. 2009; 87: 1681– 1688. [DOI] [PubMed] [Google Scholar]
- 17. Chung BA, Choi BS, Oh EJ, et al. Clinical impact of the baseline donor-specific anti-human leukocyte antigen antibody measured by Luminex single antigen assay in living donor kidney transplant recipients after desensitization therapy. Transpl Int. 2014; 27: 49– 59. [DOI] [PubMed] [Google Scholar]
- 18. Akalin E, Dinavahi R, Friedlander R, et al. Addition of plasmapheresis decrease the incidence of acute antibody-mediated rejection in sensitized patients with strong donor-specific antibodies. http://www.ncbi.nlm.nih.gov/pubmed/18337549. Clin J Am Soc Nephrol. 2008; 3: 1160– 1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reinsmoen NL, Lai C, Vo A, et al. Acceptable donor-specific antibody levels allowing for successful deceased and living donor kidney transplantation after desensitization. http://www.ncbi.nlm.nih.gov/pubmed/18813107. Transplantation. 2008; 86: 820– 825. [DOI] [PubMed] [Google Scholar]
- 20. Kannabhiran D, Lee J, Schwartz JE, et al. Characteristics of circulating donor human leukocyte antigen-specific immunoglobulin G antibodies predicative of acute antibody-mediated rejection and kidney allograft failure. http://www.ncbi.nlm.nih.gov/pubmed/25629531. Transplantation. 2015; 99: 1156– 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu P, Jin J, Everly MJ, et al. Impact of alloantibody strength in crossmatch negative DSA positive kidney transplantation. http://www.ncbi.nlm.nih.gov/pubmed/23726814. Clin Biochem. 2013; 46: 1389– 1393. [DOI] [PubMed] [Google Scholar]
- 22. Jackson AM, Kraus ES, Orandi BJ, et al. http://www.ncbi.nlm.nih.gov/pubmed/25054778. A closer look at rituximab induction on HLA antibody rebound following HLA-incompatible kidney transplantation. Kidney Int. 2015; 87: 409– 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lobashevsky AL, Higgins NG, Rosner KM, et al. Analysis of anti-HLA antibodies in sensitized kidney transplant candidates subjected to desensitization with intravenous immunoglobulin and rituximab. http://www.ncbi.nlm.nih.gov/pubmed/23778648. Transplantation. 2013; 96: 182– 190. [DOI] [PubMed] [Google Scholar]
- 24. Guthoff M, Schmid-Horch B, Weisel KC, et al. Proteasome inhibition by bortezomib: effect on HLA-antibody levels and specificity in sensitized patient awaiting renal allograft transplantation. http://www.ncbi.nlm.nih.gov/pubmed/22326708. Transpl Immunol. 2012; 26: 171– 175. [DOI] [PubMed] [Google Scholar]
- 25. Everly MJ, Terasaki PI, Trivedi HL. Durability of antibody removal following proteasome inhibitor-based therapy. http://www.ncbi.nlm.nih.gov/pubmed/22262128. Transplantation. 2012; 93: 572– 577. [DOI] [PubMed] [Google Scholar]
- 26. Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. http://www.ncbi.nlm.nih.gov/pubmed/25524446. Am J Transplant. 2015; 15: 101– 118. [DOI] [PubMed] [Google Scholar]
- 27. Macklin PS, Morris PJ, Knight SR. A systematic review of the use of rituximab for desensitization in renal transplantation. http://www.ncbi.nlm.nih.gov/pubmed/25321163. Transplantation. 2014; 98: 794– 805. [DOI] [PubMed] [Google Scholar]
- 28. Cooper JE, Gralla J, Chan L, et al. Clinical significance of post kidney transplant de novo DSA in otherwise stable grafts. http://www.ncbi.nlm.nih.gov/pubmed/22755431. Clin Transpl. 2011: 359– 364. [PubMed] [Google Scholar]
- 29. Yell M, Muth BL, Kaufman DB, et al. C1q binding activity of de novo donor-specific HLA antibodies in renal transplantation without antibody-mediated rejection. http://www.ncbi.nlm.nih.gov/pubmed/25839705. Transplantation. 2015; 99: 1151– 1155. [DOI] [PubMed] [Google Scholar]
- 30. Bartel G, Regele H, Wahrmann M, et al. Posttransplant HLA alloreactivity in stable kidney transplant recipients-Incidences and impact on long-term allograft outcomes. http://www.ncbi.nlm.nih.gov/pubmed/18853952. Am J Transplant. 2008; 8: 2652– 2660. [DOI] [PubMed] [Google Scholar]
- 31. Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. http://www.ncbi.nlm.nih.gov/pubmed/21791230. Hum Immunol. 2011; 72: 849– 858. [DOI] [PubMed] [Google Scholar]
- 32. Peacock S, Kosmoliaptsis V, Bradley AJ, et al. Questioning the added value of luminex single antigen beads to detect C1q binding donor HLA-specific antibodies. http://www.ncbi.nlm.nih.gov/pubmed/24918614. Transplantation. 2014; 98: 384– 386. [DOI] [PubMed] [Google Scholar]


