Background
Efficient islet isolation requires synergistic interaction between collagenase class I (CI) and class II (CII). The CI degradation alters the ratio between CI and CII and is responsible for batch-to-batch variations. This study compares the role of neutral protease (NP) plus clostripain (CP) with CI as essential enzymes for human islet isolation.
Methods
Human islets were isolated using 4 different enzyme mixtures composed of CII plus either intact (CI-115) or degraded CI (CI-100). Blends were administered either with or without NP/CP. Purified islets were cultured for 3 to 4 days before islet quality assessment.
Results
Whereas using intact CI-115 without NP/CP did not significantly reduce islet yield (3429 ± 631 vs 3087 ± 970 islet equivalent/g, nonsignificant), administration of degraded CI-100 without NP/CP decreased islet yield from 3501 ± 580 to 1312 ± 244 islet equivalent/g (P < 0.01), doubled the amount of undigested tissue from 11.8 ± 1.6 to 24.4 ± 1.2% (P < 0.01) and triplicated the percentage of trapped islets from 7.7 ± 2.8 to 22.5 ± 3.6% (P < 0.05). Islet yield did not vary between supplemented CI-115 and CI-100, but was increased using CI-115 when NP/CP was omitted (P < 0.05). A trend toward higher viability and increased secretory insulin response was noted in both CI-100 and CI-115 when NP/CP was not added.
Conclusions
This study suggests that NP/CP can compensate reduced CI activity. Future attempts to optimize enzyme blends should consider the possibility to increase the proportion of collagenase CI to reduce the need for potentially harmful NPs.
Transplantation of isolated human islets offers a promising therapeutic option to restore euglycemia and to normalize pathologic alterations of the protein and lipid metabolism in patients with type 1 diabetes which is essential to ameliorate progression of nephropathy, retinopathy, and cardiovascular diseases.1-3 Moreover, islet transplantation is the only minimal invasive procedure to cure hypoglycemia unawareness in prone patients with type 1 diabetes.4,5
However, the difficulty to isolate a sufficient islet mass from a single-donor pancreas is the major obstacle for the broad application of clinical islet allotransplantation. Although donor variability and low pancreas quality significantly contribute to the inconsistency of islet isolation outcome,6-9 reliable enzyme lot-to-lot consistency may be of critical importance for isolation success.10,11
Release of islets from within the acinar tissue of the pancreas essentially requires the synergistic interplay between collagenase class I (CI), class II (CII), and clostridial neutral protease (NP) or thermolysin which degrade different collagen structures during pancreas digestion. In this interaction, CII seems to play a major role in pancreas digestion, whereas CI takes the supporting part of collagen degradation.12,13 Currently, the activity of CII is measured by the Wunsch assay, whereas the CI activity is differentiated by relating the area under the curve of high-performance liquid chromatography–eluted CII protein to the area under the curve of eluted CI protein.14 As the ratio between CI and CII activity seems to be important for effective islet isolation, any shift within this delicate balance reduces the efficiency of an enzyme blend.14,15 A significant factor that can alter the proportion of CI within a blend is the degradation of CI. On the basis of previous studies, demonstrating that large amounts of NP diminish differences in the efficiency between different ratios of CII-to-CI,16 we speculated that this discrepancy may be explained by an excessive use of supplementary NP, clostripain (CP) or thermolysin.
The present study was initiated to investigate the relation between NPs and CI to elucidate the interaction and synergism between those proteolytic enzymes in a prospective approach. The aim was to prove our hypothesis that NPs can compensate reduced CI activity to isolate significant amounts of islets from the human pancreas. To be consistent with our previous study about the effect of intact and degraded CI on islet isolation outcome,17 NP and CP were combined to serve as complementary proteases.
MATERIALS AND METHODS
Islet Isolation
Pancreata were retrieved, after research consent and ethical approval, from 24 human multiorgan donors within a period of 26 months between 2009 and 2011. Once legal consent had been given, pancreata were procured from multiorgan donors with brain death using cold perfusion with University of Wisconsin (UW) solution (ViaSpan; DuPont Pharmaceuticals Ltd., Herts, United Kingdom) or with histidine-tryptophan-ketoglutarate (Custodiol; Köhler Chemie GmbH, Alsbach, Germany). Explanted pancreata were shipped to the central isolation facility (Uppsala, Sweden) in 400 mL of cold UW or Custodiol.
