Abstract
Background
The BK virus typically colonizes the lower urinary tract and is the causative agent in BK virus nephropathy (BKVN), which can progress to allograft dysfunction and graft loss. Urinary reflux in kidney allografts is induced by vesicoureteral reflux or disturbances in intrarenal reflux (IRR), believed to be associated with BKVN. This study was designed to elucidate the relationship between BKVN and IRR.
Methods
We examined 30 renal transplant recipients histologically diagnosed with BKVN using anti-Simian virus 40 immunohistochemistry and 60 clinically matched control recipients. The BKVN patients were divided into stable (n = 12) and progressive (n = 18) groups according to allograft kidney function 1 year after diagnosis. Histological rejection scores according to the pathological classification of rejection in renal allografts (Banff classification), histological BKVN stages, and histological polyomavirus load levels (pvl) proposed by the Banff working group were evaluated. The IRR was quantified by histological reflux scores defined with retention and reflux of immunostained Tamm-Horsfall protein in renal tubules and glomeruli.
Results
Higher reflux scores were observed in the BKVN group compared with that in the control group. No differences in clinical parameters were observed between the BKVN and control groups. Reflux scores and pvl were significantly higher in the progressive group than in the stable BKVN group with no significant difference in BK stage observed between groups. Reflux scores were found to be significantly correlated with pvl.
Conclusions
Our preliminary study suggested that IRR might be a predisposing and prognostic factor in BKVN.
Tubulointerstitial nephritis caused by the BK virus and subsequent interstitial fibrosis, termed BK virus nephropathy (BKVN) or polyomavirus-associated nephropathy occurs in 1% to 10%1 of kidney transplant recipients. The BKVN may progress to allograft dysfunction or loss in 15% to 50%2,3 of infected individuals. Primary BK virus infections occur early in childhood via oral and/or respiratory exposure and the virus remains latent in the renal epithelium (transitional epithelium, renal tubular epithelium, and parietal epithelium of Bowman capsule) and lymphoid cells.4 The degree of immunosuppression is likely the most important risk factor underlying BK viral infection, with BK replication considered a reliable marker of excess immunosuppression.5
However, the susceptibility of allograft kidneys to other forms of injury, such as ischemia and rejection, may explain why majority of the cases of BKVN occur after renal transplantation, and only rarely occur after liver, heart, lung, or bone marrow transplantation.6
Several important studies have established effective strategies for the management of BKVN after kidney transplantation,7-10 particularly at the timing of increasing immunosuppression: screening and preemptive monitoring of viruria using cytology (decoy cells), measurement of urinary DNA loads or urinary VP-1 mRNA loads, and measurement of viremia using plasma DNA loads.
However, allograft biopsies are still required to confirm the diagnosis of BKVN and assess for other histological types of injury, particularly concurrent acute rejection.
The BKVN is histologically diagnosed using anti-Simian virus 40 (SV40) immunohistochemistry with disease stages (A, B, and C) and histological polyomavirus load levels (pvl) proposed by the Banff working group. The BKVN disease stages (particularly stage C) and pvl have been shown to correspond with allograft outcomes.11 However, the distinction of BKVN from acute tubular necrosis, interstitial nephritis, and acute cellular rejection remains challenging.12
On the other hand, an increased incidence of BK viremia has been associated with urinary stenosis and the use of ureteral stents.13-15 These reports suggest that urinary reflux in kidney allografts is induced by vesicoureteral reflux (VUR) or intrarenal reflux (IRR), believed to be associated with BKVN.
The VUR is commonly diagnosed using a voiding cystourethrogram (VUCG), according to the classification of the International Reflux Study group (grades 1-4)16 and involves the catheterization of the bladder and radiopharmaceutical imaging of the lower and upper urinary tract (Figure 1A). Histologically reflux nephropathy associated with VUR or IRR is characterized by acquired focal renal scarring with interstitial mononuclear cell infiltration and fibrosis, particularly in medullary rays comprising proximal and distal tubules going to and from the medulla and collecting ducts (Figures 1B and C), which is termed medullary ray injury (MRI) by Kobayashi et al.17 The etiologies of MRI are considered to be ischemic and urological complications, such as atherosclerosis, calcineurin inhibitor (CNI)-associated vasculopathy, urinary tract infection (UTI), and urinary obstruction or reflux.17
FIGURE 1.
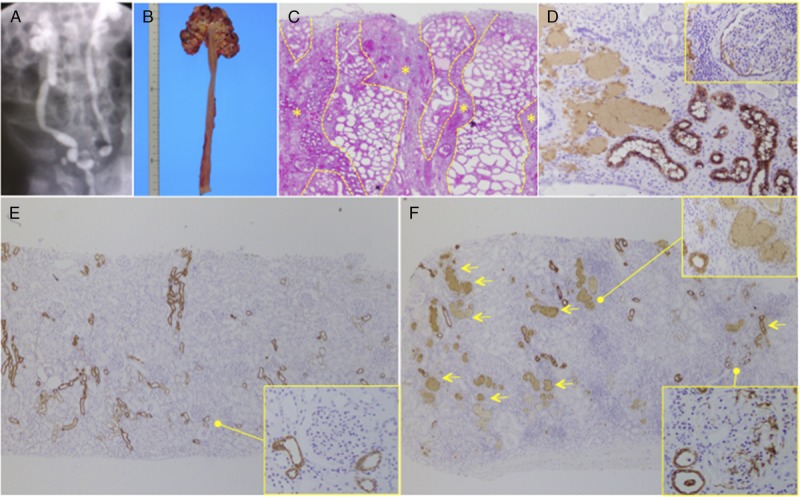
Representative image of reflux nephropathy. A, Diagnosis of VUR with a VUCG according to the classification of the International Reflux Study Group (grade 4). B, Macroimage of nephrectomy for recurrent VUR. C, Microscopic image of reflux nephropathy demonstrating medullary ray injury (yellow asterisk) according to the presence of PAS-stained casts. D, The THP immunostaining revealed THP occlusion in tubuli, deposition into the interstitium, and THP reflux into the Bowman space. E, Living donor kidney at the kidney transplantation (0-hour biopsy) demonstrated no THP obstruction of the tubuli or glomerular deposition. F, Reflux nephropathy kidney due to sever urinary stenosis at the renal autotransplantation (0-hour biopsy) demonstrating severe occlusions with THP casts in tubuli (yellow allow) and THP deposition in glomeruli. ([c] PAS stain; [d, e, f] THP immunohistochemistry). PAS, Periodic acid-Schiff.
