Supplemental digital content is available in the text.
Background
Patients undergoing orthotopic liver transplantation are at high risk of bleeding complications. Several Authors have shown that thromboelastography (TEG)-based coagulation management and the administration of fibrinogen concentrate reduce the need for blood transfusion.
Methods
We conducted a single-center, retrospective cohort observational study (Modena Polyclinic, Italy) on 386 consecutive patients undergoing liver transplantation. We assessed the impact on resource consumption and patient survival after the introduction of a new TEG-based transfusion algorithm, requiring also the introduction of the fibrinogen functional thromboelastography test and a maximum amplitude of functional fibrinogen thromboelastography transfusion cutoff (7 mm) to direct in administering fibrinogen (2012-2014, n = 118) compared with a purely TEG-based algorithm previously used (2005-2011, n = 268).
Results
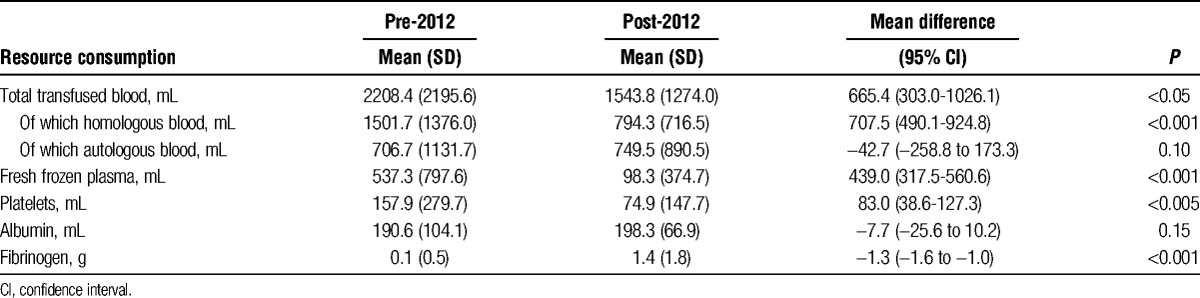
After 2012, there was a significant decrease in the use of homologous blood (1502 ± 1376 vs 794 ± 717 mL, P < 0.001), fresh frozen plasma (537 ± 798 vs 98 ± 375 mL, P < 0.001), and platelets (158 ± 280 vs 75 ± 148 mL, P < 0.005), whereas the use of fibrinogen increased (0.1 ± 0.5 vs 1.4 ± 1.8 g, P < 0.001). There were no significant differences in 30-day and 6-month survival between the 2 groups.
Conclusions
The implementation of a new coagulation management method featuring the addition of the fibrinogen functional thromboelastography test to the TEG test according to an algorithm which provides for the administration of fibrinogen has helped in reducing the need for transfusion in patients undergoing liver transplantation with no impact on their survival.
Patients undergoing orthotopic liver transplantation (OLT) are at high risk of bleeding, mainly depending on a precarious balance between procoagulant and anticoagulant factors.1 Therefore, these patients very often require transfusions of blood and blood components aimed at correcting anemia and coagulopathy. However, the transfusion of fresh frozen plasma (FFP), red blood cells, and platelets were associated with increased morbidity and mortality2 and decreased graft survival.3
Newly introduced viscoelastic coagulation tests performed bedside (point-of-care [POC]), including rotational thromboelastometry (ROTEM) and thromboelastography (TEG), produce faster and more reliable results than conventional tests. The use of transfusion algorithms based on those tests can reduce perioperative bleeding and the rate of transfusion of allogeneic blood products4-6 to such an extent that current European Society of Anesthesia7 guidelines for the management of massive bleeding recommend their use during OLT to monitor hemostasis and for coagulopathy management. Solely using the TEG method has the drawback of not being able to distinguish between the contribution of fibrinogen and that of platelets to coagulum formation.8 To address this problem, a new fibrinogen functional thromboelastography (FF-TEG) test has been recently developed; this method, when performed in addition to conventional TEG, can detect the degree to which fibrinogen contributes to coagulum strength and estimate plasma fibrinogen levels. It is a test based on the activation of the extrinsic pathway of coagulation using tissue factor. Then, platelet contribution to coagulum is inhibited through the use of a platelet inhibitor that binds to GPII/IIIa receptors. Therefore, only fibrinogen contribution to clot strength is measured. It was found that fibrinogen levels measured with this method correlate with those obtained by applying the traditional von Clauss test and that they are critical in obtaining good coagulum strength.9
In recent years, further aid in managing bleeding during OLT has come from the use of coagulation factor concentrates, which feature some advantages, such as easy storage and immediate availability for use. Their administration, often based on algorithms that involve the use of POC methods, is associated with a decrease in the need for transfusion of allogeneic blood products and with better outcomes.10-14 Among the single coagulation factors available on the market, it has been shown that the use of fibrinogen concentrate according to a transfusion algorithm based on ROTEM results in managing coagulopathy during OLT can reduce the use of allogeneic blood products.15 Another study found that the use of coagulation factor concentrates in these patients does not increase the incidence of thrombosis and ischemic events.16
At the moment, in POC-guided management of patients undergoing OLT, no unique and shared cutoffs have yet been established for the TEG parameter values to make the most appropriate choices in coagulopathy management.1,9 Indeed, the strategies adopted for coagulopathy management vary greatly depending on the centers.