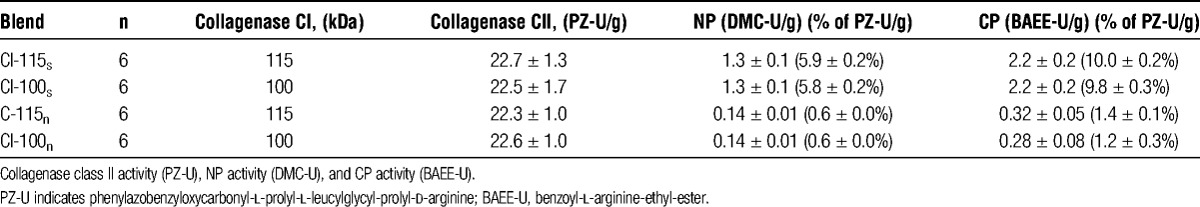
Human islets were isolated as previously described without the intention to transplant.18 The isolation procedure was always performed by the same isolation team. Briefly, after removal of the duodenum, the main pancreatic duct was cannulated at the distal part of the pancreatic head and manually distended with cold (8°C) Hank balanced salt solution (HBSS; Gibco-Invitrogen AB, Stockholm, Sweden) using a ratio of 1.2 mL/g trimmed pancreas weight. The distension solution was supplemented with collagenase, NP, and CP as shown in Table 1. The different collagenase mixtures were characterized by the presence of 22 PZ-U/g CII combined with intact or degraded CI isoforms characterized by a molecular weight of 115 kDa (CI-115) or 100 kDa (CI-100), respectively. Two lots of collagenase (LA35/10, 20090005) had to be processed to provide sufficient material of CII as well as intact CI-115 and degraded CI-100 for this study. The CII and CI, either intact or degraded, were recombined to obtain a CII-to-CI ratio of 0.7 as previously described.17 The collagenase blend CI-115s and CI-100s were completed with the same lots of 1.3 dimethyl-casein (DMC)-U/g of NP (lot 20260005) and of 2.2 benzoyl-l-arginine-ethyl-ester-U/g of CP (lot TLA 49/03) or used as nonsupplemented blends (CI-115n, CI-100n) (Table 1). Nonsupplemented blends (CI-115n, CI-100n) contained traces of NP and CP that were approximately 10-fold lower than the amount of proteases in the supplemented groups. The activities of NP and CP were also calculated as relative proportion of CII activity and are shown in brackets in Table 1. All enzymes were manufactured and provided as individual components by Serva/Nordmark Arzneimittel GmbH & Co. KG (Uetersen, Germany). The assignment of the pancreases to the experimental groups was performed in an alternating manner also considering sex and body mass index to facilitate an equal distribution of donor variables between experimental groups.
TABLE 1.