Reflux nephropathy is also associated with interstitial Tamm-Horsfall protein (THP) deposition or THP reflux into the Bowman capsule (Figures 1D and F) due to combined VUR and IRR associated with UTI, or permanently high intravesical pressures in human VUR patients18,19 or animal reflux nephropathy models.20 In kidney transplant settings, Kobayashi et al17 reported that a urinary obstruction-related MRI (THP-positive cast occlusive type) was found in 85.7% kidney recipients with a diagnosis of VUR (grades 1-3) based on VUCG. Akioka et al21 also reported interstitial THP deposition in 26.3% pediatric recipients, with 80% diagnosed with VUR (grades 1-4) based on VUCG. Thus, THP staining is believed to have utility in detecting VUR or IRR in kidney biopsy samples, particularly in kidney transplant settings.
The THP, also known as uromodulin, is a glycoprotein with a variety of N-linked and O-linked glycans that confer the ability to bind a wide range of substances.22,23 Further, THP has been shown to perform a defensive role against UTI, presumably by contributing to the binding and clearance of uropathogenic bacteria from the urinary tract.24,25 Recently, the presence of 3-dimensional THP-rich viral clusters, termed “Haufen,” have been shown to be a reliable marker of BKVN in viremic or persistently viruric patients with negative histological findings on renal biopsy.26 It is considered that “Haufen” are formed during cellular rupture and release of daughter virions into injured nephrons, with high levels of THP shown to aggregate as well as the influenza virus.27 The THP may perform both proinflammatory and antiviral roles in BKVN; however, we hypothesize that THP backflow into the tubulointerstitium and Bowman capsule occurs due to urinary reflux, which may lead to dispersion of BK virons into kidney allografts.
In the present study, we preliminary evaluated the association between BKVN and urinary reflux in kidney allografts induced by VUR or disturbances in IRR. The IRR was systemically evaluated using THP immunohistochemistry in renal biopsy samples from 30 renal transplant recipients with BKVN and 60 control recipients.
MATERIALS AND METHODS
Patients and Allograft Biopsies
We performed a retrospective analysis of 90 kidney transplant recipients who underwent allograft kidney biopsy at our center between April 2003 and March 2015. The study was approved by the local ethical committee (approval no 3367). Cases were retrieved over a 12-year period from the pathological records of the kidney center at the Tokyo Woman's Medical University. We identified 60 control renal allograft recipients matched according to age and posttransplantation period. The BK virus plasma DNA loads (threshold; >104 copies/mL) were available in 21 BKVN patients. All clinical data were retrieved from patient medical records. For the histological study, 164 kidney allograft biopsies from 30 patients with BKVN were evaluated, including preceding biopsies (n = 29), SV40-proven biopsies (n = 49), SV40-cleared biopsies (n = 26), and controls (n = 60).
Morphometric Analysis
Tissue was processed after formalin fixation using standard histological techniques. Frozen sections were prepared for immunofluorescence. Histological rejection scores according to the Banff classification 2013,28 histological BKVN stages, and pvl proposed by the Banff working group11 were independently scored by 3 pathologists. The BKVN diagnoses were confirmed by SV40 immunohistochemistry (mouse monoclonal IgG2a, Calbiochem, Merck KGaA, Darmstadt, Germany). Histological BK stages and 3-tier pvl scores (score 1, 1%; score 2, 1-10%; score 3, >10% according to the overall percentage of SV40-positive tubules) by the Banff working group11 were evaluated.
The THP immunohistochemistry using an antihuman THP antibody (mouse monoclonal IgG, Cedarlane, Burlington, ON, Canada) was performed to evaluate renal reflux with a scoring system used assessing each tubule and glomerulus in nonatrophied cortical areas. In brief, scores were assigned for (1) tubular involvement: score 0, normal THP staining in the medullary ray area, particularly the thick ascending limbs of the loop of Henle (Figure 1E); score 1, tubular luminal occlusion with THP-positive casts observed in 10% to 25% of the medullary ray lesion area; score 2, 25% to 50%; and score 3, greater than 50% and (2) glomerular involvement: score 0, no THP staining; score 1, THP reflux affecting 1% to 10% of the glomerular capsular space; 10% to 25%; and score 3, greater than 25% of the glomerular capsular space (Figure 1F). Histological reflux scores were calculated as the average of both tubular and glomerular components.
Data and Statistical Analyses
Clinical data were analyzed between 30 BKVN patients and 60 control renal allograft recipients. A separate analysis was subsequently performed for stable (n = 12) and progressive (n = 18) BKVN, defined according to the absence or presence of increased serum creatinine levels of 0.5 mg/dL or greater, respectively, over a 1-year period from initial SV40-proven diagnoses of BKVN. A total of 104 allograft biopsies from BKVN patients were evaluated including preceding biopsies (n = 29), SV40-proven biopsies (n = 49), SV40-cleared biopsies (n = 26), and 60 biopsies from control recipients. JMP Pro 11.2.0 (SAS Institute Inc, SAS Campus Drive, Cary, NC) was used for statistical analyses. Prism 6 (GraphPad Software, Inc, La Jolla, CA) was used for graph preparation. Data were presented as medians (range). Multiple comparisons were performed using the Kruskal-Wallis test followed by the Mann-Whitney test. Correlations were assessed using the nonparametric Spearman rank correlation coefficient ρ. Continuous variables were compared using the Mann-Whitney test and categorical variables were compared using Fisher exact test. The pvl scores were compared using the Jonckheere-Terpstra exact test for trend in SAS 9.4 TS 1M1 (SAS Institute Inc, SAS Campus Drive Cary, NC). In all analyses, P values less than 0.05 were considered statistically significant.