In 2012, we introduced in our center, Azienda Ospedaliero-Universitaria of Modena, Policlinico (Modena, Italy), a new transfusion algorithm, different from the previous TEG-based one: the changes consisted in the addition of the FF-TEG test to guide the administration of fibrinogen concentrate and a specific transfusion cutoff to our own TEG-based algorithm. Then, we noted an apparent decrease in transfusion. Therefore, the present study was conducted to check this impression.
We undertook this cohort single-center (Modena Polyclinic, Italy) retrospective observational study on 386 consecutive patients undergoing OLT. We assessed the impact on resource consumption of the use of this new coagulation monitoring (patients undergoing surgery between January 2012 and December 2014) compared with a previous TEG-based algorithm (patients undergoing surgery between December 2005 and December 2011).
MATERIALS AND METHODS
This study received the approval of the local ethics authority (Ethics Committee of the Province of Modena, case 139/14, approved on October 29, 2014).
Demographics
This study retrospectively includes 386 consecutive patients undergoing OLT at the Modena Polyclinic, Italy, during the period between December 2005 and December 2014. We included both those who underwent a first transplant, of liver alone or of liver and kidney, and those who experienced a retransplantation. All data were extracted from medical records. The study focused on transfused resource consumption during surgery (homologous blood, autologous blood, FFP, platelets, albumin, and fibrinogen were considered) and the outcome of the surgery in terms of patient survival.
Collection of Blood Samples
Native arterial blood samples were collected from a radial artery cannulated before induction of anesthesia and analyzed without addition of anticoagulants. However, heparinase was used only after reperfusion in all cases and from the baseline only in patients affected by acute liver failure.
Monitoring by TEG and Transfusion Algorithms
The TEG tracks were initiated within 4 minutes of drawing the blood (the machine is located in the operating room, near the patient). The tests were carried out on native blood, both before and after 2012. In the TEG test, coagulum formation was triggered through activation by contact, without the addition of activators. The TEG tests were performed at preset times: baseline (when the surgery patient first entered the operating room), laparotomy, preanhepathic, anhepatic, and 30, 60, 120, 180 minutes after reperfusion. Additional TEG tests were performed at the discretion of the anesthesiologist based on clinical conditions and surgical scope, generally when a therapeutic intervention was required due to bleeding severity to better guide and verify the administered therapy. The TEG was performed by pipetting 360 μL of blood from a 3-mL arterial sample placed in a test tube in the thromboelastograph's coagulation analyzer, and the temperature was set according to the patient's temperature.
The FF-TEG was performed according to the manufacturer's instructions, which require the addition of a platelet glycoprotein IIb/IIIa receptor inhibitor to exclude the contribution of platelets to coagulum strength.
The significance of the parameters considered referring to native blood is shown in Figure 1.
FIGURE 1.
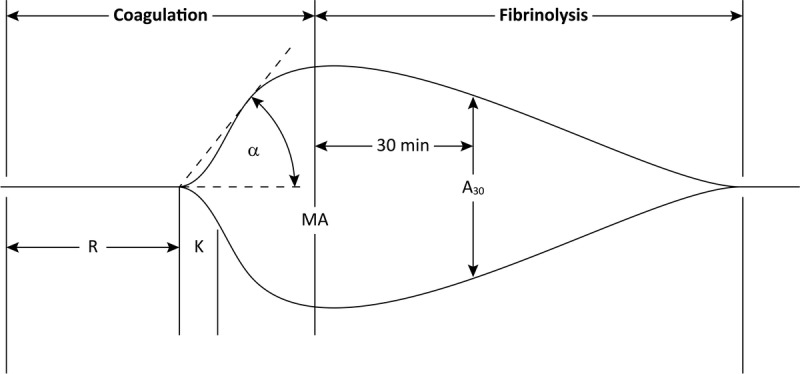
Normal TEG tracing.1,17 α angle, reflecting the rate of clot formation (normal values = 14-46°); A30, amplitude at 30 minutes (normal values = 0-5%); K, time from R until a certain clot firmness is achieved (normal values = 3-13 min); MA, maximum amplitude, reflects the strength of the coagulum (normal values = 42-63 mm); R, reaction time, represents the speed of the initial formation of fibrin (normal values = 12-26 min).
The management of coagulopathy during surgery was led by thromboelastography (TEG 5000 Hemostasis Analyzers, Haemoscope Inc.) and used by the pre-2012 transfusion algorithm shown in Figure 2 for surgeries performed before 2012 (n = 268); from 2012 onward (n = 118), we adopted hemostatic therapies guided by TEG test performed in the same way but adding the FF-TEG test with a different algorithm, based on the use of fibrinogen concentrate (Haemocomplettan, CSL Behring) for values of maximum amplitude TEG less than 30 mm and maximum amplitude of functional fibrinogen thromboelastography (MAFF) of 7 mm or less (Figure 3). The algorithm used from 2005 to 2012 was derived from Kang's one, dating back to 1985.6 Compared with this period, improvements in surgical techniques and anesthetic management led to a revision of Kang's algorithm, which brought to the definition of the algorithm commonly used before 2012. As regard the version proposed after that date, we derived the values suggested as cutoff for transfusion on the base of our experience with FF-TEG test.
FIGURE 2.