Enzyme blending
Pancreas digestion was performed in a 350-mL digestion-filtration device filled with HBSS continuously utilizing a digestion temperature of 37°C.19 During recirculation, samples were frequently assessed for amount and integrity of released islets. Digested tissue was collected in 250-mL centrifuge tubes prefilled with 25 mL of cold (4°C) newborn calf serum and centrifuged twice for 1 minute at 150×g. After 60 minutes of storage in 250 mL of 1.2-fold concentrated UW (Apoteket AB, Stockholm, Sweden)20 the digested tissue was centrifuged for 5 minutes at 3000 rpm in a Cobe 2991 cell processor (Gambro, Lakewood, CO) using a continuous hyperosmolaric Ficoll gradient for separation of islets from exocrine tissue.18 Purified islet fractions were washed twice in HBSS supplemented with 10% newborn calf serum and collected finally in 100 mL of Connaught Medical Research Laboratories 1066 supplemented with 25 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid, 1 mM pyruvate, 10 mM nicotinamide (PAA, Pasching, Austria), 10% fetal calf serum, 2.5 mM l-glutamine, 100 U/μg/mL penicillin-streptomycin (Gibco), and 20 μg/mL ciprofloxacin (Bayer, Leverkusen, Germany). Islet culture was performed in a semiclosed culture bag system (Baxter Medical AB, Stockholm, Sweden) incubated in humidified atmosphere for three to four days at 37°C.21
Islet Characterization
Subsequent to purification and after culture, yield of islet equivalents was quantified in a digital image analysis-based procedure as previously described in detail.22 As this quantification procedure does not allow assessment of unpurified islet samples, pre-purification data were not collected for the present study. Islet morphological integrity was categorized using a fragmentation score from 0 (no fragmentation) to 3 (extensive fragmentation). Isolated islets were distinguished from exocrine tissue using insulin-specific dithizone staining (Sigma-Aldrich AB, Stockholm, Sweden).23
Viability and in vitro function were assessed after 3 to 4 days of culture at 37°C. Islet viability was measured as membrane integrity using 25 μmol/L of Syto-13 (Invitrogen) and 50 μmol/L of ethidium bromide (Sigma-Aldrich) for staining of viable and dead cells, respectively.24 The fluorescence of Syto-13 and ethidium bromide was quantified at, respectively, 545 nm and 490 nm using a fluorometric plate reader.
Mitochondrial functional viability was evaluated in triplicate samples of 100 islets measuring the conversion of the tetrazolium compound 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (Promega, Mannheim, Germany) into formazan as previously described.25 Formazan formation was measured at 490 nm and expressed as optical density per ng DNA. The intracellular DNA content was determined by means of a fluorometric assay (Quant-it; Invitrogen-Molecular Probes, Stockholm, Sweden).
Islet in vitro function was assessed during static glucose incubation of islets precultured for 3 to 4 days at 37°C. Twenty hand-selected islets with an average diameter of 150 to 200 μm were sequentially incubated in duplicate first for 45 minutes in bicarbonate-free Connaught Medical Research Laboratories 1066 (Applichem GmbH, Darmstadt, Germany) supplemented with 2 mmol/L glucose followed by 45 minutes of incubation at 20 mmol/L glucose finally followed by a second 45-minute period at 2 mmol/L glucose. The glucose stimulation index was calculated by dividing the insulin release at 20 mmol/L glucose by the mean of the basal periods. After incubation, islets were recovered and sonified in acid ethanol for subsequent determination of intracellular insulin content.26 Intracellular insulin content and insulin release were normalized to islet DNA content.
Data Analysis
All statistical analyses were performed using Prism 6.0d for MacIntosh (GraphPad, La Jolla, CA). Analysis of data was carried out by the nonparametric Kruskal Wallis test followed by Dunn correction for multiple comparisons. The data for glucose-stimulated insulin release at low and high glucose concentrations were compared using the Friedman test followed by Dunn correction. Differences were considered significant at P less than 0.05. P values more than 0.05 are termed nonsignificant (NS). Results are expressed as mean ± SEM.
RESULTS
Isolation Outcome
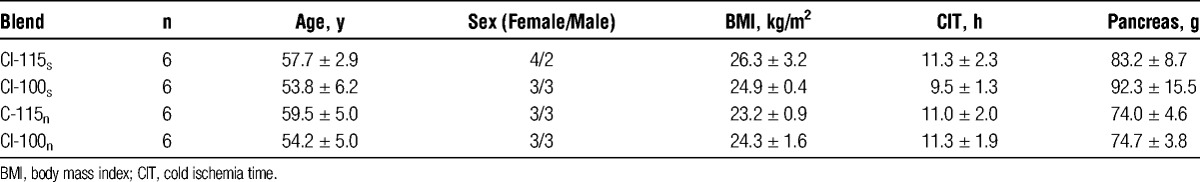
The donor characteristics are presented in Table 2. No significant differences were found between experimental groups regarding age, body mass index, sex, cold ischemia time and trimmed pancreas weight.
TABLE 2.