RESULTS
Clinical Background
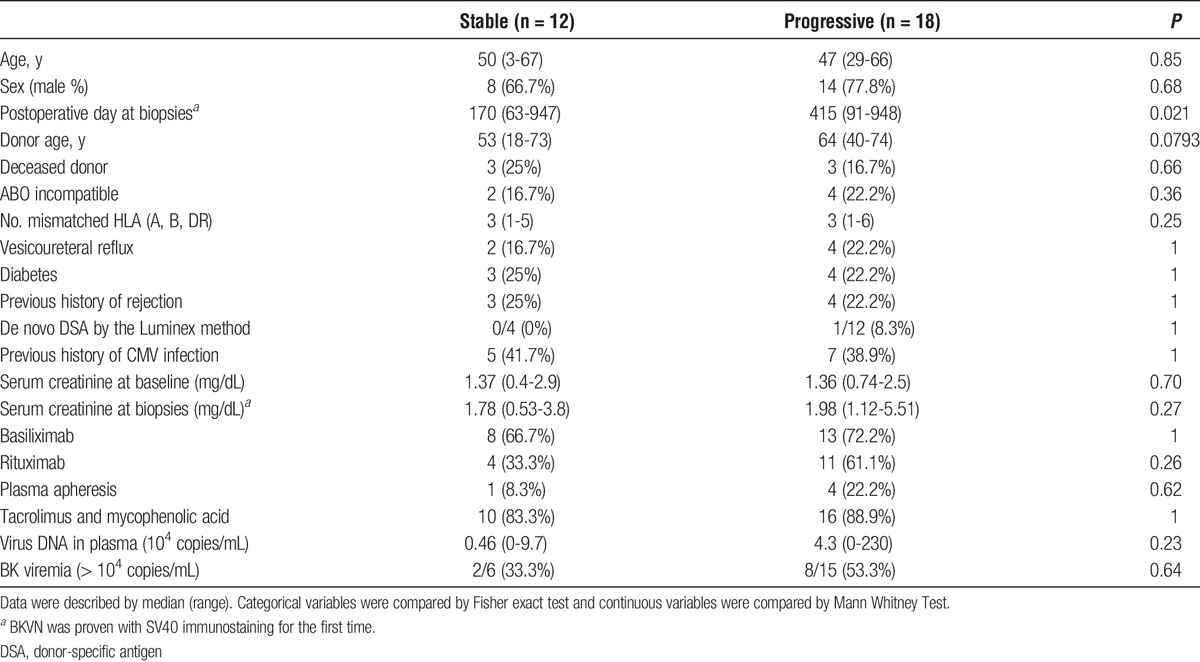
Clinical characteristics of BKVN (n = 30) and non-BKVN (n = 60) patients are shown in Table 1. Primary kidney diseases, except diabetic and congenital anomalies of kidney and urinary tract, were as follows: glomerulonephritis in 31 (34.4%) patients; other genetic kidney diseases, such as Alport syndrome, autosomal dominant polycystic kidney disease, and nephronopthisis in 4 (4.4%) patients; and other causes, such as hypertension or undefined, in 35 (38.9%) patients. No differences in clinical characteristics were observed between control recipients and BKVN patients (Table 1). In the BKVN group, ureteral obstruction was observed in 6 recipients (20%), with 3 patients requiring periodical replacement of ureteral stents. All BKVN patients were treated with reduced immunosuppression regimens, with cidofovir additionally prescribed in 3 patients (10%). Graft loss was observed in 5 (16.7%) BKVN patients due to persistent BKVN (n = 3, 10%) or combination of BKVN and rejection (n = 2, 6.7%). Two patients were found to have simultaneous acute/active AMR and 1 patient progressed to chronic, active AMR. A greater postoperative duration was observed in the progressive group compared to the stable group; however, no differences were observed in other clinical parameters, including BK viremia (Table 2). No patients died during the observation period.
TABLE 1.
Clinical characteristics of the patients in the study
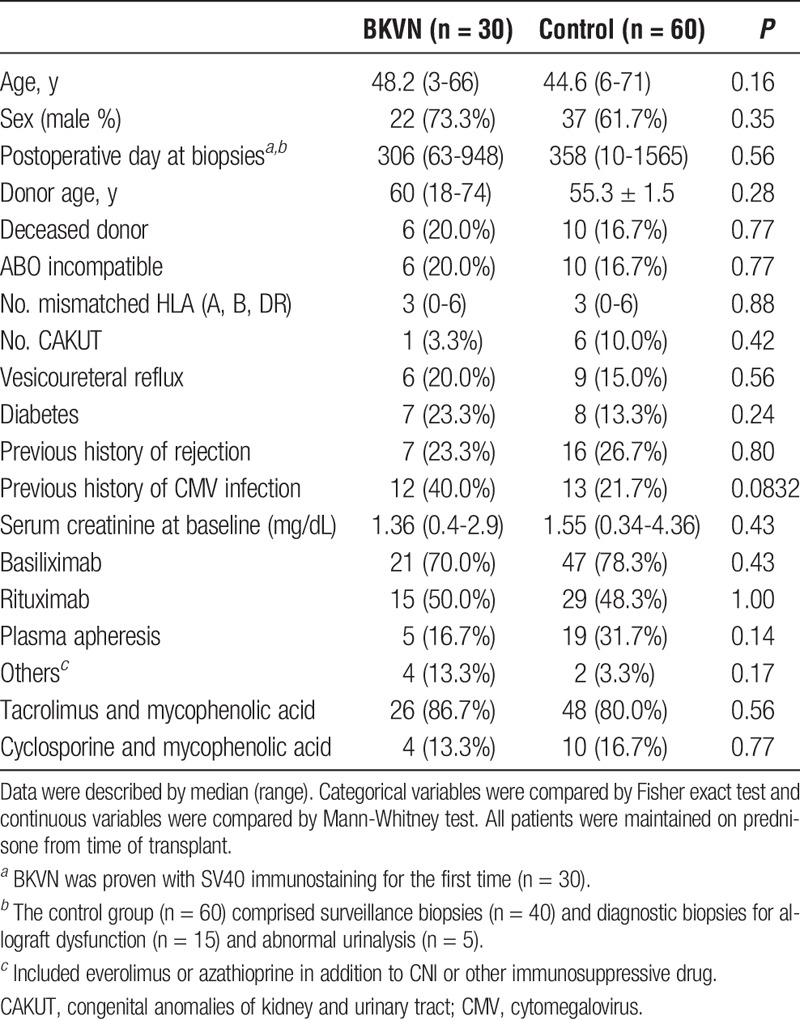
TABLE 2.