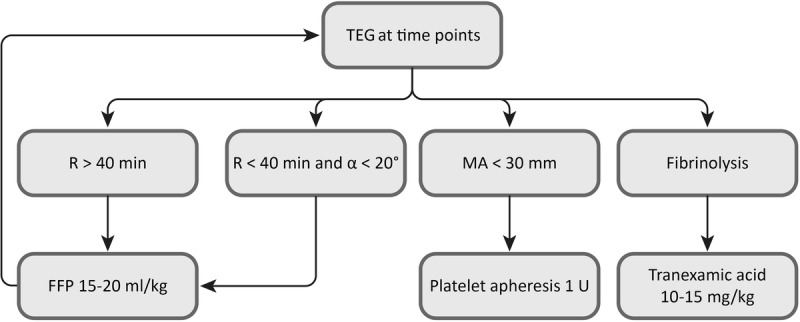
Transfusion algorithm used before 2012. FFP, fresh frozen plasma; MA, maximum amplitude; R, reaction time; TEG, thromboelastography.
FIGURE 3.
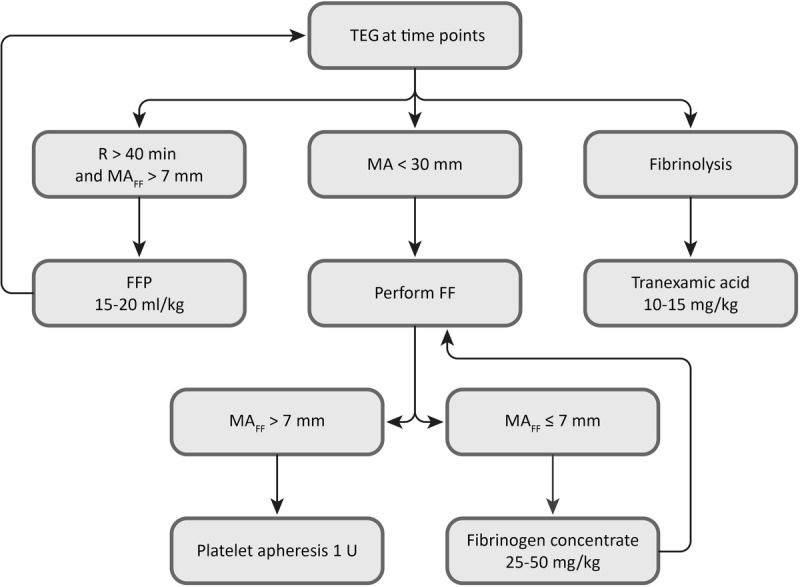
Transfusion algorithm used since 2012. FF, functional fibrinogen test TEG; FFP, fresh frozen plasma; MA, maximum amplitude; MAFF, MA obtained from functional fibrinogen test, raw data; R, reaction time; TEG, thromboelastography.
We transfused erythrocyte concentrates to maintain hemoglobin levels at 8 to 9 g/dL. This policy was consistent throughout the study period. Albumin was administered in standard volumes for all transplantations (about 200 mL) or in larger volumes if the patient experienced heavy blood loss or massive ascites.
A cell saver device was routinely used throughout the procedure, and whenever possible, cell salvage blood was transfused in place of homologous blood (we never needed to use it in recipients with malignant hepatic lesion).
Resources Consumed
Evaluation of the resources consumed was performed on: homologous blood, autologous blood, FFP, platelets, albumin, fibrinogen concentrate (Haemocomplettan, CSL Behring).
Surgical Procedure and Anesthetic Technique
All surgical procedures were performed using the piggy-back technique for graft implantation.
Patients were monitored with a 5-lead electrocardiogram, pulse oximetry, invasive systemic blood pressure (right radial) and a pulmonary Swan-Ganz continuous end diastolic volume catheter (usually advanced through a 8.5 F introducer into the right internal jugular vein for continuous CO measurement thermodilution technique), right ventricle ejection fraction, right ventricle end diastolic volume, and continuous monitoring of SvO2 and core temperature. Several times a transesophageal echocardiography was performed during liver transplantation.
General anesthesia was induced with propofol (1.5-2 mg/kg), fentanyl (1-2 μg/kg), and rocuronium (0.6 mg/kg) and maintained with desfluorane (5-6%)/O2/air in a closed circuit. After induction, a 20-G catheter (for blood sampling) was inserted in the left radial artery and an 8Fr double-lumen central venous catheter (usually) into the left internal jugular vein. Intraoperative hypothermia was prevented by forced air surface warming (Bair Hugger) and by warm fluids (Level 1 H-1025 and HOTLINE, Smiths Medical), aiming to keep body temperature above 35°C. Arterial blood gases were checked whenever we drew blood samples for TEG or when otherwise suggested. The free calcium level was kept higher than 1.0 mmol/L. During the study period, we did not make any changes in the anesthetic technique aside from transfusion algorithm.
The anesthesia team (3 anesthetists) and surgical team (2 main surgeons plus surgical fellows) did not change during the study period; every transplant procedure was performed by 2 surgeons plus 1 junior house officer and by 1 anesthesiologist plus 1 junior house officer.
Statistical Analyses
Category variables (indications for surgeries and type of surgery) were compared using the χ2 test or the Fisher exact test, whereas continuous variables were compared using the Mann-Whitney test. Factors associated with the consumption of resources during surgery were analyzed using univariate and multivariate linear regressions. Variables with P value less than 0.1 in the univariate analysis were included in the multivariate model from which we extracted only statistically significant values (P value < 0.05) using the stepwise selection technique (backward). Survival time after surgery was analyzed in both groups using the Kaplan-Meier curves and compared using the log-rank test. Results with P value less than 0.05 were considered statistically significant. All analyses were performed using statistical software R version 3.0.2.18
RESULTS
Patients were divided into three categories of surgery: liver transplantation, liver and kidney transplantation, and liver retransplantation. Overall, 13 of 386 surgeries were excluded from analysis due to missing data. Of the 373 valid data, 256 are for surgeries before 2012 and 117 for those performed later. We identified 7 categories of surgeries for liver disease (Table 1). The baseline characteristics of the 2 populations are also summarized in Table 1.