Donor characteristics
When comparing intact collagenase CI-115s with degraded collagenase CI-100s no significant differences were found with regard to digestion variables, such as recirculation time, packed tissue volume, proportion of undigested tissue and trapped islets (Table 3) as long as NP/CP were present during digestion. No alterations were also observed with respect to islet fragmentation, purity, total islet yield, and yield per gram trimmed pancreas tissue. In contrast, when NP/CP were absent during digestion, a trend toward a reduced proportion of trapped islets (NS vs CI-100n) and a lower fragmentation score (NS vs CI-100n) was noted when intact collagenase CI-115n was used for digestion. However, the differences between CI-100n and CI-115n regarding islet yield per gram pancreas were significant (P < 0.05 vs CI-100n). No effect was found with respect to recirculation time and amount of undigested tissue (Table 3).
TABLE 3.
Human islet isolation outcome
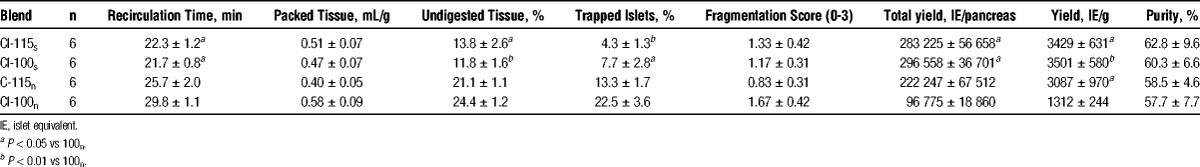
The comparison between supplemented collagenase CI-115s and nonsupplemented collagenase CI-115n demonstrated a slightly increased proportion of undigested tissue (NS vs CI-115s) and trapped islets (NS vs CI-115s) when NP/CP were not added (Table 3). Total islet yield and islet yield per gram trimmed pancreas were comparable between experimental groups.
Analyzing the isolation outcome obtained by degraded collagenase CI-100 clearly revealed that NP/CP were essentially needed to isolate acceptable islet yields per pancreas (P < 0.05 vs CI-100n) or per gram pancreas (P < 0.01 vs CI-100n). As shown in Table 3, the low islet yield obtained in the virtual absence of NP/CP correlated with the significantly increased recirculation time (P < 0.05 vs CI-100s), doubling of undigested tissue (P < 0.01 vs CI-100s) and a 3-fold increased percentage of trapped islets (P < 0.05 vs CI-100s). In contrast, none of the digestion parameters or islet yield obtained by using supplemented CI-100s was significantly altered when compared with nonsupplemented CI-115n, indicating again that the presence of NP/CP can fully compensate the lack of intact CI-115.
Islet Quality Assessment
After 3 to 4 days of culture at 37°C, islets were harvested, and quality assessment was performed. As shown in Table 4, no significant differences were observed between experimental groups in terms of islet recovery, purity, mitochondrial activity, glucose stimulation index, and intracellular insulin content comparing intact with degraded collagenase CI either in the presence (CI-115s vs CI-100s) or in the virtual absence of NP/CP (CI-115n vs CI-100n). Also, when the effect of NP/CP on intact (CI-115s vs. CI-115n) or degraded CI (CI-100s vs CI-100n) was separately analyzed, no significant variations were observed. Assessment of islet viability indicated that only islets isolated without supplementation of NP/CP had a viability that was above 70% in average. However, this trend did not reach statistical significance.
TABLE 4.
Islet quality assessment after culture
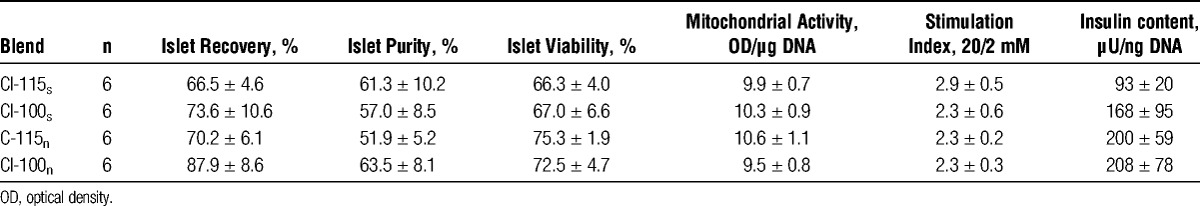
Islet in vitro function was assessed during sequential incubation at 2, 20, and again 2 mmol/L of glucose. The composition of the different enzyme blends had no significant effect on the glucose stimulation index as shown in Table 4. Nevertheless, islets isolated using NP/CP were characterized by a slightly lower intracellular insulin content compared with the other experimental groups (NS). Islets from any of the experimental groups demonstrated an insulin response toward glucose challenge. As shown in Figure 1, the magnitude of the insulin discharge varied between experimental groups. Islets secreted lower amounts of insulin during glucose challenge when isolated in the presence of NP/CP regardless whether intact collagenase CI-115s or degraded collagenase CI-100s was used for pancreas digestion (NS).