Clinical characteristics of the stable and progressive BKVN patients
Histological Characteristics of BKVN
All BKVN patients were histologically diagnosed with immunohistological staining for the SV40 antigen (Figures 2A and B). Reflux nephropathy was diagnosed according to the detection of THP in tubules and glomeruli by immunohistochemical staining (Figures 2C, D, and E). In the BKVN group, 11 patients had stage A disease, 17 patients had stage B disease, and the remaining 2 patients had stage C disease. The pvl scores according to the criteria proposed by Banff working group were 1, 2, and 3 in 8, 13, and 9 patients, respectively, at initial diagnosis before treatment. From the point of view of T cell–mediated rejection (TCMR) and antibody-mediated rejection (AMR), borderline changes, TCMR, AMR, and mixed TCMR and AMR were observed in 1, 2, 2, and 2 patients in the BKVN group, respectively. On the other hand, borderline changes, TCMR, AMR, and mixed type rejection, were observed in 3, 3, 2, and 1 patients in the control group. No significant difference in Banff scores was observed in preceding biopsies (SV40-negative) between the BKVN group (n = 29) and controls (n = 60, Figures 3A-C). On the other hand, reflux scores in preceding biopsies were higher in the BKVN group compared with the control group (Figures 3A and D). Histological reflux scores were highest in SV40-proven biopsies compared with that in all other biopsy types in the BKVN group (Figures 3A and D). Moreover, reflux scores were higher in the progressive BKVN group than in the stable group, with no difference in Banff scores observed between groups (Figure 3B).
FIGURE 2.
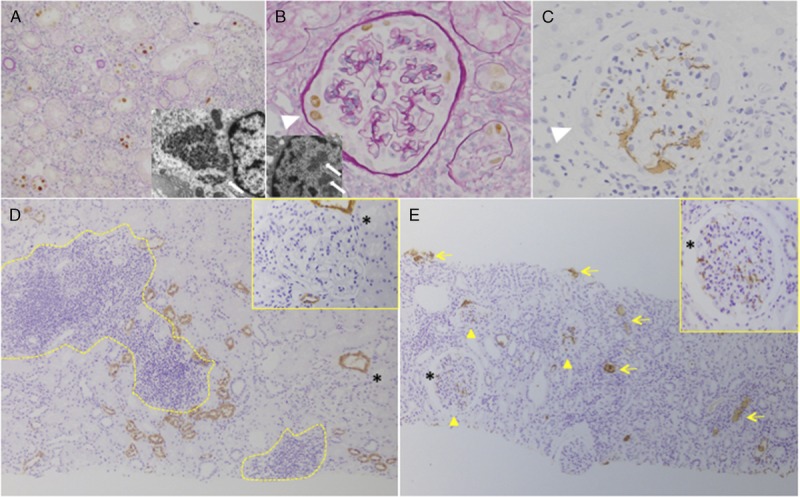
Representative BK polyoma virus nephropathy and micro urinary reflux. A, Severe tubulointerstitial nephritis with epithelial cell lysis and denudation of the TBM (BK stage B). Tubular epithelial cells were diffusely positive for SV40 staining (pvl score, 3). TEM image demonstrating BK virus particles within tubular epithelial cells (×15 000, white arrow). B, Parietal epithelial cells lining Bowman capsule demonstrated evidence of viral replication and virus particles were detected on TEM images (×20 000, white arrow). C, Urinary reflux demonstrated by THP staining in serial sections (white triangle showed same lesion). D, Transplant kidney biopsy in the control group demonstrating no THP obstruction in tubuli or deposition in glomeruli (asterisk showed same lesion) despite the presence of tubulointerstitial rejection (yellow dots). E, Reflux nephropathy detected in the BKVN group demonstrated THP cast occlusion in tubuli (yellow allow) and THP deposition in glomeruli (yellow triangle, asterisk showed same lesion). ([a, b] SV40 immunohistochemistry with PAS stain and TEM images, [c, d, e] THP immunohistochemistry). TEM, transmission electron microscopy.
FIGURE 3.