TABLE 1.
Baseline characteristics of the study population
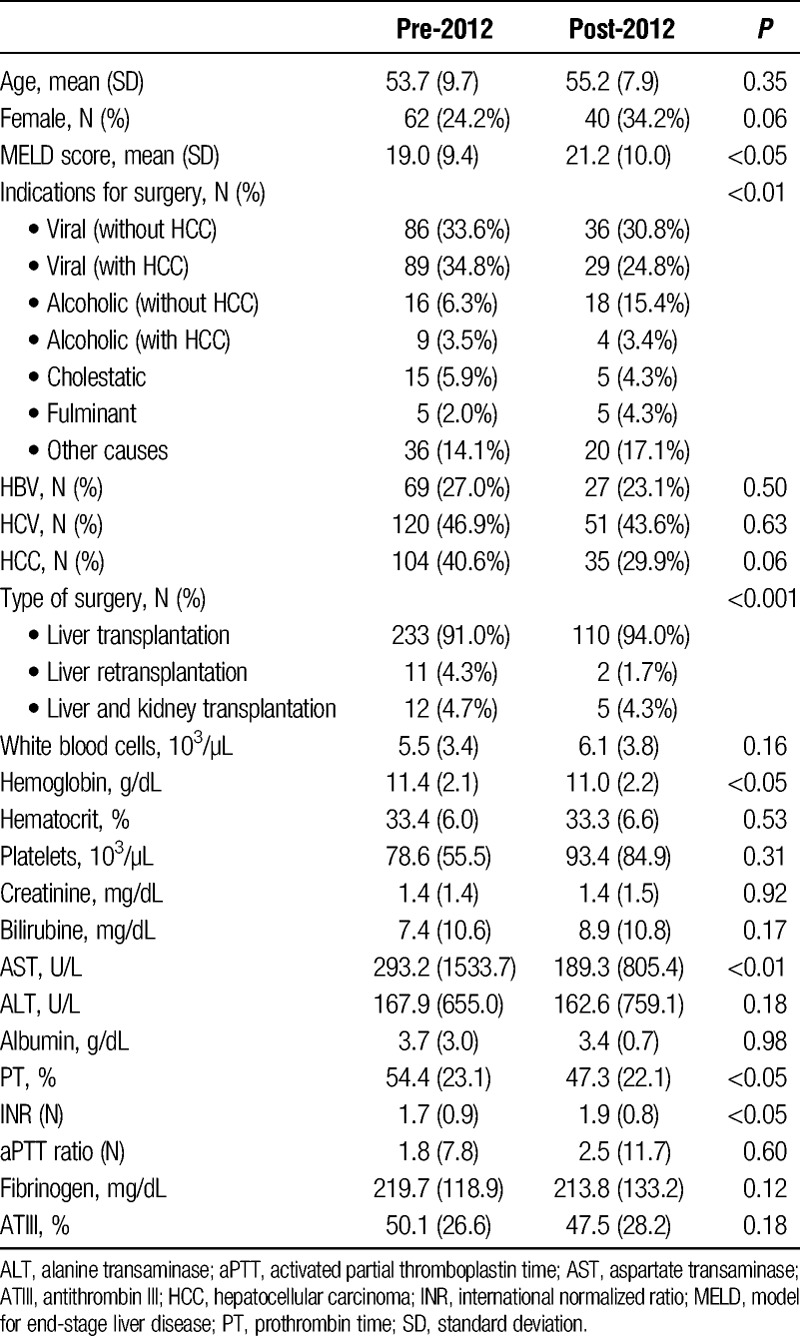
There were no statistically significant differences in terms of mean age and sex, whereas the severity level was different between the 2 groups (model for end-stage liver disease [MELD] score: 19.0 ± 9.4 before 2012 vs 21.2 ± 10.0 after 2012, P < 0.05). The number of retransplantations was higher in surgeries carried out before 2012 (4.3% vs 1.7%, P < 0.001); furthermore, we detected differences in the indications for surgery, more specifically the percentage of surgeries for cirrhosis without hepatocellular carcinoma more than doubled after 2012 (6.3% vs 15.4%, P < 0.01).
The 2 groups were also similar in most of the preoperative test results (Table 1).
We found statistically significant differences in preoperative coagulation values (prothrombin time [PT] was 54 ± 23 vs 47 ± 22, P < 0.05 and international normalized ratio [INR] was 1.7 ± 0.9 vs 1.9 ± 0.8, P < 0.05) and in hemoglobin (11.4 ± 2.1 vs 11.0 ± 2.2 g/dL, P < 0.05) and aspartate transaminase (293 ± 1534 vs 189 ± 805 U/l, P < 0.01).