FIGURE 1.
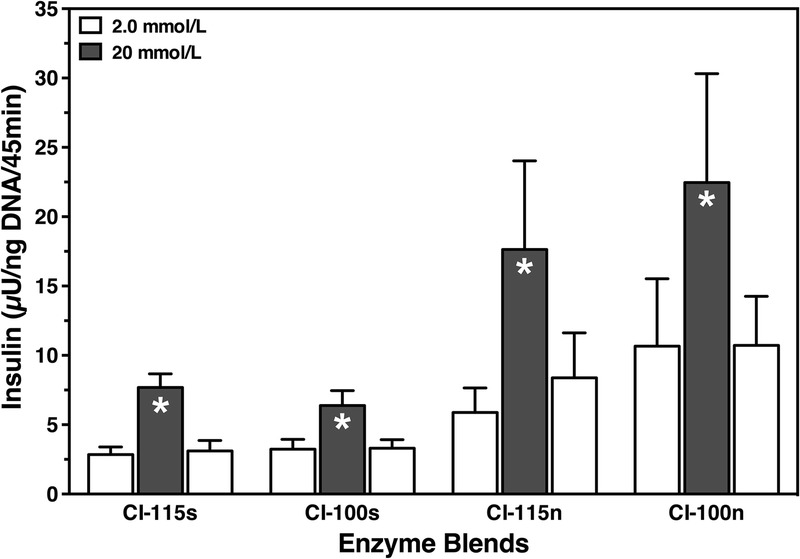
Glucose-stimulated insulin release of human islets isolated by means of intact (CI-115) or degraded (CI-100) CI in the presence (CI-115s, CI-100s) or absence (CI-115n, CI-100n) of NP and CP. After culture for 3 to 4 days at 37°C, islets were sequentially incubated for 45 minutes at 2 (white bars), 20 (grey bars), and again 2 mmol/L of glucose (n = 6). Insulin release was normalized to islet DNA content. Dunn test after Friedman test revealed *P < 0.05 for insulin secretion at 20 versus 2 mmol/L glucose as indicated.
DISCUSSION
Previous and recent studies in rats clearly suggested that the fast and effective release of islets from within the acinar tissue essentially requires the presence of collagenase CI, CII, and NPs.12,13,27 The balance between CI and CII appears to be critical for efficient islet release14 but can significantly vary from lot to lot28 and from product to product.29 An important factor that changes the proportion of CI activity is the cleavage of 1 of the 2 collagen-binding domains from the CI molecule presumably occuring during the enzyme manufacturing process.30 The loss of this collagen-binding domain does not only reduce the molecular weight of CI from 115 kDa to 100 kDa but also correlates with a 90% reduction of the specific collagen-degrading activity of CI and the reduced efficiency to successfully separate islets from the human pancreatic acinar tissue.31 However, an initial prospective study could not demonstrate any difference in human islet isolation outcome using either degraded CI-100 or intact CI-115.17 The present finding, that it is possible to successfully isolate islets from human pancreata with strongly reduced CI activity is compatible with the observation that NP can compensate a deficiency in CI activity to digest the peri-insular extracellular matrix in rat pancreata.16 Vice versa, when intact and fully active CI was used for human pancreas digestion, significant numbers of islets were isolated in the present study even in the virtual absence of NPs. These results may also explain why the amount of NPs used for complete human pancreas digestion varies enormously between different isolation centres. Unfortunately, we cannot present data collected prepurification to quantify the loss of acinar-embbeded islets during purification.