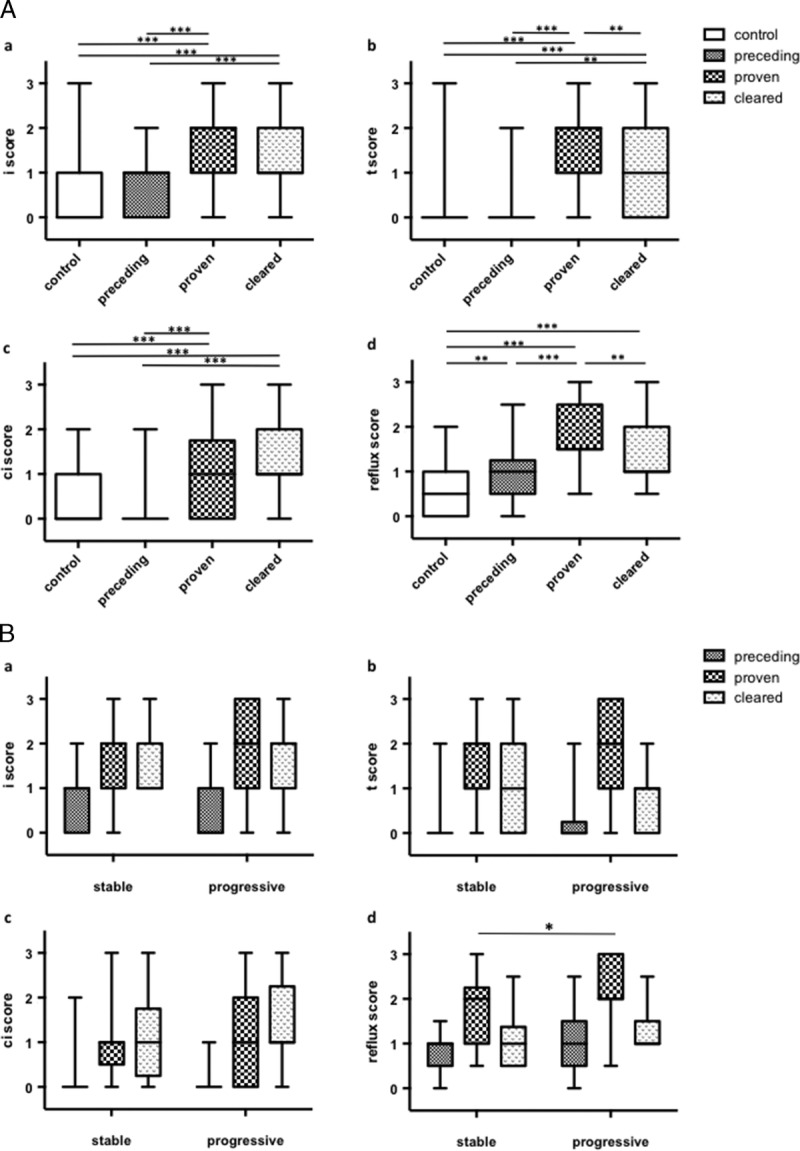
Histological analysis of BKVN according to Banff or reflux scores. A, Histological analysis of BKVN samples compared to control samples. Banff scores (i, t, ci) were higher in SV40-proven biopsies with no significant difference observed between SV40 preceding and control biopsy samples (A-C). Reflux scores according to THP immunohistochemistry in preceding and cleared phase biopsies, in addition to SV40-proven biopsies, were higher in BKVN biopsies compared with that in control biopsies (D). B, Histological analysis of stable and progressive BKVN. A-C, No significant difference in Banff scores was observed. D, Reflux scores were higher in the progressive group compared with that in the stable group in SV40-proven biopsies. Box plot displayed the full range of variation (from min to max). *P < 0.05, **P < 0.01, ***P < 0.001.
Histological Prognostic Factor in BKVN
The pvl scores at initial histological diagnosis correlated completely with BK viremia (Figure 4A); however, pvl scores were significantly higher in progressive BKVN patients than in stable BKVN patients using the Jonckheere-Terpstra exact test for trend (P = 0.0071). A significant correlation was observed between pvl scores and t scores, which reflect BK staging according to Banff criteria and reflux scores (Figures 4B and D). Reflux scores did not correlate with either t scores or ci scores according to Banff classification criteria (data not shown). These results indicate that reflux scores, in addition to pvl scores and BK staging, can be used in determining BKVN prognosis.
FIGURE 4.
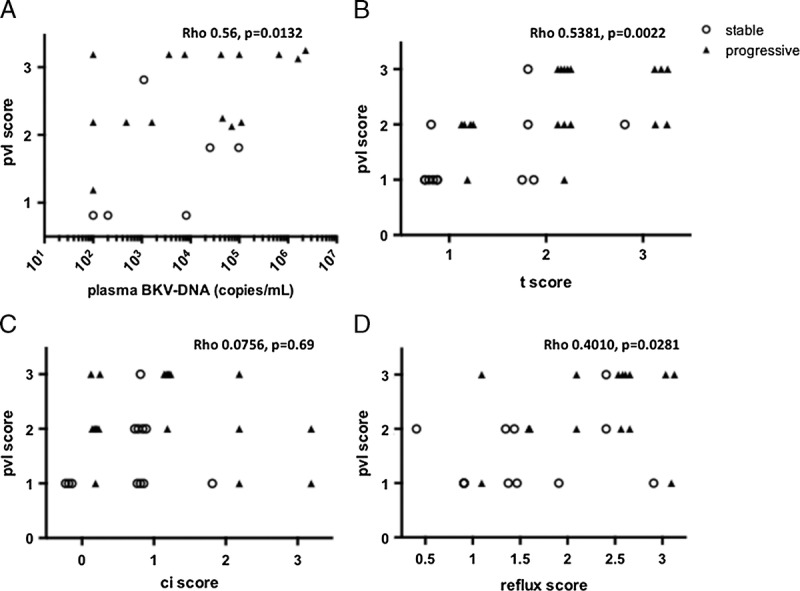
Histological pvl and stage in proven biopsies from stable and progressive BKVN cases. A, The pvl scores were found to significantly correlate with BK viremia. B-D, Significant correlations were found between pvl score and t scores of Banff classification or reflux scores.
DISCUSSION
We believe this is the first study to evaluate the association between urinary reflux and BKVN using histopathological THP immunostaining. Compared with the control group, reflux scores according to THP immunostaining in the BKVN group were higher in the preceding phase, but not in the SV40-proven stage. Reflux scores correlated with pvl scores, which reflected allograft survival in our study corroborating the observations of previous studies. Thus, reflux scores can potentially be used as a prognostic factor in BKVN patients.