Statistical analysis showed in surgeries performed after 2012, a significant decrease in the use of total blood (2208 ± 2196 vs 1544 ± 1274 mL, P < 0.05) due to the decrease in the use of homologous blood (1502 ± 1376 vs 794 ± 717 mL, P < 0.001), FFP (537 ± 798 vs 98 ± 375 mL, P < 0.001), and platelets (158 ± 280 vs 75 ± 148 mL, P < 0.005), whereas, in accordance with the newly adopted transfusion protocol, the use of fibrinogen increased (0.1 ± 0.5 vs 1.4 ± 1.8 g, P < 0.001), no significant differences were experienced in the use of autologous blood and albumin (Table 2).
TABLE 2.
Consumption of resources per patient: comparison between the surgeries carried out before and after 2012
Analysis performed per severity subgroup (MELD score ≤ or > 21) showed that this trend can be found in equal measure in sicker patients and those in less serious conditions (Tables S1 and S2, SDC, http://links.lww.com/TXD/A19).
The results of resource consumptions related to liver transplantations alone are perfectly in line with the overall analysis, because the impact of interventions other than the liver transplantations alone is imperceptible because of the small number of interventions (23 of 256 before 2012 and 7 of 117 after 2012) (Table S3, SDC, http://links.lww.com/TXD/A19).
We performed univariate and multivariate linear regression analyses for the consumption of homologous and autologous blood, FFP, platelets, albumin, and fibrinogen (detailed results in Tables S4, S5, S6, S7, S8, and S9, SDC, http://links.lww.com/TXD/A19). In these tables, variables resulting to be statistically significant in the univariate model were highlighted in yellow, whereas those that remained significant also after the multivariate analysis were highlighted in green. The MELD score of the patient undergoing surgery was statistically associated with the consumption of all resources analyzed. Female patients received more transfusions of homologous blood and FFP (in both models), with autologous blood and platelets (only in the univariate model). Lower consumption of FFP was noted in repeated transplants compared with patients who underwent OLT for the first time (in compliance with the mode of execution of the surgical procedure) whereas in liver and kidney transplants, homologous blood consumption was lower and autologous blood consumption was increased. In the univariate model, the use of autologous blood significantly decreases with the increasing age of the patient, but this loses significance when considered together with the other covariates. Figure 4 shows the average consumption of each resource used during surgery from 2006 to 2014 (2005 is not shown because the available data, relating to 2 patients, did not represent a significant sample).
FIGURE 4.
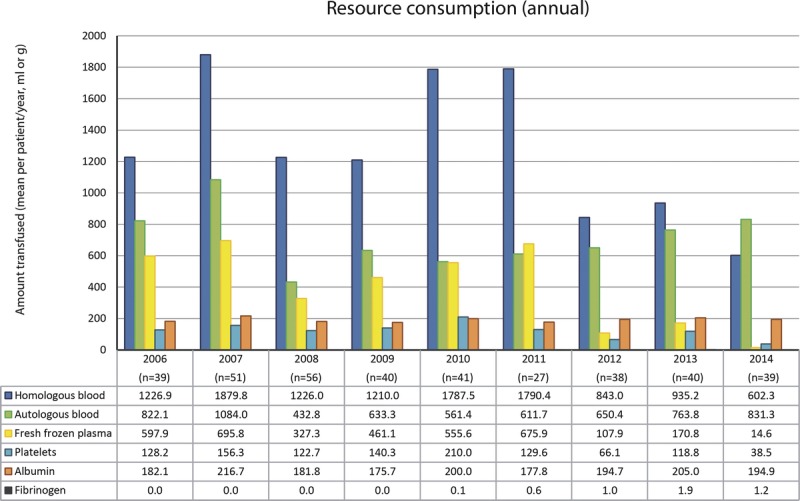
Annual consumption of resources expressed as mean consumption by patient (the 2005 data are not represented as they reflect only 2 patients and therefore do not constitute a significant sample).
Supplemental data show, for each resource consumed, all possible comparisons between the various years reviewed (Figure S1).
The mean 30-day postsurgery mortality was 2.73% for surgeries performed before 2012 and 2.56% for those performed after 2012. Six-month postsurgery mortality was considerably higher than 30-day postsurgery mortality (10.16% vs 12.82%). In both cases, the results are not statistically different (Table S10, SDC, http://links.lww.com/TXD/A19). Survival analysis using the Kaplan-Meier curves leads to the same conclusions (Figure 5). There were no significant differences in 30-day (97.5% vs 97.3%, P = 0.923) or 6-month (90.5% vs 87.6%, P = 0.405) survival.
FIGURE 5.
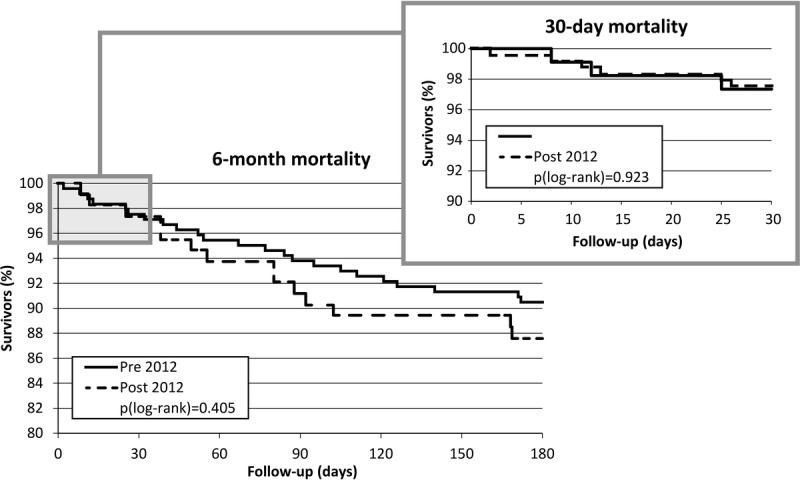
Kaplan-Meier curves for 30-day and 6-month postsurgery survival.