When the Wunsch assay is used to determine collagenase activity, degraded CI has a similar collagenase-specific activity compared to nondegraded CI. This corresponds to the daily practice in most islet isolation facilities where usually 1 vial of collagenase, equivalent to approximately 2000 to 2200 PZ-U, is used to digest a human pancreas. Nevertheless, the Wunsch assay mainly determines the CII activity32 and does not reflect the real collagen-degrading activity of CI when compared to more sensitive techniques.30,31 The variability among different collagenase products is illustrated by the variability in the amount of NPs that is needed for effective collagenase supplementation in different isolation centers. Human pancreas digestion protocols reveal a wide range of using NP in a range from 50 DMC-U33 to 400 DMC-U31 for collagenase supplementation. This indicates that the extensive use of supplementary NP, CP, or thermolysin diminishes differences in various collagenase blends particularly regarding the proportion of intact CI.16,17 It also explains why protocols for the enzymatic dissociation of human pancreata could not be standardized so far.
In the present study, the use of degraded CI-100, that is, the absence of fully active CI-115 prolonged recirculation time, increased percentage of trapped islets, and reduced islet yield when NP/CP were virtually absent during the digestion process. In contrast, the addition of these complementary proteases completely diminished the differences between CI-100 and CI-115, providing nearly identical results regarding isolation outcome. None of the comparisons between supplemented CI-100s and either supplemented CI-115n or nonsupplemented CI-115s revealed an extension of the digestion phase or a significant reduction of islet yield.
To draw the conclusion that the addition of large amounts of NPs improves the use of different enzyme blends may nevertheless be misleading, considering the potential toxicity of NPs for islet viability and morphological integrity.16,34-36 In the present study, a trend toward slightly decreased viability was observed when NP/CP were supplemented. In accordance, this trend was also found analyzing the in vitro function of isolated islets. Islets released lower amounts of insulin after glucose challenge when isolated in the presence of NP/CP. In contrast to previous studies, harmful effects of degraded CI-100 on morphological and functional integrity of isolated islets could not be confirmed in the present study.37
On the other hand, the data of the present study clearly imply that NPs can be completely avoided if fully active CI is provided in sufficient amounts. In agreement with our previous experiments in rats,16 the Edmonton group demonstrated an improvement of human islet isolation outcome when CI represent the majority of collagenase protein.38 Moreover, the retrospective analysis of 163 human islet isolations revealed that final islet purity significantly correlates with CI activity.32
In summary, for the first time, it is demonstrated that significant numbers of islets can be released from the human pancreas with significantly reduced CI activity provided that NP and CP are present during pancreas digestion. On the contrary, we show in older donors that significant islet yields can be isolated in the virtual absence of NPs as long as a sufficient proportion of fully active CI is used for pancreas digestion. These observations imply that NPs can compensate reduced collagenase CI activity to digest human pancreata for efficient islet release. Future efforts to optimize enzyme blends should consider the possibility to increase the proportion of collagenase CI to reduce the need for potentially harmful NPs. The findings of the present study may be a step forward to establish a standardized human islet isolation protocol.
ACKNOWLEDGMENTS
The authors thank Karin Andersson and Sana Asif for their technical excellent assistance.
Footnotes
Published online 15 December 2015.
Funding: This study was supported by grants from the Diabetes Wellness Network Sweden, the Swedish Medical Research Council (K2015-54X-12219-19-4) and the Juvenile Diabetes Foundation International.
H.B., D.B., P.R.J. and O.K. declare no conflict of interests. M.K. is employed by Nordmark Arzneimittel GmbH & Co. KG.
H.B. and D.B. participated in research design, performance of research, data analysis and in writing the article. M.K. participated in research design, enzyme production and in reviewing the article. P.R.J. participated in reviewing the article. O.K. participated in organ coordination and in reviewing the article.