Histologically, residual interstitial inflammation and intraepithelial lymphocyte infiltration in BKVN may persist for a prolonged period after viral clearance, mimicking the pattern observed in TCMR.12 In cases with the presence of endarteritis, fibrinoid vascular necrosis, peritubular capillaritis, or glomerulitis, in addition to C4d deposition within the peritubular capillaries, the coexistence of acute rejection should be considered.1 The correlation between tubular HLA-DR expression and interstitial inflammation prevents the histological distinction between BKVN and TCMR.12 Plasma cell infiltration in the allograft kidney is observed in other viral infections, drug-induced tubulointerstitial nephritis, IgG4-related kidney disease, plasma cell-rich rejection,29 and posttransplant lymphoproliferative disorders,30 in addition to BKVN. C4d granular deposition at tubular basement membranes in BKVN,31 negative peritubular capillary C4d staining, and a predominance of IgM-positive plasma cells in BKVN (IgG producing plasma cells typically predominate in rejection32) have also been reported. Although the diagnosis of TCMR remains challenging in BKVN in the context of tubulointerstitial nephritis, the distribution of BKVN differs from TCMR. Indeed, BKV is preferentially observed in the medulla or adjacent to the medullary ray area rather than the cortex. BK virus reactivation is thought to initiate in the lower urothelium (bladder or ureters) and the highly vascularized renal medulla, with later involvement of the rest of the kidney. In brief, BK virus infection spreads from the collecting duct and distal tubule to the neighborhood of the proximal tubule and glomerulus.33,34 The THP staining has demonstrated utility in detecting lesions because THP expression is normally limited in the TALs of long- and short-looped nephrons, according to their proximity to vascular bundles within the inner stripe of the outer medulla. In the present study, for the evaluation of reflux nephropathy or IRR by THP staining, we established a novel histological score based on the analysis of nontransplant kidney specimens from adult and pediatric VUR cases, in addition to transplant kidney biopsy samples. In the present study, the proven BKVN group had higher i, t, and ci Banff scores and reflux scores compared with the control group. However, no correlation was observed between the Banff and reflux scores in either group except for a significant correlation between the t scores and reflux scores in the proven BKVN group (Spearman ρ, 0.3437; P = 0.0180). The observed correlation between t scores and reflux scores was expected because t scores in BKVN are higher from the onset of disease.12 We also noted the different distribution of inflammation between BKVN and TCMR cases; in fact, most cases of TCMR had no sign of urinary reflux that was measured by our method, or the area of THP obstruction in tubuli or reflux into glomeruli was easily distinguished from the TCMR lesion; however, the i and t scores do not incorporate the disease etiology. Pathological examination of the VUR kidney (nephrectomy case) revealed the presence of striped inflammation and fibrosis predominantly in the medullary ray area (Figure 1). The term “interstitial fibrosis and tubular atrophy without any specific etiology” in the Banff classification includes various etiologies, such as CNI toxicity, chronic obstruction, and pyelonephritis35; however, the ci or ct scores do not incorporate the disease etiology and the differentiation of medullary ray fibrosis, required for appropriate management or nonspecific interstitial fibrosis and tubular atrophy, is known to be clinically challenging. Kobayashi et al17 established the concept of MRI with THP staining and demonstrated it to be strongly correlated with clinical background and prognosis and to have utility in determining the appropriate management approach to CNI toxicity, UTI, or VUCG for detecting VUR. Thus, we believe our developed reflux scoring system has utility in differentiating IRR from other inflammatory states and nonspecific tubulointerstitial fibrosis.
We also observed THP reflux in Bowman capsule with crescent-like formations and infection of the parietal glomerular epithelium, as previously reported36 in progressive BKVN patients, in addition to THP deposits in the tubulointerstitium which appear to derive from adjacent ruptured tubules.
The THP is extensively glycosylated, glypiated, and glycosylphosphatidylinisotol anchored at the apical plasma membrane37-39 and may contribute to various biological processes including receptor-mediated endocytosis,40,41 mechanosensation of urinary flow, Wnt signaling, cell cycle regulation, and planar cell polarity.42 The THP uses a similar mechanism to that involved in pattern recognition of pathogenic molecules, for example, transformation of immature professional antigen-presenting cells into cells with a mature phenotype by the isoreceptor of the Toll-like receptor 4.43 Moreover, after exposure of the tubulointerstitium to THP, these receptors induce costimulatory molecules (CD80 and CD86) and HLA-DR molecules and contribute to proinflammatory and autoimmune responses, such as the secretion of anti-THP antibodies into the sera.43 On the other hand, BKV uses N-linked glycoproteins to bind and enter host cells through caveolae-mediated endocytosis44 prior to BKV capsid rearrangement and nuclear entry.45 Expression of proinflammatory cytokines and chemokines is mediated by the activation of innate defense mechanisms, such as the Toll-like receptor 3 pathway.46
The presence of 3-dimensional THP-rich viral clusters, termed “Haufen,” has been recently shown to be a reliable marker of BKVN in viremic or persistently viruric patients26 and significantly correlates with histological BKVN grade.47 “Haufen” are believed to be produced by cytolysis and the release of daughter virions into injured nephrons rich in THP. The THP may perform both proinflammatory and antiviral roles; however, THP may have a paradoxical effect in promoting UTI in patients with urinary catheters through the coating of catheters and facilitation of uropathogenic bacteria binding to catheters,48 even if ureteral stenosis did not affect 10-year survival rates49 of the patient and graft. In fact, ureteric stent placement has been shown to be significantly causative for BK viremia in a number clinical studies,13-15 with speculation that the mechanical trauma associated with stent placement may injure the urothelium and allow BK viral invasion and replication through a mechanism similar to that observed in an animal model.50 A viral infection dynamic model in which replication starts in the kidney then reaches the urinary tract and is followed by bidirectional viral flux into both compartments was found to be the most compatible with clinical observations.51 Based on previous findings and those of the present study, we speculate that not only IRR but also THP backflow into the tubulointerstitium and Bowman capsule may subclinically promote the dispersion of BK virions into the allograft kidney.
The present study was limited by its retrospective nature, the lack of randomization, and the lack of multivariate analysis due to the small sample size. Moreover, the THP staining in the allograft kidney is not widely used and THP cast occlusion mainly in tubulus in medullary ray lesion and THP reflux into the Bowman capsule are not currently validated to be specific for IRR; it has reported mRNA expression of THP increased as part of the injury response of the nephron and THP was found in the specimen in other settings like acute kidney injury (AKI).52 On the other hand, cast formation in ischemia-reperfusion model of AKI was not only unaffected but was also markedly increased in THP knockout mice.53 These findings have led to suggestions that THP may be not essential for tubular cast formation in AKI even mRNA expression of THP increases in AKI.