A series of univariate logistic regressions has shown that the only factor statistically associated with mortality is patients' MELD score (Tables S11 and S12, SDC, http://links.lww.com/TXD/A19). As the MELD score increases, 30-day mortality (odds ratio, 1.071; 95% confidence interval, 1.01-1.13; P < 0.05) and 6-month mortality (odds ratio, 1.064; 95% confidence interval, 1.03-1.10; P < 0.0005) risk also increases.
DISCUSSION
For patients undergoing OLT in our center, introducing the FF-TEG test in directing the administration of fibrinogen concentrate within the TEG-based algorithm was proven useful in reducing the consumption of transfusion resources compared with the use of a TEG-based algorithm which does not involve the use of FF-TEG and fibrinogen concentrate. More specifically, we experienced a significant decrease in transfusion of homologous blood, FFP, and platelets. For obvious reasons, the consumption of fibrinogen concentrate increased. Reduced consumption of allogeneic blood resources may have very positive implications, because their use is generally associated with increased morbidity, mortality,2 and decreased survival of the transplant3 and also potentially costs.
Another significant result that surfaces from this study is the direct proportionality between the severity of liver disease (measured by MELD score) and the need for transfusion. This argument is a matter of debate because, although many studies have corroborated the severity of liver disease as a risk factor for transfusions during OLT,1,19-21 other researches, such as those by Massicotte et al and Cywinski et al,2,22 have not confirmed this link.
The use of TEG, as noted in a Cochrane review dated 201123 that analyzed 33 trials, reduces the need for transfusion in the OLT setting. On the other hand, the use of fibrinogen concentrate can also reduce perioperative bleeding and transfusions.1 In fact, in this study, the combined use of FF-TEG and fibrinogen concentrate reflected a decreased need for transfusion compared with the sole use of traditional TEG. The use of coagulation factor concentrates is sometimes criticized because of their cost. In a retrospective study by Görlinger et al,24 the implementation of a new ROTEM-based transfusion algorithm in patients undergoing visceral surgery and transplants had caused increased consumption of fibrinogen concentrate, however the decreased use of blood products (FFP, red blood cells, and platelets) had nonetheless delivered significant overall cost savings. Another study by the same group16 in patients undergoing OLT had calculated the use of fibrinogen concentrate at 3.9 g per patient. In our algorithm, however, the introduction of fibrinogen concentrate as directed by a specific test (FF-TEG) enabled us to reduce the need for blood transfusion, despite the administration of smaller quantities of fibrinogen concentrate (1.4 g per patient).
These data were robust, as confirmed by the analysis performed excluding retransplantations and transplantations of liver and kidney, which confirmed the same findings.
Lastly, in regards to surgery, during the period covered by the study, there were no changes in surgical techniques or in the makeup of the team involved. Similarly, the anesthesia care team did not undergo any changes during the study period and showed proven expertise in POC-guided coagulopathy management. However, whereas the operating teams did not undergo any changes during the study period, it is possible that the surgeons became more experienced with time, and consequently lost less blood.
Anyhow, this study found no difference in 30-day and 6-month survival among patients undergoing surgery before and after 2012. One possible explanation for this result may lie in the higher severity which characterizes the population transplanted since 2012, as indicated by the higher MELD value. With regard to survival, other studies of subjects undergoing OLT that had compared the POC methods with traditional coagulation tests, among which greater differences were expected than those between TEG and TEG + FF-TEG + the use of fibrinogen concentrate, were unable to highlight significant differences in patient survival.25,26 Finally, it is possible that we failed to show an outcome difference due to a lack of statistical power.
To our knowledge, this is the first study in the OLT setting which compares coagulopathy management by TEG with the management of coagulopathy by also adding FF-TEG and fibrinogen concentrate (conversely, there are studies which have carried out such assessments by corresponding ROTEM tests),15 and more specifically which tests how adding the FF test together with defining a MAFF transfusion cutoff for the administration of fibrinogen concentrate manages to reduce consumption of blood components. However, both in the setting of liver transplantation and in other therapeutic settings, there have been some studies which have compared transfusion strategies based on viscoelastic testing with those based on classic coagulation tests, generally finding transfusion reduction.15,24-27
Concerning how the thromboelastographic tests were conducted, we chose to use native blood. In fact, TEG tests, just like the ROTEM tests, are designed to be performed at the patient's bedside and relatively quickly (ideally within 4-6 minutes of blood sampling). Only when this is not possible, one can resort to the use of citrate and recalcification, making it possible to postpone the analysis. However, the use of native blood helps avoiding the risk of alterations in test results due to the addition of citrate and calcium, specifically in parameters r, k and α-angle, which have been observed in samples from healthy subjects in some studies.28,29 Heparinase has been used, but only on blood obtained during the reperfusion phase, in which the neutralization of heparin and heparinoids is most needed due to the heparin-like effect.1
This study introduces some new elements: first, it proposes a new management method for coagulopathy during OLT, pairing FF-TEG with TEG and fibrinogen concentrate administration. Second, it proposes a 7-mm cutoff for the administration of platelets or fibrinogen concentrate based on MAFF raw data of and not on the mg/dL fibrinogen concentration value that the test itself can provide: in fact, the latter has been assumed to overestimate actual concentrations of fibrinogen in plasma.8 That value was determined by the authors based on their experience. As noted in a 2014 study by Yang Lu,8 which for the first time applied FF-TEG to patients undergoing OLT, calculated plasma fibrinogen concentrations are reliable at baseline but tend to be overestimated after reperfusion of the transplant, when the fibrinogen generally falls to values below 100 mg/dL. Overestimation of fibrinogen levels was also found in a 2014 study by Ågren,30 in which the maximum amplitude values considered were in g/L. Those same authors suggested that the MAFF raw data expressed in mm, those used by the authors of this study, may instead be more reliable. To our knowledge, this is the first study in the OLT setting to propose a transfusion cutoff based on the FF-TEG test, as Fibtem value by the ROTEM method is most commonly used in directing the administration of fibrinogen concentrate.16 Lastly, we studied the usefulness of fibrinogen concentrate in combination with monitoring performed by FF-TEG in the management of coagulopathy of OLT.