REFERENCES
- 1. Luzi L, Perseghin G, Brendel MD, et al. Metabolic effects of restoring partial beta-cell function after islet allotransplantation in type 1 diabetic patients. Diabetes. 2001; 50: 277– 282. [DOI] [PubMed] [Google Scholar]
- 2. Fiorina P, Folli F, Zerbini G, et al. Islet transplantation is associated with improvement of renal function among uremic patients with type I diabetes mellitus and kidney transplants. J Am Soc Nephrol. 2003; 14: 2150– 2158. [DOI] [PubMed] [Google Scholar]
- 3. Warnock GL, Thompson DM, Meloche RM, et al. A multi-year analysis of islet transplantation compared with intensive medical therapy on progression of complications in type 1 diabetes. Transplantation. 2008; 86: 1762– 1766. [DOI] [PubMed] [Google Scholar]
- 4. Meyer C, Hering BJ, Grossmann R, et al. Improved glucose counterregulation and autonomic symptoms after intraportal islet transplants alone in patients with long-standing type I diabetes mellitus. Transplantation. 1998; 66: 233– 240. [DOI] [PubMed] [Google Scholar]
- 5. Ryan EA, Shandro T, Green K, et al. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004; 53: 955– 962. [DOI] [PubMed] [Google Scholar]
- 6. Benhamou PY, Watt PC, Mullen Y, et al. Human islet isolation in 104 consecutive cases. Factors affecting isolation success. Transplantation. 1994; 57: 1804– 1810. [PubMed] [Google Scholar]
- 7. Lakey JR, Warnock GL, Rajotte RV, et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996; 61: 1047– 1053. [DOI] [PubMed] [Google Scholar]
- 8. O'Gorman D, Kin T, Murdoch T, et al. The standardization of pancreatic donors for islet isolations. Transplantation. 2005; 80: 801– 806. [DOI] [PubMed] [Google Scholar]
- 9. Niclauss N, Bosco D, Morel P, et al. Influence of donor age on islet isolation and transplantation outcome. Transplantation. 2011; 91: 360– 366. [DOI] [PubMed] [Google Scholar]
- 10. Nano R, Clissi B, Melzi R, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005; 48: 906– 912. [DOI] [PubMed] [Google Scholar]
- 11. Hanley SC, Paraskevas S, Rosenberg L. Donor and isolation variables predicting human islet isolation success. Transplantation. 2008; 85: 950– 955. [DOI] [PubMed] [Google Scholar]
- 12. Wolters GH, Vos-Scheperkeuter GH, Lin HC, et al. Different roles of class I and class II Clostridium histolyticum collagenase in rat pancreatic islet isolation. Diabetes. 1995; 44: 227– 233. [DOI] [PubMed] [Google Scholar]
- 13. Fujio A, Murayama K, Yamagata Y, et al. Collagenase H is crucial for isolation of rat pancreatic islets. Cell Transplant. 2014; 23: 1187– 1198. [DOI] [PubMed] [Google Scholar]
- 14. Brandhorst H, Raemsch-Guenther N, Raemsch C, et al. The ratio between collagenase class I and class II influences the efficient islet release from the rat pancreas. Transplantation. 2008; 85: 456– 461. [DOI] [PubMed] [Google Scholar]
- 15. Kin T, Zhai X, Murdoch TB, et al. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Am J Transplant. 2007; 7: 1233– 1241. [DOI] [PubMed] [Google Scholar]
- 16. Brandhorst H, Alt A, Huettler S, et al. The ratio between class II and class I collagenase determines the amount of neutral protease activity required for efficient islet release from the rat pancreas. Transplant Proc. 2005; 37: 215– 216. [DOI] [PubMed] [Google Scholar]
- 17. Brandhorst H, Asif S, Andersson K, et al. The effect of truncated collagenase class I isomers on human islet isolation outcome. Transplantation. 2010; 90: 334– 335. [DOI] [PubMed] [Google Scholar]
- 18. Brandhorst H, Brandhorst D, Brendel MD, et al. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 1998; 7: 489– 495. [DOI] [PubMed] [Google Scholar]
- 19. Ricordi C, Lacy PE, Finke EH, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988; 37: 413– 420. [DOI] [PubMed] [Google Scholar]