In the kidney transplant setting, allograft biopsies are invasive but essential for diagnosis when allograft dysfunction or abnormal urinalysis occurs. Our aim is to associate pathological findings with appropriate management approaches in cases with allograft biopsies, and we have also clarified the pathological features of reflux nephropathy not only in transplant but also in nontransplant settings and believed the concept of medullary ray injury may compensate differentiation of pathology other than rejection.
In conclusion, our preliminary data showed IRR evaluated by THP immunostaining in the allograft kidneys might have utility in the histological evaluation of BKVN after kidney transplantation, indicating that urinary reflux might be a predisposing and prognostic factor of BKVN.
ACKNOWLEDGMENTS
The authors thank Shigeru Horita, Hideki Nakayama, and Mayuko Ohno, MT, Division of Pathology of Kidney Center, of Tokyo Women's Medical University for their technical supports. Satoru Shimizu, PhD, of the Medical Research Institute of Tokyo Women's Medical University, supported the statistical analysis in this study. The authors would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
This work was supported by JSPS KAKENHI grant 26460456.
The authors declare no conflicts of interest.
K.K., K.H., J.K., M.H., S.F., K.T., H.O., and Y.N. designed the research. K.K., K.H., J.K., and Y.N. the performed research. K.K., K.H., J.K., and Y.N. analyzed the data. K.K., K.H., and Y.N. wrote the article.
The name of the trial registry: The investigation for association between BK virus nephropathy and reflux nephropathy in a retrospective study.
Published online 15 January 2016.
REFERENCES
- 1. Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005; 79: 1277– 1286. [DOI] [PubMed] [Google Scholar]
- 2. Wadei HM, Rule AD, Lewin M, et al. Kidney transplant function and histological clearance of virus following diagnosis of polyomavirus-associated nephropathy (PVAN). Am J Transplant. 2006; 6: 1025– 1032. [DOI] [PubMed] [Google Scholar]
- 3. Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002; 13: 2145– 2151. [DOI] [PubMed] [Google Scholar]
- 4. Reploeg MD, Storch GA, Clifford DB. BK virus: A clinical review. Clin Infect Dis. 2001; 33: 191– 202. [DOI] [PubMed] [Google Scholar]
- 5. Gralla J, Huskey J, Wiseman AC. Trends in immune function assay (ImmuKnow; Cylex™) results in the first year post-transplant and relationship to BK virus infection. Nephrol Dial Transplant. 2012; 27: 2565– 2570. [DOI] [PubMed] [Google Scholar]
- 6. Bohl DL, Brennan DC. BK virus nephropathy and kidney transplantation. Clin J Am Soc Nephrol. 2007; 2: 36– 46. [DOI] [PubMed] [Google Scholar]
- 7. Buehrig CK, Lager DJ, Stegall MD, et al. Influence of surveillance renal allograft biopsy on diagnosis and prognosis of polyomavirus-associated nephropathy. Kidney Int. 2003; 64: 665– 673. [DOI] [PubMed] [Google Scholar]
- 8. Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005; 5: 582– 594. [DOI] [PubMed] [Google Scholar]
- 9. Drachenberg CB, Papadimitriou JC, Hirsch HH, et al. Histological patterns of polyomavirus nephropathy: correlation with graft outcome and viral load. Am J Transplant. 2004; 4: 2082– 2092. [DOI] [PubMed] [Google Scholar]
- 10. Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002; 347: 488– 496. [DOI] [PubMed] [Google Scholar]
- 11. Sar A, Worawichawong S, Benediktsson H, et al. Interobserver agreement for Polyomavirus nephropathy grading in renal allografts using the working proposal from the 10th Banff Conference on Allograft Pathology. Hum Pathol. 2011; 42: 2018– 2024. [DOI] [PubMed] [Google Scholar]
- 12. Menter T, Mayr M, Schaub S, et al. Pathology of resolving polyomavirus-associated nephropathy. Am J Transplant. 2013; 13: 1474– 1483. [DOI] [PubMed] [Google Scholar]
- 13. Thomas A, Dropulic LK, Rahman MH, et al. Ureteral stents: a novel risk factor for polyomavirus nephropathy. Transplantation. 2007; 84: 433– 436. [DOI] [PubMed] [Google Scholar]
- 14. Siparsky NF, Kushnir LF, Gallichio MH, et al. Ureteral stents: a risk factor for polyomavirus BK viremia in kidney transplant recipients undergoing protocol screening. Transplant Proc. 2011; 43: 2641– 2644. [DOI] [PubMed] [Google Scholar]
- 15. Hashim F, Rehman S, Gregg JA, et al. Ureteral stent placement increases the risk for developing BK viremia after kidney transplantation. J Transplant. 2014; 2014: 459747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. International Reflux Committee. Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics. 1981: 67: 392. [PubMed] [Google Scholar]
- 17. Kobayashi A, Yamamoto I, Ito S, et al. Medullary ray injury in renal allografts. Pathol Int. 2010; 60: 744– 749. [DOI] [PubMed] [Google Scholar]
- 18. Kunikata S, Ishii T, Nishioka T, et al. Clinicopathological study on end-stage reflux nephropathy in renal-transplanted children. Urol Int. 1990; 45: 70– 74. [DOI] [PubMed] [Google Scholar]
- 19. Shigematsu H, Murakami S. Intrarenal reflux and nephropathy. Nihon Jinzo Gakkai Shi. 1990; 32: 331– 337(in Japanese). [PubMed] [Google Scholar]
- 20. Andriole VT. The role of Tamm-Horsfall protein in the pathogenesis of reflux nephropathy and chronic pyelonephritis. Yale J Biol Med. 1985; 58: 91– 100. [PMC free article] [PubMed] [Google Scholar]
- 21. Akioka Y, Chikamoto H, Horita S, et al. Screening of vesicoureteral reflux in pediatric patients with kidney transplantation showing non-specific interstitial fibrosis and tubular atrophy with interstitial Tamm-Horsfall protein deposits in protocol allograft biopsy. Clin Transplant. 2009; 23: 2– 5. [DOI] [PubMed] [Google Scholar]