This study has limitations: first, it is a retrospective study and was conducted in a single center; therefore its results must certainly be confirmed by randomized controlled trials. Second, the 2 cohorts compared showed significant differences at baseline before and after the implementation of new coagulation monitoring methods. Differences were found both in indications for surgery, more specifically the percentage of surgeries for cirrhosis without hepatocellular carcinoma more than doubles after 2012 (6.3% vs 15.4%, P < 0.01), and in types of surgeries, more specifically the number of retransplantations was higher in surgeries performed before 2012 (4.3% vs 1.7%, P < 0.001). These differences may be caused by a longer study period in patients undergoing surgery before 2012 (106 months) than those operated after 2012 (35 months). Moreover, our 2 patient populations differed significantly at baseline by liver disease severity according to the MELD score (average of 19.0 for pre-2012 vs 21.2 for post-2012; P < 0.05). The greater severity of the post-2012 patients should have been related to a greater amount of blood components transfused. However, this amount is significantly lower than in pre-2012 patients, further corroborating the effectiveness of the newly proposed transfusion management method. Likewise, PT and INR also showed significant differences at baseline (54 ± 23 vs 47 ± 22, P < 0.05 for PT and 1.7 ± 0.9 vs 1.9 ± 0.8, P < 0.05 for INR), describing worse physical conditions for those patients transplanted after 2012. As one of the ways used to express PT, INR is effectively one of the parameters used to calculate the MELD score. Another difference at baseline between the 2 patient populations involves plasma hemoglobin concentrations, which are significantly lower in the post-2012 group (average: 11.4 g/dL for pre-2012 vs 11.0 g/dL for post-2012; P < 0.05). Plasma hemoglobin concentrations have been widely proven to correlate with the need for transfusion during OLT.1,2,19-21,31-33 Therefore, once again, we should expect a greater need for transfusions in the post-2012 group, which instead received fewer transfusions, confirming the validity of this newly proposed coagulation management method during OLT.
In conclusion, since 2012, the introduction of a new TEG algorithm for the management of coagulopathies during OLT, which includes using the FF-TEG and a specific transfusion cutoff (MAFF ≤ 7 mm) to direct in the use of fibrinogen concentrate, has delivered considerable savings in homologous blood, FFP, and platelets compared with surgeries performed before 2012 (statistically significant results, P <0.01) that had used traditional TEG coagulation monitoring not involving the use of the FF-TEG or of fibrinogen concentrate. This trend is similar both in less severe patients (MELD score ≤ 21) and in those with severe conditions (MELD score > 21); additionally, in the latter, we found a greater average consumption of all the resources in both analysis groups. The influence of patient MELD score on resource consumption is also confirmed by linear regression analysis results.
Conversely, no significant differences were noted in surgery outcomes: both 30-day and 6-month mortalities were not statistically different in the 2 groups, although related to the MELD score of the patient.
Further studies are needed to verify the effectiveness of the newly proposed algorithm and the use of the FF-TEG test in patients undergoing OLT.
Supplementary Material
Footnotes
Published online 15 December 2015.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
The writing of this article was funded by CSL Behring. Publishing support was provided by SEEd Medical Publishers. Statistical analyses were performed by AdRes Health Economics & Outcomes Research.
L.D.P. participated in research design, in the writing of the paper and in the performance of the research. F.R. participated in research design and in the performance of the research. A.D. participated in research design and in the performance of the research. B.B. participated in research design and in the performance of the research. V.S. participated in research design and in the performance of the research.