- 20. Robertson GS, Chadwick D, Contractor H, et al. Storage of human pancreatic digest in University of Wisconsin solution significantly improves subsequent islet purification. Br J Surg. 1992; 79: 899– 902. [DOI] [PubMed] [Google Scholar]
- 21. Goto M, Eich TM, Felldin M, et al. Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation. 2004; 78: 1367– 1375. [DOI] [PubMed] [Google Scholar]
- 22. Friberg AS, Brandhorst H, Buchwald P, et al. Quantification of the islet product: presentation of a standardized current good manufacturing practices compliant system with minimal variability. Transplantation. 2011; 91: 677– 683. [DOI] [PubMed] [Google Scholar]
- 23. Ricordi C, Gray DW, Hering BJ, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990; 27: 185– 195. [DOI] [PubMed] [Google Scholar]
- 24. Barnett MJ, McGhee-Wilson D, Shapiro AM, et al. Variation in human islet viability based on different membrane integrity stains. Cell Transplant. 2004; 13: 481– 488. [DOI] [PubMed] [Google Scholar]
- 25. Brandhorst D, Iken M, Tanioka Y, et al. Influence of collagenase loading on long-term preservation of pig pancreas by the two-layer method for subsequent islet isolation. Transplantation. 2005; 79: 38– 43. [DOI] [PubMed] [Google Scholar]
- 26. Brandhorst H, Asif S, Andersson K, et al. A new oxygen carrier for improved long-term storage of human pancreata before islet isolation. Transplantation. 2010; 89: 155– 160. [DOI] [PubMed] [Google Scholar]
- 27. Vos-Scheperkeuter GH, van Suylichem PT, Vonk MW, et al. Histochemical analysis of the role of class I and class II Clostridium histolyticum collagenase in the degradation of rat pancreatic extracellular matrix for islet isolation. Cell Transplant. 1997; 6: 403– 412. [DOI] [PubMed] [Google Scholar]
- 28. Barnett MJ, Zhai X, LeGatt DF, et al. Quantitative assessment of collagenase blends for human islet isolation. Transplantation. 2005; 80: 723– 728. [DOI] [PubMed] [Google Scholar]
- 29. Balamurugan AN, Loganathan G, Bellin MD, et al. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012; 93: 693– 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCarthy RC, Breite AG, Green ML, et al. Tissue dissociation enzymes for isolating human islets for transplantation: factors to consider in setting enzyme acceptance criteria. Transplantation. 2011; 91: 137– 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balamurugan AN, Breite AG, Anazawa T, et al. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 2010; 89: 954– 961. [DOI] [PubMed] [Google Scholar]
- 32. Antonioli B, Fermo I, Cainarca S, et al. Characterization of collagenase blend enzymes for human islet transplantation. Transplantation. 2007; 84: 1568– 1575. [DOI] [PubMed] [Google Scholar]
- 33. Cross SE, Hughes SJ, Clark A, et al. Collagenase does not persist in human islets following isolation. Cell Transplant. 2012; 21: 2531– 2535. [DOI] [PubMed] [Google Scholar]
- 34. Wolters GH, Vos-Scheperkeuter GH, van Deijnen JH, et al. An analysis of the role of collagenase and protease in the enzymatic dissociation of the rat pancreas for islet isolation. Diabetologia. 1992; 35: 735. [DOI] [PubMed] [Google Scholar]
- 35. Brandhorst H, Brendel MD, Eckhard M, et al. Influence of neutral protease activity on human islet isolation outcome. Transplant Proc. 2005; 37: 241– 242. [DOI] [PubMed] [Google Scholar]
- 36. Bucher P, Bosco D, Mathe Z, et al. Optimization of neutral protease to collagenase activity ratio for islet of Langerhans isolation. Transplant Proc. 2004; 36: 1145– 1146. [DOI] [PubMed] [Google Scholar]
- 37. Brandhorst H, Raemsch-Guenther N, Raemsch C, et al. Degraded collagenase deteriorates islet viability. Transplant Proc. 2008; 40: 370– 371. [DOI] [PubMed] [Google Scholar]
- 38. Kin T, Zhai X, O'Gorman D, et al. Detrimental effect of excessive collagenase class II on human islet isolation outcome. Transpl Int. 2008; 21: 1059– 1065. [DOI] [PubMed] [Google Scholar]