- 22. Kumar S, Muchmore A. Tamm-Horsfall protein-uromodulin (1950-1990). Kidney Int. 1990; 37: 1395– 1401. [DOI] [PubMed] [Google Scholar]
- 23. Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003; 42: 658– 676. [DOI] [PubMed] [Google Scholar]
- 24. Bates JM, Raffi HM, Prasadan K, et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004; 65: 791– 797. [DOI] [PubMed] [Google Scholar]
- 25. Mo L, Zhu XH, Huang HY, et al. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004; 286: 795– 802. [DOI] [PubMed] [Google Scholar]
- 26. Singh HK, Andreoni KA, Madden V, et al. Presence of urinary Haufen accurately predicts polyomavirus nephropathy. J Am Soc Nephrol. 2009; 20: 416– 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tamm I, Bugher JC, Horsfall FL., Jr Ultracentrifugation studies of a urinary mucoprotein which reacts with various viruses. J Biol Chem. 1955; 212: 125– 133. [PubMed] [Google Scholar]
- 28. Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014; 14: 272– 283. [DOI] [PubMed] [Google Scholar]
- 29. Desvaux D, Le Gouvello S, Pastural M, et al. Acute renal allograft rejections with major interstitial edema and plasma cell-rich infiltrates: high gamma-interferon expression and poor clinical outcome. Nephrol Dial Transplant. 2004; 19: 933– 939. [DOI] [PubMed] [Google Scholar]
- 30. Shroff R, Rees L. The post-transplant lymphoproliferative disorder—a literature review. Pediatr Nephrol. 2004; 19: 369– 377. [DOI] [PubMed] [Google Scholar]
- 31. Batal I, Zainah H, Stockhausen S, et al. The significance of renal C4d staining in patients with BK viruria, viremia, and nephropathy. Mod Pathol. 2009; 22: 1468– 1476. [DOI] [PubMed] [Google Scholar]
- 32. Kemény E, Hirsch HH, Eller J, et al. Plasma cell infiltrates in polyomavirus nephropathy. Transpl Int. 2010; 23: 397– 406. [DOI] [PubMed] [Google Scholar]
- 33. Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003; 3: 611– 623. [DOI] [PubMed] [Google Scholar]
- 34. Nickeleit V, Hirsch HH, Binet IF, et al. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol. 1999; 10: 1080– 1089. [DOI] [PubMed] [Google Scholar]
- 35. Solez K, Colvin RB, Racusen LC, et al. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant. 2007; 7: 518– 526. [DOI] [PubMed] [Google Scholar]
- 36. Celik B, Randhawa PS. Glomerular changes in BK virus nephropathy. Hum Pathol. 2004; 35: 367– 370. [DOI] [PubMed] [Google Scholar]
- 37. Rindler MJ, Naik SS, Li N, et al. Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J Biol Chem. 1990; 265: 20784– 20789. [PubMed] [Google Scholar]
- 38. Serafini-Cessi F, Malagolini N, Hoops TC, et al. Biosynthesis and oligosaccharide processing of human Tamm-Horsfall glycoprotein permanently expressed in HeLa cells. Biochem Biophys Res Commun. 1993; 194: 784– 790. [DOI] [PubMed] [Google Scholar]
- 39. Kreft B, Jabs WJ, Laskay T, et al. Polarized expression of Tamm-Horsfall protein by renal tubular epithelial cells activates human granulocytes. Infect Immun. 2002; 70: 2650– 2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sabharanjak S, Sharma P, Parton RG, et al. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002; 2: 411– 423. [DOI] [PubMed] [Google Scholar]
- 41. Kirkham M, Fujita A, Chadda R, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005; 168: 465– 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaucke F, Boehnlein JM, Steffens S, et al. Uromodulin is expressed in renal primary cilia and UMOD mutations result in decreased ciliary uromodulin expression. Hum Mol Genet. 2010; 19: 1985– 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Säemann MD, Weichhart T, Zeyda M, et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4–dependent mechanism. J Clin Invest. 2005; 115: 468– 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moriyama T, Marquez JP, Wakatsuki T, et al. Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J Virol. 2007; 81: 8552– 8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dugan AS, Eash S, Atwood WJ. Update on BK virus entry and intracellular trafficking. Transpl Infect Dis. 2006; 8: 62– 67. [DOI] [PubMed] [Google Scholar]
- 46. Ribeiro A, Wörnle M, Motamedi N, et al. Activation of innate immune defense mechanisms contributes to polyomavirus BK associated nephropathy. Kidney Int. 2012; 81: 100– 111. [DOI] [PubMed] [Google Scholar]
- 47. Singh HK, Reisner H, Derebail VK, et al. Polyomavirus nephropathy: quantitative urinary polyomavirus-Haufen testing accurately predicts the degree of intrarenal viral disease. Transplantation. 2015; 99: 609– 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Raffi HS, Bates JM, Flournoy DJ, et al. Tamm-Horsfall protein facilitates catheter associated urinary tract infection. BMC Res Notes. 2012; 26: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karam G, Hétet JF, Maillet F, et al. Late ureteral stenosis following renal transplantation: risk factors and impact on patient and graft survival. Am J Transplant. 2006; 6: 352– 356. [DOI] [PubMed] [Google Scholar]
- 50. Atencio IA, Shadan FF, Zhou XJ, et al. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol. 1993; 67: 1424– 1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Funk GA, Gosert R, Comoli P, et al. Polyomavirus BK replication dynamics in vivo and in silico to predict cytopathology and viral clearance in kidney transplants. Am J Transplant. 2008; 8: 2368– 2377. [DOI] [PubMed] [Google Scholar]
- 52. El-Achkar TM, McCracken R, Liu Y, et al. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol. 2013; 304: F1066– F1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. El-Achkar TM, Wu XR, Rauchman M, et al. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008; 295: 534– 544. [DOI] [PMC free article] [PubMed] [Google Scholar]