REFERENCES
- 1. Clevenger B, Mallett SV. Transfusion and coagulation management in liver transplantation. World J Gastroenterol. 2014; 20: 6146– 6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Massicotte L, Denault AY, Beaulieu D, et al. Transfusion rate for 500 consecutive liver transplantations: experience of one liver transplantation center. Transplantation. 2012; 93: 1276– 1281. [DOI] [PubMed] [Google Scholar]
- 3. de Boer MT, Christensen MC, Asmussen M, et al. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg. 2008; 106: 32– 44. [DOI] [PubMed] [Google Scholar]
- 4. McNicol PL, Liu G, Harley ID, et al. Blood loss and transfusion requirements in liver transplantation: experience with the first 75 cases. Anaesth Intensive Care. 1994; 22: 666– 671. [DOI] [PubMed] [Google Scholar]
- 5. Perry DJ, Fitzmaurice DA, Kitchen S, et al. Point-of-care testing in haemostasis. Br J Haematol. 2010; 150: 501– 514. [DOI] [PubMed] [Google Scholar]
- 6. Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985; 64: 888– 896. [PMC free article] [PubMed] [Google Scholar]
- 7. Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013; 30: 270– 382. [DOI] [PubMed] [Google Scholar]
- 8. Yang Lu S, Tanaka KA, Abuelkasem E, et al. Clinical applicability of rapid thrombelastography and functional fibrinogen thrombelastography to adult liver transplantation. Liver Transpl. 2014; 20: 1097– 1105. [DOI] [PubMed] [Google Scholar]
- 9. Harr JN, Moore EE, Ghasabyan A, et al. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013; 39: 45– 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahe-Meyer N, Pichlmaier M, Haverich A, et al. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009; 102: 785– 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rahe-Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009; 138: 694– 702. [DOI] [PubMed] [Google Scholar]
- 12. Schöchl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011; 15: R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weber CF, Görlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012; 117: 531– 547. [DOI] [PubMed] [Google Scholar]
- 14. Görlinger K, Dirkmann D, Hanke AA, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011; 115: 1179– 1191. [DOI] [PubMed] [Google Scholar]
- 15. Noval-Padillo JA, León-Justel A, Mellado-Miras P, et al. Introduction of fibrinogen in the treatment of hemostatic disorders during orthotopic liver transplantation: implications in the use of allogenic blood. Transplant Proc. 2010; 42: 2973– 2974. [DOI] [PubMed] [Google Scholar]
- 16. Kirchner C, Dirkmann D, Treckmann JW, et al. Coagulation management with factor concentrates in liver transplantation: a single-center experience. Transfusion. 2014; 54: 2760– 2768. [DOI] [PubMed] [Google Scholar]
- 17. Carroll RC, Craft RM, Chavez JJ, et al. Measurement of functional fibrinogen levels using the thrombelastograph. J Clin Anesth. 2008; 20: 186– 190. [DOI] [PubMed] [Google Scholar]
- 18. R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
- 19. Sabate A, Dalmau A, Koo M, et al. Coagulopathy management in liver transplantation. Transplant Proc. 2012; 44: 1523– 1525. [DOI] [PubMed] [Google Scholar]
- 20. Mangus RS, Kinsella SB, Nobari MM, et al. Predictors of blood product use in orthotopic liver transplantation using the piggyback hepatectomy technique. Transplant Proc. 2007; 39: 3207– 3213. [DOI] [PubMed] [Google Scholar]
- 21. Makroo RN, Walia RS, Aneja S, et al. Preoperative predictors of blood component transfusion in living donor liver transplantation. Asian J Transfus Sci. 2013; 7: 140– 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cywinski JB, Alster JM, Miller C, et al. Prediction of intraoperative transfusion requirements during orthotopic liver transplantation and the influence on postoperative patient survival. Anesth Analg. 2014; 118: 428– 437. [DOI] [PubMed] [Google Scholar]
- 23. Gurusamy KS, Pissanou T, Pikhart H, et al. Methods to decrease blood loss and transfusion requirements for liver transplantation. Cochrane Database Syst Rev. 2011; CD009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Görlinger K, Fries D, Dirkmann D, et al. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother. 2012; 39: 104– 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Álamo JM, León A, Mellado P, et al. Is “intra-operating room” thromboelastometry useful in liver transplantation? A case-control study in 303 patients. Transplant Proc. 2013; 45: 3637– 3639. [DOI] [PubMed] [Google Scholar]
- 26. Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010; 42: 2590– 2593. [DOI] [PubMed] [Google Scholar]
- 27. Shore-Lesserson L, Manspeizer HE, DePerio M, et al. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999; 88: 312– 319. [DOI] [PubMed] [Google Scholar]
- 28. Zambruni A, Thalheimer U, Leandro G, et al. Thromboelastography with citrated blood: comparability with native blood, stability of citrate storage and effect of repeated sampling. Blood Coagul Fibrinolysis. 2004; 15: 103– 107. [DOI] [PubMed] [Google Scholar]
- 29. Roche AM, James MF, Grocott MP, et al. Citrated blood does not reliably reflect fresh whole blood coagulability in trials of in vitro hemodilution. Anesth Analg. 2003; 96: 58– 61. [DOI] [PubMed] [Google Scholar]
- 30. Ågren A, Wikman AT, Ostlund A, et al. TEG® functional fibrinogen analysis may overestimate fibrinogen levels. Anesth Analg. 2014; 118: 933– 935. [DOI] [PubMed] [Google Scholar]
- 31. Steib A, Freys G, Lehmann C, et al. Intraoperative blood losses and transfusion requirements during adult liver transplantation remain difficult to predict. Can J Anaesth. 2001; 48: 1075– 1079. [DOI] [PubMed] [Google Scholar]
- 32. Araújo T, Cordeiro A, Proença P, et al. Predictive variables affecting transfusion requirements in orthotopic liver transplantation. Transplant Proc. 2010; 42: 1758– 1759. [DOI] [PubMed] [Google Scholar]
- 33. McCluskey SA, Karkouti K, Wijeysundera DN, et al. Derivation of a risk index for the prediction of massive blood transfusion in liver transplantation. Liver Transpl. 2006; 12: 1584– 1593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


